Introduction
The guano deposit situated at the Pabellón de Pica Mountain, 1.5 km south of the Chanabaya village, Iquique Province, Tarapacá Region, Chile (20°55′S, 70°08′W) is a unique locality among those belonging to the belt of guano occurrences of the Atacama Desert (Ericksen, Reference Ericksen1981; Pankhurst and Herve, Reference Pankhurst, Herve, Moreno and Gibbons2007; Appelton and Nothold, Reference Appleton and Nothold2002; Bojar et al., Reference Bojar, Walter, Baumgartner and Färber2010). The specific geochemical feature of this deposit is the high content of copper extracted from chalcopyrite, which is an accessory but rather abundant component of the host rock, amphibole gabbro.
The new mineral species bojarite, Cu3(N3C2H2)3(OH)Cl2⋅6H2O, described in this paper is the ninth new mineral species discovered in guano deposits at Pabellón de Pica, after ammineite CuCl2(NH3)2 (Bojar et al., Reference Bojar, Walter, Baumgartner and Färber2010), joanneumite Cu(C3N3O3H2)2(NH3)2 (Bojar et al., Reference Bojar, Walter and Baumgartner2017), chanabayaite Cu2(N3C2H2)2Cl(NH3,Cl,H2O,□)4 (Chukanov et al., Reference Chukanov, Zubkova, Möhn, Pekov, Pushcharovsky and Zadov2015a), möhnite (NH4)K2Na(SO4)2 (Chukanov et al., Reference Chukanov, Britvin, Möhn, Pekov, Zubkova, Nestola, Kasatkin and Dini2015b), shilovite Cu(NH3)4(NO3)2 (Chukanov et al., Reference Chukanov, Aksenov, Rastsvetaeva, Lysenko, Belakovskiy, Färber, Möhn and Van2015c), antipinite KNa3Cu2(C2O4)4 (Chukanov et al., Reference Chukanov, Aksenov, Rastsvetaeva, Pekov, Belakovskiy and Britvin2015d), triazolite NaCu2(N3C2H2)2(NH3)2Cl3⋅4H2O (Zubkova et al., Reference Zubkova, Chukanov, Pekov, Möhn, Giester, Yapaskurt, Lykova and Pushcharovsky2016; Chukanov et al., Reference Chukanov, Zubkova, Möhn, Pekov, Belakovskiy, Van, Britvin and Pushcharovsky2018) and ammoniotinsleyite (NH4)2Al2(PO4)2(OH)⋅2H2O (Chukanov et al., Reference Chukanov, Möhn, Pekov, Zubkova, Ksenofontov, Belakovskiy, Vozchikova, Britvin and Desor2020a). All these minerals, except antipinite, contain nitrogen, most of them contain organic groups, and in seven new minerals from this locality Cu2+ is a species-defining component.
Specimens in which bojarite was discovered were collected at Pabellón de Pica in 2019 by one of the authors (G.M.).
Bojarite is named in honour of the well-known Austrian mineralogist Dr Hans-Peter Bojar (b. 1967) who works in the Department of Mineralogy, Universalmuseum Joanneum, Graz, Austria. Dr. Bojar is a geoscientist who has contributed in many areas of Earth Science (e.g. Bojar et al., Reference Bojar, Ottner, Bojar, Grigorescu and Perşoiu2009, Reference Bojar, Bojar, Hałas and Wójtowicz2013). In particular, he is the Senior author of the discoveries of several new mineral species, including two N- and Cu-bearing minerals from Pabellón de Pica Mountain, ammineite and joanneumite (Bojar et al., Reference Bojar, Walter, Baumgartner and Färber2010, Reference Bojar, Walter and Baumgartner2017).
The new mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-037, Chukanov et al., Reference Chukanov, Möhn, Zubkova, Ksenofontov, Pekov, Agakhanov, Britvin and Desor2020b). The holotype specimen is deposited in the collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, Russia with the registration number 5574/1.
Occurrence, general appearance and physical properties
Bojarite occurs in a guano deposit on the lower part of the steep southern slope of the Pabellón de Pica Mountain. It forms fine-grained porous aggregates up to 1 mm × 3 mm × 5 mm typically combined in interrupted earthy crusts (Fig. 1), which are composed of pseudomorphs after chanabayaite aggregates (Fig. 2) and overgrow the host amphibole gabbro or granular aggregates consisting of salammoniac, halite and minor nitratine and an unidentified Mn oxide.

