Introduction
Grandviewite was described by Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008) from the Grandview mine, Arizona, USA. This mineral was found as rare greenish-blue radiating sprays of extremely acicular laths occurring in goethite-rich gossan, in association with chalcoalumite, cyanotrichite and carbonatecyanotrichite. Its chemical formula based on atomic absorption spectrometry, colorimetry, and thermogravimetric analyses was given as Cu3Al9(SO4)2(OH)29. Due to the quality of available material and crystals only 2 μm thick, the crystal structure could not be determined at that time. Possible unit-cell parameters were found by auto-indexing of powder X-ray diffraction (PXRD) data and refinement in a monoclinic setting, with a = 10.908(2), b = 6.393(3), c = 10.118(2) Å, β = 107.47° and V = 673.0(1) Å3. Possible space groups suggested by Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008) are P2, P21, Pm, P2/m, or P21/m.
A similar greenish-blue mineral was found by one of the authors (MFMZ) during a fieldtrip in 1982 in the Restauradora vein at Capillitas mine, northwestern Argentina. On the basis of incomplete analytical data, this mineral was considered as a cyanotrichite-like mineral, related to cyanotrichite and carbonatecyanotrichite (Márquez-Zavalía and Pedregosa, Reference Márquez-Zavalía and Pedregosa1994). Later, we restudied this material and found the powder X-ray diffraction data to be identical with that published for grandviewite by Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008). Nevertheless, its chemical composition was significantly different from the published one for grandviewite from the type locality (Colchester et al., Reference Colchester, Klish, Leverett and Williams2008). For these reasons, we have decided to reinvestigate the mineral grandviewite. A small part of the holotype sample of grandviewite (M50490) for our study was kindly provided from the collections of Museum Victoria, Melbourne, Australia, by Stuart J. Mills and Oskar Lindenmayer. Our reinvestigation resulted in redefinition with new chemical formula, triclinic symmetry, and determination of its crystal structure. Details of this research are presented in this paper.
This redefinition was approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA) (proposal 21-K, Miyawaki et al., Reference Miyawaki, Hatert, Pasero and Mills2022). The grandviewite samples from the Capillitas mine studied are deposited in the collections of the Department of Mineralogy and Petrology of the National Museum, Prague, Czech Republic, under the catalogue number P1P 2/2022.
Occurrence and mineral description
Occurrence in Argentina
Grandviewite occurs in the Restauradora vein of the Capillitas mine, which mines an epithermal precious- and base-metal vein deposit, located along the eastern slope of the Capillitas Range, in Catamarca province, northwestern Argentina (Fig. 1). The volcanism from which the mineralisation of the Capillitas mine and the other genetically linked deposits of the region (e.g. Farallón Negro, Alto de la Blenda, La Alumbrera, Agua Tapada, Cerro Atajo, Agua Rica) are derived (Marquez-Zavalía and Heinrich, Reference Márquez-Zavalía and Heinrich2016 and references therein), developed in a back-arc position of the Miocene–Recent volcanic arc, along the Carachipamapa–Farallón Negro transversal volcanic chain of the Central Andes, and has dominant high-K calc-alkaline to shoshonitic affinities (Viramonte et al., Reference Viramonte, Galliski, Araña Saavedra, Aparicio, García Cacho and Martín Escorza1984). The lithology comprises the upper Precambrian to lower Cambrian folded pelites of the Suncho Formation and the Buey Muerto mica schists, both intruded by the late orogenic Capillitas granite in the Ordovician to the Lower Silurian during the Famatinian Orogenic Cycle. The NE-striking structural fabrics in the Neoproterozoic – early Cambrian Pampean basement were reactivated, forming a tilt-block province. The Capillitas granite is overlain unconformably by the El Morterito Formation and the Farallón Negro Volcanic Complex Cenozoic units, and by undifferentiated Quaternary deposits.
