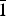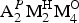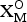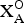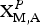Introduction
Vigrishinite, ideally NaZnTi4(Si2O7)2O3(OH)(H2O)4, is a murmanite-group mineral of the seidozerite supergroup. The forty-five seidozerite-supergroup minerals have structures based on a TS-block (TS = Titanium Silicate) (Sokolova and Cámara, Reference Sokolova and Cámara2017). The TS block consists of HOH sheets (H = heteropolyhedral and O = octahedral) and is characterized by a planar cell based on translation vectors, t1 and t2, with t 1 ~ 5.5 and t 2 ~ 7 Å and t1 ^ t2 close to 90°. The seidozerite-supergroup minerals are divided into four groups based on the content of Ti (+ Nb + Zr + Fe3+ + Mg + Mn), topology, chemical composition and stereochemistry of the TS block (Sokolova, Reference Sokolova2006, Reference Sokolova2010; Sokolova and Cámara, Reference Sokolova and Cámara2013). In the rinkite, bafertisite, lamprophyllite and murmanite groups, Ti = 1, 2, 3 and 4 apfu (atoms per formula unit). The four groups of the seidozerite supergroup correspond to Groups I, II, III and IV of Sokolova (Reference Sokolova2006). Ideal structural formulae of the murmanite-group minerals are given in Table 1.
Table 1. Ideal structural formulae of the murmanite-group minerals* (seidozerite supergroup), Ti (+ Mn + Mg) = 4 apfu.
**Structural formulae are from Sokolova and Cámara (Reference Sokolova and Cámara2013), except for betalomonosovite (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015) and calciomirmanite (Sokolova and Cámara, Reference Sokolova and Cámara2017). The invariant core of the TS block, ![]() ${\rm {\bf M}}_{\rm \bf 2}^{\rm {\bf H}} {\rm \bf M}_{\rm \bf 4}^{\rm \bf O} $(Si2O7)2
${\rm {\bf M}}_{\rm \bf 2}^{\rm {\bf H}} {\rm \bf M}_{\rm \bf 4}^{\rm \bf O} $(Si2O7)2![]() ${\rm \bf X}_{\rm \bf 4}^{\rm \bf O} $, is shown in bold;
${\rm \bf X}_{\rm \bf 4}^{\rm \bf O} $, is shown in bold; ![]() ${\rm M}_{\rm 4}^{\rm O} $ and
${\rm M}_{\rm 4}^{\rm O} $ and ![]() ${\rm M}_{\rm 2}^{\rm H} $ = cations of the O and H sheets,
${\rm M}_{\rm 2}^{\rm H} $ = cations of the O and H sheets, ![]() ${\rm A}_{\rm 2}^{P} $ = cations at the peripheral (P) sites; (
${\rm A}_{\rm 2}^{P} $ = cations at the peripheral (P) sites; (![]() ${\rm X}_{{\rm M,A}}^{\rm O} $)4 = anions of the O sheet not bonded to Si: (
${\rm X}_{{\rm M,A}}^{\rm O} $)4 = anions of the O sheet not bonded to Si: (![]() ${\rm X}_{\rm M}^{\rm O} $)2 = anions at the common vertices of 3MO and MH polyhedra; (
${\rm X}_{\rm M}^{\rm O} $)2 = anions at the common vertices of 3MO and MH polyhedra; (![]() ${\rm X}_{\rm A}^{\rm O} $)2 = anions at the common vertices of 3MO and AP polyhedra (where AP–
${\rm X}_{\rm A}^{\rm O} $)2 = anions at the common vertices of 3MO and AP polyhedra (where AP–![]() ${\rm X}_{\rm A}^{\rm O} $ < 3 Å);
${\rm X}_{\rm A}^{\rm O} $ < 3 Å); ![]() ${\rm X}_{\rm M}^{P} $ and
${\rm X}_{\rm M}^{P} $ and ![]() ${\rm X}_{\rm A}^{P} $ = apical anions of MH and AP cations at the periphery of the TS block; coordination numbers are given for non-octahedral sites in the TS block; constituents of the I block are shown in turquoise (where XP anions are ligands of P5+ cations, they are considered as part of the I block).
${\rm X}_{\rm A}^{P} $ = apical anions of MH and AP cations at the periphery of the TS block; coordination numbers are given for non-octahedral sites in the TS block; constituents of the I block are shown in turquoise (where XP anions are ligands of P5+ cations, they are considered as part of the I block).
**Structure type: B (basic) (Sokolova and Cámara, Reference Sokolova and Cámara2013); (MG = Murmanite group).
† The most recent reference on the structure: (1) Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008); (2) Lykova et al. (Reference Lykova, Pekov, Chukanov, Belakovskiy, Yapaskurt, Zubkova, Britvin and Giester2016); (3) this work; (4) Cámara et al. (Reference Cámara, Sokolova, Abdu, Hawthorne and Khomyakov2013); (5) Sokolova et al. (Reference Sokolova, Hawthorne and Abdu2013); (6) Sokolova et al. (Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015); (7) Sokolova and Hawthorne (Reference Sokolova and Hawthorne2001); (8) Sokolova et al. (Reference Sokolova, Hawthorne and Khomyakov2005).
Vigrishinite was described from Mt. Malyi Punkaruaiv, Lovozero alkaline massif, Kola Peninsula, Russia (Pekov et al., Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013). They reported its chemical composition (Table 3), the ‘simplified’ formula Zn2Ti4−x(Si2O7)2(OH,H2O,□)8 (x < 1) with Z = 2 (Table 2) and its ‘structural model’ (R 1 = 17.07%). Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported chemical compositions for vigrishinite from the two other localities in the Lovozero alkaline massif (Table 3), refined the crystal structure of vigrishinite from Mt. Alluaiv (R 1 = 11.95%) and gave the ‘ideal endmember’ formula for vigrishinite as follows: Zn2Ti4−x(Si2O7)2O2(OH,F,O)2(H2O,OH,□)4 with x < 1 (Table 2). However this is not an end-member formula because it has more than one chemical species at more than one site (Hawthorne, Reference Hawthorne2002). Further on in the paper, we will refer to this formula as the formula of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b).

Fig. 1. Relation between unit cells assigned to murmanite: [1] (Khalilov, Reference Khalilov1989; Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) and [2] (Khalilov et al., Reference Khalilov, Mamedov, Makarov and P'yanzina1965); unit cells [1] → [2] are related by the transformation matrix (100 / 0![]() $\bar 1$0 /
$\bar 1$0 / ![]() $\bar 1$0
$\bar 1$0![]() $\bar 1$). See Table 2 and text for details.
$\bar 1$). See Table 2 and text for details.
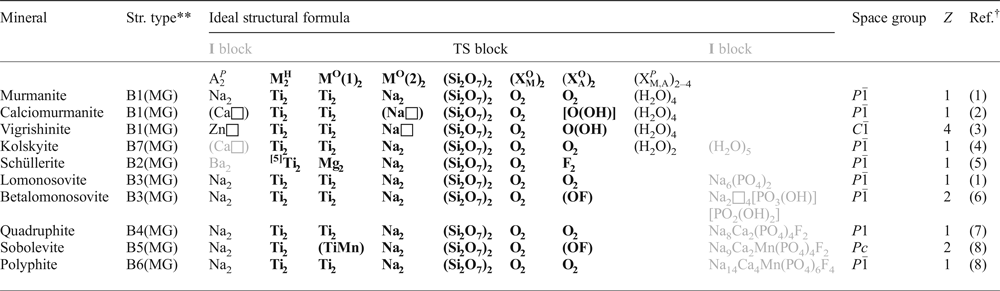
Table 2. Structural studies of murmanite and vigrishinite (previous work).*
*Samples (1,3,4,6,7) are from the Lovosero alkaline massif, Kola Peninsula, Russia; sample (2) is from Ilímaussaq, Greenland; (5) locality is unknown; unit-cell parameters are given to the third decimal;
** murmanite: ideal structural formula is given for (1,3,4) and end-member formula for (2); vigrishinite: formula (6,7);
*** for murmanite, unit cells [1] → [2] are related by the transformation matrix (100 0-10 -10-1) (see Fig. 1);
****unit cells [2] and [1] of vigrishinite are related as follows: a2= (a1 – b1)/2; b2 = (a1 + b1)/2; c2 = c1;
References: (1) Khalilov et al. (Reference Khalilov, Mamedov, Makarov and P'yanzina1965); (2) Karup-Møller (Reference Karup-Møller1986); (3) Khalilov (Reference Khalilov1989); (4) Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008); (5) Rastsvetaeva and Adrianov (Reference Rastsvetaeva and Andrianov1986); (6) Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013); (7) Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b).
Table 3. Chemical composition and unit formula* for vigrishinite.

(1) This work; (2) Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013), Mt. Malyi Punkaruaiv; (3–5) Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b): (3) Mt. Malyi Punkaruaiv,
(4) Mt. Alluaiv and (5) Mt. Karnasurt; n.a. = not analysed; n.d. = not detected; structure work done for (1,2,4).
*Formula calculated on (1) 22 (O + F) apfu, with OH + F = 1.96 pfu and H2O = 3.44 pfu and (2–5) Si + Al = 4 apfu; **calculated from crystal-structure refinement; ***modified Penfield method.
Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) stated that their structure-refinement results were better than those of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013), and hence the structure information of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) takes precedence over that of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013). Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) outlined the main features of the structural relation between vigrishinite and murmanite, ideally Na4Ti4(Si2O7)2O4(H2O)4 (Tables 1 and 2). (1) H sheet: in vigrishinite, the [6]A(1) site is occupied by Zn at 84% and two A(2) and A(2’) subsites are occupied by [4,5]Zn at 13 and 6%, respectively (Table 4); in murmanite, the [8]A site is occupied by Na; (2) O sheet: in vigrishinite, the [6]- and [5]-coordinated Na sites are occupied by Na at 70 and 50%, respectively (Table 4); in murmanite, one [6]-coordinated site is occupied by Na; (3) the a and b unit-cell parameters of vigrishinite correspond to the ab face diagonals of the unit cell of murmanite.
Table 4. Refined site-scattering, assigned site-populations and U eq for selected cation sites in vigrishinite after Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b).*

*Site-labelling in accord with Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b)
The structure work on murmanite and vigrishinite is summarized in Table 2. Khalilov et al. (Reference Khalilov, Mamedov, Makarov and P'yanzina1965) solved the crystal structure of murmanite and described its topology; their formula of murmanite is lacking Na2 apfu and hence has OH groups which are not common for murmanite. Although Karup-Møller (Reference Karup-Møller1986) did not refine the crystal structure of murmanite, he measured its unit-cell parameters and gave the end-member formula of murmanite, Na4Ti4(Si2O7)2O4(H2O)4, which is identical to the current ideal formula of the mineral (Sokolova and Cámara, Reference Sokolova and Cámara2017). Khalilov (Reference Khalilov1989) refined the crystal structure of murmanite in space group P1, reported the structure topology including a possible pattern of hydrogen bonding and wrote the structural formula of murmanite analogous to the end-member formula of Karup-Møller (Reference Karup-Møller1986) (Table 2). Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008) refined the crystal structure of murmanite in space group P ![]() $\bar 1$, confirmed the general structure topology and ideal chemical formula of murmanite of Khalilov (Reference Khalilov1989), localized the H atoms of the H2O groups and gave stereochemical details of the hydrogen bonding. Hence the correct space group for murmanite is P
$\bar 1$, confirmed the general structure topology and ideal chemical formula of murmanite of Khalilov (Reference Khalilov1989), localized the H atoms of the H2O groups and gave stereochemical details of the hydrogen bonding. Hence the correct space group for murmanite is P ![]() $\bar 1$ and elsewhere in the paper we will refer to the most recent work on murmanite by Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008). There are two choices of the unit cell for murmanite. Unit cell [2] was originally suggested by Khalilov et al. (Reference Khalilov, Mamedov, Makarov and P'yanzina1965) and then refined by Karup-Møller (Reference Karup-Møller1986). The unit cell [1] was chosen by Khalilov (Reference Khalilov1989) and subsequently used by Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008). The unit cells [1] and [2] of murmanite are related by the transformation matrix (100 / 0
$\bar 1$ and elsewhere in the paper we will refer to the most recent work on murmanite by Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008). There are two choices of the unit cell for murmanite. Unit cell [2] was originally suggested by Khalilov et al. (Reference Khalilov, Mamedov, Makarov and P'yanzina1965) and then refined by Karup-Møller (Reference Karup-Møller1986). The unit cell [1] was chosen by Khalilov (Reference Khalilov1989) and subsequently used by Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008). The unit cells [1] and [2] of murmanite are related by the transformation matrix (100 / 0![]() $\bar 1$0 /
$\bar 1$0 / ![]() $\bar 1$0
$\bar 1$0![]() $\bar 1$) (Fig. 1). For the most recent structure work of Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008), we give unit cells [1] and [2] (Table 2).
$\bar 1$) (Fig. 1). For the most recent structure work of Cámara et al. (Reference Cámara, Sokolova, Hawthorne and Abdu2008), we give unit cells [1] and [2] (Table 2).
The crystal structure of vigrishinite was solved by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986). As they did not have any chemical data for the single crystal they were working on, they were not aware that they had solved the crystal structure of a new mineral, not just reported a new structure-refinement of murmanite. Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) reported the C-centred unit cell for their ‘murmanite’ (unit cell [1], Table 2) and discussed the doubling of the a and b unit-cell parameters compared to those of murmanite (unit cell [2], Khalilov et al., Reference Khalilov, Mamedov, Makarov and P'yanzina1965). In their murmanite, Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) described the HOH layer similar to the one in murmanite of Khalilov et al. (Reference Khalilov, Mamedov, Makarov and P'yanzina1965), with the O sheet composed of Ti octahedra and [6]- and [5]-coordinated Na(1–4) polyhedra partly occupied by Na at 50%. In the H sheet, they identified the “most compact Na(5) octahedron with cation–anion distances 1.98 to 2.19 Å, mean 2.09 Å” and the “maximal electron density” requiring it “to be populated by heavy cations Mn, Fe2+, Zr, Nb and to the least degree, Na” (cf. the [6]A(1) site occupied by 0.84 Zn apfu, with bond lengths in the range from 2.086 to 2.258 Å, mean 2.14 Å; Lykova et al., Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b). Taking into account the poor quality of the vigrishinite crystals and the lack of a chemical analysis, Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) reported an adequate model of the crystal structure of vigrishinite.
It seems that Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) did not realize that Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) solved the crystal structure of vigrishinite under the name murmanite. Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) compared identical unit cells of vigrishinite and ‘murmanite’ of Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986), designating the latter as “the second triclinic variety of murmanite” [Table 2, unit cell [2] by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986)].
There is certain disagreement between the crystal-structure refinement results, chemical composition and the chemical formulae of vigrishinite based on the work of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b):
(1) Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported full occupancy for the four Ti-dominant sites in vigrishinite (Table 4), but their revised formula of vigrishinite contains Ti4–x, x < 1: Zn2Ti4–x(Si2O7)2O2(OH,F,O)2(H2O,OH,□)4 with x < 1. Note that in TS-block minerals, Ti-dominant sites are always fully occupied (cf. Sokolova and Cámara, Reference Sokolova and Cámara2017).
(2) Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported chemical analyses of vigrishinite where Zn varies from 0.82 to 1.09 apfu [Table 3, analyses (2–5)] and, in accord with their structure-refinement results, only one site is occupied by Zn at 84%, ideally giving one Zn apfu, and yet the formula has Zn2 apfu.
(3) Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported two Na sites occupied at 72 and 50% (Table 4), and chemical analyses give Na from 0.23 to 1.21 apfu [Table 3, analyses (2–5)]. However, their formula of vigrishinite does not contain any Na (Table 2).
Vigrishinite was refined in the unit cell based on the two diagonals of the planar cell based on translation vectors t1 and t2: avig = – t1 – t2 and bvig = – t1 + t2, whereas the forty-four TS-block minerals of the seidozerite supergroup have unit cells based on translation vectors t1 and t2 (Sokolova and Cámara, Reference Sokolova and Cámara2017). Such description of the crystal structure of vigrishinite complicates comparison of vigrishinite to murmanite and other TS-block structures.
The seidozerite supergroup has four groups of TS-block minerals which are defined quantitatively on the content of Ti (see above). We have re-examined vigrishinite as we wish to: (1) relate murmanite and vigrishinite via the substitution mechanism in a quantitative way; (2) understand the doubling of the t 1 and t 2 translations; and (3) write its chemical formula such that it agrees with the chemical analyses and structure-refinement results.
Here, we report the chemical composition and the refinement of the structure of vigrishinite in space group C ![]() $\bar 1$ for better comparison with all other TS-block structures, explain the doubling of the t 1 and t 2 translations of vigrishinite relative to those translations in murmanite, and revise the chemical formula of vigrishinite.
$\bar 1$ for better comparison with all other TS-block structures, explain the doubling of the t 1 and t 2 translations of vigrishinite relative to those translations in murmanite, and revise the chemical formula of vigrishinite.
Description of the sample
We obtained a ‘vigrishinite’ sample from an American mineral collector. This sample comes from the type locality, Mt. Malyi Punkaruaiv, Lovozero alkaline massif, Kola Peninsula, Russia (Pekov et al., Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013). From those fragments, we cut five crystals and based on the unit-cell parameters we identified four crystals as vigrishinite and one crystal as zvyaginite, ideally Na2ZnTiNb2(Si2O7)2O2(OH)2(H2O)4, a lamprophyllite-group mineral (Pekov et al., Reference Pekov, Lykova, Chukanov, Yapaskurt, Belakovskiy, Zolotarev and Zubkova2014; Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017). We collected single-crystal X-ray data for all four vigrishinite crystals. The crystals of vigrishinite are transparent colourless thin plates. In this paper, we report the structure-refinement results for crystal #3. Parts of the fragments from which crystals #2 and #3 had been cut were subsequently used for microprobe analysis.