Fig. 1. Blue interrupted crusts of bojarite on an aggregate of salammoniac–halite. Dark areas are very thin films of an unidentified Mn oxide. Field of view: 7 cm wide, holotype.

Fig. 2. Pseudomorphs of bojarite after radiated aggregates of chanabayaite crystals. Field of view: 1.7 mm wide, fragment of a sample collected by G.M.
Aggregates of bojarite are opaque and dull due to the fine-grained character. The colour and streak are blue. The Mohs hardness is 2 (for aggregates). Bojarite is brittle. Density could not be measured due to the porosity of the fine-grained bojarite aggregates. Density calculated using the empirical formula is 2.057 g cm–3.
Bojarite is optically isotropic and n = 1.635(2) (λ = 589 nm). Under the polarising microscope, the mineral is pale blue and non-pleochroic.
Infrared spectroscopy
In order to obtain the infrared (IR) absorption spectrum (Fig. 3), a powdered sample of bojarite was mixed with dried KBr, pelletised, and analysed using an ALPHA FTIR spectrometer (Bruker Optics) in the range 360–4000 cm–1 with a resolution of 4 cm–1. A total of 16 scans were collected. The IR spectrum of an analogous pellet of pure KBr was used as a reference.

Fig. 3. Powder infrared absorption spectrum of bojarite.
The assignment of absorption bands made in accordance with Grinshtein et al. (Reference Grinshtein, Strazdin and Grinvalde1970), Nakamoto (Reference Nakamoto2008, Reference Nakamoto2009), Chukanov et al. (Reference Chukanov, Zubkova, Möhn, Pekov, Pushcharovsky and Zadov2015a, Reference Chukanov, Zubkova, Möhn, Pekov, Belakovskiy, Van, Britvin and Pushcharovsky2018) and Chukanov and Chervonnyi (Reference Chukanov and Chervonnyi2016) is given in Table 1. Infrared bands of chanabayaite are given in Table 1 for comparison.
Table 1. Wavenumbers (cm–1) of absorption bands in the IR spectra of bojarite and chanabayaite and their assignment.

Note: w – weak band, s – strong band, sh – shoulder.
The broad bands at 1751 and 2096 cm–1 may correspond to acid groups, which could arise for example, as a result of partial protonation of triazolate anions in accordance with the dynamic equilibrium C2N3H2– + H2O ↔ C2N3H3 + OH–.
The IR spectrum of bojarite is distinct and can be used as a very reliable diagnostic feature of this mineral.
Raman spectroscopy
The Raman spectrum of a randomly oriented bojarite sample (Fig. 4) was obtained using a spectrometer based on a Horiba XploRA Raman microscope with a 532 nm 3B DPSS Nd:YAG laser. The spectrum was recorded in the range from 100 to 4000 cm–1 with a diffraction grating of 1800 gr mm–1. Laser radiation with the output power of 20–25 mW was attenuated to 1%. The diameter of the focal spot on the sample was <5 μm. The back-scattered Raman signal was collected with a 100× objective; signal acquisition time for a single scan of the spectral range was 66 s, and the signal was averaged over 4 scans. No sample damage was observed under these conditions.

