Introduction
In this paper, we continue the characterisation of new arsenate mineral species found in the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N, 160°14′E, 1200 m a.s.l.). Nineteen new minerals have been described in the previous articles of this series: yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016b), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018c), arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019a), the two isostructural minerals edtollite K2NaCu5Fe3+O2(AsO4)4 and alumoedtollite K2NaCu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Ksenofontov, Pautov, Sidorov, Britvin, Vigasina and Pushcharovsky2019b), anatolyite Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Pekov et al., Reference Pekov, Lykova, Yapaskurt, Belakovskiy, Turchkova, Britvin, Sidorov and Scheidl2019c), zubkovaite Ca3Cu3(AsO4)4 (Pekov et al., Reference Pekov, Lykova, Agakhanov, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Scheidl2019d), pansnerite K3Na3Fe3+6(AsO4)8 (Pekov et al., Reference Pekov, Zubkova, Koshlyakova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Britvin, Turchkova, Sidorov and Pushcharovsky2020a), badalovite NaNaMg(MgFe3+)(AsO4)3 (Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2020b), calciojohillerite NaCaMgMg2(AsO4)3 (Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2021a), and yurgensonite K2SnTiO2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Pushcharovsky2021b).
This paper is devoted to a new representative of the alluaudite group. This mineral, with the end-member formula NaCa2Mg2(AsO4)3, is chemically the same as the garnet-supergroup arsenate berzeliite, ideally (Ca2Na)Mg2(AsO4)3 (Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013). For this reason, we have chosen to name the new mineral paraberzeliite, from the Greek παρα for ‘near’ and the dimorphism with berzeliite. According to the nomenclature of alluaudite-group arsenates (Hatert, Reference Hatert2019), the idealised formula of paraberzeliite is written as NaCaCaMg2(AsO4)3.
Both the new mineral and its name (symbol Pbzl) have been approved by the IMA Commission on New Minerals, Nomenclature and Classification, IMA2018-001 (Pekov et al., Reference Pekov, Koshlyakova, Belakovskiy, Vigasina, Zubkova, Agakhanov, Britvin, Sidorov and Pushcharovsky2018a). The holotype specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow with the catalogue number 96193.
Occurrence and general appearance
The general characterisation of Arsenatnaya, the most mineralogically prolific of Tolbachik fumaroles, and data on the mineral parageneses zonation and distribution in this active, hot fumarole have been reported in previous papers (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b; Shchipalkina et al., Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020).
Paraberzeliite is a rare mineral found only in several specimens. The sample which became the holotype of the new mineral (Table 1, #1) was collected by us in July 2016 from the deepest, so-called anhydrite zone of the Arsenatnaya fumarole, situated at the depth of 3–4 m from the surface. In July 2017, in the same zone we found a Mn-enriched variety of paraberzeliite (Table 1, #2). Both Fe-enriched and V- and Mn-depleted varieties of the mineral (Table 1, ##3 and 4) were detected in specimens from the lower part of the middle, so-called polymineralic zone (depth of ~2 m from the surface) of the fumarole. Temperatures measured by us using a chromel–alumel thermocouple at the time of collecting (in 2015–2018) in different pockets varied from 360–380°C (middle, polymineralic zone) to 450–490°C (deepest, anhydrite zone). In both zones, paraberzeliite is a minor constituent of sublimate encrustations formed in open space in cracks and cavities (fumarole chambers). The mineral deposited presumably at temperatures not lower than 500°C. It crystallised directly from the gas phase or, more probably, formed as a result of the interaction between hot volcanic gas and basalt scoria which could be a source of Mg and Ca, the components with very low volatilities in such post-volcanic systems (Symonds and Reed, Reference Symonds and Reed1993).
Table 1. Chemical composition of paraberzeliite.
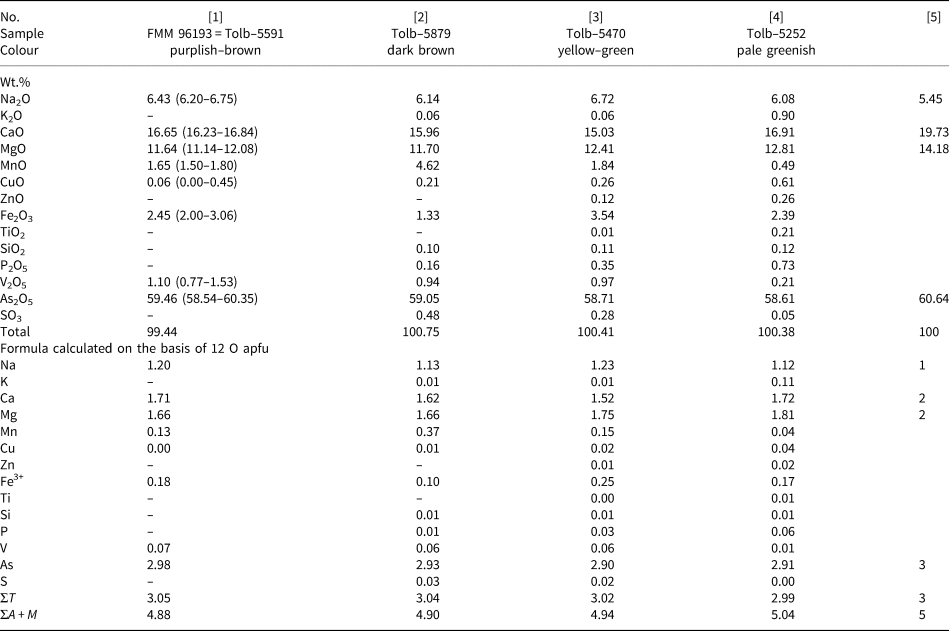
[1] holotype (average for seven spot analyses, ranges are in parentheses), [2] Mn-enriched variety, [3] Fe-enriched variety, [4] Mn- and V-depleted variety, [5] NaCa2Mg2(AsO4)3.
