Introduction
This paper continues the series of articles devoted to new arsenate minerals discovered in the active Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N 160°14′E, 1200 m a.s.l.). Eighteen new species were described in previous articles of this series, namely yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), ericlaxmanite and kozyrevskite, two polymorph modifications of Cu4O(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a), shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 related to one another in terms of crystal chemistry (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016b), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018b), arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019a), edtollite K2NaCu5Fe3+O2(AsO4)4 and its Al-dominant analogue alumoedtollite K2NaCu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Ksenofontov, Pautov, Sidorov, Britvin, Vigasina and Pushcharovsky2019b), anatolyite Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Pekov et al., Reference Pekov, Lykova, Yapaskurt, Belakovskiy, Turchkova, Britvin, Sidorov and Scheidl2019c), zubkovaite Ca3Cu3(AsO4)4 (Pekov et al., Reference Pekov, Lykova, Agakhanov, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Scheidl2019d), pansnerite K3Na3Fe3+6(AsO4)8 (Pekov et al., Reference Pekov, Zubkova, Koshlyakova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Britvin, Turchkova, Sidorov and Pushcharovsky2020a), badalovite NaNaMg(MgFe3+)(AsO4)3 (Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2020b) and calciojohillerite NaCaMgMg2(AsO4)3 (Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2021).
In this paper, we characterise the new mineral yurgensonite, ideally K2SnTiO2(AsO4)2, the first natural arsenate with species-defining tin, and the isomorphous series between yurgensonite and katiarsite.
The new mineral is named in honour of the Russian mineralogist, geochemist and specialist in studies of ore deposits Professor Georgiy Aleksandrovich Yurgenson (born 1935), an Honorary member of the Russian Mineralogical Society. He works in the Institute of Natural Resources, Ecology and Cryology of the Siberian Branch of the Russian Academy of Sciences, Chita, Russia. Prof. Yurgenson made a significant contribution to the mineralogy and geochemistry of tin deposits of the Transbaikal Region, South Siberia, and the geochemistry of arsenic in the oxidation zone of ore deposits.
Both the new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification, IMA2019–059 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Pushcharovsky2019e). The type specimens are deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow with the catalogue numbers 96702 and 96703.
Occurrence and general appearance
The Arsenatnaya fumarole, one of the most strongly mineralised and mineralogically interesting fumaroles at the Tolbachik volcano, has been described, including the data on zonation in distribution of mineral associations, in previous literature (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a; Shchipalkina et al., Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020).
