Introduction
Sepiolite [ideal formula: Mg8Si12O30(OH)4(OH2)4·8H2O] is a naturally occurring microporous clay mineral, belonging to the palygorskite–sepiolite group and originating in a wide variety of geological environments. Sepiolite can be formed by direct precipitation from highly-saline waters, or from alteration of other clay minerals, in lacustrine and marginal-marine settings, under semi-arid to arid climatic conditions (Eugster and Hardie, Reference Eugster and Hardie1975; Trauth, Reference Trauth1977; Weaver, Reference Weaver, Singer and Galàn1984; Velde, Reference Velde1985; Jones and Galán, Reference Jones, Galán and Bailey1988; Chahi et al., Reference Chahi, Fritz, Duplay, Weber and Lucas1997; Mayayo et al., Reference Mayayo, Torres-Ruiz, Gonzalez-Lopez, Lopez-Galindo and Bauluz1998; Galán and Pozo, Reference Galán, Pozo, Galán and Singer2011); authigenesis in calcareous soils under semi-arid conditions has also been reported (Galán and Pozo, Reference Galán, Pozo, Galán and Singer2011). Other known occurrences originated after seafloor hydrothermal alteration of basaltic glass, volcanoclastic and clayey sediments, or ultramafic rocks (e.g. Bonatti et al., Reference Bonatti, Craig Simmons, Breger, Hamlyn and Lawrence1983; Bach et al., Reference Bach, Banerjee, Dick and Baker2002). Sepiolite can also form in on-land hydrothermal systems by precipitation from low T (< 100°C) Si/Mg enriched fluids circulating in different rock types, including carbonate rocks (Ehlmann et al., Reference Ehlmann, Sand and Regis1962; Imai and Otsuka, Reference Imai, Otsuka, Singer and Galàn2000; Kovács-Pálffy et al., Reference Kovács-Pálffy, Kónya, Kalmár, Fehér and Földvári2016) and ultramafites/meta-ultramafites (e.g. Yalçin and Bozkaya, Reference Yalçin and Bozkaya2004). A biogenic origin induced by microorganisms (Leguey et al., Reference Leguey, Ruiz de Leòn, Ruiz and Cuevas2010; Cuevas et al., Reference Cuevas, Leguey, Ruiz, Galán and Singer2012; del Buey et al., Reference del Buey, Cabastrero, Arroyo and Sanz-Montero2018) was also reported. In the crystal structure of sepiolite (Brauner and Preisinger, Reference Brauner and Preisinger1956; Brindley, Reference Brindley1959), the octahedral (O) layer splits into ribbons elongated in the z-axis direction and pinched between two continuous, ‘waving’ tetrahedral (T) sheets, due to a periodic inversion in the apical oxygens orientation. Chessboard connected TOT ribbons alternate to nano-tunnels (maximum effective width: 10.6 Å), running along z and filled by weakly bound zeolitic H2O (Ferraris et al., Reference Ferraris, Makovicky and Merlino2008). Tightly bound, structural OH2 (Bailey et al., Reference Bailey, Alietti, Brindley, Formosa, Jasmund, Konta, Mackenzie, Nagasawa, Rausell-Colom and Zvyagin1980) completes the coordination of octahedral Mg at the ribbon borders. Exchangeable ions and/or small molecules may be hosted within the tunnels and/or on the fibre surface.
Due to its nano- and micro-porosity, sepiolite has excellent sorption properties and wide surface area, which can be exploited in a broad variety of applications such as lubricants, catalysts, adsorbents, cleaning compresses for restoration and cat litters (Àlvarez et al., Reference Àlvarez, Santarén, Esteban-Cubillo, Aparaicio, Galán and Singer2011; Lòpez-Galindo et al., Reference Lòpez-Galindo, Viseras, Aguzzi, Cerezo, Galán and Singer2011). Moreover, its markedly fibrous habit grants its use as an asbestos substitute in building and seal materials, friction compounds for automotive brakes and high-temperature insulators (Solebello, Reference Solebello2009). Sepiolite has been mined for centuries: over 90% comes from Spain (Neogene lacustrine sequences of the Madrid basin; Brell et al., Reference Brell, Doval and Caramés1985; Ordoñez et al., Reference Ordoñez, Calvo, del Cura M.A., Zarza A.M., Hoyos, Anadòn, Cabrera and Keiths1991), with 800 thousand tons annual production (O'Driscoll, Reference O'Driscoll1992); smaller deposits are located in the USA (Amargosa Desert), Kenya and Tanzania (Amboseli), China (Guanshan), Greece (Ventzia basin) and Somalia (El Bur) (Galán and Pozo, Reference Galán, Pozo, Galán and Singer2011). All commercially sold sepiolites have fibres < 5 µm long (at times, achieved after preliminary grinding) – the threshold of non-carcinogenicity fixed by the International Agency for Research on Cancer, World Health Organization (IARC, 1997). However, sepiolites with unusually long fibres (> 10 µm, due to variability in the crystallinity degree and texture; Suárez and García-Romero, Reference Suárez and García-Romero2012; García-Romero and Suárez, Reference García-Romero and Suárez2013) have been seldom signalled for their carcinogenic potential (Pott et al., Reference Pott, Bellman, Muhle, Rödelsperger, Rippe, Roller, Rosenbruch and Bignon1990; Pott et al., Reference Pott, Roller, Rippe, Germann, Bellman, Brown, Hoskins and Johnson1991; Bellman et al., Reference Bellman, Muhle and Ernst1997; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014). This study describes an atypical asbestiform sepiolite, with extremely long fibres, associated with small amounts of aliphatic hydrocarbons. Similar samples have been seldom reported in the outcrops of Northwestern Italy (e.g. Valle d'Aosta, Piemonte and Liguria: Belluso and Sandrone, Reference Belluso and Sandrone1989; Belluso et al., Reference Belluso, Compagnoni, Ferraris and di Torino1995) – only once associated with hydrocarbons (Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014).
Experimental
Materials and geological setting
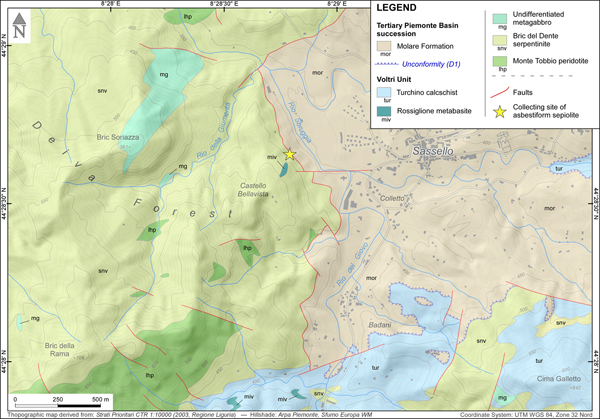
The specimen investigated was collected in 2010 by Giuseppe Pipino in a small ‘borrow pit’ in the Deiva forest (~44°28'42.6“N, 8°28'48.1”E, Sassello municipality, Liguria, NW Italy), belonging to the Beigua Geopark (Fig. 1).
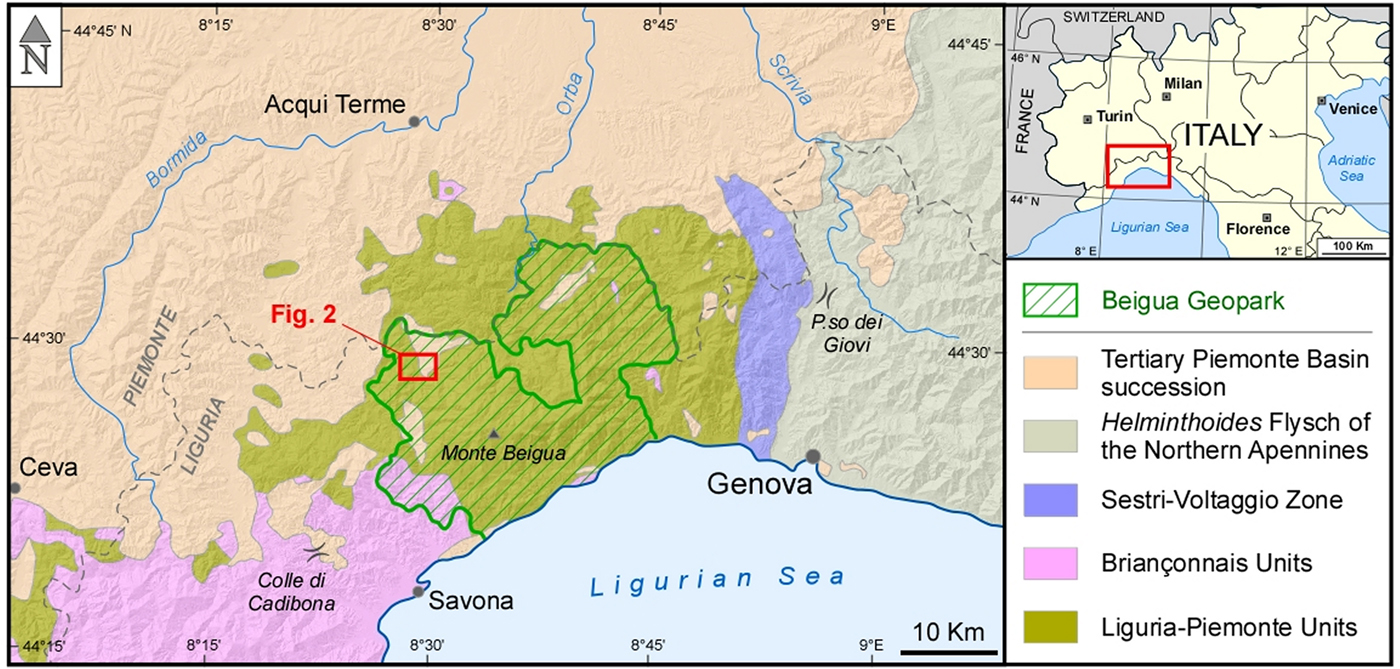
Fig. 1. Geological scheme of the Beigua Geopark and its surroundings, at the boundary between Liguria and Piemonte regions (Alps-Apennines interference zone sensu Piana et al., Reference Piana, Fioraso, Irace, Mosca, d'Atri, Barale, Falletti, Monegato, Morelli, Tallone and Vigna2017). The red rectangle indicates the position of the simplified geological map of Fig. 2.
