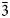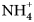Introduction
The small, long-inactive Torrecillas mine in the northern Atacama Desert of Chile was probably discovered and first worked during the guano mining boom of the early 19th century (Mortimer, et al., Reference Mortimer, Saric and Cáceres1971). The once-important Pabellón de Pica guano deposit is only ~7 km north of Torrecillas Hill. Although the veins that were exploited at Torrecillas are particularly rich in arsenic, the early miners were most likely to be working the veins for copper and, perhaps, were hoping to find gold. The deposit apparently was worked later for arsenic, as reported by Pimentel (Reference Pimentel1978), who also noted that the mine was probably finally abandoned several years prior to 1950. Based upon the very limited extent of the workings, the mine clearly was never a significant producer.
In recent years, our investigations on the minerals of this unusual deposit have yielded many new mineral species. Including the two new minerals reported herein, cuatrocapaite-(NH4) and cuatrocapaite-(K), eighteen new minerals have now been described from this deposit. All of these, except leverettite, CoCu3Cl2(OH)6 (Kampf et al., Reference Kampf, Sciberras, Williams, Dini and Molina Donoso2013), contain essential arsenic. Twelve are arsenates and five are arsenites [see Kampf et al. (Reference Kampf, Nash, Dini and Molina Donoso2019) for a complete listing]; cuatrocapaite-(NH4) and cuatrocapaite-(K) are arsenites.
The name ‘cuatrocapaite’ is in allusion to the structure, which consists of four (cuatro in Spanish) different types of layers (capa in Spanish): (1) [As2O3]; (2) [(NH4),K]; (3) [Cl6]; and (4) [(Na,Mg,)3(H2O)16]. The -(NH4) and -(K) suffixes indicate the dominant cations in the large-cation layer (2). The IPA pronunciation of cuatrocapaite is (/kwa: troʊ 'ka: pa: aɪt/). The new minerals and their names, cuatrocapaite-(NH4) (IMA2018-083, Kampf et al., Reference Kampf, Chukanov, Möhn, Dini, Molina Donoso and Friis2018a) and cuatrocapaite-(K) (IMA2018-084, Kampf et al., Reference Kampf, Chukanov, Möhn, Dini, Molina Donoso and Friis2018b), have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association. The description of cuatrocapaite-(NH4) is based upon four cotype specimens. Three are deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA with catalogue numbers 66984, 66985 and 66986. One is deposited in the collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with registration number 5255/1. The description of cuatrocapaite-(K) is based upon one holotype specimen deposited in the collections of the Natural History Museum of Los Angeles County, catalogue number 66987.
Occurrence
The Torrecillas mine is located on Torrecillas Hill, Iquique Province, Tarapacá Region, Chile (~20°58′13″S, 70°8′17″W). Four different rock units are exposed on the hill. The Coastal Range Batholith (mainly gabbros) extends from the seashore to the Pan-American Road along the base of Torrecillas Hill. At the foot of Torrecillas Hill is a small area of contact metamorphic rocks in which garnet crystals occur in metamorphosed shales. Higher on the hill, the rocks are predominantly porphyritic andesitic lavas of the Jurassic La Negra Formation (cf. Oliveros et al., Reference Oliveros, Morata, Aguirre, Féraud and Fornari2007). The Torrecillas deposit, in which the new minerals are found, consists of two main veins rich in secondary arsenic and copper minerals that intersect metamorphosed marine shales and lavas. These mineralised veins are genetically related to the aforementioned porphyritic andesitic lavas. More information on the geology and mineralogy of the area is provided by Gutiérrez (Reference Gutiérrez1975).
