Introduction
Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) published the description of calciomurmanite, (Na,□)2Ca(Ti,Mg,Nb)4[Si2O7]2O2(OH,O)2(H2O)4, a new mineral from the Lovozero and Khibiny alkaline massifs, Kola Peninsula, Russia. They described calciomurmanite as a Na–Ca ordered analogue of murmanite and related calciomurmanite to two murmanite-group minerals: murmanite, ideally Na4Ti4(Si2O7)2O4(H2O)4 (Gutkova, Reference Gutkova1930; Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) (Table 1) and kolskyite, ideally Na2CaTi4(Si2O7)2O4(H2O)7 (Cámara et al., Reference Cámara, Sokolova, Abdu, Hawthorne and Khomyakov2013a). Table 1 lists selected murmanite-group minerals with the structure type B1MG. In accord with Sokolova and Cámara (Reference Sokolova and Cámara2013), B1 = Basic structure type 1 and MG = murmanite group. Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) reported the chemical composition of calciomurmanite from three localities: (1) Mt. Flora, Lovozero (holotype, V.I. Stepanov collection, Fersman Mineralogical Museum, Moscow, Russia); (2) Mt. Eveslochorr, Khibiny (cotype, collection of the Bel'kov Museum of Geology and Mineralogy; Geological Institute, Apatity, Russia) and (3) the Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny (found by I.V. Pekov in 2008) [see chemical analyses for localities (1) and (3) in Table 2], the empirical formula Na1.34Ca1.04K0.05Mg0.49Mn0.29Fe2+0.21Nb0.36Ti2.85(Si3.87Al0.13)Σ4O16.40(OH)1.6(PO4)0.03(H2O)4.94 (holotype) with Z = 1 (Table 2) and its crystal structure (R 1 = 6.56 and 6.63% for holotype and cotype, respectively). Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) outlined the main features of the structural relation between calciomurmanite and murmanite: (1) the H sheet: in calciomurmanite (holotype and cotype), the [8]AP site is occupied by Ca at 58 and 50%; in murmanite, the [8]AP site is occupied by Na; (2) the O sheet: in calciomurmanite, the [6]M O2 site is occupied by Na at 71 and 52%; in murmanite, the [6]M O2 site is occupied by Na. Taking into account the structural information of Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) and the content of 1.34 Na and 1.04 Ca apfu (atoms per formula unit) in the empirical formula (see above), Sokolova and Cámara (Reference Sokolova and Cámara2017) wrote the ideal structural formula of calciomurmanite of the form AP2MH2MO4(Si2O7)2(XOM)2(XOA)2(XPM,A)4 as (Ca□)Ti2(Na□)Ti2(Si2O7)2O2[O(OH)](H2O)4 [Table 1, site labelling is in accord with Sokolova (Reference Sokolova2006)].
Table 1. Ideal structural formulae and crystallographic information for selected murmanite-group minerals* (seidozerite supergroup), Ti = 4 apfu.

* Ideal structural formulae are from Sokolova and Cámara (Reference Sokolova and Cámara2017); unit-cell parameters are given to the third decimal. Labelling is in accord with Sokolova (Reference Sokolova2006): MO4 and MH2 = cations of the O and H sheets, and AP2 = cations at the peripheral (P) sites; (XOM,A)4 = anions of the O sheet not bonded to Si: (XOM)2 = anions at the common vertices of 3MO and MH polyhedra; (XOA)2 = anions at the common vertices of 3MO and AP polyhedra; XPM and XPA = apical anions of MH and AP cations at the periphery of the TS block; and coordination numbers are given for non-octahedrally coordinated cation sites;
** for murmanite, unit cells [1, upper line] → [2, lower line] are related by the transformation matrix (100/0![]() $ {\bar 1} $0/
$ {\bar 1} $0/![]() $ {\bar 1} $0
$ {\bar 1} $0![]() $ {\bar 1} $);
$ {\bar 1} $);
† First reference: the discovery of the mineral; second: the most recent reference on the structure: (1) Gutkova et al. (Reference Gutkova1930); (2) Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008); (3) this work; (4) Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016); (5) Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013); (6) Sokolova and Hawthorne (Reference Sokolova and Hawthorne2018).
Table 2. Chemical composition and unit formula for MRM.

(1) This work, Mt. Pyalkimpor, Lovozero; (2 and 3) are taken from Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016): (2) Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny; (3) Mt. Flora, Lovozero (holotype); and (4) after Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008): Yubileynaya pegmatite, Mt. Karnasurt, Lovozero. 4.2% of lomonosovite intergrowth was subtracted.
n.a. = not analysed; n.d. = not detected; structure work done for (1,3,4).
* Formulae calculated on: (1) 22 (O + F) apfu, with OH = 0.11 pfu and H2O = 3.89 pfu and (2, 3) Si + Al = 4 apfu and OH–/O2– ratio calculated by charge balance; and (4) 22 (O + F) apfu, with H2O = 4 pfu.
** Determined by Mössbauer spectroscopy.
*** Calculated from crystal-structure refinement.
† Measured by the modified Penfield method (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016).
Following our previous work on murmanite, ideally Na4Ti4(Si2O7)2O4(H2O)4 (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and a murmanite-related mineral vigrishinite, ideally NaZnTi4(Si2O7)2O3(OH)(H2O)4 (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2018), we wanted to understand the details of the bond topology in the structure of calciomurmanite, especially the pattern of hydrogen bonding. In February of 2017 at the Tucson Gem and Mineral Show, we purchased a ‘calciomurmanite’ sample from Mt. Pyalkimpor, the Lovozero alkaline massif, Kola Peninsula, Russia, from Dmitriy Belakovskiy. Dmitry told us that it was a new finding of ‘calciomurmanite’ by Inna Lykova in 2016, after the approval of calciomurmanite by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2014-103, Lykova, Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2015). Our work on the new 2016-finding of ‘calciomurmanite’ has resulted in chemical composition and stereochemistry different from both calciomurmanite (holotype: Mt. Flora, Lovozero, and cotype: Mt. Eveslochorr, Khibiny) and murmanite. Here, we report the chemical composition and the refinement of the crystal structure of this murmanite-related mineral (MRM), a possible new mineral of the murmanite group, which we purchased under the name ‘calciomurmanite’.