Dash indicates that the content is below the detection limit. ΣT = Si + P+V + As + S; ΣA + M = Na + K + Ca + Mg + Mn + Cu + Zn + Fe + Ti.
In the anhydrite zone, paraberzeliite occurs in crusts mainly consisting of anhydrite with subordinate amounts of diopside, hematite, svabite and garnets of the berzeliite–schäferite series. Other associated minerals here are calciojohillerite, magnesioferrite, ludwigite, fluorapatite (As- and V-bearing variety), powellite, baryte, and rhabdoborite-group and aphthitalite-group members. In the lower part of the polymineralic zone, paraberzeliite is found in thin encrustations, which mainly consist of hematite and the arsenates calciojohillerite, badalovite, johillerite, nickenichite, tilasite and svabite. Also, in this association, fluorophlogopite, sanidine, cassiterite, anhydrite, metathénardite and belomarinaite occur.
Paraberzeliite typically forms coarse, distorted prismatic crystals up to 0.2 mm × 0.2 mm × 1 mm, often occurring in bush-like open-work aggregates, in intimate intergrowths with svabite (Fig. 1), up to 3 mm × 5 mm. Well-formed, sometimes doubly-terminated, multifaceted prismatic crystals (Fig. 2a) up to 0.15 mm long and aggregates thereof (Fig. 2b) were also observed. Paraberzeliite crystals generally contain numerous micro-inclusions of other arsenates and hematite.

Fig. 1. Open-work clusters of purplish-brown coarse prismatic, distorted crystals of paraberzeliite (holotype, catalogue number 96193) with very pale cream-coloured svabite aggregates and minor iron-black hematite; equant to tabular hexagonal whitish aphthitalite crystals overgrow aggregates of arsenates. Field of view width is 5.7 mm. Photo: I.V. Pekov & A.V. Kasatkin.
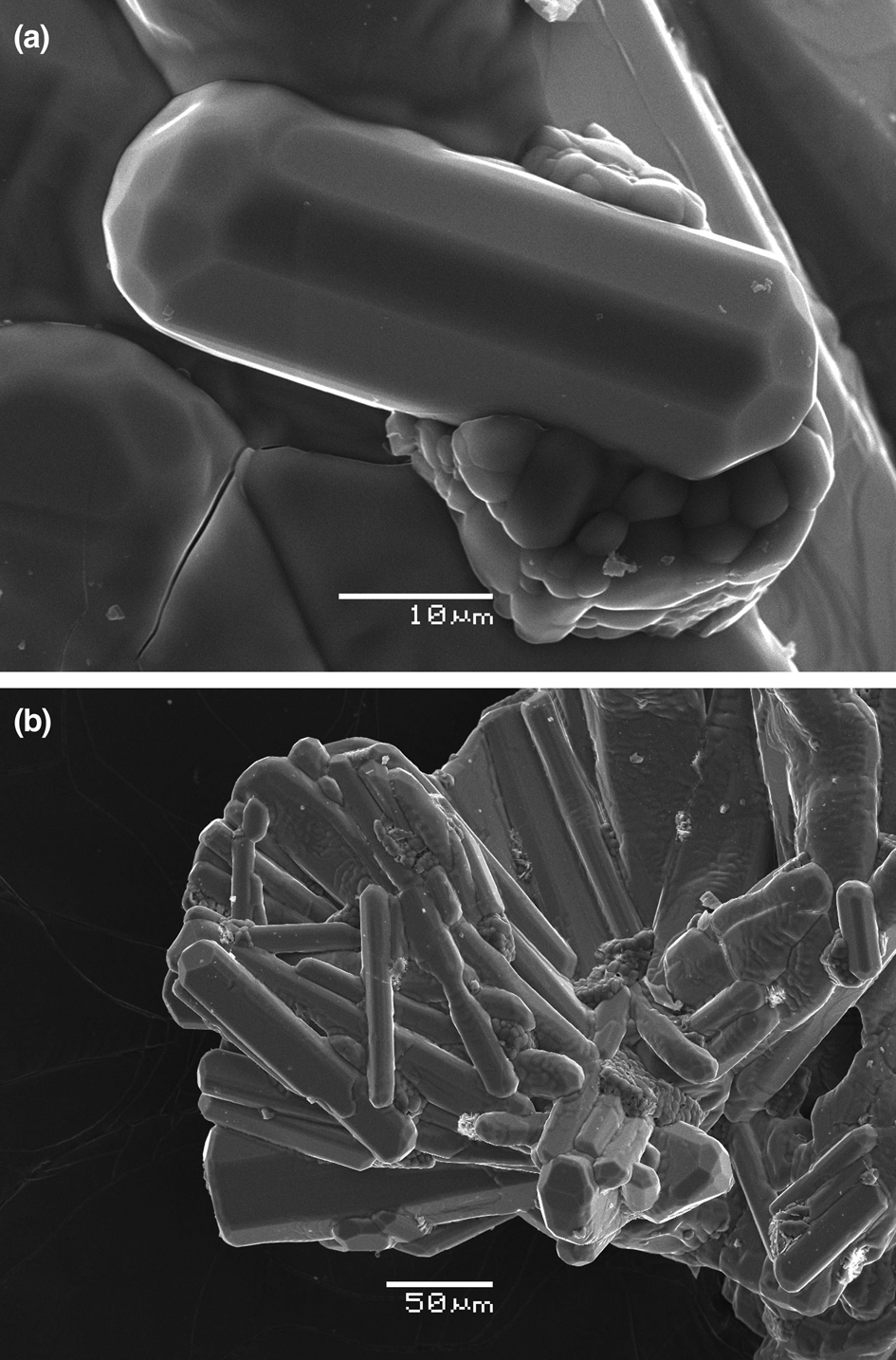
Fig. 2. Multifaceted doubly-terminated prismatic crystal (a) and aggregate of prismatic crystals (b) of paraberzeliite (holotype, catalogue number 96193) overgrowing svabite. SEM (SE) images.