All members of the isomorphous series between yurgensonite and katiarsite are minor components of the arsenate mineralisation occurring in the so-called polymineralic zone of the Arsenatnaya fumarole. They were found at a depth of 1–1.5 m from the day surface. Specimens with yurgensonite and a Sn-rich variety of katiarsite were collected by us in July 2018 from the north part of the fumarole. Temperatures measured by us using a chromel–alumel thermocouple in pockets with these minerals at the time of collecting were 360–400°C. Minerals of the katiarsite–yurgensonite series were deposited directly from the gas phase as a volcanic sublimates or, more probably, formed as a result of the interaction between fumarolic gas, an obvious carrier of As, Sn and K, with basalt scoria. The latter could be a source of Ti which has low volatility in volcanic gases, as both thermodynamic calculations (Churakov et al., Reference Churakov, Tkachenko, Korzhinskii, Bocharnikov and Shmulovich2000) and direct measurements carried out for gases of Tolbachik (Zelenski et al., Reference Zelenski, Malik and Yu2014) demonstrate.
Yurgensonite and the Sn-bearing variety of katiarsite are associated closely with one another, with other arsenates, namely badalovite, pansnerite, yurmarinite, achyrophanite, arsenatrotitanite, hatertite, khrenovite and svabite; and with sanidine, hematite, cassiterite (Ti-, Fe3+- and Sb5+-enriched varieties), rutile (Sn-, Fe3+- and Sb5+-enriched varieties) and aphthitalite-group sulfates. Katiarsite–yurgensonite series members overgrow white to yellowish sanidine crusts and yellow- or brownish-green badalovite crystals on the surface of basalt scoria altered by fumarolic gas (Fig. 1).
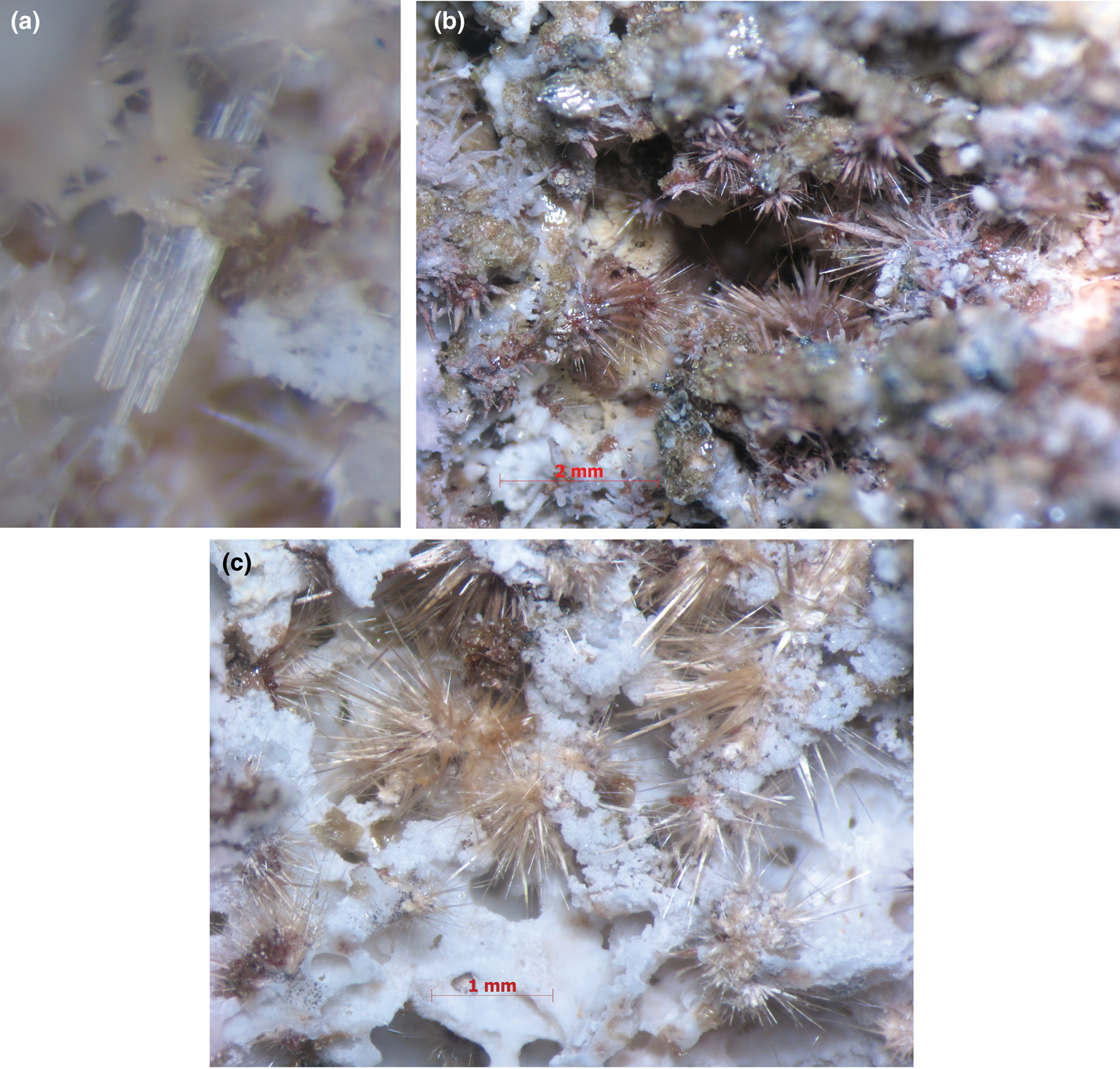
Fig. 1. Morphology of yurgensonite (a, b) and Sn-rich variety of katiarsite (c): (a) parallel intergrowth of three sword-shaped transparent colourless yurgensonite crystals with white sanidine; (b) numerous sprays of colourless acicular yurgensonite crystals, partially overgrown by reddish-brown pansnerite and milky-white aphthitalite, on aggregates of yellowish sanidine and light brownish-green badalovite; (c) numerous bush-like clusters of beige acicular crystals of Sn-rich katiarsite on white sanidine crust. The figure (a) shows the holotype specimen of yurgensonite. FOV width: (a) 0.75 mm, (b) 9 mm, (c) 5.8 mm. Photo: I.V. Pekov and A.V. Kasatkin.
Yurgensonite occurs as sword-shaped crystals (Figs 1a and 2a) up to 0.01 mm × 0.05 mm × 1 mm or acicular to hair-like individuals (Figs 1b and 2b) up to 1 mm long. They are elongated along [010]; sword-like crystals are flattened on [100]. The major forms of sword-shaped crystals (Fig. 2a) are {100}, {001} (prismatic zone) and {011} (terminations). Acicular individuals are typically split and consist of numerous crystals (subindividuals) (Fig. 2b). Sword-like crystals are commonly combined in parallel or near-parallel intergrowths (Fig. 1a) whereas acicular and hair-like individuals form radial, sheaf- or bush-like aggregates, sprays (Figs 1b and 2b) or open-work rosettes and spherulites up to 2 mm across. The Sn-enriched variety of katiarsite, visually indistinguishable from yurgensonite, also typically forms radial aggregates of acicular to hair-like crystals of the same size (Figs 1c and 2c). Epitactic overgrowing of flattened pansnerite crystals on acicular crystals of katiarsite–yurgensonite series minerals was observed (Fig. 3).
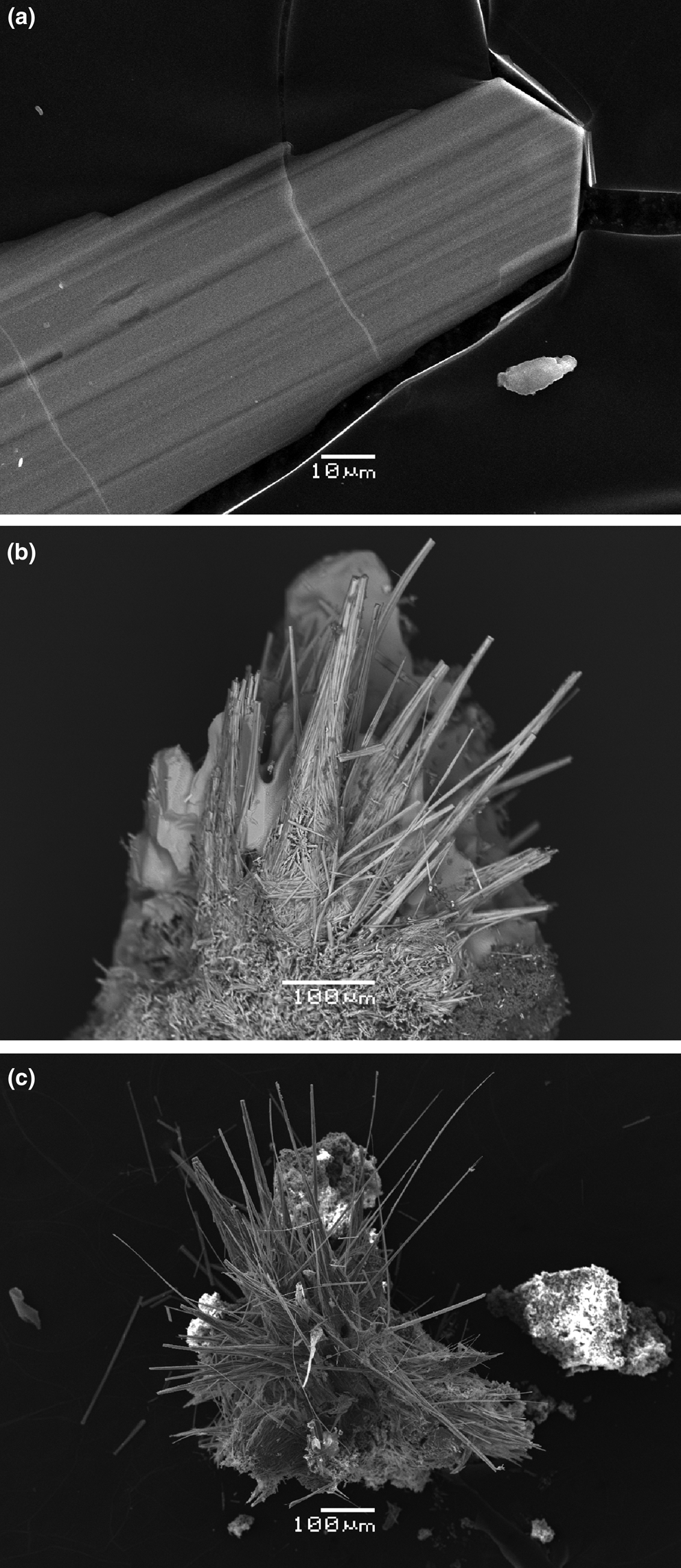
Fig. 2. Morphology of yurgensonite (a, b) and Sn-rich variety of katiarsite (c): (a) sword-shaped yurgensonite crystal extracted from the holotype specimen; (b) bush-like aggregate of acicular yurgensonite individuals (some of them are split and consist of numerous sub-individuals) on crude crystals of badalovite; (c) bush-like aggregate of acicular to hair-like individuals of Sn-rich katiarsite on sanidine. Scanning electron microscopy images, secondary electron (a, c) and back-scattered electron (b) modes.

