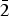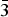Introduction
The generic name ‘hornblende’ is used widely by petrologists and mineral collectors for any black undefined Al-bearing amphibole in the calcium (and sometimes in the sodium–calcium) subgroups. Therefore, the currently approved scheme for amphibole nomenclature and classification (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012) uses the rootname hornblende for the charge arrangement A☐BCa2C(R2+4R3+)T(Si7Al)O22W(OH,F,Cl)2 where Al is dominant over R3+ cations and the prefix ‘magnesio-’ indicates Mg dominance over R2+ cations (exceptions are allowed only for riebeckite, arfvedsonite, hastingsite and hornblende).
Despite its widespread occurrence (~220 compositions listed in the mindat.org web page: https://www.mindat.org/min-2524.html, some of which should not be classified as magnesio-hornblende), the type description of an amphibole with composition corresponding to A☐BCa2C(Mg4Al)T(Si7Al)O22W(OH)2 has never been published.
In the most recent list of minerals approved by the International Mineralogical Association (IMA version July 2017; https://www.ima-mineralogy.org/Minlist.htm), magnesio-hornblende is designated as a redefined valid species (by Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012) with unknown type locality and without an original reference. Therefore, it is a potential new species which requires formal approval by the IMA Commission on New Minerals, Nomenclature and Classification (CNMNC) and publication of the mineral data. This work describes magnesio-hornblende from Lüderitz, Namibia approved by IMA-CNMNC vote 2017-059.
Occurrence and optical properties
The specimen under investigation was found in 1970 by MEC in the sand dunes of the Lüderitz, Karas Region, Namibia (~26°38′52″S, 15°09′28″E). Lüderitz is a surreal colonial relic, a true 19th century Bavarian village at the southern margin of the Namib Desert coast. It was founded in 1883 as a trading post, and in 1909 enjoyed a sudden but short period of prosperity when diamonds were discovered nearby. The Namib Desert was part of the passive margin during Early Cretaceous rifting of the South Atlantic, and extends for nearly 600 km along the Namibian coast between Lüderitz (~27°S) and the Kuiseb River (~23°S); it is 100 to 150 km wide, extending inland from the coast to the base of the Great Escarpment at the 1000 m contour. The sand sea (~34,000 km2) is dominated by large linear dunes, with areas of star-shaped dunes on its eastern margin and a belt of simple and compound transverse and barchanoid dunes (Fig. 1) along the coast (Garzanti et al., Reference Garzanti, Andò, Vezzoli, Lustrino, Boni and Vermeesch2012). Dune sands in the coastal Namib are invariably lithofeldspatho–quartzose volcaniclastic, and have a homogeneous composition from Lüderitz to Walvis Bay. They were originally deposited by the Orange River, which has its source 3000 km away in the basaltic rift highlands of Lesotho and flows into the ocean 300 km south of Lüderitz, to which sands are dragged by vigorous longshore currents (Garzanti et al., Reference Garzanti, Andò, Vezzoli, Lustrino, Boni and Vermeesch2012). Plagioclase exceeds K-feldspar. In the sand, mafic volcanic rock fragments predominate over granitoid, sedimentary (quartzose to feldspatho–quartzose siltstone/sandstone, shale, minor limestone and hybrid carbonate) and metamorphic (quartz–sericite, quartz–mica, quartz–epidote, amphibolite) grains. Samples enriched in heavy minerals are usually dominated by clinopyroxene, with subordinate opaque Fe–Ti–Cr oxides, garnet, epidote and amphibole. Landward of Lüderitz, the quartz content increases at the expense of volcanic detritus; garnet, Fe–Ti–Cr oxides, amphibole (including green and green-brown hornblende), epidote, titanite, staurolite and zircon also become more significant, suggesting contributions from the Koichab River and the locally exposed Namaqua basement rocks (Garzanti et al., Reference Garzanti, Andò, Vezzoli, Lustrino, Boni and Vermeesch2012).

Fig. 1. Barchanoid dunes near Lüderitz.
