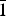Introduction
Gortdrumite was defined as a new mineral species with the chemical formula (Cu,Fe)6Hg2S5 by Steed (Reference Steed1983) during a study of the ores coming from the Gortdrum orebody of County Tipperary, Ireland. By means of X-ray powder diffraction (XRD), this author realized that the pattern did not correspond to any mineral listed in the 1974 JCPDS Powder Diffraction Database (then the Joint Committee on Powder Diffraction Standards, now the International Centre for Diffraction Data, http://www.icdd.com/). Then, with the help of A. Kato, Steed (Reference Steed1983) hypothesized an orthorhombic symmetry for gortdrumite with a = 14.958, b = 7.900 and c = 24.10 Å, and, on the basis of valence relations, he further suggested that the unit-cell content should be 4[Cu18FeHg6S16].
Interestingly, a mineral with the same physical, optical and chemical characteristics was later described by Leblhuber (Reference Leblhuber2000) during a mineralogical study of the ore bodies of the Neuschurf adit, Leogang, Salzburg, Austria. However, given the exceedingly small size of the gortdrumite crystals together with the ubiquitous intergrowth with chalcocite (with similar optical and physical characteristics), an effort to solve the structure was not attempted.
In the course of a research project dealing with the description and structural characterization of natural copper and silver sulfides/sulfosalts (e.g. Biagioni and Bindi Reference Biagioni and Bindi2017; Bindi and Menchetti Reference Bindi and Menchetti2011; Bindi et al. Reference Bindi, Evain and Menchetti2006, Reference Bindi, Evain and Menchetti2007a,Reference Bindi, Evain, Spry and Menchettib,Reference Bindi, Evain, Spry, Tait and Menchettic, Reference Bindi, Keutsch, Francis and Menchetti2009, Reference Bindi, Downs, Spry, Pinch and Menchetti2012; Evain et al. Reference Evain, Bindi and Menchetti2006), we have examined a fragment from Neuschurf adit, already studied by Leblhuber (Reference Leblhuber2000). The three samples that were used for this study originate from the mercury zone with abundant cinnabar, mercury and moschellandsbergite. Gortdrumite occurs in copper-rich vugs of a central fault zone where needle-shaped and terminated crystals up to 300 µm were found.
To help resolve the concerns relating to the structure of gortdrumite and those related to the different symmetry reported for gortdrumite in the previous literature, here we present the first crystal structure data for the mineral together with new chemical data.
Physical and optical properties of Austrian gortdrumite
Gortdrumite occurs on three samples of which one (~2 cm in size) contained the material for study. The associated minerals are cinnabar, chalcocite, and possibly cerussite and malachite.
As we are studying the structure of gortdrumite from a new occurrence, we also characterized the micro-hardness and the optical properties of the mineral in order to verify the strict analogy with the type material. In particular, the Vickers hardness number (VHN10) ranges between 190–215, averaging at 201 kg mm–2, thus nearly identical to that of type gortdrumite (Steed, Reference Steed1983). From the optical point of view, in polished section using plane-polarized light, gortdrumite is bireflectant and not pleochroic. The mineral shows no internal reflections. Between crossed polars, gortdrumite is distinctly anisotropic with colours from greyish white with a bluish tint to blue. Reflectance values were measured in air using a MPM-200 Zeiss microphotometer equipped with a MSP-20 system processor on a Zeiss Axioplan ore microscope. The filament temperature was ~3350 K. An interference filter was adjusted, in turn, to select four wavelengths for measurement (471.1, 548.3, 586.6 and 652.3 nm). Readings were taken for specimen and standard (SiC) maintained under the same focus conditions. The diameter of the circular measuring area was 0.05 mm. Reflectance values are as follows (R min, R max): 27.7, 31.1 (471.1 nm); 26.6, 30.4 (548.3 nm); 25.8, 29.4 (586.6 nm); and 25.3, 28.3 (652.3 nm), which are nearly identical to those of type gortdrumite (Criddle and Stanley, Reference Criddle and Stanley1993).