Fig. 4. Raman spectrum of bojarite.
The assignment of the Raman bands is nearly the same as the assignment of the IR bands. The bands at 3300–3500 cm–1 are attributed to O–H stretching vibrations; 3148 cm–1 to C–H stretching vibrations; and 1600–1700 cm–1 to bending vibrations of H2O molecules (the splitting of this band corresponding to a nondegenerate mode indicates the presence of two kinds of H2O molecules, in accordance with structural data). The bands at 990–1520 cm–1 are attributed to in-plane stretching and mixed vibrations of the 1,2,4-triazole ring; 899 cm–1 to in-plane bending vibrations of the 1,2,4-triazole ring; and 677 cm–1 to out-of-plane bending vibrations of the 1,2,4-triazole ring. The bands at 463, 254 and 152 cm–1 are tentatively assigned to lattice modes with significant contribution of Cu–O, Cu–N and Cu–Cl stretching vibrations, respectively.
Chemical data
Chemical data (mean of three electron microprobe analyses for Na, Mg, Fe, Cu and Cl) were obtained using an electron microprobe (energy dispersive spectroscopy mode, 20 kV, 600 pA, beam rastered on an area 10 μm × 10 μm in order to minimise unstable sample damage). Attempts to use wavelength dispersive spectroscopy, with higher beam current, were unsuccessful because of the instability of the mineral. Hydrogen, N and C were analysed by gas chromatography of products of ignition at 1200°C. Structural data and chemical tests show the absence of the CO32– anion. All carbon belongs to the triazolate anion, (N3C2H2)–. Analytical data are given in Table 2.
Table 2. Chemical composition of bojarite.

Note: Contents of other elements with atomic numbers > 6 are below detection limits.
* Calculated by stoichiometry; S.D. – standard deviation.
The empirical formula calculated based on the formula Cu3(N3C2H2)3[Cl2(H2O)4](OH)⋅2H2O obtained from the structure refinement data (see below) and three Cu+Mg+Fe+Na atoms per formula unit is (Cu2.68Mg0.17Fe0.10Na0.05)Σ3(N3C2H2)2.755[(OH)][Cl2.19(H2O)3.77(OH)0.04]Σ6⋅2.3H2O.
Excessive Na, N, Cl and H2O as well as some deficiency of triazolate anions may be due to analytical errors. The simplified formula is (Cu,Mg,Fe)3(N3C2H2)3(OH)[(H2O),Cl]6⋅2H2O. The idealised formula is Cu3(N3C2H2)3(OH)[Cl2(H2O)4]⋅2H2O which requires Cu 32.26, Cl 12.00, N 21.34, C 12.20, H 3.24, O 18.96, total 100 wt.%.
Bojarite is insoluble in water and dissolves in dilute hydrochloric acid without gas evolution. Reactions of bojarite solution in 20% HCl with potassium hexacyanoferrate(II) and potassium hexacyanoferrate(III) show that all copper in the mineral is bivalent.
X-ray diffraction and crystal structure
Single-crystal X-ray diffraction studies of bojarite could not be carried-out due to the absence of suitable single crystals: aggregates of bojarite (Figs 1, 2) are polycrystalline and consist of very small imperfect individuals. For this reason, the crystal structure of bojarite was refined based on powder X-ray diffraction data, using the Rietveld method.
Powder X-ray diffraction data of bojarite (Table 3, Fig. 5) were collected with a Rigaku R-AXIS Rapid II single-crystal diffractometer equipped with cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), at the accelerating voltage of 40 kV, current of 15 mA and exposure of 15 min. Angular resolution of the detector is 0.045° (for 2θ) and pixel size is 0.1 mm. The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017).

