Introduction
Zircons that have experienced high-temperature metamorphism (≥900°C) have been shown to contain ‘metallic-Pb nanospheres’ (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015; Whitehouse et al., Reference Whitehouse, Kusiak, Wirth and Ravindra Kumar2017). This discovery is critical for robust interpretation of radioisotopic data in metamorphic terranes, as Pb heterogeneity at the sub-micrometre scale exerts a limiting factor on the accuracy and precision of U–Pb radiometric age determination (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015, Reference Kusiak, Wilde, Wirth, Whitehouse, Dunkley, Lyon, Reddy, Berry, de Jonge, Moser, Corfu, Darling, Reddy and Tait2017; Whitehouse et al., Reference Whitehouse, Kusiak, Wirth and Ravindra Kumar2017; Ge et al., Reference Ge, Wilde, Nemchin, Whitehouse, Bellucci and Erickson2019). However, understanding of the range of environments and P–T–t paths in which metallic-Pb nanospheres form is limited, and well documented examples are confined to just two localities from ultrahigh temperature (>900°C, 0.7–1.3 GPa; UHT) metamorphic terranes. Metallic-Pb nanospheres have, to date, only been characterised using transmission electron microscopy (TEM) and are estimated to contain in excess of 139,000 Pb atoms within a ~20 nm diameter nanosphere (Kusiak et al., Reference Kusiak, Wilde, Wirth, Whitehouse, Dunkley, Lyon, Reddy, Berry, de Jonge, Moser, Corfu, Darling, Reddy and Tait2017). Investigations of Pb in zircon at the atomic scale using atom probe tomography (Valley et al., Reference Valley, Cavosie, Ushikubo, Reinhard, Lawrence, Larson, Clifton, Kelly, Wilde, Moser and Spicuzza2014, Reference Valley, Reinhard, Cavosie, Ushikubo, Lawrence, Larson, Kelly, Snoeyenbos and Strickland2015; Piazolo et al., Reference Piazolo, La Fontaine, Trimby, Harley, Yang, Armstrong and Cariney2016; Peterman et al., Reference Peterman, Reddy, Saxey, Fougerouse, Snoeyenbos and Rickard2019; Arcuri et al., Reference Arcuri, Moser, Reinhard, Langelier and Larson2020), describes ‘atomic Pb clusters’, however these are relatively small in terms of Pb concentration, typically comprising no more than a few thousand Pb atoms, and are thus distinct from metallic-Pb nanospheres.
Metallic-Pb nanospheres were first recognised in granulite-facies rocks from the Napier Complex, Enderby Land, East Antarctica (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015). This gneissic terrane experienced UHT metamorphism at ~2.55–2.48 Ga, with peak temperatures of ~1050–1120°C, and pressures of 7–11 kbar (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015). Metallic-Pb nanospheres are sparsely and randomly distributed as isolated spheres or sphere clusters, occurring either within poorly crystalline zircon, or amorphous Ti–Al-rich silicate melt inclusions (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015). At the grain-scale however, zircon appears non-metamict. Kusiak et al. (Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015) proposed a formation model in which nanoscale ‘islands’ of crystallinity within radiation-damaged zircon acted as nucleation sites, whereby lattice recrystallisation during metamorphism ‘trapped’ mobile radiogenic Pb in amorphous zircon domains. As temperatures increased, melting of amorphous domains generated locally anomalous concentrations of Pb, resulting in metal–silicate immiscibility during cooling. The ages of metallic-Pb nanospheres from the same Antarctic samples were obtained using nanoscale secondary ion mass spectrometry (Lyon et al., Reference Lyon, Kusiak, Wirth, Whitehouse, Dunkley, Wilde, Schaumlöffel, Malherbe and Moore2019), yielding model 207Pb/206Pb ages for host zircon crystallisation (3.11 Ga) and metallic-Pb nanospheres formation (2.61 Ga), substantiating that U–Pb ratios in metallic-Pb nanospheres record the timing of UHT metamorphism.
The second occurrence of metallic-Pb nanospheres, from a metamorphosed granite in the Kerala Khondalite Belt, India, underwent metamorphism (at least 900°C) at ~570 Ma (Whitehouse et al., Reference Whitehouse, Kusiak, Wirth and Ravindra Kumar2017). In this case, metallic-Pb nanospheres are considerably smaller, scarcer, and free from amorphous Si-rich phases, instead occurring in highly crystalline zircon. Such differences in occurrences raised debate about their formation and to melt immiscibility being refuted as a generic model (Whitehouse et al., Reference Whitehouse, Kusiak, Wirth and Ravindra Kumar2017). Further cases have been described briefly from tonalitic gneisses in the Saglek Block, Labrador, Canada and quartzites from the Dniester-Bug Series, Odesa quarry, Ukraine (Kusiak et al., Reference Kusiak, Wirth, Dunkley, Shumlansky, Whitehouse and Wilde2020). The example from Labrador probably formed below UHT conditions, somewhere between amphibolite and granulite facies (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse and Wilde2019).
Knowing that Pb is highly mobile in radiation-damaged zircon and that metallic Pb has a low melting point (327.5°C), metallic-Pb nanospheres should form in a wider range of geological environments and P–T–t conditions than presently acknowledged, for example, below UHT conditions. The Gawler Craton (South Australia) has a protracted geological history spanning the Archean to Mesoproterozoic and hosts metamorphism-related Au-deposits in the northwest of the craton, and iron-oxide copper gold (IOCG) deposits of the Olympic Cu–Au Province on the eastern craton margin (e.g. Reid Reference Reid2019; Fig. 1). The present work analysed zircon from the Archean–Paleoproterozoic Challenger Au deposit, in which Pb-rich nanoparticles are visible on polished grain surfaces in back-scattered electron (BSE) images. This detailed characterisation was carried out to: (1) explore the possibility of metallic-Pb nanosphere formation in ore deposits, and also outside UHT terranes; (2) investigate the possible links between metamorphism, hydrothermal alteration and ore formation with formation of metallic-Pb nanospheres, and in doing so compare modes of occurrence with previously documented examples; and (3) obtain resolvable, atomic-scale resolution images of metallic-Pb nanospheres for the first time.
