Introduction
The mineral stillwaterite, Pd8As3, was discovered in 1975 by Cabri et al. (Reference Cabri, Laflamme, Stewart, Rowland and Chen1975). A single-crystal precession study established the hexagonal symmetry of the mineral and possible space group P $\bar{3}$![]() or P3. The unit cell parameters were determined by powder X-ray diffraction (XRD) in a Gandolfi camera as a = 7.399 and c = 10.311 Å, however no more information about the structure solution was published.
or P3. The unit cell parameters were determined by powder X-ray diffraction (XRD) in a Gandolfi camera as a = 7.399 and c = 10.311 Å, however no more information about the structure solution was published.
The stillwaterite ideal formula is established as Pd8As3 (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975) and corresponds to the formula of the synthetic phase Pd2.65As reported earlier by Saini et al. (Reference Saini, Calvert, Heyding and Taylor1964). The mineral X-ray diffraction pattern for stillwaterite is very similar to those of the synthetic Pd2.65As, however there are differences in the weak intensity reflections which are not resolved in the stillwaterite XRD data. A complete crystal structure analysis has not been performed for the mineral, or for the synthetic phase.
It is important to determine the structural characteristics of stillwaterite from an economic perspective. Both chemical and crystallographic characteristics play fundamental roles in the hydro-metallurgical separation processes of these PGM minerals and particularly in the flotation of ores for separation (Shackleton et al., Reference Shackleton, Malysiak and O'Connor2007).
The stillwaterite crystal structure is interesting in the context of structural relations in a family of minerals with common formula Pd8T 3 (T = Sb and/or As): stillwaterite, Pd8As3; arsenopalladinite, Pd8As2.5Sb0.5; mertieitete-II, Pd8Sb2.5As0.5; and synthetic, Pd8Sb3. The crystal structures of arsenopalladinite, mertieitete-II and Pd8Sb3 are known today.
The Pd8Sb3 structure was solved by Wopersnow and Schubert (Reference Wopersnow and Schubert1976), and then refined by Marsh (Reference Marsh1994). It is trigonal in space group R $\bar{3}$![]() c (Table 1). The stability of the Pd8Sb3 structure type extends up to composition of Pd8Sb2.5As0.5, confirmed by the structural study of the mineral mertieite-II, Pd8Sb2.5As0.5 (Karimova et al., Reference Karimova, Zolotarev, Evstigneeva and Johanson2018). The mineral arsenopalladinite, Pd8As2.5Sb0.5, has another structure type of triclinic in space group P $\bar{1}$
c (Table 1). The stability of the Pd8Sb3 structure type extends up to composition of Pd8Sb2.5As0.5, confirmed by the structural study of the mineral mertieite-II, Pd8Sb2.5As0.5 (Karimova et al., Reference Karimova, Zolotarev, Evstigneeva and Johanson2018). The mineral arsenopalladinite, Pd8As2.5Sb0.5, has another structure type of triclinic in space group P $\bar{1}$![]() (Table 1) (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020).
(Table 1) (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020).
Table 1. Crystallographic parameters for the Pd8T 3 (T = Sb, As) compounds.

