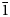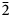Introduction
This paper continues the series of articles on new arsenate minerals from the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption 1975–1976, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N, 160°14′E, 1200 m asl). Sixteen new species have been characterised in previous articles in the series: yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016b), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016c), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018b), arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019a), the two isostructural minerals edtollite K2NaCu5Fe3+O2(AsO4)4 and alumoedtollite K2NaCu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Ksenofontov, Pautov, Sidorov, Britvin, Vigasina and Pushcharovsky2019b), anatolyite Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Pekov et al., Reference Pekov, Lykova, Yapaskurt, Belakovskiy, Turchkova, Britvin, Sidorov and Scheidl2019c), zubkovaite Ca3Cu3(AsO4)4 (Pekov et al., Reference Pekov, Lykova, Agakhanov, Belakovskiy, Vigasina, Britvin, Turchkova, Sidorov and Scheidl2019d) and pansnerite K3Na3Fe3+6(AsO4)8 (Pekov et al., Reference Pekov, Zubkova, Koshlyakova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Britvin, Turchkova, Sidorov and Pushcharovsky2020).
Alluaudite-group arsenates in the Arsenatnaya fumarole are diverse and, in some areas, abundant (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a). In the present paper we characterise a new member of this group badalovite NaNaMg(MgFe3+)(AsO4)3 (Cyrillic: бадаловит). It is named in honour of the outstanding mineralogist and geochemist Professor Stepan Tigranovich Badalov (1919–2014) who worked in the Abdullaev Institute of Geology and Geophysics, Uzbekistan Academy of Sciences, Tashkent. Prof. Badalov was an Honorary member of the Russian Mineralogical Society.
The new mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Society (IMA), IMA2016–053 (Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2016a). The holotype specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, under the catalogue number 95618.
Occurrence and general appearance
The general description of the active Arsenatnaya fumarole was given by Pekov et al. (Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a). Specimens with the new mineral were collected by us from several areas of Arsenatnaya in 2015 (holotype), 2016 and 2017. Temperatures measured using a chromel–alumel thermocouple at the time of collecting in different pockets with badalovite were 380–450°C. It seems that the new mineral was deposited directly from the gas phase as a volcanic sublimate or, more probably, formed as a result of the interaction between fumarolic gas and basalt scoria at a temperature not lower than 450°C. Basalt could be a source of Mg which has very low volatility in such post-volcanic systems at temperatures up to 400–500°C (Symonds and Reed, Reference Symonds and Reed1993).
Badalovite is a constituent of fumarolic incrustations mainly consisting of arsenates, sulfates, oxides, chlorides and silicates. It is associated with hematite, tenorite, cassiterite, johillerite, nickenichite, calciojohillerite, bradaczekite, hatertite, magnesiohatertite, metathénardite, aphthitalite, langbeinite, calciolangbeinite, sanidine (As-bearing variety), fluorophlogopite, fluoborite, tilasite, anhydrite, pseudobrookite, rutile, sylvite, halite, lammerite, urusovite, ericlaxmanite, arsmirandite, arsenowagnerite, svabite, popovite, dmisokolovite, shchurovskyite, yurmarinite, krasheninnikovite, euchlorine, wulffite, alumoklyuchevskite and sellaite.
Badalovite forms oblique-angled prismatic crystals (Figs 1 and 2) usually <1 mm long, rarely up to 1 mm × 1 mm × 5 mm. Some crystals are equant. By analogy with other alluaudite-group minerals, it could be assumed that the main forms of badalovite crystals are {100}, {010}, {011} and {20![]() $\bar{1}$}. Crystals are well-shaped (Fig. 2a) or crude, sometimes divergent, resembling sheaves (Figs 1b and 2b), commonly with numerous inclusions of grains of other minerals or particles of basalt scoria. Some badalovite crystals contain, typically in peripheral parts, zones corresponding chemically to other alluaudite-group arsenates, usually johillerite, nickenichite or calciojohillerite. This zonation commonly has an irregular, spotty character (Fig. 3). Some zoned crystals are multi-coloured: parts that correspond chemically to badalovite or calciojohillerite are pale greenish, greenish-yellow or grey; zones of Cu-enriched members of the group, i.e. johillerite or nickenichite, are blue (Fig. 1d). Badalovite crystals are typically combined in groups, open-work clusters, brushes or crusts (Figs 1 and 2) up to several hundred cm2 in area overgrowing basalt scoria altered by fumarolic gas.
$\bar{1}$}. Crystals are well-shaped (Fig. 2a) or crude, sometimes divergent, resembling sheaves (Figs 1b and 2b), commonly with numerous inclusions of grains of other minerals or particles of basalt scoria. Some badalovite crystals contain, typically in peripheral parts, zones corresponding chemically to other alluaudite-group arsenates, usually johillerite, nickenichite or calciojohillerite. This zonation commonly has an irregular, spotty character (Fig. 3). Some zoned crystals are multi-coloured: parts that correspond chemically to badalovite or calciojohillerite are pale greenish, greenish-yellow or grey; zones of Cu-enriched members of the group, i.e. johillerite or nickenichite, are blue (Fig. 1d). Badalovite crystals are typically combined in groups, open-work clusters, brushes or crusts (Figs 1 and 2) up to several hundred cm2 in area overgrowing basalt scoria altered by fumarolic gas.