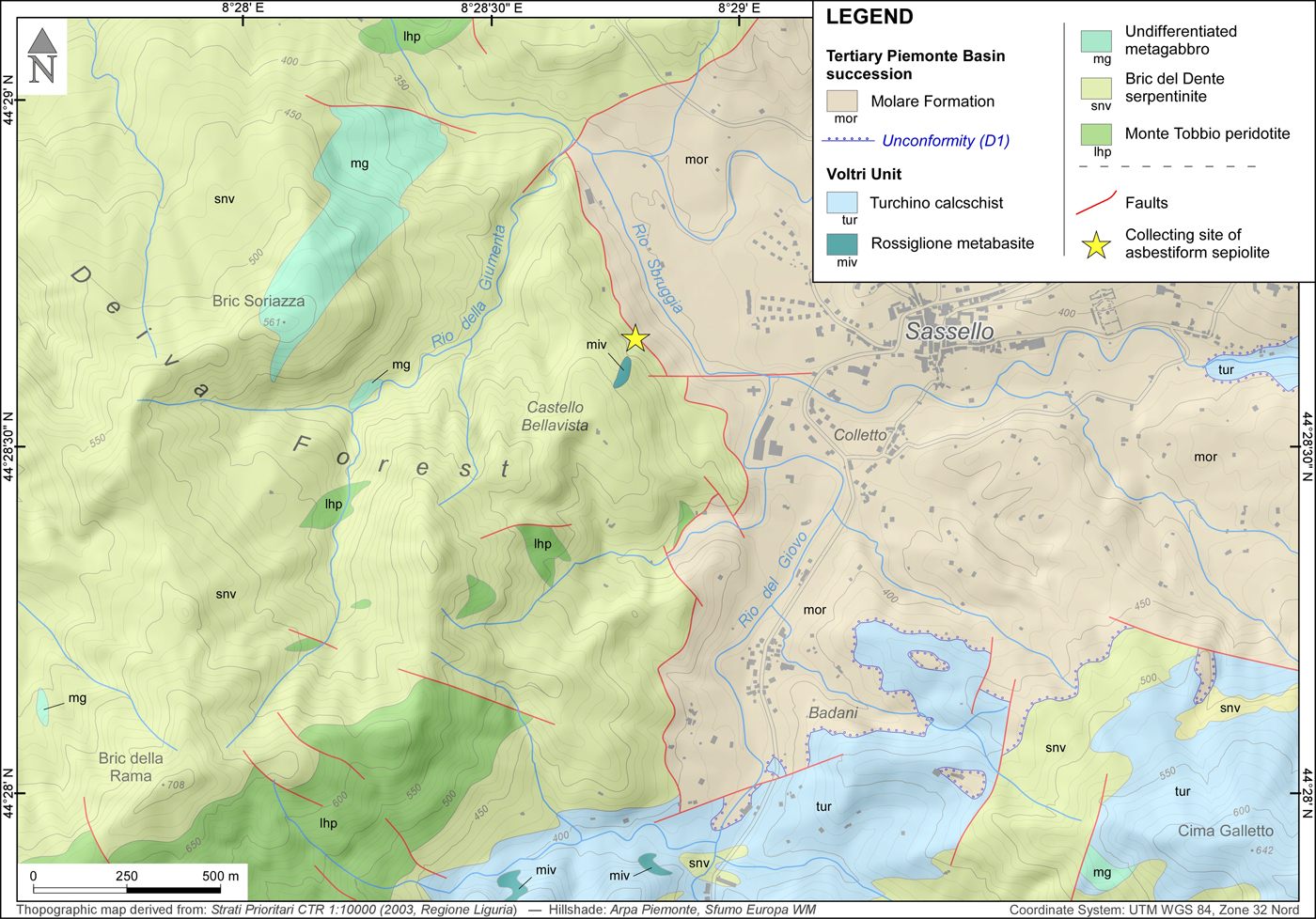
Fig. 2. Simplified geological map of the Deiva Forest–Sassello area (see position in Fig. 1), indicating the site of collection of the asbestiform sepiolite (star). Geological boundaries were redrawn from Federico et al. (Reference Federico, Crispini, Dabove, Piazza and Capponi2016); the D1 unconformity at the base of the Molare Formation is a regional unconformity of early Rupelian age which can be traced throughout the Tertiary Piemonte Basin (Piana et al., Reference Piana, Fioraso, Irace, Mosca, d'Atri, Barale, Falletti, Monegato, Morelli, Tallone and Vigna2017, and references therein).
The Sassello area is located on the northern side of the Ligurian Apennines, and belongs, from a geological point of view, to the Alps–Apennines interference zone sensu Piana et al. (Reference Piana, Fioraso, Irace, Mosca, d'Atri, Barale, Falletti, Monegato, Morelli, Tallone and Vigna2017), made up of geological units that shared, at least in part, the evolution of both orogens (Molli et al., Reference Molli, Crispini, Malusà, Mosca, Piana and Federico2010 and Piana et al., Reference Piana, Fioraso, Irace, Mosca, d'Atri, Barale, Falletti, Monegato, Morelli, Tallone and Vigna2017, with references therein). This sector is composed of metamorphic rocks derived from the Mesozoic Liguria–Piemonte oceanic domain, presently cropping out in the so-called Voltri ophiolitic ‘massif’ (Fig. 1).
The Voltri Unit, which underwent eclogite-facies Alpine peak metamorphism, makes up the greatest part of the Voltri ‘massif’, including the Sassello area. It is composed of lherzolites largely transformed into serpentinites (‘Bric del Dente’ serpentinite), associated with 100–1000 m sized bodies of metagabbro. These are followed by rocks derived from original basalts and basaltic breccias and the relevant metasedimentary cover (Chiesa et al., Reference Chiesa, Cortesogno, Forcella, Galli, Messiga, Pasquaré, Pedemonte, Piccardo and Rossi1975; Piccardo, Reference Piccardo1984; Messiga and Scambelluri, Reference Messiga and Scambelluri1991; Capponi and Crispini, Reference Capponi and Crispini2008). To the north, the metamorphic rocks of the Voltri Unit are overlain by the Cenozoic succession of the Tertiary Piemonte Basin. (Capponi et al. Reference Capponi, Crispini and Federico2013; d’Atri et al. Reference d'Atri, Irace, Piana, Tallone, Varrone, Bellino, Fioraso, Cadoppi, Fusetti, Morelli, Lanteri, Paro, Piccini, Trenkwalder and Violanti2016; Federico et al., Reference Federico, Crispini, Dabove, Piazza and Capponi2016).
This Unit underwent a polyphase tectono-metamorphic evolution (Capponi and Crispini, Reference Capponi and Crispini2002, Reference Capponi and Crispini2008). Subduction-related, eclogite facies Alpine metamorphism was followed by a diffuse re-equilibration in greenschist facies during exhumation phases, associated with two superimposed phases of isoclinal to tight folding (D1 and D2), which generated the composite, transpositive regional foliation. The D3 phase took place under greenschist- to sub-greenschist-facies conditions, generating metre- to decametre-scale open folds. The later D4 phase generated gentle to open folds (100–1000 m wavelength), associated with reverse faults and shear zones that also affected the Oligocene units of the Tertiary Piemonte Basin (Capponi et al., Reference Capponi, Crispini, Piazza and Amandola2001; Federico et al., Reference Federico, Crispini, Dabove, Piazza and Capponi2016) and were sealed by Burdigalian deposits (d’Atri et al., Reference d'Atri, Irace, Piana, Tallone, Bodrato and Roz Gastaldi1997; Piana et al., Reference Piana, d'Atri and Orione1997). Faults and shear zones related to the D4 phase, as well as younger E–W and NW–SE trending fault systems, hosted a polyphase fluid circulation accompanied by complex rock–fluid interactions, causing local metasomatic alterations of host and fault rocks and precipitation of various vein minerals (Piana et al., Reference Piana, Tallone, Cavagna and Conti2006; Spagnolo et al., Reference Spagnolo, Crispini and Capponi2007).
The investigated sepiolite comes from a quarry located west of the Sassello village, which opended up access to the ‘Bric del Dente’ antigorite serpentinites, close to the contact with the sediments of the Tertiary Piemonte Basin (Fig. 2). The quarry, completely decontaminated after the finding of asbestiform minerals, is nowadays inaccessible. In this locality, sepiolite occurred as a fibrous, yellow-to-whitish mineral, showing a slip-fibre growth in cm-thick and dm-long veins, associated with minor calcite. The specimen studied was purified before analyses, by manually removing the contaminant phases (mostly serpentine, derived from vein walls, and calcite) under a stereomicroscope. The residual fraction was dispersed in deionised water, isolating the lighter suspended portion from the heavier residual impurities (Giustetto and Chiari, Reference Giustetto and Chiari2004). Purified samples were hand-ground in an agate mortar and powdered; conventional X-ray diffraction proved sepiolite to be the only detectable phase.
