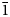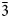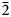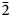Introduction
The Chelkar salt dome in the North Caspian Region, Western Kazakhstan is one of the classic localities of diverse borate mineralisation formed in evaporites. Chelkar was actively studied, including prospecting for boron, between the 1950s and 1970s based on drillcores of numerous boreholes. A geological description of the Chelkar salt dome was given by Oshakpaev (Reference Oshakpaev1974) and data on mineralogy and genesis of Chelkar borates obtained in this period have been summarised by Yarzhemskii (Reference Yarzhemskii1968, Reference Yarzhemskii1984) and Avrova et al. (Reference Avrova, Bocharov, Khalturina and Yunusova1968).
Chelkar is the type locality of several boron minerals discovered more than fifty years ago, namely aksaite (Blazko et al., Reference Blazko, Kondrat'eva and Yarzhemskii1962), halurgite (Lobanova, Reference Lobanova1962), metaborite (Lobanova and Avrova, Reference Lobanova and Avrova1964) and insufficiently studied borates strontioborite (Lobanova, Reference Lobanova1960) and chelkarite (Avrova et al., Reference Avrova, Bocharov, Khalturina and Yunusova1968). More recent data on borate minerals from Chelkar were reported by Malinko et al. (Reference Malinko, Khalturina, Ozol and Bocharov1991), Korotchenkova and Chaikovskiy (Reference Korotchenkova and Chaikovskiy2016) and Pekov et al. (Reference Pekov, Zubkova, Ksenofontov, Chukanov, Yapaskurt, Korotchenkova, Chaikovskiy, Bocharov, Britvin and Pushcharovsky2018b, Reference Pekov, Zubkova, Ksenofontov, Chukanov, Korotchenkova, Chaikovskiy, Yapaskurt, Britvin and Pushcharovsky2019).
The new mineral described in the present paper was found during the studies of core from a borehole recently drilled at the Chelkar salt dome for prospecting of potassium salts. Initially this borate was misidentified as ‘santite’, based on the electron-microprobe data (Korotchenkova and Chaikovskiy, Reference Korotchenkova and Chaikovskiy2016). Later, X-ray diffraction studies showed that it is a representative of the larderellite structure type. The new mineral, a potassium analogue of larderellite has been named yarzhemskiite (Cyrillic: яржемскиит) in honour of the Russian geologist, petrologist and mineralogist Yakov Yakovlevich Yarzhemskii (1901–?), a specialist in petrology of evaporite rocks and mineralogy and genesis of boron deposits related to evaporites. Prof. Yarzhemskii worked in the All-Union Research Institute of Halurgy (Leningrad) and made a great contribution to studies of boron-bearing sedimentary rocks and boron deposits of Western Kazakhstan, including Chelkar (see, e.g. Yarzhemskii, Reference Yarzhemskii1968, Reference Yarzhemskii1984).
Both new mineral and its name have been approved by the IMA Commission on New Minerals, Nomenclature and Classification, (IMA2018-019, Pekov et al., Reference Pekov, Zubkova, Korotchenkova, Chaikovskiy, Yapaskurt, Chukanov, Belakovskiy, Lykova, Britvin and Pushcharovsky2018a). The type specimen is deposited in the systematic collection of the Fersman Mineralogical Museum of the Russian Academy of Sciences, Moscow, with the catalogue number 96257.
Occurrence and general appearance
The new mineral was found in core of borehole #800 (depth 344–347 m) drilled at the Chelkar salt dome, near Chelkar (Shalkar) lake, Western Kazakhstan Region, Kazakhstan.
Yarzhemskiite occurs as separate crystals or grains having irregular outlines embedded in a halite–sylvite rock (sylvinite). Other associated minerals are carnallite, polyhalite, gypsum, strontioginorite, satimolite and quartz. Yarzhemskiite was formed as a result of diagenesis or of the post-diagenesis processes, probably related to salt diapirism, in boron-bearing evaporite rocks. It is likely that the formation of yarzhemskiite and associated borates accompanies the recrystallisation of sylvinite.
Crystals of the new mineral are thick tabular (flattened on [010], with the {010} pinacoid as the major crystal form), slightly elongate (short prismatic) or equant, commonly coarse, with a sculptured and/or cavernous surface. Well-formed crystals, resembling typical gypsum crystals in morphology, are up to 0.5 mm × 0.7 mm × 1 mm (typically much less) whereas grains having irregular outlines are up to 1 mm × 1.5 mm × 2 mm. Crystals and grains of yarzhemskiite, separated after dissolution of host sylvinite in water, are shown in Figs 1 and 2. Yarzhemskiite crystals typically contain inclusions of halite, sylvite and quartz.