Description of the sample
MRM occurs as large platy grains up to 5 mm x 10 mm across and up to 1 mm thick. It is opaque in large grains and cleavage plates, very pale-brown in thick fragments, and colourless and transparent in very small thin plates.
Chemical analysis
The crystal of MRM used for the structure refinement was analysed with a Cameca SX-100 electron-microprobe operating in wavelength-dispersion mode with an accelerating voltage of 15 kV, a specimen current of 5 nA, a beam diameter of 10 µm and count times on peak and background of 20 and 10 s, respectively. The following standards were used: Si and Ca: diopside; Al: andalusite; F: fluoro-riebeckite; Na: albite; Nb: Ba2NaNb5O15; Zr: zircon; Mg: forsterite, Fe: fayalite; Mn: spessartine; Sr: SrTiO3; Ti: titanite; K: orthoclase; and P: apatite. Zinc and Ta were sought but not detected. Data were reduced using the φ(ρZ) procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). The chemical composition of MRM is the mean of four determinations and is given in Table 2, analysis (1). Our chemical analysis of MRM is close to that of ‘calciomurmanite’ from the Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny [Table 2, analysis (2), from Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016], particularly for the Na2O content, 8.32 vs. 8.87 wt.%, and the Al2O3 content, 0.08 vs. 0.06 wt.%. Comparison of our chemical analysis and that of the holotype calciomurmanite from Mt. Flora, Lovozero (holotype) [Table 2, analysis (3), from Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016], shows differences in the Na2O content, 8.32 vs. 5.39 wt.%, and the Al2O3 content, 0.08 vs. 0.85 wt.%. The analyses (1), (2) and (3) (Table 2) give the following values for the content of CaO: 6.02, 6.90 and 7.61 wt.%, respectively, and these three values are much higher than that for murmanite, 1.43 wt.% [Table 2, analysis (4), from Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008]. The empirical formula of MRM, calculated on the basis of 22 (O + F), with two constraints derived from the crystal-structure refinement, OH = 0.11 pfu and H2O = 3.89 pfu, is (Na2.12K0.07Sr0.01)Σ2.20Ca0.85(Ti3.01Nb0.39Mn0.20Fe2+0.19Mg0.17Zr0.01Al0.01)Σ3.98(Si4.20O14)[O3.90F0.10]Σ4[(H2O)3.89(OH)0.11]Σ4{P0.03} with Z = 1. We suggest that {P0.03} belongs to other phases which form intergrowths with MRM. Intimate intergrowths are very common for TS-block minerals: our high-resolution transmission electron microscopy work on murmanite-group minerals lomonosovite, Na10Ti4(Si2O7)2(PO4)2O4, and betalomonosovite, Na6Ti4(Si2O7)2[PO3(OH)][PO2(OH)2]O3F (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015); zvyaginite, Na2ZnTiNb2(Si2O7)2O2(OH)2(H2O)4, a lamprophyllite-group mineral (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017), and cámaraite, NaBa3Fe2+8Ti4(Si2O7)4O4(OH)4F3, a bafertisite-group mineral (Cámara et al., Reference Cámara, Sokolova and Nieto2009), shows that these four TS-block minerals contain intergrown phases.
X-ray data collection and structure refinement
X-ray data for the MRM single crystal were collected with a Bruker APEX II ULTRA three-circle diffractometer equipped with a rotating-anode generator (MoKα radiation), multilayer optics and an APEX II 4K CCD detector. Details of data collection and structure refinement are given in Table 3. The intensities of reflections with –7 ≤ h ≤ 7, –9 ≤ k ≤ 9, –17 ≤ l ≤ 17 were collected with a frame width of 0.5° and a frame time of 30 s, and an empirical absorption correction (SADABS, Sheldrick, Reference Sheldrick2008) was applied. The crystal structure of MRM was refined using the coordinates of Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008) for murmanite in space group P ![]() $ {\bar 1} $ to R 1 = 5.72% with the Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2015). There are six cation sites in the crystal structure of MRM: the M H, AP and two Si sites of the H sheet and two M O sites of the O sheet; labelling follows Sokolova (Reference Sokolova2006). We encountered a split of the M H site into two subsites, M H1 and M H2, 0.38 Å apart. For the refinement, we constrained atoms at those two subsites of the M H site to have the same displacement parameters (using EADP constraints). The occupancies of five sites/subsites were refined with the following scattering curves: M H1,2 and M O1 sites: Ti; M O2 site: Na; and AP site: Ca. The coordinates of the H atoms were refined where the D (donor)–H distances were softly constrained to 0.98 Å. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 4, selected interatomic distances and angles in Table 5, refined site-scattering values and assigned site-populations in Table 6, bond-valence values in Table 7 and details of hydrogen bonding in Table 8. A list of observed and calculated structure factors and a Crystallography Information File (CIF) have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
$ {\bar 1} $ to R 1 = 5.72% with the Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2015). There are six cation sites in the crystal structure of MRM: the M H, AP and two Si sites of the H sheet and two M O sites of the O sheet; labelling follows Sokolova (Reference Sokolova2006). We encountered a split of the M H site into two subsites, M H1 and M H2, 0.38 Å apart. For the refinement, we constrained atoms at those two subsites of the M H site to have the same displacement parameters (using EADP constraints). The occupancies of five sites/subsites were refined with the following scattering curves: M H1,2 and M O1 sites: Ti; M O2 site: Na; and AP site: Ca. The coordinates of the H atoms were refined where the D (donor)–H distances were softly constrained to 0.98 Å. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). Final atom coordinates and equivalent displacement parameters are given in Table 4, selected interatomic distances and angles in Table 5, refined site-scattering values and assigned site-populations in Table 6, bond-valence values in Table 7 and details of hydrogen bonding in Table 8. A list of observed and calculated structure factors and a Crystallography Information File (CIF) have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 3. Miscellaneous structure-refinement data for MRM.