Crystal-structure solution and refinement
Several crystals were handpicked and tested by means of single-crystal XRD. Most of them were found to consist of multiple crystallites, fine intergrowths (mainly of gortdrumite and chalcocite), or very weakly diffracting fragments. After several trials, a small crystal fragment (20 µm × 30 µm × 60 µm) showing fairly good diffraction quality was selected for the structural study. The intensity data collection (see Table 1 for details) was carried out by means of an Oxford Diffraction Xcalibur PX Ultra single-crystal diffractometer (enhanced X-ray source, X-ray radiation CuKα, λ = 1.54184 Å) fitted with a Sapphire 2 CCD detector. A total of 1905 frames of data was collected at room temperature as seven sets of omega runs with an exposure time of 115 s per frame and a frame width of 1.00°. This afforded an overall data collection of 6371 reflections (3335 unique). The refined triclinic unit-cell parameters are: a = 9.677(4), b = 9.865(5), c = 11.992(5) Å, α = 77.85(4), β = 79.42(3), γ = 76.30(4)° and V = 1076.5(8) Å3, which are not related to the orthorhombic unit-cell inferred by Steed (Reference Steed1983) [a = 14.958, b = 7.900 and c = 24.10 Å], except for the fact that the Steed's volume is 8/3 larger than the observed volume.
Table 1. Data and experimental details for the selected gortdrumite crystal.

Data frames were processed using the CrysAlis software package (Oxford Diffraction, 2006) running on the Xcalibur PX Ultra control PC. The program ABSPACK (Oxford Diffraction, 2006) was used for the absorption correction. The merging R for the data set decreased from 16.05% before the absorption correction to 7.50% after this correction. Given the observed reflection conditions, together with the statistical tests on the distribution of |E| values that strongly indicated the presence of an inversion centre (|E 2 – 1| = 0.961), we decided to start to solve the structure in the P ![]() $\bar 1$ space group. The positions of most of the atoms (all the Hg positions and most of the Cu atoms) were determined by means of direct methods (Sheldrick, Reference Sheldrick2008). A least-squares refinement on F 2 using these heavy-atom positions and isotropic temperature factors produced an R factor of 0.205. Three-dimensional difference-Fourier synthesis yielded the position of the remaining Cu and S atoms. The program SHELXL (Sheldrick, Reference Sheldrick2008) was used for the refinement of the structure. The occupancy of all the sites was left free to vary (Hg vs. vacancy, Cu vs. vacancy, S vs. vacancy). The occupancy of the Hg sites and all the Cu sites but one (Table 2) was found to be consistent with a pure occupation by Hg and Cu, respectively, and then fixed to 1.00. One of the Cu positions showed a mean electron number of 26.1, and we decided to attribute all the Fe of the formula to this position (see below for crystal-chemical considerations). One of the S positions (S11; Table 2) was found to be half occupied and it was fixed to the value of 0.50 in the last stages of the refinement. Such a feature could indicate that what we are presenting here is actually an average structure, even if during our experiments we have not observed any hint suggesting a potential doubling of one the unit-cell parameters. Neutral-scattering curves for Hg, Cu, Fe and S were taken from the International Tables for X-ray Crystallography (Ibers and Hamilton, Reference Ibers and Hamilton1974). At the last stage, with anisotropic atomic displacement parameters for the Hg atoms and no constraints, the residual value converged at R = 0.0560 for 626 observed reflections [F o > 4σ(F o)] and 143 parameters and at R = 0.0833 for all 3335 independent reflections. Inspection of the difference-Fourier map revealed that maximum positive and negative peaks were 1.34 and 1.22 e –/Å3, respectively. Experimental details and R indices are given in Table 1. Fractional atomic coordinates and equivalent/isotropic displacement parameters are reported in Table 2, whereas the anisotropic displacement parameters of the Hg atoms are given in Table 3. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