Fig. 1. Aggregates of badalovite: (a, sample #4755) crust consisting of crude crystals with red cassiterite and iron-black hematite; (b, sample #4685) radial clusters of prismatic crystals on basalt scoria altered by fumarolic gas; (c, sample #4644) crystal crust partially covered by white aphthitalite; and (d, sample #4742) cluster of multicoloured, chemically heterogeneous crystals (red–brown colouration is caused by hematite micro-inclusions, blue areas correspond to zones composed of johillerite: see Fig. 3). FOV width: (a, b) 5.7 mm, (c) 4.4 mm, (d) 3.5 mm. Photo: I.V. Pekov & A.V. Kasatkin.

Fig. 2. Morphology of badalovite: (a) well-shaped crystals; and (b) sheaf-like clusters of divergent crystals partially covered by fine-grained hematite. Scanning electron microscopy (SEM) secondary electron images.

Fig. 3. Individual crystals of badalovite (grey: 1) with areas consisting of: johillerite (light grey: 2); 2a – johillerite chemically close to the ideal composition NaCuMg3(AsO4)3; 2b – Cu-depleted johillerite variety; and 3 – intimate intergrowth of cassiterite and hematite. Polished section, SEM back-scatter electron image.
Physical properties and optical data
Badalovite is transparent, pale green to green, greenish-grey to grey, bluish-greenish, greenish-yellow to bright yellow or honey-yellow, sometimes colourless. The streak is white to pale greenish or pale yellowish and lustre is vitreous. The mineral is brittle. Cleavage or parting was not observed, the fracture is uneven. The Mohs hardness is ca 3½. Density was not measured because crystals typically contain abundant mineral inclusions (hematite, cassiterite, different arsenates, etc.) and bubbles. Density calculated for the holotype using the empirical formula is 4.016 g cm–3.
In plane polarised transmitted light, badalovite is colourless and non-pleochroic. It is optically biaxial (–), α = 1.753(3), β = 1.757(3), γ = 1.758(3) (589 nm), 2Vmeas. = 50(10)° (estimated by the curve of the conoscopic figures on the sections perpendicular to the optical axes), 2Vcalc. = 53°. Dispersion of optical axes is strong, r > v. Optical orientation: Y = b.
Raman spectroscopy
The Raman spectrum of badalovite (Fig. 4) was obtained on a randomly oriented crystal using an EnSpectr R532 instrument (Dept. of Mineralogy, Moscow State University) with a green laser (532 nm) at room temperature. The output power of the laser beam was ~16 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines cm–1 and a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~10 μm. The backscattered Raman signal was collected with 40× objective, signal acquisition time for a single scan of the spectral range was 1500 ms and the signal was averaged over 10 scans.

