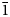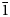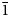Introduction
Though very limited in extent, the Torrecillas mine, a small, long-inactive mine in the northern Atacama Desert of Chile, has yielded a remarkable array of new secondary arsenic minerals. Five arsenites and twelve arsenates have now been approved as new minerals (Table 1). All of the new arsenates include hydrogen-arsenate and/or dihydrogen-arsenate groups. The last six of these arsenates, all of which occur on two closely related specimens, are described in this paper. The diversity of secondary arsenic phases also includes three already known arsenites and ten already known arsenates (Table 2).
Table 1. New secondary arsenic minerals from the Torrecillas mine.

Table 2. Other secondary arsenic minerals occurring at the Torrecillas mine.

The new mineral camanchacaite (\kɑ: mæn ˈkɑ: kɑ: aɪt\) is named for the ‘camanchaca’, a dense fog that forms along the northern Chilean coast where the Atacama Desert reaches the Pacific Ocean. The moisture particles that make up the fog are between 1 and 40 μm in diameter – too small to form raindrops. Consequently, the fog does not produce rain; however, the moisture it provides and its chemical content are likely to be responsible for at least some of the alteration of the As-bearing veins that yields secondary phases. Camanchacaite resembles the camanchaca fog in that it forms at Torrecillas (on the Atacama coast) as cloudy white balls, which contain OH but no H2O.
The name chinchorroite is for the Chinchorro culture of the inhabitants of the coastal region of northern Chile and southern Peru from 9000 to 3500 years BP that includes the area around Torrecillas. The Chinchorro people are best known for their elaborate burial practices, which involved mummification. Analysis of hair samples from Chinchorro mummies has shown that the Chinchorro people suffered extensively from arsenic poisoning due to their consumption of water contaminated by arsenic. It has been suggested that the Chinchorro practice of mummification began as a response to miscarriages and premature deaths that were caused by arsenic poisoning (c.f. Arriaza et al., Reference Arriaza, Amarasiriwardena, Cornejo, Standen, Byrne, Bartkus and Bandak2010; Byrne et al., Reference Byrne, Amarasiriwardena, Bandak, Bartkus, Kane, Jones, Yañez, Arriaza and Cornejo2010).
The name espadaite (/es ˈpa da aɪt/) is from the Spanish word espada, meaning sword, in allusion to the shape of the crystals.
The name magnesiofluckite signifies that the mineral is the Mg analogue of fluckite, CaMn2+(AsO3OH)2(H2O)2 (Bari et al., Reference Bari, Cesbron, Permingeat and Pillard1980; Catti et al., Reference Catti, Chiari and Ferraris1980), with Mg in place of Mn2+.
The name picaite (/ˈpi: kǝ aɪt/) is for the Pica (or Pica–Tarapacá) culture, which occupied the Atacama desert in northern Chile from ~900 CE until being subsumed by the Inca Empire ~1500 CE. Pica peoples were skilled at working with wood and marine-related objects, weaving and extracting rocks and minerals, used principally for decorative purposes. Pica settlements reached from the eastern Tamarugal Pampa on the east to the coast on the west and from the El Loa River on the south almost to Iquique on the north. Their coastal settlements included Salar Grande, Punta Lobos (~6 km SW of Torrecillas) and several of the small bays south of Iquique. Notably, Quebrada de Pica, a dry river canyon, reaches the Pacific Ocean immediately south of Torrecillas Hill.
The name ríosecoite (/ri: oʊ ˈseɪ koʊ aɪt/) is for Caleta Río Seco (Río Seco Cove), which is at the SW base of Torrecillas Hill, and for the small town of Río Seco, which is ~2.5 km SW of the Torrecillas mine. ‘Río seco’ means dry river in Spanish and, in this case, refers to the river that formed Quebrada de Pica, which reaches the Pacific Ocean at Caleta Rio Seco.
The new minerals and their names have been approved by the International Mineralogical Association. The type specimens upon which the descriptions are based are deposited in the collections of the Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007, USA. Specimen number 67257 is the holotype for chinchorroite, magnesiofluckite and ríosecoite, and a cotype for camanchacaite and picaite. Specimen 67285 is the holotype for espadaite and a cotype for picaite. Additional cotypes for camanchacaite are specimens 66771, 66772, 66773 and 66774.
Occurrence
The new minerals were all found on two closely related specimens collected at the Torrecillas mine, Salar Grande, Iquique Province, Tarapacá Region, Chile (~20°58'13''S, 70°8'17''W). Torrecillas Hill, on which the Torrecillas mine is located, is composed of four different rock units. The Coastal Range Batholith (mainly gabbros) extends from the seashore to the Pan-American Road along the base of Torrecillas Hill. At the foot of Torrecillas Hill is a small area of contact metamorphic rocks in which garnet crystals occur in metamorphosed shales. Higher on the hill, the rocks are predominantly porphyritic andesitic lavas of the Jurassic La Negra Formation (García, Reference García1967; Buchelt and Tellez, Reference Buchelt, Tellez, Bahlburg, Breitkreuz and Giese1988). The Torrecillas deposit, in which the new minerals were found, consists of two main veins rich in secondary arsenic and copper minerals that intersect metamorphosed marine shales and lavas. These mineralised veins are genetically related to the aforementioned porphyritic andesitic lavas of the Jurassic La Negra Formation. More information on the geology and mineralogy of the area is provided by Gutiérrez (Reference Gutiérrez1975).
The rare secondary chlorides, arsenates and arsenites were found at three main sites on the hill: an upper pit ~8 m long and 3 m deep, a lower pit ~100 m from the upper pit and ~5 m long and 3 m deep, and a mine shaft adjacent to the lower pit and lower on the hill. The two small specimens were found just inside the mine shaft by three of the authors (ARK, MD and AAMD) along with Jochen Schlüter and Joe Marty in February 2014.
The new minerals are low-temperature secondary phases occurring together on massive quartz–hematite also in association with anhydrite, gypsum, halite and talmessite. The secondary arsenic-rich assemblages at the Torrecillas deposit are interpreted as having formed under hyperarid conditions from the oxidation of native arsenic, and possibly other As-bearing primary phases, coupled with reaction with fluids rich in dissolved Na, Ca and Mg. In earlier papers, we noted these fluids as resulting from evaporating meteoric water, as is the case for some other deposits in the Atacama region (cf. Cameron et al., Reference Cameron, Leybourne and Palacios2007); however, we now are convinced that the fluids are likely, at least in part, to be related to the frequent dense coastal fogs (cf. Rech et al., Reference Rech, Quade and Hart2003; Wang et al., Reference Wang, Michalski, Seo and Ge2014). This mechanism of formation is discussed more fully below.
Appearance and properties
The physical and optical properties for all six minerals are listed in Table 3. The Mohs hardness was determined by scratch tests for all species except espadaite, which was estimated from the behaviour when broken. Densities were measured by flotation in mixtures of methylene iodide and toluene except for camanchacaite, which could not be measured because of inclusions. Optical studies for all minerals were done in white light and all, except camanchacaite, used a spindle stage for directly observing the optical indicatrix and measuring the indices of refraction. The crystal sizes, aggregations and morphologies are as follows:
Table 3. Physical and optical properties of camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

