Introduction
We present the complete characterisation of ferri-fluoro-katophorite, ideally Na(NaCa)(Mg4Fe3+)(Si7Al)O22F2, a new species of amphibole of the sodium–calcium subgroup of the amphibole supergroup. The mineral and its name were approved by the International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC), vote 2015-096. Hawthorne et al. (Reference Hawthorne, Oberti and Martin2006) characterised a series of amphiboles from the Bear Lake diggings, Bancroft area, Ontario, Canada (44°53′N, 78°19′W). Two of them (BL10 and BL11) were classified as ‘potassian-fluor-magnesio-katophorite’. According to the nomenclature rules now in force, root names are assigned to C(MgAl) compositions (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012), and hence BL10 and BL11 must now be named ferri-fluoro-katophorite. A systematic work characterising all the missing (or named; Burke and Leake, Reference Burke and Leake2004) amphibole species is in progress after the publication of that report, and sample BL11 was used to complete the mineral description for ferri-fluoro-katophorite.
Electron-microprobe analyses (EMP), secondary ion mass spectrometry (SIMS) and structure refinement (SREF) for this sample were reported by Hawthorne et al. (Reference Hawthorne, Oberti and Martin2006). In the present work, the analytical data have been optimised according to current knowledge of amphibole crystal-chemistry. The holotype material of ferri-fluoro-katophorite is deposited in the mineral collection of the Department of Natural History, Royal Ontario Museum, Canada, catalogue number M57071.
Occurrence and optical properties
The Bear Lake diggings are located 8.4 km west of Tory Hill, Monmouth Township, in the Bancroft District of southeastern Ontario, Canada. They are underlain by rocks of the Glamorgan complex, consisting mostly of quartzofeldspathic gneiss, amphibolite and marble (Armstrong and Gittins, Reference Armstrong and Gittins1968).
The complex equilibrated at amphibolite grade during the Elzevirian compressive pulse of the Grenvillian collisional orogeny, at ~1280–1300 Ma (McLelland et al. Reference McLelland, Selleck, Hamilton and Bickford2010). The supracrustal rocks, buried to 25 km or so, record a temperature of 725–750°C (Anovitz and Essene, Reference Anovitz and Essene1990; Streepey et al., Reference Streepey, Essene and Van der Pluijm1997). Towards the end of the Grenville event, at the Late Ottawan stage, the gravitational collapse of the overthickened crust led to the rise of the asthenosphere and of mantle-derived fluids, which induced the local melting of marble units. The resulting calcite dykes were emplaced between 1070 and 1040 Ma ago, at a stage of regional extension. They are steep and subparallel, and extend approximately east–west. They are typically 1 to 2 metres across, and pinch out at irregular intervals in an en échelon fashion (Vertolli et al., Reference Vertolli, Back, Fouts and Mandarino1998). The carbonatitic melt aggressively dissolved adjacent gneissic rocks, and in this way became a crustally derived silicocarbonatitic melt.
Coarse euhedral crystals of amphibole (samples BL10 and BL11), phlogopite, sanidine solid-solution (now coarsely exsolved to microcline perthite), titanite, augite, zircon and fluorapatite crystallised from this low-viscosity magma. These crystals (up to 8 cm long along c) generally protrude from the walls into the body of coarsely crystalline calcite, but they also are found away from the walls, completely enclosed by calcite, suggestive of rapid freezing of the silicocarbonatitic magma. The temperature of crystallisation of the assemblage was above the crest of the alkali feldspar solvus (i.e. above 625°C).
The refined and analysed crystal used in this work was taken from rock specimen BL11. It has a composition close to the end-member, and has the code 762 in the amphibole database of the CNR-IGG Pavia.
In the type specimen, ferri-fluoro-katophorite occurs as greenish grey prismatic to lamellar crystals with perfect {110} cleavage. The streak is grey and the tenacity is brittle.
A spindle stage was used to orient a crystal for measurement of refractive indices and 2V by extinction curves (Bartelmehs et al., Reference Bartelmehs, Bloss, Downs and Birch1992). The optical orientation was determined by transferring the crystal from the spindle stage to a single-crystal diffractometer and measuring the relative axial relations by X-ray diffraction. In transmitted plane-polarised light (λ = 590 cm–1), ferri-fluoro-katophorite is pleochroic: X = very light grey, Y = medium grey, Z = light grey, non fluorescent, and has a vitreous lustre. It is biaxial (–), α = 1.640(2), β = 1.652(2), γ = 1.658(2), 2Vmeas. = 68.9(5) and 2Vcalc. = 70.1°. The orientation is: X ∧ a = 45.4o (in β obtuse), Y || b, Z ∧ c = 30.7o (in β acute).
Single-crystal and powder diffraction analysis
Diffraction data for a prismatic single-crystal fragment 220 μm × 220 μm × 300 µm in size were collected in the θ range 2–30° using a Philips PW1100 diffractometer with graphite-monochromatised MoKα X-radiation (λ = 0.7107 Å). The scan width and the scan speed used were 2.40° and 0.06°sec–1, respectively. Two monoclinic equivalents (hkl and h-kl) were collected. Intensities were corrected for Lorentz and polarisation effects and for absorption (ψ-scan method; North et al., Reference North, Phillips and Mathews1968) and then merged, giving 1384 unique reflections (R int = 2.1%). The 1059 reflections with I o > 3σ(I) were considered as observed during unweighted full-matrix least-squares refinement on F. Scattering curves for fully ionised chemical species were used at sites where chemical substitutions occur; neutral vs. ionised scattering curves were used at the T and anion sites [except O(3)] (cf. Oberti et al. (Reference Oberti, Ungaretti, Cannillo and Hawthorne1992) for more details on the procedure). A residual corresponding to the H atom was found in the difference-Fourier map and inserted in the model with a fixed occupancy equal to 0.80 atoms per formula unit (apfu) and a fixed B eq atom-displacement parameter equal to 1.0 Å2. Full-matrix least-squares refinement on I > 3σ(I) converged to R obs = 1.7% and R all = 3.1%. Details concerning data collection and structure refinement are provided in Table 1. Refined atom coordinates and displacement parameters, and selected bond lengths and angles are given in Tables 2 and 3, respectively. Observed structure factors have been deposited together with the cif as Supplementary material (see below).
Table 1. Miscellaneous information on data collection and structure refinement of ferri-fluoro-katophorite.
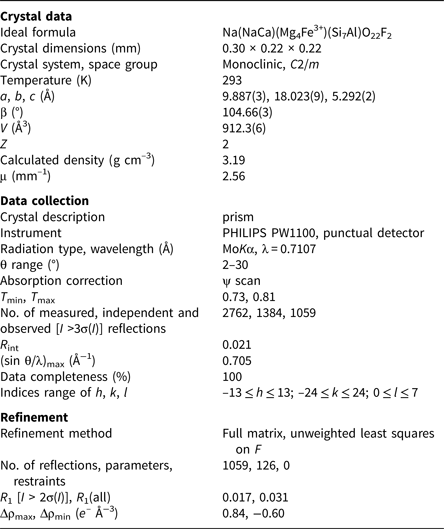
Table 2. Atom coordinates, refined site-scattering (ss, epfu) values and atom-displacement parameters (B eq, Å2;βij x 104) for ferri-fluoro-katophorite crystal 762.