Fig. 4. The Raman spectrum of badalovite.
The strongest bands in the region 750–950 cm–1 correspond to As5+–O stretching vibrations of AsO43– anions. A distinct band with a maximum at 561 cm–1 can be assigned to Fe3+–O stretching vibrations. Bands with frequencies lower than 500 cm–1 correspond to bending vibrations of AsO4 tetrahedra, Mg–O stretching vibrations and lattice modes. The absence of bands with frequencies higher than 950 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in badalovite.
Chemical composition
The chemical composition of badalovite (Table 1) was determined on a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA and a 10 μm beam diameter. The following standards were used: jadeite (Na, Al and Si); KTiOPO4 (K, Ti and P); wollastonite (Ca); olivine (Mg); MnTiO3 (Mn); Cu (Cu); ZnS (Zn and S); FeS2 (Fe); V (V); and GaAs (As). Contents of other elements with atomic numbers higher than carbon are below detection limits. H2O was not analysed because both structure data and the Raman spectrum show its absence.
Table 1. Chemical composition of badalovite.

*Average (6 spot analyses, ranges are in parentheses) data for the holotype specimen (sample numbers are field collection numbers). ‘–’ = content is below detection limit. **All Fe is calculated as Fe3+ based on the structure data and considering strongly oxidising conditions of mineral formation in the Arsenatnaya fumarole: only iron minerals with Fe3+ are found there (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a).
Different coloured samples of badalovite were found to have close chemical composition (Table 1). The empirical formula of the holotype calculated on the basis of 12 O atoms per formula unit (apfu) is Na1.67Ca0.20K0.02Mg1.92Zn0.02Mn0.02Cu0.01Fe3+0.90Al0.01(As3.01P0.03Si0.01)Σ3.05O12. The simplified formula is Na2Mg2Fe3+(AsO4)3 which requires Na2O 10.93, MgO 14.21, Fe2O3 14.08, As2O5 60.78, total 100 wt.%. The end-member formula of badalovite, according to the IMA-accepted nomenclature of the alluaudite supergroup (Hatert, Reference Hatert2019), is NaNaMg(MgFe3+)(AsO4)3.
The correctness of the obtained data is confirmed by the superior value of the Gladstone–Dale compatibility index (Mandarino, Reference Mandarino2007): 1 – (K p/K c) = 0.012, which is superior.
X-ray crystallography and crystal-structure determination
Powder X-ray diffraction data of badalovite (Table 2) were collected with a DRON-2 diffractometer using CuKα radiation. Parameters of the monoclinic unit cell calculated from the powder data are: a = 11.89(1), b = 12.799(5), c = 6.667(7) Å, β = 112.48(7)° and V = 938(2) Å3.
Table 2. Powder X-ray diffraction data of badalovite.

*For the calculated pattern, only reflections with intensities ≥1 are given. **For the unit-cell parameters calculated from single-crystal data. The strongest reflections are marked in boldtype.
Single-crystal X-ray studies of badalovite were carried out using an Xcalibur S diffractometer equipped with a CCD detector. The crystal structure was solved by direct methods and refined with the use of the SHELX-97 software package (Sheldrick, Reference Sheldrick2008) to R 1 = 0.0249 on the basis of 1538 independent reflections with I > 2σ(I). Crystal data, data collection information and structure refinement details are given in Table 3, coordinates and thermal displacement parameters of atoms and bond-valence sums in Table 4 and selected interatomic distances in Table 5. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystal data, data collection information and structure refinement details for badalovite.

Table 4. Coordinates and equivalent displacement parameters (U eq, in Å2) of atoms, bond-valence sums (BVS) and site multiplicities (Q) for badalovite.

*For occupancies of the A and M sites see Table 3. **Bond-valence parameters are taken from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). All calculations were done taking into account given cation distribution.
Table 5. Selected interatomic distances (Å) in the structure of badalovite.

