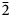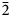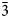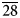Introduction
The högbomite-supergroup minerals are complex oxides that form a polysomatic series composed of spinel T 2M 4O8 (S) and nolanite TM 4O7(OH) (N) modules, where T and M represent tetrahedrally and octahedrally coordinated cations, respectively (Armbruster, Reference Armbruster1998, Reference Armbruster2002; Heiny and Armbruster, Reference Hejny and Armbruster2002). Five groups belonging to the högbomite supergroup are known. The taaffeite group includes minerals with modified nolanite BeTM 4O8 (N’) modules (Armbruster, Reference Armbruster2002). The velesite group, the only one known with iron-spinel modules (T 2Fe4O8), currently consists of one parent species zincovelesite-6N6S, Zn3(Fe3+,Mn3+,Al,Ti)8O15(OH) (Chukanov et al., Reference Chukanov, Krzhizhanovskaya, Jančev, Pekov, Varlamov, Göttlicher, Rusakov, Polekhovsky, Chervonnyi and Ermolaeva2018). The remaining three – högbomite, nigerite (Armbruster, Reference Armbruster2002) and beltrandoite (Cámara et al., Reference Cámara, Cossio, Regis, Cerantola, Ciriotti and Compagnoni2018) groups – include minerals with aluminium-spinel modules (T 2Al4O8). They are named based on the combination of the predominant cation at the largest M-centred octahedron in the N module [rootname; Ti – högbomite, Sn – nigerite (Armbruster, Reference Armbruster2002) and Fe3+ – beltrandoite (Cámara et al., Reference Cámara, Cossio, Regis, Cerantola, Ciriotti and Compagnoni2018)], the predominant T in the S module [prefix; Mg – magnesio, Fe2+ – ferro and Zn – zinco, e.g. magnesiotaaffeite] and the total number of N and S modules [hyphenated suffix; e.g. zincohögbomite-2N6S, ferronigerite-6N6S and magnesiobeltrandoite-2N3S].
Magnesiohögbomite-6N12S, (Mg,Fe)5(Al,Fe,Ti)12O23(OH), is a new member of the högbomite group described in this paper. It is named in accordance with the International Mineralogical Association (IMA) nomenclature of the högbomite supergroup (Armbruster, Reference Armbruster2002) as a mineral with Ti as the predominant cation in the N module [rootname högbomite], Mg – the predominant T in the S module [prefix magnesio-], and 6 and 12 as the total number of N and S modules, respectively [hyphenated suffix 6N12S]. Both the new mineral and the name have been approved by the Commission on New Minerals, Nomenclature and Classification of the IMA, (IMA2020-029; Lykova et al., Reference Lykova, Rowe, Poirier, Giester and Helwig2020). The holotype was deposited in the collection of the Canadian Museum of Nature, Ottawa, Canada. The catalogue number is CMNMC 87484.
Occurrence and general appearance
The mineral was found at the DeWitts Corners occurrence, lots 10 and 11, concession 1, Bathurst Township, Lanark County, Ontario, Canada (44°49′47″N, 76°21′05″W). Magnesiohögbomite-6N12S occurs, together with magnesiohögbomite-2N3S, Mg4Al9TiO19(OH), black spinel, corundum, diopside, magnesio-hastingsite, pargasite and clinochlore, in calcite “vein-dikes”. The host rocks are marble of the Central Metasedimentary Belt of the Precambrian Grenville Geological Province (Wynne-Edwards, Reference Wynne-Edwards, Price and Douglas1972). The “vein-dikes” were exposed in two adjacent shallow pits separated by a road, and thus located at different lots. The holotype of magnesiohögbomite-6N12S originates from the pit located at the lot 11; however, the new mineral was found in pits at both lot 10 and lot 11.
The origin of calcite “vein-dikes” has long been a subject of debates. However, in a recent study Martin and Schumann (Reference Martin and Schumann2019) argued that at least some of the “vein-dikes” were formed as a result of melting of Grenville marble.