Chemical analysis
The crystals of vigrishinite used for the microprobe analysis are plates measuring 0.060 mm × 0.060 mm × 0.005 mm and 0.120 mm × 0.040 mm × 0.005 mm. The crystals were analysed with a Cameca SX-100 electron-microprobe operating in wavelength-dispersion mode with an accelerating voltage of 15 kV, a specimen current of 5 nA, a beam size of 10 µm and count times on peak and background of 20 and 10 s, respectively. The following standards were used: Si and Ca: diopside; Al: andalusite; F: fluoro-riebeckite; Na: albite; Nb: Ba2NaNb5O15; Ta: Mn(Ta1.70Nb0.30)O6; Zr: zircon; Mg: forsterite, Fe: fayalite; Mn: spessartine; Zn: gahnite; Ti: titanite; K: orthoclase; Ba: baryte; and P: apatite. Strontium was sought but not detected. Data were reduced using the φ(ρZ) procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). The chemical composition of vigrishinite is the mean of 19 determinations and is given in Table 3. Our chemical analysis of vigrishinite is close to that of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013), especially for the ZnO content, 14.65 vs. 14.39 wt.% [Table 3, analyses (1) and (2)]. The empirical formula of vigrishinite, calculated on the basis of 22 (O + F), with two constraints derived from the crystal-structure refinement, OH + F = 1.96 pfu and H2O = 3.44 pfu, is: (Na0.67Zn0.21Ca0.05□1.07)Σ2(Zn0.86□1.14)Σ2 (Zn0.14□0.36)Σ0.5(Ti2.60Nb0.62Mn0.30![]() ${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14)[O2.60(OH)1.21 F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} with Z = 4. The ideal formula is NaZnTi4(Si2O7)2O3(OH)(H2O)4. It seems unlikely that cations {Zn0.23P0.03K0.03Ba0.02} belong to vigrishinite itself (see text below). We suggest that these cations belong to other phases which form intergrowths with vigrishinite. Intimate intergrowths are very common for TS-block minerals: our work using high-resolution transmission electron microscopy on the murmanite-group minerals lomonosovite and betalomonosovite (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015), and zvyaginite, a lamprophyllite-group mineral (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017), shows that these three TS-block minerals contain intergrown phases.
${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14)[O2.60(OH)1.21 F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} with Z = 4. The ideal formula is NaZnTi4(Si2O7)2O3(OH)(H2O)4. It seems unlikely that cations {Zn0.23P0.03K0.03Ba0.02} belong to vigrishinite itself (see text below). We suggest that these cations belong to other phases which form intergrowths with vigrishinite. Intimate intergrowths are very common for TS-block minerals: our work using high-resolution transmission electron microscopy on the murmanite-group minerals lomonosovite and betalomonosovite (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015), and zvyaginite, a lamprophyllite-group mineral (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017), shows that these three TS-block minerals contain intergrown phases.
X-ray data collection and structure refinement
We collected single-crystal X-ray data for vigrishinite crystals 1, 2, 3 and 4 and refined their crystal structures using atom coordinates of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) to R 1 = 16.41, 10.92, 12.20 and 13.93%, respectively. Although crystal #2 was refined to R 1 = 10.92%, R int was 7.91% and U ij of all atoms were non-positive definite (NPD). Hence we chose crystal #3 (R 1 = 12.20%) for further refinement. We felt that the refinement of the structure of vigrishinite using the unit cell based on t 1 and t 2 translations would give us an opportunity to better understand the relation between vigrishinite and murmanite. We used the transformation matrix (![]() $\bar 1$
$\bar 1$![]() $\bar 1$0 /
$\bar 1$0 / ![]() $\bar 1$10 / 00
$\bar 1$10 / 00![]() $\bar 1$) to go from the unit cell of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) to the unit cell based on t 1 and t 2 translations, space group C
$\bar 1$) to go from the unit cell of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) to the unit cell based on t 1 and t 2 translations, space group C ![]() $\bar 1$ (Table 5, in accord with the unit cell [1] of Rastsvetaeva and Andrianov, Reference Rastsvetaeva and Andrianov1986, Table 2). The unit cell of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) is a reduced cell with regard to the unit cell with space group C
$\bar 1$ (Table 5, in accord with the unit cell [1] of Rastsvetaeva and Andrianov, Reference Rastsvetaeva and Andrianov1986, Table 2). The unit cell of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) is a reduced cell with regard to the unit cell with space group C ![]() $\bar 1$. Below we give details of data collection and structure refinement using the C
$\bar 1$. Below we give details of data collection and structure refinement using the C ![]() $\bar 1$ setting.
$\bar 1$ setting.
Table 5. Miscellaneous structure-refinement data for vigrishinite.*

* The two unit-cell parameters of vigrishinite [1] are doubled where compared to the unit cells [2] or [1] of murmanite (Table 2).
**The unit cell [2] of vigrishinite, space group P ![]() $\bar 1$, is a reduced unit cell equivalent to the unit cell of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) (Table 2).
$\bar 1$, is a reduced unit cell equivalent to the unit cell of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) (Table 2).
To transform unit cell [2] into unit cell [1], we use matrix (![]() $\bar 1$
$\bar 1$![]() $\bar 1$0 /
$\bar 1$0 / ![]() $\bar 1$10 / 00
$\bar 1$10 / 00![]() $\bar 1$).
$\bar 1$).
***Calculated from the empirical formula.
X-ray data for vigrishinite were collected for crystal #3 with a Bruker APEX II ULTRA three-circle diffractometer equipped with a rotating-anode generator (MoKα), multilayer optics and an APEX II 4 K CCD detector. Details of data collection and structure refinement are given in Table 5. The intensities of reflections with –13 ≤ h ≤ 13, –17 ≤ k ≤ 17, –15 ≤ l ≤ 15 were collected with a frame width of 0.5° and a frame time of 20 s, and an empirical absorption correction (SADABS, Sheldrick, Reference Sheldrick2008) was applied. The crystal structure of vigrishinite was refined in space group C ![]() $\bar 1$ to R 1 = 12.52% with the Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2008). The occupancies of nine sites were refined with the following scattering curves: M H(1,2) and M O(1,2) sites: Ti; M O(3,4) sites: Na; AP(1,2) and A I sites (see discussion on the latter site below): Zn. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). At the last stages of the refinement, eleven subsidiary peaks MH(1A,2A,2B) (scattering curve of Ti), Si(1A, 3A, 4A) (scattering curve of Si) and Zn(A,B,C,D,E) (scattering curve of Zn) were included in the refinement; these are probably due to the presence of other minerals intergrown with vigrishinite. Final atom coordinates and equivalent displacement parameters are given in Table 6, selected interatomic distances and angles in Table 7, refined site-scattering values and assigned site-populations in Table 8, and bond-valence values for selected anions in Tables 9 and 10. A list of observed and calculated structure factors, crystallography information file (CIF) and anisotropic displacement parameters have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
$\bar 1$ to R 1 = 12.52% with the Bruker SHELXTL Version 5.1 (Sheldrick, Reference Sheldrick2008). The occupancies of nine sites were refined with the following scattering curves: M H(1,2) and M O(1,2) sites: Ti; M O(3,4) sites: Na; AP(1,2) and A I sites (see discussion on the latter site below): Zn. Scattering curves for neutral atoms were taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). At the last stages of the refinement, eleven subsidiary peaks MH(1A,2A,2B) (scattering curve of Ti), Si(1A, 3A, 4A) (scattering curve of Si) and Zn(A,B,C,D,E) (scattering curve of Zn) were included in the refinement; these are probably due to the presence of other minerals intergrown with vigrishinite. Final atom coordinates and equivalent displacement parameters are given in Table 6, selected interatomic distances and angles in Table 7, refined site-scattering values and assigned site-populations in Table 8, and bond-valence values for selected anions in Tables 9 and 10. A list of observed and calculated structure factors, crystallography information file (CIF) and anisotropic displacement parameters have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 6. Atom coordinates and equivalent displacement parameters for vigrishinite, space group C ![]() $\bar 1$.
$\bar 1$.

*U iso
Table 7. Selected interatomic distances (Å) and angles (°) in vigrishinite.

φ = O, F, OH or H2O;
Symmetry operators: a: –x+3/2, –y+½, –z + 1; b: –x + 1, –y, –z + 1; c: –x + 2, –y, –z + 1; d: x, y–1, z; e: –x + 2, –y + 1, –z + 1; f: x+½, y+½, z; g: –x + 1, –y + 1, –z + 1; h: x–½, y–½, z; i: –x+½, –y+½, z; j: x–1, y, z; k: x + 1, y, z; l: x, y + 1, z; m: –x+3/2, –y+3/2, –z + 1.
Table 8. Refined site-scattering and assigned site-populations for vigrishinite.

*Coordination numbers are shown for non-[6]-coordinated cation sites and non-4-coordinated anion sites and H2O groups; [ ] corresponding sites in Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b); φ = O, F, OH or H2O.
**Anions which do not coordinate Si.
Table 9. Bond-valence values for selected anions* in vigrishinite.

* anions which do not coordinate Si; bond-valence parameters (vu) are from Brown (Reference Brown, O'Keeffe and Navrotsky1981); bonds to oxygen were used for Ti [MO(1,2), MH(1,2)]; Na [MO(3)]; Na and Zn [MO(4)] and Zn [AP(1), AI]; site occupancies of cation sites were taken into account for all calculations.
Table 10. Bond-valence values for selected anions* involved in short-range order in vigrishinite.

*Bond-valence parameters (vu) are from Brown (Reference Brown, O'Keeffe and Navrotsky1981).