The Pd8Sb3, mertieite-II, Pd8Sb2.5As0.5, and arsenopalladinite, Pd8As2.5Sb0.5, structures have common features. They consist of alternations of layers made by pnictogen (Sb and As) atoms and layers made by palladium atoms stacked along the c axis. The pnictogen layers have the same topology in the minerals mertieite-II and arsenopalladinite (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020). The Pd8As3 structure has close relations to the structures of Pd8Sb3, mertieite-II and arsenopalladinite as evidenced by the crystallographic data in Table 1. The aim of this paper is crystal structure analysis of a synthetic analogue of stillwaterite, Pd8As3, using single-crystal X-ray diffraction data.
Materials and methods
The synthetic analogue of mineral stillwaterite Pd8As3 was prepared using the solid phase reaction method in evacuated silica glass ampoules. Pure elements were used (Pd 99.9% Reakhim, As 99.5% Reakhim). Arsenic was preliminarily purified from oxide in evacuated silica glass ampoules at 450°C for a week. As a result, the more volatile arsenic oxide was transferred to the coldest part of the ampoule and separated easily. Arsenic and palladium, taken in a stoichiometric ratio, were placed in a silica glass ampoule, evacuated to a pressure of <1 Pa, and sealed using a CH4/O2 burner. At the first step the ampoule with palladium and arsenic was heated up to temperature 850°C for seven days. It was observed that the reaction proceeds extremely slowly, therefore the sintering Pd–As piece was melted in an ampoule in a CH4/O2 flame and quenched in cold water. After that the reacted mixture was removed from the ampoule, weighed, ground in an agate mortar under acetone, placed in another silica ampoule, evacuated, and heated at 450°C for 120 days.
The crystals were mounted in an epoxy resin and a polished mount was prepared. The chemical composition of the grains was determined using a TESCAN Vega II XMU spectrometer Energy 450/XT instrument (Table 2). The energy-dispersive X-ray spectroscopy analyses were performed with an operating voltage of 20 kV and a beam current of 15 nA. Pure Pd and synthetic InAs were used as standards. The quantitative analysis on eight grains gave the empirical formula Pd7.99As3.01 calculated on the basis of 11 atoms per formula unit. Scanning electron microscopy analysis of the experimental product confirmed that it consists only of Pd8As3. The Pd8As3 forms anhedral grains 20×30 to 50×150 μm (Fig. 1).
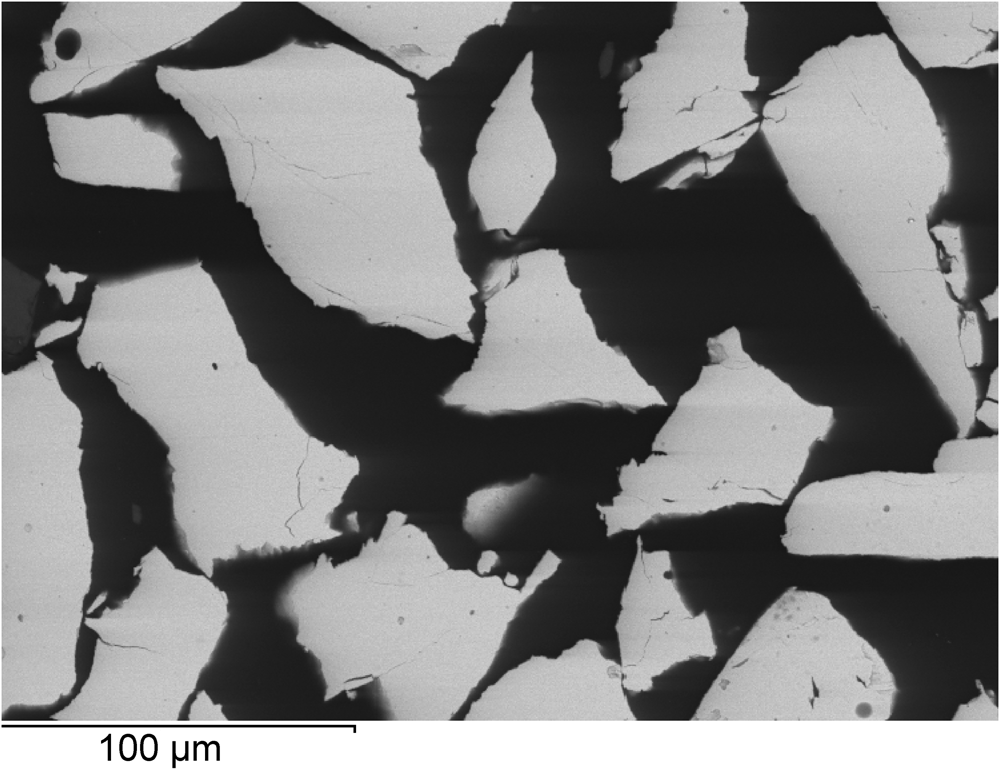
Fig. 1. Back-scattered electron image of Pd8As3 grains.
Table 2. Electron microprobe analyses of Pd8As3.

* Calculated with 11 atoms per formula unit.
Powder X-ray diffraction data of the synthetic sample were collected on a Rigaku D/MAX 2200 diffractometer using CuKα radiation. The data were indexed by the Jade 2004 program (Materials Data, Inc.) on the basis of a hexagonal unit cell with: a = 7.408 and c = 10.322 Å (Table 3).
Table 3. Powder XRD data for Pd8As3.

Single-crystal X-ray diffraction data for Pd8As3 were collected on the Xcalibur Eos diffractometer at the Center of X-ray diffraction studies at St. Petersburg State University (XRD Center SPbSU). A total of 1512 reflections were collected at 293 K using monochromatic MoKα X-radiation. The data were integrated and corrected by means of the CrysAlisPro (Agilent, 2012) program package, which was also used to apply empirical absorption correction using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The structure was solved by direct methods and refined in anisotropic approximation using SHELX programs (Sheldrick Reference Sheldrick2015a, Reference Sheldrick2015b) in the frame of the WinGX software package (Farrugia, Reference Farrugia2012). Scattering curves for neutral atoms, together with anomalous dispersion corrections, were taken from the International Tables for X-ray Crystallography (Prince, Reference Prince2004). Refinement gave full occupation for all the sites in the structure. Crystal data, experimental and refinement details are presented in Table 4. The final atomic coordinates and displacement parameters are listed in Table 5 and the selected interatomic distances are in Table 6. Table 7 shows the coordination numbers and the average values of the bond lengths. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material.
Table 4. Crystal data and structure refinement for Pd8As3.

w = 1/[\s2(Fo2) + (0.0102P)2] where P = (Fo2 + 2Fc2)/3.
Table 5. Atomic coordinates and equivalent isotropic and anisotropic displacement parameters (Å2) for Pd8As3.

