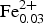Introduction
Heyerdahlite, ideally Na3Mn7Ti2(Si4O12)2O2(OH)4F(H2O)2, is a new astrophyllite-supergroup mineral from the Larvik Plutonic complex, Norway. The mineral is named heyerdahlite (pronunciation is HEY-AIR-DAL-‘AIT) after the Norwegian explorer Thor Heyerdahl (1914–2002), who was born and raised in the city of Larvik, which is within the Larvik Plutonic complex. The new mineral species and its name were approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2016-108). The holotype specimen of heyerdahlite has been deposited in the collections of Royal Ontario Museum, Toronto, Ontario, Canada; the accession number is M57516. Here we report the description and crystal structure of heyerdahlite.
The astrophyllite supergroup
General information on the astrophyllite supergroup (Sokolova et al., Reference Sokolova, Cámara, Hawthorne and Ciriotti2017a), is given here to explain the site nomenclature and details of the structure used in the sections on infrared spectroscopy, chemical analysis and structure refinement below. The HOH block is the main structural unit in all astrophyllite-supergroup structures; it consists of three H–O–H sheets where the T4O12 astrophyllite ribbons occur in the H sheets. In each structure, HOH blocks alternate with I (Intermediate) blocks along [001].
The general formula for the astrophyllite-supergroup minerals is of the form A2pBrC7D2(T4O12)2I![]() ${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{{\rm Dn}}^{\rm P} \!{\rm W}_{\!\!{\rm A2}} $, where C [cations at the M(1–4) sites in the O sheet] = Fe2+, Mn, Na, Mg, Zn, Fe3+, Ca, Zr and Li; D (cations in the H sheets) = [6,5]Ti, Nb, Zr, Sn4+, [5]Fe3+, Mg and Al; T = Si with minor Al; A2pBrIWA2 (I block) where p = 1 or 2; r = 1 or 2; A = K, Cs, Ba, H2O, Li, Rb, Pb2+, Na and □; B = Na, Ca, Ba, H2O and □; I represents the composition of the central part of the I block (excluding peripheral layers of the form A2pBrWA2), e.g. (PO4)2(CO3) (devitoite);
${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{{\rm Dn}}^{\rm P} \!{\rm W}_{\!\!{\rm A2}} $, where C [cations at the M(1–4) sites in the O sheet] = Fe2+, Mn, Na, Mg, Zn, Fe3+, Ca, Zr and Li; D (cations in the H sheets) = [6,5]Ti, Nb, Zr, Sn4+, [5]Fe3+, Mg and Al; T = Si with minor Al; A2pBrIWA2 (I block) where p = 1 or 2; r = 1 or 2; A = K, Cs, Ba, H2O, Li, Rb, Pb2+, Na and □; B = Na, Ca, Ba, H2O and □; I represents the composition of the central part of the I block (excluding peripheral layers of the form A2pBrWA2), e.g. (PO4)2(CO3) (devitoite); ![]() ${\rm X}_{\rm D}^{\rm O} $ = O;
${\rm X}_{\rm D}^{\rm O} $ = O; ![]() ${\rm X}_{\rm A}^{\rm O} $ = OH or F;
${\rm X}_{\rm A}^{\rm O} $ = OH or F; ![]() ${\rm X}_{\rm D}^{\rm P} $ = F, O, OH, H2O and □, where n = 0, 1 or 2 for (
${\rm X}_{\rm D}^{\rm P} $ = F, O, OH, H2O and □, where n = 0, 1 or 2 for (![]() ${\rm X}_{\rm D}^{\rm P} $)n; WA = H2O or □.
${\rm X}_{\rm D}^{\rm P} $)n; WA = H2O or □.
The thirteen minerals of the astrophyllite supergroup are divided into three groups based on (1) the type of self-linkage of HOH blocks, i.e. (a) HOH blocks link directly via D–![]() ${\rm X}_{\rm D}^{\rm P} $–D bridges, or (b) HOH blocks do not link directly; and (2) the dominant cation of the O sheet [the C group: C7 apfu (atoms per formula unit)]. In the astrophyllite group, HOH blocks connect via D–
${\rm X}_{\rm D}^{\rm P} $–D bridges, or (b) HOH blocks do not link directly; and (2) the dominant cation of the O sheet [the C group: C7 apfu (atoms per formula unit)]. In the astrophyllite group, HOH blocks connect via D–![]() ${\rm X}_{\rm D}^{\rm P} $–D bridges, Fe2+ is dominant at C7; in the kupletskite group, HOH blocks connect via D–
${\rm X}_{\rm D}^{\rm P} $–D bridges, Fe2+ is dominant at C7; in the kupletskite group, HOH blocks connect via D–![]() ${\rm X}_{\rm D}^{\rm P} $–D bridges, Mn2+ is dominant at C7; in the devitoite group, HOH blocks do not connect via D–
${\rm X}_{\rm D}^{\rm P} $–D bridges, Mn2+ is dominant at C7; in the devitoite group, HOH blocks do not connect via D–![]() ${\rm X}_{\rm D}^{\rm P} $–D bridges. The ideal structural formulae for the astrophyllite-supergroup minerals are given in Table 1.