Table 4. Final atom coordinates and displacement parameters (Å2) for MRM.

* U iso
Table 5. Selected interatomic distances (Å) and angles (°) in MRM.

φ = O, F, OH, H2O;
Symmetry operators (given in brackets): a: –x + 1, –y + 1, –z + 1; b: –x + 1, –y + 2, –z + 1; c: –x, –y + 2, –z + 1; d: x + 1, y, z; e: x, y – 1, z; f: x, y + 1, z; g: x – 1, y, z.
Table 6. Refined site-scattering values and assigned site-populations for MRM.

* Coordination numbers are shown for non-[6]-coordinated cation sites and non-[4]-coordinated anion sites and H2O groups; φ = O, F, OH, H2O.
** Anions which do not coordinate Si.
Table 7. Bond-valence values (vu)* for MRM.

* Bond-valence parameters (vu) are from Brown (Reference Brown, O'Keeffe and Navrotsky1981) and Brown and Altermatt (Reference Brown and Altermatt1985) for hydrogen bonding; for O atoms, coordination numbers [ ] are given where an O atom is coordinated by less than four cations; bond-valence values are calculated with cation–O parameters for: MO1 = Ti; MO2 = Ca0.60Na0.40; MH1 = Ti; MH2 = Nb and AP = Na.
Table 8. Hydrogen bonding in MRM.

Symmetry operators (in brackets): a: –x + 1, –y + 1, –z; b: –x, –y + 1, –z.
Site-population assignment
Ti-dominant sites
In the seidozerite-supergroup minerals, Ti-dominant sites are always fully occupied (Sokolova, Reference Sokolova2006; Sokolova and Cámara, Reference Sokolova and Cámara2017). In the murmanite-group minerals, Ti = 4 apfu; in the O sheet, Ti = 2 apfu (Fig. 1a) and Ti-dominant sites in the O sheet commonly contain divalent cations such as Mn, Fe2+ and Mg (Sokolova, Reference Sokolova2006; Sokolova and Cámara, Reference Sokolova and Cámara2017); in the H sheet, Ti = 2 apfu (Fig. 1b). In MRM, the [6]M H site in the H sheet, which gives 2 apfu, splits into two subsites, M H1 and M H2, with refined site-scattering values of 42(1) and 5(1) electrons per formula units (epfu) and mean bond-lengths of 1.967 and 1.96 Å, respectively (Table 6). The short distance of 0.38 Å between the two subsites indicates that these subsites can be only alternately occupied. Total refined site-scattering for the M H site is 47 epfu (more than the 44 epfu corresponding to occupancy by Ti2 apfu) and hence the M H site must be occupied by Ti plus a heavier cation, e.g. Nb, Zr, Mn and Fe2+, available from the chemical analysis (Table 2). The calculated cation radius (r) for the M H2 site is 1.96–1.38 ([4]O2–, Shannon, Reference Shannon1976) = 0.58 Å. The [6]Nb has the smallest ionic radius (0.64 Å) compared to Zr (0.72 Å), Mn (0.83 Å) and Fe2+ (0.78 Å). We assign the rest of Ti and Nb available from the chemical analysis (Table 2) plus all Zr, Mn, Fe2+ and Mg to the M O1 site in the O sheet: Ti1.13Nb0.28Mn0.20Fe2+0.19Mg0.17Zr0.01□0.02 pfu, with close agreement between refined and calculated site-scattering values, 46.2 and 48.72 epfu, respectively (Table 6).
