Introduction
The family Verrucariaceae comprises c. 43 genera and 943 species (Lücking et al. Reference Lücking, Hodkinson and Leavitt2016), the vast majority of which are lichen-forming. Important characteristics defining the family include the perithecioid ascomata, with periphyses and periphysoids but no interascal filaments, and the hemiamyloid hymenium. A wide range of growth forms is exhibited, including crustose, squamulose and foliose (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009). In two species the form is determined by the blade-like macroscopic growth form of the alga, which in these species is little altered by lichenization (Pérez-Ortega et al. Reference Pérez-Ortega, de los Ríos, Crespo and Sancho2010, Reference Pérez-Ortega, Miller and de los Ríos2018). Photobiont diversity in the family is high (Thüs et al. Reference Thüs, Muggia, Pérez-Ortega, Favero-Longo, Joneson, O'Brien, Nelsen, Duque-Thüs, Grube and Friedl2011). The family occurs worldwide, and the species inhabit a wide range of substrata, including siliceous rock and limestone (on the seashore and in freshwater), soil, and less frequently bark. The traditional generic delimitation relied heavily on spore septation and thallus growth form. Gueidan et al. (Reference Gueidan, Roux and Lutzoni2007, Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009) used molecular data from 83 taxa to propose a more natural classification but the phylogenetic position of many taxa is still unknown, including most species of the largest genus in the family, Verrucaria. There have been recent revisions of substantial species groups (e.g. Prieto et al. Reference Prieto, Martínez, Aragón and Otálora2010, Reference Prieto, Martínez, Aragón, Gueidan and Lutzoni2012; Savić & Tibell Reference Savić and Tibell2012), but no further attempts to produce a revised phylogeny for the whole family.
The crustose members of the family, especially in the genus Verrucaria, have a relatively simple morphology. This, combined with plasticity in response to environmental conditions, often makes difficult the identification and delimitation of the species using morphological characteristics alone. Despite these difficulties, or more likely because of them, a large number of species of currently uncertain status have been described. For instance, A. Zahlbruckner described 84 species, of which perhaps only 37 are currently accepted; H. Zschacke described 133 species, of which perhaps only 50 are currently accepted; M. Servít described 424 species, of which perhaps only 60 are currently accepted (unpublished data). Some recent molecular studies have revealed species which are difficult or impossible to separate using morphological characteristics alone (Savić & Tibell Reference Savić and Tibell2009; Orange Reference Orange2012, Reference Orange2020; Thüs et al. Reference Thüs, Orange, Gueidan, Pykälä, Ruberti, Lo Schiavo and Nascimbene2015). These issues provide a serious challenge to taxonomic and floristic work.
The lichens of Nepal are still underexplored. A provisional checklist contained 792 taxa (Olley Reference Olley2011), but it is estimated that at least 2000 species occur in the country (Olley & Sharma Reference Olley and Sharma2013). Before 2022, only 12 species of Verrucariaceae had been reported (Table 1); of these, 10 are mostly squamulose or foliose species which are relatively conspicuous or easy to collect.
Table 1. Verrucariaceae previously reported from Nepal.
Crustose Verrucariaceae, in particular, are likely to be under-represented in checklists and lichen collections because they are often inconspicuous, mostly grow on rock, and the taxonomy is still poorly known in many regions. In East Asia the family is well studied in Japan but poorly known elsewhere. The recent checklist of Japan (Ohmura & Kashiwadani Reference Ohmura and Kashiwadani2018) lists 77 species, of which 31 have been described from Japan since 1990 by Harada (Reference Harada1991, Reference Harada1992a, Reference Harada1993, Reference Harada1994, Reference Harada1995a, Reference Haradab, Reference Haradac, Reference Harada1996a, Reference Haradab, Reference Harada1998, Reference Harada2000, Reference Harada2003, Reference Harada2012a, Reference Haradab, Reference Harada2013a, Reference Haradab). There is no checklist for China, but at least 46 taxa of Verrucariaceae have been described from this country, most in or before 1940, for example by Zahlbruckner (Reference Zahlbruckner and Handel-Mazzetti1930) or Magnusson (Reference Magnusson and Hedin1940). In recent years, Harada & Wang (Reference Harada and Wang1996, Reference Harada and Wang2006a, Reference Harada and Wangb, Reference Harada and Wang2008) described eight species from freshwater habitats in Yunnan. Only a small number of Verrucariaceae are reported from, for instance, Bhutan (Aptroot & Feijen Reference Aptroot and Feijen2002), Taiwan (Wang-Yang & Lai Reference Wang-Yang and Lai1973), Thailand (Buaruang et al. Reference Buaruang, Boonpragob, Mongkolsuk, Sangvichien, Vongshewarat, Polyiam, Rangsiruji, Saipunkaew, Naksuwankul and Kalb2017) and Vietnam (nine species by Aptroot & Sparrius Reference Aptroot and Sparrius2006, with four more reported by Gueidan et al. Reference Gueidan, Van Do and Lu2014).
Between 2–17 October 2009, specimens of Verrucariaceae were collected in mid-altitude regions of Nepal, mostly in the Kaski District of Gandaki Pradesh, with a small number in the Kathmandu District of Bagmati Pradesh. In this short period c. 28 species were collected, suggesting that Nepal will be a rich source of Verrucariaceae. Two collections made in 2007 by an expedition from the Royal Botanic Garden Edinburgh are also treated below.
Materials and Methods
Morphology
Descriptions are of the collected material from Nepal, unless otherwise stated. Sections were cut by hand. In the descriptions, the number of spores measured and the number of specimens from which measurements were taken are given in square brackets. In muriform ascospores, the degree of septation is expressed both as the number of cells visible in optical section, and as the number of cells occurring at the apparent periphery of the spore in optical section, which is easier to determine. Where there are sufficient numbers of measurements, spore sizes are cited as: (minimum–) mean minus one standard deviation – mean – mean plus one standard deviation (–maximum).
Sequence acquisition
DNA was extracted from recently collected or frozen specimens, using the Qiagen DNeasy Plant Mini Kit; the manufacturer's instructions were followed except that warm water was used for the final elution. PCR amplification was carried out using Bioneer AccuPower PCR Premix 50 μl reaction tubes. The two internal transcribed spacer regions and the 5.8S region (ITS1-5.8S-ITS2) of the nuclear ribosomal genes, and sometimes part of the 28S ribosomal gene (LSU) and part of the small subunit of the mitochondrial ribosomal DNA (mtSSU), were amplified, using the primers ITS1F (Gardes & Bruns Reference Gardes and Bruns1993), ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990), nu-LSU-155-5ʹ (Döring et al. Reference Döring, Clerc, Grube and Wedin2000), LR3, LR5, LR7 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990), mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The PCR thermal cycling parameters followed Orange (Reference Orange2018) for all gene regions. Sequencing was performed by The Sequencing Service, College of Life Sciences, University of Dundee (www.dnaseq.co.uk).
Phylogenetic analysis
Sequences were assembled in BioEdit 7.0 (Hall Reference Hall1999) and aligned using PRANK (Löytynoja & Goldman Reference Löytynoja and Goldman2010) (http://wasabiapp.org/software/prank/) with the online interface at https://www.ebi.ac.uk/goldman-srv/webprank/. Gaps in the alignment were coded using FastGap (Borchsenius Reference Borchsenius2009).
Phylogenetic relationships and support values were investigated using maximum likelihood (ML), as implemented in RaxML (Stamatakis Reference Stamatakis2006; Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008), hosted on the CIPRES Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Analyses with RAxML used rapid bootstrapping with 1000 iterations and the GTRGAMMA substitution model; a search for the best-scoring ML tree was carried out with the bootstrap analysis in a single run. The resulting tree was visualized using MEGA v.4 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). Support values ≥ 70% ML bootstrapping were regarded as significant. Sequences used in the analyses are shown in Table 2.
Table 2. Verrucariaceae specimens generated for this study or used in the phylogenetic analyses. New sequences are in bold. * = out group taxa in Fig. 2 (Herpotrichiellaceae).

Sequences were assigned to groups of putatively related species following initial BLAST searches and broad analyses. Separate analyses were carried out for each of the following groups:
Endocarpon group (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009). A clade comprising Endocarpon, Willeya and a number of species of Verrucaria s. lat.
Staurothele group (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009). A clade comprising Catapyrenium, Placidiopsis, Staurothele (including the type species S. clopima (Wahlenb.) Th. Fries) and a number of species of Verrucaria s. lat.
Thelidium s. lat. in part. An informal group of species with colourless, 1–3-septate ascospores, including Thelidium minutulum Körb., T. pluvium Orange, T. rehmii Zschacke and T. zwackhii (Hepp) A. Massal. (Thüs & Nascimbene Reference Thüs and Nascimbene2008). The phylogenetic position of these within the family is unknown.
Verrucaria elaeomelaena group (Thüs et al. Reference Thüs, Orange, Gueidan, Pykälä, Ruberti, Lo Schiavo and Nascimbene2015). A clade comprising V. elaeomelaena (A. Massal.) Arnold, V. alpicola Zschacke, V. funckii (Spreng.) Zahlbr. and V. humida Orange. Thüs et al. (Reference Thüs, Orange, Gueidan, Pykälä, Ruberti, Lo Schiavo and Nascimbene2015) showed that it was a sister clade to the Endocarpon group.
Verrucaria hydrophila group (Pykälä et al. Reference Pykälä, Launis and Myllys2018). An informal group comprising Verrucaria hydrophila Orange, V. dolosa Hepp, V. lignicola Zschacke, V. placida Orange and V. tenebrosa Pykälä et al.
Verrucaria praetermissa group. Part of the Staurothele group of Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009), comprising Verrucaria praetermissa (Trevisan) Anzi, V. devensis (G. Salisb.) Orange, V. elaeina Borrer and V. lapidicola Orange.
Some taxa were not closely related to any other sequenced taxon and were not included in detailed analyses.
Results
Morphology
Mature ascospores could not be found in many of the collections; this is possibly due to poor drying conditions during the expedition, which took place in unseasonably damp weather.
Phylogenetic analysis
Endocarpon group, ITS analysis
ITS sequences were newly prepared for 18 specimens and an additional 50 sequences from GenBank were included in the analysis. The tree resulting from the analysis of the ITS1-5.8S-ITS2 region is shown in Fig. 1. Endocarpon is recovered as a monophyletic group with good support. The two available Endocarpon sequences from Nepal are not very closely related to other sequences in the tree. Most species of Willeya are recovered as a monophyletic group with good support, but W. irrigata is basal to a poorly supported clade comprising the remainder of Willeya together with Endocarpon, while W. honghensis is basal in a well-supported clade otherwise comprising Endocarpon species. Of the seven species of Willeya collected in Nepal, only one (W. pallidipora s. str.) is apparently conspecific with existing sequenced species. Verrucaria bella is recovered as basal to Endocarpon and Willeya species in a well-supported clade, and Verrucaria senta is shown to be not closely related to any previously sequenced species.

Fig. 1. Phylogenetic relationships of the Endocarpon group sensu Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009), based on a maximum likelihood (ML) analysis of the ITS region. Thickened lines indicate ML support values ≥ 70%. Information for all taxa is presented in Table 2. The tree is rooted using Verrucaria rosula and V. submersella.