Physical properties and optical data
In the holotype specimen, paraberzeliite is purplish-brown (Fig. 1) to brownish-purple, light brown or red–brown. The Mn-enriched variety is dark-brown to brown. The crystals from the polymineralic zone have saturated yellow–green (the Fe-richest variety) to pale greenish colour. Paraberzeliite is transparent, its streak is pale brownish to pale pinkish and the lustre is vitreous. Paraberzeliite is brittle, cleavage was not observed. The fracture is uneven. The Mohs hardness is ca. 3½. The density calculated using the empirical formula is 3.811 g cm–3.
In plane polarised transmitted light, paraberzeliite exhibits weak pleochroism with the following absorption scheme: Y (greyish purple) > Z (very pale yellowish) > X (colourless). It is optically biaxial (+), α = 1.718(4), β = 1.728(4), γ = 1.742(4) (589 nm), 2Vmeas. = 85(5)° and 2Vcalc. = 81°. Dispersion of optical axes is very strong, r < v. The partially determined optical orientation is Y = b.
Raman spectroscopy
The Raman spectrum of the holotype paraberzeliite (Fig. 3) was obtained on a randomly oriented crystal using an EnSpectr R532 instrument with a green laser (532 nm) at room temperature. The output power of the laser beam was ~10 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 4000 to 100 cm–1 with the use of a holographic diffraction grating with 1800 lines per cm–1 and a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~5 μm. The back-scattered Raman signal was collected with a 40× objective; signal acquisition time for a single scan of the spectral range was 1500 ms and the signal was averaged over 20 scans.
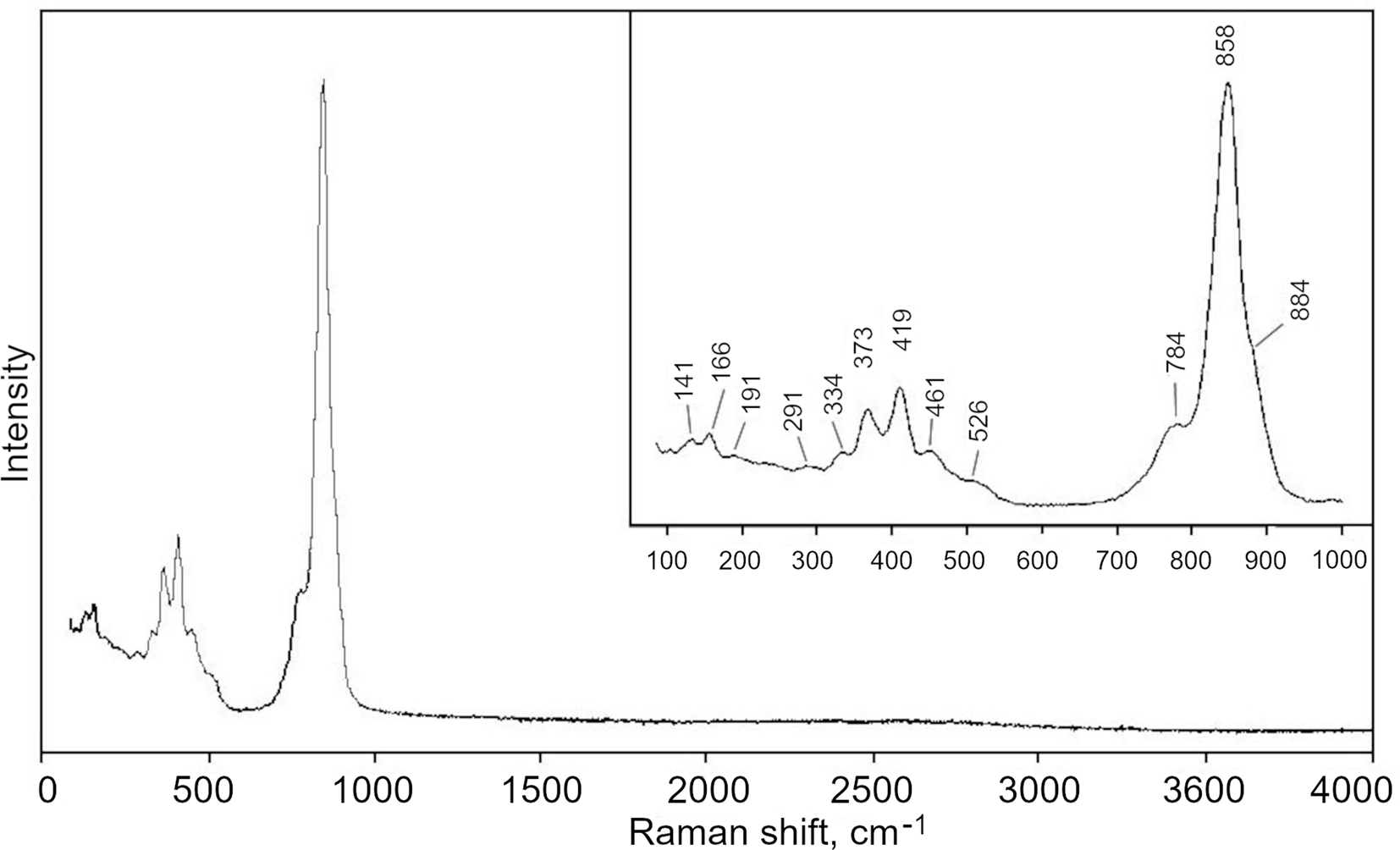
Fig. 3. The Raman spectrum of paraberzeliite.