The rare secondary chlorides, arsenates and arsenites have been found at three main sites on the hill: an upper pit measuring ~8 m long and 3 m deep, a lower pit ~100 m from the upper pit and measuring ~5 m long and 3 m deep, and a mine shaft adjacent to the lower pit and lower on the hill. Cuatrocapaite-(NH4) was found in November of 2015 in a recent excavation a few metres above the shaft by one of the authors (AAMD). Cuatrocapaite-(K) was found in January of 2016 near the upper pit by another of the authors (GM), who also collected additional cuatrocapaite-(NH4) specimens.
Both new minerals are low-temperature secondary alteration phases occurring on matrix consisting of native arsenic, arsenolite and pyrite. Cuatrocapaite-(NH4) is also associated with lavendulan, magnesiokoritnigite, torrecillasite and the I-rich NH4-analogue of lucabindiite (currently under study). Cuatrocapaite-(K) is also associated with anhydrite, gypsum, lavendulan and torrecillasite. The secondary As-rich assemblages at the Torrecillas deposit are interpreted as having formed under hyperarid conditions from the oxidation of native arsenic, and possibly other As-bearing primary phases, coupled with reaction with underground brines rich in mobile cations such as K+, Na+, ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $, Ca2+ and Mg2+ (cf. Cameron et al., Reference Cameron, Leybourne and Palacios2007). Following the exhumation of Torrecillas Hill, it is possible that long-term exposure to the frequent dense coastal fogs (cf. Rech et al., Reference Rech, Quade and Hart2003; Wang et al., Reference Wang, Michalski, Seo and Ge2014; Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2019) caused continued near-surface alteration coupled with very late-stage secondary mineral formation.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $, Ca2+ and Mg2+ (cf. Cameron et al., Reference Cameron, Leybourne and Palacios2007). Following the exhumation of Torrecillas Hill, it is possible that long-term exposure to the frequent dense coastal fogs (cf. Rech et al., Reference Rech, Quade and Hart2003; Wang et al., Reference Wang, Michalski, Seo and Ge2014; Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2019) caused continued near-surface alteration coupled with very late-stage secondary mineral formation.
Physical and optical properties
Both cuatrocapaite-(NH4) and cuatrocapaite-(K) occur as hexagonal plates, flattened on {001} and bounded by {100}, up to ~300 µm in diameter. The pyramidal forms {10ℓ} and {01ℓ} are present on some crystals, with measurements on cuatrocapaite-(NH4) plates indicating ℓ values in the 12 to 18 range. Cuatrocapaite-(NH4) plates sometimes grow in vermiform stacks (Fig. 1) and in satin-spar-like vein fillings (Fig. 2) and the mineral is also found as white powdery coatings. Most cuatrocapaite-(K) crystals have been observed in massive intergrowths, but freestanding crystals in vugs do occur (Fig. 3). No twinning was observed for either mineral. Crystals of the I-rich, NH4-analogue of lucabindiite occurring in association with cuatrocapaite-(NH4), are very similar in appearance, but exhibit apparent twinning by rotation on [001] (Fig. 4).