Staurothele group
A phylogenetic analysis of 76 selected Verrucariaceae was carried out based on mtSSU sequences (not shown). This recovered many of the clades shown in Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009), including the Staurothele group, but with low support. This group comprises Staurothele s. str., members of the Verrucaria praetermissa group, V. caerulea, and the genera Catapyrenium and Placidiopsis (Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009). In addition, a new clade not sampled by Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009) was recovered in the group. Taxa in the Staurothele group were reanalyzed using mtSSU, LSU and ITS. The resulting trees exhibited a similar topology, so the data were concatenated and analyzed together (Fig. 2). Staurothele s. str. was recovered with good support, including the type species S. clopima. Staurothele s. str. was sister to a well-supported clade of species also possessing muriform ascospores and hymenial algae but differing in the ascospores being colourless rather than (mostly) brown, and asci that are 4–8-spored rather than 2-spored. This clade is described as the new genus Nesothele below.

Fig. 2. Phylogenetic relationships of the Staurothele group sensu Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009), based on a maximum likelihood (ML) analysis of concatenated data of the ITS, LSU and mtSSU regions. Information for all taxa is presented in Table 2. Thickened lines indicate ML support values ≥ 70%. The tree is rooted using Capronia pilosella and C. semiimmersa.
Thelidium s. lat. in part, ITS analyses
Two sequences of Thelidium papulare from Nepal are nested within sequences of T. papulare from Europe (not shown). Specimens from Nepal which are morphologically similar to Thelidium minutulum are recovered as two entities: a single sequence basal to four specimens of T. minutulum from Europe, and a clade of four identical sequences sister to a clade comprising T. pluvium and T. rehmii, but with low support (Fig. 3). European specimens of T. minutulum are heterogeneous, occurring in two clades.

Fig. 3. Phylogenetic relationships of the Thelidium minutulum group, based on a maximum likelihood (ML) analysis of the ITS region. Thickened lines indicate ML support values ≥ 70%. %. Information for all taxa is presented in Table 2. The tree is rooted using Verrucaria rosula.
Verrucaria elaeomelaena group, ITS analysis
Specimens from Nepal form three well-supported clades (Fig. 4). One is nested within sequences of V. alpicola from Europe, the other two within specimens from Europe named as V. elaeomelaena. There is great variation within the ITS region of this group of species, with little morphological variation, and additional gene regions will be necessary to determine species boundaries.

Fig. 4. Phylogenetic relationships of the Verrucaria elaeomelaena group, based on a maximum likelihood (ML) analysis of the ITS region. Thickened lines indicate ML support values ≥ 70%. Information for all taxa is presented in Table 2. The tree is rooted using Verrucaria nigrescens.
Verrucaria hydrophila group, ITS analysis
An ITS tree was constructed comprising 24 publicly available and 13 unreleased sequences of V. hydrophila Orange s. lat., together with V. dolosa Hepp, V. placida Orange and V. tenebrosa Pykälä et al. (not shown). Four Nepalese specimens (Orange & Chhetri 18479, 18561, 18562 and 18563) form a well-supported clade with sequences of Verrucaria hydrophila s. lat. from Europe; another (Orange & Chhetri 18537) is basal in a well-supported clade comprising V. hydrophila s. lat., while another (Orange & Chhetri 18515) is basal to sequences of Verrucaria dolosa from Europe. Specimens attributed to V. hydrophila in Europe are morphologically uniform but there is great variation in ITS sequence, some of which may be correlated with substratum preference. Additional gene regions are needed to investigate species boundaries in this aggregate.
Verrucaria praetermissa group (part of the Staurothele group of Gueidan et al. Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009)
Two identical ITS sequences from Nepal (Orange & Chhetri 18518 and 18524) are basal to a well-supported clade of four sequences of Verrucaria praetermissa from Europe (Fig. 5).

Fig. 5. Phylogenetic relationships of the Verrucaria praetermissa group, based on a maximum likelihood (ML) analysis of the ITS region. Thickened lines indicate ML support values ≥ 70%. Information for all taxa is presented in Table 2. The tree is rooted using Verrucaria rosula.
Taxonomy
Dermatocarpon miniatum (L.) W. Mann
Thallus foliose, monophyllous, up to 23 mm diam., attached by a single central holdfast; margin shallowly lobed, sometimes thallus split to holdfast; thallus c. 280–400 μm thick. Upper surface grey to locally pale brown, pruinose; lower surface light brown in centre, dark brown or blackish near margin, slightly undulating, smooth, matt.
Ascospores 10.5–13 × 6–6.5 μm.
Specimen examined
Nepal: Bagmati Pradesh: Langtang National Park, near Ghoda Tabela, 28°12ʹ18.8ʺN, 85°29ʹ13.5ʺE, on boulder, upland temperate mixed broad-leaved forest, 2007, L. Olley L9 (E).
Dermatocarpon vellereum Zschacke
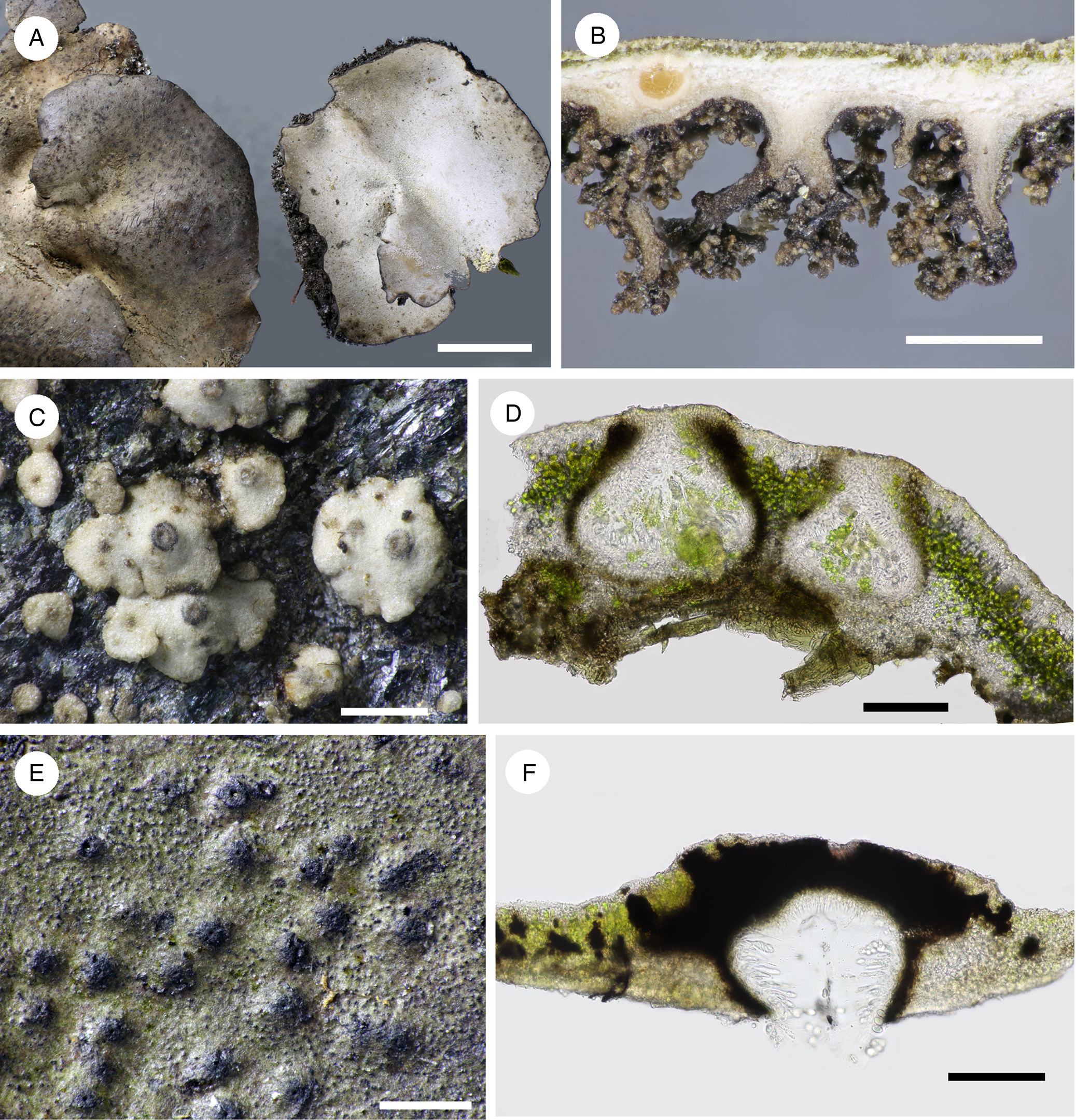
Fig. 6. A & B, Dermatocarpon vellereum (Orange & Chhetri 18504). C & D, Endocarpon Species A (Orange & Chhetri 18476). E & F, Hydropunctaria rheitrophila (Orange & Chhetri 18530). B, section of thallus showing rhizinomorphs. D & F, sections through perithecia. Scales: A = 5 mm; B, C & E = 500 μm; D & F = 100 μm. In colour online.
Thallus foliose, unifoliate, simple or somewhat lobed, up to 28 mm diam., attached by a single, central holdfast. Upper surface grey-brown, thinly to densely pruinose; lower surface dark brown, mostly obscured by densely branched rhizinomorphs.
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, Himalaya, just south-west of lodge, 28.4845°N, 83.8885°E, alt. 2840 m, on unshaded boulder by path, 2009, Orange & Chhetri 18504 (NMW.C.2012.002.161).
Endocarpon Species A
Thallus of squamules attached directly to rock, arising singly as convex buttons, sometimes contiguous, later becoming lobed, but not forming mats; squamules up to 900 μm wide, underside pale brown. Pseudocortex an alga-free zone c. 25 μm thick, of isodiametric cells, dilute brown above. Lower cortex absent.
Exciple 210 μm wide, dilute to dense brown in upper part, colourless to dilute brown below. Hymenial algal cells present, 3–3.5 × 2.5–3 μm, 1.2 times as long as wide. Asci 2-spored. Ascospores colourless, muriform, 24.5–30.5 × 10–15.5 μm.
Notes
The single sequence available is not closely related to any sequenced species (Fig. 1).
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, 0.5 km south of Ghandruk, 28°22.358ʹN, 83°48.560ʹE, alt. 1940 m, on stones at edge of lawn in garden of guest house, 2009, Orange & Chhetri 18476 (NMW).
Endocarpon Species B
Thallus squamulose, growing over moss; squamules repeatedly lobed; lobes 1.1–1.5 mm wide, to 300 μm thick, upper side light to mid-brown, slightly glossy, smooth; underside pale buff at margin, eventually blackish; no rhizines seen, but probably attached to moss by rhizohyphae. Lower cortex absent. Medulla white above, lower medulla becoming yellow, K−.
Perithecia numerous, but apparently immature; no spores seen.
Notes
An ITS sequence clusters with a sequence of Endocarpon adscendens, but it is unlikely that the two are conspecific (Fig. 1).
Specimen examined
Nepal: Bagmati Pradesh: Rasuwa District, Kyanjin Birch Wood, 28.20513°N, 85.56161°E, alt. 3810 m, boulder in juniper-Rhododendron pasture, large boulder 10 m high, 2007, A. Cross AC18 (E - E00305720).
Hydropunctaria rheitrophila (Zschacke) C. Keller, Gueidan & Thüs
Thallus light brownish green, thin, 35–75 μm thick, uncracked, rough with very numerous projecting black punctae c. 10–50 μm wide; cortex absent, thallus cells with no air spaces between them; thallus containing discrete, darkly pigmented patches (punctae) c. 15–40 μm wide.