* All sites are fully occupied by Pd and As atoms.
Table 6. Selected bonds lengths (Å) for Pd8As3.

Table 7. Coordination number (CN) together with the average bonds lengths (dm) around the different atoms in Pd8As3.

* CN(Pd) is the number of nearest Pd neighbours.
** CN(As) is the number of nearest As neighbours.
Identity of synthetic Pd8As3 to natural stillwaterite
The Pd and As content of the synthetic material is close to that reported for stillwaterite by Cabri et al. (Reference Cabri, Laflamme, Stewart, Rowland and Chen1975) using a formula calculated on 3 atoms of As. Stillwaterite grains from the Stillwater Complex type locality showed a Pd/As ratio 7.84/3.00 to 8.13/3.00 (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975). The synthetic material of our study has a Pd/As ratio 7.63/3.00 to 8.20/3.00 (Table 2) and its powder XRD pattern fits very well to the those of stillwaterite and synthetic Pd8As3 obtained by Cabri et al. (Reference Cabri, Laflamme, Stewart, Rowland and Chen1975) (Table 3).
Crystal structure description
Three palladium atoms positions are located on the 3-fold axis of symmetry (Wyckoff symbols are 2c and 2d) and the other three are general with Wyckoff symbol 6g of the space group (Table 5). Two arsenic atoms occupy sites on the 3-fold axis of symmetry: on the 1a and 2d Wyckoff sites. The third arsenic atom is in general position 6g.
The coordination polyhedra around atoms in the structure are shown in Fig. 2. The interatomic Pd–As distances varies from 2.4325 to 2.8480 Å (Table 6). The overall average Pd–As distance is 2.593 Å. These values are typical for the bonds in the crystal structures of ternary Pd–As–Sb minerals. The minimum Pd–As distance is equal to 2.465 Å in isomertieite, Pd11Sb2As2, and 2.488 Å in mertieite-II, Pd8Sb2.5As0.5 (Karimova et al., Reference Karimova, Grokhovskaya, Zolotarev and Gurzhiy2016, 2018). It is slightly less at 2.378 Å in the arsenopalladinite, Pd8As2.5Sb0.5 (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020). The maximum palladium to arsenic distance is 2.662 Å and 2.537 Å in isomertieite and mertieite-II, respectively, and very long at 2.967 Å in arsenopalladinite (Karimova et al., Reference Karimova, Grokhovskaya, Zolotarev and Gurzhiy2016, Reference Karimova, Zolotarev, Evstigneeva and Johanson2018, Reference Karimova, Zolotarev, Johanson and Evstigneeva2020). In the crystal structures of palladium arsenides the greatest variation in the palladium to arsenic distances is found in the synthetic phase Pd5As: from 2.361 Å to 3.241 Å, average 2.788 Å (Matkovic and Schubert, Reference Matkovic and Schubert1978). The mineral palladoarsenide, Pd2As, has the average Pd–As bonds length equal to 2.59 Å, the minimum is 2.39 Å and the maximum is 2.70 Å (Baelz and Schubert, Reference Baelz and Schubert1969; Begizov et al., Reference Begizov, Meshchankina and Dubakina1974). In the crystal structure of synthetic PdAs2 each palladium atom is surrounded by 6 arsenic atoms with octahedral coordination and bond lengths equal to 2.495 Å (Breese and Schnering, Reference Breese and Schnering1994).

Fig. 2. Coordination polyhedrons in the Pd8As3 structure.
We used the principles of a structure description in terms of atomic nets proposed by Pearson (Reference Pearson1972). The Pd8As3 structure consists of palladium and arsenic atoms layers alternated along the c axis (Fig. 3). Projection of the structure along the a axis is shown in Fig. 4. Topology of the layers is drawn in Fig. 5. The layers of arsenic atoms are 36 triangular nets (named ‘A’ and ‘D’). The layers of palladium atoms are triangular (‘B’), pentagon–triangular (‘C’) and the triangle–quadrangle (‘E’) nets (Fig. 5). The stacking sequence is: ABCDEEDCBA (Fig. 4). The first layer in the stacking sequence is formed by As1 and As2 atoms sites (A net, Fig. 5). It is followed by two palladium layers: highly buckled triangular net B (built up by the Pd3 site) and pentagon–triangular net C (consists of Pd1, Pd2 and Pd5 sites). The next layer is arsenic triangular net D (contains As3 sites). The Pd4 and Pd6 sites form the triangle-quadrangle E net.