Fig. 1. Cuatrocapaite-(NH4) tablets, some in vermiform stacks, with acicular torrecillasite; field of view 0.6 mm across. Specimen no. 66986.

Fig. 2. Cuatrocapaite-(NH4) satin-spar-like vein filling; field of view 1.7 mm across. Specimen no. 66984.

Fig. 3. Cuatrocapaite-(K) tablets; field of view 0.4 mm across. Specimen no. 66987.

Fig. 4. Tablets of the I-rich NH4-analogue of lucabindiite exhibiting apparent twinning by rotation on [001]; field of view 0.63 mm across. Specimen no. 66985.
Crystals are colourless and transparent, with a white streak. Crystals have a vitreous to pearly lustre, while vein fillings of cuatrocapaite-(NH4) have a silky lustre. The minerals do not fluoresce in long- or short-wave ultraviolet light. The Mohs hardness for both is 2½, based on scratch tests, and thin plates are flexible, but not elastic. Cleavage is perfect on {001}. The densities for cuatrocapaite-(NH4) and cuatrocapaite-(K), measured by flotation in methylene iodide – toluene are 2.65(2) and 2.76(2) g/cm3, respectively, and their calculated densities, based on the empirical formulas, are 2.667 and 2.771 g/cm3, respectively.
At room temperature, the minerals are not readily soluble in water or acids (including concentrated HCl, H2SO4 and HNO3). They decompose rapidly in aqueous solution of NaOH forming a nearly transparent residue of Mg(OH)2. As an added confirmation for the presence of ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ in cuatrocapaite-(NH4), a microchemical test was performed. The mineral was heated in a melting-point tube, yielding sublimates (from hot to cold) presumed to be (1) As2O3 (as arsenolite octahedra), (2) NH4Cl and (3) H2O. When placed in Na3[Co(NO2)6] solution, the presumed NH4Cl sublimate yielded an orange precipitate of (NH4)2Na[Co(NO2)6], thereby confirming the presence of
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ in cuatrocapaite-(NH4), a microchemical test was performed. The mineral was heated in a melting-point tube, yielding sublimates (from hot to cold) presumed to be (1) As2O3 (as arsenolite octahedra), (2) NH4Cl and (3) H2O. When placed in Na3[Co(NO2)6] solution, the presumed NH4Cl sublimate yielded an orange precipitate of (NH4)2Na[Co(NO2)6], thereby confirming the presence of ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $.
Optically, both minerals are uniaxial (–) and nonpleochroic. The indices of refraction for cuatrocapaite-(NH4) are ω = 1.779(3) and ε = 1.541(3) and those for cuatrocapaite-(K) are ω = 1.777(3) and ε = 1.539(3) (measured in white light). The Gladstone–Dale compatibilities 1 – (K p/K c) for the empirical formulas are both 0.019, in the range of superior compatibility (Mandarino, Reference Mandarino2007).
Infrared spectroscopy
In order to obtain infrared (IR) absorption spectra, powdered samples were mixed with anhydrous KBr, pelletised, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) at a resolution of 4 cm–1. Sixteen scans were collected for each mineral. The IR spectrum of an analogous pellet of pure KBr was used as a reference.
Absorption bands in the IR spectrum of cuatrocapaite-(NH4) (curve 1 in Fig. 5) and their assignments are (cm–1; s – strong band, w – weak band, sh – shoulder): 3440sh, 3413s, 3370sh (O–H stretching vibrations of H2O molecules), 3259 (N–H stretching vibrations of ammonium cations), 2035w (combination mode), 1652, 1630sh (bending vibrations of H2O molecules), 1425sh, 1410 (bending vibrations of ammonium cations), 1125w (broad; possibly, overlapping bands of combination modes), 673s, 593s (As3+–O stretching vibrations), 455 (Mg–O stretching vibrations combined with librational vibrations of H2O molecules). The assignment was made based in accordance with ranges for characteristic bands of ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ cations, H2O molecules, Mg–O and As3+–O bonds indicated by Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016) based on a representative collection of IR spectra of minerals (Chukanov, Reference Chukanov2014). In particular, the strongest band of Mg–O stretching vibrations is observed in the range 430–460 cm–1; the strongest band of As3+–O stretching vibrations of isolated As
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ cations, H2O molecules, Mg–O and As3+–O bonds indicated by Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016) based on a representative collection of IR spectra of minerals (Chukanov, Reference Chukanov2014). In particular, the strongest band of Mg–O stretching vibrations is observed in the range 430–460 cm–1; the strongest band of As3+–O stretching vibrations of isolated As![]() ${\rm O}_3^{3\ndash} $ groups are observed in the range 500–610 cm–1 and shift towards somewhat higher values as a result of polymerisation of AsO3 triangles. In particular, the strongest IR bands of As3+–O stretching vibrations are observed at 545 and 610 cm–1 for Pb5(AsO3)Cl7; 556, 605 and 641 cm–1 for leiteite; 628 and 654 cm–1 for manganarsite; and 532, 568 and 656 cm–1 for stenhuggarite (Chukanov, Reference Chukanov2014). It should be noted that characteristic bands of As4+–O stretching vibrations are observed in the range from 750 to 900 cm–1.
${\rm O}_3^{3\ndash} $ groups are observed in the range 500–610 cm–1 and shift towards somewhat higher values as a result of polymerisation of AsO3 triangles. In particular, the strongest IR bands of As3+–O stretching vibrations are observed at 545 and 610 cm–1 for Pb5(AsO3)Cl7; 556, 605 and 641 cm–1 for leiteite; 628 and 654 cm–1 for manganarsite; and 532, 568 and 656 cm–1 for stenhuggarite (Chukanov, Reference Chukanov2014). It should be noted that characteristic bands of As4+–O stretching vibrations are observed in the range from 750 to 900 cm–1.

