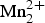Introduction
Graftonite and beusite are common late-stage accessory minerals in complex granitic pegmatites (e.g. Fransolet et al., Reference Fransolet, Keller and Fontan1986; Černý et al., Reference Černy, Selway, Ercit, Breaks, Anderson and Anderson1998; Smeds et al., Reference Smeds, Uher, Černý, Wise, Gustafsson and Penner1998; Pieczka, Reference Pieczka2007; Guastoni et al., Reference Guastoni, Nestola, Mazzoleni and Vignola2007; Reference Vignola, Diella, Oppizzi, Tiepolo and WeissVignola et al., Reference Vignola, Diella, Oppizzi, Tiepolo and Weiss2008; Galliski et al., Reference Galliski, Oyarzábal, Márquez-Zavalía and Chapman2009; Ercit et al., Reference Ercit, Tait, Cooper, Abdu, Ball, Anderson, Černý, Hawthorne and Galliski2010). They have also been found as constituents of phosphate-oxide inclusions in IIIAB iron meteorites (Bild, Reference Bild1974; Steele et al., Reference Steele, Olsen, Pluth and Davis1991; Olsen et al., Reference Olsen, Kracher, Davis, Steele, Hutcheon and Bunch1999), and Stalder and Rozendaal (Reference Stalder and Rozendaal2002) reported graftonite as a primary phase in a phosphorous-rich iron formation. Penfield (Reference Penfield1900) described graftonite, ideally [![]() ${\rm Fe}_{\rm 3}^{2 +} $(PO4)2], from a granitic pegmatite in New Hampshire. Beus (Reference Beus1950) reported a graftonite-like mineral with Mn2+ dominant over Fe2+, and Hurlbut and Aristarain (Reference Hurlbut and Aristarain1968) described beusite, ideally [
${\rm Fe}_{\rm 3}^{2 +} $(PO4)2], from a granitic pegmatite in New Hampshire. Beus (Reference Beus1950) reported a graftonite-like mineral with Mn2+ dominant over Fe2+, and Hurlbut and Aristarain (Reference Hurlbut and Aristarain1968) described beusite, ideally [![]() ${\rm Mn}_{\rm 3}^{2 +} $(PO4)2], as a distinct species from the pegmatites of the San Luis province, Argentina. The crystal structures of graftonite and beusite were solved by Calvo (Reference Calvo1968) and Hurlbut and Aristarain (Reference Hurlbut and Aristarain1968), and Steele et al. (Reference Steele, Olsen, Pluth and Davis1991) and Wise et al. (Reference Wise, Hawthorne and Černý1990) refined the structures of Ca-free and Ca-rich beusite, respectively. The structure of graftonite–beusite is a dense framework of polyhedra (Hawthorne, Reference Hawthorne1998; Huminicki and Hawthorne, Reference Huminicki, Hawthorne, Kohn, Rakovan and Hughes2002), and Tait et al. (Reference Tait, Hawthorne and Wise2013) concluded that the coordination numbers of the sites occupied by Fe2+, Mn2+ and Ca2+ (and minor Mg2+) are as follows: M(1) = [8], M(2) = [5], M(3) = [6]. As a result, there is very strong order of cations over the M(1), M(2) and M(3) sites, and Hawthorne and Pieczka (Reference Hawthorne and Pieczka2018) have introduced a new nomenclature and classification scheme for these minerals.
${\rm Mn}_{\rm 3}^{2 +} $(PO4)2], as a distinct species from the pegmatites of the San Luis province, Argentina. The crystal structures of graftonite and beusite were solved by Calvo (Reference Calvo1968) and Hurlbut and Aristarain (Reference Hurlbut and Aristarain1968), and Steele et al. (Reference Steele, Olsen, Pluth and Davis1991) and Wise et al. (Reference Wise, Hawthorne and Černý1990) refined the structures of Ca-free and Ca-rich beusite, respectively. The structure of graftonite–beusite is a dense framework of polyhedra (Hawthorne, Reference Hawthorne1998; Huminicki and Hawthorne, Reference Huminicki, Hawthorne, Kohn, Rakovan and Hughes2002), and Tait et al. (Reference Tait, Hawthorne and Wise2013) concluded that the coordination numbers of the sites occupied by Fe2+, Mn2+ and Ca2+ (and minor Mg2+) are as follows: M(1) = [8], M(2) = [5], M(3) = [6]. As a result, there is very strong order of cations over the M(1), M(2) and M(3) sites, and Hawthorne and Pieczka (Reference Hawthorne and Pieczka2018) have introduced a new nomenclature and classification scheme for these minerals.
