Introduction
This is the tenth contribution to our series of papers on new arsenate minerals from the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41'N, 160°14'E, 1200 m asl). This active fumarole, discovered by us in July 2012, was characterised by Pekov et al. (Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b). Previous papers described yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a), two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015b), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015c), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016d), pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017a), arsenowagnerite Mg2(AsO4)F (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Yapaskurt, Chukanov, Belakovskiy, Sidorov and Pushcharovsky2018a) and arsenatrotitanite NaTiO(AsO4) (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Belakovskiy, Vigasina, Yapaskurt, Sidorov, Britvin and Pushcharovsky2018c).
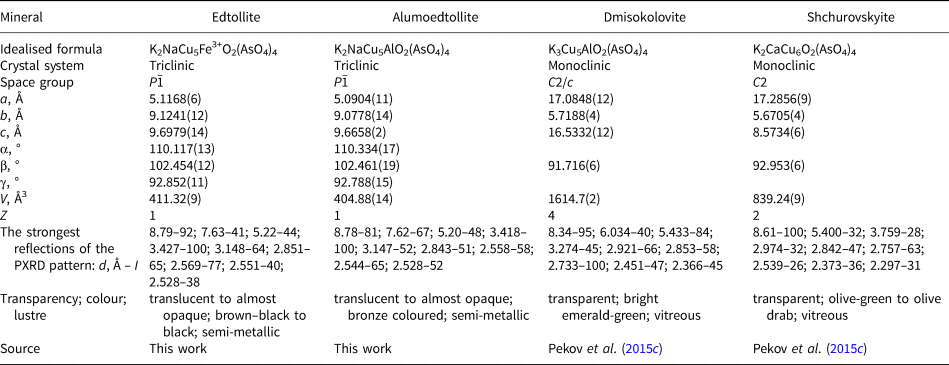
In this paper we describe two isostructural new minerals: edtollite (Cyrillic: эдтоллит), ideally K2NaCu5Fe3+O2(AsO4)4 and alumoedtollite (алюмоэдтоллит), ideally K2NaCu5AlO2(AsO4)4. Edtollitte is named in honour of the Russian geologist and Arctic explorer Eduard Vasilievich Toll (1858–1902) who made significant contributions to the geology and geography of Polar Siberia and islands in the Arctic Ocean. He had German roots and is also known as Eduard Gustav von Toll. Alumoedtollite is named as an analogue of edtollite with aluminium prevailing among trivalent cations (Al > Fe3+). Both new minerals and their names have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (CNMNC); IMA2016–010 (edtollite, Pekov et al., Reference Pekov, Zubkova, Agakhanov, Pautov, Vigasina, Sidorov, Ksenofontov, Britvin and Pushcharovsky2016c) and IMA2017–020 (alumoedtollite, Pekov et al., Reference Pekov, Zubkova, Agakhanov, Sidorov, Ksenofontov, Britvin and Pushcharovsky2017b). The type specimens are deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue numbers 95350 (edtollite) and 95906 (alumoedtollite).
Occurrence and general appearance
Specimens with both new minerals were collected by us in July 2015 at the northern area of the Arsenatnaya fumarole, from the depth of 1 m under the day surface. The temperature measured using a chromel–alumel thermocouple during collecting was 420°C in this pocket. We believe that edtollite and alumoedtollite were deposited directly from the gas phase as volcanic sublimates at temperatures not lower than 420–450°C.
Edtollite and alumoedtollite belong to the rarest Tolbachik minerals, found in negligible amounts: the former was identified in two small specimens and the latter in only one specimen. They occur in polymineralic assemblages. Edtollite is associated with orthoclase (As-bearing), sylvite, halite, hematite, tenorite, bradaczekite, johillerite, dmisokolovite, shchurovskyite, lammerite, ericlaxmanite, yurmarinite, popovite, arsmirandite (IMA2014–081, Pekov et al., Reference Pekov, Britvin, Yapaskurt, Polekhovsky, Krivovichev, Vigasina and Sidorov2015a), pharmazincite, tilasite, svabite, hatertite, nickenichite, durangite, aphthitalite, langbeinite, calciolangbeinite, anhydrite, wulffite, euchlorine, alumoklyuchevskite, krasheninnikovite, wulfenite, fluoborite, cassiterite, rutile, pseudobrookite and fluorophlogopite. In close association with alumoedtollite is sylvite, halite, tenorite, dmisokolovite, shchurovskyite, arsmirandite, johillerite, bradaczekite, tilasite, orthoclase (As-bearing), hematite and anhydrite.
Edtollite occurs as prismatic crystals up to 0.02 mm × 0.02 mm × 0.1 mm, their near-parallel (Fig. 1) or chaotic intergrowths up to 0.3 mm across. Rarely its crystals form, together with prismatic crystals of As-bearing orthoclase, open-work aggregates up to 1 mm across. In close association with bradaczekite, johillerite, dmisokolovite and hematite, the new mineral overgrows incrustations mainly consisting of As-bearing orthoclase and sylvite (Figs 1 and 2) that covers basalt scoria altered by fumarolic gas. Late-generation sylvite overgrows all the minerals listed. Alumoedtollite occurs as long-prismatic to acicular crystals up to 0.01 mm × 0.01 mm × 0.1 mm, forming bush-like clusters and open-work aggregates (up to 1 mm across) together with other arsenates, tenorite and sylvite (Figs 3 and 4).

Fig. 1. Near-parallel intergrowths of edtollite crystals on a crystal crust mainly consisting of As-bearing orthoclase and sylvite. Scanning electron microscopy (secondary electron) image.
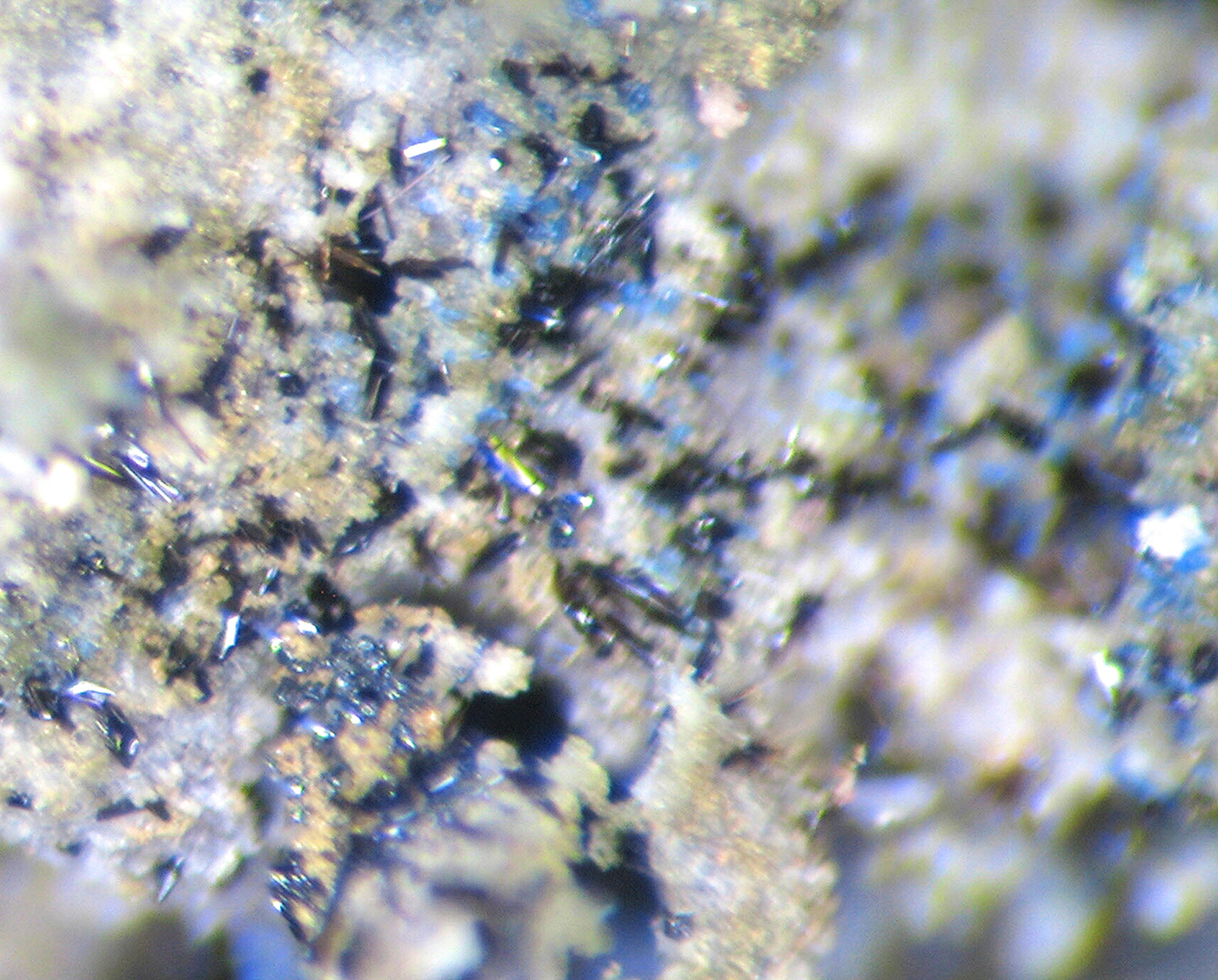
Fig. 2. Black prismatic crystals of edtollite on a crust mainly consisting of colourless As-bearing orthoclase and sylvite, with blue bradaczekite. Field of view: 1.2 mm wide. Photo: I.V. Pekov and A.V. Kasatkin.