Fig. 3. Parallel intergrowths of acicular yurgensonite crystals (white) epitaxially overgrown by elongated tabular crystals of pansnerite K3Na3Fe3+6(AsO4)8 (light grey). Back-scattered electron image.
Physical properties
Yurgensonite and a Sn-bearing variety of katiarsite are transparent in individuals and translucent in aggregates. They are colourless, white or pale beige, with white streak and vitreous lustre. Neither mineral fluoresces in UV light. They are brittle, cleavage or parting was not observed. The fracture is uneven. The hardness and density were not measured because crystals are small and thin and aggregates are open-work. Density calculated for the holotype yurgensonite using the empirical formula and unit-cell volume determined from the single-crystal X-ray diffraction (XRD) data is 3.877 g cm–3.
Optical data
In plane polarised transmitted light, yurgensonite is colourless and non-pleochroic. It is optically biaxial (–), α = 1.764(6), β = 1.780(6) and γ = 1.792(6) (589 nm); 2Vmeas. is large, close to 90°; 2Vcalc. = 81°. Dispersion of optical axes is distinct, r < v. Optical orientation is: X = b, Y = a and Z = c.
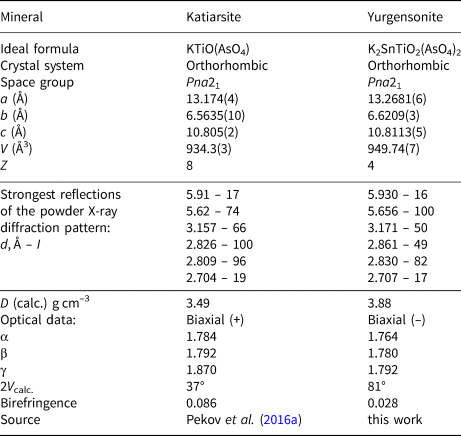
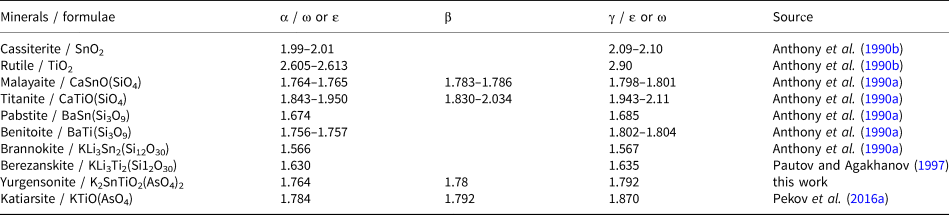
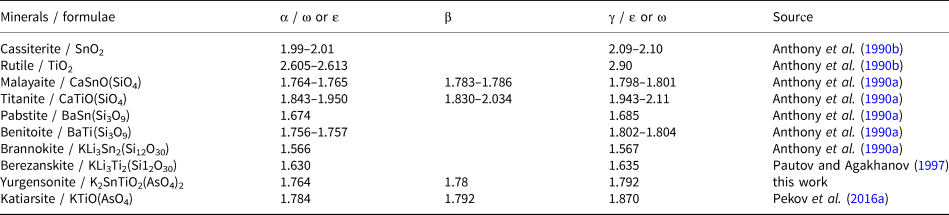
The increase of the Ti:Sn ratio in the katiarsite–yurgensonite series is accompanied by the increase of all three refractive indices and birefringence (Table 1). Yurgensonite and katiarsite demonstrate the same feature as other known pairs of isostructural minerals with Ti4+ and Sn4+: the titanium member of such a pair has significantly higher refractive indices than the tin member (Table 2). Thus, despite a much higher atomic number of tin (50) and, thus, number of electrons in comparison with titanium (atomic number 22), Ti4+ compounds have higher refractive indices than isostructural Sn4+ compounds, apparently caused by significant difference in the electron structure of Ti and Sn atoms. In addition to significant substitution of Ti4+ for Sn4+, the Ti–Sn ordering and microtwinning (see below) could cause the difference in optical sign and 2V value between katiarsite and yurgensonite (Table 1).
Table 1. Comparative data of katiarsite and yurgensonite, two natural representatives of the KTP-structure type.

Table 2. Refractive indices of isostructural Sn4+ and Ti4+ minerals.