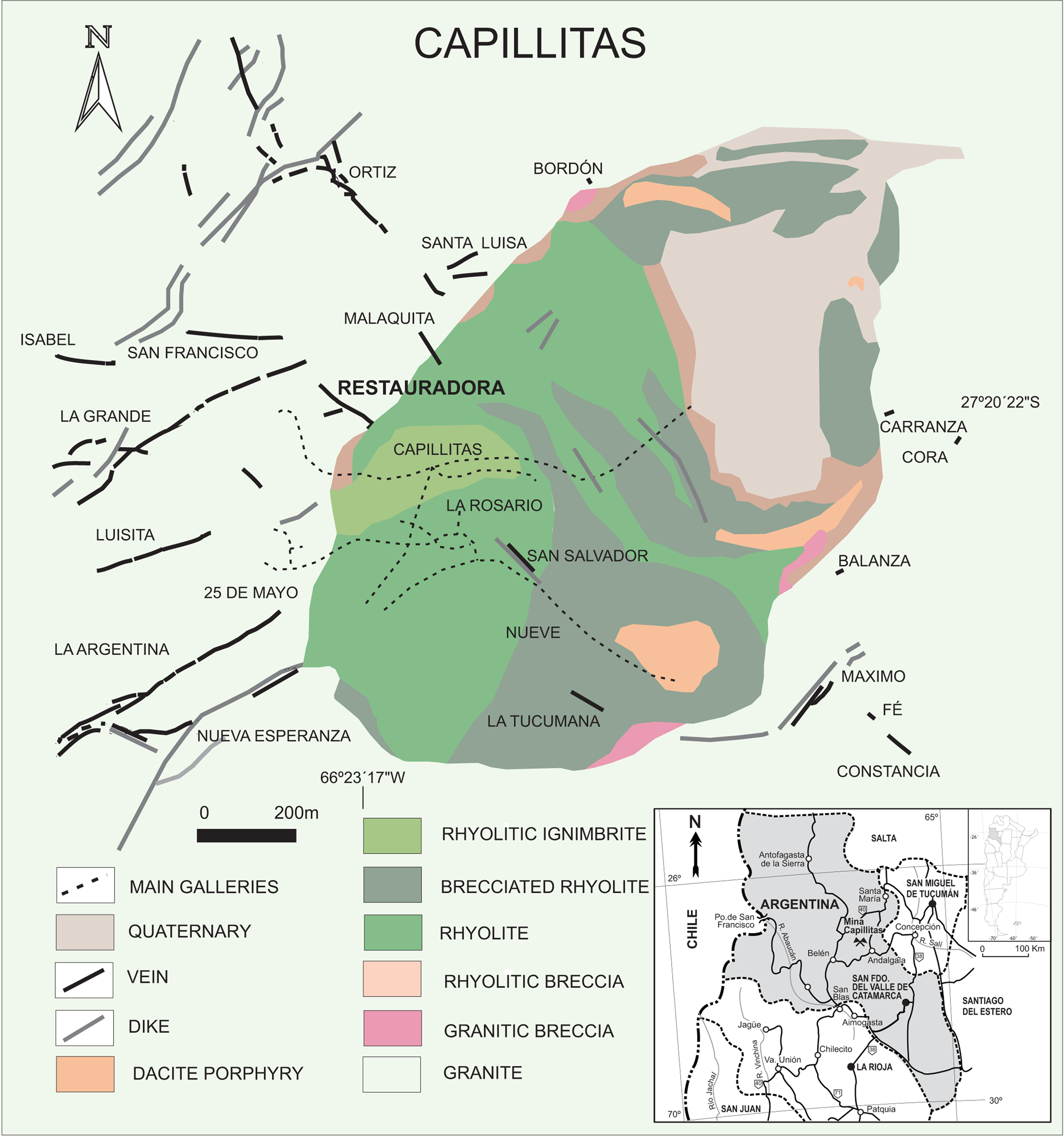
Fig. 1. Schematic geology of Capillitas, showing the location of the Restauradora vein (modified from Márquez-Zavalía et al., Reference Márquez-Zavalía, Vymazalová, Galliski, Watanabe and Murakami2020).
In the Capillitas area, the rocks of the Farallón Negro Volcanic Complex form an ellipsoidal diatreme (1500 m × 900 m), with the long axis striking NE; hydrothermal alteration is widespread, varying laterally by host-rock type. Twenty veins are hosted by the volcanic and granitic rocks in ENE and WNW directions (Márquez-Zavalía, Reference Márquez-Zavalía and Zappettini1999) and the mineralisation is very diverse with more than 120 minerals among primary and secondary species described by several authors (e.g. Márquez-Zavalía et al., Reference Márquez-Zavalía, Craig and Solberg1999, Reference Márquez-Zavalía, Vymazalová, Galliski, Watanabe and Murakami2020; Márquez-Zavalía and Craig, Reference Márquez-Zavalía and Craig2004; Márquez-Zavalía, Reference Márquez-Zavalía2006; Putz et al., Reference Putz, Paar and Topa2009). It is the type locality for five minerals: putzite (Paar et al., Reference Paar, Roberts, Berlepsch, Armbruster, Topa and Zagler2004), catamarcaite (Putz et al., Reference Putz, Paar, Topa, Makovicky and Roberts2006), ishiharaite (Márquez-Zavalía et al., Reference Márquez-Zavalía, Galliski, Drábek, Vymazalová, Watanabe, Murakami and Bernhardt2014), lislkirchnerite (Effenberger et al., Reference Effenberger, Lengauer, Libowitzky, Putz and Topa2015) and omariniite (Bindi et al., Reference Bindi, Putz, Paar and Stanley2017).
The deposit has been discontinuously mined for gold since pre-Colombian times. Extensive work was carried out over the last two centuries, interspersed with sporadic copper recovery attempts that were interrupted by metallurgical problems, mainly due to the lack of detailed knowledge of the mineralogy of the ore to be treated. Since the second part of the last century, mining has been mostly restricted to the extraction of banded – and sporadically stalactitic – rhodochrosite, the main gangue mineral in some of the veins, and for which the Capillitas mine is known worldwide.
Grandviewite was found in samples from the Restauradora vein dumps (27°20’22”S, 66°23’17”W, 3190 m a.s.l.). This vein is hosted by granite, has an average thickness of 50 cm and a total measured length of 106 m. The vein has two branches with strike and dip N10°W / N70°E and 75°E–75°W / 70°S, respectively. The works performed along this vein consist of a 100 m gallery, two inclines, and a shaft. There are two dumps, one at the gallery level and the other below it, formed from the droppings of the one above; most of the material is oxidised. The main hypogene minerals are: pyrite, sphalerite, galena, chalcopyrite, tennantite-(Zn) and tennantite-(Fe), with enargite, hübnerite, gold, stannite, stannoidite, mawsonite, silver and tellurium-bearing minerals as accessory minerals, in a gangue represented principally by quartz; grandviewite is intimately associated with carbonatecyanotrichite and azurite, with antlerite, linarite, malachite and gypsum.
Physical and optical properties
Grandviewite occurs mainly in the cracks or fissures of the host rock, developing globular masses up to a couple of millimetres in diameter, formed by very thin (up to 1 μm) platy to acicular lath-like crystals (Fig. 2), greenish-pale blue in colour, pleochroic (X = colourless, Y = very pale blue and Z = greenish-pale blue), pale blue streak, and silky to satin lustre. In Table 1, its optical data are compared with that published for the holotype from Grandview mine.