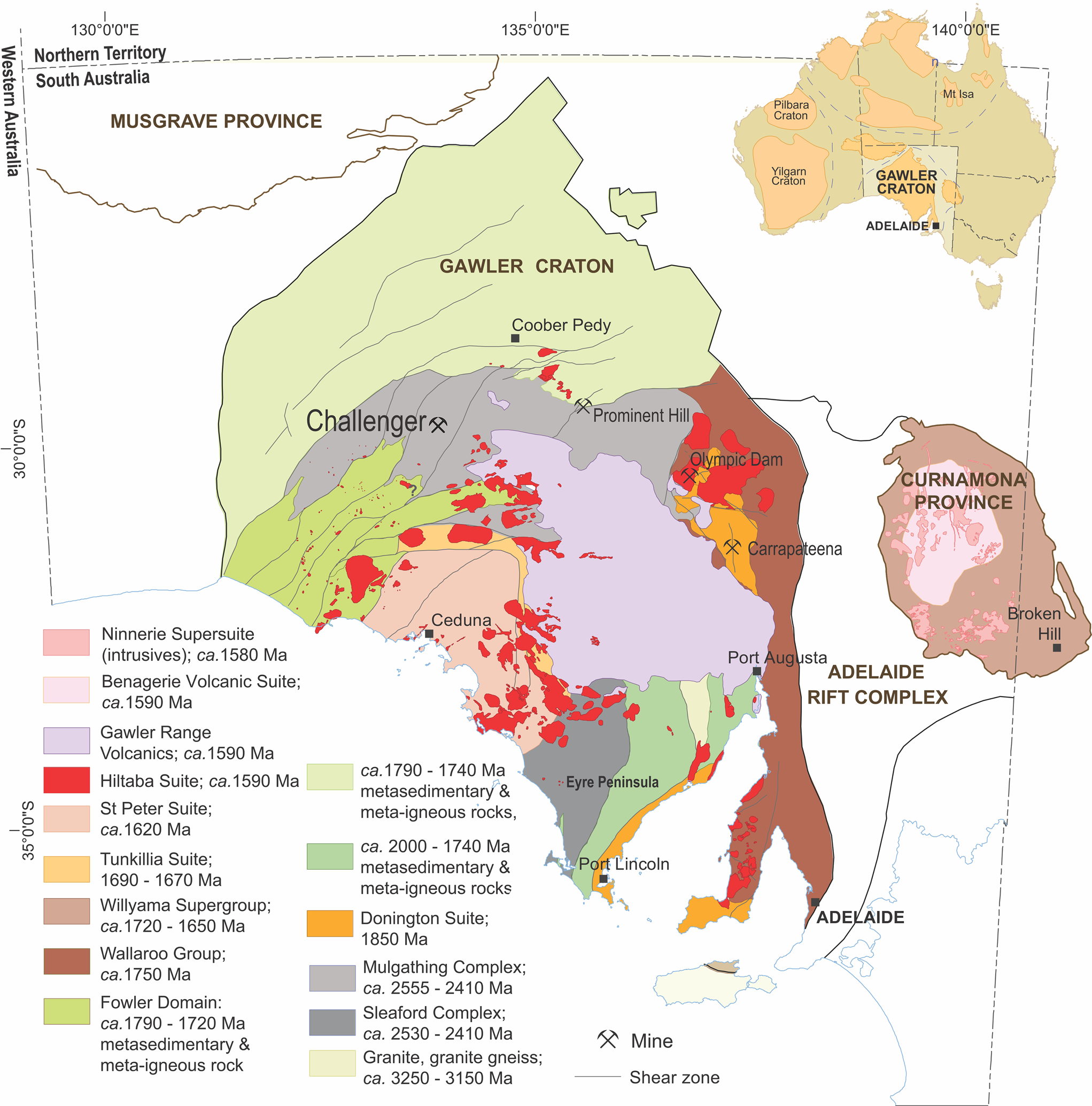
Fig. 1. Geologic map of the Gawler Craton (modified after Reid and Fabris, Reference Reid and Fabris2015) showing the main Archean to Proterozoic lithologies. The gold deposit at Challenger is hosted by the Christie Gneiss domain.
The Challenger gold deposit
The Gawler Craton consists of a Neoarchean-to-Early Paleoproterozoic core comprising metamorphic and igneous rocks of the Mulgathing and Sleaford Complexes (Betts and Giles, Reference Betts and Giles2006). Magmatism associated with a mantle plume and sedimentation in a rift or back-arc setting terminated during the ~2.5–2.4 Ga granulite facies Sleafordian Orogeny (Swain et al., Reference Swain, Woodhouse, Hand, Barovich, Schwarz and Fanning2005). The Challenger deposit occurs in the Christie Gneiss of the Mulgathing Complex, which is exposed over >25,000 km2 at the Craton's northwest margin (Fig. 1). The Christie gneiss is composed predominately of felsic migmatitic garnet–biotite gneisses of principally metasedimentary origin, however, the ‘Challenger Gneiss’, a sub-unit of the Christie gneiss and host to the Challenger deposit, is a felsic gneiss interpreted to have a volcaniclastic protolith (McFarlane et al., Reference McFarlane, Mavrogenes and Tomkins2007). Pristine magmatic zircon in gneiss proximal to the deposit suggests a protolith age of ~2.52 Ga (McFarlane et al., Reference McFarlane, Mavrogenes and Tomkins2007), whereas determination of the ages of the zircon rim and monazite constrain a metamorphic event between 2.47 and 2.41 Ga (Sleafordian orogeny; Tomkins et al., Reference Tomkins, Dunlap and Mavrogenes2004; McFarlane, Reference McFarlane2006; Reid et al., Reference Reid, Jagodzinski, Fraser and Pawley2014). Variably developed retrograde metamorphism at Challenger is expressed as a greenschist-facies overprint related to subsequent deformation during the Kimban (~1845 to 1700 Ma; Parker, Reference Parker1993) and Kararan (~1650 to 1540 Ma; Teasdale, Reference Teasdale1997) orogenies.
Challenger is a high-grade Au ore system, attributed to generation of metal-rich felsic magmas during crustal anatexis at peak metamorphic conditions (800–850°C, ~7 kbar; Tomkins and Mavrogenes, Reference Tomkins and Mavrogenes2002; Reference Tomkins and Mavrogenes2003). Four main lithologies are present: (1) granoblastic to locally magmatic garnet-biotite ± orthopyroxene distal gneisses, with sparse pyrrhotite; (2) granoblastic garnet-cordierite proximal gneisses locally with elevated As and disseminated pyrrhotite, arsenopyrite, löllingite, chalcopyrite pentlandite, gold and bismuth; (3) metatexite migmatites containing abundant (30–40%) coarse-grained stromatic leucosomes; and (4) bluish quartz-rich veins, locally intimately associated with migmatite leucosomes.
Gold mineralisation is associated with disseminated pyrrhotite, löllingite and arsenopyrite and occurs within orebodies characterised by differences in Au associations: a predominance of Au–Bi species (M1 orebody; Tomkins and Mavrogenes, Reference Tomkins and Mavrogenes2001, Reference Tomkins and Mavrogenes2002); and Au–Ag–Te associations (M2 orebody). The M2 orebody contains two distinct types of arsenopyrite, defined by different As/S ratios. Arsenopyrite geothermometry yields 600 to ~700°C and <300 to 400°C, respectively (Haese, Reference Haese2010). Much higher sulfidation is suggested for the higher temperature type, which is assumed to be melt derived. The lower temperature arsenopyrite is interpreted as a retrograde generation, consistent with grain-scale remobilisation of lattice-bound Au to form visible gold during a fluid-driven, retrograde overprint (Haese, Reference Haese2010). Replacement of löllingite by arsenopyrite is observed, with a pronounced Au–Ag–Te association characterising the reaction boundary. The low concentrations of Ag and Te in solid solution within löllingite support a scenario in which these elements were incorporated via interaction with a fluid. Coupled dissolution–reprecipitation reactions, advancing via transient porosity at the reaction front is invoked to explain petrographic relationships (Haese, Reference Haese2010), supported by electron back-scatter diffraction analysis showing that replacement of löllingite is pseudomorphic.
Methods
Zircon grains were identified within 1-inch polished blocks using a FEI Quanta 450 SEM equipped with a BSE detector. Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) U–Pb spot analyses were acquired using a RESOlution-LR 193 nm ArF excimer laser microprobe coupled to an Agilent 7900cx quadrupole ICP-MS, using a spot size of 15 μm, repetition rate of 5 Hz and constant fluence of 2.5 J.cm–2 on unknowns and reference materials. Reference materials GJ-1, Plešovice and 91500 were used to calibrate Challenger zircon data (reference material results are provided in Supplementary material – Appendix A1). Data reduction was carried out using IOLITE v.2.5 and its data reduction scheme scheme U_Pb_Geochronology3 (Paton et al., Reference Paton, Hellstrom, Paul, Woodhead and Hergt2011). No common Pb correction was applied to these data, and a 238U/235U data reduction value of 137.818 (Hiess et al., Reference Hiess, Condon, McLean and Noble2012) was applied. The Tera–Wasserburg diagram was constructed and presented using IsoplotR (Vermeesch, Reference Vermeesch2018).