Methods
A Zeiss WL Pol optical polarising microscope was used to examine 30 µm thick thin sections of sepiolite. Scanning electron microscopy (SEM) was undertaken with a JEOL JSM IT300LV (accelerating voltage = 20 kV, working distance = 10 mm; High Vacuum–Low Vacuum conditions = 10/650 Pa and 0.30–30 kV). Chemistry was obtained by electron probe microanalysis, using energy dispersive spectroscopy with an EDS Oxford INCA Energy 200, equipped with an INCA X-act SDD Thin Window detector, for detection of light elements down to boron. The samples, coated by a 5 nm thick Au layer to allow conductivity, were attached to step brass stubs to allow examination of the fibres. Due to the difficulties in analysing fibrous samples, chemical analyses were collected on 10 µm x 10 µm area on a pressed, sintered and carbon-coated sepiolite pellet, obtained with a press designed to prepare infrared-spectroscopy samples (operating conditions: counting time = 50 s, accelerating voltage = 15 kV, working distance = 25 mm and beam current = 300 pA). The collected data were processed with the Microanalysis Suite Issue 12, INCA Version 4.14 and calibrated on natural mineral standards using the ZAF correction method. The wt.% sums of oxides, showing no relevant heterogeneity, were averaged to obtain a reliable crystal-chemical formula.
Thermo-gravimetric analyses (TGA) were performed on ~10 mg specimen in an alumina crucible, using a simultaneous TGA/DSC (Differential Scanning Calorimetry) TA Instruments Q600, both in nitrogen and Ar flows (100 mL/min), from room T to 1000°C using a 10°C/min ramp. DSC data were obtained through the temperature difference between the sample pan and a reference pan.
For Fourier-Transform Infra-Red (FTIR) measurements, ~15 mg of the material were pressed into a self-supported pellet, a few μm thick, and placed in a small gold folder inside a commercial FTIR reactor cell (AABSPEC, no. 2000-A multimode), which allows recording of IR spectra under controlled temperature and gas atmosphere. FTIR spectra were recorded in transmission mode with a resolution of 2 cm–1 on a PerkinElmer System 2000 infrared spectrophotometer, equipped with a MCT detector at liquid nitrogen temperature. Two different sets of data were collected: (1) at room T, under He flow for 1 h; (2) progressively heating from room T until 400°C, with a ramp of 10°C/min in a He flow (50 cc/min).
Synchrotron powder X-ray diffraction (PXRD) data were collected at room T at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France), on the ID22 High-Resolution Powder-Diffraction beamline, using a wavelength of λ = 0.400016(2) Å. The specimen was loaded into a 1 mm diameter, spinning glass capillary, in order to limit preferred orientation effects. Collection time was 120 s. Diffracted beams were collected by a bank of nine detectors mounted on a single rotation stage (Hodeau et al., Reference Hodeau, Bordet, Anne, Prat, Fitch, Dooryhee, Vaughan and Freund1998), each preceded by a Si 111 analyser crystal, scanned vertically to measure the diffracted intensity as a function of 2θ, accessing d values from 0.774 to 22.650 Å. The GSAS software package (Larson and Von Dreele, Reference Larson and Von Dreele2007) and the EXPGUI graphical user interface (Toby, Reference Toby2001) were used for Rietveld refinement. Background was fitted using a 24-term shifted-Chebyshev function and peak profiles modelled with a pseudo-Voigt function as parameterised by Thompson et al. (Reference Thompson, Cox and Hastings1987), with asymmetry corrections according to Finger et al. (Reference Finger, Cox and Jephcoat1994). Initially, only the background, scale factor, zero and unit-cell parameters were refined. Later, fractional coordinates were refined for all atoms as well as occupancy factors for structural OH2 and zeolitic H2O, at first in alternate cycles (to minimise correlations) and finally all together. Soft constraints were imposed on tetrahedral and octahedral bond lengths and angles and progressively reduced; only the lower weighting factor (F = 10) yielding reasonable bond lengths was kept, in order to avoid unrealistic polyhedron distortions (Post et al., Reference Post, Bish and Heaney2007; Post and Heaney, Reference Post and Heaney2008; Giustetto et al., Reference Giustetto, Levy, Wahyudi, Ricchiardi and Vitillo2011a; Giustetto and Compagnoni, Reference Giustetto and Compagnoni2011; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014). Isotropic displacement parameters (U iso) for all atoms were adjusted using overall constraints for each chemical species. Residual preferred orientation for the (110) reflection, though attenuated by capillary data collection, was treated with the March–Dollase model (March, Reference March1932; Dollase, Reference Dollase1986). The consistency of the refined model was monitored constantly by checking the Goodness-Of-Fit parameters and the fit between the observed and calculated profiles; structural reliability was monitored with the ‘Moldraw’ software (Ugliengo et al., Reference Ugliengo, Viterbo and Chiari1993).
Results and discussion
Physical, optical and compositional properties
Even to the naked eye, the asbestiform habit of the Deiva forest sepiolite is manifest (Fig. 3). Macroscopically, long and apparently thick fibres (from 5 mm up to 6–7 cm) are observed, often grouped in bundles, classifiable to the V group according to García-Romero and Suárez (Reference García-Romero and Suárez2013; see fig. 2 therein). These fibres are flexible, cream to light brown in colour and readily split into thinner units when manipulated, usually crystallising parallel to the vein selvages (slip type), with a thickness from < 1 to several mm. The minerals of the palygorskite–sepiolite group are sometimes known to form compact and soft cardboard-like felts (‘mountain leather’: Grim, Reference Grim1968; Frost et al., Reference Frost, Cash and Kloprogge1998; Imai and Otsuka, Reference Imai, Otsuka, Singer and Galàn2000), in which tangled fibres form an apparently continuous mat. Asbestiform occurrences, however, have also been reported (Giustetto and Compagnoni, Reference Giustetto and Compagnoni2011; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014). Observation under the stereo-microscope confirms that these long fibres are bundles of thinner fibrils, intricately intergrown and separable by sheer mechanic stress. Polarising microscopy on thin sections (≈ 30 µm thick), cut parallel to the fibres elongation, shows a slight brown-to-yellowish pleochroism. The first colour occurs when the fibre elongation runs parallel to the polariser, implying that γ (higher refraction index) runs parallel to z. The α and β orientation, perpendicular to the fibres elongation, could not be determined. In crossed-nicols, a symmetrical extinction is observed, consistently with the orthorhombic symmetry of this phase. High and various interference colours are seemingly observed in different areas – an effect caused by the difficulty in obtaining thin sections with a constant thickness for fibrous materials. Determination of the correct interference colour is risky, but the predominance of higher ones tends to suggest moderate birefringence. Conoscopic light confirms that the mineral is biaxial, with a positive optical sign.

Fig. 3. Macroscopic appearance of the sepiolite specimen from the Deiva forest: Notice how the fibrous habit is very evident even at the macroscopic scale, with exceptionally long fibres (up to several cm; see magnification in the upper right corner).
Morphological analysis performed by SEM confirm that the cm-long fibres are indeed aggregates of thinner units (‘fibrils’ or ‘laths’: García-Romero and Suarez, Reference García-Romero and Suárez2014), mainly running parallel and often entangled (Fig. 4a). Fine measurements of their dimensions (Fig. 4b) evidenced an average thickness of ≈ 83 nm and lengths > 100 µm (IV group: very long fibres: García-Romero and Suárez, Reference García-Romero and Suárez2013). SEM observations also proved that their flexibility affects the micrometric scale.

Fig. 4. Secondary electron SEM images of the Deiva forest sepiolite: (a) the anomalously long fibres (observed by naked eye and with optical microscopy) are indeed made of bundles of thinner units (fibrils), running parallel and at times entangled; (b) the true dimensions of these fibrils (length > 100 µm; thickness < 100 nm) become evident at higher magnifications.
The chemical composition resulting from EDS spot analyses (Table 1) is consistent with previous data (Garcia-Romero and Suàrez, Reference García-Romero and Suarez2010; Suàrez and Garcia-Romero, Reference Suárez, García-Romero, Galán and Singer2011; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014). Silicon and Mg are the main components, together with moderate Fe substituting for Mg in octahedral coordination. Scarce Al (Al2O3 < 0.05 wt.%) suggests for this sepiolite an almost exclusively tri-octahedral character. The oxide sum varies between within 81 and 85%; which is consistent with the hydrous content detected by thermogravimetry (see Section on ‘Thermogravimetric analysis’ below). Low amounts of exchangeable ions (Na+ and K+), possibly located inside the tunnels, are detected occasionally.
Table 1. Chemical composition (wt.% oxides and mean value) and number of cations calculated on an anhydrous basis (32 oxygens) of the sepiolite specimen from the Deiva forest.

‘–’ = not detected
On an anhydrous basis, the crystal-chemical formula, Si12.13O32(Mg6.47Fe0.84)Σ7.31 (total iron as Fe3+), was obtained. The slight Si excess with respect to the ideal value (12) may be explained by the presence of small amounts of amorphous silica (Karakaya et al., Reference Karakaya, Karakaya and Temel2011; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014). The rather low octahedral content (< 8), not rare in sepiolite, is consistent with the amount of Fe3+, as well as the presence of vacancies (Suàrez and Garcia-Romero, Reference Suárez, García-Romero, Galán and Singer2011; Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014).
Thermogravimetric analysis
Thermogravimetric analysis (TGA/DSC) data collected on the Deiva forest sepiolite are reported in Fig. 5. All weight losses and thermal events, related to the loss of the hydrous content, are consistent with previous studies (Nagata et al., Reference Nagata, Shimoda and Sudo1974; Ruiz et al., Reference Ruiz, del Moral, Pesquera, Benito and González1996; Weir et al., Reference Weir, Kuang, Facey and Detellier2002). In sepiolite, correlations between TGA features and structural changes are known to be complex, as no strict partitioning exists between the losses of different kinds of water (Mifsud et al., Reference Mifsud, Garcia and Corma1987). Reasonable interpretations, however, can be drawn. All main events and related attributions are detailed in Table 2.