Fig. 2. Globular aggregates of grandviewite from Capillitas mine, Argentina, specimen number P1P 2/2022. The field of view = 1.8 mm, photo J. Sejkora.
Table 1. Summary of optical data for grandviewite.

Chemical composition
Samples of grandviewite were analysed with a Cameca SX-100 electron microprobe (National Museum, Prague) operating in the wavelength-dispersive mode with an accelerating voltage of 15 kV, a specimen current of 5 nA and a beam diameter of 10 μm. The following lines and standards were used: Kα: celestine (S), halite (Cl), hematite (Fe), ZnO (Zn), rhodonite (Mn), chalcopyrite (Cu), sanidine (Al, Si) and fluorapatite (P); and Lα: clinoclase (As). Peak counting times (CT) were 20 s and CT for each background was one-half of the peak time. The raw intensities were converted to concentrations automatically using the PAP (Pouchou and Pichoir, Reference Pouchou, Pichoir and Armstrong1985) matrix-correction procedure. The contents of Ba, Bi, Ca, Co, F, K, Mg, N, Na, Ni, Pb, Sb, Sr, Th, U, V and Y were sought but found to be below the detection limit (~0.05–0.20 wt.%). Water content could not be analysed directly because of the minute amount of material available. The H2O content was confirmed by Raman and infrared spectroscopy and calculated by valence balance and stoichiometry of the ideal formula from the crystal-structure refinement. Lower totals after adding the calculated water contents and a wide range of analytical totals of both studied samples reflect (1) partial dehydration of samples in the vacuum of the electron microprobe chamber or under the electron beam and (2) the thin acicular nature of grandviewite.
The chemical composition of both grandviewite samples studied (Table 2) is very similar and agrees well with the ideal formula Cu3Al2(SO4)(OH)10⋅H2O derived from the crystal-structure refinement of 3D electron diffraction data. Their empirical formulae based on 16 anions are the following: (Cu2.96Mn0.01)Σ2.97Al2.03(SO4)0.97(SiO4)0.03(AsO4)0.01(OH)9.97Cl0.01⋅H2O (Grandview mine – mean of nine analyses) and Cu2.97(Al2.03Fe0.01)Σ2.04(SO4)0.95(SiO4)0.03(AsO4)0.01(PO4)0.01(OH)9.97Cl0.01⋅H2O (Capillitas mine – mean of 23 analyses).
Table 2. Chemical composition of grandviewite.

* Part of holotype M50490 (Museums Victoria, Melbourne, Australia)
a – ideal composition; b – data published for holotype sample from Grandview mine by Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008); c – H2O contents calculated by valence balance and stoichiometry of ideal formula Cu3Al2(SO4)(OH)10⋅H2O.
Our results differ significantly from data published for grandviewite (Table 2) in the original publication of Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008) based on analyses of hand-picked bulk samples by the AAS (Cu), colorimetry (Al) and thermogravimetric analyses (S and H2O). The most likely explanation is that Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008) used an indistinguishable mixture of grandviewite and intimately associated chalcoalumite CuAl4(SO4)(OH)12⋅3H2O, or simply by mistake, confused the values of CuO and Al2O3 contents.
Powder X-ray diffraction data
Powder X-ray diffraction data for both samples were recorded at room temperature using a Bruker D8 Advance diffractometer equipped with a solid-state LynxEye detector and a secondary monochromator producing CuKα radiation housed at the Department of Mineralogy and Petrology, National Museum, Prague, Czech Republic. The instrument was operating at 40 kV and 40 mA. In order to minimise the background, the powder samples were placed on the surface of a flat silicon wafer. The powder pattern was collected in the Bragg–Brentano geometry in the range 3–70°2θ, with a step of 0.01° and a counting time of 20 s per step (total duration of the experiment was ca. 30 hours). The positions and intensities of diffractions were found and refined using the Pearson VII profile-shape function of the ZDS program package (Ondruš, Reference Ondruš1993). The peak positions in experimental patterns of both samples are very similar and agree very well with data published for grandviewite (Table 3) by Colchester et al. (Reference Colchester, Klish, Leverett and Williams2008); the observed differences in intensity of diffraction are caused by minimal amounts of samples available and preferred orientation effects. The unit-cell parameters refined by the least-squares program of Burnham (Reference Burnham1962) for both samples are comparable (Table 4). Only in the case of the a parameter was a small difference (5.749/5.713 Å) found. The observed differences in the unit-cell parameters of grandviewite obtained from the 3D ED data (Table 5) and those from the PXRD data are probably due to the temperature of measurement. The 3D ED data were recorded at 100 K to preserve the hydrated nature of the mineral under TEM vacuum, whereas the PXRD data were measured at the ambient temperature – explaining the higher unit-cell volume.
Table 3. Powder X-ray diffraction data of grandviewite.