Magnesiohögbomite-6N12S occurs as thin tabular to short-prismatic crystals up to 5 mm in size (Fig. 1). The major forms are pinacoid {0001} and hexagonal pyramid {11${\bar 2}$![]() 1}, often striated. Some crystals also show a striated hexagonal prism {11${\bar 2}$
1}, often striated. Some crystals also show a striated hexagonal prism {11${\bar 2}$![]() 0}. Most often magnesiohögbomite-6N12S forms tabular crystals as epitaxial overgrowths on spinel. The {0001} faces of magnesiohögbomite-6N12S and the {111} faces of spinel are parallel. Free standing crystals are rare and can be found on, or in, spinel and, sometimes, corundum pseudomorphs after spinel. Short-prismatic crystals with striated pyramid and prism faces have a barrel shape. Magnesiohögbomite-6N12S occurs together with visually indistinguishable magnesiohögbomite-2N3S (Fig. 1). No preference for a particular habit or association was found for either of the polysomes, and the two minerals are roughly equally abundant at the occurrence.
0}. Most often magnesiohögbomite-6N12S forms tabular crystals as epitaxial overgrowths on spinel. The {0001} faces of magnesiohögbomite-6N12S and the {111} faces of spinel are parallel. Free standing crystals are rare and can be found on, or in, spinel and, sometimes, corundum pseudomorphs after spinel. Short-prismatic crystals with striated pyramid and prism faces have a barrel shape. Magnesiohögbomite-6N12S occurs together with visually indistinguishable magnesiohögbomite-2N3S (Fig. 1). No preference for a particular habit or association was found for either of the polysomes, and the two minerals are roughly equally abundant at the occurrence.

Fig. 1. Tabular magnesiohögbomite-6N12S crystal and prismatic magnesiohögbomite-2N3S crystal on spinel. Field of view = 2 mm. Specimen CMNMC 88160. Photo: Paul Sokoloff and Inna Lykova.
Physical and optical properties
Magnesiohögbomite-6N12S is dark brown to black with brown streak and vitreous lustre. It has no cleavage or parting. The Mohs hardness is 6½. The fracture is uneven. The mineral is non-fluorescent under ultraviolet light. The density calculated using the empirical formula and unit-cell volume refined from the single-crystal XRD data is 3.87 g/cm3.
Magnesiohögbomite-6N12S is optically uniaxial (–). The refractive indices were not measured due to the lack of available high refractive index (> 1.8) immersion fluids. The mean refractive index obtained from the Gladstone–Dale relationship (Mandarino, Reference Mandarino1981) is 1.857. Pleochroism is weak; the absorption scheme is as follows: O = brown and E = pale brown.
Experimental methods
Chemical data for magnesiohögbomite-6N12S and magnesiohögbomite-2N3S were obtained using a JEOL 8230 SuperProbe electron microscope equipped with five WDS spectrometers (University of Ottawa – Canadian Museum of Nature MicroAnalysis Laboratory, Canada) with an acceleration voltage of 20 kV, a beam current of 40 nA and a beam diameter of 5 μm. The following reference materials were used: chromite (MgKα and AlKα), hematite (FeKα), gahnite (ZnKα) and rutile (TiKα). Raw intensities were converted to concentrations using the default φρZ corrections of the Probe for EMPA software package (Armstrong, Reference Armstrong and Newbury1988). Water contents were calculated from stoichiometry and included in the matrix correction.
The Fourier transform infrared (FTIR) spectrum of magnesiohögbomite-6N12S was obtained at the Canadian Conservation Institute, Canada using a Bruker Hyperion 2000 microscope interfaced to a Tensor 27 spectrometer with a wide-band mercury cadmium telluride (MCT) detector. A small fragment of magnesiohögbomite-6N12S was mounted on a low-pressure diamond anvil microsample cell and analysed in transmission mode. The spectrum was collected between 400–4000 cm–1 with the co-addition of 150 scans at a 4 cm–1 resolution.