Perithecia forming low conical-hemispherical mounds 260–335 μm wide, black at apex, covered by thallus below. Involucrellum well developed, surrounding the apex of the exciple, upper surface very irregular, grading into the black punctae of the thallus. Exciple c. 165 μm wide, dark brown. Ascospores (8.5–)9.5–10.6–11.5(–12) × (5.5–)6–6.5–7 μm, (1.4–)1.5–1.6–1.8(–1.9) times as long as wide [12/1].
Ecology and distribution
One collection on a stone in a shaded stream. Reported from Europe, North America, China (Harada & Wang Reference Harada and Wang2008), Japan (Harada Reference Harada1996c), Australia (McCarthy Reference McCarthy2003) and New Zealand (McCarthy Reference McCarthy1991).
Notes
The specimen fits the morphological concept of this species but the ITS sequence shows significant differences to material collected in Europe.
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, north-west of Ghandruk, south-east of Tadapani, 28.37866°N, 83.78715°E, alt. 2275 m, on stable stones in rivulet beside stream in forest, shaded, with Hildenbrandia sp., 2009, Orange & Chhetri 18530 (NMW.C.2002.002.167).
Nesothele Orange gen. nov.
MycoBank No.: MB 842601
Thallus crustose, usually superficial, to squamulose; ascospores hyaline, muriform, 4–8 per ascus; photobiont cells present in the hymenium.
Type species: Nesothele succedens (Rehm ex Arnold) Orange.
Thallus endolithic to more usually superficial, grey-green to brown, of lobed goniocyst-like units, or of convex areoles, or of tightly adnate squamules. Photobiont chlorococcoid.
Ascomata perithecioid, sometimes partly immersed in thallus but without a discrete thalline covering, in one species immersed in convex squamules; 300–560 μm wide, black; ostiolar region often pale and conspicuous in several species. Involucrellum present or indistinct. Hymenial gel I+ red; hamathecium of periphyses; interascal filaments absent. Hymenial algal cells 3–8.5 μm long, 1.0–4.5 times as long as wide. Ascus verrucarioid, I−, 4–8-spored. Ascospores colourless, ellipsoid to narrowly ellipsoid, 25–59.5 × 12.5–31.5 μm, muriform, without a perispore.
Conidiomata unknown.
Phylogenetic placement
Ascomycota, Verrucariales, Verrucariaceae.
Etymology
From the Classical Greek nouns Nêsos (νῆσος; island) and Thele (θηλή; nipple or teat), for the tendency of some species to have naked perithecia amongst an uneven or discontinuous thallus, both resembling islands; the name is also a reference to the morphologically similar Staurothele. The name is feminine.
Ecology and distribution
On rock, often where damp or calcareous.
Notes
In recent decades, Verrucariaceae with algae in the hymenium have usually been placed in one of two genera Staurothele for crustose species, and Endocarpon for squamulose species. In a family-level multigene analysis, Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009) showed that some Staurothele species, including the type species S. clopima, were recovered in a well-supported ‘Staurothele group’, distant from S. immersa and S. rupifraga which were recovered in a well-supported ‘Thelidium group’ together with some species currently placed in Polyblastia and Thelidium. Squamulose species attributed to Endocarpon were recovered in a well-supported ‘Endocarpon group’ which also contained a number of crustose species currently placed in Verrucaria. However, Heiðmarsson et al. (Reference Heiðmarsson, Gueidan, Miadłlikowska and Lutzoni2017) showed that the squamulose Endocarpon pulvinatum belonged in Staurothele s. str. Gueidan et al. (Reference Gueidan, Van Do and Lu2014) showed that further crustose species attributed to Staurothele formed a well-supported sister group to Endocarpon, for which the generic name Willeya was available. Nesothele represents another lineage of species with hymenial algae which was not sampled by Gueidan et al (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009).
Crustose species formerly attributed to Staurothele s. lat. are now placed in four clades: Staurothele s. str. (Staurothele group), with 2-spored asci and colourless to usually brown ascospores, thallus epilithic; Nesothele (Staurothele group), with 4–8-spored asci and hyaline ascospores, thallus endolithic to epilithic, sometimes with discrete areolae, or squamulose; Staurothele s. lat. (Thelidium group), with 2–8-spored asci and hyaline to brown ascospores, thallus often endolithic; and Willeya (Endocarpon group), with (4–)8-spored asci and hyaline ascospores, thallus epilithic, perithecia often immersed in the thallus. Staurothele caesia (Arnold) Arnold and S. guestphalica (J. Lahm ex Körb.) Arnold also belong to the Thelidium group, based on BLAST searches and alignments of ITS and mtSSU sequences, but some species of ‘Staurothele’ are not yet confidently assigned to a clade and more sampling is necessary to determine the morphological limits of the four clades.
Nesothele glebulosa Orange sp. nov.
MycoBank No.: MB 842604
Thallus lumpy, of convex areoles; perithecia naked, ascospores 29.5–41 μm long. Resembles the European Nesothele rugulosa (see below), differing in the smaller perithecia and the ITS and mtSSU sequences.
Type: Nepal, Gandaki Pradesh, Kaski District, shortly north-west of Dobhan, 28.47253°N, 83.8731°E, alt. 2530 m, on unshaded bedrock in stream, 6 October 2009, Orange & Chhetri 18502 (KATH—holotype; NMW.C.2013.001.189—isotype).
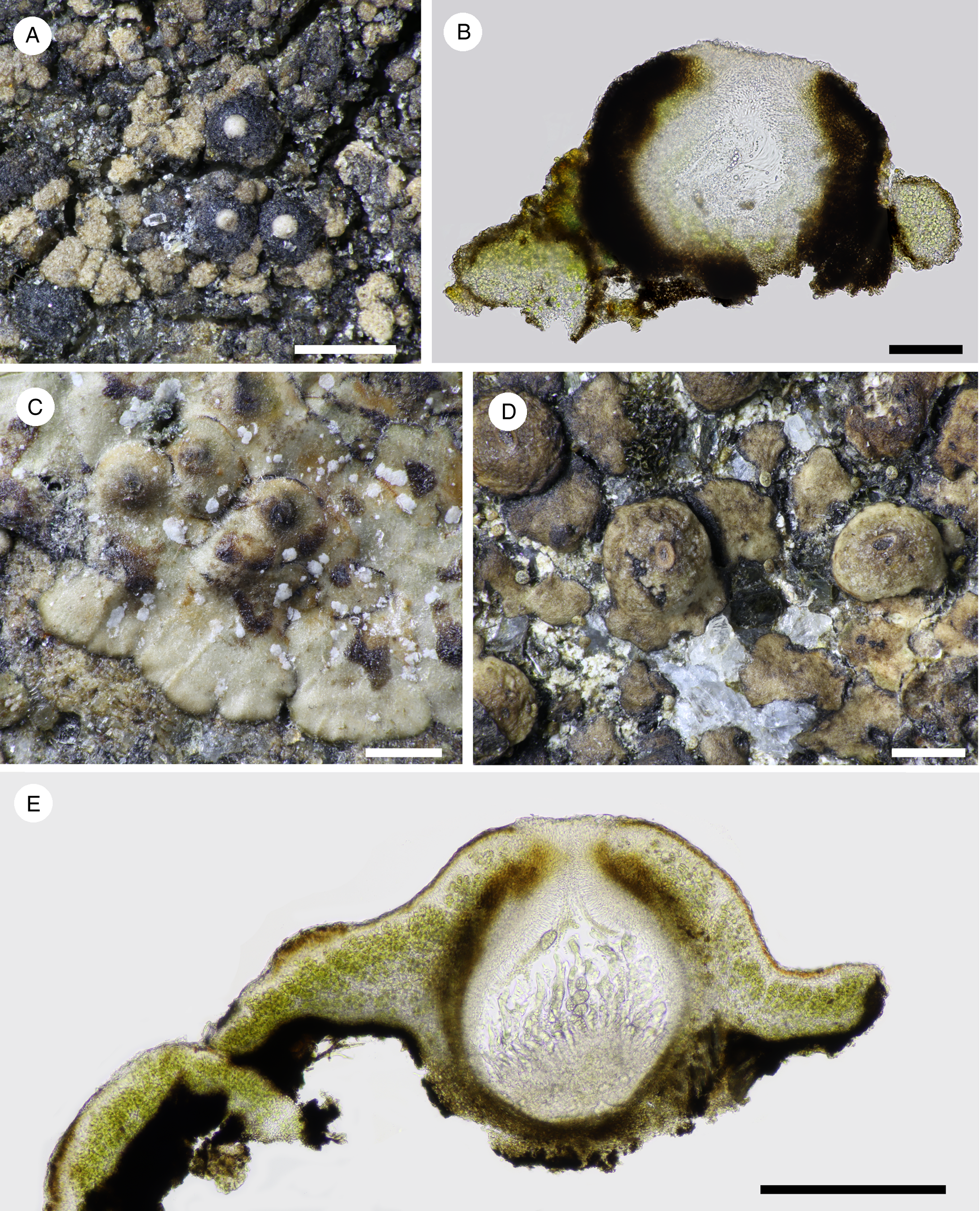
Fig. 7. A & B, Nesothele glebulosa (Orange & Chhetri 18502). C–E, Nesothele globosa (C & E, Orange & Chhetri 18498; D, Orange & Chhetri 18505). A, C & D, thallus with perithecia. B & E, sections of thallus and perithecia. Scales: A, C & D = 500 μm; B = 100 μm; E = 300 μm. In colour online.
Thallus of convex grey-brown to brown areoles 170–235 μm diam., areole surface becoming uneven.
Perithecia forming conical-hemispherical mounds 300–360 μm in diam., naked or with a small number of areoles at base, ostiole inconspicuous or visible as a convex white mound 115 μm wide. Involucrellum 50–60 μm thick at sides of exciple, appressed to exciple but spreading somewhat at base, dark brown, yellowish brown where dilute, K−, densely pigmented on the outside, cell outlines visible within; ostiolar region with a large unpigmented area when young, dark in older perithecia. Exciple 260–275 μm wide, colourless to later brown. Periphyses c. 30 μm. Hymenial algae very poorly preserved, possibly ellipsoid to rod-shaped, c. 3.5–4 × 2–3 μm. Mature asci not seen. Ascospores colourless, muriform, 29.5–41 × 12.5–15 μm [6/1], with 14–16 cells at the periphery in optical section.
Etymology
From the Latin glebulosus (lumpy), referring to the form of the thallus.
Ecology and distribution
Known from one specimen on a rock in a stream at 2530 m altitude.
Notes
Resembles the European species Nesothele rugulosa (see below) in the areolate thallus but differs in the ITS and mtSSU sequence, in the smaller perithecia and perhaps the more strongly pigmented thallus; however, more material needs to be examined to establish the morphological differences between the two species. A specimen from Great Britain (Orange 18403) is closely related (Fig. 2), but more material is needed to determine whether it is conspecific.
The following four combinations are made into Nesothele:
Nesothele globosa (H. Harada & Li S. Wang) Orange comb. nov.
MycoBank No.: MB 842606
Endocarpon globosum H. Harada & Li S. Wang, Lichenologist 28, 303 (Reference Harada and Wang1996); type: China, Yunnan, Deqin County, Meili Snow Mountain (E side), 28°24ʹN, 98°45ʹE, 3600 m alt., on non-calcareous stones submerged in a stream, 28 September 1994, H. Harada & L.-S. Wang 14563 (HKAS—holotype; CBM, TNS—isotypes[ non vidi]).