* by analogy with other alluaudite-group minerals.
Camanchacaite forms balls with compact radial structure to ~1 mm in diameter (Fig. 1). No crystal forms could be determined and no crystal fragments of adequate quality for single-crystal X-ray diffraction studies were found.

Fig. 1. Balls of camanchacaite on a yellow amorphous phase; note the broken ball of camanchacaite in the foreground. The field of view is 2.33 mm across, specimen #66772.
Chinchorroite crystals are blades (Fig. 2) to ~1 mm in maximum dimension, which occur both isolated and in massive intergrowths. Tablets are flattened on {001}, elongate on [100] and exhibit the forms {100}, {010}, {001} and {1![]() $\bar{1}$ 0} (Fig. 3). No twinning was observed.
$\bar{1}$ 0} (Fig. 3). No twinning was observed.

Fig. 2. Chinchorroite crystals with tiny currierite needles and balls of camanchacaite. The field of view is 0.7 mm across, specimen #67257.

Fig. 3. Crystal drawing of chinchorroite; clinographic projection in nonstandard orientation, a vertical.
Espadaite crystals are blades to ~0.2 mm long, forming fans, jumbled sprays and random intergrowths (Fig. 4). Blades are flattened on {001}, elongate on [100] and exhibit the forms {001}, {010}, {110} and {111} (Fig. 5). No twinning was observed.