Fig. 1. The largest found crystals (lower row) and grains with irregular outlines of yarzhemskiite. Field of view, width: 4.2 mm. Photo: I.V. Pekov and A.V. Kasatkin.

Fig. 2. Yarzhemskiite crystal. Scanning electron microscopy (secondary electron) image.
Physical properties and optical data
Yarzhemskiite is transparent, colourless, with white streak and vitreous lustre. The mineral is non-fluorescent under both ultraviolet rays and an electron beam. Yarzhemskiite is brittle. Cleavage is perfect on {100}, the fracture is stepped. The Mohs’ hardness is ca 2½. The density measured by flotation in heavy liquids (bromoform + dimethylformamide) is 2.13(1) g cm–3. The density calculated using the empirical formula is 2.112 g cm–3.
Yarzhemskiite is optically biaxial (+), α = 1.484(2), β = 1.508(2), γ = 1.546(2) (589 nm). 2Vmeas = 75(10)° and 2Vcalc = 80°. Dispersion of optical axes was not observed. Orientation: Y = b and Z ^ c = 6(1)°. In plane polarised light the mineral is colourless and non-pleochroic.
Infrared spectroscopy
The infrared (IR) absorption spectrum of yarzhemskiite was obtained for a powdered sample mixed with anhydrous KBr and pelletised. The pellet was analysed in the Institute of Problems of Chemical Physics of the Russian Academy of Sciences (Chernogolovka, Russia) using an ALPHA FTIR spectrometer (Bruker Optics) at the resolution of 4 cm–1. The sampling scan number was 16. The IR spectrum of a pure KBr disc was used as a reference.
The IR spectrum of yarzhemskiite (Fig. 3) contains bands of O–H-stretching vibrations corresponding to medium strength (at 3435 cm–1, with the shoulders at 3400 and 3275 cm–1) and strong (at 2920 cm–1) hydrogen bonds. According to the correlation between O–H stretching frequencies in IR spectra of minerals and O⋅⋅⋅O distances (from structural data) established by Libowitzky (Reference Libowitzky1999), these wavenumbers correspond to the O⋅⋅⋅O distances between O atoms of donor and acceptor groups of 2.82, 2.80, 2.73 and 2.63 Å, respectively. These values are in agreement with the D⋅⋅⋅A distances of ~2.82, 2.79, 2.68 and 2.62 Å obtained from structural data (see below).