Prothallus not apparent. Thallus arising as circular squamules, becoming shallowly lobed, dispersed, or contiguous in small groups, or confluent to produce a thallus of crustose appearance; squamules more or less plane to gently convex when sterile, to 630 μm wide, tightly adnate or margin free from the substratum; upper surface smooth, light brown, extreme margin black; squamules pseudoparenchymatous throughout, or algal layer with more or less vertically oriented hyphae. Upper cortex formed of the swollen apices of these hyphae, 2.5–3.5 μm wide, or sometimes appearing wider than tall, 5–7 × 2.5–4 μm, colourless to brown. Lower cortex dark brown; rhizohyphae sparse, brown, 4 μm wide. Fertile squamules convex to strongly convex, 630–900 μm wide.
Perithecia one per areole, almost completely immersed in convex areoles, or forming moderate to prominent mounds 380–670 μm wide when measurable, with only the ostiolar region visible as a dark ring and/or a pale spot, very rarely exposed as a conspicuous black ring. Involucrellum absent, or present as a slight pigmented thickening of the upper exciple. Centrum 360–600 μm wide. Exciple 30–40 μm thick, dark brown throughout. Periphyses with free ends c. 20–35 μm long. Hymenial algae subglobose to shortly rectangular, 3–5 × 2.5–3 μm. Asci (4?–)8-spored (4 mature asci seen). Ascospores 45–59.5 × 21.5–31.5 μm, 1.6–2.7 times as long as wide [n = 8], colourless, muriform, with c. 32–42 cells visible in optical section, 19–27 cells at the periphery.
Conidiomata not seen.
Ecology and distribution
On stones on a wet slope or beside a stream.
Notes
The thallus in this species can comprise distinct squamules with raised margins, or squamules which are tightly adnate to the margin, or confluent adnate squamules giving the appearance of a crustose thallus. The pigmented lower cortex and the presence of rhizohyphae suggest that it is convenient to regard the thallus units as squamules. The two collections differ somewhat in appearance and in ITS sequence, but they are regarded as conspecific until further material can be examined. Orange & Chhetri 18505 agrees with the protologue in the large, convex fertile squamules, but in Orange & Chhetri 18498 most squamules are sterile and thin. The protologue of Endocarpon globosum differs from the Nepalese material in the very dark brown to black squamules and the smaller ascospores, 32–42 μm long. Despite these differences, they are regarded here as conspecific until more material can be examined.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, immediately south-west of Dobhan, 28.4697°N, 83.86805°E, alt. 2510 m, on rock in stream, more or less unshaded, 2009, Orange & Chhetri 18498 (KATH, NMW); Kaski District, just south-west of Himalaya, 28.48406°N, 83.88765°E, alt. 2810 m, on stones on wet slope beside stream, 2009, Orange & Chhetri 18505 (KATH, NMW.C.2013.001.192).
Nesothele hymenogonia (Nyl.) Orange comb. nov.
MycoBank No.: MB 844555
Verrucaria hymenogonia Nyl., Actes de la Société Linnéenne de Bordeaux 21, 430 (1856).—Staurothele hymenogonia (Nyl.) Th. Fr., Botaniska Notiser 1865, 40 (1865).
Nesothele rugulosa (A. Massal.) Orange comb. nov.
MycoBank No.: MB 842607
Polyblastia rugulosa A. Massal., Memorie Lichenografiche, 139–140, fig. 171 (1855).—Staurothele rugulosa (A. Massal.) Arnold, Verhandl. zool.-bot. Gesellsch. Wien 47, 389 (1897).
Nesothele succedens (Rehm ex Arnold) Orange comb. nov.
MycoBank No.: MB 842608
Polyblastia succedens Rehm, in Arnold, Flora 53, 17 (1870).—Staurothele succedens (Rehm) Arnold, Verhandl. zool.-bot.Gesellsch. Wien 30, 149 (1880).
Thelidium aff. minutulum Körb.

Fig. 8. A & B, Thelidium cf. minutulum s. lat. (Orange & Chhetri 18522). C & D, Thelidium papulare (Orange & Chhetri 18500). E & F, Thelidium uvidulum (Orange & Chhetri 18485). Scales: A = 500 μm; B, D & F = 100 μm; C = 1 mm; E = 200 μm. In colour online.
Prothallus not apparent. Thallus very thin, c. 25 μm, translucent, pale green-brown, uncracked, smooth, apparently homogeneous within and not composed of goniocyst-like units.
Perithecia prominent, dark brown, 190–290 μm wide, with thalline covering only at base, sometimes appearing thin-walled and with sunken sides when dry; ostiole inconspicuous, visible as a small pale or dark plane dot or papilla. Involucrellum thin, appressed to exciple above, diverging slightly near base, or developed only in upper part of perithecium, 24–50 μm thick in upper part. Centrum c. 235 μm. Periphyses c. 20 μm. Asci 8-spored. Ascospores hyaline, 1-septate, (20–)21.5–23.0–24.5(–25) × (10–)10.5–11.1–12 μm, (1.9–)2–2.1–2.2 times as long as wide [11/2].
Conidiomata not seen.
Ecology and distribution
On stones in streamlets or on irrigated rocks in shade. Thelidium minutulum is reported from China (Harada & Wang Reference Harada and Wang2006b), Europe and North America.
Notes
Important features of this species are the very thin thallus, small prominent perithecia with a scarcely developed involucrellum, and 1-septate ascospores. Morphologically the material resembles Thelidium minutulum (Harada & Wang Reference Harada and Wang2006b; Thüs & Nascimbene Reference Thüs and Nascimbene2008), but an ITS tree suggests that the material is heterogeneous and is not conspecific with either Thelidium minutulum or the rather similar T. rehmii. The specimens from Nepal might represent one or two new species but formal description must await more sequenced material. Thelidium minutulum also appears to be heterogeneous in Europe.
The occurrence of an involucrellum is difficult to confirm in this and some other small Thelidium species. The exciple is a thin, hyaline layer; pigmented tissue outside this could be termed an involucrellum, but where it is closely appressed and does not diverge from the exciple it has been customary to conclude that an involucrellum is absent. In the present species the pigmented layer is variable in extent, either closely appressed to the upper part of the exciple only or extending to the base-level of the exciple and diverging slightly.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, north-west of Ghandruk, south of Tadapani, 28°23.15ʹN, 83°46.85ʹE, alt. 2450 m, on stones in stream (c. 1 m wide) in forest, shaded, 2009, Orange & Chhetri 18522 (NMW.C.2013.001.183); Kaski District, north-west of Ghandruk, south of Tadapani, 28°23.02ʹN, 83°46.85ʹE, alt. 2535 m, on irrigated rocks in ravine in forest, shaded, 2009, Orange & Chhetri 18529 (NMW.C.2013.001.181); Kaski District, north-west of Ghandruk, east of Sitkyu, 28°22.705ʹN, 83°48.02ʹE, alt. 2140 m, on stone by streamlet in scrub, Orange & Chhetri 18539 (KATH); Kaski District, south of Landruk, 0.5 km north of Tolka, 28.35°N, 83.82448°E, alt. 1775 m, on slightly damp rocks by path in scrubby forest, 2009, Orange & Chhetri 18544 (NMW); Kaski District, south of Landruk, immediately south of Tolka, alt. 1795 m, 28°20.68ʹN, 83°49.43ʹE, on stone in streamlet by path amongst fields and scrubby forest, 2009, Orange & Chhetri 18548 (NMW.C.2013.001.182).
Thelidium papulare (Fr.) Arnold
Thallus well developed, to c. 100 μm thick, pale grey-brown, cracks numerous but not delimiting discrete areoles, sides of cracks unpigmented.
Perithecia forming moderate projections 530–670 μm wide, not covered by thallus, black, apex somewhat flattened or slightly depressed, ostiolar area visible as a brown or pale disc 50–200 μm wide. Involucrellum well developed, c. 75–100 μm thick in upper two-thirds of the perithecium. Centrum c. 300–355 μm. Exciple hyaline to brown. Ascospores transversely 3-septate, with 0–1 longitudinal septum, 36–39.6–43(–48) × (11–)14–16.2–18.5(–19.5) μm, 2.1–2.5–2.9(–3.4) times as long as wide [12/2].
Ecology and distribution
Two collections were made from more or less unshaded rocks in streams.
Notes
Thelidium papulare belongs to the Verrucaria aethiobola group (Orange Reference Orange2020) rather than to Thelidium s. str., but transfer to another genus must wait for a wider study of Verrucaria s. lat.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, immediately south-west of Dobhan, 28.4696°N, 83.868°E, alt. 2510 m, on rock in stream, more or less unshaded, 2009, Orange & Chhetri 18499 (NMW.C.2013.001.188); Kaski District, just north-west of Dobhan, 28.47253°N, 83.8731°E, alt. 2530 m, on unshaded bedrock in stream, 2009, Orange & Chhetri 18500 (KATH, NMW.C.2013.001.189).
Thelidium uvidulum Orange sp. nov.
MycoBank No.: MB 842609
Thallus thin, with soralia; perithecia small, prominent, with appressed involucrellum, ascospores 1-septate, (13.5–)15.5–17.0–18.5(–19.5) μm long.
Type: Nepal, Gandaki Pradesh, Kaski District, c. 2 km south-west of Chhomrong, 28.41293°N, 83.80365°E, alt. 2175 m, on stone embedded in damp bank by path in forest, 4 October 2009, Orange & Chhetri 18485 (KATH—holotype; NMW.C.2012.002.147—isotype).
Prothallus not seen. Thallus thin, 40–70 μm thick, light grey-green to dull grey-brown, either very thin and scurfy, or thicker and composed of indistinct subunits c. 80–140 μm diam., forming an uneven crust; cracks absent, or a small number in thicker areas. Soralia usually present, 60–180 μm diam., discrete, plane, pale brownish green; soredia c. 15–29 × 15–20 μm, but difficult to measure, comprising repeating units.
Perithecia immersed only in lower 0.2–0.3 of height, forming moderately projecting mounds 200–300 μm diam., naked or with a few flecks of thallus in lower part; ostiolar region inconspicuous, or a pale dot 20–40 μm diam. Involucrellum appressed to exciple above, spreading somewhat in lower part, dark brown, K+ dark green. Centrum 185–260 μm diam. Exciple dark-pigmented or only slightly browned. Periphyses c. 20 μm long. Asci 8-spored. Ascospores colourless, 1-septate, slightly browned when overmature, (13.5–)15.5–17.0–18.5(–19.5) × (6–)6.5–7.2–7.5(–8) μm, (2.1–)2.2–2.4–2.6(–3.1) times as long as wide [37/3].
Conidiomata not seen.
Etymology
From the Latin uvidulus (damp), referring to the habitat of damp, shady rocks.
Ecology and distribution
Three collections from moist rocks in forest.
Notes
Important features include the thin thallus, the presence of soralia, prominent perithecia with a well-developed involucrellum, and small 1-septate ascospores. The specimen Orange & Chhetri 18543 differs slightly in ITS sequence from the others. There are no strongly similar sequences in a BLAST search and this species is apparently not closely related to the group of species including Thelidium minutulum. Thelidium sinense (Harada & Wang Reference Harada and Wang2006b) differs in the slightly smaller ascospores and absence of soralia. Verrucaria craterigera H. Harada is similar in having small soralia, small perithecia and small and rather narrow ascospores, but the ascospores are slightly larger and are septate only when overmature. The two taxa are not closely related.
