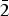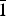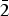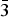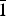Introduction
This paper continues a series of articles on new arsenate minerals from the Arsenatnaya fumarole located at the apical part of the Second scoria cone of the Northern Breakthrough of the Great Tolbachik Fissure Eruption, Tolbachik volcano, Kamchatka Peninsula, Far-Eastern Region, Russia (55°41′N, 160°14′E, 1200 m asl). This active fumarole, discovered by us in July 2012, is described in general in the first paper devoted to yurmarinite Na7(Fe3+,Mg,Cu)4(AsO4)6 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a). In other articles the following mineral species were characterized: two polymorphs of Cu4O(AsO4)2, ericlaxmanite and kozyrevskite (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2014b), popovite Cu5O2(AsO4)2 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Vigasina, Sidorov and Pushcharovsky2015a), structurally related shchurovskyite K2CaCu6O2(AsO4)4 and dmisokolovite K3Cu5AlO2(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Belakovskiy, Yapaskurt, Vigasina, Sidorov and Pushcharovsky2015b), katiarsite KTiO(AsO4) (Pekov et al., Reference Pekov, Yapaskurt, Britvin, Zubkova, Vigasina and Sidorov2016a), melanarsite K3Cu7Fe3+O4(AsO4)4 (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Polekhovsky, Vigasina, Belakovskiy, Britvin, Sidorov and Pushcharovsky2016b) and pharmazincite KZnAsO4 (Pekov et al., Reference Pekov, Yapaskurt, Belakovskiy, Vigasina, Zubkova and Sidorov2017).
This paper is devoted to the new mineral arsenowagnerite Mg2(AsO4)F (Cyrillic: арсеновагнерит). It is named as an arsenate analogue of wagnerite Mg2(PO4)F. Both the new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification (IMA2014–100). The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, under the catalogue number 95000.
Occurrence and general appearance
The first specimen of the new mineral that became the holotype was found by us in July 2014. During fieldwork in 2015 and 2016 we collected more material that gave additional information on the morphology and mineral association of arsenowagnerite.
In the holotype specimen, collected at a depth of two metres below the surface, arsenowagnerite occurs as crude tabular crystals and euhedral grains up to 0.3 mm in size combined in crusts that cover coarse spherulites mainly consisting of johillerite, tilasite and anhydrite (Figs 1a and 2). These complex sulfate–arsenate aggregates overgrow basalt scoria. The crusts of the new mineral, covering botryoidal clusters of the anhydrite–tilasite–johillerite spherulites, are up to 0.5 cm x 1 cm in area and up to 0.4 mm thick. Arsenowagnerite crystals are skeletal, typically case-like, and its aggregates are open-work. Small grains (up to 0.02 mm) of arsenowagnerite are also observed inside the anhydrite–tilasite–johillerite spherulites where they form intimate intergrowths with these three minerals (Fig. 2) and sometimes also with hematite.
Fig. 1. Morphology of arsenowagnerite aggregates. (a) The holotype: yellowish crust covering an anhydrite-tilasite–johillerite spherulite and surrounded by aggregates of blue johillerite and pinkish-brownish tilasite (in the lower part of the figure); and (b) abundant transparent pale yellowish crystals with deep red cassiterite on iron-black hematite crystal crust. Fields of view: (a) 4.4 mm; (b) 3.5 mm. Photos: I.V. Pekov and A.V. Kasatkin.

Fig. 2. Back-scattered electron image of a polished section of the holotype specimen showing a crust of skeletal, case-like, crude crystals of arsenowagnerite (1) covering an anhydrite–tilasite–johillerite aggregate with subordinate arsenowagnerite (2–4): 2 – tilasite; 3 – intimate intergrowths of johillerite (white elongated crystals), tilasite and arsenowagnerite (both light grey); 4 – anhydrite.
At depths of 2.5–3 m below the surface arsenowagnerite was found to be more abundant than at higher levels of the fumarole. In some areas it occurs as interrupted crusts up to 1.5 cm × 3 cm in area and up to 1 mm thick consisting of distorted, typically skeletal crystals up to 1 mm across (Figs 1b and 2). Well-shaped equant or tabular crystals (Fig. 3) up to 0.5 mm are rarer. Arsenowagnerite aggregates cover hematite and fluorophlogopite crystal crusts or directly overgrow basalt scoria. Other associated minerals there are cassiterite, calciojohillerite [NaCaMg3(AsO4)3, IMA2016-068], johillerite, nickenichite, svabite, berzeliite, tilasite, anhydrite, aphthitalite, metathénardite (hexagonal Na2SO4, IMA2015-102), krasheninnikovite and fluoborite. Arsenowagnerite crystals commonly contain numerous inclusions of other arsenates, hematite, cassiterite, fluorophlogopite and particles of basalt scoria.