* Part of holotype M50490 (Museums Victoria, Melbourne, Australia);
** Icalc – intensities calculated using the software PowderCell2.3 (Kraus and Nolze, Reference Kraus and Nolze1996) on the basis of the crystal-structure data given in the crystallographic information file.
Table 4. Unit-cell parameters of grandviewite (triclinic, space group P $\bar{1}$![]() ) refined from PXRD.*
) refined from PXRD.*

* dideal, dempirical – calculated for ideal and empirical formula, respectively.
Table 5. Raman and infrared spectra of grandviewite.*

* Wavenumbers in cm–1; intensity: vs – very strong, s – strong, ms – medium strong, w – weak.
Raman and infrared spectroscopy
The Raman spectra were collected in the range 4000–40 cm–1 using a DXR dispersive Raman Spectrometer (Thermo Scientific) mounted on a confocal Olympus microscope. The Raman signal was excited by an unpolarised red 633 nm He–Ne gas laser and revealed by a CCD detector. The experimental parameters were: 100× objective, 30 s exposure time, 100 exposures, 50 μm slit spectrograph aperture and 2 mW laser power level. The spectra were acquired repeatedly from different grains to obtain a representative spectrum with the best signal-to-noise ratio. In addition, the eventual thermal damage of the measured point was excluded by visual inspection of the excited surface after measurement, by observation of possible decay of spectral features at the start of excitation and by checking for thermal downshift of Raman lines. The instrument was set up by a software-controlled calibration procedure using multiple neon emission lines (wavelength calibration), multiple polystyrene Raman bands (laser frequency calibration), and standardised white-light sources (intensity calibration). Spectral manipulations were performed using Omnic 9 software (Thermo Scientific).
The infrared vibrational spectra were recorded by the attenuated total reflection (ATR) method with a diamond cell on a Nicolet iS5 spectrometer. Spectra over the 4000–400 cm–1 range were obtained by the co-addition of 64 scans with a resolution of 4 cm–1 and a mirror velocity of 0.4747 cm/s. Spectra were co-added to improve the signal-to-noise ratio.
The Raman spectra of grandviewite from both localities are very close (Fig. 3). A similar situation was observed for the infrared spectra (Fig. 4). The following interpretation (Table 5) of the spectra is based on the papers of Myneni (Reference Myneni, Alpers, Jambor and Nordstrom2000), Nakamoto (Reference Nakamoto2009), Frost et al. (Reference Frost, Sejkora, Čejka and Keeffe2009) and Čejka et al. (Reference Čejka, Sejkora, Plášil, Bahfenne, Palmer and Frost2011). Bands of low intensity, located at 3518 and 3309 cm–1 (R – Raman) and more prominent bands at 3563, 3310, 3197 and 2920 cm–1 (IR – infrared) are connected with the ν(OH) stretching vibrations of (OH) groups and hydrogen-bonded water molecules. A band at 1628 cm–1 (IR) is attributed to the ν2(δ) hydrogen-bonded water molecules. The low-intensity bands at 1197 and 1073 cm–1 (R) and strong band at 1060 with shoulders at 1109 and 1159 cm–1 (IR) are assigned to the split triply degenerate ν3(SO4)2– antisymmetric stretching vibrations. The most intensive band at 974 cm–1 (R) and medium-strong bands at 973 and 909 cm–1 (IR) are attributed to the ν1(SO4)2– symmetric stretching vibrations. Weak to medium strong bands at 668 and 611 cm–1 (R) and 667, 614 and 593 cm–1 (IR) are connected with the split triply degenerate ν4(δ)(SO4)2– bending vibrations. Weak bands at 485, 446 and 417 cm–1 (R) and strong bands 505, 450 and 422 cm–1 (IR) are assigned to the split doubly degenerate ν2(δ)(SO4)2– bending vibrations. The other observed bands at 534, 329, 271, 213, 123, 82 and 65 cm–1 (R) and 775 cm–1 (IR) are probably connected with libration modes of H2O molecules, vibrations of Cu–O and Al–O bonds, and lattice modes.

Fig. 3. Raman spectrum of holotype grandviewite (split at 2000 cm–1) from the type locality the Grandview mine, Arizona, in comparison with grandviewite from the Capillitas mine, Argentina.