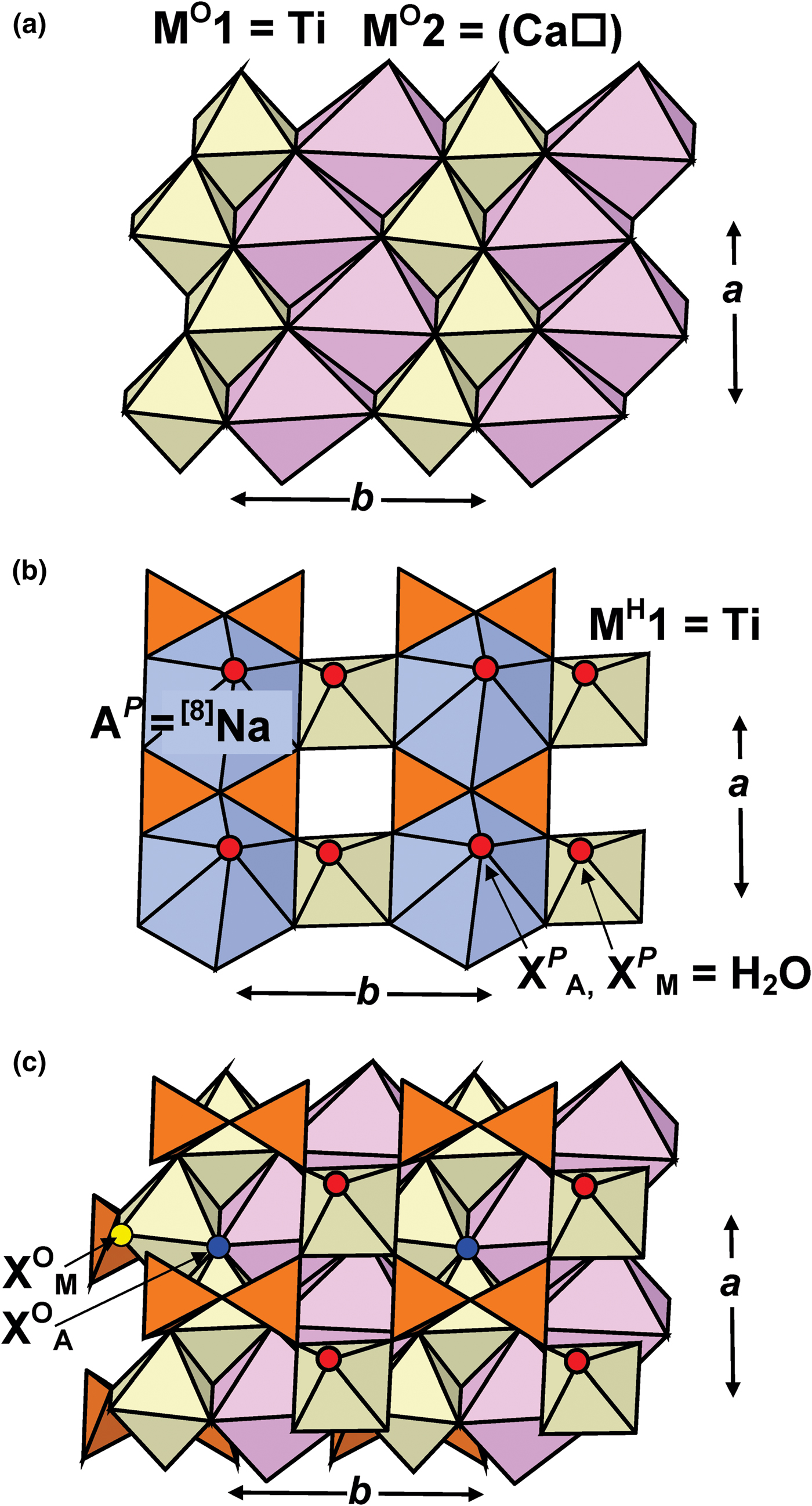
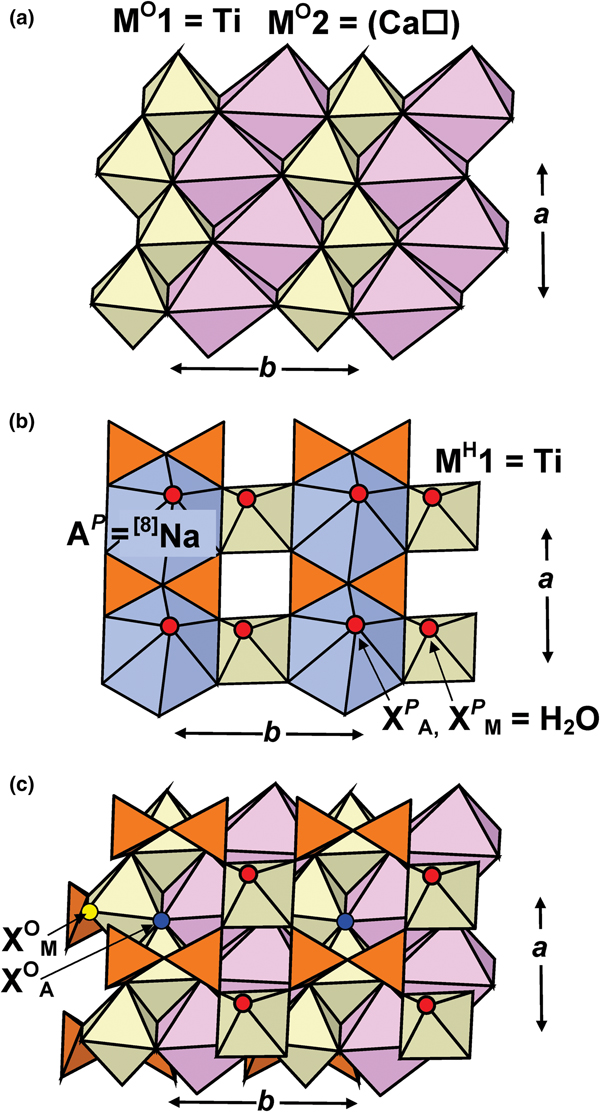
Fig. 1. Details of the TS block in MRM: the O sheet of Ti-dominant MO1 and Ca-dominant MO2 octahedra [M O2 sites are occupied at 53%] (a); the H sheet of Si2O7 groups, Ti-dominant MH octahedra and Na-dominant AP polyhedra (b); the TS block (c). Si tetrahedra are orange, Ti-dominant octahedra are yellow; Na-dominant and Ca-dominant polyhedra are navy blue and pale pink, H2O groups at the XP sites are shown as large red spheres, XOM and XOA anions are shown as yellow and dark blue spheres in (c).
Alkaline and alkali-earth sites
Chemical analysis (Table 2) gives alkali and alkali-earth cations (Na2.12Ca0.85K0.07Sr0.01)Σ3.05 apfu to assign to the AP and M O2 sites, which give 4 apfu. The [8]AP site in the H sheet of MRM has a refined site-scattering value of 24.8(1) epfu and a mean bond length of 2.558 Å (Fig. 1b, Table 6). In murmanite, the corresponding site is occupied by Na1.78Ca0.15K0.05□0.02 pfu, ideally Na2 apfu, and has a refined site-scattering value of 22.0(1) epfu and a mean bond length of 2.568 Å (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008). In the holotype calciomurmanite, (1) the corresponding site is occupied by Ca1.16□0.84 pfu, with a refined site-scattering value of 23.2 epfu and a mean bond length of 2.553 Å; (2) the two additional [7] and [8]-coordinated sites between TS blocks are occupied by Sr0.18□1.82 and K0.10□1.90 pfu with refined site-scattering values of 7.0 and 2.0 epfu, respectively (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016). In the structures of murmanite (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and MRM, there are no additional sites between TS blocks; see Table 3 for the highest peak of 1.77 e/Å3 in the difference Fourier map for MRM. To the largest [8]AP site in MRM, we assign the largest alkaline and alkali-earth cations available from the chemical analysis (Table 2), K0.07Sr0.01 apfu ([8]K: r = 1.51 Å; [8]Sr: r = 1.26 Å), with the calculated site-scattering value of 1.71 epfu. We are left with the site-scattering value 24.8–1.71 = 23.09 epfu, which cannot be compensated by all available Ca0.85 apfu, which has a calculated site-scattering value of 17 epfu. Hence we assign Na1.70Ca0.22 apfu ([8]Na: r = 1.18 Å; [8]Ca: r = 1.12 Å), with the calculated site-scattering value of 23.10 epfu. The [8]AP site in MRM is occupied by Na1.70Ca0.22K0.07Sr0.01 apfu (Table 6).