Site-population assignment
There are twelve cation sites in the crystal structure of vigrishinite: the M H(1,2), AP(1) and four Si sites of the H sheet; four M O sites of the O sheet and the interstitial A I site; except for the latter site, labelling follows Sokolova (Reference Sokolova2006).
The MH sites
In the seidozerite-supergroup minerals, Ti-dominant sites are always fully occupied (Sokolova, Reference Sokolova2006; Sokolova and Cámara, Reference Sokolova and Cámara2017). In the murmanite-group minerals, Ti = 4 apfu; in the H sheet, Ti = 2 apfu; in the O sheet, Ti = 2 apfu (Table 1) and Ti-dominant sites in the O sheet commonly contain divalent cations such as Mn, Fe2+ and Mg (Sokolova, Reference Sokolova2006; Sokolova and Cámara, Reference Sokolova and Cámara2017). In vigrishinite, the two [6]M H sites in the H sheet have refined site-scattering values of 24.1(4) and 23.3(6) epfu and equal mean bond lengths of 1.97 Å (Table 8) and we assign mainly Ti plus some Nb and minor Al to those two sites. In the O sheet, the refined site-scattering values at the M O(1) and M O(2) sites are 25.7(4) and 26.4(4) epfu and the mean bond lengths around those sites are 2.02 and 2.01 Å (Table 8); we assign remaining Ti and Nb plus Mn0.30![]() ${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Ta0.01 to the M O(1) and M O(2) sites, with calculated site-scattering values of 26.72 and 27.11 epfu (Table 8). Hence the four Ti-dominant sites are fully occupied and there is good correlation between the refined and calculated site-scattering values: 99.5 and 102.31 epfu, respectively.
${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06Zn0.05Ta0.01 to the M O(1) and M O(2) sites, with calculated site-scattering values of 26.72 and 27.11 epfu (Table 8). Hence the four Ti-dominant sites are fully occupied and there is good correlation between the refined and calculated site-scattering values: 99.5 and 102.31 epfu, respectively.
The MO sites
The two M O(3,4) sites occur in the O sheet (Fig. 2a). The refined site-scattering at the [6]M O(3) site, 8.4(7) epfu, is slightly higher than 6.1(3) epfu at the [5]M O(4) site (Table 8), and the mean bond length for the M O(3) site, 2.37 Å, is slightly longer than 2.33 Å for the M O(4) site (Table 7). There is a short distance of 2.47 Å between two MO(4) atoms related by the inversion centre (Table 7) and hence the M O(4) site must be occupied at ≤50%. In murmanite (Fig. 2b), the corresponding site is occupied by Na1.55Mn0.14Ca0.06□0.25 pfu, with mean bond length of 2.468 Å (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008). Chemical analysis gives 0.67 Na apfu (Table 3) to assign to the two M O(3,4) sites in vigrishinite; mean bond lengths for these two sites, 2.37 and 2.33 Å, are shorter than the corresponding value in murmanite and indicate that smaller cations substitute for Na at the M O(3,4) sites, e.g. Ca ([6]r = 1.00 Å, Shannon, Reference Shannon1976) and Zn ([6]r = 0.74, [5]r = 0.68 Å). We assign Na0.51Zn0.06Ca0.05□0.38 pfu to the M O(3) site and Na0.16Zn0.15□0.69 pfu to the M O(4) site, with calculated site-scattering values of 8.41 and 6.30 epfu, respectively (Table 8). Similar substitution of Zn for Na at the Na-dominant site in the O sheet (mean bond length of 2.42 Å) was reported for zvyaginite (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017).

Fig. 2. The details of the TS block: the O sheet of Ti-dominant MO(1,2) octahedra and Na-dominant MO(3) octahedra [M O(4) sites are occupied by Na and Zn at less than 50%] in vigrishinite (a) and the O sheet of Na and Ti octahedra in murmanite (b); the H sheet of Si2O7 groups, Ti-dominant MH(1,2) octahedra and Zn-dominant AP(1) octahedra (86% occupancy) in vigrishinite (c) and the H sheet of Si2O7 groups, Ti-dominant octahedra and [8]-coordinated Na-dominant polyhedra (98% occupancy) in murmanite (d); the TS block in vigrishinite (e) and murmanite (f). Si tetrahedra are orange, Ti-dominant octahedra are yellow; Na-dominant and Zn-dominant octahedra are navy blue and purple, OH groups at the ![]() ${X}_{\rm A}^{\rm O} $ sites are shown as small red spheres, H2O groups at the XP sites are shown as large red spheres, cation sites with less than 50% occupancy by Na [M O(4) site] and Zn (A I site) are shown as navy blue and purple spheres, respectively. The unit cell is shown by thin black lines in (a–d).
${X}_{\rm A}^{\rm O} $ sites are shown as small red spheres, H2O groups at the XP sites are shown as large red spheres, cation sites with less than 50% occupancy by Na [M O(4) site] and Zn (A I site) are shown as navy blue and purple spheres, respectively. The unit cell is shown by thin black lines in (a–d).
The AP sites
The [6]AP(1) site in the H sheet has a refined site-scattering value of 25.8(4) epfu and a mean bond length of 2.13 Å (Fig. 2c, Table 8). We assign Zn0.86□0.14 pfu to the AP(1) site, with a calculated site-scattering value of 25.8 epfu (Table 8).
We observed two peaks with site-scattering of 2.51 and 1.96 epfu around the AP(2) site in our structure of vigrishinite (Fig. 2c). For convenience of discussion in this section of the paper, we denote these two peaks as being at the sites AP(21) and AP(22). These peaks are 1.02(4) Å apart and have five and three distances to O atoms and H2O groups in the range AP(21): 2.02–2.26 and AP(22): 1.96–2.08 Å, respectively. The five O atoms around the AP(21) site form a tetragonal pyramid, and the AP(21) site occurs in the basal plane of the pyramid. The four O atoms around the AP(22) site form a square, and the AP(22) site occurs in the centre of that square. Therefore the anion coordination of the AP(21) and AP(22) sites is not complete. The calculated ionic radii of possible cations at the AP(21) and AP(22) sites are 0.77 and 0.73 Å.
Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) assigned minor Zn to the two [5]A(2’) and [4]A(2) sites (Table 4) which correspond to the AP(21) and AP(22) sites in our structure of vigrishinite. Based on the structure-refinement results of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b), the calculated ionic radii of Zn at the A(2’) and A(2) sites are 0.82 and 0.74 Å, respectively.
The calculated values of ionic radii both at the AP(21) and AP(22) sites in our structure of vigrishinite and at the A(2’) and A(2) sites in the structure of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) (see above) do not accord with the Shannon (Reference Shannon1976) radii for Zn: [5]r = 0.68, [4]r = 0.60 Å.
For two reasons: (1) incomplete coordination sphere of anions; and (2) disagreement between observed and calculated values of ionic radii, we do not assign Zn to the AP(21) and AP(22) sites and consider two peaks with site-scattering of 2.51 and 1.96 epfu around the AP(2) site as subsidiary peaks Zn(A) and Zn(B). Subsidiary peaks are probably due to the presence of another mineral (or minerals) which is intergrown with vigrishinite. Hence we assign a vacancy to the AP(2) site (Fig. 2c), and the AP(21) and AP(22) sites will not be considered further on in the paper. Below, we explain why Zn does not occur at the AP(2) site in vigrishinite.
The AI site
The A I site has a multiplicity of 0.5 pfu (Fig. 2e). For this site, Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported refined site-scattering values of 9.5 and 1.6 epfu and assigned site populations of Zn0.28Mg0.14□0.10 and Ca0.08□0.42 pfu, respectively [B(1) site, Table 4]. Here, the [6]A I site has a refined site-scattering value of 4.3(2) epfu and a mean bond length of 2.24 Å and we assign Zn0.14□0.36 pfu to the A I site, with calculated site-scattering value of 4.2 epfu (Table 8).
Unassigned cations
We are left with unassigned Zn0.24P0.03K0.03Ba0.02 pfu (Table 3) which we ascribe to the presence of another mineral (or minerals) intergrown with vigrishinite. In the structure of vigrishinite, there is no site that can accommodate P, K and Ba atoms so that they are properly coordinated by anions. Cations Ba2+ ([9]r = 1.47, [10]r = 1.52 Å) and K+ ([9]r = 1.55, [10]r = 1.59 Å) are too large and P5+ ([4]r = 0.17 Å) is too small to substitute for any cation in the crystal structure of vigrishinite. Note that Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) reported the presence of K, Ba and P in samples (2–5), (2) and (3,4) of vigrishinite, respectively (Table 3), but did not assign those cations either (Table 4). There is additional Zn0.24 pfu in the unit formula calculated from our chemical analysis (Table 3) that cannot be assigned to the M, AP(1) and A I sites as it exceeds the scattering observed at those sites.