Additional specimens examined
Nepal: Gandaki Pradesh: Kaski District, south of Landruk, 0.5 km north of Tolka, 28.35°N, 83.82448°E, alt. 1775 m, on slightly damp rocks by path in scrubby forest, 2009, Orange & Chhetri 18543 (|NMW.C.2012.002.148), 18545 (NMW.C.2012.002.149).
Verrucaria antepotens Orange sp. nov.
MycoBank No.: MB 842610
Thallus well developed, cracked into dark-sided areoles, growing margin thick; perithecia immersed in thallus, with weak involucrellum; ascospores (12.5–)13.5–14.6–16(–16.5) μm long.
Type: Nepal, Gandaki Pradesh, Kaski District, north-east of Chhomrong, south-west of Sinuwa, 28.43256°N, 83.83416°E, alt. 2200 m, on irrigated siliceous rocks, unshaded, overgrowing Willeya honghensis, 5 October 2009, Orange & Chhetri 18488 (KATH—holotype; NMW.C.2012.002.153—isotype).

Fig. 9. A–D, Verrucaria antepotens (A, C & D, Orange & Chhetri 18488; B, Orange & Chhetri 18492). E & F, Verrucaria bella (Orange & Chhetri 18483). G & H, Verrucaria craterigera (Orange & Chhetri 18517). Scales: A, B & E = 1 mm; C, D, F & H = 100 μm, G = 500 μm. In colour online.
Prothallus not apparent. Thallus well developed, 200–500 μm thick, non-gelatinous, pale to mid brown; growing margin of thallus thick, well delimited, often slightly lobed, continuous, but cracks rapidly appearing with age; mature thallus with extensive wide cracks, these often surrounding discrete areoles; areoles plane or with slightly ascending margins, smooth, matt, the sides black; thallus cells in vertical columns, air spaces numerous between cells; upper surface a pseudocortex with brown pigment; lower part of thallus occupied by a thick layer of strongly pigmented cells, which is already present at the young margin of the thallus.
Perithecia completely immersed in thallus, apex visible as a brown dot or a black disc 60–160 μm diam. Involucrellum weakly developed, comprising ill-defined areas of pigmented cells flanking the upper part of the exciple. Centrum 220–260 μm diam. Exciple pigmented throughout, dark brown below, partly replaced by dark green pigment in thickened upper part. Periphyses c. 30 μm long. Asci 8-spored. Ascospores (12.5–)13.5–14.6–16(–16.5) × (6–)6.5–7.1–7.5(–8) μm, (1.8–)1.9–2.1–2.2(–2.4) times as long as wide [14/1; in rather poor condition].
Pycnidia numerous, immersed in thallus, the wall colourless to brown, adjacent pycnidia sometimes with confluent loculi. Conidia rod-shaped, 5.7–6.2 × 1 μm.
Etymology
From the Latin antepotens (superior in power, strongest), referring to the thallus overgrowing other species.
Ecology and distribution
On irrigated or non-irrigated rocks, sometimes overgrowing other crustose Verrucariaceae, including Willeya honghensis.
Notes
Critical features include the thick thallus, the abrupt thallus margin, the immersed perithecia with a weakly developed involucrellum, and the small ascospores. The two specimens are overgrowing other crustose Verrucariaceae but there is no evidence that they are parasitic.
Verrucaria gongshanensis H. Harada & Li S. Wang (Harada & Wang Reference Harada and Wang2008) sometimes has largely immersed perithecia, but the involucrellum merges more completely with the basal layer, the ascospores are larger (15–20 μm long), and pycnidia have not been reported. Verrucaria luchunensis (see below) and V. nipponica are similar in having a cracked thallus, immersed perithecia and pycnidia, but they differ in the much larger ascospores. BLAST searches do not suggest any closely related species.
Additional specimen examined
Nepal: Gandaki Pradesh: Kaski District, north-east of Chhomrong, north-west of Sinuwa in direction of Kuldhigar, 28.4433°N, 83.84415°E, alt. 2390 m, on low siliceous bedrock near stream in forest, shaded, 2009, Orange & Chhetri 18492 (NMW.C.2012.002.154).
Verrucaria bella Zahlbr.
In Handel-Mazzetti, Symbolae Sinicae 3, 10 (1930); type: China, NW Yunnan, Diabasfelsen der kalttemperierte (subalpine) Stufe im Bache auf dem Nguka-la zwischen Dschungdien und Djitsung, 4125 m, 24 August 1915, Handel-Mazzetti Iter Sinense 1914–1918 no. 7769 (BM M001107027—isotype!).
Thallus well developed, 60–200 μm thick, pale brownish grey in shade to mid grey-brown when lightly shaded, non-gelatinous; marginal sterile areas sparsely cracked, fertile areas with numerous cracks, often dividing the thallus into discrete areoles; areoles mostly 160–400 μm wide (sterile areoles), plane or slightly concave, smooth, matt; thallus sometimes developing a dark basal layer; thallus cells often in vertical columns, often with air spaces between cells; upper surface a pseudocortex without pigment (in shade) or brown.
Perithecia forming low or moderately projecting mounds c. 540–800 μm diam., covered by thallus to apex, or a small area exposed at the perithecial apex; ostiole inconspicuous or appearing as a pale dot 20–40 μm diam. Involucrellum well developed, spreading basally and laterally, more weakly pigmented below, merging with dark basal layer of thallus when present. Centrum 370–435 μm diam. Exciple colourless or brown-pigmented on the outside throughout. Periphyses long, c. 60–100 μm, and branched. Ascospores (23.5–)27–31.7–43 × (12.5–)14–15.0–16(–17) μm, (1.6–)1.8–2.1–2.4(–2.8) times as long as wide [25/2], perispore not seen.
Pycnidia numerous, conspicuous, scattered over the thallus, isodiametric to elongated, 80–300 × 80–300 μm, brown, plane or slightly projecting; ostiole brown, locule often invaginated. Conidia rod-shaped, 5–6.5 × c. 0.8 μm.
Ecology and distribution
On siliceous rock; beside streams and occasionally to frequently submerged, or on shaded rock beside a path, at altitudes of 2055–2205 m.
Notes
Characteristic features of this species include the well-developed, cracked thallus, perithecia forming projecting mounds, a well-developed and spreading involucrellum, large ascospores, and the presence of numerous pycnidia. The contrast between the large fertile and small sterile areoles is a distinctive feature of some specimens, but it is not clear in others. LSU and ITS analyses suggest that this belongs in the Endocarpon group of Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009) (Fig. 1). The identification as Verrucaria bella remains provisional, as the protologue of V. bella does not mention pycnidia, although they are conspicuous in the Nepalese specimens, and also suggests that the ascospores are eventually brownish (Zahlbruckner Reference Zahlbruckner and Handel-Mazzetti1930). Verrucaria honghensis H. Harada & Li S. Wang (Harada & Wang Reference Harada and Wang2008) is similar, but it lacks the conspicuous pycnidia of this species and has slightly smaller ascospores. Verrucaria gongshanensis H. Harada & Li S. Wang (Harada & Wang Reference Harada and Wang2008) has a brown, cracked thallus but much smaller ascospores, while V. nipponica H. Harada (Harada Reference Harada1992b, Reference Harada2012b) differs in the involucrellum being weakly developed above and merging with a black basal layer.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, Kyumnu, north of Kyumnu Khola, 28.4088°N, 83.79945°E, alt. 2055 m, on siliceous boulder by path in forest, 2009, Orange & Chhetri 18483 (KATH, NMW.C.2012.002.132); Kaski District, north-east of Chhomrong, near Tilche, 28.43208°N, 83.83268°E, alt. 2100 m, on siliceous rock in dry streamlet, in light shade, 2009, Orange & Chhetri 18486 (NMW.C.2012.002.133); Kaski District, north-east of Chhomrong, north-west of Sinuwa in direction of Kuldhigar, 28.4433°N, 83.84415°E, alt. 2390 m, on low siliceous bedrock near stream in forest, shaded, 2009, Orange & Chhetri 18490 (KATH, NMW.C.2013.001.173); Kaski District, west of Ghandruk, 28.37845°N, 83.79056°E, alt. 2205 m, on rock in small stream in forest, shaded, frequently submerged, 2009, Orange & Chhetri 18532 (KATH, NMW.C.2012.002.134).
Verrucaria craterigera H. Harada
Lichenology 10, 119 (2012); type: Japan, Honshu, Chiba-ken, Kimitsu-shi, 2002, Harada 20424 (CBM-FL 15028—holotype [non vidi]).
Prothallus not seen. Thallus thin, 60–105 μm thick, non-gelatinous, pale grey-green to pale grey-brown, surface slightly uneven; uncracked or locally with short cracks; thallus cells more or less irregularly arranged (but not in goniocyst-like units). Soralia present, discrete, 140–200 μm diam., plane, pale brownish green, often with slightly raised, ragged rim; soredia 16–40 × 16–22 μm, outer layer slightly irregular, but with more or less compact layer of isodiametric cells c. 3.7–4.5 μm diam., some cells projecting slightly; free hyphae absent or few and very short.
Perithecia 0.3–0.5-immersed in the thallus, forming moderately projecting mounds 200–300 μm diam., black or partly grey, sometimes with a slight covering of thallus at the base, but mostly naked; ostiolar region a pale spot. Involucrellum well developed, not much or slightly diverging from exciple below, occupying upper 0.66 or the entire height of the exciple, dark brown, K+ dark green-brown. Centrum 165–200 μm diam. Exciple colourless or slightly browned at sides and base. Periphyses c. 15 μm long. Asci 8-spored, c. 80 × 20 μm. Ascospores (16.5–)18.5–20.2–22(–24.5) × (6.5–)7–7.4–8(–8.5) μm, (2.2–)2.5–2.7–3(–3.2) times as long as wide [20/1; poor condition], some spores 1-septate when overmature; perispore not seen.
Conidiomata not seen.
Ecology and distribution
One collection from rock in forest.
Notes
Significant characters include the thin thallus with well-defined soralia, small perithecia, a well-developed involucrellum, and small ascospores. The material from Nepal resembles the protologue of Verrucaria craterigera (Harada Reference Harada2012b), which was described from a single specimen from Japan, differing only in the slightly thicker thallus and slightly longer ascospores (16–18 × 6–8 μm fide Harada). The identification is provisional, and further material collected from Nepal and elsewhere is required. Vegetative propagules are uncommon in Verrucariaceae, especially well-defined soralia as occur in this species; these were termed goniocystangia by Harada (Reference Harada2012b). The single specimen is very similar in appearance to Thelidium uvidulum, especially in the presence of soralia, but the ascospores are smaller in that species and the ITS sequence differs considerably.
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, north-west of Ghandruk, near Tadapani, 28.39333°N, 83.77066°E, alt. 2640 m, on boulder in forest, 2009, Orange & Chhetri 18517 (KATH, NMW.C.2012.002.155).
Verrucaria elaeomelaena (A. Massal.) Arnold

Fig. 10. A & B, Verrucaria elaeomelaena s. lat. (Orange & Chhetri 18503). C–F, Verrucaria hydrophila s. lat. (C & D, Orange & Chhetri 18561; E & F, Orange & Chhetri 18479). A, C & E, thallus and perithecia. B, D & F, sections of perithecia. Scales: A, C & E = 500 μm; B, D & F = 100 μm. In colour online.