Raman spectroscopy
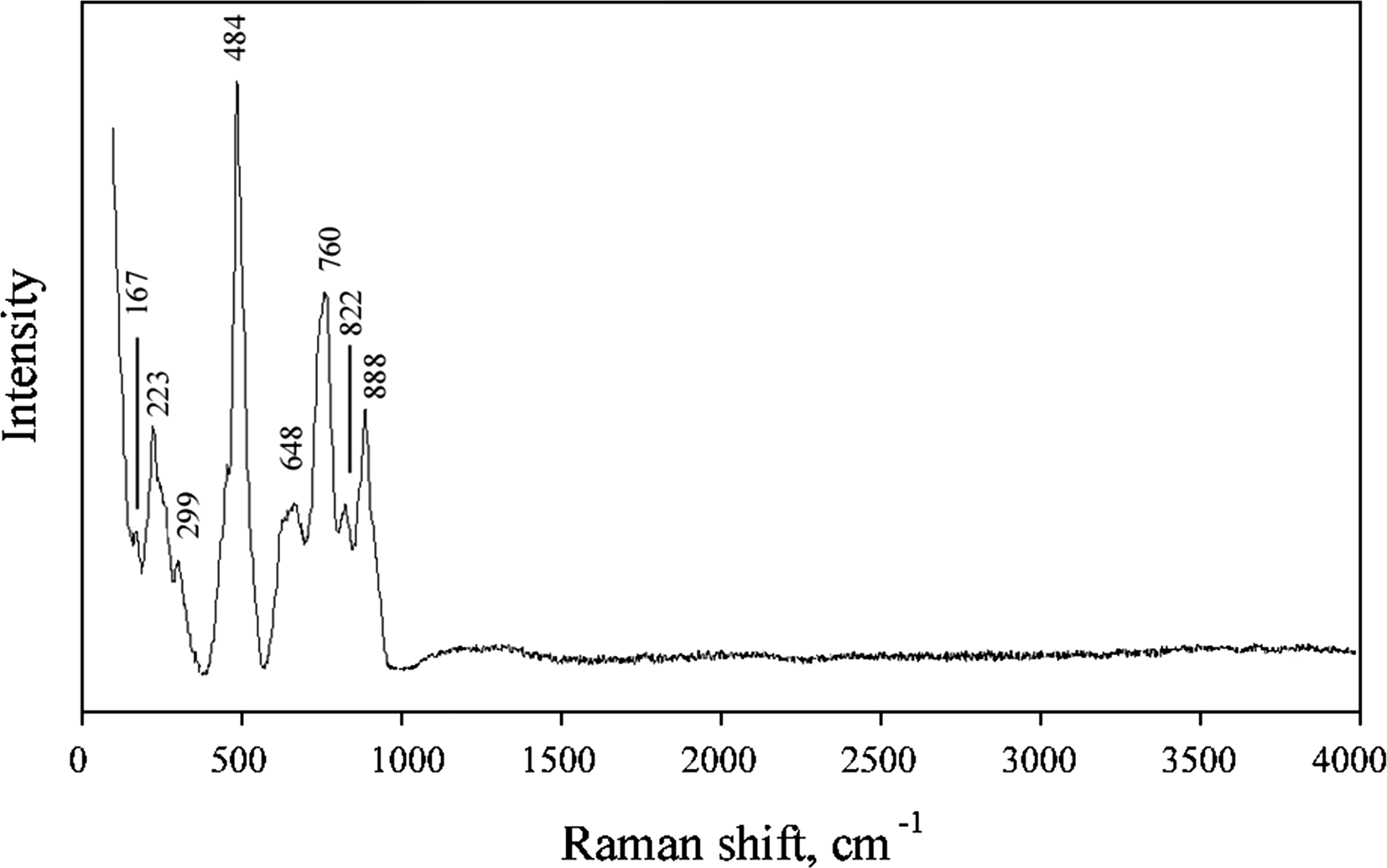
The Raman spectrum of yurgensonite (Fig. 4) was obtained on an aggregate of randomly oriented acicular crystals using an EnSpectr R532 instrument with a green laser (532 nm) at room temperature. The output power of the laser beam was ~14 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines cm–1 and a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~25 μm. The back-scattered Raman signal was collected with 40× objective; signal acquisition time for a single scan of the spectral range was 2000 ms and the signal was averaged over 6 scans.
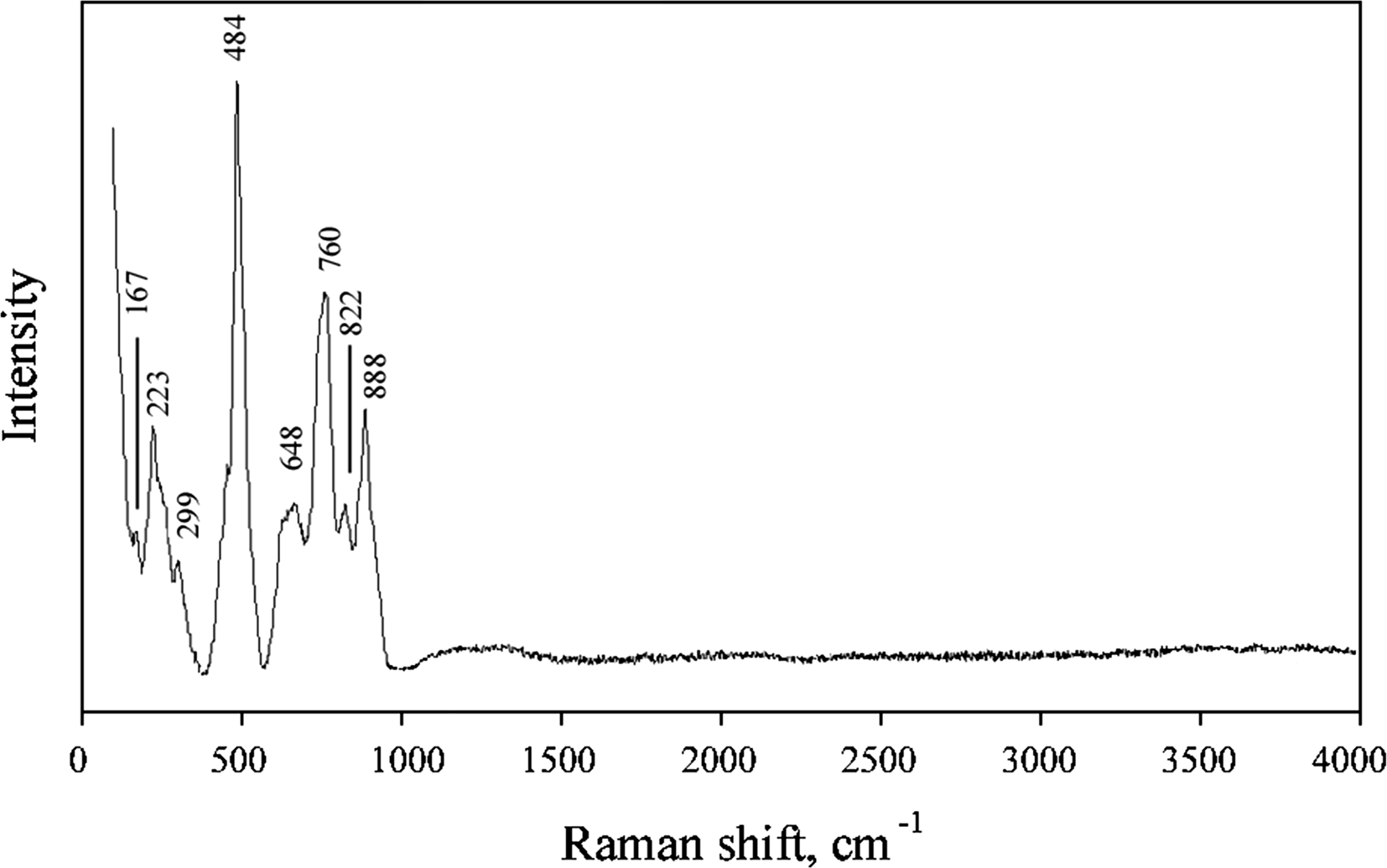
Fig. 4. The Raman spectrum of yurgensonite.