$\bar 1$ space group. The positions of most of the atoms (all the Hg positions and most of the Cu atoms) were determined by means of direct methods (Sheldrick, Reference Sheldrick2008). A least-squares refinement on F 2 using these heavy-atom positions and isotropic temperature factors produced an R factor of 0.205. Three-dimensional difference-Fourier synthesis yielded the position of the remaining Cu and S atoms. The program SHELXL (Sheldrick, Reference Sheldrick2008) was used for the refinement of the structure. The occupancy of all the sites was left free to vary (Hg vs. vacancy, Cu vs. vacancy, S vs. vacancy). The occupancy of the Hg sites and all the Cu sites but one (Table 2) was found to be consistent with a pure occupation by Hg and Cu, respectively, and then fixed to 1.00. One of the Cu positions showed a mean electron number of 26.1, and we decided to attribute all the Fe of the formula to this position (see below for crystal-chemical considerations). One of the S positions (S11; Table 2) was found to be half occupied and it was fixed to the value of 0.50 in the last stages of the refinement. Such a feature could indicate that what we are presenting here is actually an average structure, even if during our experiments we have not observed any hint suggesting a potential doubling of one the unit-cell parameters. Neutral-scattering curves for Hg, Cu, Fe and S were taken from the International Tables for X-ray Crystallography (Ibers and Hamilton, Reference Ibers and Hamilton1974). At the last stage, with anisotropic atomic displacement parameters for the Hg atoms and no constraints, the residual value converged at R = 0.0560 for 626 observed reflections [F o > 4σ(F o)] and 143 parameters and at R = 0.0833 for all 3335 independent reflections. Inspection of the difference-Fourier map revealed that maximum positive and negative peaks were 1.34 and 1.22 e –/Å3, respectively. Experimental details and R indices are given in Table 1. Fractional atomic coordinates and equivalent/isotropic displacement parameters are reported in Table 2, whereas the anisotropic displacement parameters of the Hg atoms are given in Table 3. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 2. Atoms, site occupancy factors (s.o.f.), fractional atomic coordinates and equivalent/isotropic displacement parameters (Å2) for the selected gortdrumite crystal.

Table 3. Anisotropic displacement parameters of the atoms for the selected gortdrumite crystal.

Chemical composition
The chemical composition was determined using wavelength dispersive analysis (WDS) by means of an electron microprobe on the same crystal used for the structural study. Concentrations of major and minor elements were determined at an accelerating voltage of 20 kV and a beam current of 10 nA. For the WDS analyses the following lines were used: SKα, FeKα, CuKα and HgMα. The standards employed were: Cu5S9 for Cu, FeAsS for Fe and HgS for S and Hg. The crystal fragment was found to be homogeneous within analytical uncertainty. The average chemical compositions (six analyses on different spots), together with ranges of wt.% of elements, are reported in Table 4. On the basis of 58 atoms, the chemical formula for gortdrumite can be written as Cu24.83Fe1.73Hg9.09S22.35, or, according to the revised chemical formula obtained on the basis of the structural results below, Cu24Fe2Hg9S23. The new formula is also in satisfactory agreement with the original data given by Steed (Reference Steed1983), which, when normalized to 58 atoms, give an average formula of Cu25.4–26.3Fe1.1–1.8Hg8.7–8.8S21.9–22.3.
Table 4. Electron microprobe data (means and ranges in wt.% of elements), atomic ratios (on the basis of 58 atoms) with their standard deviations (σ) for the selected gortdrumite crystal.

The density could not be determined because of paucity of available material and the penetrative intergrowth with other phases (mainly chalcocite). Using the unit-cell parameters from X-ray single-crystal work and the empirical formula, the calculated density is 6.500 g.cm–3. Using the ideal Cu24Fe2Hg9S23 formula, the density is 6.443 g cm–3.
Description of the structure and discussion
The crystal structure of gortdrumite (Fig. 1) represents a new structure type in the Cu–(Fe)–Hg–S system. Although most of the Hg–Cu atoms exhibit bonds with S atoms, there are also some important metal–metal contacts (especially Cu–Cu contacts; Table 5) which play an important role in some coordination environments. Thus, gortdrumite should be considered as a very peculiar sulfide (close to an intermetallic compound) and, therefore, the classic polyhedral description and bond-valence considerations are hardly applicable.