Fig. 4. Espadaite blades with balls of camanchacaite. The field of view is 0.84 mm across, specimen #67285.

Fig. 5. Crystal drawing of espadaite; clinographic projection in nonstandard orientation, a vertical.
Magnesiofluckite crystals are tablets and short blades to ~1 mm in maximum dimension, which are often grouped in tightly intergrown aggregates (Fig. 6). Tablets are flattened on {010}, slightly elongate on [001] and exhibit the forms {100}, {010}, {001}, {1![]() $\bar{1}$0}, {101} and {10
$\bar{1}$0}, {101} and {10![]() $\bar{1}$} (Fig. 7). No twinning was observed.
$\bar{1}$} (Fig. 7). No twinning was observed.

Fig. 6. Magnesiofluckite crystals with ball of camanchacaite. The field of view is 0.65 mm across, specimen #67257.

Fig. 7. Crystal drawing of magnesiofluckite; clinographic projection in standard orientation.
Picaite crystals are thick blades to ~1 mm long, typically in parallel intergrowths (Fig. 8). Blades are flattened on {100}, elongate on [001] and exhibit the forms {100}, {010}, {011}, {11![]() $\bar{1}$} and {021} (Fig. 9). No twinning was observed.
$\bar{1}$} and {021} (Fig. 9). No twinning was observed.

Fig. 8. Picaite crystals with balls of camanchacaite. The field of view is 1.1 mm across, specimen #67285.

Fig. 9. Crystal drawing of picaite; clinographic projection in standard orientation.
Ríosecoite crystals are prisms to ~1 mm long, elongated and striated on [100] with irregular terminations that commonly come to a somewhat rounded point. Only the prism forms {010} and {001} were observed. The prisms are often intergrown in subparallel bundles (Fig. 10). No twinning was observed.

Fig. 10. Ríosecoite crystals with balls of camanchacaite. The field of view is 1 mm across, specimen #67257.
Raman spectroscopy
Raman spectra from 4000 to 100 cm–1 were recorded using a Horiba XploRA PLUS with a 532 nm diode laser. The spectra for all phases were featureless between 2500 and 950 cm–1. The background-removed spectra in the ranges 3700–2500 cm–1, 965–715 cm–1 and 600–100 cm–1 are shown in stacked format in Fig. 11. The complete spectra in text format have been deposited with the Principal Editors of Mineralogical Magazine and are available as Supplementary material (see below). A listing of the positions of all fitted bands, including generalised mode assignments, is given in Table 4. Detailed mode characterisation, including comparisons with other arsenates, is beyond the scope of this paper; however, because we were unable to obtain a structure refinement for camanchacaite, it is worth mentioning that its Raman spectrum is quite similar to that of calciojohillerite, NaCaMg3(AsO4)3 (Igor Pekov, Pers. Comm.; Pekov et al., Reference Pekov, Koshlyakova, Agakhanov, Zubkova, Belakovskiy, Vigasina, Turchkova, Sidorov and Pushcharovsky2016), which is an unprotonated alluaudite-group mineral of similar composition.

Fig. 11. Raman spectra of camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.
Table 4. Raman shifts (cm–1) for camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

1 Bold numbers indicate the most intense band in each spectrum.
2 Compared to austinite, adamite, paradamite, kottigite, mimetite, wendelwilsonite from Caracas and Bobocioiu (Reference Caracas and Bobocioiu2011).
3 Compared to synthetic Sr5(As2O7)2(AsO3OH) from Mihajlović et al. (Reference Mihajlović, Libowitzky and Effenberger2004).
4 Due to the complexity of the crystal structures, we are unable to assign all bands to specific Raman mode vibrational types.
Composition
Quantitative analyses were done at the University of Utah on a Cameca SX-50 electron microprobe with four wavelength-dispersive spectrometers using Probe for EPMA software. Analytical conditions were: 15 kV accelerating voltage, 10 nA beam current and a beam diameter of 10 μm. Counting times were 30 s on peak and 15 s on background for each element. In some cases, Na showed a time-dependent decrease in intensity under the electron beam, which was accounted for by an exponential fit to the intensity vs. time measurements and extrapolation to zero-time intensity. No other elements were detected by energy-dispersive spectroscopy. Other likely elements were sought by wavelength-dispersive spectroscopy scans, but none were above the detection limits. Raw X-ray intensities were corrected for matrix effects with a φ(ρz) algorithm (Pouchou and Pichoir, Reference Pouchou, Pichoir, Heinrich and Newbury1991). Because in all instances, insufficient material was available for a direct determination of H2O, the amount of water was calculated on the basis of the crystal-structure analysis. Analytical data are given in Table 5 and empirical formulas are given in Table 6.
Table 5. Chemical analyses [mean (std. dev.) range in wt.%] for camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