Fig. 3. (a,b) Scanning electron microscopy images of crystals of arsenowagnerite.
Temperatures measured by us using a chromel-alumel thermocouple in areas with arsenowagnerite at the time of its collecting were 360–450°C. We believe that the new mineral was formed at temperatures not lower than 450°C as a result of the interaction between fumarolic gas (an obvious source of As, O and F) and basalt scoria, the most probable source of Mg which has low volatility at temperatures at least of 500°C (Symonds and Reed, Reference Symonds and Reed1993).
Physical properties and optical data
Arsenowagnerite is transparent with vitreous lustre. It is typically light yellow to lemon-yellow, sometimes pale greenish-yellow or colourless. Its streak is white. Arsenowagnerite demonstrates weak orange-red fluorescence in short-wave (λ = 245 nm) ultraviolet (UV) light and does not fluoresce under long-wave (330 nm) UV irradiation. The mineral is brittle. One direction of distinct cleavage was observed under the microscope; by analogy with structurally related wagnerite and sarkinite, we assume that its direction is {001}. The fracture is uneven. The Mohs hardness is ~5. Density could not be measured correctly because of the cavernous, case-like character of individuals of the mineral (Fig. 2) and numerous inclusions in them. Density calculated using the empirical formula is 3.698 g cm–3.
In plane polarized light arsenowagnerite is colourless and non-pleochroic. It is optically biaxial (+), α = 1.614(2), β = 1.615(2), γ = 1.640(2) (589 nm), 2Vmeas = 25(5)° and 2Vcalc = 23°. Dispersion of optical axes was not observed.
Infrared spectroscopy
In order to obtain the infrared (IR) absorption spectrum (Fig. 4), a powdered sample of arsenowagnerite was mixed with dried KBr, pelletized, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) with a resolution of 4 cm–1 and 16 scans accumulated. The IR spectrum of an analogous pellet of pure KBr was used as a reference.

Fig. 4. The powder IR absorption spectrum of arsenowagnerite.
Wavenumbers of absorption bands in the IR spectrum of arsenowagnerite and their assignments (cm–1; s – strong band, sh – shoulder) are: 900sh, 874s, 861s, 840sh, 820sh (stretching vibrations of As![]() ${\rm O}_{\rm 4}^{3\ndash} $ groups); 561, 525sh, 507s, 491s, 470s, 443, 417 (bending vibrations of As
${\rm O}_{\rm 4}^{3\ndash} $ groups); 561, 525sh, 507s, 491s, 470s, 443, 417 (bending vibrations of As![]() ${\rm O}_{\rm 4}^{3\ndash} $ groups combined with Mg–O-stretching vibrations); 375 (lattice mode, possibly involving Mg–F-stretching vibrations). Weak shoulders at 1090 and 1140 cm–1 correspond to trace amounts of the sulfate anion.
${\rm O}_{\rm 4}^{3\ndash} $ groups combined with Mg–O-stretching vibrations); 375 (lattice mode, possibly involving Mg–F-stretching vibrations). Weak shoulders at 1090 and 1140 cm–1 correspond to trace amounts of the sulfate anion.
Numerous bands in the ranges from 800 to 900 and from 400 to 600 cm–1 reflect the presence of numerous non-equivalent sites of As![]() ${\rm O}_{\rm 4}^{3\ndash} $ groups and Mg2+ cations in the crystal structure of arsenowagnerite (see below). Bands corresponding to O–H, C–O and B–O bonds are absent in the IR spectrum of the mineral.
${\rm O}_{\rm 4}^{3\ndash} $ groups and Mg2+ cations in the crystal structure of arsenowagnerite (see below). Bands corresponding to O–H, C–O and B–O bonds are absent in the IR spectrum of the mineral.
Chemical composition
Chemical composition of arsenowagnerite was determined on a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Dept. of Petrology, Moscow State University), with an acceleration voltage of 20 kV, a beam current of 20 nA and a 3 µm beam diameter. The standards used are: CaWO4 (Ca), diopside (Mg, Si), MnTiO3 (Mn, Ti), CuFeS2 (Cu, Fe), ZnS (Zn, S), GaP (P), V (V), FeAsS (As) and MgF2 (F).
The chemical composition of the new mineral is consistent and only slightly varies in different samples. The average (over six spot analyses) chemical composition of the holotype specimen (wt.%, ranges are in parentheses) is: MgO 38.72 (38.04–39.28), CaO 0.23 (0.12–0.38), MnO 0.32 (0.08–0.61), CuO 0.60 (0.23–0.85), ZnO 0.05 (0.00–0.14), Fe2O3 0.11 (0.07–0.22), TiO2 0.03 (0.00–0.06), SiO2 0.08 (0.04–0.09), P2O5 0.18 (0.00–0.40), V2O5 0.03 (0.00–0.07), As2O5 54.96 (54.27–56.29), SO3 0.10 (0.00–0.44), F 8.91 (8.61–9.20), –O=F –3.75, total 100.57. Admixed iron is considered as Fe3+ because of the extremely oxidizing conditions of mineral formation in the Arsenatnaya fumarole (Pekov et al., Reference Pekov, Zubkova, Yapaskurt, Belakovskiy, Lykova, Vigasina, Sidorov and Pushcharovsky2014a). Contents of other elements with atomic numbers higher than carbon are below detection limits.
The empirical formula, calculated on the basis of 5 anions (O + F) per formula unit, is: (Mg1.98Cu0.02Mn0.01Ca0.01)Σ2.02(As0.99P0.01)Σ1.00O4.03F0.97. The idealized formula is Mg2(AsO4)F, which requires MgO 39.03, As2O5 55.64, F 9.20, –O=F –3.87, total 100.00 wt.%.
The Gladstone-Dale compatibility index 1 – (Kp/Kc) = –0.018, superior (Mandarino, Reference Mandarino2007).
X-ray crystallography and crystal structure
Powder X-ray diffraction data of arsenowagnerite (Table 1) were obtained using a camera RKU-114.6 (Debye-Scherrer geometry, d = 114.6 mm, FeKα-radiation). Monoclinic unit-cell parameters calculated from the powder data are: a = 9.858(2), b = 12.964(2), c = 12.332(3) Å, β = 109.32(2)° and V = 1487.2(8) Å3.
Table 1. Powder X-ray diffraction data of arsenowagnerite.