Powder X-ray diffraction data were collected at the Canadian Museum of Nature, Canada using a Bruker D8 Discover microdiffractometer equipped with a DECTRIS EIGER2 R 500K detector and IμS microfocus X-ray source (λCuKα = 1.54184 Å). The instrument was calibrated at a sample-to-detector distance of 175 mm using a statistical calibration method (Rowe, Reference Rowe2009). A powder ball 200 μm in diameter, mounted on a fibre pin mount, was analysed with continuous Phi rotation and 10° rocking motion along the Psi axis of the Centric Eulerian Cradle stage.
Single-crystal X-ray studies were carried out at T = 296 K using a Bruker Kappa X8 APEX diffractometer equipped with a CCD detector and with an Incoatec Microfocus Source IμS (30 W, multilayer mirror, MoKα) at the Institute of Mineralogy and Crystallography, University of Vienna, Austria. Eighteen sets of phi and omega scans with 0.75° scanwidth were measured up to 80°2θ (full sphere) at a crystal-detector distance of 50 mm. The absorption was corrected by evaluation of multi-scans (Krause et al. Reference Krause, Herbst-Irmer, Sheldrick and Stalke2015).
Results
Chemical data
Chemical data for magnesiohögbomite-6N12S and magnesiohögbomite-2N3S are given in Table 1. The data on the holotype of magnesiohögbomite-6N12S (6 analyses) were collected on a crystal later used for the crystal-structure study. The empirical formula calculated on the basis of 17 cations, excluding H+, is (Mg2.95Fe2+1.51Al0.49Zn0.05)Σ5(Al10.71Fe3+0.78Ti0.51)Σ12O23(OH). The Fe2+/Fe3+ ratio was calculated by the charge balance. For comparison, the formula calculated based on an average of 25 analyses of six crystals from different specimens is (Mg3.01Fe2+1.46Al0.49Zn0.04)Σ5(Al10.66Fe3+0.83Ti0.51)Σ12O23(OH). The data show that the mineral is homogeneous. The empirical formula of magnesiohögbomite-2N3S based on an average of 31 analyses of six crystals and calculated on the basis of 14 cations, excluding H+, is (Mg2.36Fe2+1.08Al0.52Zn0.04)Σ4(Al8.69Fe3+0.83Ti0.48)Σ10O19(OH).
Table 1. Chemical data (wt.%) for magnesiohögbomite-6N12S and magnesiohögbomite-2N3S from the DeWitts Corners.

* Fe2+/Fe3+ ratio was calculated based on the charge balance.
** Calculated from the stoichiometry.
The simplified formula of magnesiohögbomite-6N12S is (Mg,Fe)5(Al,Fe,Ti)12O23(OH). The end-member formula is Mg5Al11TiO23(OH), which requires MgO 23.68, Al2O3 65.88, TiO2 9,38, H2O 1.06, total 100 wt.%.
Magnesiohögbomite-6N12S does not react with a diluted aqueous HCl solution at room temperature.
Infrared spectroscopy
The IR spectrum of magnesiohögbomite-6N12S (Fig. 2) shows IR bands of O–H-stretching (at 3365 cm–1) vibrations and M–O-stretching (in the range from 440 to 680 cm–1) vibrations where M = Mg, Fe, Al, Ti and Zn. The band assignment was made in accordance with Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016).

Fig. 2. Infrared spectrum of magnesiohögbomite-6N12S.
Characteristic bands of H–O–H bending vibrations of H2O molecules (in the range 1550–1750 cm–1) and CO32– anions (in the range 1350–1550 cm–1) are absent in the IR spectrum of magnesiohögbomite-6N12S.
X-ray diffraction data
The indexed powder X-ray diffraction data are given in Table 2. Parameters of the trigonal unit cell refined from the powder data are as follows: a = 5.7262(1), c = 83.1262(6) Å and V = 2360.51(2) Å3.
Table 2. Powder X-ray diffraction data (d in Å) for magnesiohögbomite-6N12S. The strongest lines are in bold.