The holotype specimen (Fig. 2) consists of a friable block of subhedral to anhedral magnesio-hornblende crystals up to a few mm in size. The crystals are green to dark green with good cleavage parallel to {110}. A spindle stage was used to orient a crystal for measurement of refractive indices and 2V by extinction curves (Bartelmehs et al., Reference Bartelmehs, Bloss, Downs and Birch1992). The optical orientation was determined by transferring the crystal from the spindle stage to a single-crystal diffractometer and measuring the relative axial relations by X-ray diffraction. In transmitted plane-polarized light, magnesio-hornblende is pleochroic (X = pale yellow, Y = bluish green, Z = dark green) with Z > Y > X. Magnesio-hornblende is non fluorescent and has a vitreous lustre. It is biaxial (–), with α = 1.640(2), β = 1.654(2), γ = 1.666(2) (measured with gel-filtered Na light, λ = 589.9 nm), 2V (meas.) = 82(1)° and 2V (calc.) = 84.9°. The dispersion is weak (v > r), and the orientation is: X ^ a = 33.7° (in β obtuse), Y || b, Z ^ c = 18.2° (in β acute). The density calculated based on the chemical formula and the unit-cell parameters is 3.137 g·cm–3. The compatibility index [1 – (K p/K c)] (Mandarino, Reference Mandarino2007) is 0.010 (superior).

Fig. 2. The holotype specimen studied in this work. Scale in cm.
The holotype material is deposited in the mineralogical collections of the Museo di Mineralogia, Sistema Museale di Ateneo, University of Pavia, under the catalogue number 2017-01. The refined and analysed crystal of this work has the code 1325 in the amphibole database of the CNR-IGG Pavia.
Single-crystal diffraction and powder-diffraction analysis
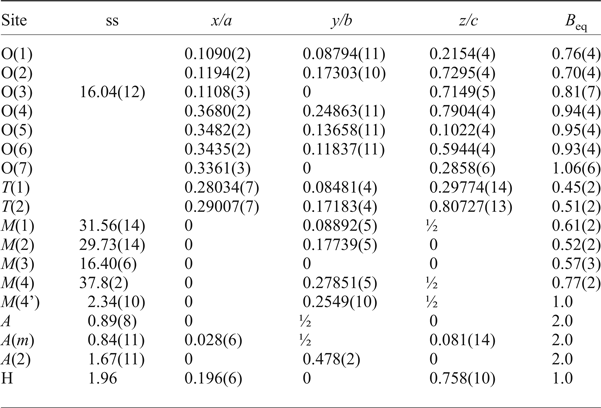
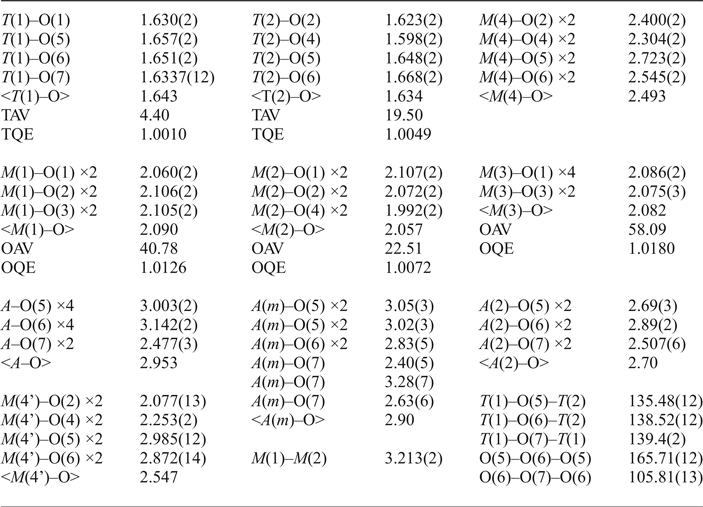
The crystal studied is 0.60 mm × 0.45 mm × 0.10 mm in size. The unit-cell parameters are a = 9.8308(7), b = 18.0659(11), c = 5.2968(4) Å, β = 104.771(6)° and V = 909.64 (11) Å3, and were calculated by a least-squares procedure using the d* values measured for reflections belonging to 60 selected rows of reciprocal space and occurring in the θ range –30° to + 30°. Two monoclinic equivalents were collected in the 2θ range 4–60° by a Philips PW1100 4-circle diffractometer using MoKα radiation; absorption and Lorentz and polarization corrections were applied. Merging of equivalent reflections gave R sym = 2.60% on 1290 pairs. Reflections with I > 3σI were considered as observed during unweighted full-matrix least-squares refinement on F using a program locally written to deal with complex solid-solutions (Cannillo et al., Reference Cannillo, Germani and Mazzi1983). Scattering curves for fully ionized chemical species were used at sites where chemical substitutions occur; neutral vs. ionized scattering curves were used at the T and at the anion sites [except O(3)]. More details on the refinement strategy can be found in Oberti et al. (Reference Oberti, Ungaretti, Cannillo and Hawthorne1992) and Oberti et al. (Reference Oberti, Della Ventura, Boiocchi, Zanetti and Hawthorne2017). The final disagreement indexes are R obs = 2.72% (1244 reflections) and R all = 3.15% (1380 reflections). Refined atom coordinates and displacement parameters, and selected bond lengths and angles are given in Tables 1 and 2, respectively. Crystallographic information files with embedded structure factors have been deposited with the Principal Editors of Mineralogical Magazine and are available as supplementary material (see below). The a:b:c ratio calculated from the unit-cell parameters is 0.544:1:0.293.