Description of the structure
Cation and anion sites
Here we consider twelve cation sites in the crystal structure of vigrishinite: the M H(1,2), AP(1) and four Si sites of the H sheet; four M O sites of the O sheet and the interstitial A I site; and eight anion sites: two ![]() ${X}_{\rm M}^{\rm O} $ = anion sites at the common vertices of 3MO and MH polyhedra; two
${X}_{\rm M}^{\rm O} $ = anion sites at the common vertices of 3MO and MH polyhedra; two ![]() ${X}_{\rm A}^{\rm O} $ = anion sites at the common vertices of 3MO and AP polyhedra or 3MO polyhedra where the AP(2) site is vacant; three
${X}_{\rm A}^{\rm O} $ = anion sites at the common vertices of 3MO and AP polyhedra or 3MO polyhedra where the AP(2) site is vacant; three ![]() ${X}_{{\rm M,A}}^{P} $ = anion sites at the apical vertices of two MH octahedra and one AP(1) octahedron at the periphery of the TS block and the
${X}_{{\rm M,A}}^{P} $ = anion sites at the apical vertices of two MH octahedra and one AP(1) octahedron at the periphery of the TS block and the ![]() ${X}_{\rm A}^{P} $(2) site above the vacant AP(2) site; labelling is in accord with Sokolova (Reference Sokolova2006).
${X}_{\rm A}^{P} $(2) site above the vacant AP(2) site; labelling is in accord with Sokolova (Reference Sokolova2006).
In the O sheet, the Ti-dominant M O(1,2) sites (Table 8) are coordinated by four O atoms, an (O,OH) anion (O > OH) at the ![]() ${X}_{\rm A}^{\rm O} $(1) site and an OH group at the
${X}_{\rm A}^{\rm O} $(1) site and an OH group at the ![]() ${X}_{\rm A}^{\rm O} $(2) site (Fig. 2a). The ideal composition of the two M O(1,2) sites is Ti2 apfu. In murmanite, the Ti-dominant site is coordinated by six O atoms (Fig. 4b). The M O(3) site is 62% occupied primarily by Na plus minor Zn and Ca (Table 8, Fig. 2a), and is coordinated by five O atoms and an (O,OH) anion (O > OH) at the
${X}_{\rm A}^{\rm O} $(2) site (Fig. 2a). The ideal composition of the two M O(1,2) sites is Ti2 apfu. In murmanite, the Ti-dominant site is coordinated by six O atoms (Fig. 4b). The M O(3) site is 62% occupied primarily by Na plus minor Zn and Ca (Table 8, Fig. 2a), and is coordinated by five O atoms and an (O,OH) anion (O > OH) at the ![]() ${X}_{\rm A}^{\rm O} $(1) site, with < MO(3)–φ> = 2.37 Å (φ = anion and/or H2O group) (Table 7). The ideal composition of the M O(3) site is Na apfu. The □-dominant [5]M O(4) site is less than 50% occupied by Na and Zn (Table 8, Fig. 2a). The M O(4) site is coordinated by four O atoms and an OH group at the
${X}_{\rm A}^{\rm O} $(1) site, with < MO(3)–φ> = 2.37 Å (φ = anion and/or H2O group) (Table 7). The ideal composition of the M O(3) site is Na apfu. The □-dominant [5]M O(4) site is less than 50% occupied by Na and Zn (Table 8, Fig. 2a). The M O(4) site is coordinated by four O atoms and an OH group at the ![]() ${X}_{\rm A}^{\rm O} $(2) site, with < MO(4)–φ> = 2.33 Å (Table 7); the sixth O atom occurs at a distance of 2.93(2) Å. The [5]Na atoms in the O sheet were reported for betalomonosovite, another murmanite-group mineral (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015). The ideal composition of the M O(4) site is □ pfu (Table 8). In murmanite, the Na atom is coordinated by six O atoms (Fig. 2b). The sum of the cations at the M O(1–4) sites gives the ideal composition of the O sheet as Na□Ti2 pfu.
${X}_{\rm A}^{\rm O} $(2) site, with < MO(4)–φ> = 2.33 Å (Table 7); the sixth O atom occurs at a distance of 2.93(2) Å. The [5]Na atoms in the O sheet were reported for betalomonosovite, another murmanite-group mineral (Sokolova et al., Reference Sokolova, Abdu, Hawthorne, Genovese, Cámara and Khomyakov2015). The ideal composition of the M O(4) site is □ pfu (Table 8). In murmanite, the Na atom is coordinated by six O atoms (Fig. 2b). The sum of the cations at the M O(1–4) sites gives the ideal composition of the O sheet as Na□Ti2 pfu.
In the H sheet, there are four tetrahedrally coordinated Si(1–4) sites occupied by Si. There are two Ti-dominant [6]M H(1,2) sites; each M H site is coordinated by five O atoms and an H2O group at the ![]() ${X}_{\rm M}^{P} $ site as in murmanite (Figs 4c,d). The M H(1,2) sites ideally give Ti2 apfu. The [6]AP(1) site is 86% occupied by Zn; ideally it gives Zn apfu (Table 8). Zinc at the AP(1) site is coordinated by four O atoms, an (O,OH) anion (O > OH) at the
${X}_{\rm M}^{P} $ site as in murmanite (Figs 4c,d). The M H(1,2) sites ideally give Ti2 apfu. The [6]AP(1) site is 86% occupied by Zn; ideally it gives Zn apfu (Table 8). Zinc at the AP(1) site is coordinated by four O atoms, an (O,OH) anion (O > OH) at the ![]() ${X}_{\rm A}^{\rm O} $(1) site and an H2O group at the
${X}_{\rm A}^{\rm O} $(1) site and an H2O group at the ![]() ${X}_{\rm A}^{P} $(1) site, with <AP(1)–φ> = 2.13 Å (Table 7). The AP(2) site is vacant (Table 8, Fig. 2c). The ideal composition of the AP + M H sites is Zn□Ti2 pfu.
${X}_{\rm A}^{P} $(1) site, with <AP(1)–φ> = 2.13 Å (Table 7). The AP(2) site is vacant (Table 8, Fig. 2c). The ideal composition of the AP + M H sites is Zn□Ti2 pfu.
We write the cation part of the TS block as the sum of cations of the 2H and O sheets: ideally Zn□Ti2Na□Ti2 pfu, with a total charge of 19+.
The [6]A I site is occupied 28% by Zn; ideally it gives □0.5 pfu (Table 8, Fig. 2e). Zinc at the A I site is coordinated by two O atoms and H2O and OH groups at the four XP (M,A) sites (see discussion below), with < AI –φ> = 2.24 Å (Table 7). There is no such interstitial site in murmanite (Fig. 4f). We do not count the contribution of this site towards the cation part of the ideal formula as its occupancy is <50% and it does not affect the topology of vigrishinite when compared to murmanite.
The four Si(1–4) atoms and fourteen O(1–14) atoms that coordinate the Si atoms give (Si2O7)2 pfu (Tables 6,7). Anions at the ![]() ${X}_{\rm M}^{\rm O} $(1 and 2) sites receive bond valences from four cations: MH(1 and 2); MO(2 and 1); 2MO(4); and 2MO(3), with total bond-valence sums of 1.80 and 1.81 vu (valence units) (Table 9) and they are O atoms, giving O2 apfu (Table 8). Anions at the [4]
${X}_{\rm M}^{\rm O} $(1 and 2) sites receive bond valences from four cations: MH(1 and 2); MO(2 and 1); 2MO(4); and 2MO(3), with total bond-valence sums of 1.80 and 1.81 vu (valence units) (Table 9) and they are O atoms, giving O2 apfu (Table 8). Anions at the [4]![]() ${X}_{\rm A}^{\rm O} $(1) and [3]
${X}_{\rm A}^{\rm O} $(1) and [3]![]() ${X}_{\rm A}^{\rm O} $(2) sites receive bond valences from four and three cations, respectively. The [4]
${X}_{\rm A}^{\rm O} $(2) sites receive bond valences from four and three cations, respectively. The [4]![]() ${X}_{\rm A}^{\rm O} $(1) anion receives bond valences from MO(1,2,3) and AP(1) cations, with total bond-valence sum of 1.52 vu (Table 9). Note that M O(3) and AP(1) sites are occupied by Na and Zn at 62 and 86%, respectively. Consider the possible short-range-order (SRO) arrangements around the
${X}_{\rm A}^{\rm O} $(1) anion receives bond valences from MO(1,2,3) and AP(1) cations, with total bond-valence sum of 1.52 vu (Table 9). Note that M O(3) and AP(1) sites are occupied by Na and Zn at 62 and 86%, respectively. Consider the possible short-range-order (SRO) arrangements around the ![]() ${\rm X}_{\rm A}^{\rm O} $(1) anion. SRO~60% occurs where the M O(3) and AP(1) sites are fully occupied by Na and Zn, i.e. the [4]
${\rm X}_{\rm A}^{\rm O} $(1) anion. SRO~60% occurs where the M O(3) and AP(1) sites are fully occupied by Na and Zn, i.e. the [4]![]() ${\rm X}_{\rm A}^{\rm O} $(1) anion receives a total bond valence of 1.63 vu (Table 10) and is an O atom, i.e. the [4]
${\rm X}_{\rm A}^{\rm O} $(1) anion receives a total bond valence of 1.63 vu (Table 10) and is an O atom, i.e. the [4]![]() ${X}_{\rm A}^{\rm O} $(1) site gives 0.60 O apfu. SRO ~ 40% occurs where the M O(3) site is vacant and the AP(1) site is 50% occupied by Zn and 50% vacant. Hence the [3]
${X}_{\rm A}^{\rm O} $(1) site gives 0.60 O apfu. SRO ~ 40% occurs where the M O(3) site is vacant and the AP(1) site is 50% occupied by Zn and 50% vacant. Hence the [3]![]() ${\rm X}_{\rm A}^{\rm O} $(1) anion receives a total bond valence of 1.43–1.16 vu (Table 10) and is a monovalent anion: an OH group or F, i.e. the [3]
${\rm X}_{\rm A}^{\rm O} $(1) anion receives a total bond valence of 1.43–1.16 vu (Table 10) and is a monovalent anion: an OH group or F, i.e. the [3]![]() ${X}_{\rm A}^{\rm O} $(1) site gives (OH, F)0.40 pfu. We assign O0.60(OH)0.21F0.19 to the
${X}_{\rm A}^{\rm O} $(1) site gives (OH, F)0.40 pfu. We assign O0.60(OH)0.21F0.19 to the ![]() ${X}_{\rm A}^{\rm O} $(1) site, ideally O1.00 apfu (Table 8). The O atom at the
${X}_{\rm A}^{\rm O} $(1) site, ideally O1.00 apfu (Table 8). The O atom at the ![]() ${X}_{\rm A}^{\rm O} $(2) site receives a total bond valence of 1.31 vu (Table 9) and we assign an OH group to the
${X}_{\rm A}^{\rm O} $(2) site receives a total bond valence of 1.31 vu (Table 9) and we assign an OH group to the ![]() ${X}_{\rm A}^{\rm O} $(2) site (Table 8). The
${X}_{\rm A}^{\rm O} $(2) site (Table 8). The ![]() ${X}_{\rm A}^{\rm O} $(1) and
${X}_{\rm A}^{\rm O} $(1) and ![]() ${X}_{\rm A}^{\rm O} $(2) sites ideally give O(OH) pfu. The four (
${X}_{\rm A}^{\rm O} $(2) sites ideally give O(OH) pfu. The four (![]() ${X}_{{\rm M,A}}^{\rm O} $)4 sites ideally give O3(OH) pfu.