The bands in the Raman spectrum of paraberzeliite were assigned according to Nakamoto (Reference Nakamoto1986). The bands with maxima at 884, 858 (the strongest band) and 784 cm–1 correspond to As5+–O stretching vibrations of AsO43– anions and the weak band with maximum at 526 cm–1 probably corresponds to Fe3+–O stretching vibrations from the small amount of admixed Fe3+. Bands with frequencies lower than 470 cm–1 correspond to bending vibrations of AsO4 tetrahedra, Mg–O stretching vibrations and lattice modes. The absence of bands with frequencies higher than 900 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds.
Chemical composition
The chemical composition of paraberzeliite was studied using a Jeol JSM–6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA and a 10 μm beam diameter. The following standards were used: jadeite (Na and Si), KTiOPO4 (K, Ti and P), wollastonite (Ca), olivine (Mg), MnTiO3 (Mn), Cu (Cu), ZnS (Zn and S), FeS2 (Fe), V (V) and GaAs (As). Contents of other elements with atomic numbers higher than carbon were below detection limits.
The chemical compositions of different varieties of paraberzeliite are given in Table 1. The empirical formula of the holotype specimen calculated on the basis of 12 O atoms per formula unit (apfu) is (Na1.20Ca1.71Mg1.66Mn0.13Fe3+0.18)Σ4.88(As2.98V0.07)Σ3.05O12. Taking into account all analyses reported in Table 1 and structural data for the holotype (see below), the simplified formula of paraberzeliite can be presented as (Na,Ca)(Ca,Na)(Ca,Mn)(Mg,Fe3+,Mn)2(AsO4)3 and the idealised, end-member formula is NaCaCaMg2(AsO4)3.
X-ray crystallography and crystal structure determination
The powder X-ray diffraction (XRD) data for holotype paraberzeliite (Table 2) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with cylindrical image plate detector (radius 127.4 mm) using Debye–Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and 12 min exposure. Angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). Parameters of the monoclinic unit cell refined from the powder data are: a = 12.313(8), b = 13.077(3), c = 6.772(4) Å, β = 113.75(4)° and V = 998(1) Å3.
Table 2. Powder X-ray diffraction data of paraberzeliite.
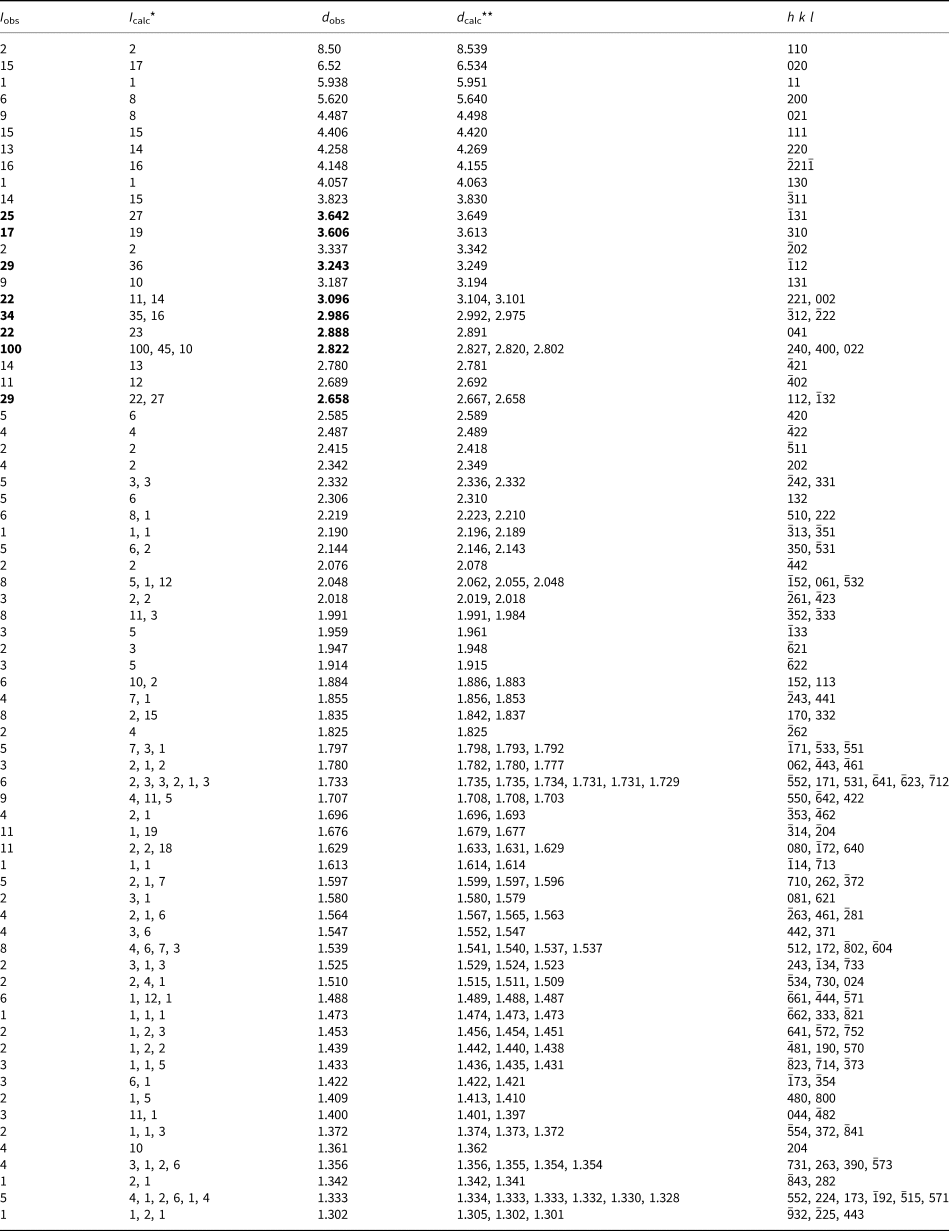
*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data; the strongest reflections are marked in bold type.