Prothallus not apparent. Thallus very thin, up to 30 μm thick, smooth, uncracked, pale greenish brown in herbarium, green and translucent when fresh and wet.
Perithecia forming irregular, shallowly to rather strongly conical projections 220–560 μm wide, black or concolorous with the thallus, mostly covered by thallus to the apex, but the covering may be patchy or very thin and inconspicuous. Involucrellum conical, reaching to substratum, c. 50 μm thick in upper part of perithecium. Centrum c. 200–330 μm. Exciple c. 13 μm thick at sides and base, hyaline or lightly browned, or dilute dull green near the ostiole. Periphyses c. 20–25 μm long. Ascospores (20.5–)23–26.0–29(–29.5) × (9–)10.5–12.0–13.5(–16.5) μm, (1.6–)2–2.2–2.4(–2.5) times as long as wide [34/7; poor condition].
Conidiomata not seen.
Ecology and distribution
On stones in small streams, rarely on irrigated rocks. This aggregate species was reported from Japan by Harada (Reference Harada2012b) (as Verrucaria andesiatica).
Notes
The species is treated here as an aggregate due to the unresolved taxonomy in this group (Thüs et al. Reference Thüs, Orange, Gueidan, Pykälä, Ruberti, Lo Schiavo and Nascimbene2015) and the small quantities of material available from Nepal. Important features of the aggregate species include the very thin, uncracked thallus and the conical involucrellum. The Nepalese specimens are recovered in three clades within an ITS analysis of the V. elaeomelaena group (Fig. 4). Two specimens (18503 and 18528) are nested within sequences from Europe identified as V. alpicola Zschacke, and the relatively large ascospores, 25.5–29.5 μm long, approach this taxon in size. However, specimen 18497 also has relatively large ascospores but it is nested within V. elaeomelaena s. lat. Collections 18536 and 18560 are also nested within V. elaeomelaena s. lat. and the ascospores are comparatively small, 20.5–24.5 μm long. However, further analysis is not possible due to the small quantity of material and the poor condition of the spores. It will not be possible to resolve this complex using the ITS region alone, and a careful analysis using several gene regions is needed.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, north-east of Bamboo, 28.46646°N, 83.86521°E, alt. 2460 m, on stone in intermittent streamlet in forest, 2009, Orange & Chhetri 18497 (KATH, NMW.C.2013.001.178); Kaski District, just north-west of Dobhan, 28.47306°N, 83.8735°E, alt. 2540 m, on stone in streamlet by path in forest, in light shade, 2009, Orange & Chhetri 18503 (NMW.C.2013.001.176); Kaski District, north-west of Ghandruk, south of Tadapani, 28.38366°N, 83.78083°E, alt. 2535 m, on irrigated rocks in ravine in forest, shaded, 2009, Orange & Chhetri 18528 (KATH, NMW.C.2013.001.177); Kaski District, north-west of Ghandruk, south of Tadapani, 28.3885°N, 83.77833°E, alt. 2450 m, on stones in stream (c. 1 m wide) in forest, shaded, 2009, Orange & Chhetri 18520 (NMW.C.2013.001.179); Kaski District, north-west of Ghandruk, south of Tadapani, 28.38518°N, 83.78018°E, alt. 2640 m, on stone in streamlet in forest, shaded, not submerged at present, 2009, Orange & Chhetri 18526 (NMW.C.2013.001.180); Kaski District, north-west of Ghandruk, east of Sitkyu, 28.37833°N, 83.80026°E, alt. 2140 m, on stone by streamlet in scrub, 2009, Orange & Chhetri 18536 (NMW.C.2012.002.157); Kaski District, south of Pothana, 28.31003°N, 83.8291°E, alt. 1940 m, on stone beside small stream in open forest, shaded, 2009, Orange & Chhetri 18560 (NMW.C.2012.002.158).
Verrucaria hydrophila Orange s. lat.
Thallus thin, up to 20 μm thick, often extensive, pale grey-green to mid grey-brown, uncracked, without dark basal layer; thallus often bright green and translucent when wet and fresh.
Perithecia forming low to moderately projecting conical to conical-hemispherical mounds 300–400 μm diam., covered by thin layer of thallus to apex, or covering sometimes patchy, or lost in upper part; apex of perithecium rounded, the ostiolar region inconspicuous or visible as a pale dot or papilla 20–60 μm wide. Involucrellum conical, densely pigmented at upper surface, in lower part weakly pigmented or grading into colourless tissue at base of exciple. Exciple 140–200 μm diam., colourless at sides and base. Periphyses c. 18–22 μm long. Ascospores (17.5–)21–24.0–27(–29.5) × (9–)9.5–10.3–11(–11.5) μm, (1.9–)2.1–2.3–2.6(–2.8) times as long as wide [31/4], lacking a perispore.
Ecology and distribution
On rocks and stones in or beside shaded streams.
Notes
Important characters include the very thin uncracked thallus and the more or less conical involucrellum. Verrucaria elaeomelaena is very similar in morphology, though not closely related; it tends to have larger perithecia and spores, but some specimens can be impossible to place without sequencing. Verrucaria hydrophila as defined here is probably a species complex, with much variation in the ITS region but very little morphological variation. In Europe, some ITS clades are associated with specimens from non-aquatic rock or bark by streams. The specimens from Nepal occur in at least two clades within the complex. An analysis using several gene regions is needed to resolve this group.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, c. 1 km north-west of Ghandruk, Chharnami, 28.3820°N, 83.7987°E, alt. 1975 m, on rock by stream, unshaded, 2009, Orange & Chhetri 18479 (NMW.C.2013.001.191); Kaski District, north-west of Ghandruk, east of Sitkyu, 28.37833°N, 83.80026°E, alt. 2140 m, on stone by streamlet in scrub, 2009, Orange & Chhetri 18537 (NMW.C.2012.002.159); Kaski District, south of Pothana, 28.31003°N, 83.8291°E, alt. 1940 m, on stone in small stream in open forest, frequently submerged, shaded, 2009, Orange & Chhetri 18561 (|KATH, NMW.C.2013.001.175), 18562 (NMW), 18563 (|NMW.C.2013.001.174), 18564 (NMW.C.2012.002.160).
Verrucaria lactea Orange sp. nov.
MycoBank No.: MB 842611
Thallus very pale grey, very sparsely cracked; perithecia immersed, involucrellum shallowly conical. Resembles the European Verrucaria praetermissa but differs in the larger ascospores and strongly deviating ITS sequence.
Type: Nepal, Bagmati Pradesh, Kathmandu District, Shivapuri, 27.78933°N, 85.37666°E, alt. 1740 m, on shaded rock by stream in forest, probably inundated during high flows, 17 October 2009, Orange & Chhetri 18572 (KATH—holotype; NMW.C.2013.001.193—isotype).

Fig. 11. A–C, Verrucaria lactea (Orange & Chhetri 18572). D & E, Verrucaria luchunensis (Orange & Chhetri 18569). A & D, thallus. B, C & E, sections of thallus and perithecia. Scales: A & D = 500 μm; B, C & E = 100 μm. In colour online.
Thallus crustose, extensive, 35–175 μm thick, very pale grey, smooth, matt; cracks sparse, not delimiting areoles, sides of cracks unpigmented; thallus locally with a black basal layer.
Perithecia mostly immersed in the thallus, not projecting, or forming low and indistinct projections in the thallus; apex visible as a black dot up to 90 μm wide, or projecting as a small black, often rough mound up to 220 μm wide; ostiolar region inconspicuous or visible as a plane pale dot up to 65 μm wide. Involucrellum shallowly conical, densely pigmented at surface, paler and sometimes colourless below, cells with large oil droplets; involucrella confluent, forming a dark basal layer locally in the thallus. Exciple colourless or lightly browned, 180 μm wide. Ascospores simple, colourless, (23.5–)26.5–29.1–32(–33) × (9–)9.5–10.5–11.5 μm, (2.4–)2.6–2.8–3.0(–3.1) times as long as wide [18/1], but measured spores possibly a little immature; gelatinous perispore present, 4–5 μm thick in water.
Conidiomata not found.
Etymology
From the Latin adjective lacteus (milk-white), referring to the extensive, whitish thallus.
Ecology and distribution
A single collection from shaded rock near a stream.
Notes
This species resembles Verrucaria praetermissa in the pale thallus with an unpigmented cortex, immersed perithecia and a shallowly conical involucrellum, but the very pale and scarcely cracked thallus would be unusual for that species. The ITS sequence of the holotype differs widely from V. praetermissa, and a BLAST search does not return any closely related species. The ascospores of the new species are larger than in the two specimens of V. praetermissa from Nepal (although the latter were in poor condition) and are larger than the size of (16–)18–25(–28) × (6.5–)7–10(–10.5) μm given for European material by Orange (Reference Orange2000).
Verrucaria luchunensis H. Harada & Li S. Wang
Lichenology 7, 17 (2008); type: China, Yunnan, 2005, Harada 21401 (CBM-FL-16560—holotype; KUN-L—isotype [non vidi]).
Prothallus not seen. Thallus well developed, thick, 100–370 μm, non-gelatinous, very pale brownish grey to pale brown; margin entire, soon becoming cracked, mature thallus extensively cracked, the cracks sometimes completely surrounding discrete areoles; areoles with sides pale or dark brown, the surface smooth, plane, matt; thallus cells irregularly arranged or in indistinct vertical columns, air spaces between cells numerous; upper surface a pseudocortex with or without brown pigment, cells sometimes broken but not forming an epinecral layer; lower part of thallus with extensive dark brown pigment, forming a dark basal layer, cells containing large oil droplets.
Perithecia completely immersed in the thallus, the ostiolar region visible as a pale grey-brown spot or disc 40–160 μm, often lying in a small depression; dark-pigmented tissue not reaching thallus surface. Involucrellum irregular in shape, comprising areas of brown-pigmented tissue flanking the upper exciple, and merging below with the dark basal layer of the thallus. Centrum 175–285 μm diam. Exciple colourless when young, later brown to greenish brown at the sides and base, pigment K+ brown or dark green. Asci 8-spored. Ascospores (22.5–)24.5–27.1–29.5(–33) × (8–)9–10.9–12.5(–15.5) μm, (1.8–)2.3–2.5–2.8(–3.0) times as long as wide [30/3], with a well-defined gelatinous perispore 0.8–1.6 μm thick (in K); overmature spores light brown.
Conidiomata not seen.
Ecology and distribution
Three collections on shady rocks near streams, but never inundated.
Notes
Important features include the thick, cracked thallus, completely immersed perithecia, large ascospores, and presence of a perispore. The perispore is easily visible as a smooth gelatinous coating in most spores; when thin, it can be mistaken for a thickened spore wall. The exciple is unusual amongst other Verrucaria species described here, in that it is more deeply pigmented than the adjacent involucrellum. The green pigment usually present in parts of the exciple can be striking, especially in K, though traces of a similar pigment can occur in other species. The upper exciple is weakly pigmented and the involucrellum is weakly developed, so that no dark pigments are visible in surface view of the thallus. In the field this species could be mistaken for an unrelated, sterile, crustose lichen from another family.