A single 20 μm long, ~8 μm wide and <100 nm thick, TEM foil was prepared using dual-beam focused ion beam SEM methods by ion beam (Ga+) milling and attached to a Cu-grid on a FEI Helios Nanolab 600 platform. Parameters for coarse milling of zircon required energy settings between 2.5 and 6.5 nA (30 kV) and fine polishing at 20 pA (5 kV). High-angle annular dark field scanning transmission electron microscopy (HAADF STEM) imaging and energy-dispersive X-ray spectrometry (EDS), in both spot analysis and mapping modes, were conducted with an ultra-high-resolution, probe-corrected, FEI Titan Themis S/TEM operated at 200 kV. This instrument is equipped with the X-FEG Schottky source and Super-X EDS geometry. The Super-X EDS detector provides geometrically symmetric EDS detection with an effective solid angle of 0.8 Sr. Probe correction delivered sub-Ångstrom spatial resolution and an inner collection angle greater than 50 mrad was used for HAADF experiments using the Fischione HAADF detector. Diffraction indexing was performed using Winwulff© 1.5.2 software (JCrystalSoft, http://www.jcrystal.com/) and publicly available data from the American Mineralogist Crystal Structure Database (http://rruff.geo.arizona.edu/AMS/amcsd.php).
Results
Zircon from a sulfide-arsenide-rich sample of migmatite, typical of the M2 orebody at Challenger (Fig. 2) was investigated. The specimen, obtained from mining level 560 m RL, consists of finely banded leucosome and melanosome (Fig. 2b, c) and is formed by various proportions of quartz, feldspar and biotite ± garnet with minor Ti–Fe-oxides (ilmenite, rutile). No pyroxene is present. This sample is relatively rich in ore minerals, (up to 5 vol.%), comprising mainly pyrrhotite (Fe1–xS), löllingite (FeAs2) and arsenopyrite (FeAsS) (Figs 2c, 3a). Gold and electrum (~Au85Ag15) are associated with fluid-assisted reactions between löllingite and pyrrhotite producing arsenopyrite (Fig. 3b). Extensive Au–Ag-enrichment occurs at the direct contacts between fine-grained arsenopyrite grains protruding into löllingite (Fig. 3c, d). In this case, hessite (Ag2Te) and electrum form either single- or binary-phase associations at the tips/edges of arsenopyrite grains (Fig. 3d–f).

Fig. 2. (a) Photographs of leucosomes (highlighted in white) and pegmatitic veins in the mine wall (level 760 m RL) at Challenger; (b) sampled migmatite from mine level 560 m RL; (c) hand specimen photograph of sample CH18 (visible pyrrhotite in quartz) which contains the zircon investigated.
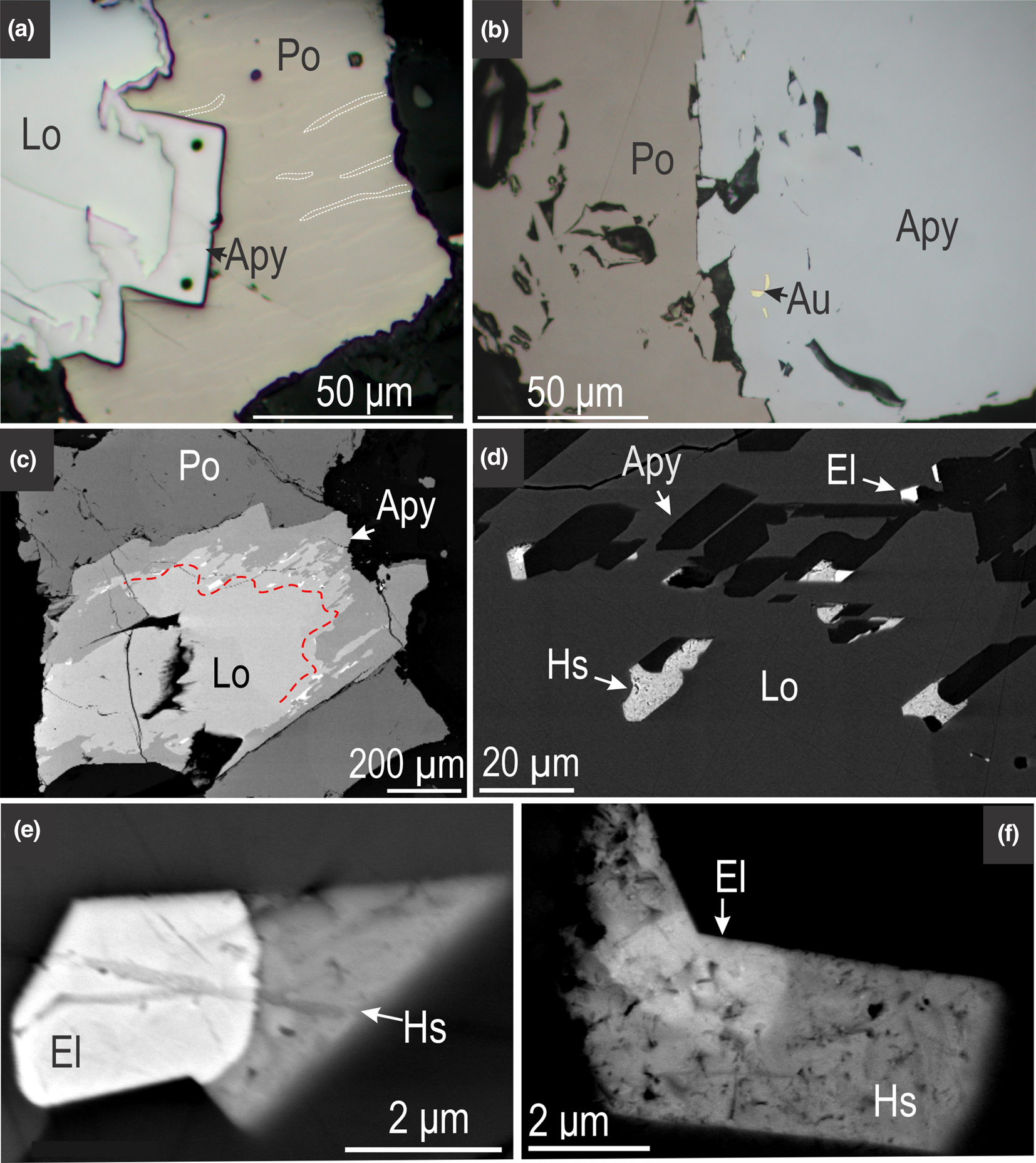
Fig. 3. (a) Reflected-light microscope photomicrographs (a, b) and BSE images (c–f) showing aspects of ore mineral assemblages from the M2 orebody at Challenger. (a) Typical löllingite (Lo) – arsenopyrite (Apy) – pyrrhotite (Po) association with pyrrhotite displaying lamellar exsolutions (examples outlined in white); (b) visible gold in arsenopyrite close to the contact to pyrrhotite; (c, d) Au–Ag-enrichment ‘front’ at the boundary between löllingite and arsenopyrite (marked by red line); (d) detail from (c) showing electrum (El) and hessite (Hs) along the edges of small, euhedral arsenopyrite.;(e, f) binary-phase associations comprising electrum and hessite, typical of (sulfo)arsenide re-equilibration via fluid-assisted reactions during retrograde metamorphism.