*ss – site scattering in electrons per forumla unit (epfu).
**Kept fixed to the value obtained from the analysis.
Table 3. Selected interatomic distances (Å), angles (o), tetrahedral and octahedral angle variances (TAV, OAV, °^2) and quadratic elongations (TQE, OQE) according to Robinson et al. (Reference Robinson, Gibbs and Ribbe1971) for ferri-fluoro-katophorite crystal 762.

The a:b:c ratio calculated from the unit cell parameters is 0.549:1:0.294
Powder X-ray diffraction data (CuKα, λ = 1.54178 Å) were obtained using the XPREP utility of SAINT (Bruker, 2003), which generates a 2D powder diffractogram (Debye-Scherrer technique) starting from the F 2 collected on the single crystal and taking into account solely the information concerning the unit-cell dimensions and the Laue symmetry. No Lorentz and polarisation corrections were applied. Data are given in Table 4.
Table 4. Powder X-ray diffraction data for ferri-fluoro-katophorite crystal 762.

Note: the strongest eight reflections are in bold. Only peaks with I rel ≥ 5 are reported.
Chemical analysis
Chemical analysis (10 points) on the crystal used for structure refinement was done using a Cameca SX-100 electron microprobe (wavelength-dispersive spectroscopy mode, 15 kV, 20 nA, count time 20 s and 5 µm beam diameter). The Li content was measured by SIMS analysis (Ottolini et al., Reference Ottolini, Bottazzi and Vannucci1993). The standards used were: Si and Ca: diopside (TAP); Ti: titanite (LPET); Al: andalusite (TAP); Fe: fayalite (LLiF); Mn: spessartine (LLiF); Mg: forsterite (LTAP); Zn: gahnite (LLiF); Ni: pentlandite (LLiF); Na: albite (TAP); K: orthoclase (LPET); F: fluoro-riebeckite (TAP); and Cl: tugtupite (LPET). Both Cl and Ni were below the detection limits. The Fe3+/Fetot ratio was calculated based on SREF results for the A-site occupancy (and hence for the total number of cations), and the amount of H2O used in the calculation is that needed to obtain F + OH + Cl = 2 apfu (atoms per formula unit). The oxide wt.% and the calculated unit-formula are reported in Table 5. The proposed empirical formula for crystal 762 is: A(Na0.55K0.32)Σ0.87B(Ca1.18Na0.79Mn2+0.03)Σ2.00C(Mg3.29Fe2+1.19Fe3+0.31Al0.09Ti4+0.08Mn2+0.02Li0.02)Σ5.00T(Si7.39Al0.61)Σ8.00O22W[F1.23(OH)0.77]Σ2.00 (where the dominant cations or anions in the relevant homovalent series are in bold). The simplified end-member formula is ANaB(NaCa)C(Mg4Fe3+)T(Si7Al)O22WF2, which requires SiO2: 49.33, Al2O3: 5.98, Fe2O3: 9.36, MgO: 18.90, Na2O: 7.27, F: 4.46, F=O: –1.88, total = 100.00 wt.%.
Table 5. Chemical composition (average of 10 points) and unit formula* for ferri-fluoro-katophorite (762), and a comparison between observed and calculated site-scattering values.