Table 1. Atom coordinates, refined site-scattering values* (ss, epfu), and equivalent atom-displacement parameters (B eq, Å2) for magnesio-hornblende crystal 1325.

*Hawthorne et al. (Reference Hawthorne, Ungaretti and Oberti1995); ‘epfu’ – electrons per formula unit.
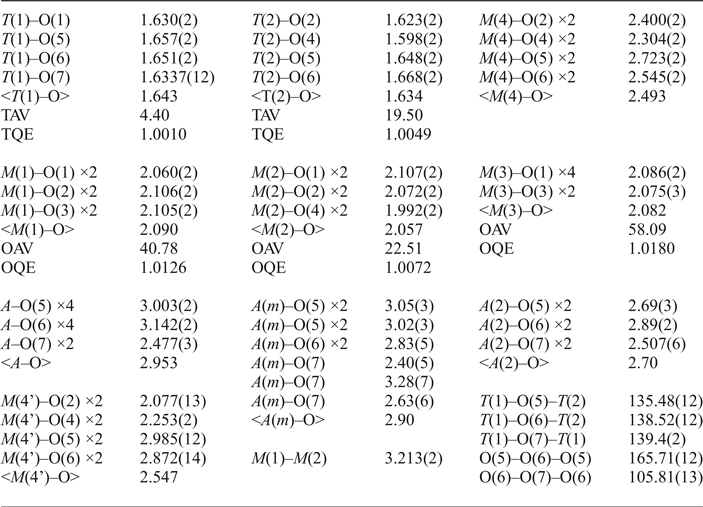
Table 2. Selected interatomic distances (Å), angles (°), tetrahedral and octahedral angle variances (TAV, OAV, °^2) and quadratic elongations (TQE, OQE) according to Robinson et al. (Reference Robinson, Gibbs and Ribbe1971) refined for magnesio-hornblende crystal 1325.
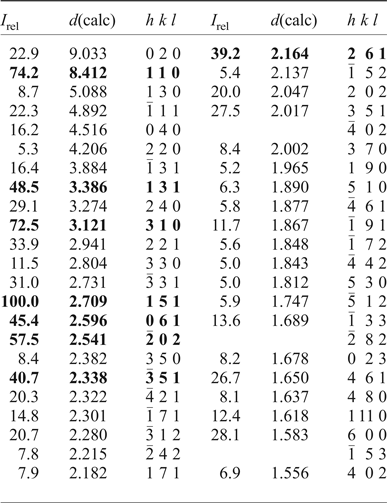
Powder X-ray diffraction data (CuKα, λ = 1.54178 Å) were obtained using the XPREP utility of SAINT (Bruker, Reference Bruker2003), which generates a 2D powder diffractogram (Debye-Scherrer technique) starting from the F 2obs collected on the single crystal and taking into account solely the information concerning the unit-cell dimensions and the Laue symmetry. No Lorentz and polarization corrections were applied. Data are given in Table 3.