a Calculated from the crystal structure determination, only reflections with intensities >1 are given.
b For the unit-cell parameters calculated from single-crystal data.
The single-crystal X-ray diffraction data were indexed in the R ${\bar 3}$![]() m space group with the following unit-cell parameters: a = 5.7194(2), c = 83.069(5) Å and V = 2353.3(2) Å3. The structure was solved and refined to R 1 = 0.022 on the basis of 1891 independent reflections with I > 2σ(I) using the SHELXL-2018/3 program package (Sheldrick, Reference Sheldrick2015). Crystal data, data collection and structure refinement details are given in Table 3, atom coordinates, equivalent displacement parameters, site occupancy factors in Table 4, selected interatomic distances in Table 5 and bond valence sums (BVS) in Table 6.
m space group with the following unit-cell parameters: a = 5.7194(2), c = 83.069(5) Å and V = 2353.3(2) Å3. The structure was solved and refined to R 1 = 0.022 on the basis of 1891 independent reflections with I > 2σ(I) using the SHELXL-2018/3 program package (Sheldrick, Reference Sheldrick2015). Crystal data, data collection and structure refinement details are given in Table 3, atom coordinates, equivalent displacement parameters, site occupancy factors in Table 4, selected interatomic distances in Table 5 and bond valence sums (BVS) in Table 6.
Table 3. Crystal data, data collection information and structure refinement details for magnesiohögbomite-6N12S.

Table 4. Coordinates and equivalent displacement parameters (U eq, in Å2) of atoms and site occupancies for magnesiohögbomite-6N12S.

aWyckoff site; bU iso.
* The Mg5 site occupancy was refined assuming full occupancy and refining Mg (including the similarly light Al) against Fe, the best agreement was obtained with Mg0.803(4)Fe0.197(4). In the final refinement cycles the occupancy was fixed as Mg0.43Al0.40Fe0.17 on the e ref value [14.8], electron microprobe data, interatomic distances and bond valence calculations.
** The Ti12 site was refined as Ti0.519(7)Fe0.481(7).
Table 5. Selected interatomic distances (Å) in the structure of magnesiohögbomite-6N12S.

Table 6. Bond valence calculations* for magnesiohögbomite-6N12S.

* Bond-valence parameters were taken from Brese and O'Keeffe (Reference Brese and O'Keeffe1991) and, for H bonding, Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988). Bond-valence sums were calculated taking into account site occupancies.
The crystallographic information file for magnesiohögbomite-6N12S has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below). It was also deposited in the Inorganic Crystal Structure Database (ICSD; #CSD 2046917).
Discussion
Crystal structure
Magnesiohögbomite-6N12S, 6 × [(Mg,Fe)5(Al,Fe,Ti)12O23(OH)], is a new polysome of the högbomite group formed by alternation of N and S modules in the sequence 3 × (NSSNSS) leading to the general formula 6 × T 5M 12O23(OH). The sequence of cubic ‘c’ and hexagonal ‘h’ closed-packed oxygen layers is 3 × (cccccchcccch). The structure of a potentially new 6N12S polysome of the högbomite supergroup was predicted to have the sequences 3 × (NNSSSS) and 3 × (cccccccchcch) (Armbruster, Reference Armbruster2002). As our study shows 6N12S polysomes have a different sequence of the c and h layers that has not been observed in the högbomite supergroup minerals before.