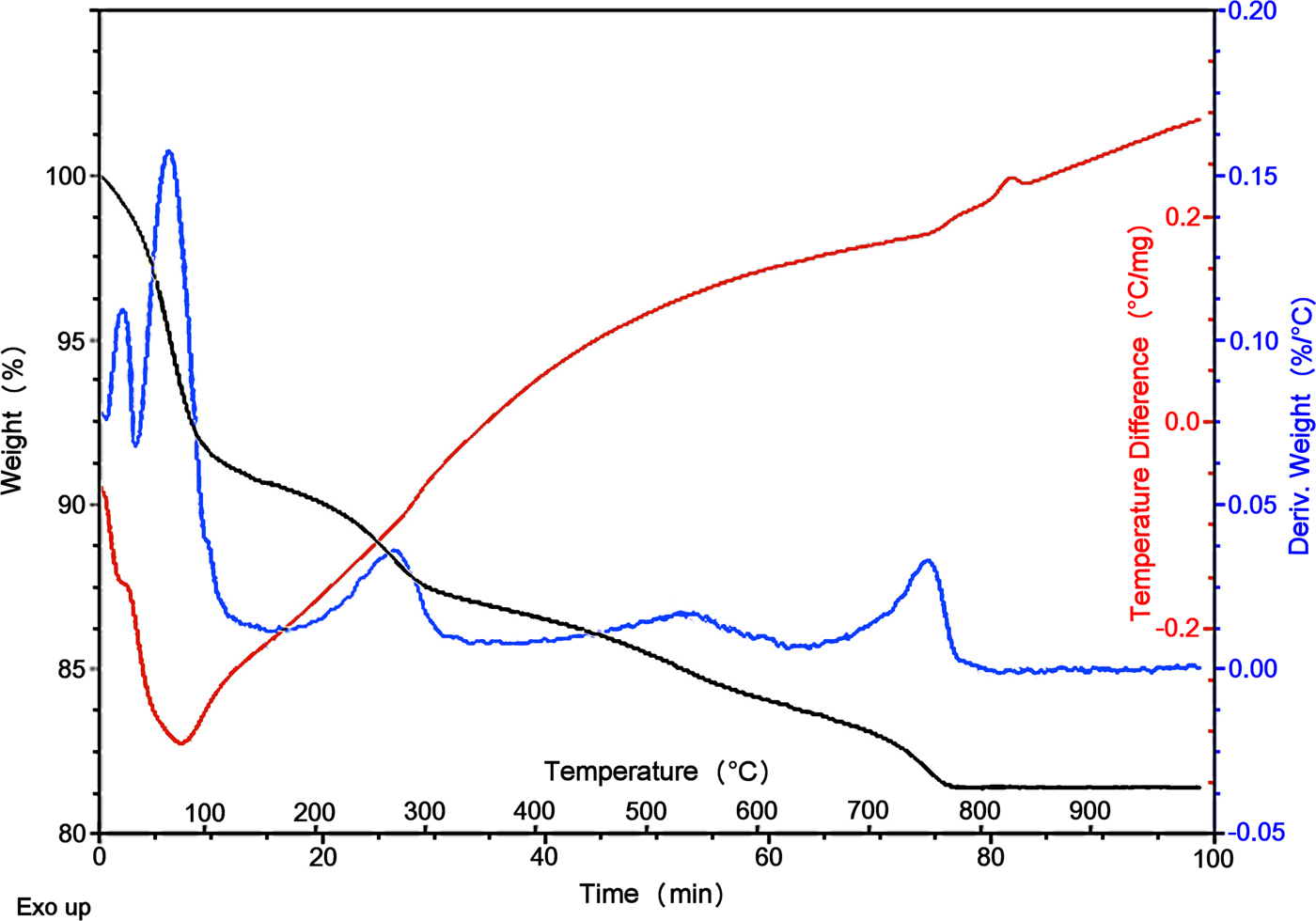
Fig. 5. Observed TGA/derivative weight and DSC curves for the Deiva forest sepiolite.
Table 2. TGA weight losses and related attributions for the Deiva forest sepiolite.
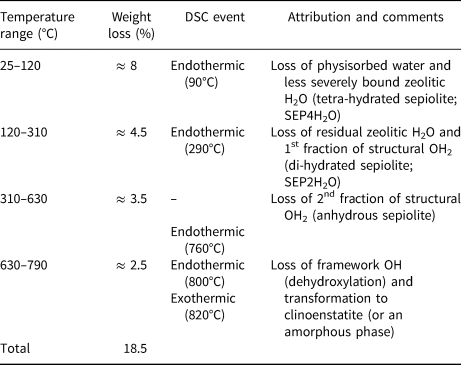
A significant weight loss is observed between room T and 120°C (≈ 8%), related to the departure of pellicular, physisorbed water (from the fibre surface) and of zeolitic H2O positioned at the edges of the tunnels. Such a loss is consistent, albeit slightly lower, with literature data (10–11 wt.%, as observed by Jones and Galán, Reference Jones, Galán and Bailey1988; Frost and Ding, Reference Frost and Ding2003). An analogous decrease was recently observed on a similar sepiolite occurrence (Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014) and attributed to the presence of small amounts of hydrocarbons on the fibre surface, which may possibly limit the adhesion of pellicular water. A further weight loss (≈ 4.5%) occurs until 310°C, due to the departure of the residual zeolitic H2O together with the first fraction of structural OH2. Loss of the remaining OH2 (3.5%) and subsequent dihydroxylation (2.5%) proceed until 790°C, when clinoenstatite (or an amorphous phase) appears. The overall weight loss (≈ 18.5%) is similar to that recorded in the literature (i.e. 18.5–19.5%: Frost et al., Reference Frost, Kristòf and Horvàth2009; Giustetto et al., Reference Giustetto, Wahyudi, Corazzari and Turci2011c).
Fourier-transform infrared spectroscopy
The collected FTIR patterns are basically consistent with literature data (Frost et al., Reference Frost, Locos, Ruan and Kloprogge2001; Ovarlez et al., Reference Ovarlez, Giulieri, Chaze, Delamare, Raya and Hirschinger2009; Bukas et al., Reference Bukas, Tsampodimou, Gionis and Chryssikos2013), although slight shifts in positions might depend on different environments due to crystal-chemical compositions (Ahlrichs et al., Reference Ahlrichs, Serna and Serratosa1975) or to the strength of H bonds between OH2 and H2O (Mendelovici, Reference Mendelovici1973; Mendelovici and Portillo, Reference Mendelovici and Portillo1976; Giustetto et al., Reference Giustetto, Seenivasan, Bonino, Ricchiardi, Bordiga, Chierotti and Gobetto2011b). A detailed attribution of the sepiolite vibrational modes and their evolution with He flux and T rise is reported in Table 3.
Table 3. FTIR active vibrational modes and related attributions at different temperatures for the Deiva forest sepiolite and the associated aliphatic hydrocarbons.
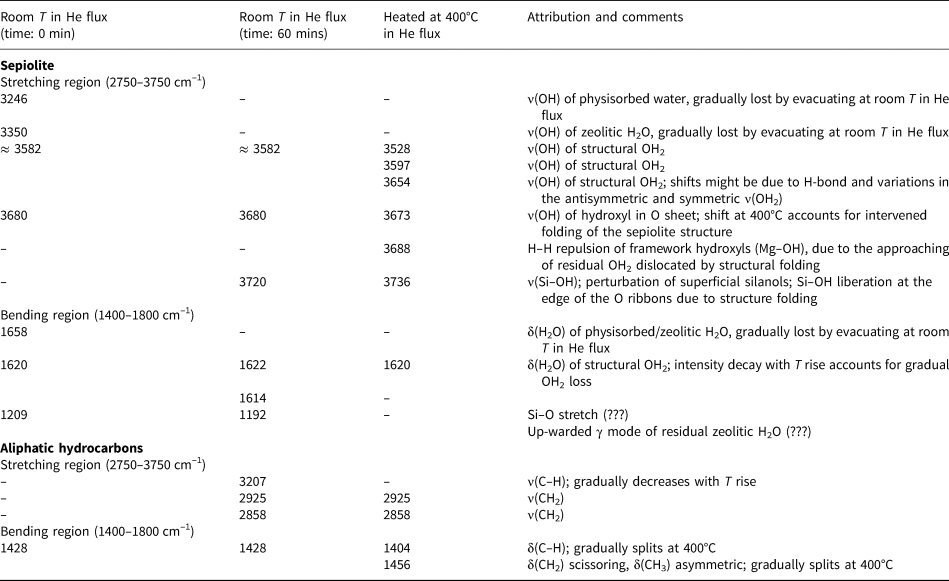
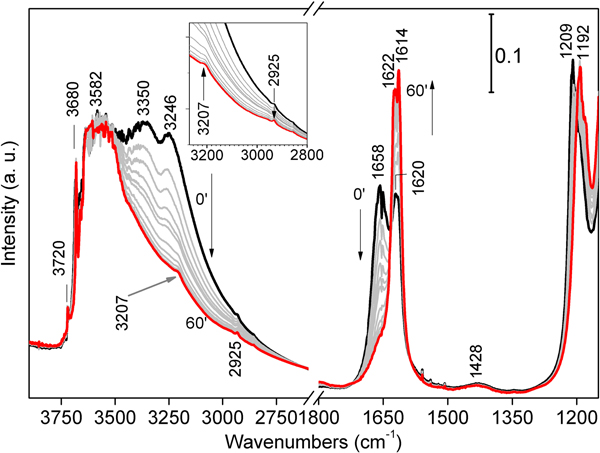
Fourier-Transform IR spectra were first collected at room T in He flow for 1 h, to induce a gradual loss of the more weakly bound H2O molecules at a constant temperature. In these spectra, three main signals appear in the stretching region (Fig. 6, left-hand side) at 3246, 3350 and 3582 cm–1, followed by another one at 3680 cm–1. The former two – related to physisorbed and zeolitic H2O, respectively – gradually decrease as the He flow proceeds; the latter two maintain their position and intensity throughout all the experiment duration, consistently with the permanence in the framework of structural OH2 and hydroxyls in the O sheet, respectively. As dehydration proceeds, two faint bands appear at 3207 and 2925 cm–1: these bands should not be related to sepiolite, but rather to tiny amounts of aliphatic hydrocarbons. In the bending region (Fig. 6, right-hand side), two similar bands appear at 1658 and 1620 cm–1, related to physisorbed/zeolitic H2O and Mg-coordinated OH2, respectively (Hayashi et al., Reference Hayashi, Otsuka and Imai1969). In time, these signals tend to coalesce forming a narrow doublet (1622 and 1614 cm–1), mainly related to OH2. A significant change affects the 1209 cm–1 signal, which red-shifts at 1192 cm–1 after treatment, thus suggesting its dependence from the mineral hydrous content. A faint signal at 1428 cm–1 shows no apparent change with dehydration; again, this band should not be related to sepiolite, but rather to the presence of modest amounts of hydrocarbons (Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014).