Fig. 4. Infrared spectrum of holotype grandviewite (split at 2000 cm–1) from the type locality Grandview mine, Arizona, in comparison with grandviewite from the Capillitas mine, Argentina.
Gladstone–Dale compatibility
The Gladstone–Dale compatibility (Mandarino, Reference Mandarino1981) 1–(KP/KC), calculated from the unit-cell parameters refined from PXRD data, is superior for both occurrences; Grandview mine, Arizona: 0.004 and 0.005 for ideal and empirical formula, respectively; Capillitas mine, Argentina: –0.016 and –0.014 for ideal and empirical formula, respectively.
Crystal structure of grandviewite
3D Electron diffraction analysis
Due to the nature of the crystals (very thin laths), transmission electron microscopy has been chosen to investigate the structural properties of grandviewite from the Capillitas mine.
The 3D electron diffraction (ED) data were collected in an FEI Tecnai 02 transmission electron microscope (TEM) (acceleration voltage of 200 kV, LaB6) equipped with a side-mounted CCD camera Olympus SIS Veleta with a 14bit dynamic range. The sample was crushed in a mortar without solvent and deposited on a Au-grid coated by a thin film of holey amorphous carbon. To preserve the hydrated structure of the mineral under the high vacuum in the TEM, the grid was plunged into liquid nitrogen and transferred to the TEM using a Gatan 626 cryo-transfer holder (Gemmi et al., Reference Gemmi, Mugnaioli, Gorelik, Kolb, Palatinus, Boullay, Hovmöller and Abrahams2019; Mugnaioli et al., Reference Mugnaioli, Lanza, Bortolozzi, Righi, Merlini, Cappello, Marini, Athanassiou and Gemmi2020; Steciuk et al., Reference Steciuk, Majzlan and Plášil2021a, Reference Steciuk, Sejkora, Čejka, Plášil and Hloušek2021b). The PEDT technique was chosen to collect stepwise 3D ED data at 100 K. For each selected crystal area (Fig. 5), a series of non-oriented patterns were collected sequentially by a 1° step on the accessible tilt range of the goniometer (Kolb et al., Reference Kolb, Gorelik, Kübel, Otten and Hubert2007, Reference Kolb, Gorelik and Otten2008; Mugnaioli et al., Reference Mugnaioli, Gorelik and Kolb2009) automated by the in-house software RATS, including the tracking of the crystal following the procedure described by Plana-Ruiz (Reference Plana-Ruiz, Portillo, Estradé, Peiró, Nicolopoulos and Kolb2018). To reduce the dynamical effects, the 3D ED (Gemmi and Lanza, Reference Gemmi and Lanza2019; Gemmi et al., Reference Gemmi, Mugnaioli, Gorelik, Kolb, Palatinus, Boullay, Hovmöller and Abrahams2019) was coupled with precession electron diffraction (PED) using the precession device Nanomegas Digistar (Vincent and Midgley, Reference Vincent and Midgley1994). The precession semi-angle was set to 1°. Low illumination settings were used to minimise the beam-induced damage on the crystals. 3D ED data reduction was performed using the computer program PETS2 (Palatinus et al., Reference Palatinus, Brázda, Jelínek, Hrdá, Steciuk and Klementová2019). From all crystals measured, the four best 3D ED data sets were merged and treated together for the structure analysis, and two more were used to evaluate the unit-cell parameters. The result of the data reduction was a hkl-type file obtained from merging the four data sets with associated intensities and estimated standard deviations (Rint(obs/all) = 0.1703/0.3380). This file was used in the subsequent structure solution. For the refinement, considering the dynamical effects, the data sets were processed again separately to give hkl-type files where each ED frame is considered independent (Palatinus et al., Reference Palatinus, Corrêa, Steciuk, Jacob, Roussel, Boullay, Klementová, Gemmi, Kopeček, Domeneghetti, Cámara and Petříček2015a, Reference Palatinus, Petříček and Correâ2015b). The structure was solved using Superflip (Palatinus and Chapuis, Reference Palatinus and Chapuis2007; Palatinus, Reference Palatinus2013) in Jana2020 (Petříček et al., Reference Petříček, Dušek and Palatinus2020) and refined using DYNGO (Palatinus et al., Reference Palatinus, Petříček and Correâ2015b) and Jana2020. The data collection details are presented in Table 6.

Fig. 5. Four grandviewite crystals used for the 3D ED analysis and sections of the reciprocal space with the two triclinic indexings in the subcell (green) and the supercell (red). The dotted red circles on each crystal represent the area selected and the beam size during data collections.
Table 6. Crystallographic parameters from 3D ED at 100 K for grandviewite.