Fig. 5. Powder IR absorption spectra of (1) cuatrocapaite-(NH4) and (2) cuatrocapaite-(K).
Absorption bands in the IR spectrum of cuatrocapaite-(K) (curve 2 in Fig. 5) and their assignments are (cm–1): 3530sh, 3478sh, 3406s, 3355sh (O–H stretching vibrations of H2O molecules), 3254 (N–H stretching vibrations of ammonium cations), 2035w (combination mode), 1652, 1622 (bending vibrations of H2O molecules), 1419w (bending vibrations of ammonium cations), 1125w (broad; possibly, overlapping bands of combination modes), 670s, 598s (As3+–O stretching vibrations), and 455 (Mg–O stretching vibrations combined with librational vibrations of H2O molecules).
For both minerals, splitting of the band of bending vibrations of H2O molecules (a non-degenerate mode) indicates the presence of locally non-equivalent water molecules in the structure. The IR spectrum of cuatrocapaite-(NH4) differs from that of cuatrocapaite-(K) in much higher intensities of the ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ bands.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ bands.
Composition
Chemical analyses (3 points for each mineral) were carried out using an electron microprobe (EDS mode, 20 kV and 600 pA. Attempts to use WDS mode, with higher beam current, were unsuccessful because of the instability of the minerals. For cuatrocapaite-(NH4), the beam was rasterised on an area 16 µm × 16 µm to minimise sample damage. Also for cuatrocapaite-(NH4), N was analysed by gas chromatography of products of ignition at 1200°C in oxygen flow, by means of a Vario Micro cube analyser (Elementar GmbH, Germany). For cuatrocapaite-(K), is was possible to estimate the content of NH4 from the IR spectrum using cuatrocapaite-(NH4) as a standard because in both minerals ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ has identical local environments.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ has identical local environments.
For both minerals, H2O was calculated from the idealised formula. Contents of other elements with atomic numbers >8 are below detection limits. Analytical data are given in Table 1.
Table 1. Analytical data (wt.%) for cuatrocapaite-(NH4) and cuatrocapaite-(K).

* Measured by gas chromatography for cuatrocapaite-(NH4); estimated by IR spectroscopy for cuatrocapaite-(K)
§ Calculated based on the ideal formula
S.D. – Standard deviation
The charge-balanced empirical formulas for cuatrocapaite-(NH4) and cuatrocapaite-(K) (based on 12 As atoms per formula unit) are (NH4)2.48Na1.66Mg0.87K0.09(As12O18.05)Cl5.88·16.02H2O and K2.68Na1.33Mg0.93(NH4)0.31(As12O18.01)Cl6.16·16.04H2O, respectively. The simplified formula for both minerals is (NH4,K)3(Na,Mg,□)3(As2O3)6Cl6·16H2O (with K preceding NH4 in the formula of the K-dominant phase). The ideal formula for cuatrocapaite-(NH4) is (NH4)3NaMg□(As12O18)Cl6·16H2O, which requires (NH4)2O 4.37, Na2O 1.73, MgO 2.25, As2O3 66.34, Cl 11.89, H2O 16.11, –O = Cl –2.68, total 100.00 wt.%. The idealised formula for cuatrocapaite-(K) is K3NaMg□(As12O18)Cl6·16H2O, which requires K2O 7.63, Na2O 1.67, MgO 2.18, As2O3 64.07, Cl 11.48, H2O 15.56, –O = Cl –2.59, total 100.00 wt.%.
X-ray crystallography and structure refinement
Powder X-ray studies were carried out using a Rigaku R-Axis Rapid II curved imaging plate microdiffractometer, with monochromatic MoKα radiation. A Gandolfi-like motion on the ϕ and ω axes was used to randomise the samples and observed d values and intensities were derived by profile fitting using JADE 2010 software (Materials Data, Inc.). The powder data for cuatrocapaite-(NH4) and cuatrocapaite-(K), presented in Tables 2 and 3, respectively, are quite similar and show good agreement with the patterns calculated from the structure refinements. Unit-cell parameters refined from the powder data using JADE 2010 with whole pattern fitting are a = 5.2501(7), c = 46.639(8) Å and V = 1113.3(4) Å3 for cuatrocapaite-(NH4) and a = 5.2637(16), c = 46.228(19) Å and V = 1109.2(8) Å3 for cuatrocapaite-(K).
Table 2. Powder X-ray data (d in Å) for cuatrocapaite-(NH4).

The strongest lines are given in bold
Table 3. Powder X-ray data (d in Å) for cuatrocapaite-(K).

