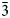Introduction
In this paper, we continue to characterise new arsenate mineral species found in the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N 160°14′E, 1200 m asl). Arsenatnaya is one of the largest and hottest fumaroles at this scoria cone, a monogenetic volcano formed in 1975 (Fedotov and Markhinin, Reference Fedotov and Markhinin1983). This fumarole was thus named due to the abundance of arsenate minerals as described by Pekov et al. (Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a). In particular, 13 new arsenates from this locality have been characterised: yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015b), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015c), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016b), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016c), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018b), arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2019a), and isostructural minerals edtollite K2NaCu5Fe3+O2(AsO4)4 and alumoedtollite K2NaCu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Ksenofontov, Pautov, Sidorov, Britvin, Vigasina and Pushcharovsky2019b).
This paper describes the new mineral anatolyite, Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Cyrillic: анатолиит) named in honour of the outstanding Russian crystallographer, mineralogist and mathematician Anatoly Kapitonovich Boldyrev (1883–1946), Professor of Crystallography and Mineralogy in the Leningrad Mining Institute. Both new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (IMA2016–040, Pekov et al., Reference Pekov, Lykova, Yapaskurt, Belakovskiy, Turchkova, Britvin, Sidorov and Scheidl2016a). The type specimens are deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue numbers 95620 and 95913.
Occurrence, mineral association and morphology
Material with the new mineral was collected by us in July 2015 from the northern area of the Arsenatnaya fumarole, at a depth of 1.5 m under day surface. The temperature, measured using a chromel–alumel thermocouple, was 420°C at the time of collecting. Anatolyite was deposited directly from the gas phase as a volcanic sublimate or, more likely, formed as a result of the interaction between hot gas and basalt scoria at a temperature not lower than 420–450°C. The volcanic gas seems to be a carrier of As, Na and Fe while the basalt scoria is the most probable source of Mg, Al and Ca which have low volatilities in volcanic gases (Symonds and Reed, Reference Symonds and Reed1993).
Anatolyite is one of the rarest minerals of fumarolic encrustations in the polymineralic zone of Arsenatnaya (Pekov et al., Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018a). Closely associated minerals are potassic feldspar (As-bearing), hematite, tenorite, cassiterite, johillerite, tilasite, ericlaxmanite, lammerite, arsmirandite (IMA2014–081, Pekov et al., Reference Pekov, Britvin, Yapaskurt, Polekhovsky, Krivovichev, Vigasina and Sidorov2015a), sylvite, halite, aphthitalite, langbeinite, anhydrite, wulffite, krasheninnikovite, fluoborite, pseudobrookite and fluorophlogopite.
Anatolyite occurs as clusters (up to 0.3 mm × 0.5 mm × 1.2 mm) or open-work aggregates (up to 2 mm across) of crystals overgrowing crusts of As-bearing potassic feldspar that cover basalt scoria altered by volcanic gas (Fig. 1). The crystals (up to 0.2 mm across) are rhombohedral–prismatic, equant or slightly elongated along [001]. They are well-shaped or, more commonly, crude and blocky, with rough surfaces (Fig. 2). Goniometric measurements were not performed due to the small size of anatolyite crystals, however, based on the scanning electron image (Fig. 2) and by analogy with the isostructural mineral yurmarinite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), the observed crystal forms could be assigned to the pinacoid {001}, hexagonal prisms {100} and {110} and rhombohedra {101} and {011}.

Fig. 1. Clusters of pale brownish–pinkish crystals of anatolyite (marked by arrows) with iron-black hematite on a crust of As-bearing potassic feldspar covering the surface of basalt scoria altered by fumarolic gas. FOV width: 3.6 mm. Photo: I.V. Pekov and A.V. Kasatkin, specimen #4739.