Single-crystal XRD studies of the holotype paraberzeliite were performed using an Xcalibur S diffractometer equipped with a CCD detector. A full sphere of three-dimensional data was collected. Intensity data were corrected for Lorentz and polarisation effects. The crystal structure of the new mineral was solved by direct methods and refined using the SHELX software package (Sheldrick, Reference Sheldrick2015). Crystal data, data collection information and structure refinement details are given in Table 3, coordinates and thermal displacement parameters of atoms and bond-valence sums in Table 4 and selected interatomic distances in Table 5. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystal data, data collection information and structure refinement details for paraberzeliite.

Table 4. Coordinates, equivalent displacement parameters (U eq, Å2) and bond-valence sums (BVS) for atoms in the structure of paraberzeliite.
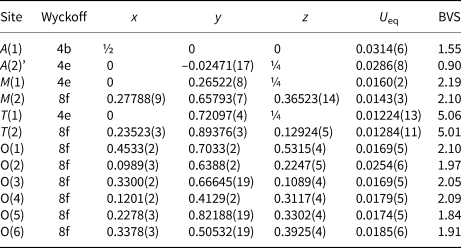
Bond-valence parameters were taken from Brese and O'Keefe (Reference Brese and ÒKeeffe1991). Bond-valence sums were calculated taking into account cation distribution (see Table 3).
Table 5. Selected interatomic distances (Å) in the structure of paraberzeliite.
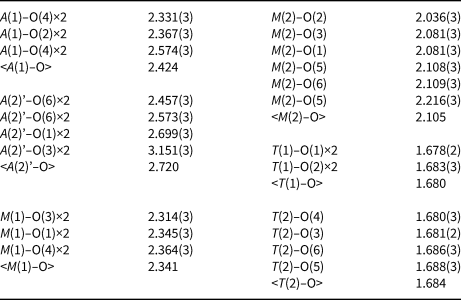
Unit-cell parameters obtained from the single-crystal XRD data for the Fe-rich variety of paraberzeliite (sample Tolb–5470: Table 1, #3) are: a = 12.307(17), b = 13.015(14), c = 6.758(11) Å, β = 113.52(18)° and V = 993(2) Å3.
Discussion
Paraberzeliite possesses the alluaudite-type structure based on a three-dimensional heteropolyhedral pseudo-framework composed of zig-zag chains of edge-sharing M(1)O6 and M(2)O6 octahedra connected with T(1)O4 and T(2)O4 tetrahedra (Fig. 3a). The M-octahedral chains consist of [M(2)2O10] dimers of distorted M(2)O6 octahedra connected via distorted M(1)O6 octahedra isolated from one another (Fig. 4). T(1)O4 tetrahedra share all vertices with the M-centred octahedra to form the (010) heteropolyhedral layers, while each T(2)O4 tetrahedron shares three vertices with the MO6 octahedra of one layer and the fourth vertex with the octahedron of adjacent layer, thus linking the layers to a three-dimensional M–T–O pseudo-framework. Large-cation positions A(1) and A(2)' are situated in channels of two types running through the pseudo-framework parallel to [001] (Fig. 3a). The first channel can be described as a chain of distorted cubes A(1)O8 sharing common faces, and the second as a chain of A(2)'O8 polyhedra connected via common edges (Fig. 4). The M and A cation sites are labelled according to Hatert (Reference Hatert2019).