* The poor quality of the espadaite crystal surfaces and significant beam damage provided low analyses for all components especially Na; consequently, Na2O was calculated from the structure and the other components were normalised to a total of 100%.
§ H2O was calculated for all analyses based upon the structures.
Table 6. Empirical formulas of camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

X-ray crystallography and structure refinement
Powder and single-crystal X-ray diffraction studies were done using a Rigaku R-Axis Rapid II curved-imaging-plate microdiffractometer, with monochromatic MoKα radiation. For the powder diffraction (PXRD) studies, a Gandolfi-like motion on the φ and ω axes was used to randomise the polycrystalline samples and observed d values and intensities were derived by profile fitting using JADE 2010 software (Materials Data, Inc.). For all species except camanchacaite, PXRD d values and intensities were calculated using JADE 2010 based on the results of the single-crystal structure refinements (see below). For camanchacaite, calculated d values and intensities were obtained from a whole-pattern-fitting Rietveld refinement using JADE 2010 based on the isotypic structure of magnesiocanutite as a starting point with the cations sites assigned as A = Na, M1 = Ca and M2 = Mg. Detailed listings of observed and calculated PXRD data for all six new species have been deposited with the Principal Editors of Mineralogical Magazine and are available as Supplementary material (see below).
Single-crystal structure studies were done for all species except camanchacaite. The Rigaku CrystalClear software package was used for processing the structure data, including the application of empirical multi-scan absorption corrections using ABSCOR (Higashi, Reference Higashi2001). Initial structure models for espadaite, picaite and riosecoite were obtained by the charge-flipping method using SHELXT (Sheldrick, Reference Sheldrick2015a), while those for chinchorroite and magnesiofluckite were obtained by direct methods using SIR2011 (Burla et al., Reference Burla, Caliandro, Camalli, Carrozzini, Cascarano, Giacovazzo, Mallamo, Mazzone, Polidori and Spagna2012). SHELXL-2016 (Sheldrick, Reference Sheldrick2015b) was used for the refinement of the structures. Full occupancies for the Na, Mg and Ca sites were assumed, except for the Na site in espadaite. The Na site refined to an occupancy of 0.891(12). Because the electron probe microanalysis results indicated a significant excess of Mg over what the Mg site can accommodate, the excess was assigned to the Na site and the Na occupancy was refined yielding a site occupancy of (Na0.826(12)Mg0.0625□0.111). Difference-Fourier maps revealed the location of all H atoms in the structures of chinchorroite, magnesiofluckite, picaite and ríosecoite, but not for the structure of espadaite because the small size of the crystal used gave a much smaller data set. Hydrogen atom positions were refined with soft restraints of 0.82(3) Å on the O–H distances and 1.30(3) Å on the H–H distances and with the U eq of each H of the OH groups set to 1.5 times that of the donor O atom and the U eq of each H of the H2O groups set to 1.2 times that of the donor O atom. Cell parameters are reported in Table 7. Data collection and refinement details are given in Table 8. Atom coordinates and displacement parameters have been deposited with the Principal Editors of Mineralogical Magazine and are available as Supplementary material (see below). Selected bond distances are reported in Table 9 and bond-valence analyses in Table 10.
Table 7. Crystallographic properties of camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

Table 8. Details of structure refinements for chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

Table 9. Selected bond distances (Å) and angles (°) for chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite.

Table 10. Bond-valence analyses for chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite. Values are expressed in valence units.