${X}_{{\rm M,A}}^{\rm O} $)4 sites ideally give O3(OH) pfu.
Consider the four ![]() ${X}_{{\rm (M,A)}}^{P} $ sites at the periphery of the TS block (Figs 2e,3). Figure 3 shows a possible pattern of hydrogen bonding between H2O groups at the
${X}_{{\rm (M,A)}}^{P} $ sites at the periphery of the TS block (Figs 2e,3). Figure 3 shows a possible pattern of hydrogen bonding between H2O groups at the ![]() ${X}_{{\rm (M,A)}}^{P} $ sites of two adjacent TS blocks. This pattern is similar to that in murmanite (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008), epistolite [ideally Na4TiNb2(Si2O7)2O2(OH)2(H2O)4, Sokolova and Hawthorne, Reference Sokolova and Hawthorne2004] and zvyaginite (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017). In these structures, H2O groups form a ribbon which extends along a (t1). The O atom of the H2O group at the
${X}_{{\rm (M,A)}}^{P} $ sites of two adjacent TS blocks. This pattern is similar to that in murmanite (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008), epistolite [ideally Na4TiNb2(Si2O7)2O2(OH)2(H2O)4, Sokolova and Hawthorne, Reference Sokolova and Hawthorne2004] and zvyaginite (Sokolova et al., Reference Sokolova, Genovese, Falqui, Hawthorne and Cámara2017). In these structures, H2O groups form a ribbon which extends along a (t1). The O atom of the H2O group at the ![]() ${X}_{\rm M}^{P} $(2) site receives 0.37 vu from Ti at the M H(2) site (Table 9, Fig. 3) and we assign an H2O group to the
${X}_{\rm M}^{P} $(2) site receives 0.37 vu from Ti at the M H(2) site (Table 9, Fig. 3) and we assign an H2O group to the ![]() ${X}_{\rm M}^{P} $(2) site (Table 8). The
${X}_{\rm M}^{P} $(2) site (Table 8). The ![]() ${X}_{\rm A}^{P} $(2) site occurs just above the vacant AP(2) site and is not bonded to any cation (Figs 2c,3); it splits into two subsites,
${X}_{\rm A}^{P} $(2) site occurs just above the vacant AP(2) site and is not bonded to any cation (Figs 2c,3); it splits into two subsites, ![]() ${X}_{\rm A}^{P} $(21) and
${X}_{\rm A}^{P} $(21) and ![]() ${X}_{\rm A}^{P} $(22), with 70 and 30% occupancy by O atoms of H2O groups; the
${X}_{\rm A}^{P} $(22), with 70 and 30% occupancy by O atoms of H2O groups; the ![]() ${X}_{\rm A}^{P} $(21,22) sites give (H2O)1.00 pfu (Tables 8,9).
${X}_{\rm A}^{P} $(21,22) sites give (H2O)1.00 pfu (Tables 8,9).

Fig. 3. A general scheme of possible hydrogen bonding in vigrishinite. O atoms of H2O groups and OH groups at the XP sites are shown as large and small red spheres, respectively; Zn atoms at the AP(1) site are shown as purple spheres, Ti atoms at the M H sites are shown as yellow spheres and Zn atoms at the A I site are shown as white spheres with purple rims; Zn–O(H2O) and Ti–O(H2O) bonds are shown as solid black lines; possible directions of hydrogen bonds are shown as dashed black lines and their lengths are given in Å. Red rectangles show possible short-range-order arrangements around the A I site: SRO 72%: A I = □, ![]() ${\rm X}_{\rm M}^{P} $(1) = H2O,
${\rm X}_{\rm M}^{P} $(1) = H2O, ![]() ${\rm X}_{\rm A}^{P} $(1) = H2O and (inset) SRO 28%: A I = Zn,
${\rm X}_{\rm A}^{P} $(1) = H2O and (inset) SRO 28%: A I = Zn, ![]() ${\rm X}_{\rm M}^{P} $(1) = OH,
${\rm X}_{\rm M}^{P} $(1) = OH, ![]() ${\rm X}_{\rm A}^{P} $(1) = OH.
${\rm X}_{\rm A}^{P} $(1) = OH.
To assign H2O and OH groups to the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites we need to consider SRO arrangements involving the A I site which is 28% occupied by Zn (Fig. 3, Tables 9,10). SRO 72% occurs where the A I site is vacant and the O atoms at the
${X}_{\rm A}^{P} $(1) sites we need to consider SRO arrangements involving the A I site which is 28% occupied by Zn (Fig. 3, Tables 9,10). SRO 72% occurs where the A I site is vacant and the O atoms at the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites receive bond valence only from one cation, 0.46 vu from Ti at the M H(1) site and 0.25 vu from Zn at the AP(1) site, respectively (Table 9). Hence at SRO 72%, the
${X}_{\rm A}^{P} $(1) sites receive bond valence only from one cation, 0.46 vu from Ti at the M H(1) site and 0.25 vu from Zn at the AP(1) site, respectively (Table 9). Hence at SRO 72%, the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites are occupied by H2O groups (Fig. 3, upper red rectangle). SRO 28% occurs where the A I and AP(1) sites are locally 100% occupied by Zn and the O atoms at the
${X}_{\rm A}^{P} $(1) sites are occupied by H2O groups (Fig. 3, upper red rectangle). SRO 28% occurs where the A I and AP(1) sites are locally 100% occupied by Zn and the O atoms at the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites each receive bond valence from two cations, 0.67 vu from the MH(1) plus AI cations and 0.61 vu from the AP(1) plus AI cations, respectively (Table 10). Hence at SRO 28%, the
${X}_{\rm A}^{P} $(1) sites each receive bond valence from two cations, 0.67 vu from the MH(1) plus AI cations and 0.61 vu from the AP(1) plus AI cations, respectively (Table 10). Hence at SRO 28%, the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites are occupied by OH groups (Fig. 3, lower red rectangle). In accord with the two SRO arrangements, SRO 72% and SRO 28%, we assign (H2O)0.72(OH)0.28 pfu, to each of the
${X}_{\rm A}^{P} $(1) sites are occupied by OH groups (Fig. 3, lower red rectangle). In accord with the two SRO arrangements, SRO 72% and SRO 28%, we assign (H2O)0.72(OH)0.28 pfu, to each of the ![]() ${X}_{\rm M}^{P} $(1) and
${X}_{\rm M}^{P} $(1) and ![]() ${X}_{\rm A}^{P} $(1) sites each; these two sites ideally give (H2O)2.00 pfu (Table 8). We sum compositions of the four
${X}_{\rm A}^{P} $(1) sites each; these two sites ideally give (H2O)2.00 pfu (Table 8). We sum compositions of the four ![]() ${X}_{{\rm (M,A)}}^{P} $ sites as follows: (H2O)0.72(OH)0.28[
${X}_{{\rm (M,A)}}^{P} $ sites as follows: (H2O)0.72(OH)0.28[![]() ${X}_{\rm M}^{P} $(1)] + (H2O)1.00[
${X}_{\rm M}^{P} $(1)] + (H2O)1.00[![]() ${X}_{\rm M}^{P} $(2)] + (H2O)0.72(OH)0.28[
${X}_{\rm M}^{P} $(2)] + (H2O)0.72(OH)0.28[![]() ${X}_{\rm A}^{P} $(1)] + (H2O)1.00[
${X}_{\rm A}^{P} $(1)] + (H2O)1.00[![]() ${X}_{\rm A}^{P} $(2)] = (H2O)3.44(OH)0.56, ideally (H2O)4 pfu (Table 8).