There are five independent T sites and seven independent M sites in the structure. Mg, Fe2+, Fe3+, Al and Ti are distributed among these sites based on the microprobe data and refined site-scattering factors (e ref, in electrons per site) taking into account bond valence sums (BVS) and interatomic distances (Tables 4–6). The smallest tetrahedron Mg(5)O4 with the average <Mg5–O> distance of 1.838 Å and the largest octahedron Ti(12)O6 with the average <Ti12–O> distance of 2.036 Å in the structure belong to the N module (Fig. 3). The site occupancy at the Mg5 site was refined, assuming full occupancy, as Mg0.803(4)Fe0.197(4) [e ref = 14.8]. In the final refinement cycles the occupancy was fixed as Mg0.43Al0.40Fe3+0.17. The Ti12 site is occupied by 0.52Ti + 0.39Fe3+ + 0.09Fe2+. The proposed distribution is supported by the observed bond-valence sums at these sites [2.64 valence units (vu) at the Mg5 site and 3.21 vu at the Ti12 site, Table 6]. The other four T sites are Mg/Fe2+ mixed-occupancy sites with preferential occupancy of Mg (68, 61, 70 and 62% at the Mg1, Mg2, Mg3 and Mg4 sites, respectively; Table 4). M sites Al6, Al7, Al8, Al9 and Al10 are fully occupied by Al atoms, while there is an admixture of Fe3+ atoms (8% of the site occupancy) at the Al11 site. The ideal composition for the N module is Mg(Al3Ti)O7(OH) and for the S module is Mg2Al4O8.

Fig. 3. General view of the crystal structure of magnesiohögbomite-6N12S. Al-centred octahedra are light blue, Ti-centred octahedra are dark blue, Mg-centred tetrahedra are orange and small purple spheres are H. The sequence of cubic ‘c’ and hexagonal ‘h’ closed-packed oxygen layers is shown. S: spinel T 2M 4O8 module; N: nolanite TM 4O7(OH) module. The unit cell is outlined.
Cámara et al. (Reference Cámara, Cossio, Regis, Cerantola, Ciriotti and Compagnoni2018) who studied Fe2+/Fe3+ distribution between T and M sites in magnesiobeltrandoite-2N3S, Mg3Al10Fe3+O19(OH), showed that, although Fe2+ is mostly ordered at the T sites, whereas Fe3+ is at the M sites, some Fe3+ atoms are present at the tetrahedral site of the N module and some Fe2+ atoms are observed at the largest octahedron of the N module. Their data are in a good agreement with the proposed cation distribution in magnesiohögbomite-6N12S.
The BVS at the O1 site (1.22 vu) indicates that it is occupied by OH– groups. H atoms were localised in the structure; the O1–H1 distance is 0.89 Å, the H1⋅⋅⋅O8 distance is 2.31 Å and the O1⋅⋅⋅O8 distance is 3.01 Å. The remaining 11 anion sites are occupied by O2– anions. The band at 3365 cm–1 in the IR spectrum of magnesiohögbomite-6N12S confirms the presence of hydroxyl groups (Fig. 2). Both BVS and IR data indicate the absence of H2O0 in magnesiohögbomite-6N12S.
The proton position was not very reliably determined, so we used the correlation of O–H stretching frequencies with O⋅⋅⋅O and H⋅⋅⋅O bond lengths, established by Libowitzky (Reference Libowitzky1999), to extract more information about the actual distances. The O⋅⋅⋅O bond length was estimated as 2.78 Å and the H⋅⋅⋅O bond length as 1.89 Å.
The resulting structural formula of magnesiohögbomite-6N12S (Mg3.04Fe2+1.39Al0.40Fe3+0.17)Σ5.00(Al10.76Fe3+0.63Ti0.52Fe2+0.09)Σ12.00O23(OH) is in a good agreement with its empirical formula (Mg2.95Fe2+1.51Al0.49Zn0.05)Σ5.00(Al10.71Fe3+0.78Ti0.51)Σ12.00O23(OH).