Bond-valence parameters are from Gagné and Hawthorne (Reference Gagné and Hawthorne2015). Hydrogen-bond strengths are based on O–O bond lengths from Ferraris and Ivaldi (Reference Ferraris and Ivaldi1988). For espadaite, multiplicities are indicated by ×↓→ and hydrogen-bond contributions are not included.
Descriptions of the structures
Camanchacaite
Camanchacaite is a member of the alluaudite group and is isostructural with compounds with the protonated alluaudite-type structure, including the minerals o'danielite, NaZn3[AsO4][AsO3(OH)]2 (Keller and Hess, Reference Keller and Hess1988), groatite, NaCaMn2[PO4][PO3(OH)]2 (Cooper et al., Reference Cooper, Hawthorne, Ball, Ramik and Roberts2009), canutite, NaMn3[AsO4][AsO3(OH)]2 (Kampf et al., Reference Kampf, Mills, Hatert, Nash, Dini and Molina Donoso2014b), and magnesiocanutite, NaMnMg2[AsO4]2[AsO2(OH)2] (Kampf et al., Reference Kampf, Nash, Dini and Molina Donoso2017c). The general formula of these phases is AM1M22[T1O4][T2O3(OH)]2 {T = P or As}, where the M1 and M2 octahedra link by edge-sharing to form staggered chains, T1 and T2 tetrahedra cross-link the chains, and A cations occupy large channel sites. In camanchacaite, A = Na, M1 = Ca and M2 = Mg. The crystal structure of canutite is shown in Fig. 12.

Fig. 12. The crystal structure of camanchacaite (based on that of isostructural canutite) viewed down [001]. Unit cell shown by dashed line. Na–O bonds shown as sticks. Hydrogen bonds shown as solid lines.
Chinchorroite
The structure of chinchorroite contains two types of arsenate groups. The As1 and As2 tetrahedra share a common vertex (O4) forming an As2O7 pyroarsenate (diarsenate) group. The As3 tetrahedron is an AsO3OH acid (protonated) arsenate group. Another unusual feature of the chinchorroite structure is an abbreviated chain of five edge-sharing MgO6 octahedra that includes one Mg1, two Mg2 and two Mg3 octahedra, all of which exhibit regular geometry. Both the As2O7 and AsO3OH groups share corners with octahedra in this five-member chain segment (Fig. 13). An edge-sharing dimer of distorted Na octahedra shares edges with Mg2 and Mg3 octahedra in adjacent chain segments, linking them into a complex thick slab of edge-sharing Mg and Na octahedra parallel to {10![]() $\bar{1}$} (Fig. 14). The As2O7 and AsO3OH groups further link the octahedra within these sheets and a single vertex (O7) of the As2 tetrahedron links to a Mg2/Mg3 octahedron vertex in an adjacent sheet, thereby creating a heteropolyhedral framework (Fig. 15).
$\bar{1}$} (Fig. 14). The As2O7 and AsO3OH groups further link the octahedra within these sheets and a single vertex (O7) of the As2 tetrahedron links to a Mg2/Mg3 octahedron vertex in an adjacent sheet, thereby creating a heteropolyhedral framework (Fig. 15).

Fig. 13. The abbreviated chains of edge-sharing octahedra with surrounding polyhedra in the structures of chinchorroite and matulaite.

Fig. 14. The thick {10![]() $\bar{1}$} sheet of edge-sharing octahedra with surrounding pyroarsenate and hydrogen-arsenate groups in chinchorroite. Note that O7 vertices link to adjacent sheets. View is down [010].
$\bar{1}$} sheet of edge-sharing octahedra with surrounding pyroarsenate and hydrogen-arsenate groups in chinchorroite. Note that O7 vertices link to adjacent sheets. View is down [010].