Bands in the region between 740 and 900 cm–1 in the Raman spectrum of yurgensonite correspond to As5+–O antisymmetric stretching vibrations of AsO4 groups. A broad band with the main maximum at 648 cm–1 can be assigned to Ti–O and Sn–O stretching vibrations in MO6 octahedra (see below). A strong band at 484 cm–1 (with distinct low-frequency shoulder) probably corresponds to stretching vibrations of non-bridging Ti–O/Sn–O bonds and antisymmetric vibrations of Ti–O–Sn/Ti–O–Ti/Sn–O–Sn bridges. Bands with frequencies lower than 400 cm–1 correspond to lattice modes involving As–O, Sn–O and Ti–O bending and K–O stretching vibrations. The bands in the spectrum are assigned according to Nakamoto (Reference Nakamoto1986) and Watson (Reference Watson1991).
The Raman spectrum of yurgensonite is, in general, similar to the spectra of katiarsite (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a) and its synthetic analogue KTA (Watson, Reference Watson1991). However, all bands with frequencies lower than 740 cm–1 (i.e. involving vibrations in octahedra) in the spectrum of the new mineral are shifted to lower frequencies in comparison with katiarsite, due to the substitution of ~50% of Ti4+ for the heavier and larger Sn4+ cation in yurgensonite.
The absence of bands with frequencies higher than 900 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in yurgensonite.
Chemical composition
The chemical composition of the katiarsite–yurgensonite series minerals was determined in two laboratories. In the Fersman Mineralogical Museum (FMM), the minerals were studied using a Jeol 733 electron microprobe instrument and in the Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University (MSU), a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer was used. In both cases WDS mode was used, with an acceleration voltage of 20 kV, a beam current of 20 nA and a 3 μm beam diameter. The following standards were used (FMM / MSU): Na (albite / albite), K (microcline / microcline), Rb (Rb2Nb4O11 / Rb2Nb4O11), Cu (Cu metal/ Cu metal), Al (Al2O3 / Al2O3), Fe (InAs / FeAsS), Si (microcline / diopside), Ti (ilmenite / MnTiO3), Sn (SnO2 / SnS), P (LaPO4 / GaP), V (V metal / V metal), As (InAs / FeAsS), Sb (Sb / Sb2S3) and S (ZnS / ZnS). Contents of other elements with atomic numbers higher than carbon are below detection limits.
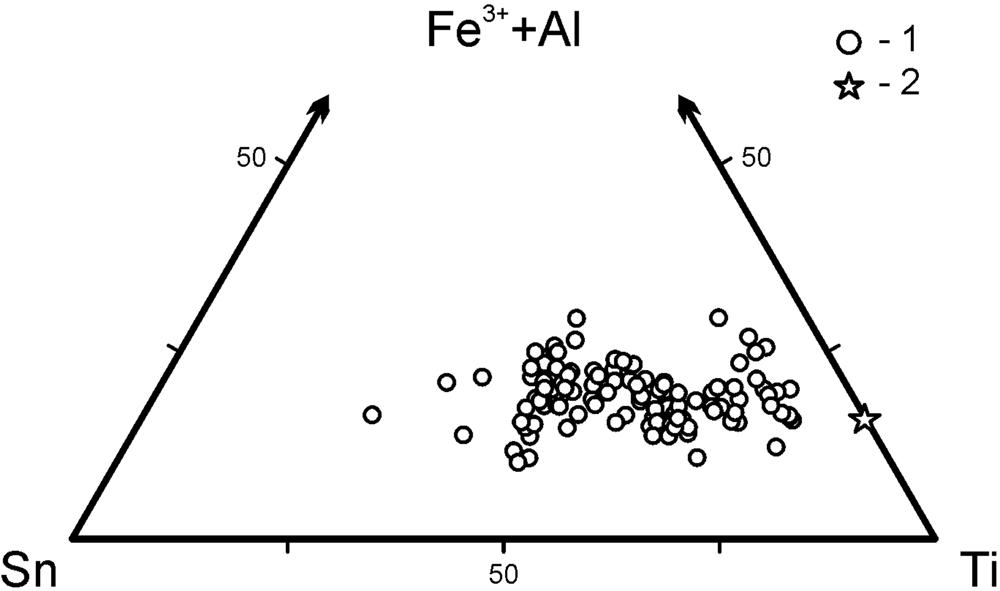
A representative selection of electron-microprobe analyses of minerals belonging to the katiarsite–yurgensonite series are given in Table 3 and the ratios of the major octahedrally coordinated components in these arsenates are shown in Fig. 5.