${\rm X}_{\rm D}^{\rm P} $–D bridges. The ideal structural formulae for the astrophyllite-supergroup minerals are given in Table 1.
Table 1. Ideal structural formulae of the form A2pBrC7D2(T4O12)2I![]() ${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{{\rm Dn}}^{\rm P} $WA2 for the astrophyllite-supergroup minerals*.
${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{{\rm Dn}}^{\rm P} $WA2 for the astrophyllite-supergroup minerals*.

*After Sokolova et al. (Reference Sokolova, Cámara, Hawthorne and Ciriotti2017a);
**References (the most recent work on the structure): (1) Cámara et al. (Reference Cámara, Sokolova, Abdu and Hawthorne2010); (2) Sokolova and Hawthorne (Reference Sokolova and Hawthorne2016); (3) Stepanov et al. (Reference Stepanov, Bekenova, Levin, Sokolova, Hawthorne and Dobrovolskaya2012);
(4) Agakhanov et al. (Reference Agakhanov, Pautov, Sokolova, Abdu and Karpenko2016); (5) Piilonen et al. (Reference Piilonen, McDonald and Lalonde2001); (6) Piilonen et al. (Reference Piilonen, Lalonde, McDonald and Gault2000); (7) this work; (8) Kampf et al. (Reference Kampf, Rossman, Steele, Pluth, Dunning and Walstrom2010); (9) Khomyakov et al. (Reference Khomyakov, Cámara, Sokolova, Hawthorne and Abdu2011); (10) Sokolova and Cámara (Reference Sokolova and Cámara2008); (11) Sokolova et al. (Reference Sokolova, Cámara, Hawthorne, Semenov and Ciriotti2017b).
Occurrence and mineral association
Heyerdahlite occurs in the Bratthagen nepheline syenite pegmatite of the Larvik Plutonic complex, Norway (Larsen Reference Larsen and Larsen2010, Reference Larsen2013; Khomyakov et al., Reference Khomyakov, Cámara, Sokolova, Hawthorne and Abdu2011; Oberti et al., Reference Oberti, Boiocchi, Hawthorne and Kristiansen2014). The pegmatite is situated in a road cut on the main road RV8, ~200 m SE of the Bratthagen farm (59°09′26″N 10°00′39″E) in Lågendalen, Hedrum, Vestfold County, Norway.
The lardalite/foyaite rocks at Lågendalen contain the last segment of the Larvik Plutonic complex. The foyaites are younger than the lardalites and belong to the igneous rock complex of the Oslo region. The extreme mineral association in the Bratthagen syenite pegmatite shows mineralogical and geochemical features rather different from most of the syenite pegmatites in the Larvik Plutonic complex. Textural features suggest that these pegmatites are related to the host foyaite, which is younger than the lardalites. The mineral association is diagnostic of agpaitic pegmatites (Dahlgren, Reference Dahlgren and Larsen2010). The locality has been protected by law since 1984, and mineral collecting is now prohibited.
Heyerdahlite formed as a late-stage hydrothermal mineral in a nepheline-syenite pegmatite. It occurs as radiating fans up to 2 mm in diameter, consisting of transparent colourless to pale-brown elongated lath-like crystals (Figs 1a,b). The heyerdahlite crystals are ~1 mm long and only 50 µm wide. Associated minerals are albite, aegirine, hastingsite/magnesio-hastingsite, kupletskite, lorenzenite and pyrophanite.

Fig. 1. (a) A fan of transparent colourless to pale-brown elongated crystals of heyerdahlite on albite, the black crystal is aegirine; (b) scanning electron microscopy image of heyerdahlite crystals.
Almost 60 different species have been characterized in this area, many of which are typical of late-stage mineralization in highly evolved agpaitic intrusions (Oberti et al., Reference Oberti, Boiocchi, Hawthorne and Kristiansen2014).