Zircon is generally found within feldspars, quartz and garnet (Fig. 4a, b), though some finer grains (~10 μm) are associated closely with Au-bearing sulfides (Fig. 4c). Zircon is generally subhedral to rounded, and displays varying degrees of alteration related to both hydrothermal activity and metamictisation (Fig. 4d–f). Three texturally distinct types of zircon are observed: (1) >50 μm sized grains containing clear zonation patterns probably relating to (magmatic) growth (Fig. 4e); (2) small (<40 μm) rounded zircon containing overgrowths of probable metamorphic origin (Fig. 4d); (3) very small (<20 μm) grains entirely hosted by sulfide minerals (Fig. 4f). Lead-bearing nanoparticles (bright on BSE images) are visible within fractures on the surface of most zircons of all types in BSE images (Fig. 4d–f).
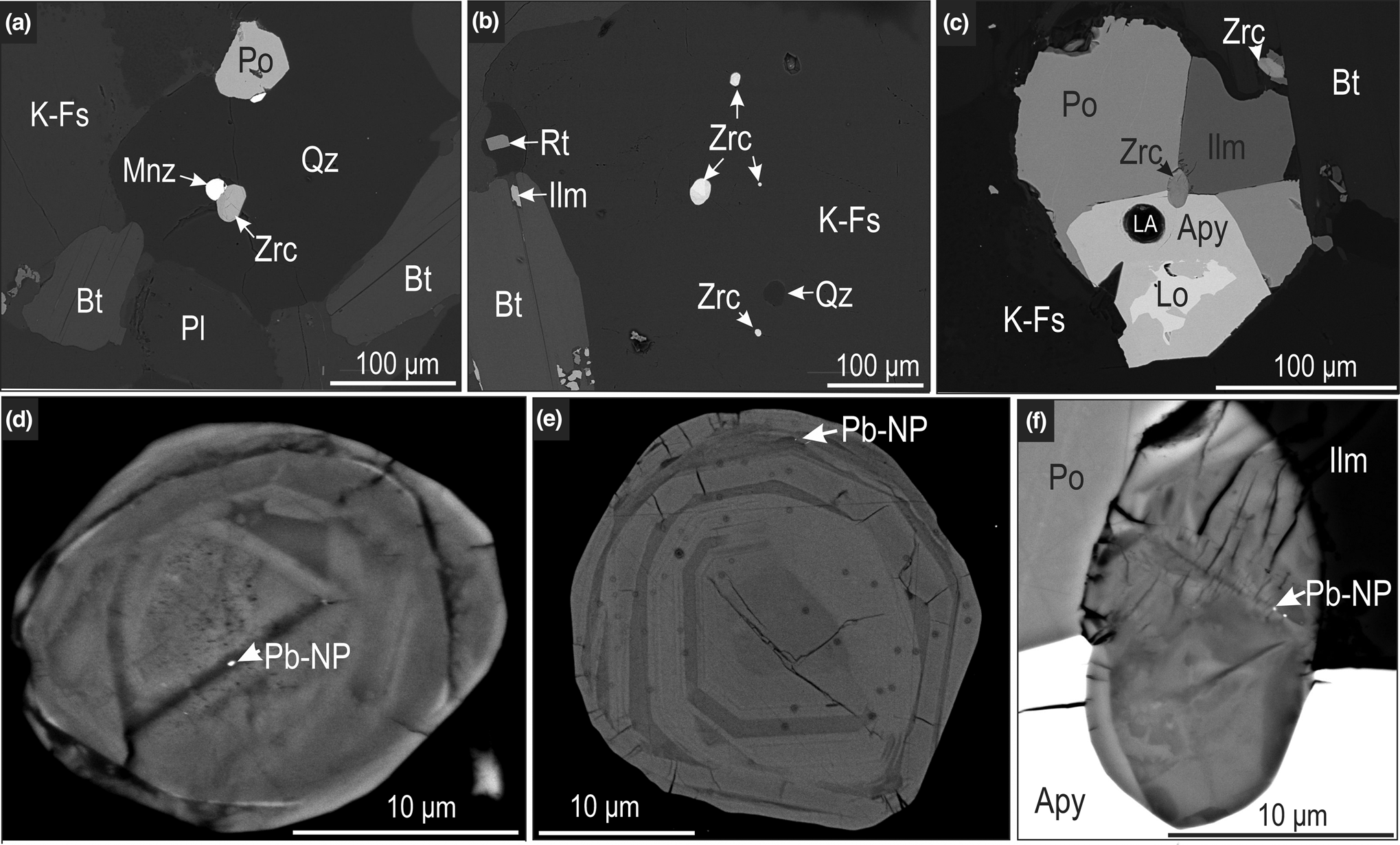
Fig. 4. BSE images of zircon associations at Challenger: (a, b) mineralogical neighbourhood of sub-rounded to rounded zircon grains within quartz (Qz) and K-feldspar (K-Fs) grains; (c) fan-like aggregate of pyrrhotite (Po), arsenopyrite (Apy), löllingite (Lo) and ilmenite (Ilm) surrounding a small zircon grain. Higher resolution BSE images of selected zircon grains displaying varying zonation patterns and arrowed Pb-bearing nanoparticles. Abbreviations: Bt – biotite; LA – laser ablation crater; Pl – plagioclase; Mnz – monazite; Zrc – zircon; Pb-Np – Pb-nanoparticle.
Radiometric U–Pb age determination using LA-ICP-MS comprising six spots across three grains within a single polished block yielded a Neoarchean upper intercept (2592±100 Ma; Fig. 5a, DR1). Uranium concentrations range from 387 to 2140 ppm. This anaylsis was undertaken to constrain broadly the ages of the zircon, rather than provide information on the effects of metallic-Pb nanospheres upon U–Pb systematics, due to the low spatial resolution of the method relative to grain sizes. The U–Pb data are low precision, probably due to mixing of grain domains in spot analyses, apparent Pb-loss (discordance), resulting from radiation damage, and possibly the effects of Pb-mobility relating to formation of metallic-Pb nanospheres. Individual 207Pb/206Pb ages and corresponding spot locations are provided in Fig. 5b. Younger individual 207Pb/206Pb ages probably relate to Pb-mobility. It should however be noted that the upper intercept age is probably somewhat leveraged by an apparent 432 Ma lower intercept. This might be related to the regional, low-temperature ~500 Ma Delamerian orogeny observed in zircon elsewhere in the Gawler Craton (e.g. Courtney-Davies et al., Reference Courtney-Davies, Ciobanu, Verdugo-Ihl, Slattery, Cook, Dmitrijeva, Keyser, Wade, Domnick, Ehrig, Xu and Kontonikas-Charos2019), or be an inaccurate lower intercept. Due to small grain sizes and inclusions, further grains within the same polished blocks were not considered for age determination techniques.