Fig. 5. Observed and calculated powder X-ray diffraction patterns of bojarite with admixed belloite (B) and halite (H). The solid line corresponds to calculated data. The crosses correspond to the observed pattern. The vertical bars mark all possible Bragg reflections. The difference between the observed and calculated patterns is shown by the curve at the bottom.
Table 3. Powder X-ray diffraction data of bojarite.
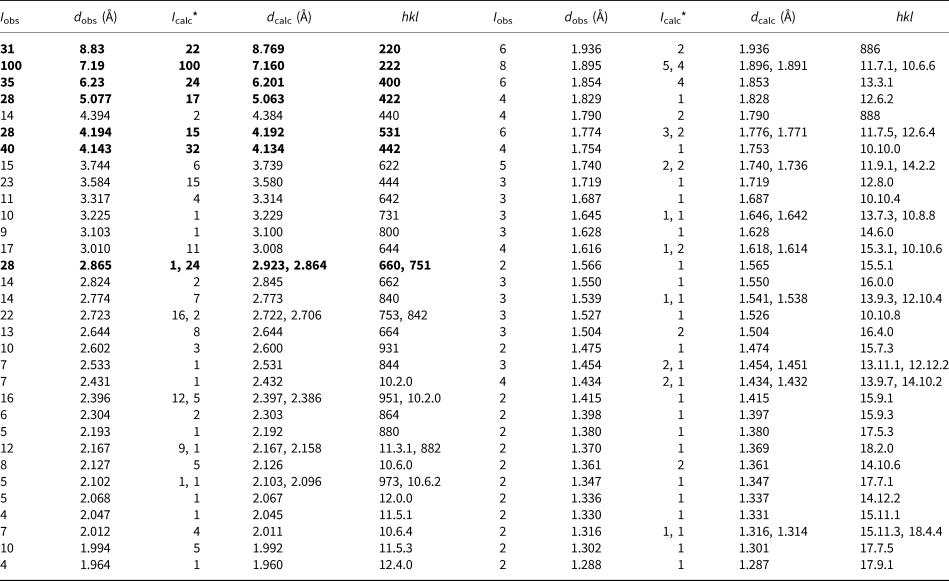
* For the calculated pattern only reflections with intensities ≥1 are given. The strongest lines are given in bold.
Refinement of the crystal structure of bojarite was performed by the Rietveld method using the model for synthetic [Cu3(trz)3(OH)]Cl2·6H2O (trz = 1,2,4-triazole anion N3C2H2–) (Yamada et al., Reference Yamada, Maruta and Takeda2011) as the starting point. Hydrogen atoms were not located. Data treatment and the Rietveld structure analysis were carried using the JANA2006 program package (Petříček et al., Reference Petříček, Dušek and Palatinus2006). The profiles were modelled using a pseudo-Voigt function. The structure was refined in isotropic approximation of atomic displacements. Only Cu and Cl sites were allowed to move whereas U iso was refined for all atomic sites. The structural analysis was complemented by addition of halite, NaCl, (Abrahams and Bernstein, Reference Abrahams and Bernstein1965) and belloite, Cu(OH)Cl, (Effenberger, Reference Effenberger1984) as impurities, to account for a few low-intensity diffraction peaks in the powder pattern. The relative contents of the three minerals in the powder mixture are: 92% of bojarite, 6% of belloite and 2% of halite. The final agreement factors are: R p = 0.0225, Rw p = 0.0310 and R obs = 0.0417. The refined parameters of the cubic unit cell are a = 24.8047(5) Å and V = 15,261.6(5) Å3; space group Fd ![]() $\bar{3}$c and Z = 32. The observed and calculated powder XRD diagrams demonstrate a good agreement (Fig. 5). Coordinates and thermal displacement parameters of atoms are given in Table 4 and selected interatomic distances in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
$\bar{3}$c and Z = 32. The observed and calculated powder XRD diagrams demonstrate a good agreement (Fig. 5). Coordinates and thermal displacement parameters of atoms are given in Table 4 and selected interatomic distances in Table 5. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 4. Сoordinates and isotropic parameters (U iso in Å2) of atoms and site occupancy factors (s.o.f.) in the structure of bojarite.

Table 5. Selected interatomic distances (Å) in the structure of bojarite.

Discussion
Bojarite is a natural analogue of the synthetic compound [Cu3(trz)3(OH)]Cl2⋅6H2O and is isostructural with [Cu3(trz)3(OH)]Br2·6H2O where trz denotes 1,2,4-triazolate anion (Yamada et al., Reference Yamada, Maruta and Takeda2011). The crystal structure of bojarite is built by equilateral triangular units involving Cu2+ cations: three Cu2+ cations of the unit are linked by an oxygen atom (O1 = OH with the bond-valence sum of 1.32) at the centre of the equilateral triangle and connected to two nitrogen atoms of the triazole ring (Fig. 6), leading to the formation of the [Cu3(trz)3(OH)]2+ building block reported for the synthetic compound by Yamada et al. (Reference Yamada, Maruta and Takeda2011). The third nitrogen atom of each trz group of the triangular unit coordinates to an adjacent triangular unit, leading to the formation of a three-dimensional network. The Cu2+ cation is coordinated by three N atoms, one O atom (of the OH– group) and, in a statistically mixed arrangement (see Table 4), either two Cl– anions (site occupancy factor = 0.333) or, in the adjacent O2 site, two H2O molecules (s.o.f. = 0.667), with elongate Cu–(Cl,H2O) distances (Table 5). The fragment of the crystal structure containing the [Cu3(trz)3(OH)]2+ building block with additional Cl anions and three additional trz ligands is shown in Fig. 6. Two additional H2O molecules per formula unit are located at the O3 site, in cavities of the positively charged three-dimensional network formed by the [Cu3(trz)3(OH)]2+ building blocks. These extra-framework H2O molecules can be deleted from the structure on heating to 150°C without destruction of the framework (Yamada et al., Reference Yamada, Maruta and Takeda2011).