${X}_{\rm A}^{P} $(2)] = (H2O)3.44(OH)0.56, ideally (H2O)4 pfu (Table 8).
The anions and H2O groups sum as follows: (Si2O7)2[O(1–14)] + O2 + O(OH) + (H2O)4 = (Si2O7)2O2O(OH)(H2O)4 pfu, with a total charge of 19– .
We write the ideal structural formula of vigrishinite as the sum of cation and anion parts: Zn□Ti2Na□Ti2 + (Si2O7)2O2O(OH)(H2O)4 = Zn□Ti2Na□Ti2(Si2O7)2O2O(OH)(H2O)4, Z = 4. A short form of the ideal structural formula is NaZnTi4(Si2O7)2O3(OH)(H2O)4.
Structure topology of vigrishinite
The main structural unit in the crystal structure of vigrishinite is a TS block that consists of HOH sheets.
The O sheet is composed of Ti-dominant MO(1,2) octahedra which form brookite-like chains along a and Na-dominant MO(3) octahedra, with the □-dominant M O(4) site occupied mainly by Na and Zn at <50% (Fig. 2a). Hence there is order of Na and □ over two M O(3,4) sites in the O sheet of vigrishinite. In murmanite, the Ti- and Na-dominant octahedra which constitute the O sheet each form brookite-like chains (Fig. 2b). Ideal compositions of the O sheet in vigrishinite, [Na□Ti2O3(OH)]2+ pfu, and murmanite, [Na2Ti2O4]2+ apfu (Cámara et al., Reference Cámara, Sokolova, Hawthorne and Abdu2008) are related by the following substitution: O(Na+)mur + O(O2–)mur ↔ O(□)vig + O[(OH)–]vig.
In vigrishinite, the H sheet is built of Si2O7 groups, Ti-dominant MH(1,2) and Zn-dominant AP(1) octahedra, with a vacant AP(2) site (Fig. 2c). Hence there is order of Zn and □ over the two AP sites in vigrishinite. In murmanite, there is only one [8]AP site, occupied mainly by Na at 98% (Fig. 2d). Ideal compositions of the H sheets in vigrishinite, [Zn□Ti2(Si2O7)2(H2O)4]2– pfu, and murmanite, [Na2Ti2(Si2O7)2(H2O)4]2– pfu, are related by the following substitution: H(![]() ${\rm Na}_{\rm 2}^{\rm +} $)mur ↔ H(Zn2+)vig + H(□)vig.
${\rm Na}_{\rm 2}^{\rm +} $)mur ↔ H(Zn2+)vig + H(□)vig.
In vigrishinite and murmanite, the topology of the TS block is as in the murmanite group of TS-block minerals, where Ti (+ Mn + Mg) = 4 apfu per (Si2O7)2: Si2O7 groups link to two Ti octahedra of the O sheet adjacent along t1 (Figs 2e,f; 4a,c). In the crystal structures of vigrishinite and murmanite, TS blocks parallel to (001) link via hydrogen bonds between H2O groups at apical vertices [![]() $X^{P}_{\lpar \rm M,A\rpar} $ sites] of MH and AP polyhedra (Fig. 4a ,c; for the pattern of hydrogen bonding, see Fig. 3).
$X^{P}_{\lpar \rm M,A\rpar} $ sites] of MH and AP polyhedra (Fig. 4a ,c; for the pattern of hydrogen bonding, see Fig. 3).

Fig. 4. The crystal structure of vigrishinite: a general view (a) and the arrangement of Zn octahedra [AP(1) site] and □ [AP(2) site] in the H sheets (b); the crystal structure of murmanite: a general view (c) and the arrangement of [8]Na polyhedra [AP site] in the H sheets (d); cations and anions at sites with occupancy less than 50% are not shown in (a) and (c). Legend as in Fig. 2; the vacancy at the AP(2) site is shown as a white circle with a black rim in (b). In (d), unit cells [1] and [2] are centred on the [8]Na polyhedra (shown by thick red lines), and in (c), the unit cell [2] is centred on Zn octahedra (shown by thick red lines) and d = 12.176 Å (shown by thick dashed red lines) connects Zn octahedron and □ [AP(2) site].
Figures 4b and 4d show the arrangement of Zn octahedra [AP(1) site] and □ [AP(2) site] in vigrishinite and the arrangement of Na polyhedra [AP site] in murmanite, respectively. The arrangement of Na polyhedra in murmanite (Fig. 4d) obeys unit cells [1], with c 1 = 12.176 Å, and [2], with c 2 = 11.699 Å (shown by solid red lines) (Table 2). The arrangement of Zn octahedra and □ in vigrishinite (Fig. 4b) supports only unit cell [1], with c 2 = 11.699 Å (shown by solid red lines) as reported by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986), whereas d [AP(1)–AP(2)] = 12.176 Å (shown by a dashed red line) is not a valid translation because of the order of Zn and □ (Table 2).
We conclude that: (1) the general topology of the crystal structure of zvyaginite described above is in accord with Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986), Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b); (2) the stereochemistry of Zn and Na in the TS block is different from that reported by Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b); (3) doubling of the t 1 and t 2 translations of vigrishinite relative to those of murmanite, a vig = 10.530, b vig = 13.833 Å, a mur = 5.388, b mur = 7.058 Å, is due to the order of Zn and □ in the H sheet and Na and □ in the O sheet of vigrishinite.
On the occurrence of Zn in the H sheet of vigrishinite and validity of Zn-exchanged “murmanite”
On the occurrence of Zn in the H sheet of vigrishinite
First, consider the H sheet in murmanite, where [8]Na occurs at the AP site in the centre of the six-membered ring of four Si tetrahedra and two Ti octahedra (Fig. 5a). In accord with Sokolova and Cámara (Reference Sokolova and Cámara2016), we will call this six-membered ring the AP ring as it hosts the AP site. The t 1 translation is approximately the sum of lengths of edges of the Ti and Si polyhedra: t 1 ≈ [(2.70 + 2.70) + (2.73 + 2.66)]/2 ≈ 5.395 Å (cf. a mur = 5.388 Å, Table 2). The t 2 translation depends on the dimensions of a Ti octahedron and an Si2O7 group along t2, i.e. t 2 is the sum of the length of an edge of a Ti octahedron and the distance between O atoms of Si tetrahedra along t2 [(O–O)H as defined by Sokolova, Reference Sokolova2006]; hence t 2 ≈ [(2.74 + 2.70) + (4.38 + 4.37)]/2 ≈ 7.095 Å (cf. b mur = 7.058 Å, Table 2). A similar consideration applies to the O sheet in murmanite.

Fig. 5. Details of the topology of the H sheet in murmanite (a) and vigrishinite (b). Legend as in Fig. 2, Na atoms at the AP site in murmanite are shown as navy blue spheres, Zn octahedra are purple, all linear dimensions are in Å.
Next, consider the H sheet in vigrishinite, where Zn occurs in the centre of the AP(1) ring (Fig. 5b). As Zn ([6]r = 0.74 Å, [8]r = 0.90 Å) is a smaller cation than Na ([6]r = 1.02 Å, [8]r = 1.18 Å), Zn cannot be [8]-fold coordinated (cf. [8]-coordinated Na in murmanite, Fig. 2d) because of the size of the AP(1) ring, and therefore Zn is [6]-coordinated in the H sheet of vigrishinite. To maintain the six-membered AP(1) ring, two edges of the Zn octahedron parallel to t2 must match the (O–O)H distances of Si2O7 groups (Fig. 5b). Hence SiO4 tetrahedra of Si2O7 groups in that ring rotate towards each other in the plane of the ring, with O–O–O angles of ~78.6 and 79.0°, and the (O–O)H distances decrease to 3.31–3.33 Å (Fig. 5b). To compensate for the shortening of the AP(1) ring along t2, the AP(2) ring elongates along t2, with its (O–O)H distances increasing to 5.05 and 5.09 Å, and the SiO4 tetrahedra of Si2O7 groups in that ring rotate away from each other in the plane of the ring, resulting in O–O–O angles of ~155.7 and 156.9° (Fig. 5b). Different distortions of the AP(1) and AP(2) rings allow maintenance of matching t 1 and t 2 translations of the H and O sheets. The t 2 translation is the sum of the lengths of an edge of two Ti octahedra and two (O–O)H distances characterizing the dimensions of two Si2O7 groups along t2; hence t 2 ≈ [(2.76 + 2.76) + (2.78 + 2.68) + (5.05 + 5.09) + (3.33 + 3.31)]/2 ≈ 13.87 Å (cf. b vig = 13.833 Å; b mur = 7.058 Å, Table 2).
Elongation of the AP(2) ring makes it too large to accommodate Zn or Na, and hence the AP(2) site is vacant (Fig. 5b, Table 8). Different distortions of the AP rings correspond to the order of Zn and □ in the H sheet of vigrishinite which causes doubling of the t 1 and t 2 translations of vigrishinite relative to those of murmanite.