Fig. 2. Crystals of anatolyite forming a crust on the surface of dense cluster of the same mineral. Scanning electron microscopy (secondary electron) image, specimen #4739.
Physical properties and optical data
Anatolyite is transparent, pale brownish–pinkish, with white streak and vitreous lustre. It is brittle, cleavage or parting was not observed, and fracture is uneven. The Mohs’ hardness is ~4½. Density calculated using the empirical formula is 3.872 g cm–3.
The mineral is optically uniaxial (–), ω = 1.703(4) and ε = 1.675(3) (589 nm). In transmitted, plane-polarised light, anatolyite is colourless and non-pleochroic.
Chemical composition
The chemical composition of anatolyite was determined using a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Department of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA and a beam diameter of 3 µm. The chemical composition of anatolyite (average of 6 spot analyses) and the standards used are given in Table 1. Contents of other elements with atomic numbers higher than carbon are below detection limits.
Table 1. Chemical composition of anatolyite.

S.D. – standard deviation
The empirical formula calculated on the basis of 24 O apfu is Na6.03K0.10Ca0.50Mg1.63Mn0.03Cu0.10Zn0.08Al1.11Fe3+1.12Ti0.03Sn0.01Si0.01P0.09As5.97O24 or, after the most probable assignment of constituents to positions in accordance with the structure refinement data (see below): (Na5.90K0.10)Σ6.00(Ca0.50Na0.13Zn0.08Mn0.03)Σ0.74(Mg1.63Fe3+1.12Al0.15Cu0.10)Σ3.00(Al0.96Ti0.03Sn0.01)Σ1.00(As5.97P0.09Si0.01)Σ6.07O24. The simplified formula is Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 (Z = 6). The formula Na6Ca(Mg2Fe3+)Al(AsO4)6 requires Na2O 16.27, CaO 4.91, MgO 7.05, Al2O3 4.46, Fe2O3 6.99, As2O5 60.32, total 100.00 wt.%.
X-ray crystallography and crystal structure
Powder X-ray diffraction data of anatolyite (Table 2) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA, and exposure time of 15 min. Angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The hexagonal unit-cell parameters of calculated from the powder data are: a = 13.672(1), c = 18.265(3) Å and V = 2957(1) Å3.
Table 2. Powder X-ray diffraction data of anatolyite.

*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
Single-crystal X-ray studies of anatolyite were carried out using a STOE StadiVari diffractometer equipped with a Dectris PILATUS 300K pixel detector. The crystal structure was solved by direct methods and refined with the use of the SHELX-97 software package (Sheldrick, Reference Sheldrick2008) to R = 0.0477. The crystal data and the experimental details are given in Table 3, atom coordinates and displacement parameters in Table 4, selected interatomic distances in Table 5 and bond-valence calculations in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Crystal data, data collection information and structure refinement details for anatolyite.

Table 4. Coordinates and thermal displacement parameters (U, Å2) of atoms and site occupancies and multiplicities (Q) for anatolyite.

*Site occupancy was refined as 14% vacant taking into account chemical data (possible minor constituents, such as Mn or Zn, were not taken into consideration during refinement) and presence of vacancies in the A1 site in synthetic arsenates and phosphates with the same structure type (Masquelier et al., Reference Masquelier, d'Yvoire and Collin1995; Belam et al., Reference Belam, Madani, Driss and Daoud2000); **the M2 site was refined assuming full occupancy and refining Mg (including the similarly light Al) against Fe3+, the best agreement was obtained with Mg0.560(14)Fe3+0.440(14). In the final refinement cycles the occupancy was fixed as Mg0.51Fe0.43Al0.06 based on the e ref value [18.16] and electron microprobe data.
Table 5. Selected interatomic distances (Å) in the structure of anatolyite.

Table 6. Bond-valence calculations* for anatolyite.

* Bond-valence parameters were taken from (Brese and O'Keeffe, Reference Brese and O'Keeffe1991).
The crystal structure of anatolyite is based on octahedral clusters M 4O18 (Fig. 3a) consisting of M1- and M2-centred octahedra. A regular M1O6 octahedron, occupied predominantly by Al, shares three edges with three slightly distorted mixed-occupied octahedra M2O6 [Mg and Fe3+ are major M2 cations and Mg > Fe3+: Tables 1 and 4]. The clusters linked via AsO4 tetrahedra to form a heteropolyhedral framework (Fig. 3b); each AsO4 tetrahedron shares two oxygen vertices with one cluster and two other vertices with two adjacent clusters, one per each. Two large cation sites A1 and A2 are located in the voids of the framework: the regular A1O6 octahedron is partially occupied with predominance of Ca whereas the A2O8 polyhedron, with distances varying in the range 2.303(6)–2.910(6) Å, is Na-centred (Table 4). The prevalence of Al in M1 and Ca in A1 is clearly confirmed by cation–anion distances in corresponding polyhedra (Table 5) and bond-valence calculations (Table 6), as well as mixed occupancy of M2 by bivalent and trivalent cations. The A2 site is partially (14%) vacant. In general, the structure refinement results are in good agreement with chemical data obtained for anatolyite using the electron microprobe (Table 1).