Fig. 3. Clusters of long-prismatic crystals of alumoedtollite on sylvite crust. Scanning electron microscopy (secondary electron) image.

Fig. 4. Aggregates of small bronze-coloured prismatic crystals of alumoedtollite close associated with green dmisokolovite, blue bradaczekite, black tenorite and colourless sylvite. Field of view 1.8 mm wide. Photo: I.V. Pekov and A.V. Kasatkin.
Physical properties and optical data
Both new minerals are translucent to almost opaque, with a semi-metallic lustre. Edtollite is brown–black to black, some crystals have an olive-green hue, and the streak is light brown. Alumoedtollite is bronze coloured with a light yellow streak. Both minerals are brittle, cleavage or parting was not observed, and the fracture is uneven (observed under the scanning electron microscope). The Mohs’ hardness and density could not be measured because of the small size of crystals and the open-work character of aggregates. Density values calculated using the empirical formulae and the unit-cell parameters from single-crystal X-ray diffraction data are 4.264 g cm–3 for edtollite and 4.280 g cm–3 for alumoedtollite.
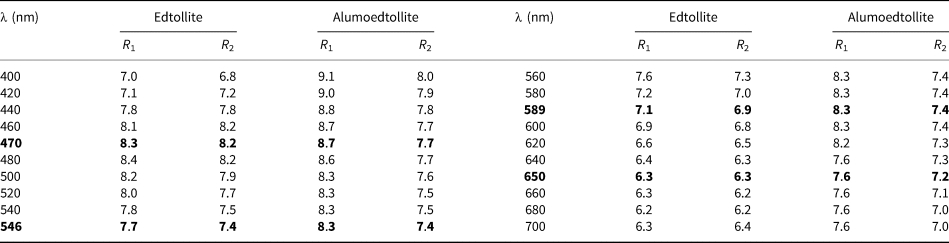
Optical properties of the new minerals were examined in reflected light. Both are grey, and pleochroism was not observed. Anisotropism is distinct. Bireflectance is very weak for edtollite (ΔR = 0.2%) and weak for alumoedtollite (ΔR = 0.9%) (589 nm). Internal reflections are weak, brown for edtollite and yellowish for alumoedtollite. The reflectance values for both minerals measured by means of the UMSP-50 Opton microspectrophotometer using the SiC standard are given in Table 1.
Table 1. Reflectance data (R, %) for edtollite and alumoedtollite.
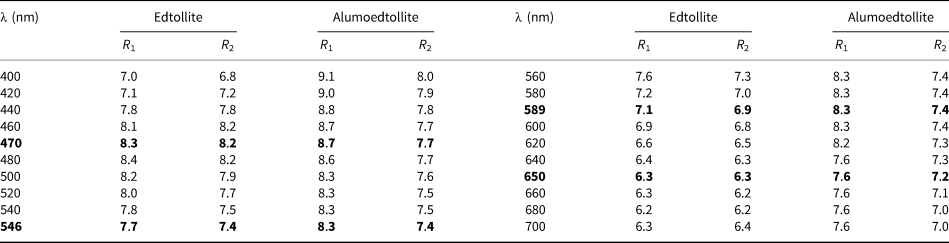
Data for wavelengths recommended by the IMA Commission on ore microscopy (COM) are marked in boldtype.
Raman spectroscopy
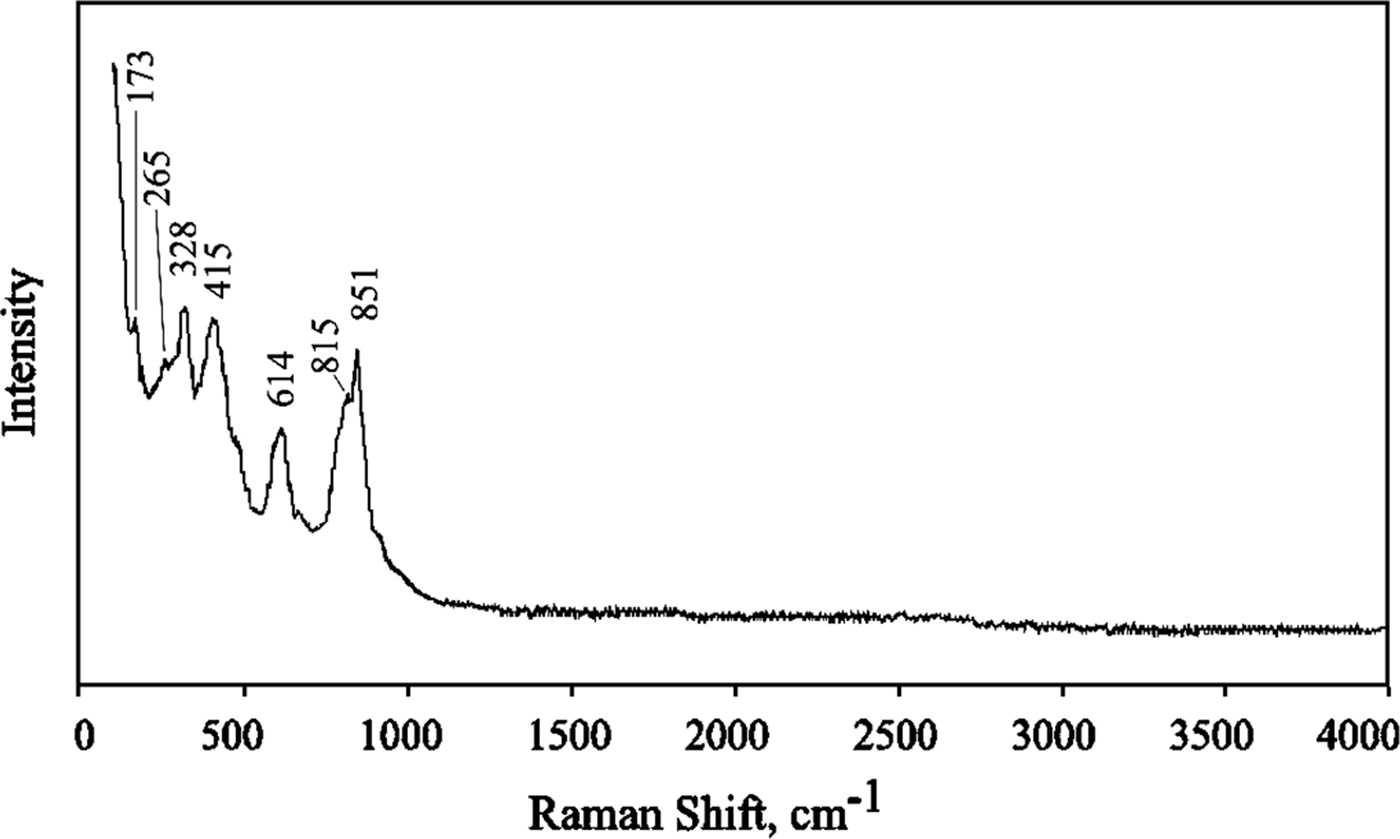
The Raman spectrum of edtollite (Fig. 5) was obtained on a crystal cluster using an EnSpectr R532 instrument (Dept. of Mineralogy, Moscow State University) with a green laser (532 nm) at room temperature. The output power of the laser beam was ~15 mW. The spectrum was processed using the EnSpectr expert mode program in the range from 100 to 4000 cm–1 with the use of a holographic diffraction grating with 1800 lines cm–1 and a resolution of 6 cm–1. The diameter of the focal spot on the sample was ~10 μm. The back-scattered Raman signal was collected with 40× objective; signal acquisition time for a single scan of the spectral range was 1000 ms and the signal was averaged over 50 scans.