*For the calculated pattern, only reflections with intensities ≥1 are given; **for the unit-cell parameters calculated from single-crystal data.
Single-crystal X-ray studies of the new mineral were carried out using an Xcalibur S diffractometer equipped with a CCD detector. A full sphere of three-dimensional data was collected. Data reduction was performed using CrysAlisPro Version 1.171.37.34 (Agilent Technologies, 2014). The data were corrected for Lorentz and polarization effects. The crystal structure of arsenowagnerite was solved by direct methods and refined with the use of the SHELX-97 software package (Sheldrick, Reference Sheldrick2008) to R = 0.0485. The unit-cell parameters and the experimental details are presented in Table 2, atom coordinates and equivalent displacement parameters in Table 3, selected interatomic distances in Table 4 and bond-valence calculations in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 2. Crystal data, data collection information and structure refinement details for arsenowagnerite.

Table 3. Сoordinates and equivalent displacement parameters (U eq, in Å2) of atoms for arsenowagnerite.

*U iso.
Table 4. Selected interatomic distances (Å) in the structure of arsenowagnerite.

* ϕ = unspecified ligand.
Table 5. Bond-valence calculations* for arsenowagnerite.

*Bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O`Keeffe1991).
In the crystal structure of arsenowagnerite (Fig. 5) almost regular AsO4 tetrahedra, distorted MgO4F2 octahedra and distorted MgO4F trigonal bipyramids share either edges or vertices to build up a complex three-dimensional network. The new mineral belongs to the wagnerite-Ma2bc structure type (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014; Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014). Its structure is close to those described for wagnerite, ideally