On the validity of Zn-exchanged ‘murmanite’
Lykova et al. (Reference Lykova, Pekov, Zubkova, Chukanov, Yapaskurt, Chervonnaya and Zolotarev2015a) refined Ag-exchanged forms of murmanite using the unit cell of murmanite; they did not observe any superstructure reflections. Ag+ ([6]r = 1.15 Å, [8]r = 1.28 Å) is a relatively large cation and it substitutes for Na ([6]r = 1.02 Å, [8]r = 1.18 Å) both in the O and H sheets of murmanite.
Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) refined crystal structures of two ‘Zn-exchanged forms of murmanite’ (Zn = 0.15 and 0.85 apfu). They observed additional systematic reflections in the reciprocal space of ‘Zn-exchanged forms of murmanite’ and chose ‘larger unit cells’ identical to the unit cell of ‘the second triclinic variety of murmanite’ of Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) and vigrishinite (Pekov et al., Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013). Above, we showed that Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) solved the crystal structure of vigrishinite under the name murmanite. Note that Zn ([6]r = 0.74 Å, [8]r = 0.90 Å) is a much smaller cation compared to Ag+ and Na. In the first paragraph of this section, we showed that incorporation of Zn in vigrishinite results in distortions of the six-membered rings of polyhedra in the H sheet, where the smaller ring contains Zn and the larger ring contains a vacancy. We are confident that ion-exchange of Zn with murmanite resulted in the formation of vigrishinite-like phases, not ‘Zn-exchanged forms of murmanite’.
If Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) realized that Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) had solved the crystal structure of vigrishinite, they would have been able to evaluate their results of ion-exchange in a different way.
Until our work on vigrishinite, we did not consider the structure work of Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) as it was not accompanied by a chemical analysis. Description of vigrishinite by Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013), with questionable partly occupied Ti-dominant sites, forced us to have a closer look at the structure work in question, and now we recognize the excellent work on vigrishinite by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986).
Summary
(1) Electron-microprobe analysis of vigrishinite from the type locality, Mt. Malyi Punkaruaiv, Lovozero alkaline massif, Kola Peninsula, Russia, is in accord with the analogous results of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013). The empirical and ideal formulae of vigrishinite have been revised as follows: (Na0.67Zn0.21Ca0.05□1.07)Σ2(Zn0.86□1.14)Σ2(Zn0.14 □0.36)Σ0.5(Ti2.60Nb0.62Mn0.30![]() ${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06 Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14)[O2.60(OH)1.21F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} and NaZnTi4(Si2O7)2O3(OH)(H2O)4 with Z = 4 [cf. formula of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b): Zn2Ti4–x(Si2O7)2O2 (OH,F,O)2(H2O,OH,□)4, x < 1 with Z = 2].
${\rm Fe}_{{\rm 0}{\rm. 23}}^{{\rm 2 +}} $Mg0.10Zr0.06 Zn0.05Al0.03Ta0.01)Σ4(Si4.02O14)[O2.60(OH)1.21F0.19]Σ4[(H2O)3.44(OH)0.56]Σ4{Zn0.24P0.03K0.03Ba0.02} and NaZnTi4(Si2O7)2O3(OH)(H2O)4 with Z = 4 [cf. formula of Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b): Zn2Ti4–x(Si2O7)2O2 (OH,F,O)2(H2O,OH,□)4, x < 1 with Z = 2].
(2) The crystal structure of vigrishinite has been refined in space group C ![]() $\bar 1$, a = 10.530(2), b = 13.833(3), c = 11.659(2) Å, α = 94.34(3), β = 98.30(3), γ = 89.80(3)°, V = 1675.5(2.1) Å3, R 1 = 12.52% with Z = 4. The general topology of the crystal structure is in accord with Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013): it is an array of TS blocks connected via hydrogen bonds between H2O groups. However the stereochemistry of the TS block is different from that of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b): there is order of Na and □ in the O sheet of the composition [Na□Ti2O3(OH)]2+ and order of Zn and □ in the two H sheets of the composition [Zn□Ti2(Si2O7)2(H2O)4]2–.
$\bar 1$, a = 10.530(2), b = 13.833(3), c = 11.659(2) Å, α = 94.34(3), β = 98.30(3), γ = 89.80(3)°, V = 1675.5(2.1) Å3, R 1 = 12.52% with Z = 4. The general topology of the crystal structure is in accord with Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013): it is an array of TS blocks connected via hydrogen bonds between H2O groups. However the stereochemistry of the TS block is different from that of Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b): there is order of Na and □ in the O sheet of the composition [Na□Ti2O3(OH)]2+ and order of Zn and □ in the two H sheets of the composition [Zn□Ti2(Si2O7)2(H2O)4]2–.
(3) Vigrishinite is a TS-block mineral of the murmanite group (seidozerite supergroup) where Ti + Mn + Mg = 4 apfu. Vigrishinite has an ideal structural formula of the form ![]() ${\rm A}_{2}^{P} {\rm M}_{\rm 2}^{\rm H} {\rm M}_{\rm 4}^{\rm O} $(Si2O7)2(
${\rm A}_{2}^{P} {\rm M}_{\rm 2}^{\rm H} {\rm M}_{\rm 4}^{\rm O} $(Si2O7)2(![]() ${\rm X}_{\rm M}^{\rm O} $)2(
${\rm X}_{\rm M}^{\rm O} $)2(![]() ${\rm X}_{\rm A}^{\rm O} $)2(
${\rm X}_{\rm A}^{\rm O} $)2(![]() ${\rm X}_{{\rm M,A}}^{P} $)4: Zn□Ti2Na□Ti2(Si2O7)2O2O(OH)(H2O)4, Z = 4. Vigrishinite is a Zn-bearing, Na-poor and OH-rich analogue of murmanite, ideally Na2Ti2Na2Ti2(Si2O7)2O2O2(H2O)4.
${\rm X}_{{\rm M,A}}^{P} $)4: Zn□Ti2Na□Ti2(Si2O7)2O2O(OH)(H2O)4, Z = 4. Vigrishinite is a Zn-bearing, Na-poor and OH-rich analogue of murmanite, ideally Na2Ti2Na2Ti2(Si2O7)2O2O2(H2O)4.
(4) Murmanite and vigrishinite are related by the following substitution in the H and O sheets of the TS-block: H(![]() ${\rm Na}_{\rm 2}^{\rm +} $)mur + O(Na+)mur + O(O2−)mur ↔ H(Zn2+)vig + H(□)vig + O(□)vig + O[(OH)–]vig. Doubling of the t 1 and t 2 translations of vigrishinite, a vig = 10.530, b vig = 13.833 Å, relative to those of murmanite: a mur = 5.388, b mur = 7.058 Å, is due to the order of Zn and □ in the H sheet and Na and □ in the O sheet of vigrishinite.
${\rm Na}_{\rm 2}^{\rm +} $)mur + O(Na+)mur + O(O2−)mur ↔ H(Zn2+)vig + H(□)vig + O(□)vig + O[(OH)–]vig. Doubling of the t 1 and t 2 translations of vigrishinite, a vig = 10.530, b vig = 13.833 Å, relative to those of murmanite: a mur = 5.388, b mur = 7.058 Å, is due to the order of Zn and □ in the H sheet and Na and □ in the O sheet of vigrishinite.
(5) The crystal structure of vigrishinite was originally solved and refined by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986). They reported two sets of the unit-cell parameters: [1] a C-centred unit cell, a = 10.535, b = 13.884, c = 11.688 Å, α = 94.31, β = 98.62, γ = 89.81° and V = 1685.43 Å3, where they discussed the doubling of the a and b unit-cell parameters compared to those of murmanite, and [2] a reduced unit cell, space group P1, a = 8.700, b = 8.728, c = 11.688 Å, α = 94.31, β = 98.62, γ = 105.62o and V = 838.84 Å3. In the H sheet, they identified the [6]Na(5) site, with < Na(5)–O> = 2.09 Å. Based on a high value of site scattering at the Na(5) site, they suggested assignment of Mn2+, Fe2+, Zr, Nb and ‘to the least degree’, Na, to that site. For the structure of their murmanite, Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) described an HOH layer similar to that of murmanite. Pekov et al. (Reference Pekov, Britvin, Zubkova, Chukanov, Bryzgalov, Lykova, Belakovskiy and Pushcharovsky2013) and Lykova et al. (Reference Lykova, Pekov, Zubkova, Yapaskurt, Chervonnaya, Zolotarev and Giester2015b) erroneously denoted the mineral studied by Rastsvetaeva and Andrianov (Reference Rastsvetaeva and Andrianov1986) as ‘the second triclinic variety of murmanite’.
Acknowledgements
We are grateful to reviewer Fernando Cámara and Associate Editor Peter Leverett for their comments and to Principal Editor Peter Williams for handling the manuscript. We thank Mark A. Cooper for collection of single-crystal X-ray data for the four vigrishinite crystals. ES acknowledges financial support by a Canada Research Chair in Crystallography and Mineralogy to FCH. FCH acknowledges support by a Canada Research Chair in Crystallography and Mineralogy and by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada, and by Innovation Grants from the Canada Foundation for Innovation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/minmag.2017.081.060