Fig. 3. Powder IR absorption spectra of (1) yarzhemskiite and (2) larderellite from its type locality, Larderello, Pisa Province, Tuscany, Italy (spectrum B91 in: Chukanov, Reference Chukanov2014).
The band at 1624 cm–1 is due to H–O–H bending vibrations of H2O molecules. The bands in the ranges 1200–1500 and 1000–1100 cm–1 correspond to B–O-stretching vibrations of BO3 and BO4 polyhedra, respectively. The band at 938 cm–1 with the shoulder at 950 cm–1 is assigned to B–O–H bending vibrations (the splitting is due to resonance between two neighbouring BOH groups). The bands in the range 600–800 cm–1 are mainly due to O–B–O bending vibrations. The bands below 600 cm–1 correspond to lattice modes involving vibrations of large structural fragments and librational vibrations of H2O molecules. Weak bands in the range 1900–2500 cm–1 are overtones and combination modes.
The IR spectrum of larderellite (Fig. 3) is similar to that of yarzhemskiite, but contains additional strong bands at 3240 cm–1 (stretching vibrations of NH4+ cations), 1444 cm–1 (taking into account a high width, this band is to be assigned to a superposition of mixed modes involving B–O-stretching vibrations of BO3 polyhedra and bending vibrations of NH4+ cations), as well as the distinct band at 692 cm–1 tentatively assigned to librational vibrations of NH4+ cations. Based on these features, larderellite and yarzhemskiite can be easily distinguished.
Chemical composition
Chemical data for yarzhemskiite were obtained using a Jeol JSM-6480LV scanning electron microscope equipped with an INCA-Wave 500 wavelength-dispersive spectrometer (Laboratory of Analytical Techniques of High Spatial Resolution, Department of Petrology, Moscow State University), with an acceleration voltage of 20 kV and a beam current of 20 nA. The electron beam was rastered to the 5 μm × 5 μm area. The following standards were used: albite (Na), microcline (K), diopside (Ca) and BN (B). Contents of other elements with atomic numbers higher than carbon are below their detection limits.
The average (for five spot analyses) chemical composition of yarzhemskiite (wt.%, ranges are in parentheses) is: Na2O 0.01 (0.00–0.02), K2O 17.84 (17.77–17.88), CaO 0.07 (0.03–0.10), B2O3 67.21 (66.64–67.76), H2Ocalc 13.91, total 99.04. H2O content was calculated by stoichiometry for (OH)2(H2O), according to structural data.
The empirical formula calculated on the basis of 10 O atoms per formula unit is K0.98B5.005O7(OH)2⋅H2O. The idealised formula is KB5O7(OH)2⋅H2O which requires K2O 18.31, B2O3 67.68, H2O 14.01, total 100 wt.%.
X-ray crystallography
Powder X-ray diffraction (XRD) data of yarzhemskiite (Table 1) were collected with a Rigaku R-AXIS Rapid II diffractometer (X-Ray Diffraction Resource Center, St. Petersburg State University, St. Petersburg, Russia) equipped with a cylindrical image plate detector (radius 127.4 mm) using Debye-Scherrer geometry, CoKα radiation (rotating anode with VariMAX microfocus optics), 40 kV, 15 mA and an exposure time of 10 min. The angular resolution of the detector is 0.045°2θ (pixel size 0.1 mm). The data were integrated using the software package Osc2Tab (Britvin et al., Reference Britvin, Dolivo-Dobrovolsky and Krzhizhanovskaya2017). Parameters for the monoclinic unit cell calculated from the powder data are: a = 9.473(3), b = 7.521(2), c = 11.422(3) Å, β = 97.37(3)° and V = 807.0(7) Å3.
Table 1. Powder X-ray diffraction data (d in Å) of yarzhemskiite.

*For the calculated pattern, only reflections with intensities ≥2 are given (for ≥1 see supplementary material); **for the unit-cell parameters calculated from single-crystal data; the strongest reflections are marked in boldtype.
Single-crystal XRD studies of yarzhemskiite were carried out using an Xcalibur S diffractometer (Faculty of Geology, Moscow State University) equipped with a CCD detector. A full sphere of three-dimensional data was collected. The data were corrected for Lorentz and polarisation effects. The crystal structure was solved by direct methods and refined using the SHELX-97 software package (Sheldrick, Reference Sheldrick2015) to R = 0.0336 for 2220 unique reflections with I > 2σ(I). The H atoms of OH groups and H2O molecule were localised from the difference-Fourier synthesis. Crystal data, data collection information and structure refinement details are presented in Table 2, atom coordinates and displacement parameters in Table 3 and 4, selected interatomic distances in Table 4, data on hydrogen-bond geometry in Table 5 and bond-valence calculations in Table 6. The crystallographic information files have been deposited with the Principal Editor of Mineralogical Magazine and are available as Supplementary material (see below).
Table 2. Crystal data, data collection information and structure refinement details for yarzhemskiite.

Table 3. Сoordinates, equivalent and anisotropic displacement parameters (U eq, in Å2) of atoms in yarzhemskiite.

*U iso. The positions of H atoms were localised from the difference-Fourier map and refined with O–H and H–H distances softly restrained to 0.85(1) and 1.37(2) Å, respectively, to hold near-optimal geometry.
Table 4. Selected interatomic distances (Å) in the structure of yarzhemskiite.

Table 5. Hydrogen-bond geometry (Å,°) in the structure of yarzhemskiite.

Table 6. Bond-valence calculations for yarzhemskiite.