Physical properties
Heyerdahlite is colourless to pale brown, it is transparent and has a vitreous lustre and a pale-brown streak. Cleavage is perfect parallel to {001}, heyerdahlite is brittle and has a hackly fracture. The Mohs hardness is 3. Heyerdahlite does not fluoresce under ultraviolet light. We did not measure density due to paucity of material, D calc. = 3.245 g/cm3 (from the empirical formula). A spindle stage was used to orient a crystal for measurement of refraction indices and 2V by the extinction-curve method (Bartelmehs et al., Reference Bartelmehs, Bloss, Downs and Birch1992). Heyerdahlite is biaxial (+), with refractive indices (λ = 589 nm) α = 1.694(2), β = 1.710(5), γ = 1.730(5); 2Vmeas. = 80(4)° and 2Vcalc. = 84.5°. The optical orientation was determined by transferring the crystal from the spindle stage to a single-crystal diffractometer and measuring the relative axial relations by X-ray diffraction; these values are given in Table 2. The dispersion is strong, r > v. It is pleochroic according to the scheme X > Y > Z, where X = yellowish brown, Y = brownish yellow and Z = pale yellow. The compatibility index (1 – Kp/Kc) = 0.028 (for D calc.) is rated as excellent (Mandarino, Reference Mandarino1981).
Table 2. Optical orientation (o).

Infrared spectroscopy
The Fourier-transform infrared (FTIR) spectra of heyerdahlite (Fig. 2) were collected on crystal fragments using a Bruker Hyperion 2000 IR microscope equipped with a liquid-nitrogen-cooled MCT detector (University of Manitoba). A single-crystal fragment was pressed into a homogenous film with a thickness of 10 µm with a diamond anvil and used to collect the powder spectrum shown in Fig. 2a. Single-crystal fragments with thicknesses of ~50 µm were used to collect the spectra shown in Figs 2b and 2c. Data over the range 4000–650 cm–1 were obtained by averaging 100 scans with a resolution of 4 cm–1. Base-line correction was carried out using the OPUS spectroscopic software (Bruker Optic GmbH).

Fig. 2. FTIR spectra of heyerdahlite collected on (a) powder and (b,c) a single crystal.
In the powder (Fig. 2a) and single-crystal (Figs. 2b,c) FTIR spectra, two strong OH bands are observed at ~3620 cm–1 (with a shoulder at ~3634 cm–1) and ~3556 cm–1 (with a shoulder at ~3582 cm–1) (Figs 2a,b). These two bands correspond to principal OH stretching bands where OH groups occur at the ![]() ${\rm X}_{\rm A}^{\rm O} $(1) and
${\rm X}_{\rm A}^{\rm O} $(1) and ![]() ${\rm X}_{\rm A}^{\rm O} $(2) sites. There is a sharp peak at ~1631 cm–1 (Fig. 2c) that may be assigned to an H–O–H bending mode of an H2O group. The corresponding H2O stretching bands are extremely broad (at ~3440 cm–1) (Fig. 2b) and underlie the sharper OH-stretching bands. In the lower-frequency region (Fig. 2a), two strong bands at ~1040 and ~940 cm–1 may be assigned to symmetric and asymmetric Si–O stretches and a less intense, broad band at ~635 cm–1 (with shoulder at ~685 cm–1) is due to an Si–O–Si deformation. Multiple low-intensity bands observed in the range 2380–2301 cm–1 are due to atmospheric CO2 transitions (Fig. 2a).
${\rm X}_{\rm A}^{\rm O} $(2) sites. There is a sharp peak at ~1631 cm–1 (Fig. 2c) that may be assigned to an H–O–H bending mode of an H2O group. The corresponding H2O stretching bands are extremely broad (at ~3440 cm–1) (Fig. 2b) and underlie the sharper OH-stretching bands. In the lower-frequency region (Fig. 2a), two strong bands at ~1040 and ~940 cm–1 may be assigned to symmetric and asymmetric Si–O stretches and a less intense, broad band at ~635 cm–1 (with shoulder at ~685 cm–1) is due to an Si–O–Si deformation. Multiple low-intensity bands observed in the range 2380–2301 cm–1 are due to atmospheric CO2 transitions (Fig. 2a).