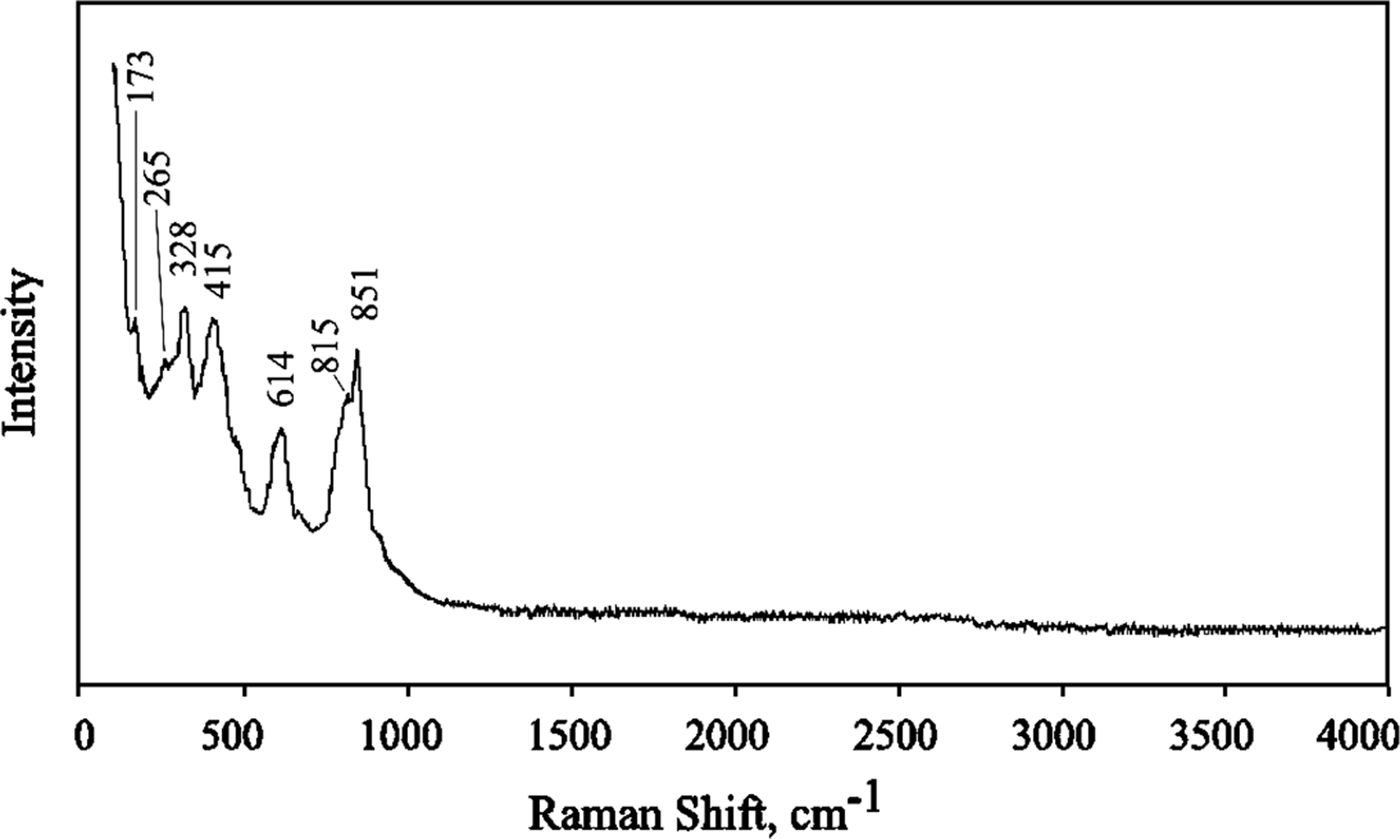
Fig. 5. The Raman spectrum of edtollite.
The strong band with maximum at 851 cm–1 (with a shoulder at 815 cm–1) corresponds to As5+–O stretching vibrations of AsO43– anionic groups. Bands with frequencies <700 cm–1 correspond to bending vibrations of AsO4 tetrahedra, Cu2+–O and Fe3+–O stretching vibrations and lattice modes. The absence of bands with frequencies >900 cm–1 indicates the absence of groups with O–H, C–H, C–O, N–H, N–O and B–O bonds in the mineral.
Chemical composition
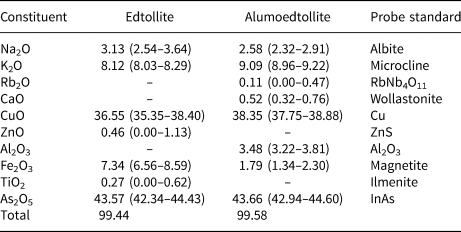
Chemical data for both new minerals were obtained using a Jeol 733 electron microprobe instrument (wavelength-dispersive mode, acceleration voltage of 20 kV, beam current of 20 nA and 3 μm beam diameter). The chemical composition is given in Table 2. Contents of other elements with atomic numbers higher than carbon are below detection limits.
Table 2. Average chemical composition (wt.%, ranges are in parentheses) of edtollite and alumoedtollite.

‘–' = below detection limit.
The empirical formulae calculated on the basis of 18 O apfu for edtollite is: K1.83Na1.07Cu4.88Zn0.06Fe3+0.98Ti0.04As4.03O18; and for alumoedtollite is: K2.02Rb0.01Na0.87Ca0.10Cu5.06Al0.72Fe3+0.24As3.99O18.
After the grouping of constituents in accordance with the structure data (see below), the empirical formulae could be presented as follows for edtollite: (K1.83Na0.07)Σ1.90Na1.00(Cu3.90Zn0.06)Σ3.96(Cu0.98Fe3+0.98Ti0.04)Σ2.00O1.88(AsO4)4.03; and for alumoedtollite: (K1.98Rb0.01)Σ1.99(Na0.87Ca0.10K0.03)Σ1.00Cu4.02(Cu1.04Al0.72Fe3+0.24)Σ2.00O2.04(AsO4)3.99.