Mg2(PO4)F (Coda et al., Reference Coda, Giuseppetti, Tadini and Carobbi1967), its OH-dominant analogue hydroxylwagnerite Mg2(PO4)(OH,F) (Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014), synthetic β-Mg2(PO4)OH (Raade and Rømming, Reference Raade and Rømming1986), sarkinite, ideally ![]() ${\rm Mn}_{\rm 2}^{2 +} $(AsO4)(OH) (Dal Negro et al., Reference Dal Negro, Giuseppetti and Pozas1974), and its synthetic analogue (Stock et al., Reference Stock, Stucky and Cheetham2002).
${\rm Mn}_{\rm 2}^{2 +} $(AsO4)(OH) (Dal Negro et al., Reference Dal Negro, Giuseppetti and Pozas1974), and its synthetic analogue (Stock et al., Reference Stock, Stucky and Cheetham2002).
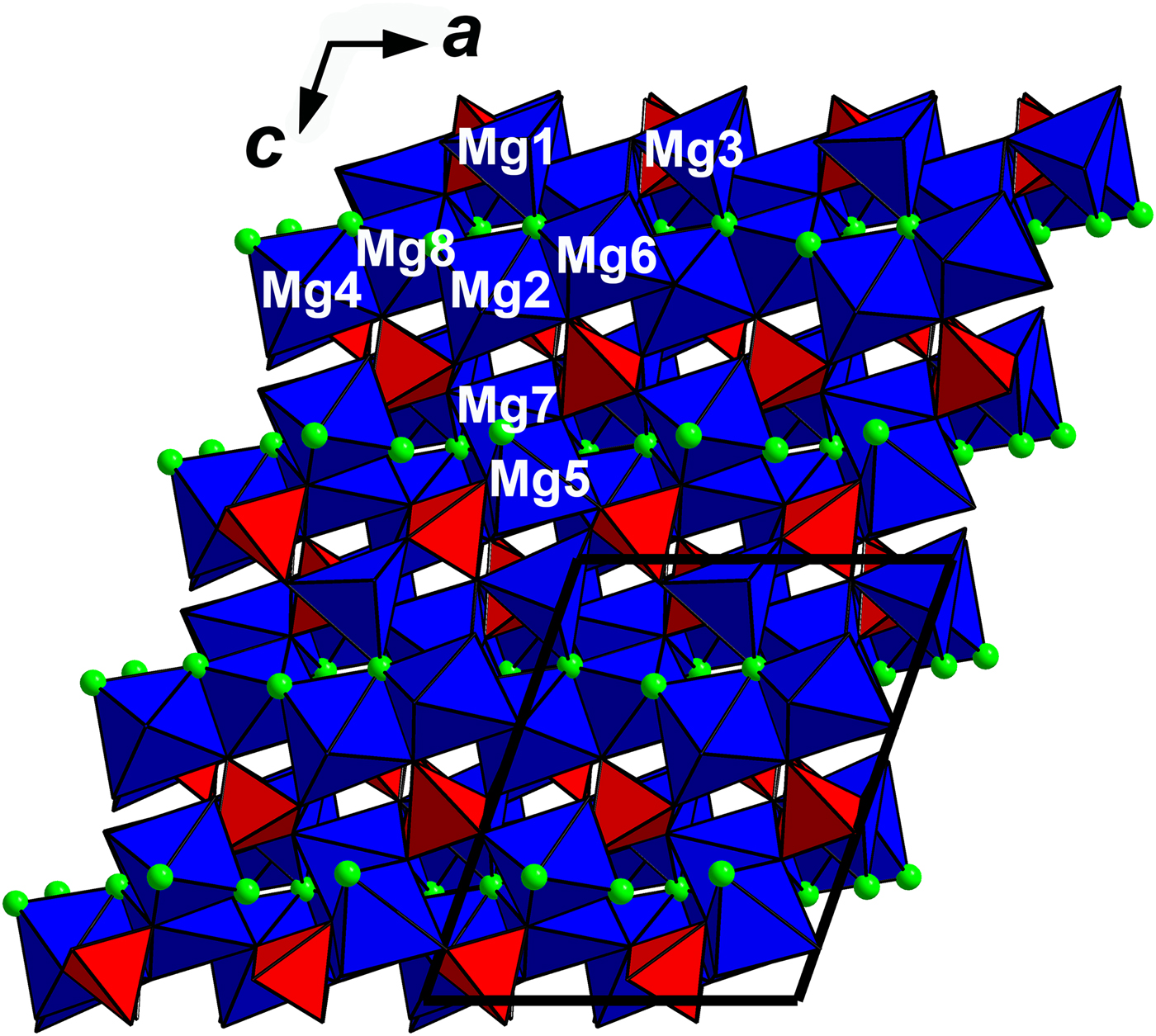
Fig. 5. The crystal structure of arsenowagnerite. Mg-centred polyhedra are blue, red tetrahedra are AsO4 groups and green circles are F atoms. The numbers of Mg polyhedra correspond to those in Tables 3–5. The unit cell is outlined.
Similarly to synthetic β-Mg2(PO4)(OH), some deficiency in the valence sums for Mg(6) and Mg(8) was revealed (Table 5). According to Raade and Rømming (Reference Raade and Rømming1986), it is related to a higher degree of distortion of these two pseudo-octahedra with the two longest Mg–O distances in the structure (Table 4): Mg(6)–O(4) = 2.156 and Mg(8)–O(2) = 2.205 Å.
Discussion
Arsenowagnerite Mg2(AsO4)F is a member of the triplite group belonging to the triplite–triploidite supergroup (Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014). It is an arsenate analogue of wagnerite Mg2(PO4)F and a magnesium and fluorine analogue of sarkinite ![]() ${\rm Mn}_{\rm 2}^{2 +} $(AsO4)OH. Comparative data for these three minerals are given in Table 6.
${\rm Mn}_{\rm 2}^{2 +} $(AsO4)OH. Comparative data for these three minerals are given in Table 6.
Table 6. Comparative data of arsenowagnerite, wagnerite and sarkinite.