The strongest lines are given in bold
Initial single-crystal X-ray studies were carried out for both cuatrocapaite-(NH4) and cuatrocapaite-(K) using the same diffractometer and radiation used for the powder studies. Crystals of cuatrocapaite-(K) are generally of poorer quality than those of cuatrocapaite-(NH4) and our structure data for cuatrocapaite-(K), while confirming it to be isostructural with cuatrocapaite-(NH4), yielded a much poorer refinement (R 1 = 6.97% for 263 reflections with I o > 2σI). Rather than report this refinement, herein we provide the details only for the structure refinement for cuatrocapaite-(NH4). Nevertheless, we confirm that the refinement for cuatrocapaite-(K) is consistent with nearly full occupancy of K at the (K,NH4) site.
The final cuatrocapaite-(NH4) structure data were collected on a Rigaku Synergy-S diffractometer equipped with a HyPix-6000HE detector at the Natural History Museum, University of Oslo using monochromatised MoKα radiation. The Rigaku CrysAlisPro software package was used for processing the structure data, including the application of a shape-based absorption correction. An initial structure model was obtained by the charge-flipping method using SHELXT (Sheldrick, Reference Sheldrick2015a) in the space group R ![]() ${\bar 3}$m. Refinement proceeded by full-matrix least-squares on F 2 using SHELXL-2016 (Sheldrick, Reference Sheldrick2015b). A difference-Fourier map failed to reveal the locations of the H atoms associated with the
${\bar 3}$m. Refinement proceeded by full-matrix least-squares on F 2 using SHELXL-2016 (Sheldrick, Reference Sheldrick2015b). A difference-Fourier map failed to reveal the locations of the H atoms associated with the ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ cation or with the two partially occupied OW sites. The H sites associated with
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ cation or with the two partially occupied OW sites. The H sites associated with ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ are presumed to be disordered, as is commonly the case. The only difference-Fourier peak > 0.42 e–/A3 has a magnitude of 1.21 e–/A3 (which refines to <5% occupancy by O). This peak at [⅔, ⅓, 0.391], in the centre of the ‘cavities’ in the As2O3 (arsenite) sheet and only 1.42 Å from the Cl site, is assumed to be a refinement artefact. The data collection and refinement details are given in Table 4, atom coordinates and displacement parameters in Table 5 and selected bond distances in Table 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ are presumed to be disordered, as is commonly the case. The only difference-Fourier peak > 0.42 e–/A3 has a magnitude of 1.21 e–/A3 (which refines to <5% occupancy by O). This peak at [⅔, ⅓, 0.391], in the centre of the ‘cavities’ in the As2O3 (arsenite) sheet and only 1.42 Å from the Cl site, is assumed to be a refinement artefact. The data collection and refinement details are given in Table 4, atom coordinates and displacement parameters in Table 5 and selected bond distances in Table 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Data collection and structure refinement details for cuatrocapaite-(NH4).

R int = Σ|F o2–F o2(mean)|/Σ[F o2]. GoF = S = {Σ[w(F o2–F c2)2]/(n–p)}1/2. R 1 = Σ||F o|–|F c||/Σ|F o|. wR 2 = {Σ[w(F o2–F c2)2]/Σ[w(F o2)2]}1/2; w = 1/[σ2(F o2) + (aP)2 + bP] where a is 0.0305, b is 0 and P is [2F c2 + Max(F o2,0)]/3.
* The structural formula reflects the refined site occupancies; no effort has been made to balance the net charge of this formula, which has a net charge of +0.3.
Table 5. Atom coordinates and displacement parameters (Å2) for cuatrocapaite-(NH4)*.

* Site occupancies: N = 0.884(9)N/0.116(9)K, Na = 0.287(9), Mg = 0.264(10), OWa = 0.303(13), OWb = 0.277(8).
Table 6. Selected bond distances (Å) for cuatrocapaite-(NH4).