*The lattice parameters are averaged from six 3D ED data sets collected on six different crystals.
Crystal-structure determination
Grandviewite is described in the triclinic supercell a = 6.002(3) Å, b = 10.54(3) Å, c = 11.249(8) Å, α = 72.14(17)°, β = 81.56(60)°, γ = 86.10(79)° and V = 669.8(12) Å3 in space group P $\bar{1}$![]() . The 3D ED data first revealed a strong subcell with parameters a’ = ½ab = 10.54(3) Å, c = 11.249(8) Å, α = 72.14(17)°, β = 81.56(60)°, γ = 86.10(79)° and V’ = ½V = 334.9(12) Å3 in space group P $\bar{1}$
. The 3D ED data first revealed a strong subcell with parameters a’ = ½ab = 10.54(3) Å, c = 11.249(8) Å, α = 72.14(17)°, β = 81.56(60)°, γ = 86.10(79)° and V’ = ½V = 334.9(12) Å3 in space group P $\bar{1}$![]() . The weaker supercell is obtained by doubling the a’ parameters (a = 6.002(3) Å). Depending on the crystal, supercell reflections are more or less weak and/or diffuse, showing that some crystals are close to being disordered and some possess a more pronounced ordering. This work focuses on the description of the supercell. However, in order to shed light on the origin of the ordering and as only a few supercell reflections are observed (for I≥σ(I)) with a rather diffuse profile, the structure was first determined in the subcell (see Fig. 6). Results obtained for the subcell are shown for comparison only, however we do not provide any additional details for the refinement. The superstructure has been solved ab initio from the merged data to increase the data completeness up to ~94.93% for a sinθ/λ = 0.72 Å–1 resolution shell (see Table 6). The most important experimental parameters are listed in Table 6. The initial solution shows three Cu sites in pyramidal coordination, two Al sites in octahedral coordination, and two sites related to partially occupied SO4. At this step, the solution in the subcell helped interpret the initial solution for the superstructure. In Fig. 6, the electrostatic potential map of the SO4 chain is given in the subcell and the supercell. Though the copper- and the aluminium-centred polyhedra are not involved in the supercell ordering, the unique S and O7 sites from the subcell, which from the refinement result are half occupied, are clearly ordered in the supercell. In the subcell, the apical O8 site appears disordered between two positions (Fig. 6). This split, already visible from the structure solution, was later confirmed by the subcell refinement. In the supercell, the electrostatic potential corresponding to O8_1 and O8_2 is only visible close to the most occupied S site (labelled S1_2) and not visible at the 2σ[ΔV(r)] level in the vicinity of the least occupied S1_1 site due to too low occupancy (Fig. 6). The comparison of the two solutions confirms the origin of the supercell due to the SO4 ordering. The refinement of the supercell structure from 3D ED data has been carried out considering the dynamical effects (so-called ‘dynamical refinement’). For well-crystallised samples, including the multiple scattering (dynamical effects) in the refinement of 3D ED a significant improvement in terms of structural parameters and R-factors (Palatinus et al., Reference Palatinus, Corrêa, Steciuk, Jacob, Roussel, Boullay, Klementová, Gemmi, Kopeček, Domeneghetti, Cámara and Petříček2015a, Reference Palatinus, Petříček and Correâ2015b; Blum et al., Reference Blum, Housset, Clabbers, Van Genderen, Bacia-Verloop, Zander, McCarthy, Schoehn, Ling and Abrahams2021) is obtained. Soft restraints on S–O and O–O distances were added to stabilise the refinement with the weak superstructure reflections. The sum of S1_1 and S1_2 occupancies is set to 1 in addition to all the oxygen sites involved in the SO4 ordering: O7_1/O7_2, O8_1/O8_2 and O8b_1/O8b_2. The occupancy of oxygen atoms involved in the tetrahedral coordination of SO4 is set to the occupancy of the corresponding S. After a few cycles, including non-hydrogen atoms, a residual electrostatic potential map (equivalent to a difference-Fourier map) was generated to reveal the hydrogen positions and their bond lengths (Table 7). Ten expected hydrogen sites are very visible (Fig. 7), with significant isosurface levels (Table 7). In the absence of the S1_1 or S2_2 atoms, O6_1 or O6_2 become H2O molecules. However, the corresponding hydrogen sites forming H2O are not visible due to their partial occupancies and are probably shadowed by the SO4 disorder. All O–H distances were restrained to 1.03 Å according to the average value of the apparent O–H distances obtained from the difference-potential map (Table 7) (Clabbers et al., Reference Clabbers, Gruene, van Genderen and Abrahams2019), and the isotropic displacement parameters of hydrogen atoms were set as riding with extension factor 1.5 (Palatinus et al., Reference Palatinus, Brázda, Boullay, Perez, Klementová, Petit, Eigner, Zaarour and Mintova2017). The introduction of hydrogen in the refinement improved the R-factor by ~1% before the frame orientation optimisation. The last refinement steps led to R(obs)/wR(obs) = 0.1304/0.1316 for 6401/31007 observed reflections with I ≥ 3σ(I) and 118 structural parameters (Table 6). Higher R-factors are obtained for superstructure reflections h = 2n + 1 (R(obs) = 0.2386 for 754 observed reflections) coming from the supercell because they are weaker and less sharp than the reflections h = 2n (R(obs) = 0.1222 for 5648 observed reflections) of the sub-structure. The refined formula is Cu3Al2(SO4)(OH)10(H2O) for Z = 2, which is in line with results from the electron microprobe analyses. The refinement details are given in Table 6 and selected interatomic distances in Table 8. The positional and atomic displacement parameters are given in Table 9. Structural parameters are provided in the crystallographic information files which have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