Fig. 1. The crystal structure of gortdrumite down [001], perspective view. The horizontal direction corresponds to the a axis. Light blue, dark blue, red and yellow spheres refer to Cu, Fe, Hg and S atoms, respectively. The unit-cell is outlined.
Table 5. Bond distances (in Å) in the structure of gortdrumite.

Symmetry codes: (i) −x, −y + 1, −z + 1; (ii) x, y − 1, z; (iii) x − 1, y, z; (iv) x, y, z + 1; (v) −x + 1, −y + 1, −z + 1; (vi) −x + 1, −y + 2, −z; (vii) −x + 1, −y + 2, −z + 1; (viii) −x + 1, −y + 1, −z + 2; (ix) −x, −y + 1, −z + 2; (x) x, y − 1, z + 1.
In the crystal structure of gortdrumite there are 12 independent S sites and 18 metal (Hg, Cu and Fe) sites with Z = 1. Mercury is hosted at five different sites (one with halved multiplicity; Wyckoff position 1b), mostly displaying a typical linear coordination. Only Hg4 has an additional ligand at distances <3 Å [i.e. Hg4–S6 = 2.72(1) Å]. The overall mean bond distance of the five Hg sites (in linear coordination) is 2.386 Å, comparable to those found in cinnabar (i.e. 2.368 Å; Auvray and Genet, Reference Auvray and Genet1973). In addition, Hg coordination can be compared with those found in imiterite (2.376 Å; Guillou et al., Reference Guillou, Monthel, Picot, Pillard, Protas and Samama1985) as well as in the lead sulfosalts rouxelite (2.381 Å; Orlandi et al., Reference Orlandi, Meerschaut, Moëlo, Palvadeau and Léone2005) and marrucciite (2.361 and 2.386 Å at Hg1 and Hg2 sites, respectively; Orlandi et al., Reference Orlandi, Moëlo, Campostrini and Meerschaut2007), and in the silver sulfosalt fettelite (2.403 and 2.393 Å at Hg1 and Hg2 sites, respectively; Bindi et al., Reference Bindi, Keutsch, Francis and Menchetti2009).
Copper cations are found in various low-coordination (2, 3 and 4) sites, in agreement with the Cu preference for such environments, and Fe in tetrahedral coordination. Cu12 exhibits an almost linear coordination with S atoms [S8–Cu12–S10 = 176.8(6)°], but there are two Cu–Cu contacts that complete the coordination sphere of the atom. Cu4, Cu5, Cu7, Cu9, Cu10 and Cu11 show a distorted triangular coordination with S atoms. The overall mean bond distance of the six Cu atoms is 2.38 Å, which is only slightly larger than that found for triangularly-coordinated Cu in the crystal structure of Cu12Sb4S13 (2.26 Å; Pfitzner et al., Reference Pfitzner, Evain and Petricek1997) and in Cu10.4Zn1.2Fe0.3S13 (2.30 Å; Wuensch, Reference Wuensch1964). The slight lengthening of the IIICu–S bond distances (where the prefixed Roman numeral corresponds to the coordination number) could be due to a minor Hg/Fe → Cu substitution not detected by the refinement of the electron density at the different sites. Furthermore, Cu4, Cu5, Cu7, Cu9, Cu10 and Cu11 have at least one contact with another Cu atom in their coordination sphere. Noteworthy, the short cation–cation distances observed for Cu10 [Cu1–Cu10 = 2.402(6) Å] and Cu11 [Cu3–Cu11 = 2.500(7) Å] are even shorter that the Cu–Cu distance in metallic copper, i.e. 2.55 Å (Suh et al., Reference Suh, Ohta and Waseda1988). Similar features have been observed in the structure of brodtkorbite (Cu–Cu up to 2.53 Å – Sejkora et al., Reference Sejkora, Škácha, Laufek and Plášil2017), of weissite (Bindi et al., Reference Bindi, Carbone, Belmonte, Cabella and Bracco2013), which shows even shorter Cu–Cu [2.282(3) Å] distances, and cameronite (Bindi and Pinch, Reference Bindi and Pinch2014) with the shortest Cu–Cu distance of 2.4603(4) Å.