Fig. 15. The structure of chinchorroite (a) viewed down [100] and (b) viewed down [010].
Pyroarsenate groups have been reported previously in the structures of only two minerals: petewilliamsite, (Ni,Co)30(As2O7)15 (Roberts et al., Reference Roberts, Burns, Gault, Criddle and Feinglos2004) and theoparacelsite, Cu3(OH)2As2O7 (Sarp and Černy, Reference Sarp and Černy2001). However, no other mineral structure has been reported to contain both pyroarsenate and hydrogen-arsenate groups and we are aware of only one synthetic phase Sr5(As2O7)2(AsO3OH) containing both of these structural units (Mihajlović et al., Reference Mihajlović, Libowitzky and Effenberger2004). The chain of five edge-sharing Mg octahedra is similar to the five-member chain segment of edge-sharing Al octahedra in the structure of matulaite, Fe3+Al7(PO4)4(PO3OH)2(OH)8(H2O)8·8H2O, (Kampf et al., Reference Kampf, Mills, Rumsey, Spratt and Favreau2012), although in matulaite, each end of the chain segment has an additional corner-linked Al octahedron and the PO4 and PO3OH tetrahedra decorating the chain are configured differently (Fig. 13).
Espadaite
The structure of espadaite contains one NaO6 polyhedron (highly distorted octahedron with a split OW2 vertex), one MgO6 octahedron, two different CaO8 polyhedra and three different hydrogen arsenate groups. Although H atom sites could not be located in the structure refinement, it was possible to derive a complete hydrogen-bonding scheme (see Table 9), and resultant bond-valence sums (BVS) allowed assignment of O, OH and H2O to each of the O sites (see Table 10). The OH3 site, in particular, presents an unusual situation. The OH3 site receives normal hydrogen bonds from OH8 (0.23 valence units) and OH12 (0.19 vu), and its close approach (2.472 Å) to an adjacent OH3 site is indicative of a symmetrical hydrogen bond between them, presumably sharing an H atom sited at the special position (½,½,0). The result is that OH3 has ½ O and ½ OH character. Assigning the As2 and As3 arsenate groups as dihydrogen arsenates and the As1 group as a half dihydrogen and half monohydrogen arsenate, results in an ideal formula with [AsO2.5(OH)1.5]4[AsO2(OH)2]814–, or alternatively [AsO3(OH)]2[AsO2(OH)2]1014–, as the anionic component, thereby balancing the charge of the ideal cation content of [Na4Ca3Mg2]14+.
The MgO6 octahedron and CaO8 polyhedra link by sharing edges. The arsenate tetrahedra share their non-OH vertices with MgO6 octahedra and CaO8 polyhedra, thereby further bridging these polyhedra. The resulting heteropolyhedral sheet parallel to {001} (Fig. 16) of formula {Ca3Mg2[AsO3(OH)]2[AsO2(OH)2]10}4− is pseudohexagonal in general aspect and contains large voids that accommodate the partly occupied OW4a and OW4b H2O sites. The NaO6 polyhedron is located in the interlayer region, and two NaO6 polyhedra share an edge to form a dimer. The shared O vertices of the dimer are H2O groups (OW1 and OW2; the OW2 being split into two half-occupied sites), and the other five vertices of each Na octahedron (OH4, OH7, OH8 and OH12) are OH groups of the three arsenate groups in the sheets. The Na2(OH)8(H2O)2 dimer thereby links adjacent sheets together in the [001] direction (Fig. 17). An additional isolated H2O group (OW3) is also located in the interlayer region. The heteropolyhedral sheet and the structure of espadaite as a whole appear to be unique.

Fig. 16. The heteropolyhedral layer in the structure of espadaite, viewed along [001].

Fig. 17. The structure of espadaite, viewed down [100].
Magnesiofluckite
Magnesiofluckite is isostructural with fluckite, CaMn2+(AsO3OH)2(H2O)2. In the structure of magnesiofluckite (Fig. 18), CaO6 and MgO6 octahedra each occur as edge-sharing dimers, with equivalent octahedra on either side of a centre of symmetry; Ca2O10 dimers are centred at (0,0,0) and Mg2O10 dimers are centred at (½,0,½). CaO6 and MgO6 octahedra also share edges with one another, thereby forming edge-sharing chains parallel to [10![]() $\bar{1}$] and composed of alternating Ca2O10 and Mg2O10 dimers. These chains are linked in the [101] direction by AsO3OH groups to form sheets parallel to {010}. The sheets are linked to each other via hydrogen bonds.
$\bar{1}$] and composed of alternating Ca2O10 and Mg2O10 dimers. These chains are linked in the [101] direction by AsO3OH groups to form sheets parallel to {010}. The sheets are linked to each other via hydrogen bonds.