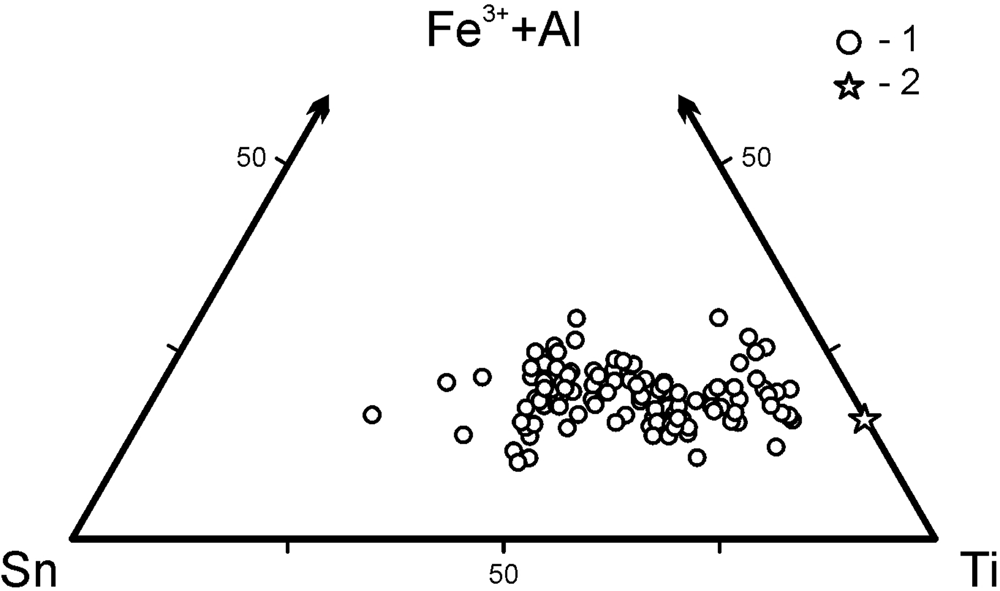
Fig. 5. Ratios of major octahedrally coordinated cations (M) in minerals of the katiarsite–yurgensonite series: 1 – this work, 2 – holotype katiarsite (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a).
Table 3. Chemical composition of minerals of the katiarsite–yurgensonite isomorphous series.

1–8 – katiarsite [1 – calculated values for KTiO(AsO4); 2 ht – holotype specimen: Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a], 9–14 – yurgensonite [11 ht – holotype specimen, averaged values for four spot analyses, ranges are in parentheses; 14 – calculated values for K2SnTiO2(AsO4)2]. Analyses are ordered by increase of Sn content. Dash means that the content of a constituent is below detection limit. *Total also includes 0.48 wt.% Nb2O5 that corresponds to 0.02 Nb apfu.
The empirical formula of the holotype yurgensonite (#11 in Table 3) calculated on the basis of 10 O atoms per formula unit (apfu) is (K1.92Na0.09Rb0.01)Σ2.02(Sn0.81Ti0.71Fe3+0.30Sb5+0.17Al0.03)Σ2.02(As1.945Si0.03S0.02P0.01V0.01)Σ2.015O10. The idealised formula, written taking into account the ordering of Sn and Ti (see below), is K2SnTiO2(AsO4)2.
Iron is the major admixed component in katiarsite–yurgensonite series minerals: all the samples studied contain from 3.3 to 5.4 wt.% Fe2O3 that corresponds to 0.2–0.35 Fe apfu. Antimony can be a significant admixture: in some samples, up to 6.0 wt.% Sb2O5 was found (up to 0.2 Sb apfu); however, other samples are Sb-free. Both Fe and Sb seem to be the constituents which substitute Ti and/or Sn at the octahedrally coordinated M sites (see below), as well as minor Al and Cu. Unlike iron, for which the content does not depend on the Ti:Sn ratio in minerals of this series, antimony is mainly concentrated in tin-rich samples (Table 3). Among tetrahedrally coordinated components T, arsenic strongly prevails: the contents of admixed V, P, S and Si together do not exceed 0.13 apfu (#9 in Table 3). Large cations A are represented mainly by potassium whereas admixed sodium content is up to 0.25 apfu = 1.5 wt.% Na2O (#5 in Table 3).
X-ray crystallography and crystal structure determination
Powder XRD data of the holotype yurgensonite (Table 4) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and exposure 15 min. Angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). Parameters of the orthorhombic unit cell calculated from the powder data are: a = 13.276(3), b = 6.629(2), c = 10.823(4) Å and V = 952.5(8) Å3.
Powder XRD patterns of yurgensonite and Sn-free katiarsite (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a) are very close, the difference is only in values of d spacings which are smaller for the latter (Table 1).
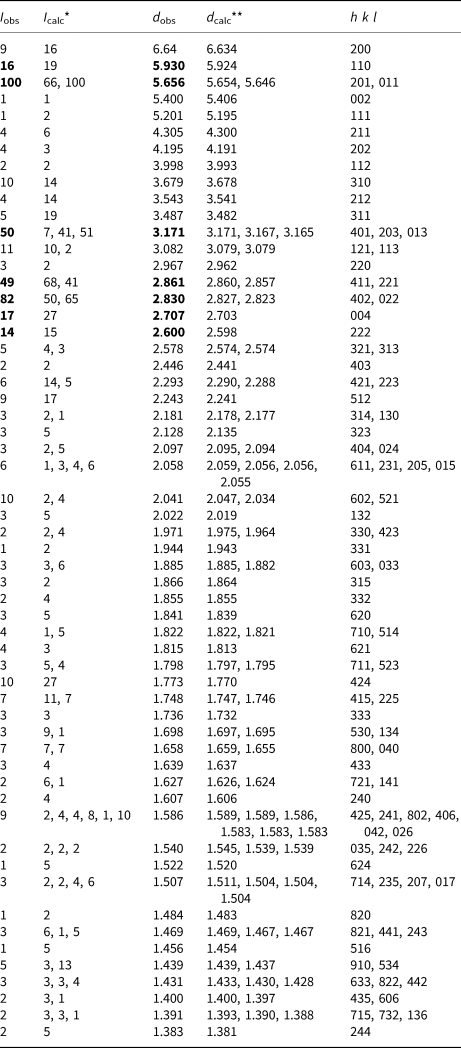
Table 4. Powder X-ray diffraction data (d in Å) of yurgensonite.