Fig. 6. A fragment of the crystal structure of bojarite. Cu atoms are light blue, O red, Cl green, N dark blue and C black.
Note that the [Cu3(trz)3(OH)]2+ building block has been found in a number of synthetic compounds (Ouellette et al., Reference Ouellette, Jones and Zubieta2011 and references therein). Thus, for example, the same building block, a topologically similar three-dimensional framework, the same space group and close unit cell parameters were reported for the compounds [CuII3(trz)3(OH)3(H2O)4]⋅4.5H2O (cubic, space group Fd ![]() $\bar{3}$c, a = 24.7233(4) Å, V = 15,111.9(4) Å3 and Z = 32) and [CuII3(trz)3OH][CuI2Br4] (cubic, space group Fd
$\bar{3}$c, a = 24.7233(4) Å, V = 15,111.9(4) Å3 and Z = 32) and [CuII3(trz)3OH][CuI2Br4] (cubic, space group Fd ![]() $\bar{3}$c, a = 24.5326(6) Å, V = 14,764.9(6) Å3 and Z = 32) which are structurally close to bojarite and are characterised by the same cationic framework substructure {Cu3(OH)(trz)3}n2n+ (Ouellette et al., Reference Ouellette, Jones and Zubieta2011).
$\bar{3}$c, a = 24.5326(6) Å, V = 14,764.9(6) Å3 and Z = 32) which are structurally close to bojarite and are characterised by the same cationic framework substructure {Cu3(OH)(trz)3}n2n+ (Ouellette et al., Reference Ouellette, Jones and Zubieta2011).
A review of metal coordination compounds with 1,2,4-triazole derivatives as ligands is given by Haasnoot (Reference Haasnoot2000). In the 5-membered 1,2,4-triazole ring all C–N and N–N bonds are conjugated and have fractional bond orders between 1 and 2. As a result, C–N and N–N bond lengths are rather short (typically, between 1.30 and 1.38 Å). Copper and zinc show high affinity for the tetrazolate ligand, and halogen anions F–, Cl– and Br– are known to stabilise crystal structures of triazolate complexes.
Bojarite is a member of the transformation series triazolite NaCu2(N3C2H2)2(NH3)2Cl3⋅4H2O → chanabayaite Cu2(N3C2H2)2Cl(NH3,Cl,H2O,□)4 → bojarite Cu3(N3C2H2)3(OH)Cl2⋅6H2O. On the first stage of this transformation (pseudomorphisation), partial dehydration, loss of NaCl and evolution of NH3 took place: chanabayaite replaces triazolite (Chukanov et al., Reference Chukanov, Zubkova, Möhn, Pekov, Belakovskiy, Van, Britvin and Pushcharovsky2018). On the second stage, removal of the rest of NH3 was accompanied by hydration: a pseudomorph of bojarite after chanabayaite is formed. Thus, the 1,2,4-triazolate anion is the most stable and immobile unit in these minerals.
Acknowledgements
The authors are grateful to the referees Peter Leverett and Sándor Szakáll for useful comments. This work was supported by the Russian Foundation for Basic Research, grant no. 18-29-12007-mk in part of the crystal structure study. A part of this work (IR spectroscopy and chemical analyses) was carried-out in accordance with the state task, state registration number ААAА-А19-119092390076-7. The authors thank the X-ray Diffraction Centre of Saint-Petersburg State University for instrumental and computational resources.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.85