. The weaker supercell is obtained by doubling the a’ parameters (a = 6.002(3) Å). Depending on the crystal, supercell reflections are more or less weak and/or diffuse, showing that some crystals are close to being disordered and some possess a more pronounced ordering. This work focuses on the description of the supercell. However, in order to shed light on the origin of the ordering and as only a few supercell reflections are observed (for I≥σ(I)) with a rather diffuse profile, the structure was first determined in the subcell (see Fig. 6). Results obtained for the subcell are shown for comparison only, however we do not provide any additional details for the refinement. The superstructure has been solved ab initio from the merged data to increase the data completeness up to ~94.93% for a sinθ/λ = 0.72 Å–1 resolution shell (see Table 6). The most important experimental parameters are listed in Table 6. The initial solution shows three Cu sites in pyramidal coordination, two Al sites in octahedral coordination, and two sites related to partially occupied SO4. At this step, the solution in the subcell helped interpret the initial solution for the superstructure. In Fig. 6, the electrostatic potential map of the SO4 chain is given in the subcell and the supercell. Though the copper- and the aluminium-centred polyhedra are not involved in the supercell ordering, the unique S and O7 sites from the subcell, which from the refinement result are half occupied, are clearly ordered in the supercell. In the subcell, the apical O8 site appears disordered between two positions (Fig. 6). This split, already visible from the structure solution, was later confirmed by the subcell refinement. In the supercell, the electrostatic potential corresponding to O8_1 and O8_2 is only visible close to the most occupied S site (labelled S1_2) and not visible at the 2σ[ΔV(r)] level in the vicinity of the least occupied S1_1 site due to too low occupancy (Fig. 6). The comparison of the two solutions confirms the origin of the supercell due to the SO4 ordering. The refinement of the supercell structure from 3D ED data has been carried out considering the dynamical effects (so-called ‘dynamical refinement’). For well-crystallised samples, including the multiple scattering (dynamical effects) in the refinement of 3D ED a significant improvement in terms of structural parameters and R-factors (Palatinus et al., Reference Palatinus, Corrêa, Steciuk, Jacob, Roussel, Boullay, Klementová, Gemmi, Kopeček, Domeneghetti, Cámara and Petříček2015a, Reference Palatinus, Petříček and Correâ2015b; Blum et al., Reference Blum, Housset, Clabbers, Van Genderen, Bacia-Verloop, Zander, McCarthy, Schoehn, Ling and Abrahams2021) is obtained. Soft restraints on S–O and O–O distances were added to stabilise the refinement with the weak superstructure reflections. The sum of S1_1 and S1_2 occupancies is set to 1 in addition to all the oxygen sites involved in the SO4 ordering: O7_1/O7_2, O8_1/O8_2 and O8b_1/O8b_2. The occupancy of oxygen atoms involved in the tetrahedral coordination of SO4 is set to the occupancy of the corresponding S. After a few cycles, including non-hydrogen atoms, a residual electrostatic potential map (equivalent to a difference-Fourier map) was generated to reveal the hydrogen positions and their bond lengths (Table 7). Ten expected hydrogen sites are very visible (Fig. 7), with significant isosurface levels (Table 7). In the absence of the S1_1 or S2_2 atoms, O6_1 or O6_2 become H2O molecules. However, the corresponding hydrogen sites forming H2O are not visible due to their partial occupancies and are probably shadowed by the SO4 disorder. All O–H distances were restrained to 1.03 Å according to the average value of the apparent O–H distances obtained from the difference-potential map (Table 7) (Clabbers et al., Reference Clabbers, Gruene, van Genderen and Abrahams2019), and the isotropic displacement parameters of hydrogen atoms were set as riding with extension factor 1.5 (Palatinus et al., Reference Palatinus, Brázda, Boullay, Perez, Klementová, Petit, Eigner, Zaarour and Mintova2017). The introduction of hydrogen in the refinement improved the R-factor by ~1% before the frame orientation optimisation. The last refinement steps led to R(obs)/wR(obs) = 0.1304/0.1316 for 6401/31007 observed reflections with I ≥ 3σ(I) and 118 structural parameters (Table 6). Higher R-factors are obtained for superstructure reflections h = 2n + 1 (R(obs) = 0.2386 for 754 observed reflections) coming from the supercell because they are weaker and less sharp than the reflections h = 2n (R(obs) = 0.1222 for 5648 observed reflections) of the sub-structure. The refined formula is Cu3Al2(SO4)(OH)10(H2O) for Z = 2, which is in line with results from the electron microprobe analyses. The refinement details are given in Table 6 and selected interatomic distances in Table 8. The positional and atomic displacement parameters are given in Table 9. Structural parameters are provided in the crystallographic information files which have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).