Beusite-(Ca), CaMn2(PO4)2, is a Ca analogue of beusite with Ca2+ completely ordered at the M(1) site. The name is in accord with the nomenclature scheme for the graftonite group approved by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (memorandum 66–SM/17 Hålenius et al. (Reference Hålenius, Hatert, Pasero and Mills2017)). The holotype sample is deposited in the mineral collection of the Department of Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, D.C., 20560, USA, catalogue number 177054.
Occurrence
Beusite-(Ca) occurs in a small pegmatite dyke in the regionally zoned Peg swarm of the Archean Yellowknife pegmatite field, and is located between Upper Ross Lake and Redout Lake, 75 km northeast of Yellowknife and 3.5 km east of the Redout granite, Canada (62°44′37′′N, 113°6′26′′W, Wise and Černý, Reference Wise and Černý1990). The pegmatite is lenticular, strikes N–S, dips 45–70°E and cuts an interlayered sequence of amphibolite and granodiorite. The pegmatite is of the beryl–columbite–phosphate subtype of rare-element pegmatites (Černý, Reference Černý, Möller, Černý and Saupe1989) and shows well-developed, although not continuous, internal zonation; much of the primary zonation is obscured by albite units. The border zone is a fine-grained muscovite + quartz assemblage, followed by a fine-grained microcline perthite + quartz + muscovite + ‘cleavelandite’ wall-zone. Most of the pegmatite is composed of a coarse-grained microcline perthite (graphic) + quartz + muscovite + ‘cleavelandite’ + beryl zone that hosts most of the accessory minerals. The core is discontinuous and consists of coarse-grained quartz-microcline + accessory beryl.
Accessory minerals include ‘biotite’, yellow-green beryl, columbite-(Fe)–tantalite-(Fe), tapiolite-(Fe), almandine and pyrite.
Physical properties
Beusite-(Ca) forms pale-brown lamellae 0.1–1.5 mm wide, intergrown with triphylite lamellae (Fig. 1a); associated beusite-(Ca) lamellae are in optical orientation with each other. The beusite–triphylite intergrowths occur as a 6 cm × 5 cm × 3 cm nodule (Fig. 1b). Beusite-(Ca) is pale brown and transparent with a vitreous lustre and a very pale-brown streak; it does not fluoresce in either longwave or shortwave ultraviolet light. It is brittle with an irregular fracture, and has a Mohs hardness of 5. Cleavage is good on both {010} and {100}, there is no parting, and the calculated density is 3.610 g/cm3. To measure the optical properties, a crystal was mounted on a Bloss spindle stage and the extinction curves were measured using white light. The resulting measurements were processed using Excalibr II (Bartelmehs et al., Reference Bartelmehs, Bloss, Downs and Birch1992) and the 2V angle was derived. Excalibr II also provided the setting angles for measurement of refractive indices: α = 1.685(2), β = 1.688(2), γ = 1.700(5), α; 2Vobs = 46.0(5)°, 2Vcalc = 53°; the dispersion is r < v, weak. No pleochroism was observed, small crystals (<50 µm) are colourless. The optic orientation was measured by transferring the crystal and goniometer head from the spindle stage to a single-crystal diffractometer and orienting the crystallographic axes: X || b; Y ˄ a = 40.3° in β obtuse; Z ˄ a = 49.7° in β acute.