The atoms Cu1, Cu2, Cu3, Cu6 and Cu9 show a distorted tetrahedral coordination with S atoms. The overall mean bond distance for the five Cu atoms is 2.36 Å, which compares favourably with that found for tetrahedrally-coordinated Cu in the crystal structure of Cu12Sb4S13 (2.31 Å; Pfitzner et al., Reference Pfitzner, Evain and Petricek1997) and in Cu10.4Zn1.2Fe0.3S13 (2.34 Å; Wuensch, Reference Wuensch1964). As already noticed for the IIICu–S-polyhedra, for the tetrahedral Cu positions the coordination sphere is also filled by at least one Cu–Cu contact. The only CuS4 tetrahedron without any other contact <3 Å is Cu6. Finally, the position that we think could probably be occupied by Fe exhibits a tetrahedral coordination with a mean bond distance of 2.46 Å, a value only slightly larger than that (2.42 Å) computed according to the parameters of Brese and O'Keeffe (Reference Brese and O'Keeffe1991).
Although not a layered compound, the gortdrumite structure can be seen as a succession of layers on (110), with layers made of Hg atoms and Cu4–Cu5–Cu6 polyhedra, and layers made only of Cu/Fe-coordination polyhedra (Fig. 2a). In this regard, gortdrumite exhibits similarities with the recently determined brodtkorbite (Cu2HgSe2) structure (Sejkora et al., Reference Sejkora, Škácha, Laufek and Plášil2017). Brodtkorbite consists of layers of edge-sharing [CuSe4] distorted tetrahedra that form mackinawite-like layers parallel to (100). The layers show the AA type of stacking and are connected by linear Se–Hg–Se bonds. Each Cu atom shows three short contacts with three adjacent Cu atoms forming a pseudo-hexagonal net of metal–metal interactions within one layer (Fig. 2b). If we focus our attention on the ‘Hg layer’ in Fig. 2, it appears evident that gortdrumite is nearly identical to brodtkorbite except for the fact that in gortdrumite there is an alternation along [110] of two leftward linear S–Hg–S bonds and one rightward S–Hg–S surrounded by Cu4–Cu5–Cu6 polyhedra. Such a distribution accounts for the Cu enrichment observed in gortdrumite with respect to brodtkorbite.

Fig. 2. (a) The crystal structure of gortdrumite as seen on (110), Cu/Fe atoms are depicted as light-blue polyhedra, whereas Hg atoms are given as red spheres; (b) Polyhedral representation of the crystal structure of brodtkorbite emphasizing the mackinawite-type layers of edge-sharing [CuSe4] tetrahedra. The linear Se–Hg–Se bonds are indicated. Colours as for the gortdrumite structure.
The powder X-ray pattern calculated using the structural data (Table 2) obtained in this study is shown in Table 6. It appears very similar to that reported by Steed (Reference Steed1983) for type gortdrumite.
Table 6. Powder XRD data for gortdrumite.

Notes: 1 = Calculated powder pattern and indexing for gortdrumite on the basis of a = 9.677(4), b = 9.865(5), c = 11.992(5) Å, α = 77.85(4), β = 79.42(3) and γ = 76.30(4)°, and with the atom coordinates reported in Table 2. Intensities were calculated using XPOW software, version 2.0 (Downs et al., Reference Downs, Bartelmehs, Gibbs and Boisen1993). 2 = observed powder pattern originally reported by Steed (Reference Steed1983).
Acknowledgements
X-ray intensity data were collected at CRIST, Centro di Cristallografia Strutturale, University of Florence, Italy. Thanks are due to Dan Topa for the first analyses of gortdrumite and to Hubert Putz for the loan of the material. The paper benefited by the reviews made by Peter Leverett, Jiří Sejkora and one anonymous reviewer. Associate Editor Andrew Christy is thanked for his efficient handling of the manuscript. This work was funded by “Progetto d'Ateneo 2014” issued by the University of Florence (Italy) to LB.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.065