Elongated A(1)–O distances are given in italics.
Discussion
Badalovite is a mineral of the alluaudite group belonging to the alluaudite supergroup and the cation sites in its structure are labelled below in accordance with the nomenclature of this supergroup (Hatert, Reference Hatert2019).
The crystal structure of badalovite (Fig. 5a), like structures of other members of the alluaudite group (Moore, Reference Moore1971; Moore and Ito, Reference Moore and lto1979; Krivovichev et al., Reference Krivovichev, Vergasova, Filatov, Rybin, Britvin and Ananiev2013; Hatert, Reference Hatert2019 and references therein), contains zig-zag chains of edge-sharing M(1)O6 and M(2)O6 octahedra. The M-octahedral chains consist of [M(2)2O10] dimers of distorted M(2)O6 octahedra connected via distorted M(1)O6 octahedra that are isolated from one another. As(1)O4 tetrahedra share all vertices with the M-centred octahedra forming the (010) heteropolyhedral layers while each As(2)O4 tetrahedron shares three vertices with the M-centred octahedra of one layer (Fig. 5b) and the fourth vertex with the octahedron of adjacent layer, thus linking the layers to a three-dimensional framework (Fig. 5a). In badalovite Mg strongly prevails in the M(1) site (Wyckoff symbol: 4e) whereas the M(2) site (8f) contains approximately equal amounts of Mg and Fe3+. The presence of Fe in a trivalent state is undoubtedly confirmed by the mean M(2)–O distance [2.056 Å, whereas mean M(1)–O distance is 2.134: Table 5] and bond-valence sum (2.49, Table 4). This is in agreement with the strongly oxidising conditions of mineral deposition in the Arsenatnaya fumarole (see above).
Channels of two types in the framework contain the A(1) and A(2)’ sites predominantly occupied by Na in badalovite. The A(1) site is coordinated by six O atoms with the distances in the range from 2.357(2) to 2.540(2) Å, two elongated A(1)–O(2) bonds [2.916(2) Å] could also be included in the coordination sphere of A(1). The A(2)’ site centres eight-fold oxygen polyhedron with A(2)’–O distances in the range from 2.437(2) to 3.005(3) Å (Table 5). A minor admixture of Cu (0.01 apfu) found by electron microprobe could be located in M(2) or in the A(1)’ site that typical for Cu2+ cations in alluaudite-type arsenates (Krivovichev et al., Reference Krivovichev, Vergasova, Filatov, Rybin, Britvin and Ananiev2013; Koshlyakova et al., Reference Koshlyakova, Zubkova, Pekov, Giester and Sidorov2018; Hatert, Reference Hatert2019): a small peak with x = 0.0, y = 0.5072 and z = 0.25 on the residual Fourier synthesis (1.02 e –/Å3) revealed during the refinement of the badalovite structure corresponds to the A(1)’ site.
The formula of the crystal studied derived from structure refinement is A (1)(Na0.80Ca0.20)A (1)’□A (2)□A (2)’(Na0.90□0.10) M (1)(Mg0.85Ca0.15)M (2)(Mg1.08Fe3+0.92)(AsO4)3 (□ – vacancy). The simplified crystal chemical formula of badalovite can be written as A (1)NaA (1)’□A (2)□A (2)’NaM (1)MgM (2)(Mg0.5Fe3+0.5)2(AsO4)3, or, in the shortened form, A[NaNa]M[Mg(MgFe3+)](AsO4)3 (Z = 4).
Another alluaudite-group arsenate, yazganite (Sarp and Černý, Reference Sarp and Černý2005) has the same species-defining cations as badalovite, however, (1) yazganite is a hydrous mineral and (2) the cation ratios in these minerals are significantly different. The simplified crystal chemical formula of yazganite is A (1)NaA (1)’□A (2)□ A (2)’(H2O)M (1)MgM (2)(Fe3+)2(AsO4)3 and its end-member formula, according to the actual nomenclature of the alluaudite supergroup, is NaMgFe3+2(AsO4)3⋅H2O (Hatert, Reference Hatert2019).
Acknowledgements
We thank an anonymous referee, Oleg Siidra and Structures Editor Daniel Atencio for valuable comments. This study was supported by the Russian Science Foundation, grant no. 19-17-00050.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.43.