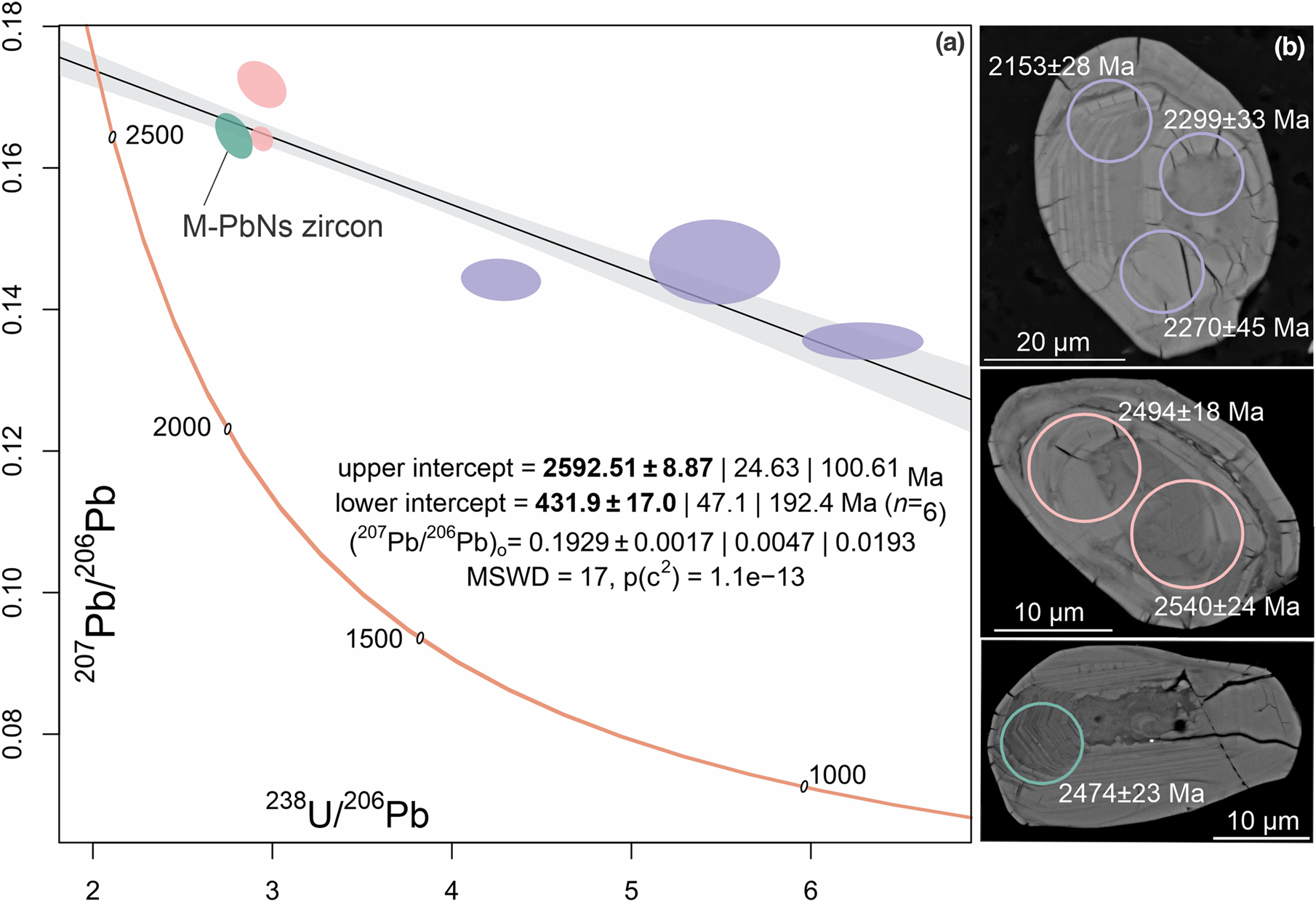
Fig. 5. (a) Tera–Wasserburg diagram displaying zircon U–Pb LA-ICP-MS spot data. Error ellipse colours correspond to spot locations overlaid on zircon BSE images (b). Ages adjacent to spot locations are individual 207Pb/206Pb values.
Zircon studied at the nanoscale contains primary, less-altered domains, which are crosscut by fractures hosting alteration and micrometre-sized inclusions of galena (Fig. 6a, b). Boundaries between altered and unaltered domains vary from scalloped to sharp (Fig. 6a, b). A single TEM foil was extracted perpendicular to zonation patterns and intersecting surface inclusions of galena (Fig. 6b, c). The HAADF STEM image of the TEM foil contains a darker, altered domain to the left, and a brighter, ‘unaltered’ domain to the right (Fig. 6c). Zircon is highly crystalline throughout least-altered domains becoming poorly crystalline in the altered domains. Galena nanoparticles occur in altered zircon domains (Fig. 6c), whereas Pb-only nanoparticles, more spherical and coarser (5–30 nm), are distributed, ~500 nm apart from one another, throughout unaltered domains (Fig. 6c, d).
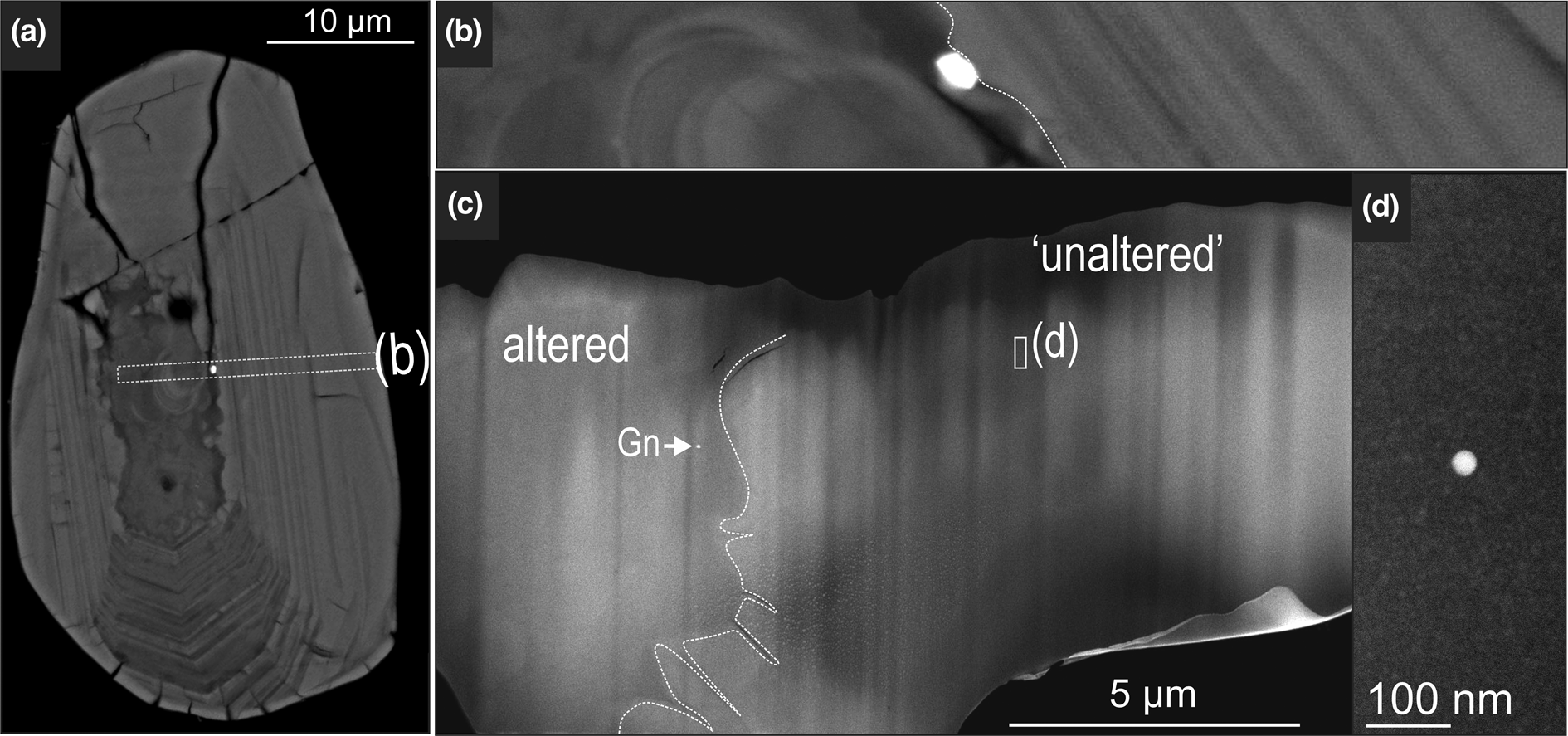
Fig. 6. BSE (a, b) and HAADF STEM images (c) of zircon showing altered and unaltered domains on the surface and in depth. The FIB extraction location is marked by dashed rectangle in (a). Note presence of galena (Gn) in the left side of the altered zircon domain in (c) and bright, spherical, Pb-bearing NP in (d).