*calculated based on 15.87 cations and 24 (O, OH, F, Cl) with (OH + F + Cl) = 2 apfu.
esd – estimated standard deviation.
Based on this empirical formula, the calculated density of ferri-fluoro-katophorite BL11 is 3.19 g/cm3. The compatibility index (1 – (K P/K C); Mandarino, Reference Mandarino1981) is –0.007 (superior).
Comments on the crystal-chemistry of ferri-fluoro-katophorite
Site populations were derived from the unit formula and the results of the structure refinement and are given in Table 6. There is excellent agreement between the refined site-scattering values (ss, electrons per formula unit) and the corresponding mean bond lengths (mbl, Å) and those calculated based on the assigned site-populations
Table 6. Site populations* for ferri-fluoro-katophorite, crystal 762.
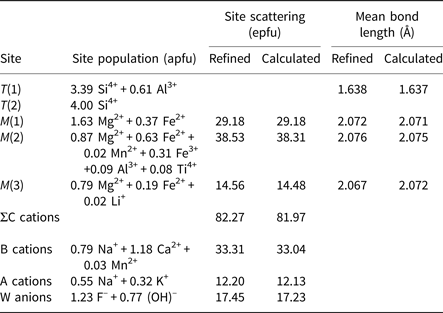
*Hawthorne et al. (Reference Hawthorne, Ungaretti and Oberti1995).
The results show that Ti4+ is ordered at the M(2) site, together with all the other high-charge cations, which is in accord with the absence of an oxo component (O2–) at the O(3) site; this result validates the constraint (OH,F,Cl) = 2 apfu used to calculated the unit formula. As already discussed in Hawthorne et al. (Reference Hawthorne, Oberti and Martin2006), and explained on the basis of ‘local’ bond-valence requirements, the A cations are strongly ordered at the A(m) subsite, where the interactions with F are stronger (Oberti et al., Reference Oberti, Hawthorne, Cannillo, Cámara, Hawthorne, Oberti, Della Ventura and Mottana2007).
Relation with other species and a review of related compositions
The sample designated ‘ferri-katophorite’ by Pushcharovski et al. (Reference Pushcharovski, Lebedeva, Pekov, Ferraris, Novakova and Ivaldi2003) has the formula A(Na0.87K0.13)B(Na1.18Ca0.82)C[M(1)(Mg1.41 Fe3+0.42Ti0.17)M(2)(Fe3+1.31Mg0.69)M(3)(Mg0.60Fe2+0.38Mn0.02)]T[T(1)(Si3.16Al0.84)T(2)Si4] O22 W(O1.05OH0.66F0.29), which according to current nomenclature (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012) is a member of the oxo-amphiboles group, and should, in principle, deserve a new rootname. However, this mineral has never been officially approved by IMA–CNMNC, and the proposed formula has major crystal-chemical problems because the sum of the highly charged cations at the M(1) site does not compensate for the oxo component.
Therefore, the only published composition corresponding to katophorite is the recently approved katophorite from Myanmar (IMA 2013-140; Oberti et al., Reference Oberti, Boiocchi, Hawthorne, Ball and Harlow2015), which has the unit formula A(Na0.85K0.04)Σ0.89B(Ca1.22Na0.78)Σ2.00C(Mg3.76Al0.43Fe3+0.30Cr3+0.27Fe2+0.18Li0.05Ti4+0.01)Σ5.00T(Si7.21Al0.79)Σ8.00O22W[(OH)1.67O0.30F0.03)Σ2.00.
A comparison is provided in Table 7 between the optical and crystallographic properties of these two known species related to the rootname katophorite.
Table 7. Comparison of the optical and crystallographic properties for the two known amphiboles related to the root name katophorite.

Acknowledgements
We thank Raymond A. McDougall, who introduced RFM to the fascinating Bear Lake diggings, and who provided the crystals from his collection. Fast and useful reviews by Igor Pekov, Peter Leverett and an anonymous reviewer are also acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.130