*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data. The strongest reflections are marked in boldtype.
The powder XRD pattern of a Ti-rich variety of yurgensonite was obtained under the same conditions (specimen chemically close to the border with katiarsite: #9 in Table 3). It is very similar to the patterns of Sn-free katiarsite (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a) and the Sn-rich variety of yurgensonite (holotype: Table 4); in comparison with them, this intermediate in the Ti:Sn ratio sample demonstrates intermediate d spacings. Its unit-cell parameters calculated from the powder data are: a = 13.178(8), b = 6.583(2), c = 10.826(7) Å and V = 939(1) Å3.
Single-crystal XRD studies of the holotype yurgensonite were carried out using an Xcalibur S diffractometer equipped with a CCD detector. A full sphere of three-dimensional data was collected. Crystal data, data collection information and structure refinement details are given in Table 5. Data reduction was performed using CrysAlisPro Version 1.171.37.35 (Agilent, 2014). The data were corrected for Lorentz and polarisation effects. The structure was solved by direct methods and refined with the use of SHELX software package (Sheldrick, 2015) on the basis of 2235 independent reflections with I > 2σ(I) to the final R = 0.0502. The twinning by merohedry Class I (Nespolo and Ferraris, Reference Nespolo and Ferraris2000) with an inversion centre as a twinning operator was found in the crystal studied. The twin domains ratio is 54/46. Atom coordinates and displacement parameters are presented in Table 6, selected interatomic distances in Table 7 and bond valence calculations in Table 8. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 5. Crystal data, data collection information and structure refinement details for yurgensonite.

Table 6. Сoordinates and equivalent displacement parameters (U eq, in Å2) of atoms and site occupancy factors (s.o.f.) for yurgensonite.

*Admixed Fe and Sb (see Table 3), close in electron numbers to Ti and Sn, respectively, were not taken into account during refinement; **U iso.
Table 7. Selected interatomic distances (Å) in the structure of yurgensonite.
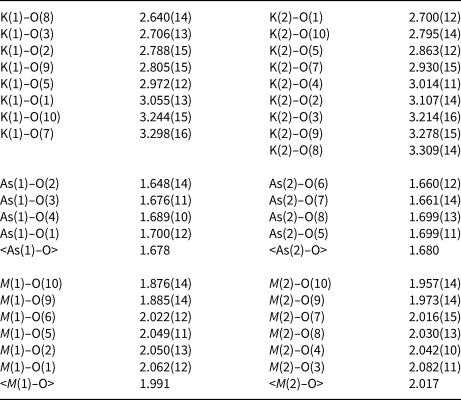
Table 8. Bond valence calculations for yurgensonite.

Bond-valence parameters are taken from Gagné and Hawthorne (Reference Gagné and Hawthorne2015).
Discussion
Yurgensonite K2SnTiO2(AsO4)2 is a Ti,Sn-ordered isostructural analogue of katiarsite KTiO(AsO4) (Table 1). Both minerals, found only in the Arsenatnaya fumarole, are representatives of the well-known KTP [KTiO(PO4)] structure type. The KTP family contains more than forty isostructural (Pna21) synthetic phosphates and arsenates with the general formulae A +M 4+O(T 5+O4) [T 5+ = P and As; M 4+ = Ti, Ge, V, Sn, Zr, (Ga3+0.5Nb5+0.5), (Fe3+0.5Nb5+0.5), (Mn3+0.5Nb5+0.5) and (Mg2+0.33Nb5+0.67); A + = K, Na, Rb, Cs, Ag, NH4 and Tl] and KM 3+(F,OH)(T 5+O4) [T 5+ = P and As; M 3+ = Ga and Fe]. Some representatives of this family belong to very important optical crystalline materials. They are unique in overall qualifications for second-order non-linear and electro-optic processes with a large hyperpolarisability, an excellent temperature window, a wide wavelength range for phase matching, and outstanding crystal stability. Some members of this family are characterised by the unique combination of high non-linear susceptibility and ferroelectric superionic conductivity (Novikova et al., Reference Novikova, Sorokina, Verin, Alekseeva, Orlova, Voronkova and Tseitlin2018).
The crystal structure of KTP-type compounds (see, e.g. Fig. 6) contains undulating chains of corner-linked alternating crystallographically non-equivalent octahedra M(1) and M(2). The chains are cross-linked by TO4 tetrahedra and thus a heteropolyhedral framework is formed. The A + cations occupy the channels of the framework (Stucky et al., Reference Stucky, Phillips and Gier1989; Phillips et al., Reference Phillips, Harrison, Stucky, McCarron III, Calabrese and Gier1992; Northrup et al., Reference Northrup, Parise, Cheng, Cheng and McCarron1994; Weber, Reference Weber2003).

Fig. 6. The crystal structure of yurgensonite projected along the b axis. M(1) = Ti and M(2) = Sn (see Table 6). The unit cell is outlined.
Yurgensonite is the second mineral belonging to the KTP-structure type, after katiarsite (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a) which is a natural analogue of synthetic compound KTA, one of the most important non-linear optical materials (Mayo et al., Reference Mayo, Thomas, Teat, Loiacono and Loiacono1994; Northrup et al., Reference Northrup, Parise, Cheng, Cheng and McCarron1994; Weber, Reference Weber2003). In yurgensonite, Sn4+ prevails in one of two crystallographically non-equivalent octahedrally coordinated sites [M(2)] while another octahedron M(1)O6 is Ti4+-dominant (Tables 6 and 7). Potassium cations occupy two sites in the channels of the Sn–Ti–As–O heteropolyhedral framework (Fig. 6). The crystal chemical formula of yurgensonite could be also written in the form K2(SnO)(TiO)(AsO4)2.