The protologue of V. luchunensis H. Harada & Li S. Wang (Harada & Wang Reference Harada and Wang2008) from Yunnan agrees with the present species in the immersed perithecia, weakly developed involucrellum, rather large ascospores, the presence of a perispore and the absence of pycnidia. It differs in the slightly smaller ascospores and in the dark tissue of the exciple reaching the thallus surface. This last feature may simply be absent in the specimens from Nepal because of the shady habitat. Distinctly halonate ascospores, as found in the Nepalese specimens, are uncommon in Verrucariaceae. The similarities outlined here are sufficient to provisionally identify the Nepalese material as this species, but confirmation by molecular methods would be desirable. Verrucaria nipponica Zahlbruckner grows on periodically inundated rocks in Japan; it has a similar ascospore size but differs in the presence of pycnidia and the consistently pale sides of the areoles (Harada Reference Harada1992b). In addition, the occurrence of a perispore is not mentioned (Harada Reference Harada1992b).
BLAST searches do not suggest any closely related species.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, north-east of Chhomrong, north-west of Sinuwa in direction of Kuldhigar, 28.44091°N, 83.8423°E, alt. 2340 m, on boulder in forest, shaded, near a stream but never inundated, 2009, Orange & Chhetri 18489 (KATH, NMW.C.2012.002.150); ibid., 28.4433°N, 83.84415°E, alt. 2390 m, on low siliceous bedrock near stream in forest, shaded, 2009, Orange & Chhetri 18491 (KATH, NMW.C.2012.002.151). Bagmati Pradesh: Kathmandu District, Shivapuri, 27.79216°N, 85.37508°E, alt. 1750 m, shady rocks near stream in forest, 2009, Orange & Chhetri 18569 (KATH, NMW.C.2012.002.152).
Verrucaria parvipeltata Orange sp. nov.
MycoBank No.: MB 842612
Thallus comprising basally constricted areoles distributed on an extensive dark prothallus; perithecia sometimes partly surrounded by areoles, otherwise free of thallus.
Type: Nepal, Gandaki Pradesh, south-east of Tolka, Bhedi Kharka, 28.33616°N, 83.8302°E, alt. 1890 m, on rock in retaining wall of stone platform by path in forest, shaded, 12 October 2009, Orange & Chhetri 18555 (KATH—holotype; NMW.C.2013.001.186—isotype).

Fig. 12. A–D, Verrucaria parvipeltata (A & B, Orange & Chhetri 18555; C & D, Orange & Chhetri 18512). E & F, Verrucaria praetermissa (Orange & Chhetri 18518). G & H, Verrucaria senta (Orange & Chhetri 18542). A, E & G, thallus and perithecia. B, D, F & H, sections of thallus and perithecia. C, section of areole. Scales: A & E = 500 μm; B, D, F & H = 100 μm; C = 50 μm; G = 1 mm. In colour online.
Prothallus conspicuous, dark brown. Areoles arising singly on the prothallus, grey to pale brown, convex, enlarging and generally becoming flattened and shallowly lobed, up to 330–500 μm wide, constricted below, sometimes the surface becoming nodular; areoles paraplectenchymatous throughout; upper surface with a pseudocortex of brown cells, lower cortex absent.
Perithecia brown, forming convex mounds 265–400 μm wide, without a thalline covering, sometimes partly surrounded by areoles, otherwise free of thallus. Involucrellum conical-hemispherical, reaching down to the substratum, dark brown, K+ dull dark green or dull brownish green. Centrum 175–185 μm wide. Exciple hyaline. Ascospores 20.5–26 × 8.5–10 μm, 2.1–3 times as long as wide [13/2; rather poorly developed].
Conidiomata not seen.
Etymology
From the Latin parvus (small) and peltatus (peltate), referring to the basally constricted areoles.
Ecology and distribution
On rock, shaded or unshaded.
Notes
This is a striking species which will be readily recognized in the field by its extensive dark prothallus incompletely covered by brown areoles of subsquamulose appearance. The perithecia sometimes occur separated from the areoles and arising on the prothallus. BLAST searches of ITS and LSU do not return any closely related species.
Additional specimens examined
Nepal: Gandaki Pradesh: Kaski District, south of Ghandruk, by Chane Kola, 28.36446°N, 83.80403°E, alt. 1710 m, on stone in vegetated block scree, 2009, Orange & Chhetri 18474 (NMW.C.2013.001.187); Kaski District, south-west of Chhomrong, 28.41066°N, 83.797°E, alt. 2200 m, on damp boulder by path in deciduous forest, 2009, Orange & Chhetri 18508 (KATH, NMW.C.2013.001.184); Kaski District, north-west of Ghandruk, north of Chuile, just south of Kyumnu Khola, 28.40773°N, 83.77653°E, alt. 2045 m, on unshaded stone by path, 2009, Orange & Chhetri 18512 (NMW.C.2013.001.185).
Verrucaria praetermissa (Trevis.) Anzi
Prothallus white, non-fimbriate. Thallus well developed, 60–200 μm thick, non-gelatinous, pale grey-green, continuous or usually with sparse to numerous cracks, these locally extensive but only rarely completely surrounding discrete areoles; cracks pale-sided (except when adjacent to the basal layer); thallus cells irregularly arranged or in very weakly delimited vertical columns; upper surface an unpigmented pseudocortex; a darkly pigmented basal layer locally present, sometimes probably part of a widely spreading involucrellum.
Perithecia immersed in the thallus, at most producing very low mounds that are too poorly delimited to measure; perithecium visible at first by grey ostiolar region, later the apex exposed as a black ring or disc 100–200 μm diam.; ostiolar region visible as a pale dot usually 20–40 μm diam., later sometimes concave, to 80 μm. Involucrellum well developed, conical, often wide-spreading, densely pigmented at the surface, in lower part weakly pigmented with dark cell outlines visible, or locally colourless near the base of the exciple; involucrella often coalescing and merging with the concolorous basal layer. Centrum 170–210 μm wide. Exciple colourless. Periphyses c. 20 μm long, in a common gel. Asci 8-spored. Ascospores c. 18–20 × 8–8.5 μm, but only a small number of old or immature spores seen.
Conidiomata not seen.
Ecology and distribution
Two collections on stones in shaded streams. Reported from Europe, North America, Japan (Harada Reference Harada2012b), China (Yunnan; Harada & Wang Reference Harada and Wang2008), Australia (McCarthy Reference McCarthy1995b) and New Zealand (Malcolm & Galloway Reference Malcolm and Galloway1997).
Notes
Significant characteristics include the pale, well-developed thallus, cracks with unpigmented sides, immersed perithecia and conical involucrellum. The collections are very similar in morphology to European specimens, although ascospore size could not be determined accurately in the Nepalese material. However, the two ITS sequences from Nepal form a sister clade to sequences from Europe (Fig. 5). Further related collections should be studied before any new species are described in this group.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, north-west of Ghandruk, south of Tadapani, 28.3885°N, 83.77833°E, alt. 2450 m, on stones in stream (c. 1 m wide) in forest, shaded, 2009, Orange & Chhetri 18518 (KATH, NMW.C.2012.002.145); ibid., 28.3885°N, 83.77866°E, alt. 2570 m, submerged in stream in forest (water pH 8.3, conductivity 223 μS cm−2), shaded, 2009, Orange & Chhetri 18524 (NMW.C.2012.002.146).
Verrucaria senta Orange sp. nov.
MycoBank No.: MB 842613
Thallus brown, cracked into dark-sided areoles; perithecia prominent, naked, ascospores (21.5–)25.5–31(–33) μm long. Differing from superficially similar species with a brown thallus by the ITS sequence and occurrence of pycnidia.
Type: Nepal, Gandaki Pradesh, Kaski District, 1 km south of Landruk, 28.36833°N, 83.826166°E, alt. 1650 m, on siliceous rock on unshaded, occasionally irrigated face, 11 October 2009, Orange & Chhetri 18542 (KATH—holotype; NMW C.2012.002.144—isotype).
Prothallus not seen. Thallus well developed, 80–220 μm thick, greenish mid brown to dark brown, extensively cracked, the cracks often delimiting discrete areoles; sterile areoles mostly 140–500 μm wide, fertile areoles mostly 350–900 μm wide, thallus occasionally with a marked division into sterile and fertile areoles, sometimes most areoles fertile and the distinction not obvious; areoles with upper surface matt, uneven, marked with faint lines (at least in lightly shaded specimen) denoting subunits mostly 90–220 μm wide; sides of areoles black; upper surface a pseudocortex with brown pigment; lower part of thallus often with extensive darkly pigmented tissue, which ascends the areole margin.
Perithecia mostly 1–3(–6) per fertile areole, forming moderately projecting mounds 240–500 μm diam., black, more or less smooth to rough, often with thin patches of thallus in lower half, but mostly naked; ostiole mostly inconspicuous, sometimes visible as a plane dot 20–40 μm diam. Involucrellum well developed, spreading laterally and downwards, merging with the dark basal layer of the thallus. Centrum 295–355 μm diam. Exciple slightly to strongly pigmented in outer layers. Periphyses c. 30–36 μm long. Asci 8-spored. Ascospores (21.5–)25.5–28.2–31(–33) × (10.5–)12–12.8–13.5(–14.5) μm, (1.8–)1.9–2.2–2.4(–2.7) times as long as wide [30/2; mostly in poor condition].
Pycnidia scattered, mostly immersed, apex projecting, dark brown, of irregular outline, 50–120 μm diam.; wall brown throughout. Conidia rod-shaped, 4.1–4.5 × c. 0.8 μm.
Etymology
From the Latin sentus (rough, rugged, uneven), a reference to the appearance of the thallus.
Ecology and distribution
Two collections from rocks that are dry, or irrigated only in wet weather.
Notes
Important features include the well-developed thallus, black-sided areoles subdivided into (inconspicuous) smaller units, and the projecting naked perithecia. Pycnidia were conspicuous in one specimen but not seen in the other. LSU and ITS analyses suggest that this belongs in the Endocarpon group of Gueidan et al. (Reference Gueidan, Savić, Thüs, Roux, Keller, Tibell, Prieto, Heiðmarsson, Breuss and Orange2009) (Fig. 1). This is a relatively nondescript species, superficially similar to a number of others with a brown thallus and medium to large ascospores. Verrucaria macrostoma Dufour ex DC. has a brown thallus and similar ascospore size but differs in the larger areoles. Verrucaria nigrescens Pers. has smaller ascospores, (17–)19–27(–30) × 8–14 μm, and V. fusconigrescens Nyl. also has smaller ascospores (17–)19.5–23.5(–26) μm long. These three species also differ in ITS sequence and in the absence of pycnidia.
Additional specimen examined
Nepal: Gandaki Pradesh: west-south-west of Ghandruk, north-west of Landruk, east side of Modi Khola, 28.37463°N, 83.82185°E, alt. 1390 m, on rock amongst rice terraces, unshaded, 2009, Orange & Chhetri 18541a (NMW.C.2012.002.143).
Verrucaria Species A

Fig. 13. A & B, Verrucaria Species A (Orange & Chhetri 18546). C & D, Willeya honghensis (Orange & Chhetri 18487). E & F, Willeya cf. japonica (Orange & Chhetri 18510). G & H, Willeya Species A (Orange & Chhetri 18554). A, C, E & G, thallus and perithecia. B, D, F & H, sections of thallus and perithecia. Scales: A, E & G = 500 μm; B, D, F & H = 100 μm; C = 1 mm. In colour online.