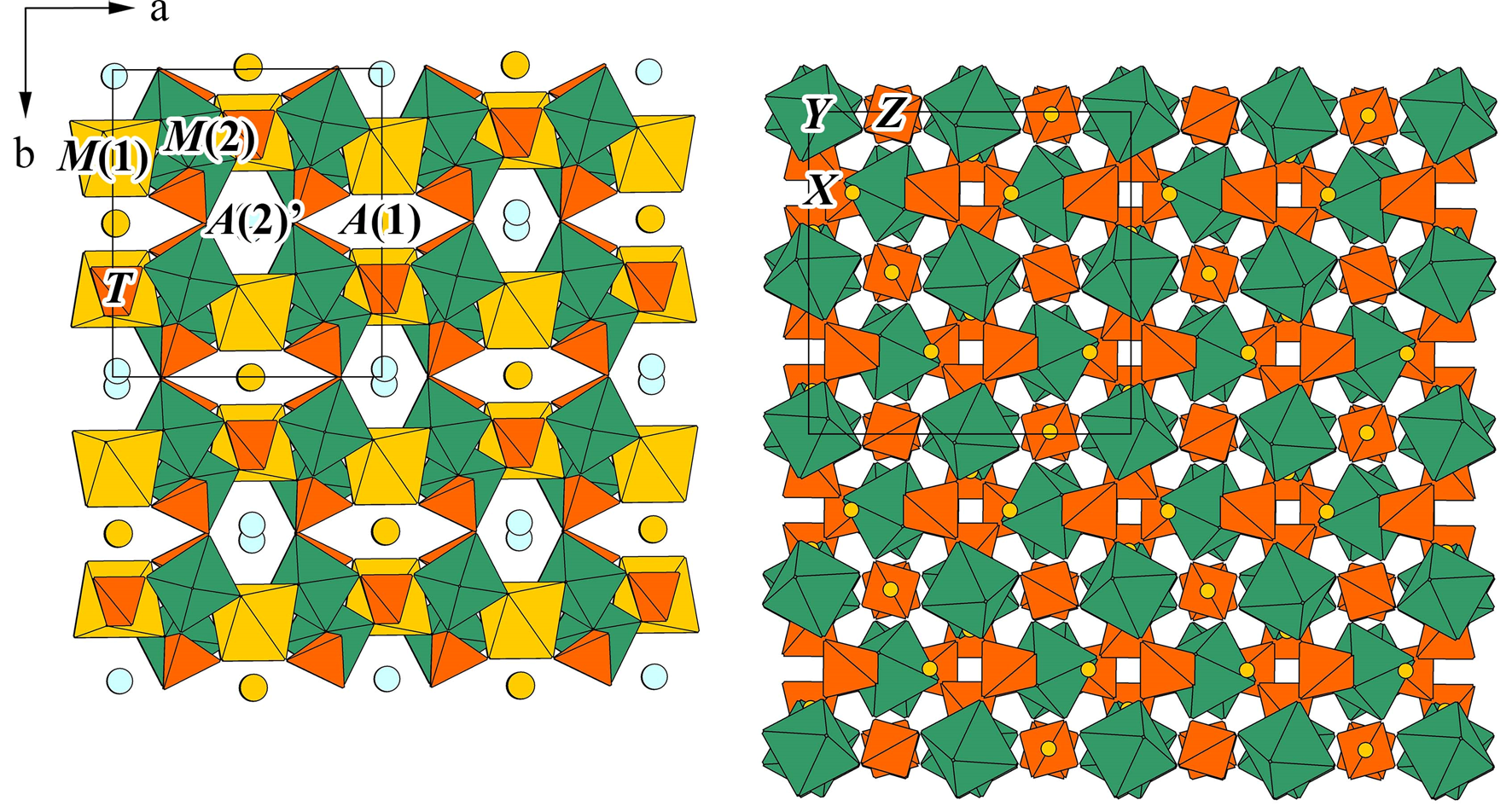
Fig. 4. Crystal structures of dimorphous paraberzeliite (left) and berzeliite (right, drawn based on data of Hawthorne (Reference Hawthorne1976); the X site marked as yellow circle contain Ca and Na, Y – Mg and Z – As5+). Unit cells are outlined.