The M O2 site in the O sheet of MRM (Fig. 1a), has a refined site-scattering value of 16.3 epfu and a mean bond length of 2.454 Å. To the M O2 site, we assign the remaining Ca and Na, Ca0.63Na0.42□0.95 pfu, where Ca > Na. The refined and calculated site-scattering values for the M O2 site of 16.3 and 17.22 epfu, respectively, are in good agreement.
Description of the structure
Cation and anion sites
Here we consider six cation sites in the crystal structure of MRM: the M H, AP and two Si sites of the H sheet and the two M O sites of the O sheet; and six anion sites: X OM and X OA = anion sites at the common vertices of 3MO and MH polyhedra and 3MO and AP polyhedra, respectively; two X P(M,A) = anion sites at the apical vertices of MH octahedron and one [8]AP polyhedron at the periphery of the TS block; labelling is in accord with Sokolova (Reference Sokolova2006). The specification of anions will be given at the end of this section.
In the O sheet, the Ti-dominant M O1 site is coordinated by four O atoms and two XOA anions of the following composition (O0.95F0.05), with < MO1–φ> = 2.015 Å (φ = unspecified anion) (Tables 5,6; Figs 1a,c). The ideal composition of the M O1 site is Ti2 apfu (Table 6). In murmanite, the Ti-dominant site is coordinated by six O atoms. The M O2 site is 53% occupied by Ca and Na (Ca > Na) (Table 6, Fig. 1a), and is coordinated by five O atoms and an XOA anion, with < MO2–φ> = 2.454 Å (Table 5). The ideal composition of the M O2 site is (Ca□) pfu (Table 6). In murmanite, the corresponding site is occupied by Na1.55Mn0.14Ca0.06□0.25 pfu, ideally Na2 apfu, with a mean bond length of 2.468 Å (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008). In holotype and cotype calciomurmanite, the corresponding site is occupied by Na1.42□0.58 and Na1.02□0.98 pfu, respectively, ideally (Na□) pfu, with mean bond lengths of 2.454 and 2.467 Å, respectively (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016). Note that for the holotype calciomurmanite, the chemical analysis gives only 1.34 Na apfu [Table 2, analysis (3)]. The ideal composition of the M O2 + M O1 sites is (Ca□)Ti2 apfu.
In the H sheet, there are two tetrahedrally coordinated sites (Si1, Si2) occupied by Si. There is one Ti-dominant [6]M H site which splits into two subsites; each M H1,2 subsite is coordinated by five O atoms and an H2O group or an OH group at the X PM site, with < MH1,2–φ> = 1.967 and 1.96 Å (Figs 1b,c), respectively. The M H1 and M H2 subsites are 0.38 Å apart and they are occupied by Nb0.11□1.89 and Ti1.88Al0.01□0.11 pfu, respectively (Table 6). Positional Ti–Nb disorder within one site has been reported for several Ti-silicates, e.g. in the O sheet of fogoite-(Y), ideally Na3Ca2Y2Ti(Si2O7)2OF3 (Cámara et al., Reference Cámara, Sokolova, Abdu, Hawthorne, Charrier, Dorcet and Carpentier2017), a rinkite-group mineral (seidozerite supergroup), and in the H sheet of veblenite, K2□2Na(Fe2+5Fe3+4Mn2+7□)Nb3Ti(Si2O7)2(Si8O22)2O6(OH)10(H2O)3 (Cámara et al., Reference Cámara, Sokolova, Hawthorne, Rowe, Grice and Tait2013b). The M H site ideally gives Ti2 apfu. The [8]AP site is occupied mainly by Na, less Ca, and minor K and Sr, and is ideally Na2 apfu (Table 6). The AP site is coordinated by six O atoms, an (O,F) anion (O >> F) at the X OA site and an H2O group at the X PA site, with < AP–φ> = 2.558 Å (Figs 1b,c; Table 5). The ideal composition of the AP + M H sites is Na2Ti2 apfu.
We write the cation part of the TS block as the sum of cations of the 2H and O sheets: ideally Na2Ti2(Ca□)Ti2 pfu, with a total charge of 20+.
The two Si1,2 atoms and seven O(1–7) atoms that coordinate the Si atoms give (Si2O7)2 pfu (Tables 4,5). An anion at the X OM site (Fig. 1c) receives bond valences from four cations: MH1, MH2, MO2 and MO1, with a total bond-valence sum of 2.01 vu (valence units) (Table 7); thus it is an O atom, giving O2 apfu (Table 6). An anion at the X OA site (Fig. 1c) receives bond valences from four cations: 2(MO1), MO2 and AP, with a total bond-valence sum of 1.67 vu (Table 7). We assign O1.90F0.10 to the X OA site, ideally O2.00 apfu (Table 6). Joint occurrence of O and F atoms at the X OA site was reported for the murmanite-group minerals sobolevite, Na6(Na2Ca)(NaCaMn)Na2Ti2Na2(TiMn)(Si2O7)2(PO4)4O2(OF)F2 (Sokolova et al., Reference Sokolova, Egorov-Tismenko and Khomyakov1988; Sokolova et al., Reference Sokolova, Hawthorne and Khomyakov2005) and betalomonosovite, Na2□4Na2Ti2Na2Ti2(Si2O7)2[PO3(OH)][PO2(OH)2]O2(OF) (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015). The two (X OM,A)2 sites ideally give O4 apfu.