Bond-valence parameters for the K–O and B–O bonds were taken from (Brese and O'Keeffe, Reference Brese and ÒKeeffe1991) and those for H bonding from (Ferraris and Ivaldi, Reference Ferraris and Ivaldi1988).
Discussion
The crystal structure of yarzhemskiite K[B5O7(OH)2]⋅H2O (Fig. 4), as well as its synthetic analogue (Zhang et al., Reference Zhang, Zhang, Zheng and Yang2005) and the isostructural mineral larderellite (NH4)[B5O7(OH)2]⋅H2O (Merlino and Sartori, Reference Merlino and Sartori1969), is based on the infinite chains built by boron-centred polyhedra, which are running along the b axis (Fig. 5a). The basic structural unit of the chain is a double ring B5O7(OH)2 consisting of one BO4 tetrahedron and four BO3 triangles; within the chain, each unit is linked with the adjacent one through the 21 symmetry operation. K+ cations centre ten-fold polyhedra which form, together with the borate chains [B5O7(OH)2]–∝, layers parallel to (100) (Fig. 5b,c). Adjacent layers are linked only via H bonds resulting in the perfect {100} cleavage of the mineral.

Fig. 4. The crystal structure of yarzhemskiite in three projections with the outlined unit cell (a, b, c) and the fragment of the structure with shown H-bonding scheme (d). Key shown in (a).

Fig. 5. Infinite chain built by BO3 triangles and BO4 tetrahedra (a) and the layers formed by these chains and ten-fold K-centred polyhedra (b, c) in the structure of yarzhemskiite. The unit cell is outlined in (b, c). For legend see Fig. 4.
According to the classification of fundamental building blocks (FBB) in borates, yarzhemskiite, as well as larderellite, contains the following FBB: 4Δ1□:<2Δ1□>–<2Δ1□>. This code means that four B-centred triangles (Δ) and one B-centred tetrahedron (□) form a cluster consisting of two borate rings <2Δ1□> linked via the tetrahedron belonging to both rings (Burns et al., Reference Burns, Grice and Hawthorne1995; Grice et al., Reference Grice, Burns and Hawthorne1999). The symmetry of this borate fragment is ![]() $\bar{4}$m2 (Belokoneva, Reference Belokoneva2005). In yarzhemskiite and larderellite these FBBs are connected via common O vertices to form chains [B5O7(OH)2]n –n (Fig. 5a). In other minerals such FBB occurs as the isolated pentaborate ion [B5O6(OH)4]–, namely in santite K[B5O6(OH)4]⋅2H2O (Zachariasen and Plettinger, Reference Zachariasen and Plettinger1963; Merlino and Sartori, Reference Merlino and Sartori1970), sborgite Na[B5O6(OH)4]⋅3H2O (Merlino and Sartori, Reference Merlino and Sartori1972), ramanite-(Cs) Cs[B5O6(OH)4]⋅2H2O and ramanite-(Rb), Rb[B5O6(OH)4]⋅2H2O (Behm, Reference Behm1984; Penin et al., Reference Penin, Seguin, Gérand, Touboul and Nowogrocki2002; Thomas et al., Reference Thomas, Davidson and Hahn2008). In ammonioborite (NH4)3[B15O20(OH)8]⋅4H2O (Merlino and Sartori, Reference Merlino and Sartori1971), three units B5O6(OH)4– are connected to form the anion [B15O20(OH)8]3– with FBB = 12Δ3□:3(<2Δ1□>–<2Δ1□>) (Grice et al., Reference Grice, Burns and Hawthorne1999).
$\bar{4}$m2 (Belokoneva, Reference Belokoneva2005). In yarzhemskiite and larderellite these FBBs are connected via common O vertices to form chains [B5O7(OH)2]n –n (Fig. 5a). In other minerals such FBB occurs as the isolated pentaborate ion [B5O6(OH)4]–, namely in santite K[B5O6(OH)4]⋅2H2O (Zachariasen and Plettinger, Reference Zachariasen and Plettinger1963; Merlino and Sartori, Reference Merlino and Sartori1970), sborgite Na[B5O6(OH)4]⋅3H2O (Merlino and Sartori, Reference Merlino and Sartori1972), ramanite-(Cs) Cs[B5O6(OH)4]⋅2H2O and ramanite-(Rb), Rb[B5O6(OH)4]⋅2H2O (Behm, Reference Behm1984; Penin et al., Reference Penin, Seguin, Gérand, Touboul and Nowogrocki2002; Thomas et al., Reference Thomas, Davidson and Hahn2008). In ammonioborite (NH4)3[B15O20(OH)8]⋅4H2O (Merlino and Sartori, Reference Merlino and Sartori1971), three units B5O6(OH)4– are connected to form the anion [B15O20(OH)8]3– with FBB = 12Δ3□:3(<2Δ1□>–<2Δ1□>) (Grice et al., Reference Grice, Burns and Hawthorne1999).
Yarzhemskiite K[B5O7(OH)2]⋅H2O and larderellite (NH4)[B5O7(OH)2]⋅H2O (Table 7) are isostructural, however, there is no evidence that they form a solid-solution series. Larderellite does not contain admixed potassium (Palache et al., Reference Palache, Berman and Frondel1951; Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2003) and our electron-microprobe and IR spectroscopy data show the absence of ammonium in a detectable amount in yarzhemskiite. The crystal structures of these minerals and the synthetic analogue of yarzhemskiite are very close in character to B-centred polyhedra. Mean <B–O> distances vary from 1.369 to 1.373 Å for BO3 triangles and 1.471 Å for tetrahedra in yarzhemskiite; the corresponding values are 1.36–1.38 and 1.47 Å in larderellite (Merlino and Sartori, Reference Merlino and Sartori1969) and 1.364–1.372 and 1.470 Å in the synthetic analogue of yarzhemskiite (Zhang et al., Reference Zhang, Zhang, Zheng and Yang2005). Potassium cations in yarzhemskiite and its synthetic analogue are ten-coordinated. K–O distances vary from 2.777 to 3.264 Å (mean <K–O> 2.986 Å) in yarzhemskiite and from 2.775 to 3.267 Å (mean <K–O> 2.987 Å) in synthetic K[B5O7(OH)2]⋅H2O while ammonium cations in larderellite occupy larger ten-fold polyhedra with NH4–O distances varying from 2.86 to 3.35 Å with a mean <NH4–O> distance of 3.07 Å. This results in the larger values of the unit-cell dimensions and volume of larderellite [V = 835.4 Å3] (Merlino and Sartori, Reference Merlino and Sartori1969) compared with those in yarzhemskiite [807.0 Å3] and its synthetic analogue [807.2 Å3] (Zhang et al., Reference Zhang, Zhang, Zheng and Yang2005).
Table 7. Comparative data for yarzhemskiite, larderellite and santite.