Chemical analysis
A crystal of heyerdahlite was analysed with a Cameca SX-100 electron-microprobe (University of Manitoba) operating in wavelength-dispersion mode with an accelerating voltage of 15 kV, a specimen current of 15 nA, a beam size of 10 µm and count times on peak and background of 20 and 10 s, respectively. The following standards were used: Si and Ca: diopside; F: fluoro-riebeckite; Na: albite; Nb: Ba2NaNb5O15; Fe: fayalite; Mn: spessartine; Zr: zircon; Ti: titanite; Pb: PbTe; Mg: forsterite; Zn: gahnite; Cs: pollucite; K: orthoclase; and Rb: Rb-leucite. Tantalum, Al, Sn and Cr were sought but not detected. Data were reduced using the φ(ρZ) procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). The chemical composition of heyerdahlite is the mean of 8 determinations and is given in Table 3. The amount of H2O was calculated from structure refinement. Table 3 gives the empirical formula unit based on 32.18 (O + F) apfu, with constraints OH + F = 5 pfu and H2O = 1.18 pfu: (Na1.18K0.68Rb0.12Cs0.01Pb0.01)Σ2Na1.00(Mn6.29Zn0.23Mg0.07Zr0.04![]() ${\rm Fe}_{{\rm 0}{\rm. 03}}^{{\rm 2 +}} $Ca0.01Na0.34)Σ7.01(Ti1.78Nb0.17Mg0.03Zr0.02)Σ2(Si8.03O24)O2[(OH)3.92F0.08]Σ4F1.00[(H2O)1.18□0.82]Σ2 for Z = 1. The basis of 32.18 (anions + H2O groups) for calculation of the empirical formula unit was derived from the structure-refinement results: 31 anions (the established basis for calculation of the formula unit for the astrophyllite and kupletskite groups of the astrophyllite-supergroup minerals, Piilonen et al., Reference Piilonen, Lalonde, McDonald, Gault and Larsen2003) plus 1.18 H2O pfu. Note that 1.18 H2O pfu at the W A site is necessary to complete the coordination of Na1.18 apfu at the A(2) site (see below).
${\rm Fe}_{{\rm 0}{\rm. 03}}^{{\rm 2 +}} $Ca0.01Na0.34)Σ7.01(Ti1.78Nb0.17Mg0.03Zr0.02)Σ2(Si8.03O24)O2[(OH)3.92F0.08]Σ4F1.00[(H2O)1.18□0.82]Σ2 for Z = 1. The basis of 32.18 (anions + H2O groups) for calculation of the empirical formula unit was derived from the structure-refinement results: 31 anions (the established basis for calculation of the formula unit for the astrophyllite and kupletskite groups of the astrophyllite-supergroup minerals, Piilonen et al., Reference Piilonen, Lalonde, McDonald, Gault and Larsen2003) plus 1.18 H2O pfu. Note that 1.18 H2O pfu at the W A site is necessary to complete the coordination of Na1.18 apfu at the A(2) site (see below).
Table 3. Chemical composition (wt.%) and unit formula* (apfu) for heyerdahlite.

*Unit formula calculated on 32.18 (O + F) apfu, with OH + F = 5 pfu and H2O = 1.18 pfu;
**calculated from the structure-refinement results.
X-ray data collection and structure refinement
X-ray data for heyerdahlite were collected from a twinned crystal with a single-crystal Bruker D8 three-circle diffractometer equipped with a rotating-anode generator (MoKα radiation), multilayer optics and an APEX-II detector (University of Manitoba). Powder X-ray diffraction data were obtained by collapsing experimental data from the twinned crystal into two dimensions and are presented in Table 4. Details of data collection and structure refinement are given in Table 5. The intensities of reflections were collected with a frame width of 0.3° and a frame time of 6 s, and an empirical absorption correction (TWINABS, Sheldrick, Reference Sheldrick2008) was applied. CELL_NOW (Sheldrick, Reference Sheldrick2004) was used to obtain an HKLF5 file, and the Bruker SHELXTL Version 5.1 was used for the refinement of the crystal structure in space group P ![]() $\bar 1$ using the atom coordinates of zircophyllite (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2016); coordinates of the A(2) and WA atoms were taken from the difference-Fourier map. The crystal structure of heyerdahlite was refined to R 1 = 4.44%. The twin law is 180o rotation around the real axis [120] and the twin ratio is 0.572(3):0.428(3). The occupancies of eight cation sites were refined with the following scattering curves: M(1–4) and D sites: Mn and Ti; A(1) and A(2) sites: K and Na; B site: Na; and the W A site: O. We observed disorder of K and Na at the A(1) and A(2) sites, with K–Na = 0.754 Å. The refinement of the site occupancy for the B site converged to 1.0 and was fixed. At the last stages of the refinement, occupancies of the A(1), A(2) and W A sites were fixed in accord with chemical composition (Table 3). Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 6, selected interatomic distances and angles in Table 7, refined site-scattering values and assigned site-populations in Table 8, details of hydrogen bonding in Table 9 and bond-valence values for selected anions in Table 10. A list of observed and calculated structure factors, Crystallography Information File (CIF) and a table of anisotropic displacement parameters have been deposited as Supplementary material (see below).