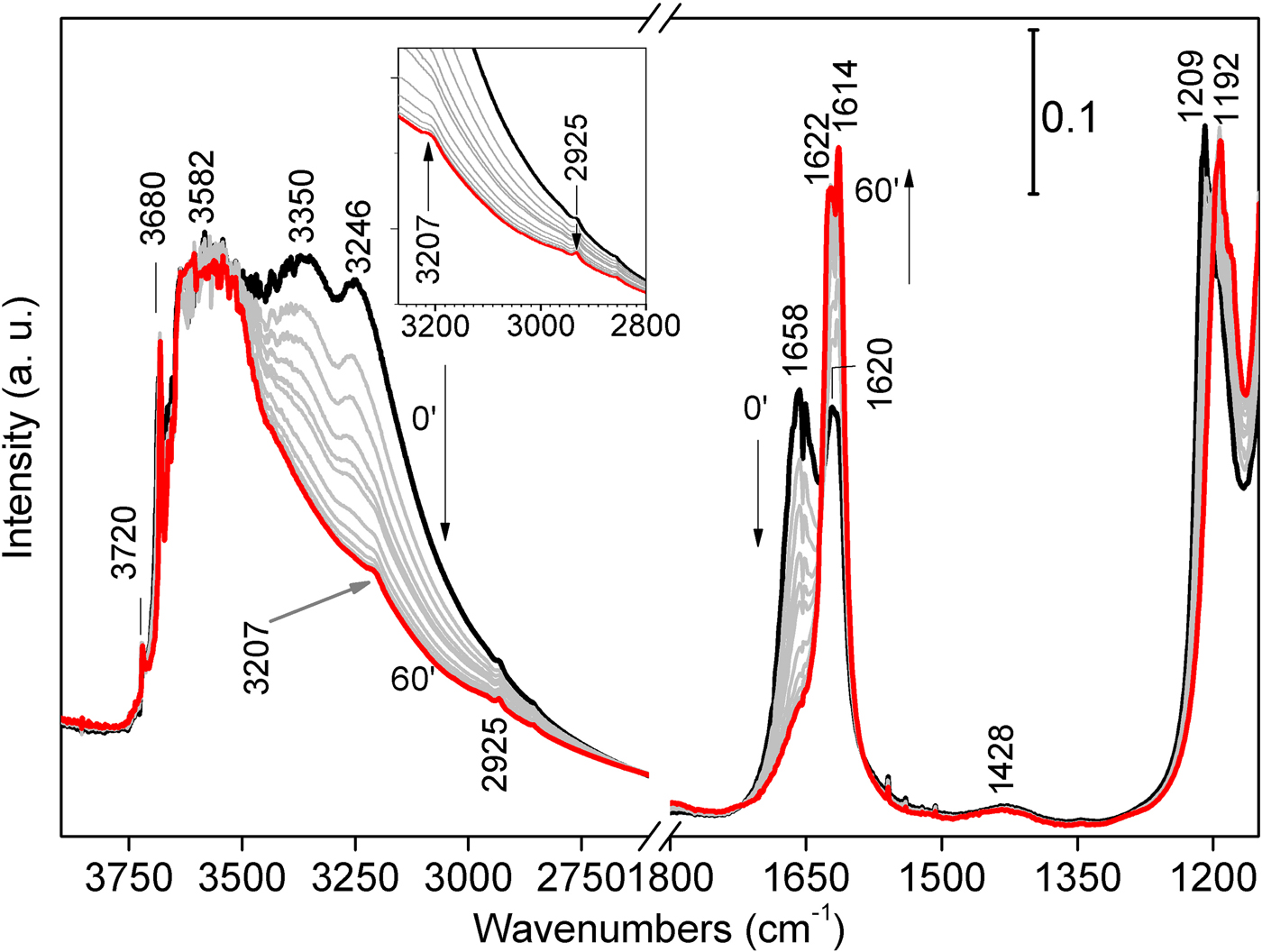
Fig. 6. FTIR spectra collected on the Deiva forest sepiolite at room T in He flux for 1 h, in order to favour the progressive loss of zeolitic H2O at a constant temperature; black and red spectra: 0 and 60 mins, respectively; grey curves: intermediate time. The stretching region of those bands related to aliphatic hydrocarbons are shown in detail in the inset.
The specimen was then heated from room T to 400°C under the same He flow, to study the consequent structural changes and dehydration steps. A general loss in intensity is observed throughout the entire spectrum, consistently with the further loss of the hydrous fraction (residual zeolitic H2O and some structural OH2). In the stretching region (Fig. 7, left-hand side magnification), the broad band centred at ~3582 cm–1 progressively decreases and splits, revealing three separate maxima (3528, 3597 and 3654 cm–1) all related to the ν-OH of structural OH2. The band related to hydroxyls in the O sheet (3680 cm–1) slightly red-shifts at 3673 cm–1, while a new weak feature appears at 3688 cm–1 (left inset of Fig. 7). This splitting accounts for structural folding after formation of di-hydrated sepiolite (SEP 2H2O), due to partial loss of structural OH2 (Hayashi et al., Reference Hayashi, Otsuka and Imai1969; Ovarlez et al., Reference Ovarlez, Giulieri, Delamare, Sbirrazzuoli and Chaze2011); the remaining OH2 gets closer to the hydroxyls, exerting a mutual H–H repulsion (Serna et al., Reference Serna, Ahlrichs and Serratosa1975). The same situation is confirmed by the blue-shift of the narrow band at 3720 cm–1, related to Si–OH stretch (Cannings, Reference Cannings1968; Jung and Grange, Reference Jung and Grange2004; Ovarlez et al., Reference Ovarlez, Giulieri, Chaze, Delamare, Raya and Hirschinger2009), which reaches 3736 cm–1 at 400°C. The persistence of the two maxima at 3597 and 3528 cm–1 (assigned to OH2 anti-symmetric and symmetric stretching frequencies, respectively), confirms that at 400°C some structural OH2 still occupies the framework. It is relevant that two weak features, positioned at 2925 and 2858 cm–1 (asymmetric and symmetric stretching of methylene groups, respectively; Fig. 7), become more and more evident with T increase. Again, these bands are not related to sepiolite, but rather to the CH2 stretch of small amounts of aliphatic hydrocarbons. The band at 3207 cm–1, possibly also related to organic compounds, tends instead to decrease with T rise.