*Given here in standard setting; original data were reported by Merlino and Sartori (Reference Merlino and Sartori1969) for space group P21/a and, therefore, for unit cell with changed c and a parameters.
**Powder XRD data are given for the synthetic analogue of santite studied by Clark and Christ (Reference Clark and Christ1959).
Yarzhemskiite differs from structurally related larderellite and chemically related santite (Table 7) in the genetic aspect. Both larderellite and santite were first discovered at Larderello, Tuscany, Italy in hot lagoons where boron-rich volcanic fumaroles meet with water (Bechi, Reference Bechi1854; Palache et al., Reference Palache, Berman and Frondel1951; Merlino and Sartori, Reference Merlino and Sartori1970). Later both these minerals were found in deposits of moderately hot fumaroles at the Vulcano island, Aeolian Archipelago, Sicily, Italy (Campostrini et al., Reference Campostrini, Demartin, Gramaccioli and Russo2011). Santite is also mentioned, in association with Na, Ca and Mg borates, in deposits of the thermal spring at Eagle Borax Spring, Death Valley, California, USA (Crowley, Reference Crowley1996; Anthony et al., Reference Anthony, Bideaux, Bladh and Nichols2003), and as a daughter phase in fluid inclusions in minerals of boron-enriched granitic pegmatites from three localities: Il Prado pegmatite, San Piero in Campo, Elba Island, Italy (Thomas et al., Reference Thomas, Davidson and Hahn2008) and the Vezdarinskaya and Leskhozovskaya pegmatite veins, Shakhdara River, SW Pamirs, Tajikistan (Smirnov, Reference Smirnov2015). Thus, larderellite and santite in all known cases were deposited from the hot gas or hot water solutions. Unlike them, yarzhemskiite was formed as a result of diagenetic or post-diagenetic processes in boron-bearing evaporites (see: Yarzhemskii, Reference Yarzhemskii1984).
Acknowledgements
We thank two anonymous referees for valuable comments. This study was supported by the Russian Foundation for Basic Research, grant no. 18-05-00332. The technical support by the SPbSU X-Ray Diffraction Resource Center in powder XRD study of the mineral is acknowledged.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.80