$\bar 1$ using the atom coordinates of zircophyllite (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2016); coordinates of the A(2) and WA atoms were taken from the difference-Fourier map. The crystal structure of heyerdahlite was refined to R 1 = 4.44%. The twin law is 180o rotation around the real axis [120] and the twin ratio is 0.572(3):0.428(3). The occupancies of eight cation sites were refined with the following scattering curves: M(1–4) and D sites: Mn and Ti; A(1) and A(2) sites: K and Na; B site: Na; and the W A site: O. We observed disorder of K and Na at the A(1) and A(2) sites, with K–Na = 0.754 Å. The refinement of the site occupancy for the B site converged to 1.0 and was fixed. At the last stages of the refinement, occupancies of the A(1), A(2) and W A sites were fixed in accord with chemical composition (Table 3). Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 6, selected interatomic distances and angles in Table 7, refined site-scattering values and assigned site-populations in Table 8, details of hydrogen bonding in Table 9 and bond-valence values for selected anions in Table 10. A list of observed and calculated structure factors, Crystallography Information File (CIF) and a table of anisotropic displacement parameters have been deposited as Supplementary material (see below).
Table 4. Powder X-ray diffraction data* for heyerdahlite.

*Powder data were obtained by collapsing the experimental data from the single-crystal experiment into two dimensions. The most intense reflection (001) was covered by the beam stop. Therefore intensity was taken from the calculated powder diffraction pattern and all intensities rescaled accordingly. Intensities with I est. < 10 are not listed.
Table 5. Miscellaneous refinement data for heyerdahlite.

Table 6. Atom coordinates and equivalent displacement parameters (Å2) for heyerdahlite.

*U iso.
Table 7. Selected interatomic distances (Å) and angles (o) for heyerdahlite.
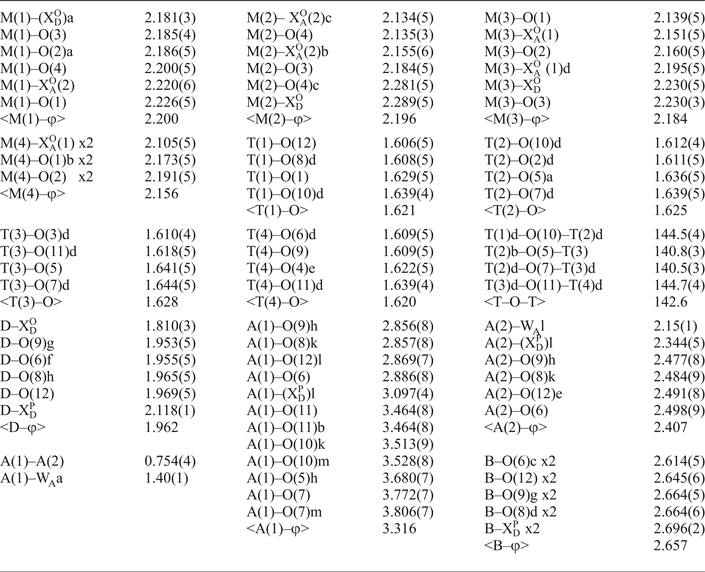
*φ = O, OH, F.
Operators for generating equivalent atoms: a: x + 1, y, z; b: x–1, y, z; c: –x + 1, –y, –z + 1; d: –x + 1, –y + 1, –z + 1; e: x, y + 1, z; f: –x, –y, –z + 1; g: x, y–1, z; h: –x, –y + 1, –z + 1; k: –x, –y + 1, –z + 2; ; l: x, y, z + 1; m: –x + 1, –y + 1, –z + 2.
Table 8. Refined site-scattering values and assigned site-populations for heyerdahlite.

*coordination number is given for non-[6]-coordinated sites;
**Ct = cation, φ = O, OH, F, H2O.
Table 9. Hydrogen bonding in heyerdahlite.