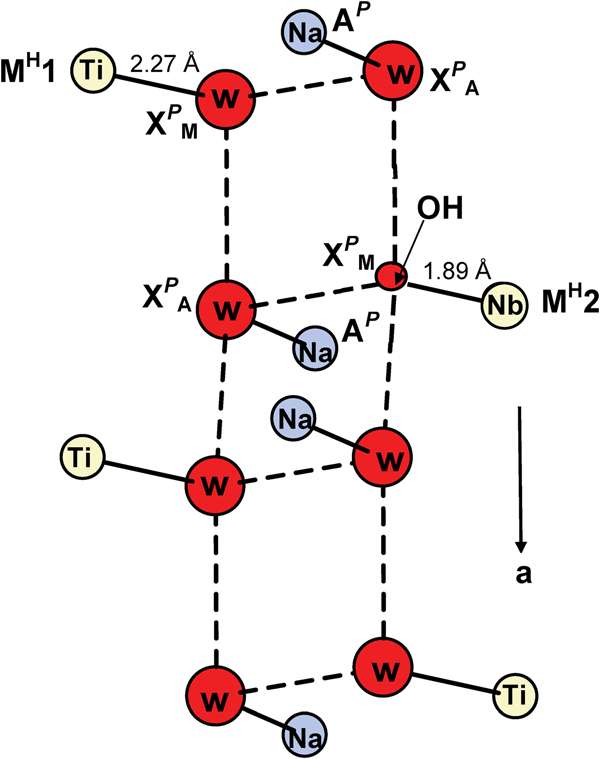
Consider the two XP (M,A) sites at the periphery of the TS block (Figs 1c, 2). Figure 3 shows the general pattern of hydrogen bonding between H2O groups at the X P(M,A) sites of two adjacent TS blocks. Details of the hydrogen bonding are given in Table 8. This pattern is analogous to that in murmanite-group minerals: murmanite, originally proposed by Khalilov (Reference Khalilov1989) and described in detail (including positions of H atoms) by Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008); in vigrishinite, NaZnTi4(Si2O7)2O3(OH)(H2O)4 (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2018); and in the lamprophyllite-group minerals: epistolite, Na4TiNb2(Si2O7)2O2(OH)2(H2O)4 (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2004) and zvyaginite, Na2ZnTiNb2(Si2O7)2O2(OH)2(H2O)4 (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017). In these structures, H2O groups form a ribbon which extends along a (t1) (Fig. 3). The O atom of the H2O group at the X PA site receives 0.22 vu from Na at the AP site (Table 7, Fig. 3) and we assign an H2O group to the X PA site (Table 6). To assign species to the X PM site, we need to consider short-range order (SRO) arrangements involving the M H1 and M H2 subsites which are 95% occupied by Ti (and minor Al) and 5% occupied by Nb, respectively (Fig. 3). SRO-95% occurs where the M H1 subsite is occupied by Ti and the M H2 subsite is vacant, and the O atom at the X PM site receives bond valence only from one cation: 0.31 vu from Ti at the M H1 subsite, with MH1–O = 2.267 Å (Table 5). Hence at SRO-95%, the X PM site is occupied by H2O groups (Fig. 3), giving 1.89 H2O pfu (Table 6). SRO-5% occurs where the M H1 is vacant and the M H2 subsite is occupied by Nb, and the O atom at the X PM site receives bond valence only from one cation: 1.00 vu from Nb at the M H2 subsite, with MH2–O = 1.89 Å (Table 5). Hence at SRO-5%, the X PM site is occupied by OH groups (Fig. 3), giving 0.11 OH pfu (Table 6). In accord with the two SRO arrangements, SRO-95% and SRO-5%, we assign (H2O)1.89(OH)0.11 pfu to the X PM site (Table 6). We sum the compositions of the two X P(M,A) sites as follows: (H2O)1.89(OH)0.11 pfu [X PM] + (H2O)2.00 [X PA] = (H2O)3.89(OH)0.11, ideally (H2O)4 pfu (Table 6).
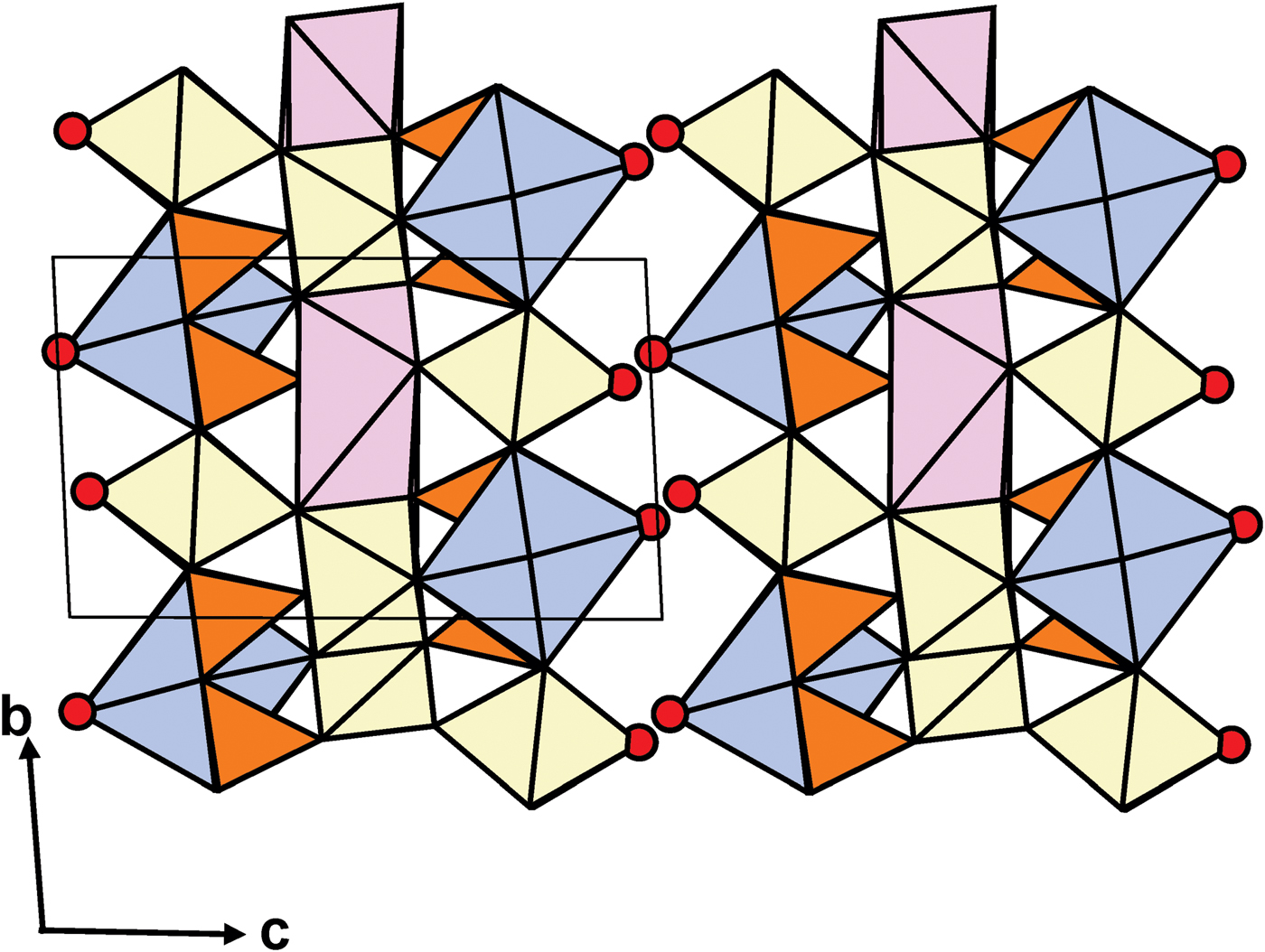
Fig. 2. A general view of the crystal structure of MRM. Legend as in Fig. 1.