STEM EDS mapping and high-resolution imaging show the distinct character of inclusions in the two types of zircon domains (Figs 7, 8). Spherical, Pb-nanoparticles in unaltered domains also display enrichment in Sc, expressed as zones, several nm in width, partially mantling the spheres (Fig. 7a, b). In fact, every single Pb-NP imaged is paired by such Sc-rich, Si-depleted nanodomains of zircon as shown by the EDS spectrum (Fig. 7c). The nanoparticles in altered zircon domains are more varied in shape and composition, generally composed of clustered galena grains (Fig. 7d).
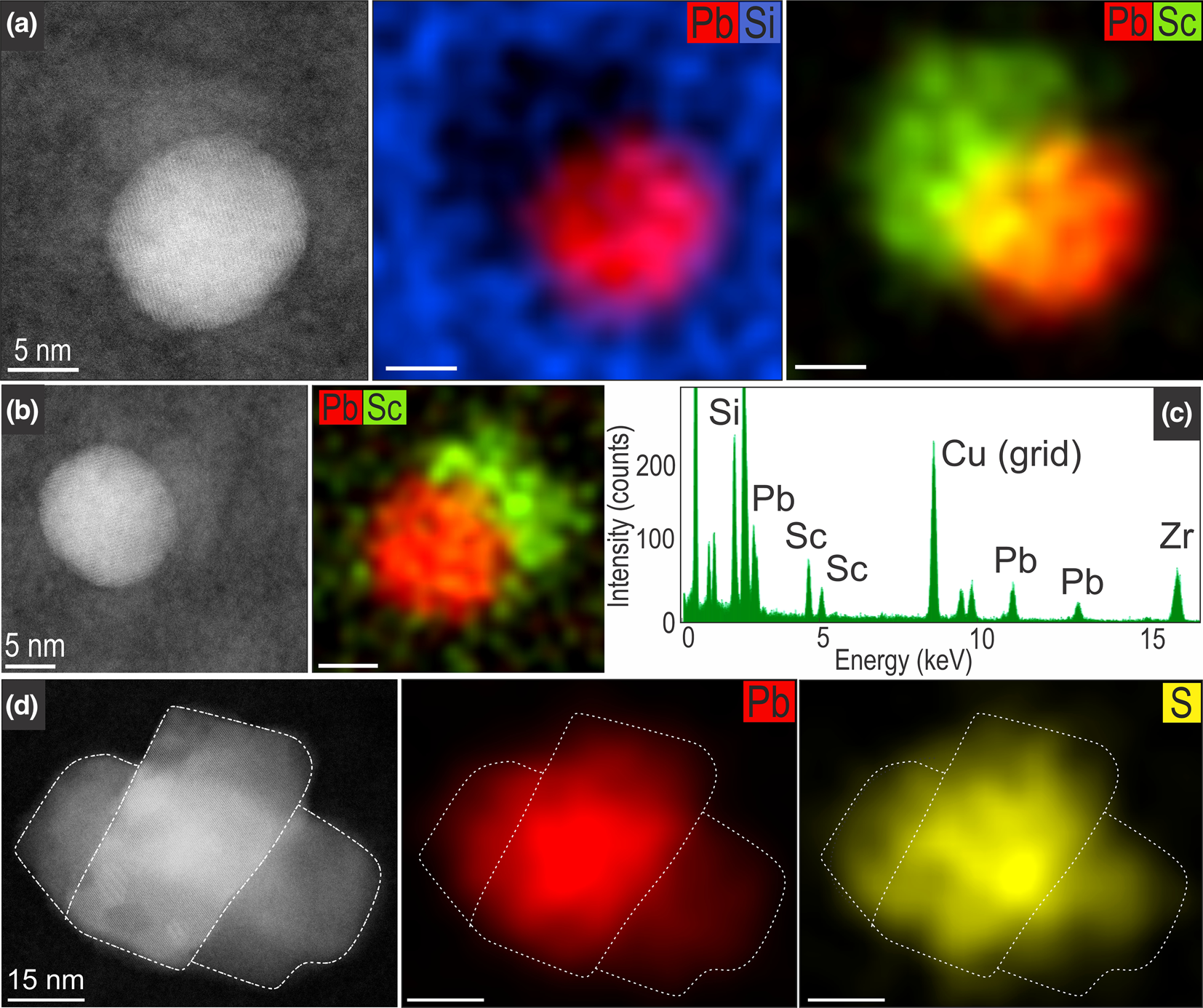
Fig. 7. HAADF STEM images and EDS STEM maps showing metallic-Pb nanospheres in unaltered zircon (a–c) and larger galena nanoparticles in altered zircon (d). Note the spherical morphology of metallic-Pb nanospheres and the presence of Sc haloes, with corresponding EDS spectra (c) taken from a Pb-sphere/Sc halo interface in map (b).