a: –x, –y + 1, –z + 1; b: x + 1, y, z; c: –x + 1, –y + 1, –z + 1.
Table 10. Selected bond-valence values* for heyerdahlite.

* Bond-valence parameters (in valence units) are from Brown (Reference Brown, O'Keeffe and Navrotsky1981).
** M = Mn; D = Ti; A(1) = K, 50% occupancy; A(2) = Na, 50% occupancy; B = Na; ![]() ${\rm X}_{\rm D}^{\rm O} $ = O;
${\rm X}_{\rm D}^{\rm O} $ = O; ![]() ${\rm X}_{\rm A}^{\rm O} $(1,2) = OH;
${\rm X}_{\rm A}^{\rm O} $(1,2) = OH; ![]() ${\rm X}_{\rm D}^{\rm P} $ = F.
${\rm X}_{\rm D}^{\rm P} $ = F.
Structure description
In the crystal structure of heyerdahlite, there are four T sites occupied by Si (Table 6) and tetrahedrally coordinated by O atoms, with < Si–O> = 1.624 Å (Table 7). There is one Ti-dominant D site coordinated by five O atoms and an ![]() ${\rm X}_{\rm D}^{\rm P} $ anion [
${\rm X}_{\rm D}^{\rm P} $ anion [![]() ${\rm X}_{\rm D}^{\rm P} $ = F1.00], with < D–ϕ> 1.962 Å (ϕ = O, F); and its composition is (Ti1.78Nb0.17Mg0.03Zr0.02), ideally Ti2 apfu (Table 7). There are four Mn-dominant M sites octahedrally coordinated by O atoms and OH groups (plus minor F) at the
${\rm X}_{\rm D}^{\rm P} $ = F1.00], with < D–ϕ> 1.962 Å (ϕ = O, F); and its composition is (Ti1.78Nb0.17Mg0.03Zr0.02), ideally Ti2 apfu (Table 7). There are four Mn-dominant M sites octahedrally coordinated by O atoms and OH groups (plus minor F) at the ![]() ${\rm X}_{\rm A}^{\rm O} $(1,2) sites (Tables 6–8), with < M(1–4)–ϕ> = 2.200–2.156 Å (ϕ = O, OH). These four M sites give (Mn6.29Na0.33Zn0.23Mg0.07Zr0.04
${\rm X}_{\rm A}^{\rm O} $(1,2) sites (Tables 6–8), with < M(1–4)–ϕ> = 2.200–2.156 Å (ϕ = O, OH). These four M sites give (Mn6.29Na0.33Zn0.23Mg0.07Zr0.04![]() ${\rm Fe}_{{\rm 0}{\rm. 03}}^{{\rm 2 +}} $Ca0.01)Σ7, ideally Mn7 apfu (Table 2). Details of hydrogen bonding involving H atoms of OH groups are given in Table 9. M(1–4) octahedra share common edges to form a trioctahedral O sheet. As in most astrophyllite-supergroup structures, the sizes of M octahedra follow the pattern M(1) > M(2) > M(3) > M(4) due to the different linkages of M octahedra and the polyhedra of the H sheets (Sokolova, Reference Sokolova2012). The T tetrahedra share vertices to form the T4O12(Si4O12) astrophyllite ribbon along [100]. Astrophyllite ribbons and D octahedra share common vertices to form an H sheet. Two H and one O sheets form an HOH block identical to that in kupletskite (Fig. 3a). In the heyerdahlite structure, D octahedra share a common
${\rm Fe}_{{\rm 0}{\rm. 03}}^{{\rm 2 +}} $Ca0.01)Σ7, ideally Mn7 apfu (Table 2). Details of hydrogen bonding involving H atoms of OH groups are given in Table 9. M(1–4) octahedra share common edges to form a trioctahedral O sheet. As in most astrophyllite-supergroup structures, the sizes of M octahedra follow the pattern M(1) > M(2) > M(3) > M(4) due to the different linkages of M octahedra and the polyhedra of the H sheets (Sokolova, Reference Sokolova2012). The T tetrahedra share vertices to form the T4O12(Si4O12) astrophyllite ribbon along [100]. Astrophyllite ribbons and D octahedra share common vertices to form an H sheet. Two H and one O sheets form an HOH block identical to that in kupletskite (Fig. 3a). In the heyerdahlite structure, D octahedra share a common ![]() ${\rm X}_{\rm D}^{\rm P} $ anion where
${\rm X}_{\rm D}^{\rm P} $ anion where ![]() ${\rm X}_{\rm D}^{\rm P} $ = F (Tables 8,10).