Fig. 18. The structure of magnesiofluckite, viewed down [101].
Picaite
The structure of picaite contains NaO6 and CaO6 octahedra and As1O3(OH) and As2O2(OH)2 tetrahedra. Both the NaO6 and CaO6 octahedra link by sharing edges to form Na2O10 and Ca2O10 dimers, respectively; edge sharing between alternating Na2O10 and Ca2O10 dimers results in a zigzag chain along [101]. The As1O3(OH) and As2O2(OH)2 tetrahedra each share one corner with a NaO6 octahedron and one corner with a CaO6 octahedron within the same chain of edge-sharing octahedra, resulting in a heteropolyhedral chain (Fig. 19). The remaining two corners of each As1O3(OH) and As2O2(OH)2 tetrahedron link to NaO6 and CaO6 octahedra in adjacent chains, resulting a framework (Fig. 20). Hydrogen bonds provide additional linkage within this framework. The structure of picaite is unique and we are unaware of any other structure to which it is very similar.

Fig. 19. The chain of edge-sharing octahedra with bridging As1O3(OH) and As2O2(OH)2 tetrahedra in the structure of picaite.

Fig. 20. The structure of picaite viewed down [101], the direction of the chains.
Ríosecoite
The structure of ríosecoite contains one MgO6 octahedron, one Ca1O8 polyhedron, one Ca2O7 polyhedron and three different AsO3(OH) tetrahedra (centred by As1, As2 and As3). Two Ca1O8 and two Ca2O7 polyhedra share multiple edges to form a tetramer (Fig. 21). Each MgO6 octahedron shares one edge with a Ca1O8 polyhedron and one edge with a Ca2O7 polyhedron, each in a different tetramer, resulting in a chain of edge-sharing polyhedra along [100]. The As1O3(OH) tetrahedra further link the polyhedra within the chain. The complex chains composed of MgO6 octahedra, Ca1O8 polyhedra, Ca2O7 polyhedra, and AsO3(OH) tetrahedra (Fig. 22) are linked to each other by As–O–Mg and As–O–Ca corner links to form a framework (Fig. 23). Hydrogen bonds provide additional linkages within this framework. The structure of ríosecoite is unique and we are unaware of any other structure to which it is very similar.

Fig. 21. The tetramer of edge-sharing Ca1O8 and Ca2O7 polyhedra in the structure of ríosecoite.

Fig. 22. The complex chain (along a) of edge-sharing CaO7 and CaO8 polyhedra and MgO6 octahedra with bridging AsO3(OH) tetrahedra in the structure of ríosecoite. O–H bonds are shown as sticks and hydrogen bonds as thin lines. The unit cell outline is shown with dashed lines.