Fig. 3. A general scheme of hydrogen bonding in MRM, only H2O and OH groups are shown, O atoms involved in hydrogen bonding are omitted. O atoms of H2O groups and OH groups at the XP sites are shown as large and small red spheres, respectively; Na atoms at the AP site are shown as navy blue spheres, and Ti and Nb atoms at the M H1 and M H2 subsites are shown as yellow spheres; Ti–O(H2O), Nb–O(OH) and Na–O(H2O) bonds are shown as solid black lines. D(donor)–A(acceptor) directions are shown as dashed lines.
The anions and H2O groups sum as follows: (Si2O7)2 [O(1–7)] + O2 [X OM] + O2 [X OA] + (H2O)4 [X P(M,A)] = (Si2O7)2O4(H2O)4 pfu, with a total charge of 20–.
We write the ideal structural formula of MRM as the sum of cation and anion parts: Na2Ti2(Ca□)Ti2 + (Si2O7)2O4(H2O)4 = Na2Ti2(Ca□)Ti2(Si2O7)2O4(H2O)4 with Z = 1. A short form of the ideal structural formula is Na2CaTi4(Si2O7)2O4(H2O)4.
Structure topology of MRM
The crystal structure of the MRM is topologically identical to the structures of murmanite (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and calciomurmanite (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) and is related to vigrishinite (Sokolova and Hawthorne, Reference Sokolova and Hawthorne2018). The main structural unit in the crystal structure of MRM is a TS block that consists of HOH sheets. In accord with Sokolova and Cámara (Reference Sokolova and Cámara2013), it is a basic structure, structure type B1MG.
The O sheet is composed of Ti-dominant MO1 octahedra and Ca-dominant MO2 octahedra occupied at 53%; MO1 and MO2 octahedra each form brookite-like chains along a (Fig. 1a). Ideal compositions of the O sheet in MRM, calciomurmanite and murmanite are [(Ca□)Ti2O4]2+ pfu, [(Na□)Ti2O3(OH)]2+ pfu and [Na2Ti2O4]2+ apfu, respectively.
Murmanite, MRM and calciomurmanite are related by the following substitutions in the O sheet:
In MRM, the H sheet is built of Si2O7 groups, Ti-dominant [6]MH octahedra and Na-dominant [8]AP polyhedra (Fig. 1b). Ideal compositions of the H sheets in MRM and murmanite are identical: [Na2Ti2(Si2O7)2(H2O)4]2– apfu; ideal composition of the H sheets in calciomurmanite is [Ca□Ti2(Si2O7)2(H2O)4]2– pfu. Murmanite + MRM and calciomurmanite are related by the following substitution in the H sheet:
In MRM, the topology of the TS block is as in the murmanite group of TS-block minerals [Ti + Mg + Mn = 4 apfu]: Si2O7 groups link to two Ti octahedra of the O sheet adjacent along t1 (Fig. 1c). In the crystal structure of MRM, TS blocks parallel to (001) link via hydrogen bonds between H2O groups at apical vertices [XP (M,A) sites] of MH and AP polyhedra (Fig. 2; for the pattern of hydrogen bonding, see Fig. 3).