Table 3. Powder X-ray diffraction data (d in Å) for magnesio-hornblende crystal 1325.
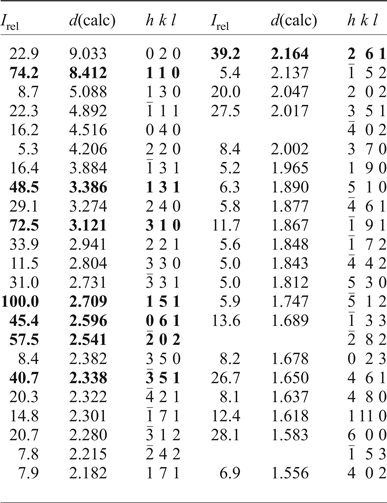
The strongest eight reflections are in bold. Only peaks with I rel ≥ 5 are reported.
Chemical analysis
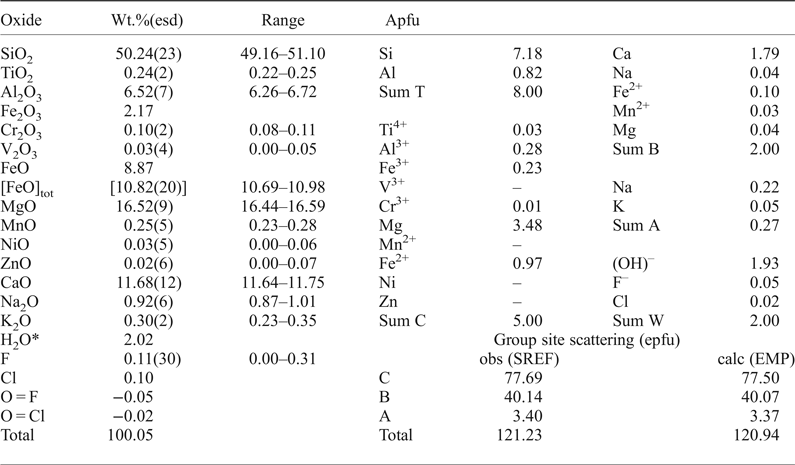
Chemical analysis (10 points) on the crystal used for structure refinement was undertaken with a Cameca SX-100 electron microprobe (WDS mode, 15 kV, 20 nA, count time 20 s and 5 µm beam diameter). The standards used are as follows: Si and Ca: diopside (TAP); Ti: titanite (LPET); Al: andalusite (TAP); Cr: chromite (LPET); Fe: fayalite (LLiF); Mn: spessartine (LLiF); Mg: forsterite (LTAP); Zn: gahnite (LLiF); Ni: pentlandite (LLiF); Na: albite (TAP); K: orthoclase (LPET); F: fluoro-riebeckite (TAP) and Cl: tugtupite (LPET). H2O was estimated based on 2 = (OH,F,Cl) atoms per formula unit (apfu) and taking into account the constraints on the group sites based on stoichiometry and site-scattering values (for A, B and C cations) obtained from the structure refinement; this method allows calculation of Fe3+/Fe2+. The oxide wt.% and the calculated unit-formula are reported in Table 4. The proposed empirical formula for crystal 1325 is: A(□0.73Na0.22K0.05)Σ1.00B(Ca1.79Fe2+0.10Mg0.04Mn2+0.03Na0.04)Σ2.00C(Mg3.48Fe2+0.97Al0.28Fe3+0.23Cr3+0.01Ti0.03)Σ5.00T(Si7.18Al0.82)Σ8.00O22W[(OH)1.93F0.05Cl0.02]Σ2.00 (where the dominant cations/anions relevant to nomenclature issues are in bold). The simplified end-member formula of magnesio-hornblende is A□BCa2C(Mg4Al)T(Si7Al)O22W(OH)2, which requires SiO2 51.68, Al2O3 12.53, MgO 19.80, CaO 13.78, H2O 2.21, total 100.00 wt.%.
Table 4. Chemical composition (10 analytical points), unit formula (based on 24 anions and 15.27 cations) and a comparison between observed and calculated site scattering for magnesio-hornblende (1325).