Fig. 3. The main building unit, an octahedral cluster M 4O18, with connected AsO4 tetrahedra (a) in the crystal structure of anatolyite (b; the unit cell is outlined). For legend see Table 4.
Discussion
Anatolyite Na6(Ca,Na)(Mg,Fe3+)3Al(AsO4)6 is a structural analogue of yurmarinite, Na7(Fe3+,Mg,Cu)4(AsO4)6, for comparison see Table 7. Both minerals are isostructural with various synthetic trigonal arsenates and phosphates (space group R ![]() $\bar{3}$c, a = 13.35–13.8 and c = 18.3–18.6 Å for arsenates and a = 13.4 and c = 17.85–17.9 Å for phosphates). Their general formula is (Na,□)7M 4(T 5+O4)6, with T = As or P. All the synthetic compounds, as well as yurmarinite, contain trivalent cations (Fe3+ or Al) as strongly prevailing in the M sites. However, if the M sites are completely occupied by trivalent cations then
$\bar{3}$c, a = 13.35–13.8 and c = 18.3–18.6 Å for arsenates and a = 13.4 and c = 17.85–17.9 Å for phosphates). Their general formula is (Na,□)7M 4(T 5+O4)6, with T = As or P. All the synthetic compounds, as well as yurmarinite, contain trivalent cations (Fe3+ or Al) as strongly prevailing in the M sites. However, if the M sites are completely occupied by trivalent cations then ![]() ${\scale51%{\bf {\vskip-6.2 pt 1}}\!} {\vskip-6pt {{\rotate166 /}}}\!{\scale51% {\bf 7}}$ of Na sites should be vacant, i.e. the general formula of such compounds is (Na6□1)Σ7M 3+4(T 5+O4)6, or Na3M 3+2(T 5+O4)3. The examples are synthetic II-Na3Fe3+2(AsO4)3 (d'Yvoire et al., Reference d'Yvoire, Bretey and Collin1988), Na3(Al1.89Y0.11)(AsO4)3 (Belam et al., Reference Belam, Madani, Driss and Daoud2000) and Na3Fe3+2(PO4)3 (Belokoneva et al., Reference Belokoneva, Ruchkina, Dimitrova and Stefanovich2002). However, whereas in Na3(Al1.89Y0.11)(AsO4)3 the vacant site is A1, in II-Na3Fe3+2(AsO4)3 and Na3Fe3+2(PO4)3 the A2 site is partially vacant while the A1 site is fully occupied. The vacancy at the A1 site of Na3(Al1.89Y0.11)(AsO4)3 could have resulted in underbonding at the O4 site as it does not coordinate M sites. At the same time, the admixture of Y distorts the structure and results in the shortening of the A2–O4 bonds. Thus, the underbonding is compensated by the bond incidence contribution of the A2 site. A topologically close structure characterised by monoclinic distortion (space group C2, a = 14.576, b = 13.409, c = 9.728 Å and β = 96.95°) was reported for α-Na3Al2(AsO4)3, while the high-temperature (> 44°C) β-Na3Al2(AsO4)3 phase is rhombohedral and isotypic with II-Na3Fe3+2(AsO4)3 (Masquelier et al., Reference Masquelier, d'Yvoire and Collin1995). All these compounds containing vacancies in Na sites are sodium ion conductors, which are interesting for materials science. The full occupancy of the Na sites is possible only if trivalent cations in the M sites are partially substituted by bivalent cations as in synthetic Na7(Fe3+3Fe2+)(AsO4)6 (Masquelier et al., Reference Masquelier, d'Yvoire and Collin1995) and Na7(Fe3+3Fe2+)(PO4)6 (Lii, Reference Lii1996). Thus, the general formula of the abovementioned synthetic compounds can be written as (Na7−x□x)(M 3+3+xM 2+1–x)(T 5+O4)2 with T = As or P, M 3+ = Fe or Al (±Y) and M 2+ = Fe and 0 ≤ x ≤ 1.