Fig. 3. The crystal structure of Pd8As3, perspective view along c axis. Black circles – As atoms, red circles – Pd atoms.

Fig. 4. The crystal structure of Pd8As3, projection along the c axis. Colour code as in Fig. 3.

Fig. 5. Atomic nets in the crystal structure of Pd8As3. Colour code as in Fig. 3.
Discussion
The Pd8T 3 (T = As and/or Sb) family includes minerals mertieite-II, Pd8Sb2.5As0.5, arsenopalladinite, Pd8As2.5Sb0.5, stillwaterite, Pd8As3, and synthetic Pd8Sb3. The structural motive is the same in these compounds: nets of pnictogen atoms are the base of the structures. The alternation of the arsenic and antimony nets along the c axis in the Pd8T 3 compounds is shown on Fig. 6.

Fig. 6. Pnictogen nets in the structures of the Pd8T 3 (T = As, Sb) compounds. The blue balls are antimony atoms, the black balls are arsenic atoms. The palladium atoms are omitted.
The common scheme of the pnictogen nets is represented on Fig. 7. The pnictogen nets are triangular with 36 topology. They contain three atomic sites possibly hosting arsenic or antimony atoms: T 1, T 2 and T 3.

Fig. 7. The idealised plan of the pnictogen nets in the Pd8T 3 (T = As, Sb) compounds.
The distribution of pnictogen atoms on the triangle nets sites in the structures of Pd8T 3 compounds is given in Table 8. Four variants of pnictogen site occupation are represented: all T-sites are occupied by antimony (Pd8Sb3); all T-sites are filled by arsenic (stillwaterite); only one T-site is filled by arsenic (mertietite-II); and only one T-site is filled by antimony (arsenopalladinite) (Table 8).
Table 8. Occupation of the sites in pnictogen (As/Sb) triangular nets in Pd8T 3, (T = As, Sb).

* The crystal of mertieite-II from Kaareoga River placer is enriched with arsenic and occupation of the T2 site is Sb0.96As0.04 (Karimova et al., Reference Karimova, Zolotarev, Evstigneeva and Johanson2018).
** The T2 site in arsenopalladinite is divided into two sites (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020).
Synthetic Pd8Sb3 (Pd8Sb2.5Sb0.5) and mertieite-II, Pd8Sb2.5As0.5 – the antimony end-members of the Pd8T 3 (T = As, Sb) family – are isostructural. They have the highest symmetry of the structures (Table 1) and the highest symmetry of the pnictogen nets among the named compounds (Table 8). Incorporation of arsenic atoms in the T3 site of the Pd8Sb3 pnictogen nets does not affect the structure symmetry in the case of mertieite-II, Pd8Sb2.5As0.5.
In contrast, arsenopalladinite, Pd8As2.5Sb0.5, contains pnictogen nets constituted mainly by arsenic atoms, except for one site (Table 8). The symmetry of the structure changes from trigonal in mertieite-II to triclinic in arsenopalladinite (Karimova et al., Reference Karimova, Zolotarev, Johanson and Evstigneeva2020).
Stillwaterite, Pd8As3 – the arsenic end-member of the Pd8T 3 (T = As, Sb) family – has the same 36 topology of the pnictogen nets: arsenic atoms occupy all atomic sites of the pnictogen nets. The pnictogen nets symmetry of the Pd8As3 structure is lower than those of Pd8Sb3 (Table 8). The main difference between the structure of stillwaterite, Pd8As3, and structures of Pd8T 3 compounds is the topology of palladium layers. Triangular and pentagon–triangular palladium nets are found in the Pd8Sb3, Pd8Sb2.5As0.5 and Pd8As2.5Sb0.5 structures (Karimova et al., Reference Karimova, Zolotarev, Evstigneeva and Johanson2018, Reference Karimova, Zolotarev, Johanson and Evstigneeva2020). The Pd8As3 structure contains these types of palladium layers too (named the B and C nets), however it has also triangle–quadrangle (E) layers which are not found in other Pd8T 3 structures.
Acknowledgments
We are grateful to Principal Editor Dr. Stuart Mills, Associated Editor Dr. František Laufek, Structural Editor Prof. Peter Leverett, and two anonymous Reviewers for valuable and helpful comments. We acknowledge the Resource Centre of X-ray diffraction studies of St. Petersburg State University for collection of the experimental single crystal XRD data. This work is carried out in the framework of the Russian State Assignment for Fundamental Research (granted to Institute of Geology of Ore Deposits RAS). Support by President Grant to the leading scientific schools of the Russian Federation through Project NSh–2394.2022.1.5 is acknowledged. Authors thank A.N. Nekrasov (IEM RAS) for the SEM/EDXS analyses.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2022.57
Competing interests
The authors declare none.