${\rm X}_{\rm D}^{\rm P} $ = F (Tables 8,10).

Fig. 3. The crystal structure of heyerdahlite: (a) general view; and (b) a possible short-range-order arrangement of K and Na atoms at the A(1) and A(2) sites, respectively. Mn-dominant and Ti-dominant octahedra are magenta and yellow, respectively; Si tetrahedra are orange; Na and K atoms at B, A(2) and A(1) sites are shown as medium blue and large green spheres; F atoms [![]() ${\rm X}_{\rm D}^{\rm P} $ site], O atoms of OH groups [
${\rm X}_{\rm D}^{\rm P} $ site], O atoms of OH groups [![]() ${\rm X}_{\rm A}^{\rm O} $ sites] and H2O groups [W A site] are shown as small yellow, small turquoise and medium red spheres, respectively; H atoms of OH groups are shown as small grey spheres, O–H bonds are shown as black lines; the unit cell is shown with thin black lines.
${\rm X}_{\rm A}^{\rm O} $ sites] and H2O groups [W A site] are shown as small yellow, small turquoise and medium red spheres, respectively; H atoms of OH groups are shown as small grey spheres, O–H bonds are shown as black lines; the unit cell is shown with thin black lines.
In the I block between adjacent HOH blocks, there are two interstitial cation sites, A and B, and a W A site partly occupied by H2O. The A site splits into two sites, [12]-coordinated A(1) and [6]-coordinated A(2), separated by a short distance of 0.754 Å (Table 7). The A(1) site is coordinated by eleven O atoms and a F atom at the ![]() ${\rm X}_{\rm D}^{\rm P} $ site, and the A(2) site is coordinated by four O atoms, an
${\rm X}_{\rm D}^{\rm P} $ site, and the A(2) site is coordinated by four O atoms, an ![]() ${\rm X}_{\rm D}^{\rm P} $ anion and an H2O group at the W A site. There is a short distance A(1)–WA = 1.40 Å (Table 7) and hence the A(2) + W A sites and the A(1) site cannot all be locally occupied. The A(1) and A(2) sites are occupied by (□1.18K0.68Rb0.12Cs0.01Pb0.01) pfu (41% occupancy) and (Na1.18□0.82) pfu (59% occupancy), respectively (Table 8). As the A(1) site is occupied at 41%, the W A site can be occupied by H2O at 59%, i.e. by 1.18 H2O pfu which is in accord with 1.18 Na apfu at the A(2) site (Table 8). A possible short-range-order arrangement of K and Na atoms where they fully occupy the A(1) and A(2) sites, respectively, is shown in Fig. 3b. The aggregate content of the A site is (Na1.18K0.68Rb0.12Cs0.01Pb0.01)Σ2, ideally Na2 apfu. Note that the ideal composition of the A site, Na2 apfu, (where it is fully occupied by Na) requires full occupancy of the W A site: (H2O)2 pfu; otherwise the coordination of the Na atom is not complete. The [10]-coordinated B site is occupied by Na (Table 8), giving Na apfu.
${\rm X}_{\rm D}^{\rm P} $ anion and an H2O group at the W A site. There is a short distance A(1)–WA = 1.40 Å (Table 7) and hence the A(2) + W A sites and the A(1) site cannot all be locally occupied. The A(1) and A(2) sites are occupied by (□1.18K0.68Rb0.12Cs0.01Pb0.01) pfu (41% occupancy) and (Na1.18□0.82) pfu (59% occupancy), respectively (Table 8). As the A(1) site is occupied at 41%, the W A site can be occupied by H2O at 59%, i.e. by 1.18 H2O pfu which is in accord with 1.18 Na apfu at the A(2) site (Table 8). A possible short-range-order arrangement of K and Na atoms where they fully occupy the A(1) and A(2) sites, respectively, is shown in Fig. 3b. The aggregate content of the A site is (Na1.18K0.68Rb0.12Cs0.01Pb0.01)Σ2, ideally Na2 apfu. Note that the ideal composition of the A site, Na2 apfu, (where it is fully occupied by Na) requires full occupancy of the W A site: (H2O)2 pfu; otherwise the coordination of the Na atom is not complete. The [10]-coordinated B site is occupied by Na (Table 8), giving Na apfu.