The simplified, end-member formulae are for edtollite K2NaCu5Fe3+O2(AsO4)4 (which requires Na2O 2.92, K2O 8.87, CuO 37.43, Fe2O3 7.52, As2O5 43.26, total 100.00 wt.%) and for alumoedtollite K2NaCu5AlO2(AsO4)4 (which requires Na2O 3.00, K2O 9.11, CuO 38.48, Al2O3 4.93, As2O5 44.48, total 100.00 wt.%).
X-ray crystallography
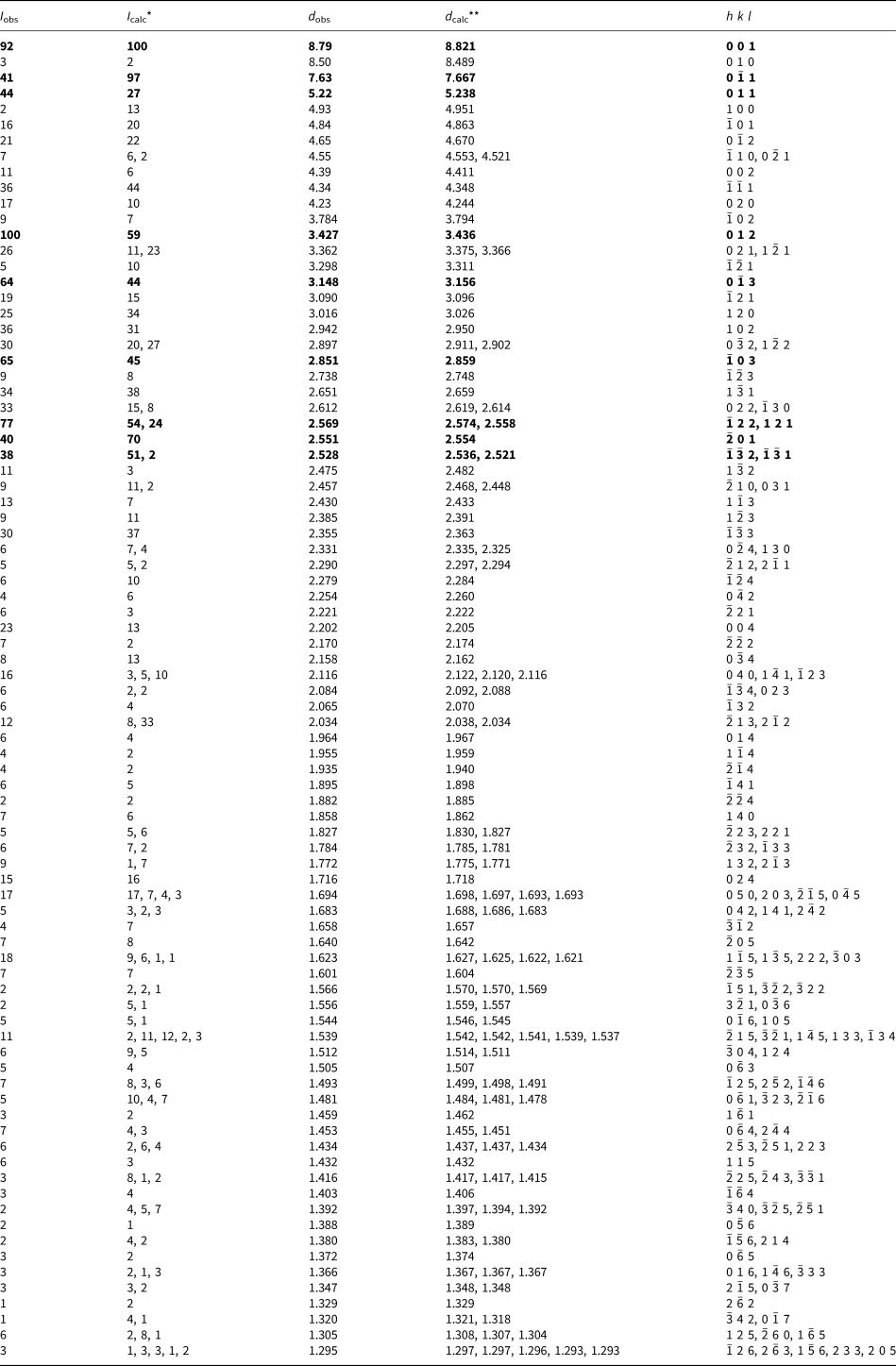
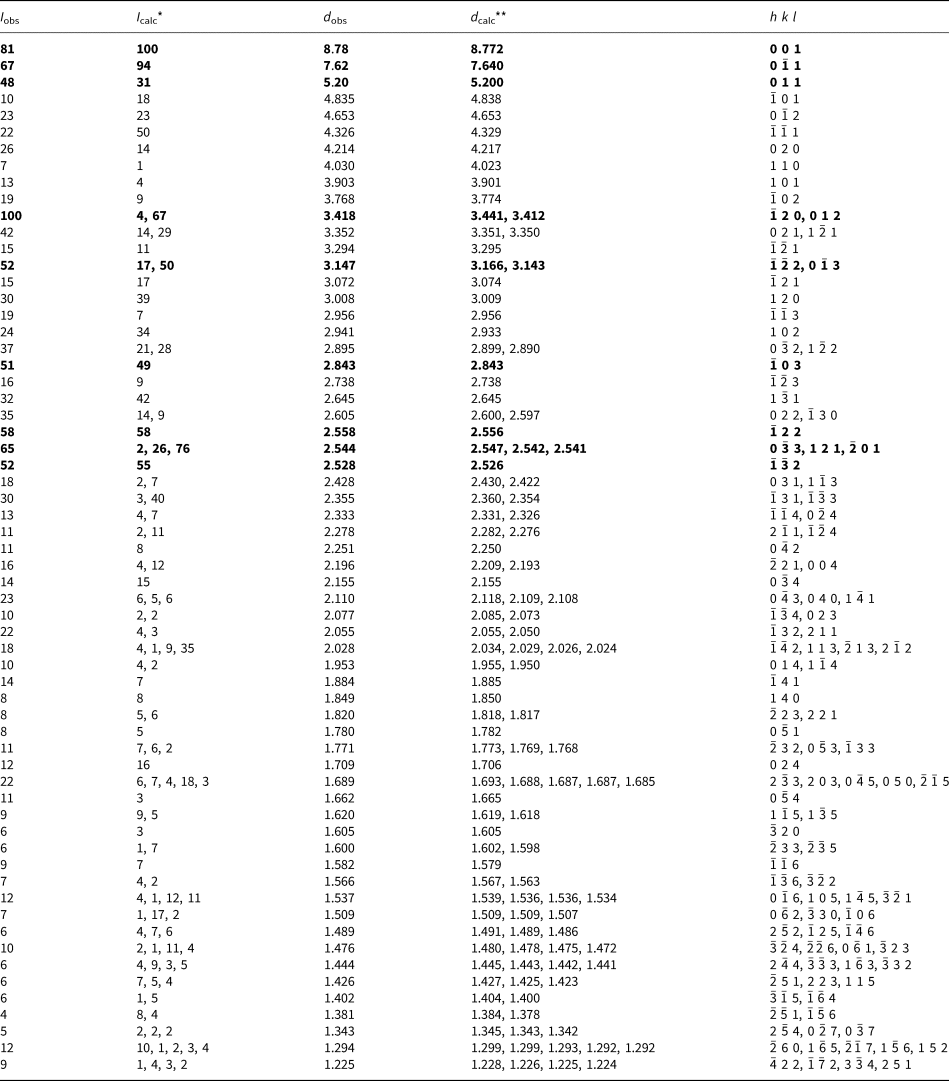
Powder X-ray diffraction (PXRD) data for both minerals were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with a cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA, and exposure = 15 min. Angular resolution of the detector is 0.045°2θ (pixel size = 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). The PXRD data for edtollite are given in Table 3 and for alumoedtollite in Table 4. Parameters of triclinic unit cells calculated from the powder data for edtollite are: a = 5.12(1), b = 9.12(2), c = 9.68(2) Å, α = 110.04(5), β = 102.37(4), γ = 92.87(4)° and V = 410.4(5) Å3; and for alumoedtollite are: a = 5.09(1), b = 9.07(2), c = 9.66(2) Å, α = 110.32(7), β = 102.43(7), γ = 92.77(7)° and V = 405.2(6) Å3.
Table 3. Powder X-ray diffraction data (d in Å) for edtollite.
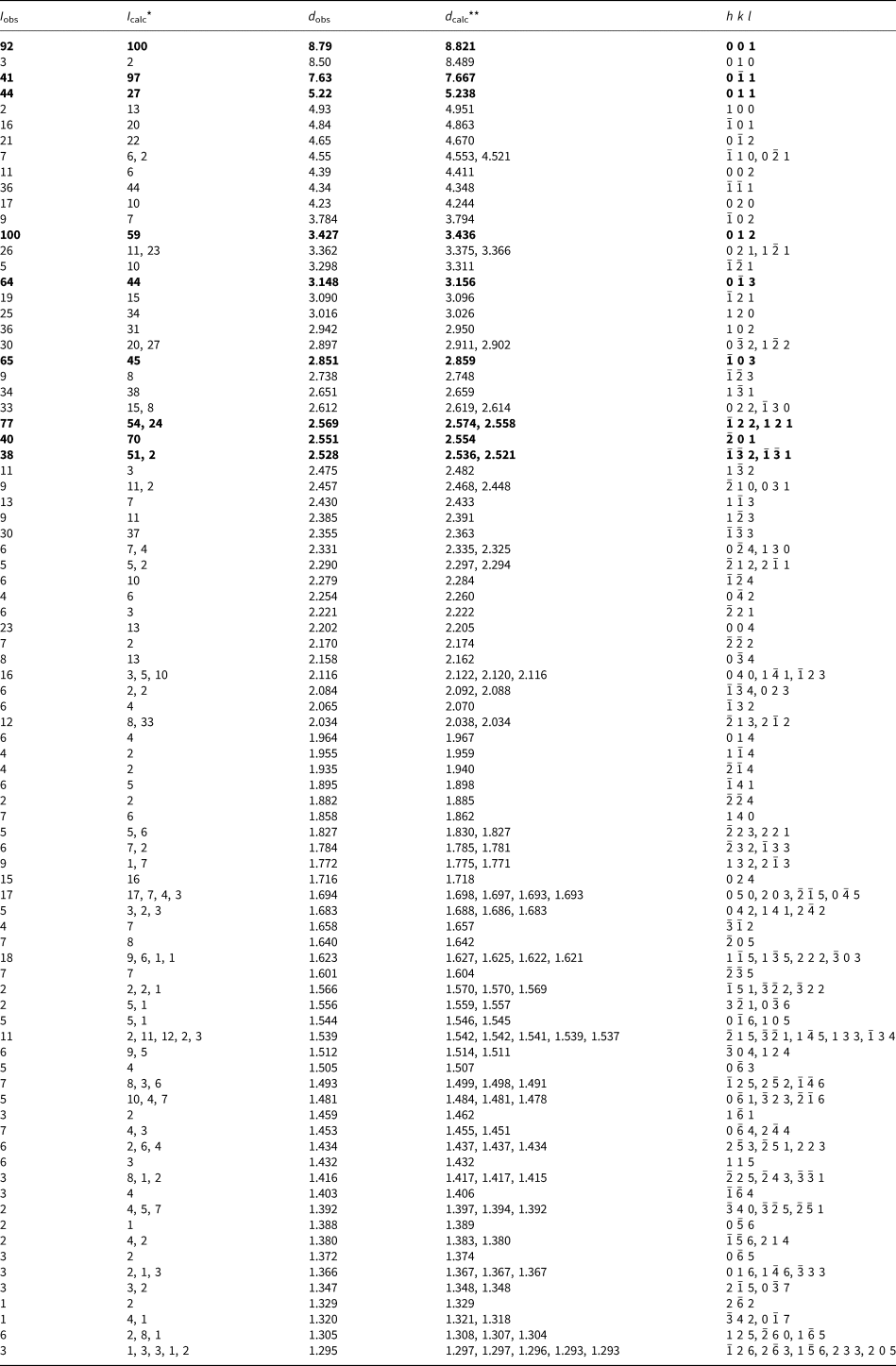
*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
Table 4. Powder X-ray diffraction data (d in Å) for alumoedtollite.