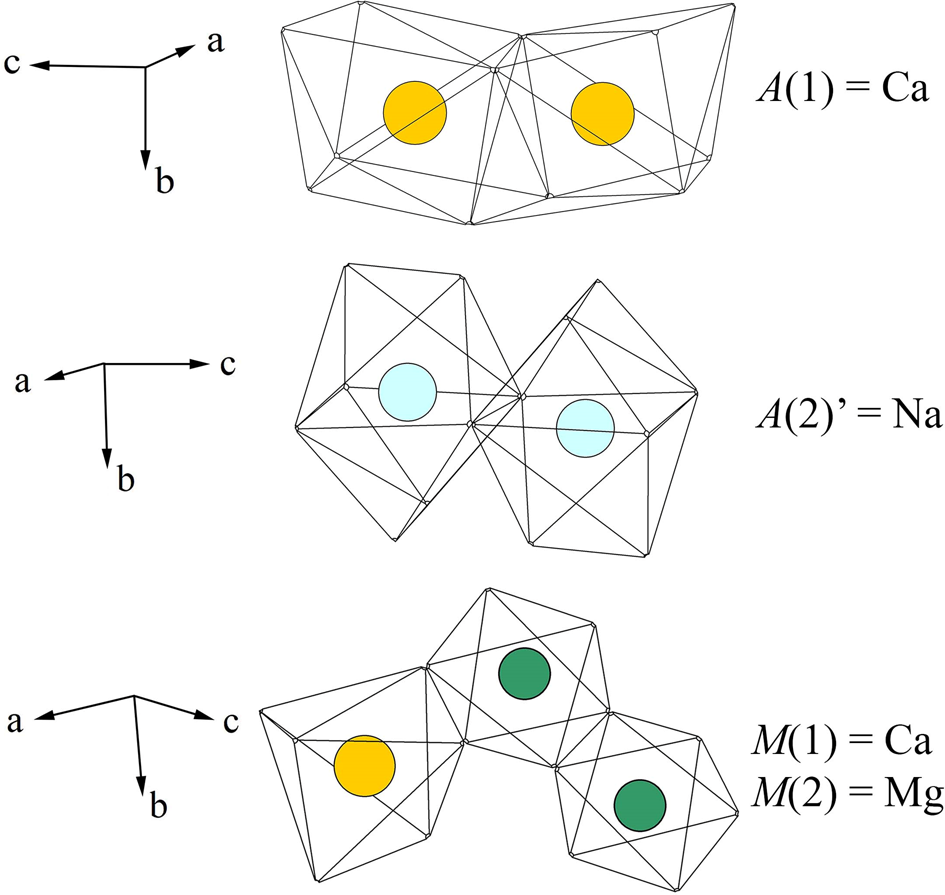
Fig. 5. Motifs of AO8 and MO6 polyhedra in the crystal structure of paraberzeliite. Colours of circles showing different cations correspond to Fig. 4, left.
The refinement of the site occupancies in holotype paraberzeliite was performed taking into account electron microprobe data (Table 1, #1); minor admixture of V in the T sites was ignored and Na vs. Ca were refined for both A positions. The refinement of M(1) site occupancy with the Ca scattering curve showed 1.01 apfu Ca (probably due to very minor Mn admixture) and was fixed at 1.0 Ca for further refinement. Mg, Fe3+ and Mn were assigned to the M(2) site with the sum of site occupation factors restrained to be 1.0. The ratio between Na+ and Fe3+ was assumed from the charge-balance requirement, taking into account the only significant heterovalent substitution (see below), which leads to the series with the general simplified formula Na1+xCa2(Mg,Mn)2–xFe3+x(AsO4)3.
In paraberzeliite, the A(1) site is predominantly occupied by Ca and A(2)' by Na. The average A(1)–O distance is 2.42 and A(2)'–O = 2.72 Å. None of the other sites in the channels known in natural or synthetic alluaudite-type compounds [A(1)', A(1)'', A(2), A(2)' and A(2)'': Krivovichev et al., Reference Krivovichev, Vergasova, Filatov, Rybin, Britvin and Ananiev2013; Ðorðevic et al., Reference Ðorðevic, Wittwer and Krivovichev2015] are occupied. The M(1) site is occupied by Ca [M(1)–O = 2.34 Å]. The M(2) site is occupied by Mg with admixtures of Fe3+ and Mn [M(2)–O = 2.11 Å].
The full crystal chemical formula of the structurally studied paraberzeliite crystal can be written as (□ = vacancy): A (1)(Ca0.60Na0.40)Σ1.00 A (1)'□ A (1)''□2 A (2)□ A (2)'(Na0.81Ca0.19)Σ1.00 A (2)''□2 M (1)Ca1.00 M (2)(Mg1.55Mn0.24Fe0.21)Σ2.00 TAs3.0O12 (Tables 3 and 5), which is close to the average composition of the holotype sample (Table 1, #1). The structural formula of paraberzeliite simplified to the species-defining constituents is A (1)CaA (2)'NaM (1)CaM (2)Mg2(TAsO4)3=A[NaCa]M[CaMg2](TAsO4)3 = NaCaCaMg2(AsO4)3.
Unlike paraberzeliite, in the structure of its analogue with Mn prevailing in the M(2) site, caryinite, ideally NaCa2Mn2(AsO4)3, ACa is concentrated in the A(1)'' site but not in A(1). Thus, the simplified structural formula of caryinite is A (1)''CaA (2)'NaM (1)CaM (2)Mn2(TAsO4)3. Notably, caryinite from a hydrothermal mineral assemblage in the famous Långban skarn deposit in Varmland, Sweden, contains significant admixture of Mg concentrated in the M(2) site: M (2)(Mn1.45Mg0.54Fe0.01)Σ2.00 (Ercit, Reference Ercit1993). Some specimens of paraberzeliite contain up to 0.4 apfu Mn (Table 1, #2) and the existence of a hypothetical isomorphous series between caryinite and paraberzeliite is possible, such as is known for the Långban series between berzeliite (Ca2Na)Mg2(AsO4)3 and manganberzeliite (Ca2Na)Mn2(AsO4)3 (Holtstam and Langhof, Reference Holtstam and Langhof1999); the arsenate garnets are dimorphous with these alluaudite-group minerals. A related pair of alluaudite-group minerals, arseniopleite NaCaMn3(AsO4)3 (the sample from the Långban-type deposit Sjö in Sweden was studied: Tait and Hawthorne, Reference Tait and Hawthorne2003) and calciojohillerite NaCaMg3(AsO4)3 (from the Arsenatnaya fumarole: Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2021a), demonstrates the same features of ACa distribution: their simplified structural formulae are A (1)''Ca A (2)'NaM (1)MnM (2)Mn2(AsO4)3 and A (1)CaA (2)'NaM (1)MgM (2)Mg2(AsO4)3, respectively.