Fig. 6. Visualisation of the SO4 chain in the subcell and in the supercell from the charge-flipping algorithm. In both cases, the electrostatic potential map with isosurface level ≥ 3σ[ΔV(r)] (yellow) and ≥ 2σ[ΔV(r)] (white) and the model are represented.

Fig. 7. Determination of the hydrogen sites from the residual electrostatic potential map (equivalent to a difference-Fourier map) in the subcell and in the supercell. The residual map is represented as isosurface with levels >3σ[ΔV(r)] (yellow) and >2.5σ[ΔV(r)] (white). SO4, AlO6 and CuO5 polyhedra are represented in yellow, grey and blue, respectively.
Table 7. Hydrogen distances (d in Å) from the difference-Fourier map (without refinement) for grandviewite.*

* Oi = donor, Oj = acceptor.
Table 9. Site occupation factors (S.o.f.), positional and atomic displacement parameters (in Å2) for grandviewite.

Structure and topological description
The structure possesses three Cu sites in [4 + 2] and [4 + 1] coordinations, two Al sites in octahedral coordination, two partially occupied S sites in tetrahedral coordination, and sixteen oxygen atoms (two are disordered) involved in the polyhedral coordination. Ten hydrogen sites are responsible for ten hydroxyl groups in the structure (Fig. 8). Cu1 is in tetragonal bipyramidal coordination [4 + 2] and Cu2_1 and Cu2_2 are both in coordination [4 + 1] as tetragonal pyramids. All Cu1 are connected to each other through edges of the square plane of the bipyramid, thus creating infinite chains along a. The same connectivity is observed between Cu2_1 and Cu2_2 and between the AlO6 octahedra. In the (b,c) plane AlO6–Cu1–AlO6 form a flat slab that extends as an infinite chain along a, with Cu1-centred polyhedra connected via two edges on the top and two at the bottom with adjacent AlO6 octahedra. Two chains of polyhedra centred by Cu2 (Cu2_1 and Cu2_2) are connected to the AlO6 octahedra on both ends of the AlO6–Cu1–AlO6 slab through one vertex (O1_1 or O1_2). SO4 tetrahedra form a disordered chain along a, alternated with occupancies of 30%/70% for S1_1/S1_2 as well as the terminal O7_1/O7_2 oxygen atoms and the O8 atoms forming the tetrahedra. They are connected to the Cu2-centered (Cu2_1 and Cu2_2) pyramids via the apical oxygen of the [4+1]CuO5 polyhedra and are otherwise stabilised by strong hydrogen bonds with surrounding units (Fig. 8b). All the polyhedra bonded via covalent bonds form a unit (pink outline in Fig. 8) with the formula Cu3Al2(SO4)(OH)10 that is extended infinitely along a and connected in the b,c plane via strong hydrogen bonds.

Fig. 8. (a) Grandviewite structure with partially occupied SO4 chain. (b) (001) projection of grandviewite in four unit cells showing the fundamental building unit (FBU) (pink area) of the grandviewite structure linked to the other units by strong hydrogen bonds (dashed lines). (c) Topology of the FBU. SO4, AlO6 and CuO5 polyhedra are represented in yellow, grey and blue, respectively.
Grandviewite and related minerals
Grandviewite can be defined as part of the ternary system CuO–SO4–AlO1.5 with minerals such as chalcoalumite, cyanotrichite, spangolite, and some potential end-members such as brochantite, posnjakite, or aluminite (Fig. 9b). Among them, chalcoalumite and spangolite with the highest H2O moieties (H2O + OH) content (and, to some extent, chalcophyllite Cu18Al2(AsO4)4(SO4)3(OH)24⋅36 H2O) exhibit layered structures with (SO4)-free copper-aluminium(-arsenate) layers and the (SO4)2– tetrahedra located in the thick interlayer. For other reported minerals in the system, the structures are slab-based where the SO4 tetrahedra are directly bonded to the Al–Cu units (except for the Cu-free end-member aluminite). For brochantite, representing a copper end-member, slabs of CuO6 dimers are not independent and are interconnected via their vertices, and via the vertices of neighbouring SO4. The concept of a fundamental building unit (FBU) appears for grandviewite and cyanotrichite where the connectivity between adjacent FBU is only ensured via strong hydrogen bonds (Fig. 8b and c). In terms of composition, cyanotrichite, Cu4Al2(SO4)(OH)12(H2O)2 (Mills et al., Reference Mills, Christy, Colombo and Price2015), is the closest known mineral to grandviewite, Cu3Al2(SO4)(OH)10(H2O) (Fig. 9a and b). As mentioned above, grandviewite was mistaken for a cyanotrichite-like mineral related to cyanotrichite and carbonatecyanotrichite because of their similar colour and rather close composition. Although distinct, their structures nevertheless possess similar features. Both minerals are built from parallel FBU, extending along the last direction to the infinite. The main differences lie (1) in the different FBU topology due to different Cu:Al ratio and, more importantly, (2) in the presence of a characteristic SO4 ordering in grandviewite whereas SO4 remains disordered in cyanotrichite.

Fig. 9. (a) Graph with Cu, S Al, OH and H2O contents in grandviewite and related minerals. * For brochantite/posnjakite, 1H2O only corresponds to the amount in posnjakite. (b) Ternary diagram CuO–SO4–AlO1.5 (molar units) showing grandviewite compared to related minerals.
Conclusion
New chemical and crystallographic data collected on grandviewite from the holotype (Grandview mine, Arizona) and additional specimens found at the Capillitas mine (Argentina) proved that both are identical within analytical uncertainties and this mineral is a hydrated copper-aluminium hydroxo-sulfate, having the significantly revised ideal formula Cu3Al2(SO4)(OH)10⋅H2O (Z = 2) and a triclinic symmetry (space group P $\bar{1}$![]() ).
).
Acknowledgements
The authors thank Stuart J. Mills and Oskar Lindenmayer (Museums Victoria, Melbourne, Australia) for the kind provision of a small part of the holotype sample of grandviewite for our study. The helpful comments of an anonymous reviewer, Peter Leverett, Uwe Kolitsch, associated editor Charles Geiger, and Principal Editor Stuart Mills are greatly appreciated. The study was financially supported by the Ministry of Culture of the Czech Republic (long-term project DKRVO 2019-2023/1.II.d; National Museum, 00023272) for JS and ZD, the PIP 112-20120100554-CO, and PIP 112-20200101489-CO CONICET grants for MFMZ.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.59
Competing interests
The authors declare none.