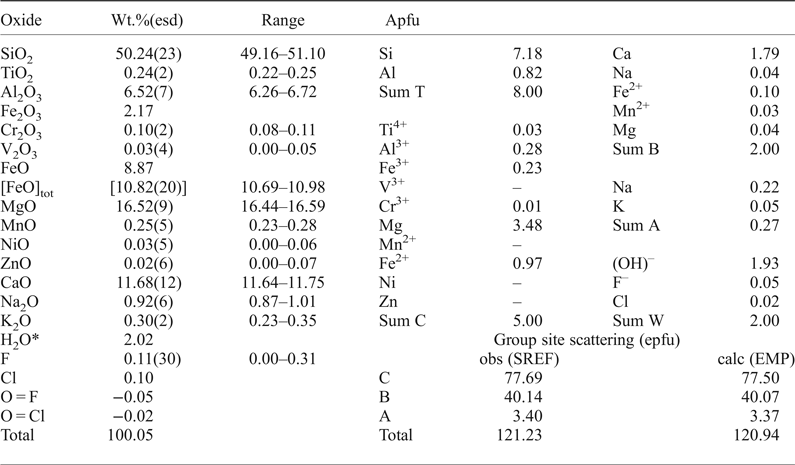
* calculated based on 24 (O, OH, F, Cl) with (OH + F + Cl) = 2 apfu.
The crystal chemistry of magnesio-hornblende
Site populations were obtained taking into account the experimental mean bond lengths and site-scattering values for the individual sites (Hawthorne et al., Reference Hawthorne, Ungaretti and Oberti1995; Oberti et al., Reference Oberti, Hawthorne, Cannillo, Cámara, Hawthorne, Oberti, Della Ventura and Mottana2007). The results are reported in Table 5. As expected, TAl is ordered at the T(1) site, smaller B cations (Mn,Fe,Mg) are ordered at the M(4’) site and CR3+ cations are almost ordered at the M(2) site but for a small amount of Al occurring at M(3). This was assumed to improve the agreement between the observed and calculated mean bond lengths at the M(2) and M(3) sites, is in accord with the high Mg content of the sample and may suggest high-T crystallization (Oberti et al., Reference Oberti, Hawthorne, Ungaretti and Cannillo1995).
Table 5. Site populations for magnesio-hornblende, crystal 1325. There is close agreement between the refined values of site-scattering (ss, epfu) and mean bond lengths (mbl, Å) and those calculated based on the proposed site-populations*.
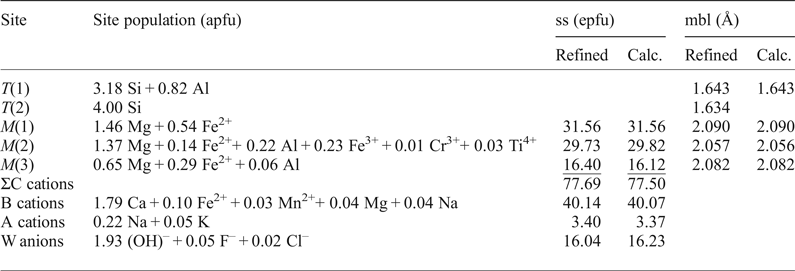
*Hawthorne et al. (Reference Hawthorne, Ungaretti and Oberti1995)
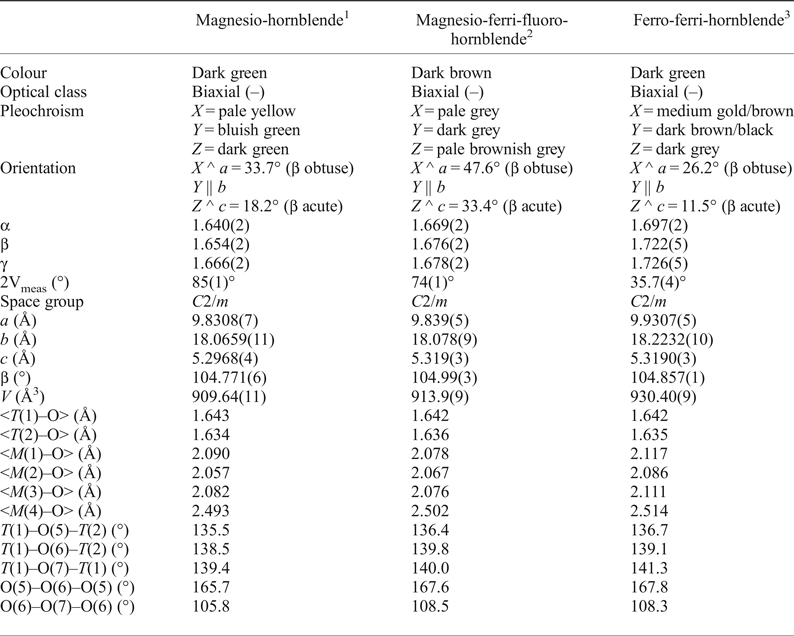
A comparison of the optical and crystallographic properties of the three members related to the hornblende rootname that have been characterized recently is provided in Table 6. In these three hornblende compositions the 2V value drops significantly with increasing Fe content, a feature which might be used for preliminary mineral characterization from petrographic sections. Moreover, changes in the mean bond lengths measured in the strip of octahedra are in accord with changes in the composition of the samples. The CAl1CFe3+1 exchange (occurring at the M(2) site) and the W(OH)–1WF1 exchange (occurring at the O(3) site coordinated by two M(1) and one M(3) cations) describe the transition from magnesio-hornblende