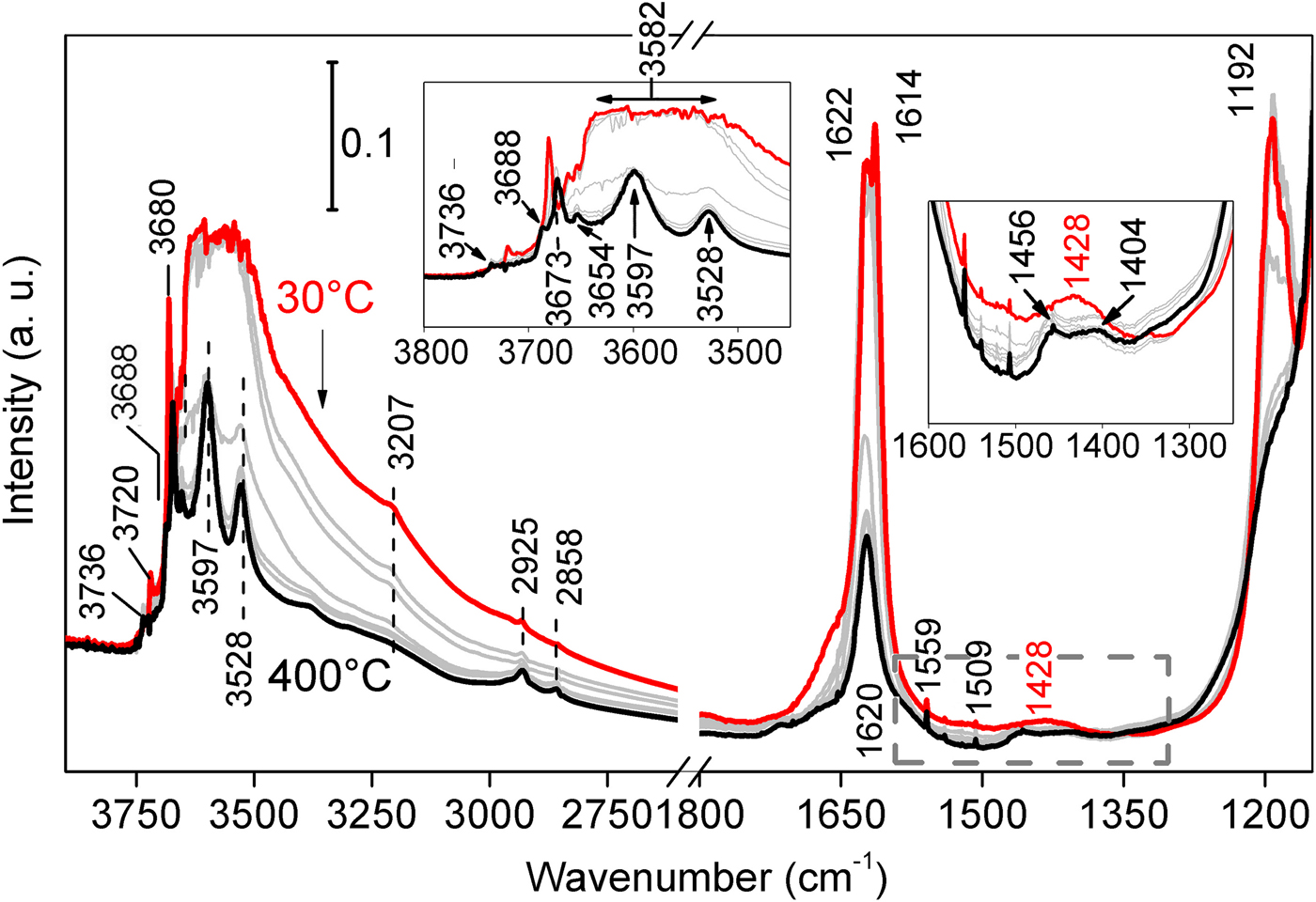
Fig. 7. FTIR spectra collected on the Deiva forest sepiolite by gradually heating from room T (red) to 400°C (black) under He flux, in order to appreciate consequent structural changes and further dehydration. Spectra measured at intermediate temperatures are shown in grey. The two insets show magnifications of the OH stretching at higher wavenumbers (on the left) and of hydrocarbon maxima in the bending region (on the right, highlighted with a dashed square).
In the bending zone (Fig. 7, right hand side), heating from room T to 400°C causes the abovementioned narrow doublet to recoalesce in a single peak [structural OH2 δ(OH): 1620 cm–1], which decreases in intensity as T increases, consistently with loss of OH2 and structure folding. The component at 1192 cm–1 almost disappears at 400°C, implying that such a feature, rather than being related to framework stretching modes (Si-O stretch; Frost et al., Reference Frost, Locos, Ruan and Kloprogge2001), could be linked here to the up-warded γ mode of some residual zeolitic H2O (Giustetto et al., Reference Giustetto, Seenivasan, Bonino, Ricchiardi, Bordiga, Chierotti and Gobetto2011b). The weak hump at 1428 cm–1 tends to break down in two separate features as T rises (Fig. 7, right-hand side magnification), at 1404 and 1456 cm–1 respectively; besides, a narrower mode appears at 1509 cm–1. All these bands correspond to similar ones detected on gabbroid or serpentinised-carbonated mantle-derived ultramafic xenoliths or saponite clays of hydrothermal origin, typical of aliphatic hydrocarbons (Ciliberto et al., Reference Ciliberto, Crisafulli, Manuella, Samperi, Scirè, Scribano, Viccaro and Viscuso2009; Sciré et al., Reference Sciré, Ciliberto, Crisafulli, Scribano, Bellatreccia and Della Ventura2011; Manuella et al., Reference Manuella, Carbone and Barreca2012). While in the bending region (~1200–1600 cm–1) the bands related to organic matter might be overlapped with overtone of skeletal bands of sepiolite (or oxides) and structural OH groups, this not expected to occur in the stretching zone (~2600–3100 cm–1). In the studied case, those signals appearing in both the stretching and bending regions of organic compounds (i.e., observed at 1404, 1428, 1456 and 1509 cm–1, coupled to those at 2925, 2858 and 3207 cm–1; Table 3), and the related intensity changes, indicate that they can reasonably be ascribed to hydrocarbons.
Crystal structure refinement
It is well known that the structural refinement of sepiolite – a highly defective clay mineral with fibrous habit, moderate crystallinity and unequal grain size – is troublesome (Krekeler and Guggenheim, Reference Krekeler and Guggenheim2009; Guggenheim and Krekeler, Reference Guggenheim, Krekeler, Galán and Singer2011). All past results, based on synchrotron data of powdered specimens, with broad diffraction peaks and convoluted background (Post et al., Reference Post, Bish and Heaney2007; Giustetto et al., Reference Giustetto, Levy, Wahyudi, Ricchiardi and Vitillo2011a, Reference Giustetto, Seenivasan and Belluso2014), were affected by low accuracy (i.e. one order of magnitude higher than those typical of single-crystal refinements; Guggenheim and Krekeler, Reference Guggenheim, Krekeler, Galán and Singer2011). Only the work by Sanchez del Rio et al. (Reference Sanchez del Rio, Garcia-Romero, Suarez, da Silva, Fuentes Montero and Martinez-Criado2011) presented pioneering XRD data collected on single-crystals. In the present work, the crystal structure of the Sassello sepiolite was refined with the Rietveld method on synchrotron PXRD data. Starting fractional atomic coordinates were taken from Giustetto et al. (Reference Giustetto, Seenivasan and Belluso2014), in view of the apparent analogies between the analysed specimens. The octahedral (O) sheet was considered occupied only by Mg, ignoring the subordinate Fe content (see Section on ‘Compositional properties’ above) as well as the tiny amounts of exchangeable ions in the tunnels.
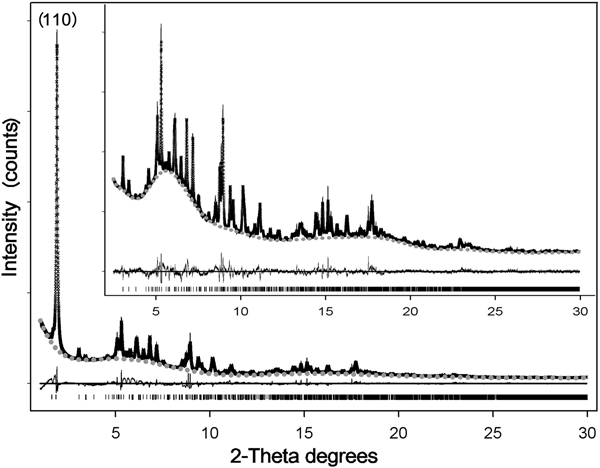
Reasonable results in the Rietveld refinement of this highly defective mineral often involve fixing (or constraining) some atomic parameters (Guggenheim and Krekeler, Reference Guggenheim, Krekeler, Galán and Singer2011). The positions and occupancies of zeolitic H2O and structural OH2 were refined on the entire pattern (Fig. 8) – thus including the stronger (110) reflection at lower angles (d110 ≈ 12.1 Å), symptomatic of the channel content (Ovarlez et al., Reference Ovarlez, Giulieri, Chaze, Delamare, Raya and Hirschinger2009). Once such a procedure converged, this reflection – marked by strong asymmetry – was excluded (Chiari et al., Reference Chiari, Giustetto and Ricchiardi2003; Post et al., Reference Post, Bish and Heaney2007; Post and Heaney, Reference Post and Heaney2008; Giustetto et al., Reference Giustetto, Levy, Wahyudi, Ricchiardi and Vitillo2011a, Reference Giustetto, Seenivasan and Belluso2014) and the atomic coordinates and occupancy factors were blocked for any kind of water. The refinement was then resumed in the 2.5–50°2θ interval, by adjusting the fractional coordinates of all residual atoms in the structure. Such a protocol allowed reliable structural data to be obtained on the polyhedrons sites, while preserving realistic positions and occupancies for all kinds of water (Guggenheim and Krekeler, Reference Guggenheim, Krekeler, Galán and Singer2011). The observed and calculated PXRD patterns for the Sassello sepiolite – and related difference curve, are shown in Fig. 8 [in the magnification: excluding the (110) reflection]. Final refinement data, agreement factors and unit-cell parameters are listed in Table 4. Atomic fractional coordinates, occupancy factors and displacement parameters are reported in Table 5. The related crystallographic information file was deposited with the Principal Editors of Mineralogical Magazine and is available as Supplementary material (see below). Despite all the above mentioned limitations, the standard deviations on atomic coordinates are quite low, compared to literature data. Figure 9 shows the refined structural model.