Crystal structures and physical properties of synthetic KTP-type phosphates in which Ti4+ is completely or partially substituted by Sn4+ are well-studied and, in particular, a continuous solid-solution series KTi1-xSnxOPO4 has been reported (Krotova et al., Reference Krotova, Sorokina, Verin, Voronkova, Yanovskii and Simonov2003 and references therein). The compounds with approximately equal contents of Ti and Sn, namely KTi0.47Sn0.53OPO4 (Krotova et al., Reference Krotova, Sorokina, Verin, Voronkova, Yanovskii and Simonov2003), KTi0.5Sn0.5OPO4 (Crennell et al., Reference Crennell, Owen, Cheetham, Kaduk and Jarman1991), K0.5Na0.5Ti0.5Sn0.5OPO4, Na0.5Rb0.5Ti0.5Sn0.5OPO4 and K0.5Rb0.5Ti0.5Sn0.5OPO4 (Crennell et al., Reference Crennell, Cheetham, Kaduk and Jarman1992), demonstrate significant ordering of Ti and Sn with Ti predominance in the M(1) site whereas Sn prevails in M(2). For example, the following distribution of Ti and Sn between these sites was found for KTi0.47Sn0.53OPO4: M (1)(Ti0.645Sn0.335)M (2)(Sn0.696Ti0.304) (Krotova et al., Reference Krotova, Sorokina, Verin, Voronkova, Yanovskii and Simonov2003). For K0.5Rb0.5Ti0.5Sn0.5OPO4 the distribution is as follows: M (1)(Ti0.67Sn0.33)M (2)(Sn0.67Ti0.33) (Crennell et al., Reference Crennell, Cheetham, Kaduk and Jarman1992). Yurgensonite is characterised by similar distribution of species-defining Sn and Ti: M (1)(Ti0.67Sn0.33)M (2)(Sn0.64Ti0.36) (Table 6), which is confirmed by average interatomic distances: M(1)–O = 1.991 and M(2)–O = 2.017 Å (Table 7).
A synthetic titanium-free tin analogue of KTA, KSnOAsO4 was reported by Lin et al. (Reference Lin, Lin and Lii1995).
Katiarsite and yurgensonite in the Arsenatnaya fumarole form a continuous solid-solution series almost without gaps (Table 3, Fig. 5). The XRD data for its members with different composition together with data on synthetic KTP-type compounds allow us to conclude that all representatives of this series are isostructural and, thus, the katiarsite–yurgensonite series can be considered to be an isomorphous series. We believe that the M(1) site in all members of this series is Ti-dominant whereas the M(2) site is characterised by wide variation of the Ti:Sn ratio. The formal border between katiarsite, ideally KTiO(AsO4), and yurgensonite, ideally K2SnTiO2(AsO4)2, should be at the point with composition K2Sn0.5Ti1.5O2(AsO4)2 [= K2(Sn0.5Ti0.5O)(TiO)(AsO4)2]. Real composition of the minerals is more complicated due to the presence of significant amounts of admixed M cations, and samples with Sn > 0.5 Sn apfu are considered as corresponding to the mineral species yurgensonite (##9–13 in Table 3).
Iron and antimony are the common substituents in the majority of the samples of katiarsite–yurgensonite series minerals studied (Table 3). It is impossible to quantify the distribution of Fe and Sb between the M(1) and M(2) sites due to the insufficient amounts of these admixed components and similarity of their X-ray scattering power to Ti and Sn, respectively. We suggest that these cations reside in the structure as Fe3+ and Sb5+. This conclusion is based on (1) general crystal chemical features of KTP-type compounds (see above), (2) strongly oxidising conditions of mineral formation in the Arsenatnaya fumarole (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a; Shchipalkina et al., Reference Shchipalkina, Pekov, Koshlyakova, Britvin, Zubkova, Varlamov and Sidorov2020) and (3) the presence of rutile and cassiterite strongly enriched by the tripuhyite component, i.e. Fe3+ and Sb5+ which together substitute Ti4+ (Sandalov et al., Reference Sandalov, Pekov, Koshlyakova, Yapaskurt, Agakhanov, Sidorov and Britvin2020), in close association with katiarsite–yurgensonite series members. The substitution scheme with participation of Sb for these KTP-type arsenate minerals seems the same: Fe3+ + Sb5+ → 2M 4+ (M = Sn and Ti). It is noteworthy that the substitution M 3+ + M 5+ ↔ 2M 4+ is in general typical for KTP-type compounds (Weber, Reference Weber2003). This is also in agreement with the ionic radii which are (in octahedral coordination) as follows: Sn4+ 0.69, Ti4+ 0.605, Sb5+ 0.60 and Fe3+ 0.645 Å whereas ionic radii of Sb3+ and Fe2+ are 0.76 and 0.78 Å, respectively (Shannon, Reference Shannon1976). However, some samples of minerals of the katiarsite–yurgensonite series exhibit a substantial, up to 0.4 apfu, admixture of Fe+Al (with Fe >> Al), without concomitant incorporation of Sb5+ (Table 3). As the presence of Fe4+ can be ruled out under natural (terrestrial) oxidation conditions, and neither F– nor OH– were detected in the samples of katiarsite and yurgensonite, the sole mechanism which can be invoked for compensation of the emerging charge imbalance is the introduction of oxygen vacancies. The trivalent centres (Fe, Cr, Ti, In and Sc) and associated oxygen vacancies are well-known in both synthetic KTiOPO4 (KTP) and KTiOAsO4 (KTA) (e.g. Bulka et al., Reference Bulka, Vinokurov, Nizamutdinov and Khasanova1987; Gaite et al., Reference Gaite, Stenger, Dusausoy, Marnier and Rager1991; Mashkovtsev and Isaenko, Reference Mashkovtsev and Isaenko1996). The vacancies arise via paired substitution expressed as: 2M 4+ + O2- → 2M 3+ + □. The concentration of trivalent cations in the synthetic samples of KTP and KTA not exceeded 0.0n atoms per formula with 5 oxygen atoms, as the laser-grade KTP and KTA are grown as high-purity crystals. If the proposed substitution mechanism is also valid for minerals of the katiarsite–yurgensonite series, they might contain up to 0.08 oxygen vacancies per formula with 5 oxygen atoms. This is among the highest vacancy contents ever detected in the compounds belonging to the KTP-structure type.
Acknowledgements
We thank anonymous referees for valuable comments. This study was supported by the Russian Science Foundation, grant no. 19-17-00050. The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.47