to magnesio-ferri-fluoro-hornblende, and lead to an increase in the <M(2)–O> distance and a decrease in both <M(1)–O> and <M(3)–O> distances. In contrast, the CMg–1CFe2+1 exchange (occurring at all the M(1,3) sites) and the CAl−1CFe3+1 exchange (occurring at the M(2) site) describe the transition from magnesio-hornblende to ferro-ferri-hornblende and increases all the <M(1,2,3)–O> distances. All these changes are consistent with the differences in ionic radii (Shannon, Reference Shannon1976) of the constituent cations.
Table 6. A comparison of the optical and crystallographic properties available for magnesio-hornblende (this work), magnesio-ferri-fluoro-hornblende (Oberti et al., Reference Oberti, Boiocchi, Hawthorne, Ball and Chiappino2016a), and ferro-ferri-hornblende (Oberti et al., Reference Oberti, Boiocchi, Hawthorne, Ball, Cámara, Pagano and Pagano2016b).
1 A(□0.73Na0.22K0.05)Σ1.00B(Ca1.79Fe2+0.10Mn2+0.03Mg0.04Na0.04)Σ2.00C(Mg3.48Fe2+0.97Al0.28Fe3+0.23 Cr3+0.01Ti0.03)Σ5.00T(Si7.18Al0.82)Σ8.00 O22W[(OH)1.93F0.05Cl0.02]Σ2.00
2 A(□0.63Na0.15K0.22)Σ1.00B(Ca1.66Mn0.09Na0.25)Σ1.00C(Mg2.20Fe2+1.94Mn0.01Zn0.01Fe3+0.72Ti0.13)Σ5.01T(Si6.89Al1.11)Σ8.00O22W[F1.55(OH)0.37Cl0.08)Σ2.00
3 A(□0.77Na0.10K0.13)Σ1.00B(Ca1.93Na0.07)Σ2.00C(Mg1.16Fe2+3.21Mn0.06Fe3+0.45Al0.12Ti0.01)Σ5.01T(Si7.26Al0.74)Σ8.00O22W(OH1.89F0.01Cl0.10)Σ2.00
The extension of the double chain of tetrahedra along c, as measured by the O(5)–O(6)–O(5) angle, is lower in magnesio-hornblende than in ferro-ferri-hornblende; the more extended double chain of tetrahedra promotes linkage with the strip of octahedra where Mg and Al are replaced, respectively, by the larger Fe2+ and Fe3+ cations. However, the O(5)–O(6)–O(5) angle in magnesio-ferri-fluoro-hornblende (where the TAl content is 0.37 apfu larger) is very similar to that in ferro-ferri-hornblende (Oberti et al., Reference Oberti, Boiocchi, Hawthorne, Ball, Cámara, Pagano and Pagano2016b).
Acknowledgements
The authors wish to thank Fernando Cámara and Peter Leverett for their kind and useful reviews.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.093