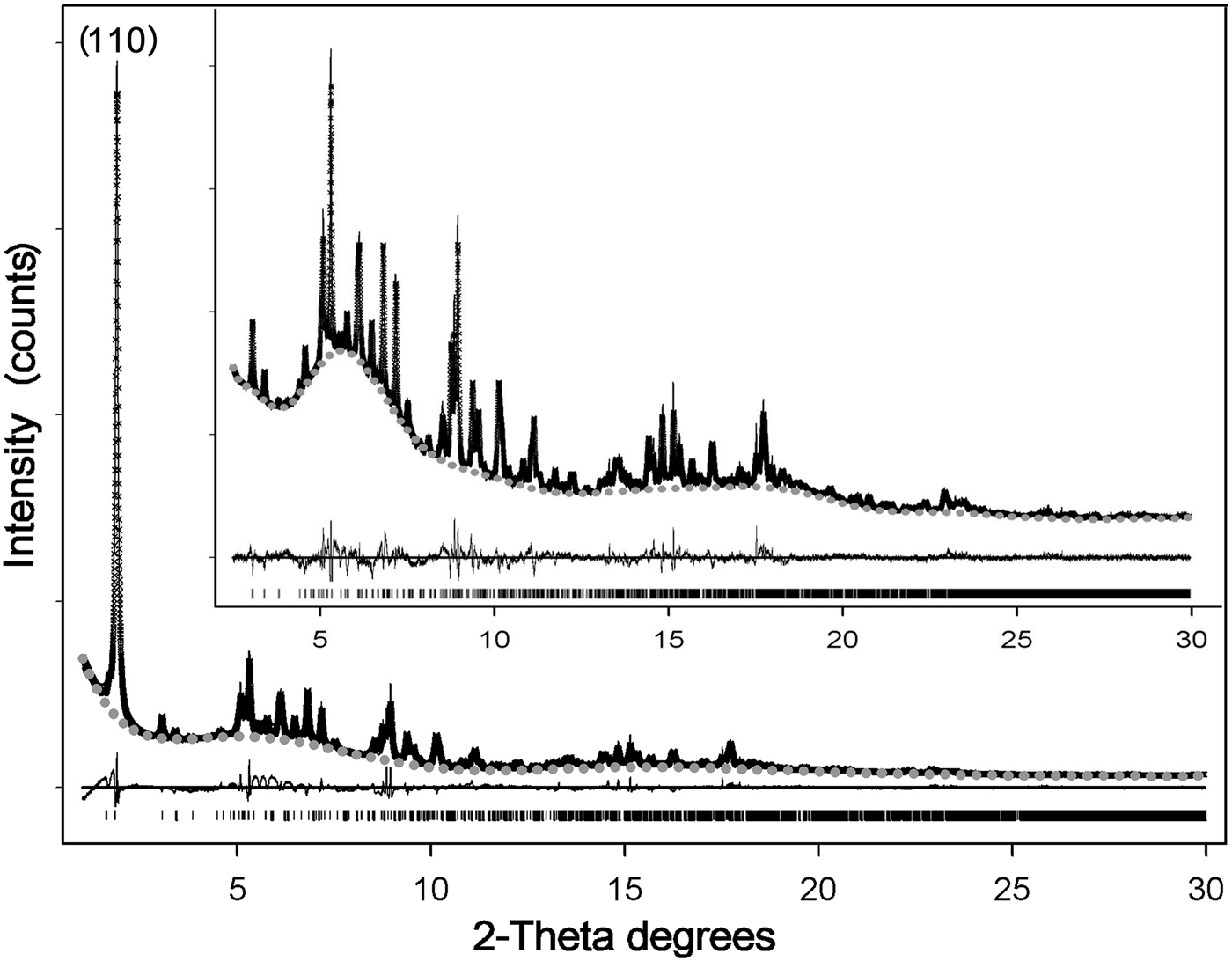
Fig. 8. Crystal-structure refinement with the Rietveld method of the Deiva forest sepiolite: observed (crosses) and calculated (solid line) diffraction patterns, background (grey dots) and related difference curve (lower line). Magnification (upper image) shows the diffraction patterns by excluding the stronger (110) reflection.
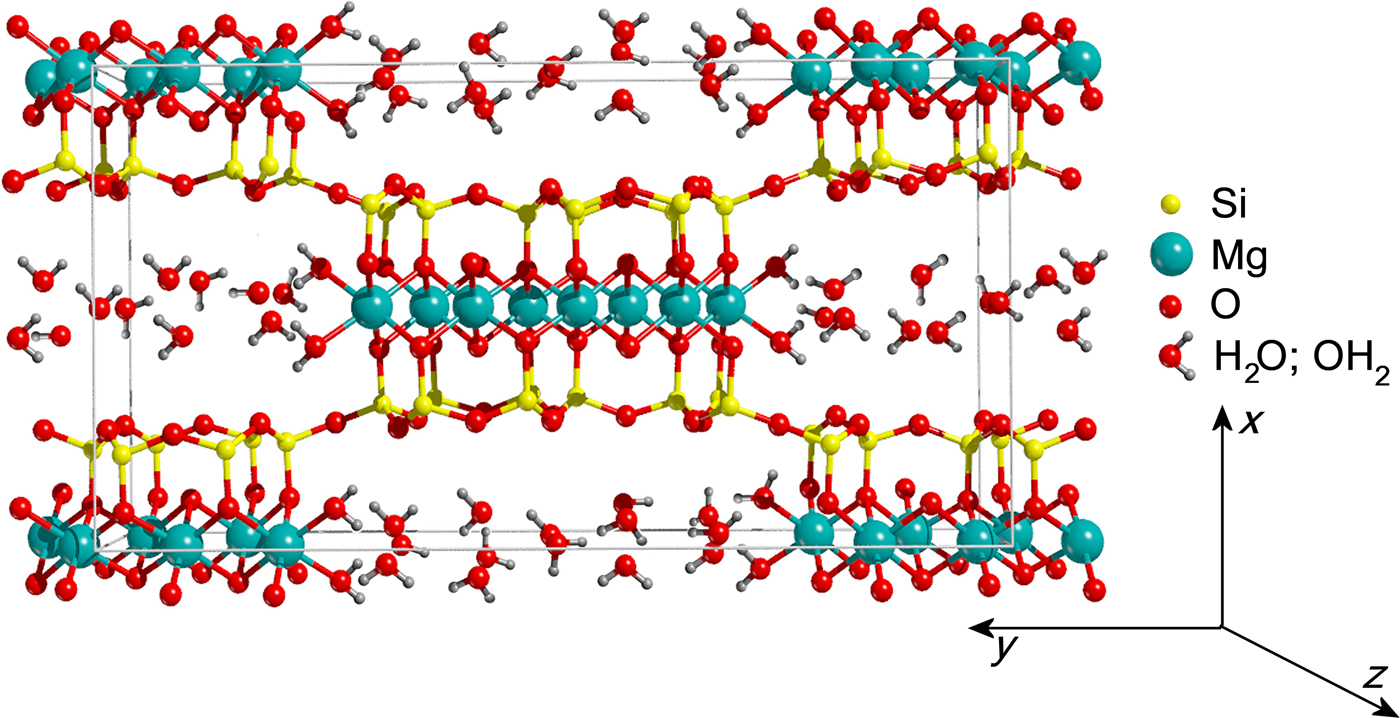
Fig. 9. Refined crystal structure for the Deiva forest sepiolite (unit cell outlined as thin grey bars).
Table 4. Crystal-chemical formula, space group, cell parameters and refinement data for the Deiva forest sepiolite.

Table 5. Refined fractional atomic coordinates, occupancy factors and isotropic displacement parameters for the Deiva forest sepiolite.
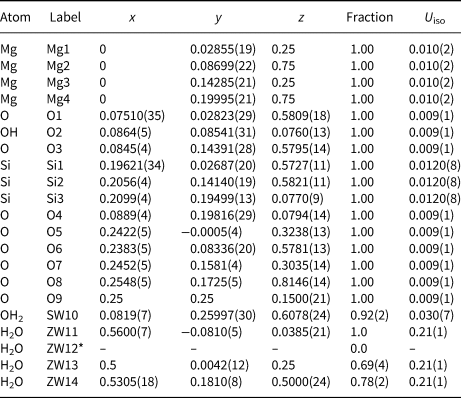
*The occupancy of ZW12 dropped to 'zero' during refinement
No spurious experimental reflections, not envisaged by the simulated pattern, were observed during refinement, thus confirming the reliability of the adopted sepiolite structural model (something recently called into question for palygorskite; Garcìa-Rivas et al., Reference García-Rivas, Sánchez del Río, García-Romero and Suárez2017). Although the Goodness-Of-Fit is affected by the disorder and multiple defects affecting this and similar clay minerals (Giustetto and Chiari, Reference Giustetto and Chiari2004; Krekeler and Guggenheim, Reference Krekeler and Guggenheim2009), the quality of the refined model is consistent with previous works (Artioli and Galli, Reference Artioli and Galli1994; Chiari et al., Reference Chiari, Giustetto and Ricchiardi2003; Post et al., Reference Post, Bish and Heaney2007; Post and Heaney, Reference Post and Heaney2008; Giustetto et al., Reference Giustetto, Levy, Wahyudi, Ricchiardi and Vitillo2011a, Reference Giustetto, Seenivasan and Belluso2014). Without discussing the finer structural details (something prevented by the relatively high σ), the unit-cell parameters are consistent with previous models (i.e. Post et al., Reference Post, Bish and Heaney2007; Giustetto et al., Reference Giustetto, Levy, Wahyudi, Ricchiardi and Vitillo2011a, Reference Giustetto, Seenivasan and Belluso2014) – the related differences varying within 0.01 Å. Structural OH2 has an almost full occupancy [0.92(2)]. The occupancy of the ‘ZW12’ H2O molecule dropped to zero while refining (Table 5), proving this site to be empty. Such an absence, however, does not affect the global zeolitic H2O content: almost 16.7 H2O molecules occupy the unit cell – slightly exceeding the value expected by the ideal formula (16; Preisinger, Reference Preisinger1959). A continuous web of H bonds connects all water molecules one to another and to the structural OH2 (Fig. 9): the mutual O···O distances in the tunnel vary between 2.271 and 2.765 Å (Jeffrey, Reference Jeffrey1997). The detection, in the XRD pattern, of broad humps centred at ~6.5 and 17.0°2θ, though also related to technical issues (i.e. use of a glass capillary during data collection), suggests possible presence of an additional amorphous phase – presumably silica, as hinted by EDS analyses (see section on ‘Compositional properties’ above).