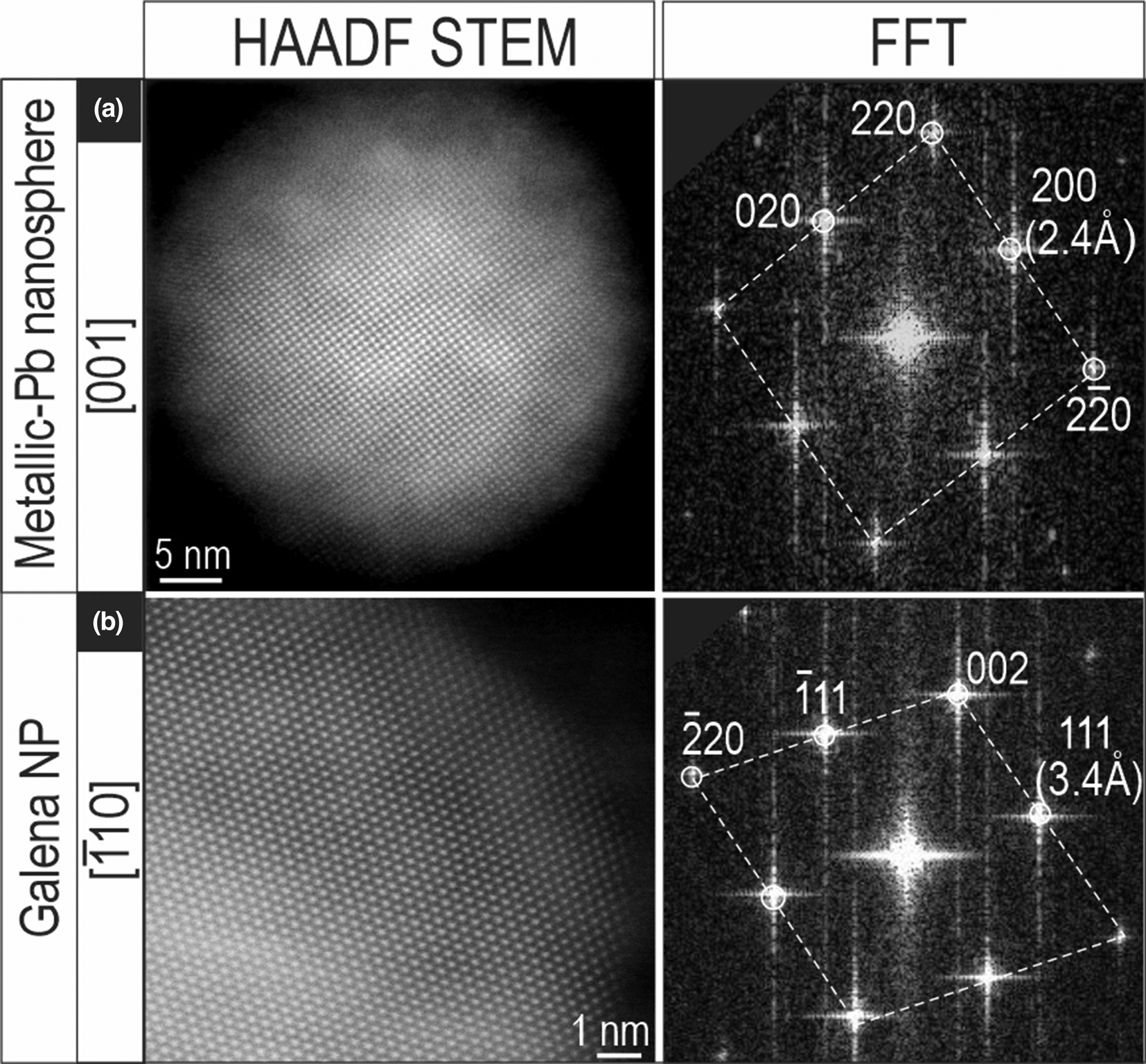
Fig. 8. HAADF STEM images and corresponding fast Fourier transform (FFT) patterns showing a metallic-Pb nanospheres (a) and galena NP (b) from unaltered and altered zircon domains, respectively.
The Pb-only nanoparticles are distinct from galena, on the basis of HAADF STEM imaging and Fast Fourier Transform (FFT) patterns (Fig. 8). The two Pb-bearing nanoparticles were imaged on two main zone axes, [001] and $[ {\bar{1}10} ]$![]() . The Pb-bearing species share the same Fm3m cubic symmetry however they are discriminated readily by their measured d 111 spacings: 2.4 Å in metallic lead; and 3.4 Å in galena.
. The Pb-bearing species share the same Fm3m cubic symmetry however they are discriminated readily by their measured d 111 spacings: 2.4 Å in metallic lead; and 3.4 Å in galena.
Discussion
Zircon containing metallic-Pb nanospheres originates from an Archean lithology whereby U–Pb age determination at ~2.59 Ga (Fig. 5) falls within the uncertainty of the previous zircon age of 2.52 Ga attributed to the volcaniclastic sequence, which is considered the protolith for Christie Gneiss at Challenger (McFarlane et al., Reference McFarlane, Mavrogenes and Tomkins2007).
Formation of Pb-bearing nanoparticles in the Challenger zircon is shown schematically in Fig. 9. Subsequent migmatisation of Challenger host rocks led to formation of Au–Bi melts (Tomkins and Mavrogenes Reference Tomkins and Mavrogenes2003; Fig. 9a). Prograde metamorphism, with Au–Bi-melts formed at peak metamorphism at ~800°C (Fig. 9b), also provided the heat source that allowed trapping and nucleation of radiogenic Pb during thermal annealing of damaged zircon domains. Radiation damage accumulation occurring in the period between zircon crystallisation and metamorphism at ~2.47–2.41 Ga (Tomkins et al., Reference Tomkins, Dunlap and Mavrogenes2004; McFarlane, Reference McFarlane2006; Reid et al., Reference Reid, Jagodzinski, Fraser and Pawley2014) allowed time for radiogenic Pb to mobilise within nm-scale domains of the damaged zircon lattice.
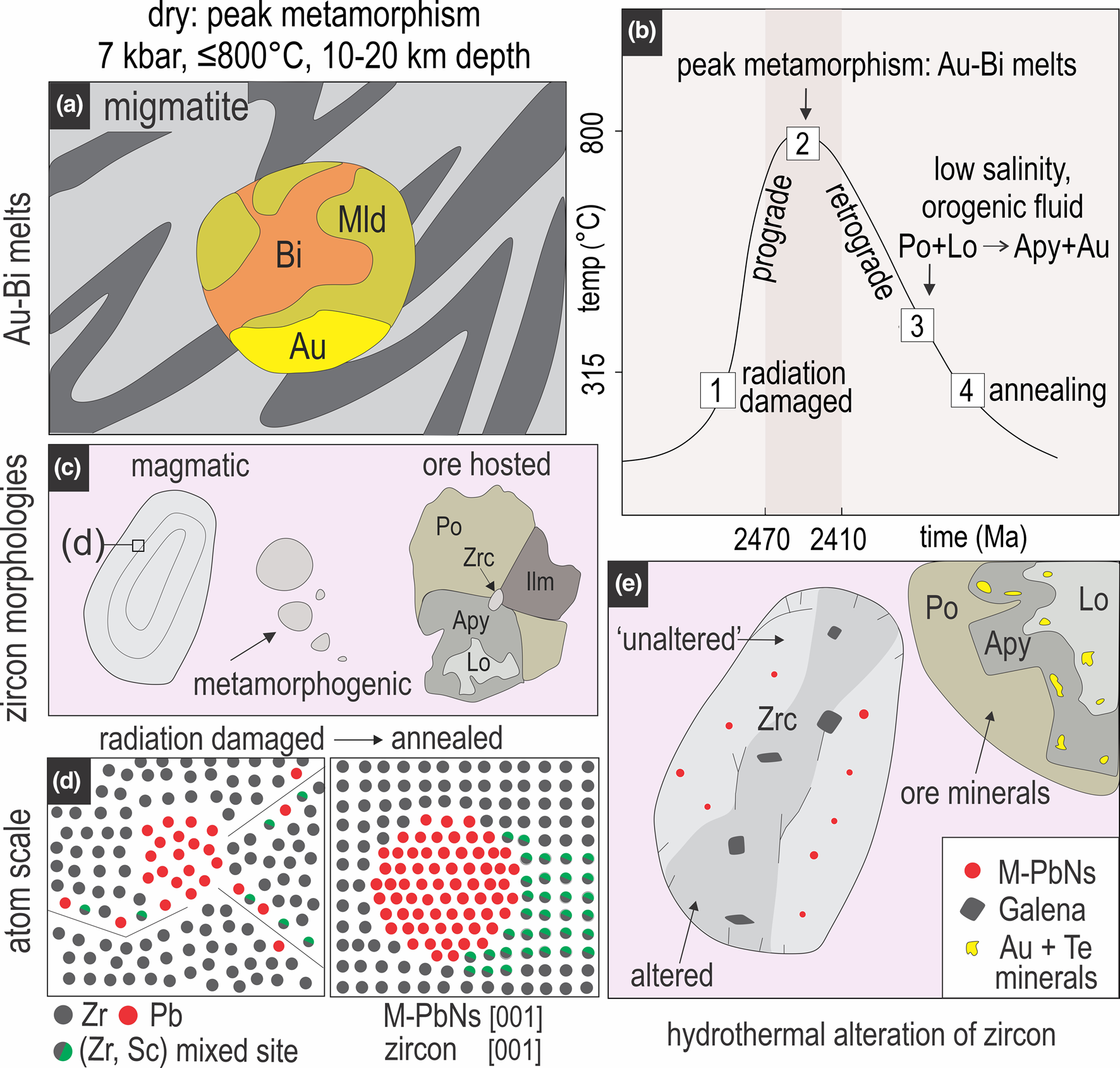
Fig. 9. Schematic representation of metallic-Pb nanosphere formation at Challenger: (a) development of Au–Bi–Mld (maldonite) melts during crustal anatexis; (b) temperature versus time plot (based on arsenopyrite geothermometry; Haese, Reference Haese2010) showing how the processes discussed here relate to prograde and retrograde metamorphic pathways; (c) examples of different types of zircon identified at Challenger; (d) inset of atomic structure in ‘magmatic’ zircon, depicting radiation damage in zircon lattice and mobilisation of Pb and Sc atoms (left), and the same area post annealing, whereby Pb atoms have concentrated to form metallic-Pb nanospheres with Sc-rich nanodomains of zircon forming haloes; (e) final schematic image depicting ore minerals proximal to zircon, which is altered via interaction with hydrothermal fluids, possibly leading to transformation of metallic-Pb nanospheres into other phases such as galena.