Similar disorder at the A site was reported for the astrophyllite-group minerals bulgakite and nalivkinite (Agakhanov et al., Reference Agakhanov, Pautov, Sokolova, Abdu and Karpenko2016) where the ideal composition of the A site is Li2 apfu (Table 1) and nafertisite, ideally Na3Fe2+10Ti2(Si6O17)2O2(OH)6F(H2O)2 (Cámara et al., Reference Cámara, Sokolova, Abdu and Hawthorne2014).
The ideal formula of heyerdahlite
For the ideal structural formula of heyerdahlite of the form A2BC7D2(T4O12)2![]() ${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{\rm D}^{\rm P} $WA2 (Sokolova et al., Reference Sokolova, Cámara, Hawthorne and Ciriotti2017a), we sum the ideal compositions of the following sites: C7 = Mn7; D = [6]Ti2; T4 = Si4; A2 = Na2; B = Na;
${\rm X}_{{\rm D2}}^{\rm O} {\rm X}_{{\rm A4}}^{\rm O} {\rm X}_{\rm D}^{\rm P} $WA2 (Sokolova et al., Reference Sokolova, Cámara, Hawthorne and Ciriotti2017a), we sum the ideal compositions of the following sites: C7 = Mn7; D = [6]Ti2; T4 = Si4; A2 = Na2; B = Na; ![]() ${\rm X}_{\rm D2}^{\rm O} $ = O2;
${\rm X}_{\rm D2}^{\rm O} $ = O2; ![]() ${\rm X}_{{\rm A4}}^{\rm O} $ = (OH)4;
${\rm X}_{{\rm A4}}^{\rm O} $ = (OH)4; ![]() ${\rm X}_{\rm D}^{\rm P} $ = F, WA2 = (H2O)2 (Table 8). Hence the ideal structural formula of heyerdahlite is Na2NaMn7Ti2(Si4O12)2O2(OH)4F(H2O)2, Z = 1; the ideal formula of heyerdahlite is Na3Mn7Ti2(Si4O12)2O2(OH)4F(H2O)2.
${\rm X}_{\rm D}^{\rm P} $ = F, WA2 = (H2O)2 (Table 8). Hence the ideal structural formula of heyerdahlite is Na2NaMn7Ti2(Si4O12)2O2(OH)4F(H2O)2, Z = 1; the ideal formula of heyerdahlite is Na3Mn7Ti2(Si4O12)2O2(OH)4F(H2O)2.
Heyerdahlite and kupletskite are related by the following substitution ![]() ${}_{}^{\rm A} {\rm Na}_{\rm 2}^{\rm +} $ + W(H2O)2 ↔
${}_{}^{\rm A} {\rm Na}_{\rm 2}^{\rm +} $ + W(H2O)2 ↔ ![]() ${}_{}^{\rm A} {\rm K}_{\rm 2}^{\rm +} $ + W□.
${}_{}^{\rm A} {\rm K}_{\rm 2}^{\rm +} $ + W□.
Summary
Heyerdahlite, ideally Na3Mn7Ti2(Si4O12)2O2(OH)4F(H2O)2, is a new mineral of the kupletskite group (astrophyllite supergroup). The formula of heyerdahlite is of the form A2BC7D2(T4O12)2O2(OH)4![]() ${\rm X}_{\rm D}^{\rm P} $WA2, where ideally C7 = Mn7 at the M(1–4) sites; D2 = Ti2; T4 = Si4; A2 = Na2; B = Na;
${\rm X}_{\rm D}^{\rm P} $WA2, where ideally C7 = Mn7 at the M(1–4) sites; D2 = Ti2; T4 = Si4; A2 = Na2; B = Na; ![]() ${\rm X}_{\rm D}^{\rm P} $ = F; WA2 = (H2O)2.
${\rm X}_{\rm D}^{\rm P} $ = F; WA2 = (H2O)2.
The heyerdahlite structure consists of HOH blocks which alternate with interstitial sites of the I block along [001]. The topology of the I block is the same as that in bulgakite and nalivkinite where Li is the dominant cation at the A site.
Acknowledgements
We are grateful to Fernando Cámara and Henrik Friis for extremely thorough reviews and to Structure Editor Peter Leverett and Associate Editor Ed Grew for valuable comments. We thank Harald Folvik, Natural History Museum, University of Oslo, for the scanning electron microscopy image of heyerdahlite crystals. We thank Mark A. Cooper, University of Manitoba, for collection of the single-crystal X-ray data for heyerdahlite. This work was supported by a Canada Research Chair in Crystallography and Mineralogy and by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada to FCH, and by Innovation Grants from the Canada Foundation for Innovation to FCH.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.051