The synthetic analogue of the end-member paraberzeliite was reported and dimorphism between alluaudite- and garnet-type synthetic arsenates with the same general formula NaCa2M 2+2(AsO4)3 (M = Mg, Ni and Co) was discussed by Khorari et al. (Reference Khorari, Rulmont and Tarte1997).
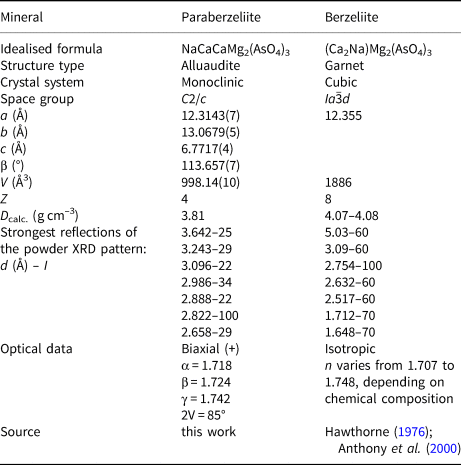
The garnet-type structure of berzeliite X(Ca2Na)YMg2(ZAsO4)3 is significantly denser compared to that of paraberzeliite. In particular, the distances (Ca,Na)–O in the dodecahedrally coordinated X site is 2.47 Å and Mg–O in the octahedrally coordinated Y site is 2.10 Å (Hawthorne, Reference Hawthorne1976). The unit-cell volume of berzeliite is 1886 Å3 (Z = 8), whereas the corresponding, i.e. doubled value of unit-cell volume of paraberzeliite is 998 × 2 = 1996 Å3. These minerals significantly differ from one another in powder XRD patterns, physical properties including optical data (Table 6) and crystal morphology. Notably, in the Arsenatnaya fumarole, these dimorphs, even though they occur together in the anhydrite zone, clearly differ from one another in colour: paraberzeliite is brown (from light to dark brown, sometimes with purple or red hue) or brownish-purple, whereas berzeliite is yellow (from orange- or lemon-yellow to pale yellowish, almost colourless) or bright orange.
Table 6. Comparative data for paraberzeliite and berzeliite.
Garnets of the continuous isomorphous series berzeliite (Ca2Na)Mg2(AsO4)3 – schäferite (Ca2Na)Mg2(VO4)3 from the Arsenatnaya fumarole have been studied by us in detail (Koshlyakova et al., Reference Koshlyakova, Pekov, Zubkova, Agakhanov, Turchkova, Kartashov, Sidorov and Pushcharovsky2020). Berzeliite from this locality differs distinctly from paraberzeliite in the composition of impurities. Berzeliite contains very few admixed X and Y constituents; in particular, it is Mn- and Fe-poor (0.1–0.5 wt.% MnO and 0.00–0.5 wt.% Fe2O3 that corresponds to 0.01–0.03 apfu Mn and 0.00–0.03 apfu Fe) and the K content in all analyses of these garnets is below the detection limit. Compared to paraberzeliite, berzeliite is Si-enriched: in the majority of samples of this garnet, SiO2 content varies from 0.4 to 2.0 wt.% (= 0.03–0.17 apfu Si) and in some cases reaches 3.8 wt.% (= 0.35 apfu Si). The Na content in Tolbachik berzeliite is less than 1.0 apfu (in the Si-enriched variety it decreases to 0.70 apfu as a result of the major heterovalent substitution TSi4+ + XCa2+ → TAs5+ + XNa+), whereas in all the samples of paraberzeliite investigated, the Na content is higher than 1.1 apfu due to the major heterovalent substitution MFe3+ + ANa+ → MMg2+ + ACa+ [a hypothetic isomorphous series paraberzeliite NaCaCaMg2(AsO4)3 – magnesiohatertite NaNaCa(MgFe3+)(AsO4)3 can be suggested]. Correspondingly, the Ca content in berzeliite from Arsenatnaya is higher than 1.95 apfu (typically > 2.0 apfu), whereas in paraberzeliite it is less than 1.75 apfu.
Both berzeliite and paraberzeliite occur in the same paragenesis in the anhydrite zone of the Arsenatnaya fumarole; however, the former is an abundant mineral here, sporadically composing up to 10 vol.% of fumarolic encrustations, whereas the latter is rare and was only detected in small amounts in several specimens. The dense garnet-type structure of berzeliite is probably more stable than the alluaudite-type structure under the high-temperature and low-pressure conditions characteristic of fumarolic systems.
Acknowledgements
We thank referees Anthony R. Kampf and Peter Leverett for their valuable comments. This study was supported by the Russian Science Foundation, grant no. 19-17-00050. The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.82