MRM and murmanite are related by the following substitution:
MRM and calciomurmanite are related by the following substitution:
 $$\eqalign{& ^{\rm O} ({\rm C}{\rm a}^{2 +} {\squ})_{{\rm MRM}} + ^{\rm H}\!\!\lpar {{\rm N}{\rm a}^ +_2} \rpar _{{\rm MRM}} + ^{\rm O}\!\!\lpar {{\rm O}^{2-}} \rpar _{{\rm MRM}}\cr & \quad \leftrightarrow ^{\rm O}\!\!({\rm N}{\rm a}^ + {\squ})_{{\rm cal}} + ^{\rm H}\!\!({\rm C}{\rm a}^{2 +} {\squ})_{{\rm cal}} + ^{\rm O}\!\![\lpar {{\rm OH}} \rpar ^{-} ]_{{\rm cal}}.}$$
$$\eqalign{& ^{\rm O} ({\rm C}{\rm a}^{2 +} {\squ})_{{\rm MRM}} + ^{\rm H}\!\!\lpar {{\rm N}{\rm a}^ +_2} \rpar _{{\rm MRM}} + ^{\rm O}\!\!\lpar {{\rm O}^{2-}} \rpar _{{\rm MRM}}\cr & \quad \leftrightarrow ^{\rm O}\!\!({\rm N}{\rm a}^ + {\squ})_{{\rm cal}} + ^{\rm H}\!\!({\rm C}{\rm a}^{2 +} {\squ})_{{\rm cal}} + ^{\rm O}\!\![\lpar {{\rm OH}} \rpar ^{-} ]_{{\rm cal}}.}$$We conclude that: (1) the general topology of the crystal structure of MRM described above is in accord with the topology of murmanite (Khalilov, Reference Khalilov1989; Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and calciomurmanite (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016); and (2) the stereochemistry of Ca and Na in the TS block is different from that reported for calciomurmanite (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016): disorder of Ca and □ in the O sheet of MRM versus disorder of Ca and □ in the H sheet of calciomurmanite (Table 1). A similar stereochemistry of Zn and □ was described for two TS-block minerals: the order of Zn and □ in the O sheet of zvyaginite (Pekov et al., Reference Pekov, Lykova, Chukanov, Yapaskurt, Belakovskiy, Zolotarev and Zubkova2014; Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017) and the order of Zn and □ in the H sheet of vigrishinite (Pekov et al., Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013; Sokolova and Hawthorne, Reference Sokolova and Hawthorne2018).
Summary
Electron-microprobe analysis of MRM from the Mt. Pyalkimpor, Lovozero alkaline massif, Kola Peninsula, Russia, is in accord with that of ‘calciomurmanite’ from the Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016, Table 1, analysis 6). The empirical formula of MRM, (Na2.12K0.07Sr0.01)Σ2.20Ca0.85(Ti3.01Nb0.39Mn0.20Fe2+0.19Mg0.17Zr0.01Al0.01)Σ3.98(Si4.20O14)[O3.90F0.10]Σ4[(H2O)3.89(OH)0.11]Σ4{P0.03} with Z = 1, gives the sum of alkali and alkali-earth cations as 3.05 apfu and Na:Ca ≈ 2 : 1. The incomplete empirical formula of ‘calciomurmanite’ from the Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny [Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016, Table 1, analysis 6; this paper, Table 2, analysis (2)] gives a sum of alkali and alkali-earth cations, (Na2.32K0.11)Σ2.43Ca1.00, with Σ = 3.43 apfu and Na:Ca ≈ 2:1. For the holotype calciomurmanite, alkali and alkali-earth cations are as follows (Na1.34K0.05)Σ1.39Ca1.04, Σ = 2.43 apfu and Na:Ca ≈ 1:1.
The crystal structure of MRM has been refined in space group P ![]() $ {\bar 1} $, a = 5.363(2), b = 7.071(2), c = 12.176(5) Å, α = 92.724(3), β = 107.542(7), γ = 90.13(2)°, V = 439.7(4) Å3, R 1 = 5.72% and Z = 1. The general topology of the crystal structure of MRM is in accord with the topology of murmanite (Khalilov, Reference Khalilov1989; Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and calciomurmanite (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016): it is an array of TS blocks connected via hydrogen bonds between H2O groups. However the stereochemistry of the TS block is different from that in the calciomurmanite of Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016). In MRM, there is disorder of Ca and □ at the M O2 site in the O sheet of the composition [(Ca□)Ti2O4]2+. In calciomurmanite, there is disorder of Ca and □ at the AP site in the H sheets of the composition [(Ca□)Ti2(Si2O7)2(H2O)4]2–.
$ {\bar 1} $, a = 5.363(2), b = 7.071(2), c = 12.176(5) Å, α = 92.724(3), β = 107.542(7), γ = 90.13(2)°, V = 439.7(4) Å3, R 1 = 5.72% and Z = 1. The general topology of the crystal structure of MRM is in accord with the topology of murmanite (Khalilov, Reference Khalilov1989; Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and calciomurmanite (Lykova et al., Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016): it is an array of TS blocks connected via hydrogen bonds between H2O groups. However the stereochemistry of the TS block is different from that in the calciomurmanite of Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016). In MRM, there is disorder of Ca and □ at the M O2 site in the O sheet of the composition [(Ca□)Ti2O4]2+. In calciomurmanite, there is disorder of Ca and □ at the AP site in the H sheets of the composition [(Ca□)Ti2(Si2O7)2(H2O)4]2–.
MRM has an ideal structural formula of the form AP2MH2MO4(Si2O7)2(XOM)2(XOA)2(XPM,A)4: Na2Ti2(Ca□)Ti2(Si2O7)2O4(H2O)4, in a shorter form Na2CaTi4(Si2O7)2O4(H2O)4, Z = 1.
MRM is a Ca-rich and Na-poor analogue of murmanite, ideally Na2Ti2Na2Ti2(Si2O7)2O2O2(H2O)4. MRM is a Na-rich and OH-poor analogue of calciomurmanite, ideally (Ca□)Ti2(Na□)Ti2(Si2O7)2O2[O(OH)](H2O)4. MRM and (murmanite and calciomurmanite) are related by the following substitutions: O(Ca2+□)MRM ↔ O(Na+2)mur and O(Ca2+□)MRM + H(Na+2)MRM + O(O2–)MRM ↔ O(Na+□)cal + H(Ca2+□)cal + O[(OH)–]cal.
MRM is a possible new mineral of the murmanite group (seidozerite supergroup) where Ti + Mg + Mn = 4 apfu. We feel it is more appropriate that Inna Lykova proposes MRM as a new mineral as she found it in the field (personal communication, Dmitriy Belakovskiy), and because Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016) reported a composition very similar to MRM from the Shcherbakovitovoe pegmatite, Mt. Koashva, Khibiny.
Acknowledgements
We are grateful to reviewers Fernando Cámara and an anonymous reviewer and to Associate Editor Ed Grew for the comments which helped to improve the manuscript. We thank Mark A. Cooper for collection of single-crystal X-ray data for the MRM crystals. This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada, and by Innovation Grants from the Canada Foundation for Innovation to FCH.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.119