The variety of zircon sizes and textures, and their distribution within the rocks and ores (Fig. 4) suggest that together with magmatic zircon inherited from the protolith, the smallest grains, some of which are ore-hosted, could be of syn-metamorphic origin (Fig. 9c). Moreover, the presence of zircon containing Pb-nanoparticles encapsulated within sulfides (Fig. 4f) that were concentrated during prograde metamorphism constrains growth and mineralisation of metallic-Pb nanospheres as near-coeval at the same dry metamorphic conditions (Fig. 9b, c). The suprasolidus state of Christie Gneiss for at least 30 Ma (Halpin and Reid, Reference Halpin and Reid2016) might have allowed for maturation of initial, smaller atomic Pb clusters into large metallic-Pb nanospheres, which are far more dispersed (e.g. 500 nm apart) and free from the immiscibility-related amorphous silica-rich and/or Ti-Al-phases described elsewhere (Kusiak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015). The absence of Si-rich melt inclusions here, supports a formation mechanism whereby the annealing of Pb-enriched amorphous domains allowed Pb atoms to concentrate into spherical, metallic-Pb concentrations (Fig. 9d).
Mobilisation of trace elements contemporaneous with Pb clustering is evidenced by the development of ubiquitous, marginal Sc domains, in some cases as large as the metallic-Pb nanospheres themselves (Fig. 7a). However, unlike Pb nucleation from a possible melt (Kuisak et al., Reference Kusiak, Dunkley, Wirth, Whitehouse, Wilde and Marquardt2015), the high melting point of Sc (1541°C) suggests solid-state diffusion and concentration around the metallic-Pb nanospheres nuclei. This is supported by the observed Sc-enrichment within the zircon lattice at direct contacts with metallic-Pb nanospheres rather than forming discrete Sc-bearing phases. The zircon itself is considered the source of the Pb forming metallic-Pb nanospheres, hence the most likely source of Sc is the same host zircon. The reasoning for anomalous Sc concentrations forming adjacent to metallic-Pb nanospheres, coupled with no obvious enrichment in other trace elements, might be due to the similar atomic radii of Pb and Sc (154 and 184 pm, respectively), whereby the two elements behave similarly during high-temperature metamorphism.
Although Au could have been introduced during peak metamorphism, prevailing assemblages indicate a significant retrograde overprint. Tomkins and Mavrogenes (Reference Tomkins and Mavrogenes2001) argued for diffusional processes controlling gold behaviour during both prograde and retrograde metamorphism, however the petrographic evidence for a retrograde metamorphic fluid is not only compelling (löllingite–arsenopyrite relationships suggestive of coupled dissolution–reprecipitation reactions, gold remobilisation; Haese, Reference Haese2010) but is complemented by the observation of biotite as rims on metamorphic garnet and orthopyroxene (Tomkins and Mavrogenes, Reference Tomkins and Mavrogenes2002). Even though supra- and/or subsolidus reactions could be invoked to explain the formation of the two arsenopyrite generations, on the basis of phase relations in the system Fe–As–S (Clark, Reference Clark1960), the observed pseudomorphic replacement of löllingite by arsenopyrite via fluid-driven reactions coupling dissolution with reprecipitation rates is more feasible. Formation of small, euhedral, grains of reprecipitated arsenopyrite (Fig. 3c, d), together with other phases at the grain tips is indicative of a reaction front that propagates via transient porosity. The porosity is not visible, however, as it has been obliterated by precipitation of Au–Ag–Te minerals.
The reaction between pyrrhotite and Au-bearing löllingite facilitated by fluids led to release of Au (Haese, Reference Haese2010; Fig. 9b), possibly also triggering interaction between metallic-Pb nanospheres and S-bearing fluids to form galena nanoparticles occurring along fractures and/or altered domains in zircon (Fig. 9e). Small amounts of interstitial fluid can be assumed to have been present, for example during formation of biotite, facilitating remobilisation of Au within the M2 orebody.
Conclusions
Our new findings substantiate ideas that metallic-Pb nanospheres are common components of high-temperature rocks, extending the range of conditions at which they can form to below UHT facies, and the range of environments in which they might be identified. The diversity of metallic-Pb nanosphere sizes (e.g. <5 to >50 nm), pronounced trace-element associations (e.g. Al, Ti and Sc), and the physical form these elements take (e.g. melt inclusions and solid state ‘haloes’) demonstrates that a single mechanism for metallic-Pb nanosphere formation is unlikely. Internal zircon features such as primary zircon composition, texture and radiation dose/time, coupled with external forces such as host rocks, metamorphic grades and timescales of metamorphism probably combine to control how mobile radiogenic Pb is concentrated into metallic-Pb nanospheres in different geological settings.
The presence of metallic-Pb nanospheres and Pb-bearing nanoparticles in zircon is unlikely to be restricted to the Challenger deposit. Future, or previous studies, attempting to determine the age of zircon from ore bodies experiencing high metamorphic grades should thoroughly screen zircon for nanoscale inclusions if high accuracy and precision ages are required. Although the quantitative effects of metallic-Pb nanospheres upon zircon age determinations are not yet fully resolved, and are likely to be highly variable from case to case, the geochronological and S/TEM data presented here demonstrates that Pb has been mobilised. The relative significance of radiation damage versus metallic-Pb nanosphere formation is nonetheless uncertain, however, both processes and products probably combine to reduce age resolution.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.81
Acknowledgements
This work was funded by the ‘FOX’ project (Trace elements in iron oxides: deportment, distribution and application in ore genesis, geochronology, exploration and mineral processing), supported by BHP Olympic Dam and the South Australian Government Mining and Petroleum Services Centre of Excellence. Samples were collected by Royce Haese as part of a 2010 honours project. We gratefully acknowledge constructive reviews from James Darling and an anonymous reviewer, which helped us to improve this manuscript. Anonymous reviewers are thanked for comments on an earlier version of this contribution.