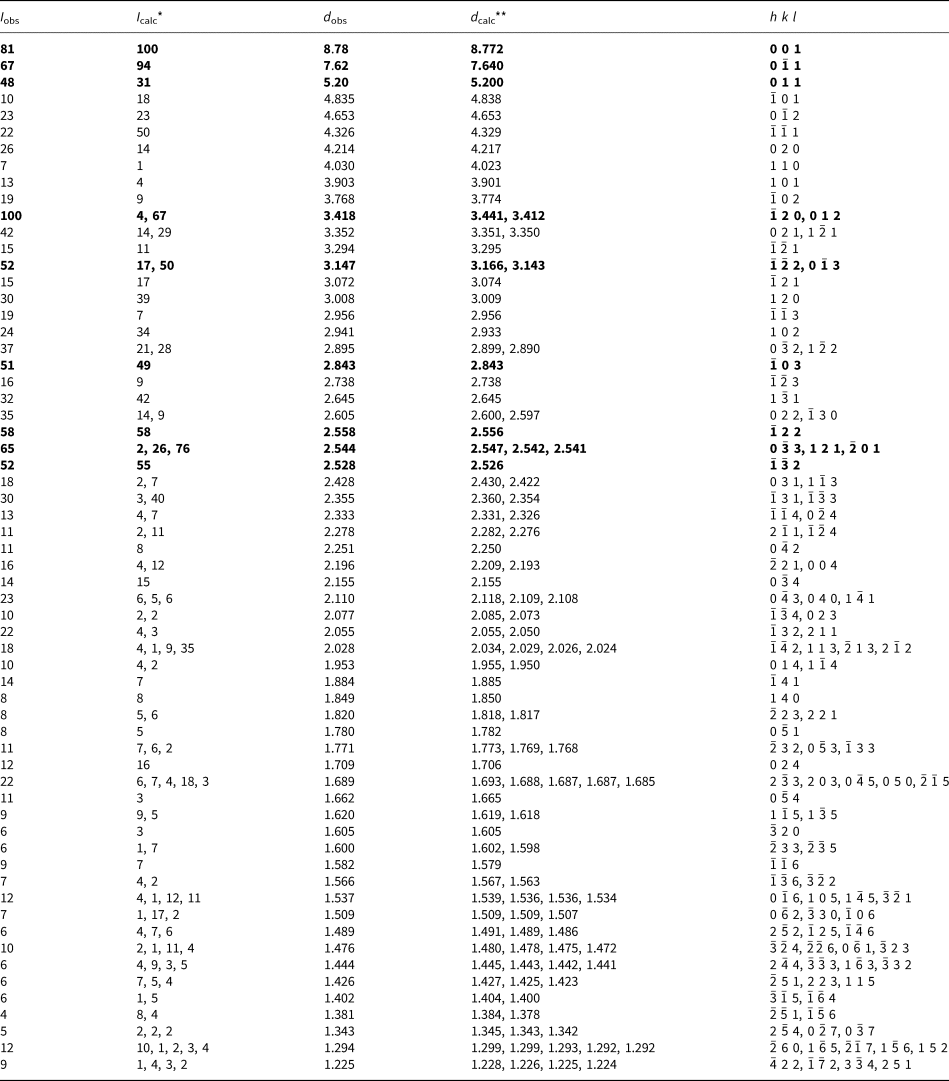
*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
The strongest lines are given in bold.
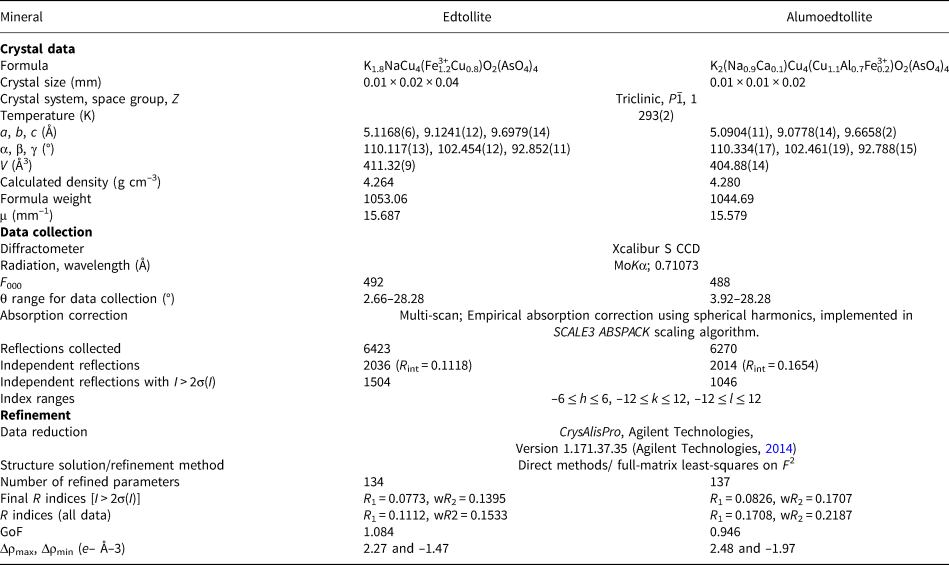
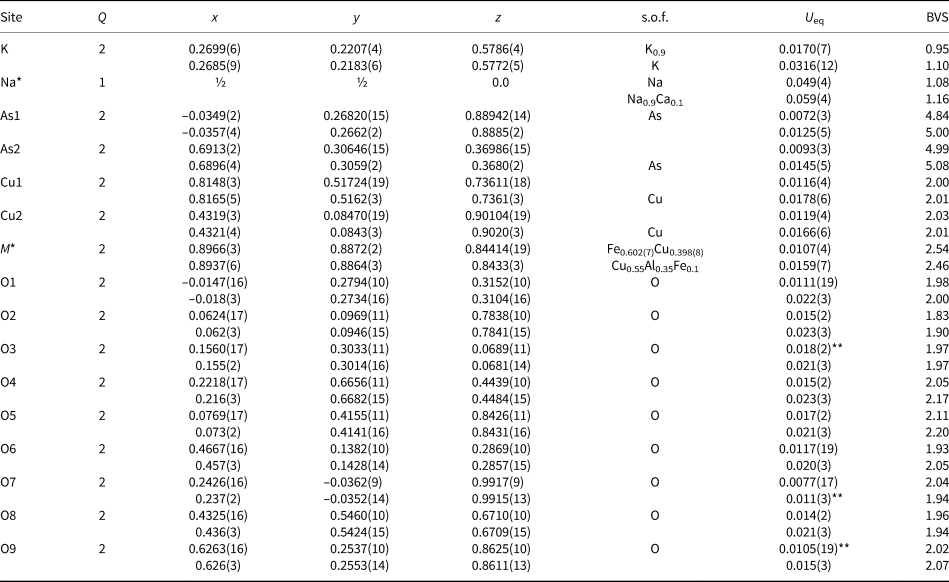
Single-crystal X-ray studies of both new minerals were carried out using an Xcalibur S diffractometer equipped with a CCD detector with a sample-to-detector distance of 45 mm, scan width (Δω) of 1° per frame during the data collection, and an exposure time of 200 s for edtollite and 300 s for alumoedtollite. The crystal structures were solved by direct methods and refined with the use of SHELX-97 software package (Sheldrick, Reference Sheldrick2008) using the neutral atom scattering factors. The refinement was performed in anisotropic approximation for all atoms except O3 and O9 sites in edtollite and O7 in alumoedtollite. Site occupancy factors for the K site in edtollite and Na and M sites in alumoedtollite were refined and fixed on the last cycles of the refinement in accordance with refined numbers of electrons, electron microprobe data and the requirement of charge-balanced formulae. The crystal data, data collection information and structure refinement details for edtollite and alumoedtollite are given in Table 5, fractional atomic coordinates and displacement parameters of atoms and bond-valence sums in Table 6, and selected interatomic distances in Table 7. Detailed bond-valence calculations are given in Supplementary material Tables S1a and S1b (see below).
Table 5. Crystal data, data collection information and structure refinement details for edtollite and alumoedtollite.