Genesis of the Sassello sepiolite and origin of the associated aliphatic hydrocarbons
The occurrence of sepiolite in the serpentinites of the Voltri Unit was also reported by Spagnolo et al. (Reference Spagnolo, Crispini and Capponi2004, Reference Spagnolo, Crispini and Capponi2007), who observed this mineral to form mm- to cm-thick “layers”, genetically related to fluid circulation both in reverse shear zones, associated to the earliest Miocene D4 deformation event, and within post-Burdigalian transtensives and strike-slip fault systems (see section on ‘Geological setting’ above).
It can be reasonably supposed that the studied asbestiform sepiolite is related to the same genetic context, i.e. a low-temperature hydrothermal circulation that developed along late-orogenic tectonic structures. However, the structural relationships of the sepiolite veins could not be investigated during sampling nor later, due to reclamation of the borrow pit where the mineral had been found.
Magnesium and Si probably derived from aqueous fluids with negligible Al activity, interacting with serpentinites and partly serpentinised peridotites; Fe from interaction with iron-bearing minerals (e.g. magnetite in serpentinites or olivine and clinopyroxene in peridotites). A slight SiO2 excess in solution, which is expected to favour sepiolite precipitation (Birsoy, Reference Birsoy2002), may explain the tiny quantities of amorphous silica hinted at by the crystal-chemical formula (see section on ‘Compositional properties’ above).
The sepiolite studied is associated with long-chain aliphatic hydrocarbons. The abiotic genesis of these organic compounds after hydrothermal activity in mafic to ultramafic rocks has been reported from different geological settings (Charlou et al., Reference Charlou, Fouquet, Bougault, Donval, Etoubleau, Jean-Baptiste, Dapoigny, Appriou and Rona1998; Proskurowski et al., Reference Proskurowski, Lilley and Seewald2008; Ciliberto et al., Reference Ciliberto, Crisafulli, Manuella, Samperi, Scirè, Scribano, Viccaro and Viscuso2009; Dos Anjos et al., Reference Dos Anjos, Meunier, Guimaraès and El Albani2010; Sciré et al., Reference Sciré, Ciliberto, Crisafulli, Scribano, Bellatreccia and Della Ventura2011; Etiope and Sherwood Lollar, Reference Etiope and Sherwood Lollar2013), and has been documented to occur even at great depth during subduction of ophicarbonates (Vitale Brovarone et al., Reference Vitale Brovarone, Martinez, Elmaleh, Compagnoni, Chaduteau, Ferraris and Esteve2017). Their generation is interpreted commonly as the result of the Fischer–Tropsch-type (FT-t) reaction (MacDonald and Fyfe, Reference Macdonald and Fyfe1985; Taran et al., Reference Taran, Kliger and Sevastianov2007; Konn et al., Reference Konn, Charlou, Donval, Holm, Dehairs and Bouillon2009), an exothermic reduction of CO and CO2 by gaseous H2, catalysed by group VIII ions (such as Fe, Co, Ni) or oxides. Oxides of C mainly derive from mantle CO2 dissolved in hydrothermal fluids or liberated from olivine fluid inclusions; H2 originates after the hydration of peridotite minerals during serpentinisation (Charlou et al., Reference Charlou, Donval, Fouquet, Jean-Baptiste and Holm2002; Ciliberto et al., Reference Ciliberto, Crisafulli, Manuella, Samperi, Scirè, Scribano, Viccaro and Viscuso2009; Marcaillou et al., Reference Marcaillou, Muñoz, Vidal, Parra and Harfouche2011).
Considerable amounts of hydrocarbons (mostly methane, with minor ethane and propane) are released by present-day alkaline, Ca–OH springs from the Voltri ophiolitic ‘massif’ (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli, Longinelli, Marini, Ottonello and Vetuschi Zuccolini2002; Cipolli et al., Reference Cipolli, Gambardella, Marini, Ottonello and Vetuschi Zuccolini2004; Boschetti et al., Reference Boschetti, Etiope and Toscani2013; Chavagnac et al., Reference Chavagnac, Monnin, Ceuleneer, Boulart and Hoareau2013; Schwarzenbach et al., Reference Schwarzenbach, Lang, Früh-Green, Lilley, Bernasconi and Méhay2013), which discharge fluids from meteoric waters modified by the prolonged interaction with the host serpentinites and peridotites (Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli, Longinelli, Marini, Ottonello and Vetuschi Zuccolini2002; Cipolli et al., Reference Cipolli, Gambardella, Marini, Ottonello and Vetuschi Zuccolini2004; Boschetti et al., Reference Boschetti, Etiope and Toscani2013). These hydrocarbons, mostly of abiotic origin (as indicated by the C and H isotopic compositions), formed during low-temperature serpentinisation of the host ultramafic rocks by the same meteoric-derived fluids, either through FT-t reactions or direct production of CH4 (without intermediate H2 generation) by CO2-rich fluids (Boulart et al., Reference Boulart, Chavagnac, Monnin, Delacour, Ceuleneer and Hoareau2012; Boschetti et al., Reference Boschetti, Etiope and Toscani2013).
Bruni et al. (Reference Bruni, Canepa, Chiodini, Cioni, Cipolli, Longinelli, Marini, Ottonello and Vetuschi Zuccolini2002) modelled the reaction path of meteoric waters with Voltri serpentinites and proposed a two-step evolution, in which Ca–OH waters are formed by low-temperature interactions with the host rocks, passing through an intermediate Mg–HCO3 composition (springs with this composition are also common in the area; Bruni et al., Reference Bruni, Canepa, Chiodini, Cioni, Cipolli, Longinelli, Marini, Ottonello and Vetuschi Zuccolini2002). Interestingly, in this model the switch from Mg–HCO3 to Ca–OH waters is caused by precipitation of sepiolite. As a matter of fact, the serpentinites surrounding modern alkaline springs of the Voltri ‘massif’ are pervaded locally by thin veins of sepiolite, whose formation has been suggested to be related to the alkaline fluids themselves (Schwarzenbach et al., Reference Schwarzenbach, Lang, Früh-Green, Lilley, Bernasconi and Méhay2013). A similar mechanism, involving the low-temperature interaction of fluids with serpentines and peridotites, could be also envisaged for the local precipitation of the studied sepiolite as well as the generation of the related hydrocarbons.
The association of sepiolite with abiotic aliphatic hydrocarbons has been reported only once by Giustetto et al. (Reference Giustetto, Seenivasan and Belluso2014), who proved this coupling to favour defibrillation of the sepiolite crystals, with a consequent increase in the interfibre porosity (open texture) and modification of the fibre morphology, enhancing its length vs. thickness ratio from ‘high’ to ‘very high’. Obviously, a similar phenomenology can be hypothesised also for the Sassello sepiolite. The thinner and exceptionally long fibrils (rods and/or laths; García-Romero and Suárez, Reference García-Romero and Suárez2013) may thus become potentially dangerous for human health, if aero-dispersed and inhaled.
Conclusions
An asbestiform sepiolite occurrence – in cm-thick veins within the serpentinites of the Voltri Unit cropping out near Sassello – was studied, in order to characterise its morphological habit and structural features and thus evaluate its possible hazard for human health. This sepiolite is formed by pluricentimetric flexible fibres that, when observed with SEM, appear as bundles of thinner intertwined fibrils (length > 100 µm). FTIR analyses show the presence of moderate amounts of aliphatic hydrocarbons (yet to be identified thoroughly) coupled to these fibres – an atypical association reported only once in the literature (Giustetto et al., Reference Giustetto, Seenivasan and Belluso2014).
These long and thin fibrils (or laths) might cause this sepiolite specimen to be potentially dangerous for human health, due to their latent carcinogenic risk if aero-dispersed and inhaled in high doses. Furthermore, the contextual presence of aliphatic hydrocarbons may alter the fibrils surface reactivity, altering their capability of interacting with the pulmonary epithelium. The toxicity of occasional inhalation, skin absorption or ingestion of aliphatic hydrocarbons has also been acknowledged (Farinha et al., Reference Farinha, Assunção and Vinhas2011). In the most recent report of the International Agency for Research on Cancer (IARC, 1997), an “inadequate evidence in humans for the carcinogenicity of sepiolite” is assessed. However, a “limited evidence in experimental animals for the carcinogenicity of long fibres” (> 5 µm) was suggested. Sepiolite fibres were included by IARC in ‘Group 3’, implying that further investigations are needed to evaluate their effective noxiousness (IARC, 2012). Fortunately, the risk of inhaling high doses of the Sassello sepiolite fibres was averted by reclamation of the outcrop where the sample had been found.
Impending studies will be aimed at evaluating and comparing the potential carcinogenic risk of this and other similar sepiolite occurrences, in view of the peculiar association with the organic compounds that might affect their reactivity towards living cells and/or tissues.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.159
Acknowledgements
The authors are indebted to Cesare Atzori, for his help in collecting TGA data, and to Emanuele Costa and Elena Belluso, for their contributions in collecting and interpreting SEM-EDS data. Roberto Compagnoni is thanked for his help in interpreting analyses on thin sections at the polarising microscope. Special thanks go to Carlotta Giacobbe and Rossella Arletti, for their support in collecting synchrotron PXRD diffraction patterns. We would like to thank the reviewers of this paper (Fabio Carmelo Manuella and an anonymous reviewer), who helped – with their suggestions and comments – to significantly improve the scientific weight of the manuscript. Special thanks go to Marco Ciriotti, Giuseppe Pipino and Roberto Cabella, without whom this work could not have been.