Fig. 23. The structure of ríosecoite viewed down [100], the direction of the chains. O–H bonds are shown as sticks and hydrogen bonds as thin lines. The unit-cell outline is shown with dashed lines.
Discussion
The high levels of arsenic in northern Chile are well documented (cf. Tapia et al., Reference Tapia, González, Townley, Oliveros, Álvarez, Aguilar, Menzies and Calderón2018). The arsenic, originating most notably from volcanic activity, hot springs and porphyry and epithermal ore deposits over the past 70 million years, once mobilised by weathering, makes its way into drainage systems and groundwater, where it has contributed to deleterious health effects on humans and other organisms dating back at least to the Chinchorro culture (but undoubtedly much earlier) and continuing to this day.
The Torrecillas deposit consists of volcanogenic/hydrothermal veins, variably rich in Cu and/or As, that have been emplaced in metamorphosed marine shales and lavas, perhaps as a skarn-type deposit. Alteration of parts of the veins composed largely of native arsenic has produced an array of arsenites and arsenates (Tables 1 and 2). The hyperarid climate of the Atacama Desert is an important factor in the formation of the secondary mineralisation at Torrecillas, as it is in virtually every ore deposit in the Atacama Desert. The oxidation of native arsenic, and possibly other As-bearing primary phases in the veins, was followed by reaction with fluids rich in dissolved salts. In the Atacama Desert, these saline brines are generally derived from evaporating meteoric water (cf. Cameron et al., Reference Cameron, Leybourne and Palacios2007); however, considering the proximity of the Torrecillas deposit to the Pacific Ocean, it seems possible that the frequent dense coastal camanchaca fogs have also played a role in the alteration of the veins and the formation of the secondary minerals, particularly in the recent past since the exhumation of the deposit well above sea level on Torrecillas Hill. The capacity of salt-rich fog to weather rocks under conditions prevalent in the Atacama Desert has been demonstrated (cf. Goudie et al., Reference Goudie, Wright and Viles2002). Isotopic evidence has shown that significant amounts of Ca and SO4 in Atacama soils are derived from marine aerosols even in inland areas (100 km or more from the coast) reached by the coastal fogs (Rech et al., Reference Rech, Quade and Hart2003). The fogs along the northern Chilean coast are relatively rich in dissolved Na+, Cl– and SO42–, and also contain significant amounts of Ca2+, Mg2+, K+, NH4+ and NO3– (Wang et al., Reference Wang, Michalski, Seo and Ge2014). The frequency of the fogs (up to 189 days per year; Cereceda and Schemenauer, Reference Cereceda and Schemenauer1991), coupled with the long-term concentration of their salt content under hyperarid conditions, increases the likelihood that they may be a significant factor in the development of the secondary mineralisation at Torrecillas.
Among the As-rich assemblages, some consist of only arsenites, some of both arsenites and arsenates, and some of only arsenates. The localised conditions of Eh, pH and chemical constituents determine the minerals in each assemblage, but none can be considered an equilibrium assemblage. Each assemblage can be considered a snapshot of a dynamically (if slowly) progressing open-system alteration process.
Although arsenites typically predate arsenates, it is not always easy to determine crystallisation sequences. An unusual feature of the Torrecillas secondary arsenic mineral assemblages is the recognition, so far, of twelve new arsenates, all of which contain hydrogen (and/or dihydrogen) arsenate groups; although, among the already known arsenates, there are also normal arsenates. All of the secondary arsenic minerals occurring on the two specimens containing the six new minerals described in this paper are arsenates. Besides the new species camanchacaite, chinchorroite, espadaite, magnesiofluckite, picaite and ríosecoite, other arsenates found on these specimens are talmessite and currierite. The essential cations in these minerals are limited to Ca, Mg and Na. These elements could all be derived from the fog; however, these elements, sourced from altered country rocks, are common constituents of the saline brines noted above. The sequence of crystallisation of the arsenates in the Torrecillas deposit is difficult to determine completely and unambiguously. Talmessite and currierite seem to be earliest, followed by magnesiofluckite and picaite. Camanchacaite seems to occur next in the sequence, but observations suggest that it crystallises over a range and both precedes and postdates the crystallisation of ríosecoite and chinchorroite. The last of these phases to form is espadaite.
It is tempting to try to relate the crystallisation sequence to differences in the compositions and/or structures of the arsenates. Unfortunately, previous studies (cf. Magalhães et al., Reference Magalhães, de Jesus and Williams1988; Majzlan et al., Reference Majzlan, Drahota, Filippi, Bowell, Alpers, Jamieson, Nordstrom and Majzlan2014) do not provide much insight that is directly applicable to doing so for the phases at hand. The only straightforward conclusion that can be drawn is that the presence of hydrogen arsenate groups is indicative of crystallisation from solutions of relatively low pH. In this respect, it is worth observing that the saline brines in the region have close to neutral pH (Guillermo Chong Díaz, pers. comm.) and the coastal fogs usually have pH in the neutral to somewhat acid range (Wang et al., Reference Wang, Michalski, Seo and Ge2014). When these fluids interact with reduced phases, such as native As, oxidation tends to produce acids, such as arsenous acid, H3As3+O3 (less oxidised), and arsenic acid, H3As5+O4 (more oxidised). The formation of the hydrogen arsenates described herein can be attributed to the evaporation of solutions containing H3As5+O4, which are also rich in Na+, Ca2+ and Mg2+.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.28
Acknowledgements
Reviewers Frank Hawthorne and Elena Zhitova are thanked for their constructive comments on the manuscript. Guillermo Chong Díaz provided valuable insights, especially regarding the geology and mineral formation. A portion of this study was funded by the John Jago Trelawney Endowment to the Mineral Sciences Department of the Natural History Museum of Los Angeles County.