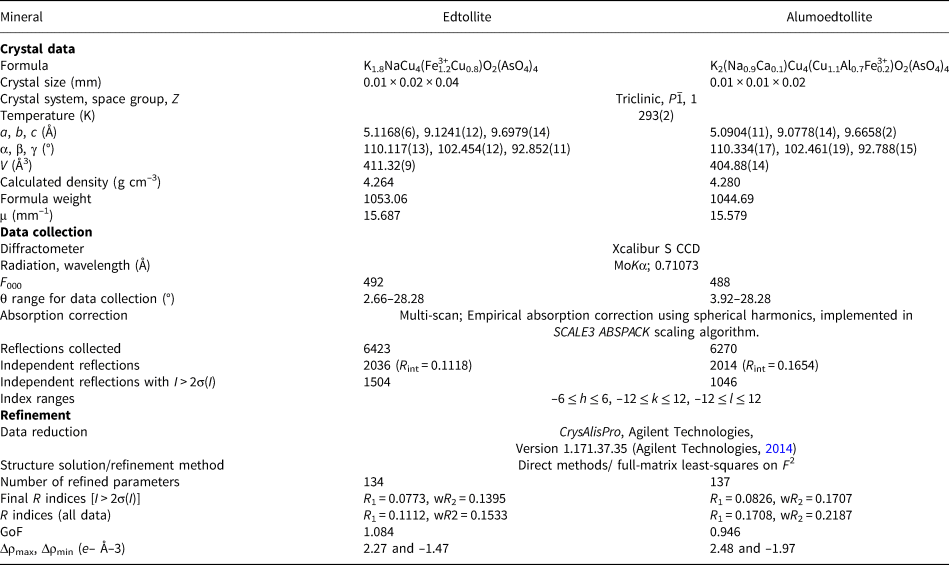
Table 6. Coordinates, site multiplicities (Q), site occupancy factors (s.o.f.), equivalent displacement parameters (U eq, in Å2) of atoms, and bond-valence sums (BVS) for edtollite (first line of each row) and alumoedtollite (second line of each row).
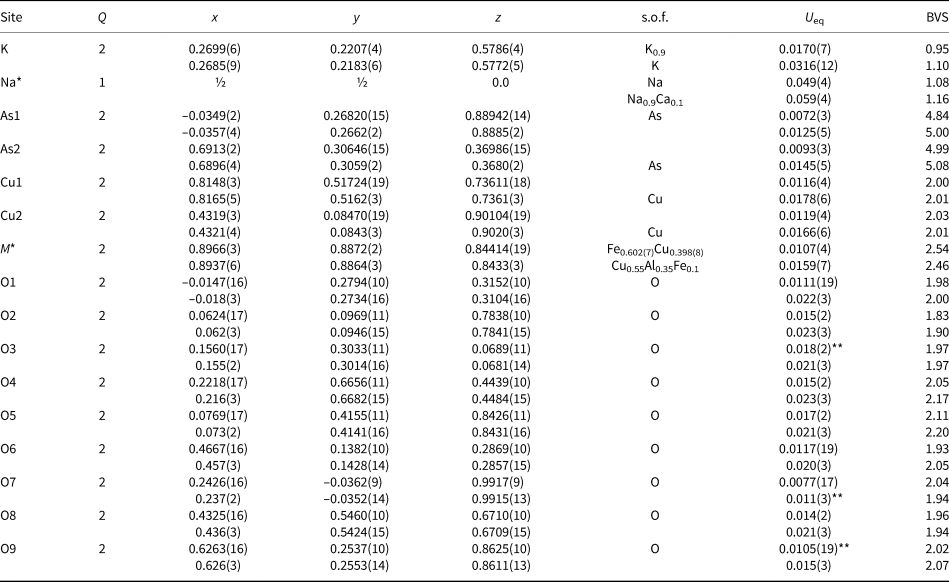
*Site occupancy factors for the K site in edtollite and the Na and M sites in alumoedtollite were fixed on the last stages of the refinement in accordance with refined numbers of electrons and electron microprobe data; **U iso. Parameters for bond valence calculations were taken from (Brese and O'Keeffe, Reference Brese and O`Keeffe1991).
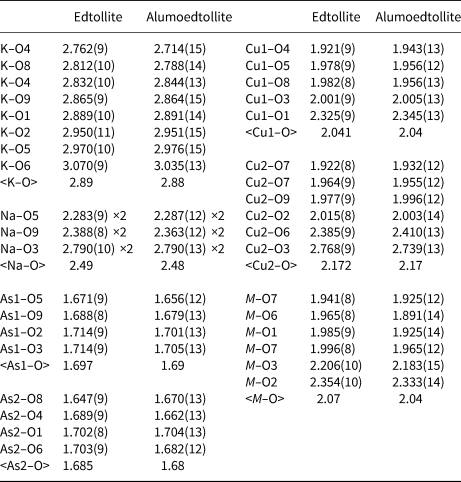
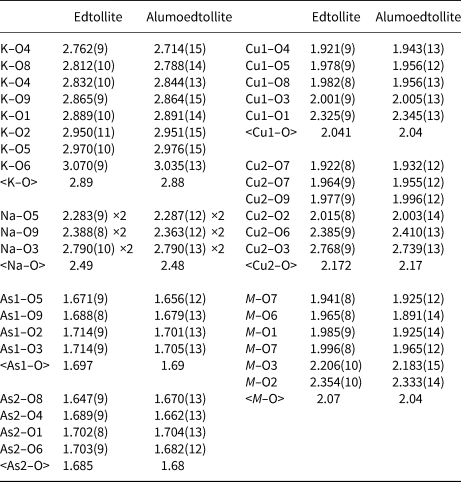
Table 7. Selected interatomic distances (Å) in the structures of edtollite and alumoedtollite.
Crystal structure
Edtollite and alumoedtollite show a novel structure type. They are isostructural and, thus, we give the shared crystal-structure drawings in Figs 6 and 7a, and use the generalised term ‘edtollites’ below if both minerals are meant. Their crystal structure (Fig. 6a) is based on the heteropolyhedral pseudo-framework having, as the main structural unit, a heteropolyhedral column (Fig. 6b) elongated along a and built as follows: Cu2-centred distorted octahedra with [4+1+1] Cu2+ coordination of which is four short Cu2–O bonds, one elongated bond and one strongly elongated bond; distorted octahedra MO6 with [4+2] coordination of which is four short M–O bonds and two elongated bonds; distorted Cu1-centred square pyramids; and isolated AsO4 tetrahedra. Cu2O6 and MO6 octahedra compose the axial part of the column (Fig. 7a). Cu2O6 octahedra are characterised by strong Jahn–Teller distortion, but this is not so significant (Table 7) for MO6 octahedra. Cu1-centred polyhedra share common edges with MO6 octahedra (Fig. 6a). The formula of the heteropolyhedral pseudo-framework of both minerals can be written as [Cu4M 2O2(AsO4)4]3–. Large cations K+ and Na+ are located in channels of the pseudo-framework. In detail, K occurs in wide channels and the centre of eight-fold polyhedra, whereas Na, along with minor Ca admixtures, is in the centre of distorted octahedra in narrow channels (Fig. 6a) that are isolated from each other.
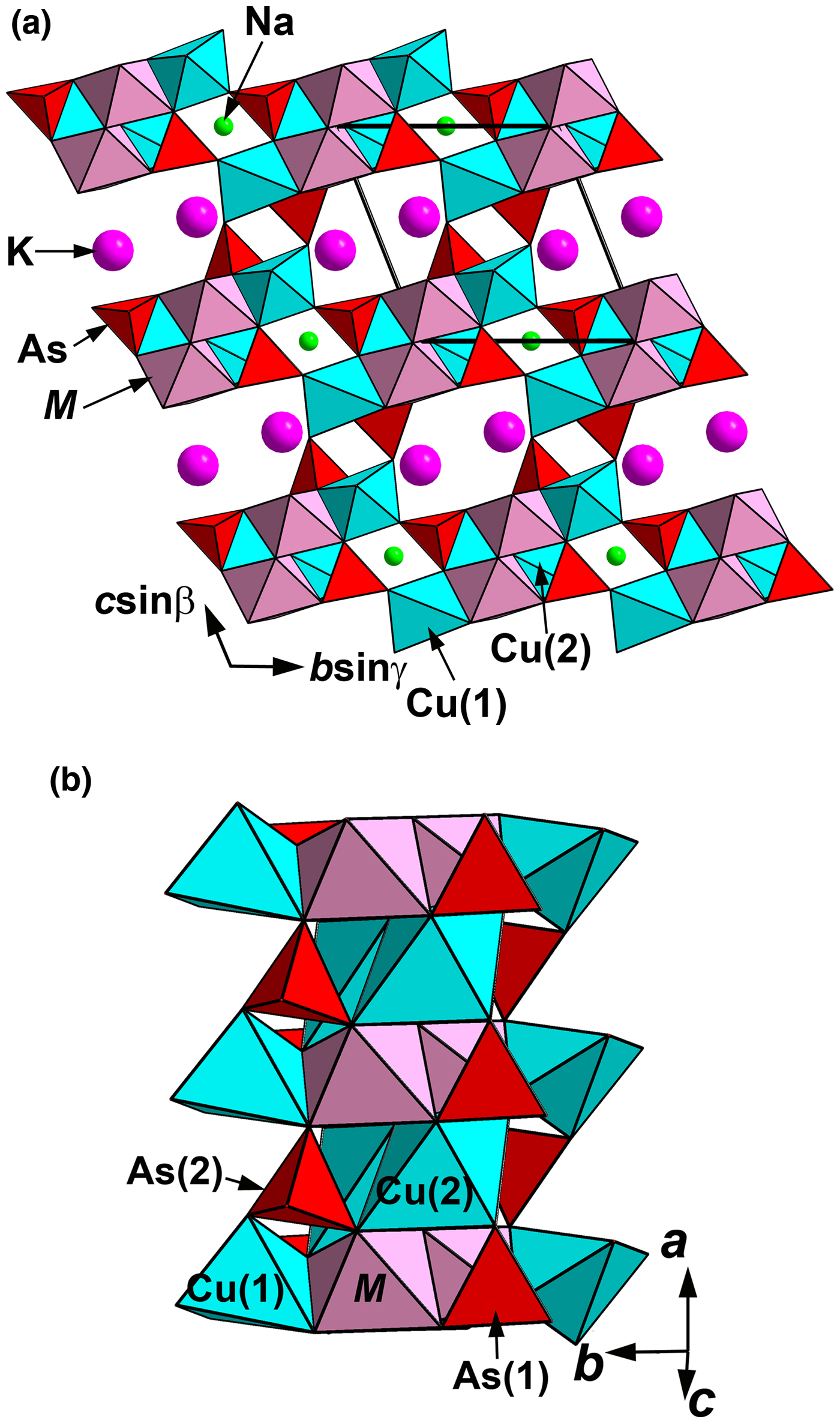
Fig. 6. The crystal structure of edtollite/alumoedtollite projected along the a axis (a) and its main structural unit, a heteropolyhedral Cu–M–As–O column (b). For legend see text and Table 6. The unit cell is outlined in (a).