Fig. 1. (a) Back-scatter electron image showing intergrowths of beusite (light) and triphylite (dark); (b) the nodule (6 cm × 5 cm × 3 cm) in which beusite-(Ca) was discovered.
Chemical composition
Crystals were analysed with a Cameca SX100 electron microprobe operated in wavelength-dispersive mode at 15 kV and 20 nA, using a beam diameter of 2 µm. The following standards were used: apatite (P and Ca), chromite (Fe and Mg) and MnF2 (Mn). The concentration of Mg was below the detection limit. Data reduction was done using the φ(ρZ) procedure of Pouchou and Pichoir (Reference Pouchou, Pichoir and Armstrong1985). Table 1 gives the chemical composition (mean of ten points). The empirical formula unit, calculated on the basis of 8 anions per formula unit, is (Ca0.94Mn1.14Fe0.92)Σ3.00(PO4)2.00, ideally CaMn2(PO4)2.
Table 1. Chemical composition (wt.%) of beusite-(Ca).

nd – not detected.
Raman spectroscopy
The Raman spectrum of beusite-(Ca) was collected in back-scattered mode with a HORIBA Jobin Yvon-LabRAM ARAMIS integrated confocal micro-Raman system equipped with a 460 mm focal-length spectrograph and a multichannel air-cooled (–70°C) CCD detector. A magnification of 100× was used with an estimated spot size of 1 µm, a 1800 gr/mm grating, an excitation radiation of 532 nm, and laser power between 5 and 12.5 mW. Calibration was done using the 520.7 cm–1 line of Si metal, data were collected over the range 100–1200 cm–1 for 20 s, and the final spectrum is the average of two scans. In the Raman spectrum (Fig. 2), the peaks at 961, 1008 and 1027 cm–1 (strong), 1054 (w = weak) and 1090 and 1104 cm–1 (m = medium) may be assigned to stretching vibrations of the PO4 groups. The peaks at 592 (vw = very weak), 562 (m), 548 (w), 473 (vw), 458 (w) and 416 (w) cm–1 are due to the bending vibrations of PO4 and stretching vibrations of CaO8 and MnO6 polyhedra. The peaks at 347 (vw), 261 (w), 231 (vw), 212 (w), 182 (vw), 160 (vw), 140 (vw) and 115 (vw) cm–1 are due to angular deformations of the CaO8 and MnO6 polyhedra.

Fig. 2. The Raman spectrum of holotype beusite-(Ca).
Mössbauer spectroscopy
Mössbauer spectroscopy was done in transmission geometry at room temperature (RT) using a 57Co(Rh) point source. For preparing the Mössbauer absorber, the powdered sample of beusite-(Ca) was mixed with sugar and loaded into a Pb disk with 5 mm inner diameter. The spectrum was analysed in terms of a Voigt-function-based quadrupole-splitting distribution (QSD) (Rancourt and Ping, Reference Rancourt and Ping1991) using the RECOIL® software package. The centre shift (CS) is given relative to α-Fe at RT. The Mössbauer spectrum of beusite-(Ca) is shown in Fig. 3. It was fitted to a QSD model having two generalized QSD sites, one for Fe2+ in beusite-(Ca) (with two Gaussian components) and the other for Fe2+ in triphylite (with one Gaussian component) (Fig. 3a). The Mössbauer parameters are given in Table 2. The QSD curve for Fe2+ in beusite-(Ca) shows two well-resolved Gaussian components centred at 1.81 mm/s and 2.27 mm/s (Fig. 3b). Following previous Mössbauer work on (Fe,Mn)3(PO4)2 solid solutions (Nord and Ericsson, Reference Nord and Ericsson1982), the component with a QS = 1.81 mm/s (relative area of 54%) is assigned to Fe2+ at the M2 site and that with a QS = 2.27 mm/s (relative area = 9%) to Fe2+ at the M3 site. Thus, in the beusite-(Ca) studied, 86% of Fe2+ occurs at the M2 site and 14% at the M3 site. This is in accord with the results of Nord and Ericsson (Reference Nord and Ericsson1982) that Fe2+ preferentially enters the [5]-coordinated M2 site.

Fig. 3. The Mössbauer spectrum of a mixture of holotype beusite-(Ca) and triphylite., see text for details
Table 2. Mössbauer parameters for beusite-(Ca)–triphylite mixture.

CS – centre shift; QS – quadropole splitting.
Powder X-ray diffraction
Beusite-(Ca) is intimately intergrown with exsolved lamellae of triphylite and cannot be separated. Thus we collapsed the single-crystal data to produce an experimental diffraction pattern (for CuKα) that simulates that of a powder pattern (in much the same way as a Gandolfi apparatus). The pattern is given Table 3.
Table 3. X-ray powder diffraction pattern for beusite-(Ca).