*Wagnerite-Ma2bc (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014; Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014). **In the original paper (Dal Negro et al., Reference Dal Negro, Giuseppetti and Pozas1974), the space group P21/a was given; we have changed the setting for better comparison with wagnerite and arsenowagnerite.
Wagnerite is represented in nature by several polytype modifications (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014, and references therein). Their diversity is caused by partial substitutions of Fe2+, Mn2+, Ca2+, Ti4+ or Fe3+ for Mg2+ and of OH– or O2– for F– which causes significant variations in bond lengths. As a result of this, individual coordination polyhedra around cation sites are locally modified with regards to coordination number and geometry, and this may affect the geometry of the whole structure (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014). The structural relationship between wagnerite and triplite, ideally ![]() ${\rm Mn}_{\rm 2}^{2 +} $(PO4)F, with b ≈ 6.45 Å (Waldrop, Reference Waldrop1969) led to the proposal to consider wagnerite as a polytypic series based on the smallest, triplite-type unit cell. In the crystal structure and unit-cell dimensions, arsenowagnerite is close to the polytype modification of wagnerite with b ≈ 13 Å (~2b of triplite) now considered as wagnerite-Ma2bc (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014; Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014). It should be noted that this polytype is the only known for wagnerite samples chemically close to the end-member Mg2(PO4)F (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014). We do not exclude the same correspondence between chemical composition and structure for arsenowagnerite.
${\rm Mn}_{\rm 2}^{2 +} $(PO4)F, with b ≈ 6.45 Å (Waldrop, Reference Waldrop1969) led to the proposal to consider wagnerite as a polytypic series based on the smallest, triplite-type unit cell. In the crystal structure and unit-cell dimensions, arsenowagnerite is close to the polytype modification of wagnerite with b ≈ 13 Å (~2b of triplite) now considered as wagnerite-Ma2bc (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014; Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014). It should be noted that this polytype is the only known for wagnerite samples chemically close to the end-member Mg2(PO4)F (Lazic et al., Reference Lazic, Armbruster, Chopin, Grew, Baronnet and Palatinus2014). We do not exclude the same correspondence between chemical composition and structure for arsenowagnerite.
No data on synthetic Mg2(AsO4)F were found by us in the literature and databases. The crystal structures of synthetic ![]() ${\rm Fe}_{\rm 2}^{2 +} $(AsO4)F (Berrocal et al., Reference Berrocal, Mesa, Pizarro, Urtiaga, Arriortua and Rojo2006) and Cd2(AsO4)F (Engel, Reference Engel1989) are generally close to that of triplite (Waldrop, Reference Waldrop1969) and synthetic Mn2(PO4)F (Rea and Kostiner, Reference Rea and Kostiner1972). Thus, arsenowagnerite is the first fluoroarsenate belonging to the wagnerite-Ma2bc structure type. In accordance with the nomenclature of polytypes of triplite-group members (Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014), the new mineral could be considered as arsenowagnerite-Ma2bc.
${\rm Fe}_{\rm 2}^{2 +} $(AsO4)F (Berrocal et al., Reference Berrocal, Mesa, Pizarro, Urtiaga, Arriortua and Rojo2006) and Cd2(AsO4)F (Engel, Reference Engel1989) are generally close to that of triplite (Waldrop, Reference Waldrop1969) and synthetic Mn2(PO4)F (Rea and Kostiner, Reference Rea and Kostiner1972). Thus, arsenowagnerite is the first fluoroarsenate belonging to the wagnerite-Ma2bc structure type. In accordance with the nomenclature of polytypes of triplite-group members (Chopin et al., Reference Chopin, Armbruster, Grew, Baronnet, Leyx and Medenbach2014), the new mineral could be considered as arsenowagnerite-Ma2bc.
Acknowledgements
We thank Evgeny Galuskin, Peter Leverett and an anonymous referee for valuable comments. This study was supported by the Russian Foundation for Basic Research, grant no. 17-05-00179.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.067