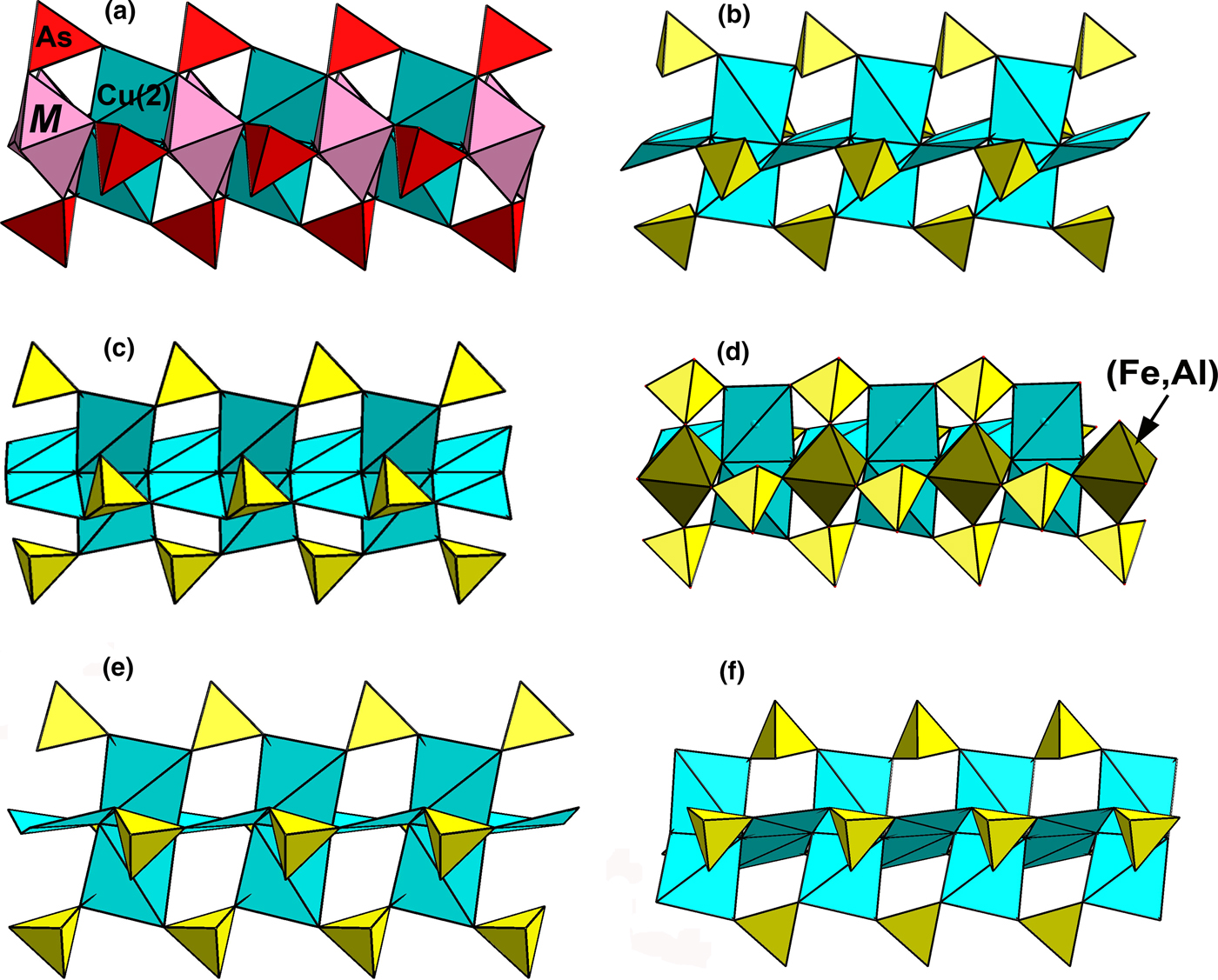
Fig. 7. The heteropolyhedral chains in edtollite/alumoedtollite (our data; only the axial part of the column presented in Fig. 6b is shown there) (a); and structurally related sulfates: eleomelanite (b: our data); piypite (c: drawn using data from Effenberger and Zemann, Reference Effenberger and Zemann1984); klyuchevskite (d: drawn using data by Gorskaya et al., Reference Gorskaya, Filatov, Rozhdestvenskaya and Vergasova1992); wulffite (e); and parawulffite (f) (drawn using data from Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Chukanov, Lykova, Saveliev, Sidorov and Pushcharovsky2014c). Cu-centred polyhedra are blue, SO4 tetrahedra are yellow.
Interatomic distances (Table 7) and bond-valence calculations (weighted bond-valence sums: Bosi, Reference Bosi2014) for the M site (Table 6) clearly indicate the occurrence of both divalent (Cu2+) and trivalent (Fe3+ and Al) cations in approximately equal amounts. The refined number of electrons in the M site in alumoedtollite (e −ref = 22.9) clearly shows that Al occurs there. The presence of Fe3+ in edtollites is consistent with strongly oxidising conditions in the Arsenatnaya fumarole (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a, Reference Pekov, Koshlyakova, Zubkova, Lykova, Britvin, Yapaskurt, Agakhanov, Shchipalkina, Turchkova and Sidorov2018b).
The general crystal chemical formula of edtollites is KK2NaNaCu(1–2)Cu4M(CuM 3+)O2(As(1–2)AsO4)4, where the superscript symbols show structure positions (Table 6); M 3+ = Fe or Al in edtollite or alumoedtollite, respectively. The distribution of cations in the empirical formulae reported above is made in accordance with this formula. The species-defining feature that causes a difference between edtollite and alumoedtollite is in the occupancy of the M site: for their end-members, it should be written as (CuAl) and (CuFe3+), respectively. Both electron microprobe and structure refinement data show that in edtollite the content of the M site is (Fe3+0.6Cu0.4) while in alumoedtollite it is (Cu0.55Al0.35Fe0.1). Thus these minerals are different in terms of both approaches, (1) using general predominance of a constituent in a site (M is Fe3+-dominant in edtollite and Cu2+-dominant in alumoedtollite) and (2) using the valency-imposed double site-occupancy principle: (Cu2+Fe3+) in edtollite and (Cu2+Al3+) in alumoedtollite. The latter approach is in use as a nomenclature rule accepted by the IMA CNMNC for such cases (Hatert and Burke, Reference Hatert and Burke2008: pp. 719–720). In particular, the name alumoedtollite was given in accordance with this rule. The simplified crystal-chemical formulae of edtollite and alumoedtollite can be written as K2NaCu4(CuFe3+)O2(AsO4)4 and K2NaCu4(CuAl)O2(AsO4)4, respectively.
The presence of admixed Ca in the Na site in alumoedtollite allows the full substitution of Na for Ca in this position to be assumed. It could result in the absence of a trivalent cation in the M site with the formation of the hypothetic phase with the end-member formula K2CaCu6O2(AsO4)4, a dimorph of shchurovskyite (see below) with the edtollite-type structure. Moreover, both Na and M sites share the oxygen atom O3 and this bond is the longest for the Na-centred polyhedron and one of the longest for the M polyhedron. Possible dominance of Ca at the Na site probably would reduce the Na(Ca)–O3 distance, while the M–O3 becoming longer would be in a good accordance with the dominance of Cu at the M site.