Crystal structure
A single crystal (30 µm × 30 µm × 60 µm) was attached to a tapered glass fibre and mounted on a Bruker D8 three-circle diffractometer equipped with a rotating-anode generator (MoKα X-radiation), multilayer optics and an APEX-II detector. In excess of a Ewald sphere of data was collected to 60°2θ using 4 s per 0.2° frame with a crystal-to-detector distance of 5 cm. Empirical absorption corrections (SADABS; Sheldrick, Reference Sheldrick2008) were applied and equivalent reflections were merged, resulting in 1852 unique reflections. Unit-cell dimensions (Table 4) were obtained by least-squares refinement of the positions of 4083 reflections with I > 10σI. In principle, three scattering species cannot be refined over three sites in a crystal structure (Hawthorne, Reference Hawthorne1983), and site assignment becomes more difficult where scattering species of similar atomic number (i.e. Fe and Mn) are involved. We dealt with this problem by: (1) freely refining site-scattering values at the three M sites; (2) assigning all Ca to M(1) on the basis of the observed bond-lengths and the resulting bond-valence sums; and (3) assigning Fe to M(2) and M(3) on the basis of the Mössbauer results (Table 2). The structure was refined to an R 1 index of 1.55%. Miscellaneous information concerning structure solution and refinement is listed in Table 4. Atom positions and equivalent isotropic-displacement parameters are given in Table 5, selected interatomic distances in Table 6, refined site-scattering values (Hawthorne et al., Reference Hawthorne, Ungaretti and Oberti1995) in Table 7, and a bond-valence calculation is shown in Table 8. Observed and calculated structure-factors and a crystallographic information file have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 4. Miscellaneous information for beusite-(Ca).

R 1 = Σ(|F o| – |F c|) / Σ|F o|; wR 2 = [Σw (F o2 – F c2)2/Σw (F o2)2]½, w = 1/[σ2(Fo2) + (0.0241P)2 + 0.53P] where P = (Max(F o2,0) + 2F c2)/3
Table 5. Atom coordinates and anisotropic displacement parameters for beusite-(Ca).

Table 6. Selected interatomic distances (Å) in beusite-(Ca).

Table 7. Site-scattering values (epfu) and site populations (apfu) in beusite-(Ca).

epfu – electrons per formula unit; apfu – atoms per formula unit; [CN] – coordination number.
Table 8. Bond-valence* (valence units) table for beusite-(Ca).

*Calculated from the parameters of Gagné and Hawthorne (Reference Gagné and Hawthorne2015).
Beusite-(Ca) is isostructural with the rest of the minerals of the graftonite group. As indicated in Table 7, Mn2+ is strongly ordered at the [6]-coordinated M(3) site, and a minor amount of Mn2+ occurs at M(1). Note that the ordering of Mn2+ and Fe2+ over the M(2) and M(3) sites is not part of the classification criteria for this group as it requires crystal-structure refinement and Mössbauer spectroscopy to determine the site populations of M(2) and M(3) (Hawthorne and Pieczka, Reference Hawthorne and Pieczka2018). With regard to classification, the formula is written as (Ca0.94Mn1.13Fe0.92)Σ3(PO4)2 which gives the ideal formula: CaMn2(PO4)2. The position of the holotype composition of beusite-(Ca) in the classification scheme for the minerals of the graftonite group (Hawthorne and Pieczka, Reference Hawthorne and Pieczka2018) is shown in Fig. 4.

Fig. 4. The chemical composition of holotype beusite-(Ca) (green square, mean of 10 analyses) using the classification scheme of Hawthorne and Pieczka (Reference Hawthorne and Pieczka2018).
Origin
Beusite-(Ca) is a primary phase in a beryl–columbite–phosphate rare-element pegmatite, and formed during crystallization of the inner intermediate-zone and core of the pegmatite. Beusite-(Ca) is commonly intergrown with triphylite–lithiophilite and is thought to result from exsolution from a high-temperature (Li,Ca)-rich graftonite-like parent phase. The beusite-(Ca)–triphylite intergrowths are in sharp contact with blocky metasomatically altered pink microcline, and incipient Na-metasomatism is indicated by the presence of a small patch of alluaudite-group minerals near the intergrowths of beusite-(Ca) and triphylite. Minor ferrisicklerite is present as a weathering product of triphylite.
Acknowledgements
This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council of Canada and by grants from the Canada Foundation for Innovation to FCH. AP was supported by the AGH UST grant 11.11.140.319, and AW by the National Science Centre (Poland) grant 2015/17/N/ST10/02666.
We thank Fernando Colombo, Pete Leverett and Frédéric Hatert for their comments on this paper.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2018.120