* Too short for cation–O bonds.
Description of the structure
The structure of cuatrocapaite-(NH4) (Fig. 6) contains four types of layers: (1) a planar neutral As2O3 (arsenite) sheet with hexagonal symmetry (Fig. 7a); (2) an ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ layer that links adjacent arsenite sheets via bonds to their O atoms; (3) a Cl– layer placed on the As side of each arsenite sheet with the Cl atoms forming long bonds to the As atoms; and (4) a layer containing partially occupied Na, Mg and H2O sites (Fig. 7b,c,d) that is flanked on either side by Cl layers. The Cl–As2O3–NH4–As2O3–Cl layer sequence is identical to the Cl–As2O3–K–As2O3–Cl layer sequence in the structures of lucabindiite (Garavelli et al., Reference Garavelli, Mitolo, Pinto and Vurro2013) and gajardoite (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2016). In lucabindiite, this sequence repeats itself with no intervening layers, while gajardoite incorporates a disordered Ca–H2O layer and cuatrocapaite-(NH4) incorporates a disordered Na–Mg–H2O layer. Based on the low occupancies of the sites in the Na–Mg–H2O layer and the placements of the H2O groups around the cations (Fig. 7b,c,d), the Na and Mg coordination polyhedra in this layer appear to be isolated, that is, not to be linked to one another. Note that the short Mg–OWa distance of 1.985 Å and the short Na–OWb distance of 1.721 Å do not correspond to bonds; these OW sites are vacant when the nearby cation sites are occupied.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $ layer that links adjacent arsenite sheets via bonds to their O atoms; (3) a Cl– layer placed on the As side of each arsenite sheet with the Cl atoms forming long bonds to the As atoms; and (4) a layer containing partially occupied Na, Mg and H2O sites (Fig. 7b,c,d) that is flanked on either side by Cl layers. The Cl–As2O3–NH4–As2O3–Cl layer sequence is identical to the Cl–As2O3–K–As2O3–Cl layer sequence in the structures of lucabindiite (Garavelli et al., Reference Garavelli, Mitolo, Pinto and Vurro2013) and gajardoite (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2016). In lucabindiite, this sequence repeats itself with no intervening layers, while gajardoite incorporates a disordered Ca–H2O layer and cuatrocapaite-(NH4) incorporates a disordered Na–Mg–H2O layer. Based on the low occupancies of the sites in the Na–Mg–H2O layer and the placements of the H2O groups around the cations (Fig. 7b,c,d), the Na and Mg coordination polyhedra in this layer appear to be isolated, that is, not to be linked to one another. Note that the short Mg–OWa distance of 1.985 Å and the short Na–OWb distance of 1.721 Å do not correspond to bonds; these OW sites are vacant when the nearby cation sites are occupied.

Fig. 6. The structure of cuatrocapaite-(NH4).

Fig. 7. Selected layer components in the structure of cuatrocapaite-(NH4) viewed down c: (a) the As2O3 (arsenite) layer, (b) the Na–Mg–H2O layer, (c) the Na–H2O portion of the Na–Mg–H2O layer, (d) the Mg–H2O portion of the Na–Mg–H2O layer. The unit-cell outline is shown.
The bond-valence sums (BVS) based on the As3+–O parameters from Gagné and Hawthorne (Reference Gagné and Hawthorne2015), As3+–Cl from Brese and O'Keeffe (Reference Brese and O'Keeffe1991) and ![]() ${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $–O from García-Rodríguez et al. (Reference García-Rodríguez, Rute-Pérez, Piñero and González-Silgo2000) are As1 = 2.98, As2 = 3.00, O = 2.08, N(NH4) = 1.00 and Cl = 0.24 valence units (vu). The Cl BVS does not include hydrogen bond contributions from the partially occupied H2O groups; however, very low BVS values are also observed for Cl in the lucabindiite (0.31 vu) and gajardoite (0.24 vu) structures, a phenomenon probably caused by the repulsive effect of the lone electron pair of the As3+. The calculation of the BVS values for the components of the Na–Mg–H2O layer is problematic because of the disorder and partial occupancies. Note that, while the Na and Mg site assignments are based upon bond lengths, the Mg–OWb distance (2.230 Å) suggests that some Na may occupy this site, as well.
${\rm NH}_{\rm 4}^{\vskip -2pt\rm \scale65% +} $–O from García-Rodríguez et al. (Reference García-Rodríguez, Rute-Pérez, Piñero and González-Silgo2000) are As1 = 2.98, As2 = 3.00, O = 2.08, N(NH4) = 1.00 and Cl = 0.24 valence units (vu). The Cl BVS does not include hydrogen bond contributions from the partially occupied H2O groups; however, very low BVS values are also observed for Cl in the lucabindiite (0.31 vu) and gajardoite (0.24 vu) structures, a phenomenon probably caused by the repulsive effect of the lone electron pair of the As3+. The calculation of the BVS values for the components of the Na–Mg–H2O layer is problematic because of the disorder and partial occupancies. Note that, while the Na and Mg site assignments are based upon bond lengths, the Mg–OWb distance (2.230 Å) suggests that some Na may occupy this site, as well.
Acknowledgements
An anonymous reviewer and Structures Editor Peter Leverett are thanked for their constructive comments on the manuscript. Infrared spectroscopic studies and determination of the chemical composition were carried out with the support of the Russian Foundation for Basic Research, grant no. 17-05-00179. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.26.