Relationship to other minerals
The axial part of the above-discussed heteropolyhedral column in edtollites formed by Cu2- and M-centred octahedra and AsO4 tetrahedra (Fig. 7a) is close to that found in several oxysulfates, namely eleomelanite (K2Pb)Cu4O2(SO4)4 (IMA2015-118, Pekov et al., Reference Pekov, Zubkova, Agakhanov, Chukanov, Belakovskiy, Sidorov, Britvin and Pushcharovsky2016b), klyuchevskite K3Cu3Fe3+O2(SO4)4 (Gorskaya et al., Reference Gorskaya, Filatov, Rozhdestvenskaya and Vergasova1992), alumoklyuchevskite K3Cu3AlO2(SO4)4 (Krivovichev et al., Reference Krivovichev, Filatov and Cherepansky2009; Siidra et al., Reference Siidra, Nazarchuk, Zaitsev, Lukina, Avdontseva, Vergasova, Vlasenko, Filatov, Turner and Karpov2017), wulffite K3NaCu4O2(SO4)4, parawulffite K5Na3Cu8O4(SO4)8 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Chukanov, Lykova, Saveliev, Sidorov and Pushcharovsky2014c), piypite K8Cu9O4(SO4)8Cl2 = K8Cu8O4(SO4)8·CuCl2 (Effenberger and Zemann, Reference Effenberger and Zemann1984) (Fig. 7), and two synthetic piypite-like phases K4Cu4O2(SO4)2·KCl (Effenberger, Reference Effenberger1985) and Na4Cu4O2(SO4)2·MeCl (Me = Na, Cu or □) (Kahlenberg et al., Reference Kahlenberg, Piotrowski and Giester2000). Structures of the above-listed minerals are shown in Supplementary material Fig. S1; for better clarity and comparison all Cu-centred polyhedra are shown as approximately planar squares whereas elongated Cu–O bonds are not included in the polyhedra, and only the axial part of the heteropolyhedral column for edtollites is shown in polyhedral presentation whereas Cu1 sites are given as circles. Close topology of the heteropolyhedral chains in edtollites, eleomelanite, piypite, wulffite, parawulffite, klyuchevskite and alumoklyuchevskite causes a comparable value of the unit-cell parameter in the direction parallel to the chain: 5.09 Å in alumoedtollite, 5.12 Å in edtollite and 4.9–5.0 Å in the sulfates.
Edtollite, ideally K2NaCu5Fe3+O2(AsO4)4, and alumoedtollite, ideally K2NaCu5AlO2(AsO4)4, demonstrate the same stoichiometry and the same ratios between large (K, Na or Ca) and medium-size (Cu, Fe or Al) metal cations as two other arsenate minerals closely associated with edtollites, namely shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015c). The latter and alumoedtollite have very similar idealised formulae, with the only difference being in the large-cation part: K3 in dmisokolovite and K2Na in alumoedtollite. However, the structural difference between edtollites and the pair of arsenates closely related to each other – shchurovskyite and dmisokolovite – is significant. Edtollites differ from shchurovskyite and dmisokolovite in atomic arrangement that causes strong differences in unit-cell dimensions and PXRD patterns. These structure features also strongly influence the physical properties: shchurovskyite and dmisokolovite are transparent green minerals with a vitreous lustre while edtollite is brown–black and alumoedtollite is bronze coloured, both with a semi-metallic lustre (Table 8).
Table 8. Comparative data of four arsenates with the same stoichiometry: edtollite, alumoedtollite, dmisokolovite and shchurovskyite.
It is worth noting the crystal chemical relationship (see Figs 7 and S1) between the oxyarsenate pair edtollite K2NaCu5Fe3+O2(AsO4)4–alumoedtollite K2NaCu5AlO2(AsO4)4 and the oxysulfate pair klyuchevskite K3Cu3Fe3+O2(SO4)4–alumoklyuchevskite K3Cu3AlO2(SO4)4 occurring in the same Arsenatnaya fumarole.
The crystal structure of edtollites could also be described in terms of anion-centred (oxocentred) tetrahedra (Krivovichev et al., Reference Krivovichev, Mentre, Siidra, Colmont and Filatov2013). O7 atoms are bonded only with Cu2 and M to form the [O2Cu2M 2]∞ chains built by edge-sharing OCu2M 2 tetrahedra and running along the a axis (Fig. 8). Similar chains built by tetrahedra [OFeCu3] and [OAlCu3] were described in klyuchevskite (Gorskaya et al., Reference Gorskaya, Filatov, Rozhdestvenskaya and Vergasova1992) and alumoklyuchevskite (Krivovichev et al., Reference Krivovichev, Filatov and Cherepansky2009), respectively; this feature emphasises the above-mentioned similarity of edtollites and these sulfates. Topologically the same chains built by OCu4 tetrahedra were found in eleomelanite (Pekov et al., Reference Pekov, Zubkova, Agakhanov, Chukanov, Belakovskiy, Sidorov, Britvin and Pushcharovsky2016b), piypite (Effenberger and Zemann, Reference Effenberger and Zemann1984), its synthetic sodium analogue Na2Cu2O(SO4)·MCl (M = Na, Cu or □) (Kahlenberg et al., Reference Kahlenberg, Piotrowski and Giester2000), coparsite, Cu4O2[(As,V)O4]Cl (Starova et al., Reference Starova, Krivovichev and Filatov1998), wulffite and parawulffite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Chukanov, Lykova, Saveliev, Sidorov and Pushcharovsky2014c). Unlike them, in shchurovskyite and dmisokolovite the motif of oxocentred tetrahedra is represented by isolated (from each other) dimers, [O2Cu6] and [O2AlCu5], respectively (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015c).
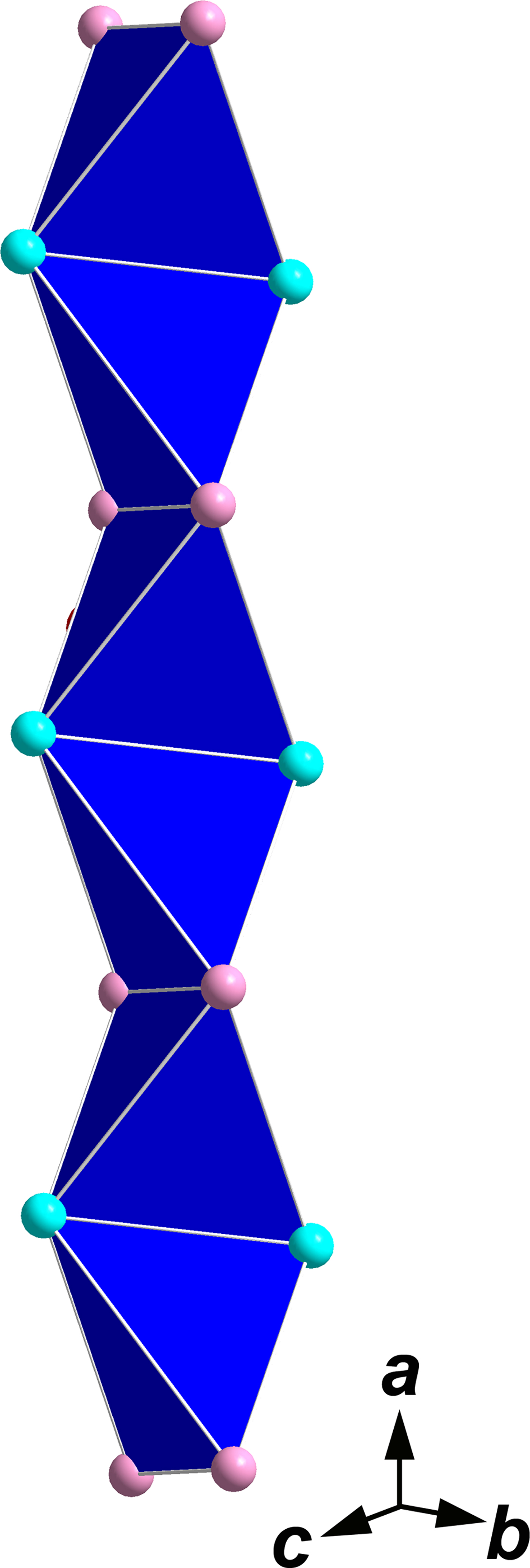
Fig. 8. Chain [O2Cu2M 2]∞ formed by oxocentred tetrahedra OCu2M 2 in the crystal structure of edtollite/alumoedtollite. Turquoise-coloured circles are Cu2+ cations and pink circles are M cations (see Table 6).
Acknowledgements
We thank referees Peter Leverett, John M. Hughes and Fernando Cámara, and Associate Editor Ferdinando Bosi for valuable comments. This study was supported by the Russian Foundation for Basic Research, grant no. 17-05-00179. The technical support by the SPbSU X-Ray Diffraction Resource Center in the PXRD study is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.155