Relationships with other högbomite-supergroup minerals
Magnesiohögbomite-6N12S is a member of the magnesiohögbomite subgroup composed of högbomite-group minerals with Mg as the predominant tetrahedrally coordinated cation in the S module. Four polysomatic members of the subgroup were known prior to this study: magnesiohögbomite-2N2S, magnesiohögbomite-2N3S, magnesiohögbomite-6N6S (Armbruster, Reference Armbruster2002) and magnesiohögbomite-2N4S (Shimura et al., Reference Shimura, Akai, Lazic, Armbruster, Shimizu, Kamei, Tsukada, Owada and Yuhara2012; Table 7). The reasons for the crystallisation of a specific polysome are still not known. Heiny and Armbruster (Reference Hejny and Armbruster2002) reviewed available chemical data on different polysomes and concluded that there is no correlation between Ti content and type of polysome. Our data corroborate their findings. There is no substantial difference in the content of Ti between magnesiohögbomite-6N12S (0.51 atoms per formula unit (apfu); Table 1) and magnesiohögbomite-2N3S (0.48 apfu). Moreover, the observed small differences in their chemistry (Table 1) seem to reflect the differences in the N : S, and thus T : M ratio, only. Magnesiohögbomite-2N3S (T : M = 4 : 10) is characterised by slightly lower contents of Mg and Fe2+, predominantly concentrated at the T sites, than magnesiohögbomite-6N12S (T : M = 5 : 12). It means that both polysomes used very similar combinations of ions to build N and S modules but followed one of the two stacking patterns with no apparent preference. Out of 23 analysed magnesiohögbomite crystals from the DeWitts Corners, 12 turned out to be magnesiohögbomite-2N3S, nine are magnesiohögbomite-6N12S and two showed the presence of both polysomes. No correlation between crystal habits and/or associations and type of polysome was observed. It is probable that both stacking periodicities are formed under similar conditions and either one or the other is realised randomly during crystallisation, which would explain their equal abundance at the DeWitts Corners occurrence. Why magnesiohögbomite-6N12S was formed and not its hexagonal polymorph magnesiohögbomite-2N4S (Table 7) is unclear.
Table 7. Comparative data for magnesiohögbomite-6N12S and other members of the magnesiohögbomite subgroup.

*Powder X-ray diffraction patterns calculated from the crystal structure data by Hejny and Armbruster (Reference Hejny and Armbruster2002). **Powder X-ray diffraction pattern obtained on magnesiohögbomite-2N3S from the DeWitts Corners locality, our data.
1M: Mg, Fe, Al; T: Al, Fe, Ti; 2 S: spinel T 2M 4O8 module; N: nolanite TM 4O7(OH) module.
Magnesiohögbomite-6N12S is the first 6N12S polysome of the högbomite supergroup studied in detail. A högbomite-group mineral with 6N12S as the total number of N and S (also known as a polytype 36R) was previously found at the Dentz Farm, Gravelotte, Murchison Range, Limpopo, South Africa (Nel, Reference Nel1949; McKie, Reference McKie1963). It was included in the revised nomenclature of the högbomite-group minerals as a potentially new mineral ‘ferrohögbomite-6N12S’ (Armbruster, Reference Armbruster2002), but never properly examined. The Dentz Farm, where cores of spinel are rimmed by chlorite, corundum and hogbomite in aluminous lenses in serpentines, is one of the type localities of magnesiohögbomite-2N3S (Nel, Reference Nel1949; McKie, Reference McKie1963, Armbruster, Reference Armbruster2002), and so, possibly, the second locality where both 2N3S and 6N12S polysomes occur together. While more work is required on the prospective 6N12S polysome to confirm this conjecture, such an occurrence would support the idea that magnesiohögbomite-6N12S and magnesiohögbomite-2N3S are formed under the same conditions.
Both magnesiohögbomite-6N12S and magnesiohögbomite-2N3S from the DeWitts Corners are characterised by a relatively low Ti content (0.51 and 0.48 apfu, respectively; Table 1). However, because Fe2+ atoms are distributed between both the T sites and the Ti site (see above), both Fe3+ and Fe2+ atoms are present at the Ti site, and so Ti atoms are the predominant component at the site, not Fe3+, even when the total Fe occupancy slightly exceeds the Ti occupancy. Thus, both minerals belong to the högbomite group with the Ti dominant N module, not to the beltrandoite group with the Fe3+ dominant N module.
Acknowledgements
We thank anonymous referees for their valuable comments. IL thanks John Montgomery for bringing the DeWitts Corners occurrence to her attention. We are grateful to Paul Sokoloff for his help in obtaining the colour photo.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2021.31