${\scale51%{\bf {\vskip-6.2 pt 1}}\!} {\vskip-6pt {{\rotate166 /}}}\!{\scale51% {\bf 7}}$ of Na sites should be vacant, i.e. the general formula of such compounds is (Na6□1)Σ7M 3+4(T 5+O4)6, or Na3M 3+2(T 5+O4)3. The examples are synthetic II-Na3Fe3+2(AsO4)3 (d'Yvoire et al., Reference d'Yvoire, Bretey and Collin1988), Na3(Al1.89Y0.11)(AsO4)3 (Belam et al., Reference Belam, Madani, Driss and Daoud2000) and Na3Fe3+2(PO4)3 (Belokoneva et al., Reference Belokoneva, Ruchkina, Dimitrova and Stefanovich2002). However, whereas in Na3(Al1.89Y0.11)(AsO4)3 the vacant site is A1, in II-Na3Fe3+2(AsO4)3 and Na3Fe3+2(PO4)3 the A2 site is partially vacant while the A1 site is fully occupied. The vacancy at the A1 site of Na3(Al1.89Y0.11)(AsO4)3 could have resulted in underbonding at the O4 site as it does not coordinate M sites. At the same time, the admixture of Y distorts the structure and results in the shortening of the A2–O4 bonds. Thus, the underbonding is compensated by the bond incidence contribution of the A2 site. A topologically close structure characterised by monoclinic distortion (space group C2, a = 14.576, b = 13.409, c = 9.728 Å and β = 96.95°) was reported for α-Na3Al2(AsO4)3, while the high-temperature (> 44°C) β-Na3Al2(AsO4)3 phase is rhombohedral and isotypic with II-Na3Fe3+2(AsO4)3 (Masquelier et al., Reference Masquelier, d'Yvoire and Collin1995). All these compounds containing vacancies in Na sites are sodium ion conductors, which are interesting for materials science. The full occupancy of the Na sites is possible only if trivalent cations in the M sites are partially substituted by bivalent cations as in synthetic Na7(Fe3+3Fe2+)(AsO4)6 (Masquelier et al., Reference Masquelier, d'Yvoire and Collin1995) and Na7(Fe3+3Fe2+)(PO4)6 (Lii, Reference Lii1996). Thus, the general formula of the abovementioned synthetic compounds can be written as (Na7−x□x)(M 3+3+xM 2+1–x)(T 5+O4)2 with T = As or P, M 3+ = Fe or Al (±Y) and M 2+ = Fe and 0 ≤ x ≤ 1.
Table 7. Comparative data for yurmarinite and anatolyite.

*Wyckoff site symbols are given in square brackets.
Anatolyite is the first representative of this structure type in which bivalent cations prevail in both M2 [Mg] and A1 [Ca] sites. The simplified scheme of cation substitutions defining the relationship between yurmarinite and anatolyite can be written as A1Na+ + M1Fe3+ + M2Fe3+ ↔ A1Ca2+ + M1Al3+ + M2Mg2+. The substitution of significant amount of Fe for Al and Mg and part of Na for Ca causes lower unit-cell dimensions, density and refractive indices of anatolyite in comparison with yurmarinite (Table 7). The substitution of Na for Ca at the A1 site could have led to overbonding at the O4 site, but partial occupancy of the cation site alleviates the issue.
Acknowledgements
We thank Peter Leverett, Fernando Camara and anonymous referees for valuable comments. This study was supported by the Russian Foundation for Basic Research, grants nos. 17-05-00179 (mineralogical and structural studies) and 18-29-12007 (crystal chemical analysis). The technical support by the SPbSU X-Ray Diffraction Resource Center in the powder XRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.11.