Prothallus not seen. Thallus very thin, c. 20–30 μm thick, mid greenish brown, smooth, uncracked, apparently composed of inconspicuous, indistinct, coalescing units c. 100–200 μm wide; upper surface a pseudocortex with brown pigment.
Perithecia forming low to moderately projecting mounds 200–460 μm diam., sometimes covered to the apex by the thallus, but usually the thalline cover patchy, no more than 50%. Involucrellum well developed, shallowly conical, densely pigmented at the surface, internal parts more weakly pigmented with dark cell outlines visible. Centrum 110–250 μm diam. Exciple colourless. Periphyses c. 15 μm long. Asci 8-spored. Ascospores (24.5–)27–29.2–31.5(–33.5) × (11–)11.5–12.7–14(–15) μm, (2.1–)2.2–2.3–2.5(–2.6) times as long as wide [16/1]; perispore not seen.
Conidiomata not seen.
Ecology and distribution
One collection from a stone in a shady stream.
Notes
Important features include the very thin, smooth, uncracked thallus, the conical involucrellum and the large ascospores. The thallus is composed of small, obscurely delimited subunits, most easily seen in heavily shaded parts of the specimen, or on the perithecia, where they contribute to the patchy appearance of the partial thalline cover. This is in contrast to species including the superficially similar Verrucaria hydrophila, where the thallus appears to be continuous. However, the difference is slight and needs to be confirmed in additional specimens. It resembles three species described from Europe: V. hydrophila and V. elaeomelaena also have a very thin thallus and conical involucrellum, and V. margacea (Wahlenb.) Wahlenb. agrees in the slightly patchy thallus, conical involucrellum and large ascospores. However, LSU and ITS sequences do not suggest a close relationship with any of these taxa. Due to the sparse material, the single specimen is not formally described here.
Specimen examined
Nepal: Gandaki Pradesh: south of Landruk, 1 km south-east of Tolka, 28.3405°N, 83.83033°E, alt. 1775 m, on stone in small stream in forest (water pH 7.4, conductivity 34 μS cm−1), shaded, 2009, Orange & Chhetri 18546 (NMW.C.2012.002.156).
Willeya Müll. Arg.
The genus Willeya was resurrected by Gueidan et al. (Reference Gueidan, Van Do and Lu2014) for a number of species with pale ascospores formerly placed in Staurothele s. str. Staurothele s. str. was restricted to those species with two dark brown ascospores per ascus. Twelve species have been accepted in Willeya, and further species of Staurothele s. lat. are likely to belong there. The thallus is epilithic (partly endolithic in one taxon), cracked and often distinctly areolate; the perithecia are typically immersed in the thallus but may protrude to some extent. There are 2–8 spores per ascus, and in most species ascospores are in the size range 18–32 μm long (rarely to 52 μm). The described species differ in relatively minor features such as thallus colour (which seems to be rather variable even in one species), ascospore size and the degree of protrusion of the perithecia. Pycnidia are reported for only two species. Because of the small amount of material available, the lack of firm morphological differences and the lack of DNA data, the status of some of the earlier described species is uncertain.
Willeya eminens Orange sp. nov.
MycoBank No.: MB 842614
Perithecia prominent, with an uneven thallus cover. Willeya protrudens Gueidan differs in ITS sequence.
Type: Nepal, Gandaki Pradesh, Kaski District, south of Syauli Bajar, 28.34706°N, 83.8036°E, alt. 1135 m, on siliceous stone in drystone retaining wall by path, 2 October 2009, Orange & Chhetri 18533 (NMW.C.2012.002.137—holotype).

Fig. 14. A & B, Willeya irrigata (Orange & Chhetri 18540). C & D, Willeya pallidipora (Orange & Chhetri 18478). E & F, Willeya nepalensis (Orange & Chhetri 18480). G & H, Willeya eminens (Orange & Chhetri 18533). Scales: A, C & E = 500 μm; B, D, F & H = 100 μm; G = 1 mm. In colour online.
Thallus thin, c. 40 μm thick, light brown, with occasional cracks that do not delimit areoles; dark basal layer absent, except near perithecia; epinecral layer absent.
Perithecia forming irregularly conical-hemispherical projections 560–670 μm wide, with an uneven cover of thallus below, but with the black apex visible above; ostiolar area forming a pale dot 85–105 μm wide. Involucrellum conical. Centrum c. 325 μm wide. Exciple brown. Asci 8-spored. Ascospores colourless, 22.5–29 × 9–12.5 μm, 2.1–2.4 times as long as wide [14/1], muriform, c. 17–25 cells visible in optical section. Hymenial algae 5–10 × 1.5 μm, oblong to cylindrical.
Etymology
From the Latin eminens (projecting), referring to the perithecial mounds.
Ecology and distribution
One collection from a wall.
Notes
The rather large and prominent perithecia distinguish this species from all other Willeya species in Nepal, but W. protrudens also has projecting perithecia. The ITS tree (Fig. 1) suggests they are not closely related, but more material needs to be collected before the range of variation in each can be established. The thallus of the type specimen grows immediately adjacent to one of W. nepalensis (the remainder of that species separated as Orange & Chhetri 18472). The latter differs in the smaller, less prominent perithecia, although without close examination the two thalli might be considered part of the same colony.
Willeya honghensis (H. Harada & Li S. Wang) Orange comb. nov.
MycoBank No.: MB 842615
Staurothele honghensis H. Harada & Li S. Wang, Lichenology 5, 15 (2006); type: China, Yunnan, Hekou-xian,Manhao, 22°59ʹ23ʺN, 103°24ʹ19ʺE, 13 January 2005, Harada 21262 (CBM-FL-16423—holotype; KUN-L—isotype [non vidi]).
Prothallus not seen. Thallus well developed, 200–230 μm thick, non-gelatinous, mid grey-brown, cracks numerous, often enclosing discrete areoles; upper surface of areoles more or less plane, smooth, matt, with black sides; thallus margin apparently of discrete areoles that rapidly become confluent (but little free margin was seen); thallus cells in weak vertical columns, with numerous air spaces between cells; upper surface a pseudocortex with dull brown pigment; lower parts of thallus often without living algae, with brown-pigmented areas. Photobiont cells 4.5–9 × 5–7.5 μm.
Perithecia almost completely immersed in the thallus, not forming mounds, the naked apex forming a low black protrusion 70–140 μm diam.; ostiolar region inconspicuous, sometimes visible as a small pale dot. Involucrellum rather weakly developed, scarcely diverging from exciple, sometimes merging with pigmented basal layer of thallus. Centrum 160–255 μm diam. Periphyses c. 25 μm long. Hymenial algal cells broadly ellipsoid, 3.3–4.1 × 2.5–3.3 μm, solitary or dividing by a transverse septum. Asci not observed. Ascospores c. 32–38 × 10–13 μm, colourless, muriform (but only a small number of poorly developed spores seen).
Pycnidia immersed in thallus, ostiole brown, wall colourless. Conidia rod-shaped, 5.3–7.0 × c. 1.2 μm.
Ecology and distribution
One collection from irrigated siliceous rocks. Also found in China (Yunnan).
Notes
Important features of this species include the well-developed, cracked thallus, black-sided areoles, and immersed perithecia with only the extreme apex visible. The specimen from Nepal is identified provisionally as Willeya honghensis (Harada & Wang Reference Harada and Wang2006a). In fig. 1G of the protologue of that species, the perithecia are shown as forming rather distinct projecting mounds, though a section shown in fig. 3A suggests this character is not always well developed. Although pycnidia are present in the Nepalese collection, they were not found in the type specimen. The few overmature ascospores in the Nepal collection are larger than in W. honghensis. The significance of all these features is difficult to assess since W. honghensis was described from only a single collection. The Nepalese specimen also resembles Staurothele kwapiensis Zahlbr. (Zahlbruckner Reference Zahlbruckner and Handel-Mazzetti1930) in the short and broad hymenial algae and in the ascospore length but, according to Harada & Wang (Reference Harada and Wang2006a), an involucrellum is absent in S. kwapiensis. Staurothele yunnana H. Harada & Li S. Wang differs in the perithecia forming mounds (Harada & Wang Reference Harada and Wang2006a). These two species probably belong in Willeya but as material has not been examined in the course of the present study, the transfer is not made. Willeya tetraspora Aptroot (Aptroot Reference Aptroot2016) has ascospores (27–)30–32–35(–37) μm long, and it possesses pycnidia, but it grows on non-irrigated limestone.
In the ITS tree (Fig. 1), W. honghensis is recovered as basal to a well-supported clade comprising Endocarpon species, and not amongst other Willeya species, but more gene regions are needed to recover a reliable phylogeny.
Specimens examined
Nepal: Gandaki Pradesh: Kaski District, north-east of Chhomrong, south-west of Sinuwa, 28.43256°N, 83.83416°E, alt. 2200 m, on irrigated siliceous rocks, unshaded, 2009, Orange & Chhetri 18487 (NMW.C.2012.002.135).
Willeya irrigata Orange sp. nov.
MycoBank No.: MB 842616
Thallus cracked into black-sided areoles; perithecia immersed, ascospores (28.5–)29.5–36(–40) μm, hymenial algal cells oblong.
Type: Nepal, Gandaki Pradesh, Kaski District, west-south-west of Ghandruk, west side of Modi Khola, 28.374°N, 83.82145°E, alt. 1360 m, on irrigated rock face beside fields, 10 October 2009, Orange & Chhetri 18540 (KATH—holotype).
Prothallus not seen. Thallus well developed, 195–560 μm thick, non-gelatinous, pale to mid brown; thallus margin entire, soon becoming cracked; the cracks later extensive, and often delimiting discrete areoles, areoles more or less plane, 330–670 μm wide, with black sides; thallus cells in weakly delimited vertical columns, air spaces present between some cells; upper surface a pseudocortex with dull brown pigment; lower part of thallus often darkly pigmented, sometimes forming a thick dark basal layer, cells containing large oil droplets.
Perithecia almost completely immersed in the thallus, apex visible as a black disc or black convex structure 120–300 μm diam. Involucrellum fairly well developed, more or less conical, densely pigmented at the surface, more weakly coloured below, merging with dark basal layer of thallus. Centrum 150–280 μm diam. Exciple weakly pigmented on outer side. Periphyses c. 15–26 μm long, unbranched, enclosed in a common gel. Hymenial algal cells oblong, 3.3–7 × 2.1–2.5 μm, 1.3–3.4 times as long as wide, often dividing by a transverse septum, cells occasionally forming chains of several cells. Asci not seen when mature. Ascospores colourless, muriform, (28.5–)29.5–32.8–36(–40) × (9–)11.5–12.7–14(–14.5) μm, (2.1–)2.2–2.7–3.3(–4.0) times as long as wide [12/2; in poor condition].
Pycnidia immersed in thallus, visible as a small brown dot. Conidia rod-shaped, 7–7.8 × c. 1 μm.
Etymology
From the Latin irrigatus (watered, irrigated), referring to the habitat.
Ecology and distribution
Two collections from occasionally irrigated rock faces.
Notes
Important features of this species include the well-developed thallus which is extensively cracked into black-sided areoles, the mostly immersed perithecia, oblong hymenial algae and the comparatively large ascospores (most species of Willeya have much smaller ascospores). Willeya tetraspora has globose hymenial algae. Staurothele kwapiensis also has globose hymenial algae and it lacks pycnidia, while Willeya honghensis differs in its ellipsoid hymenial algae and ITS sequence.
Additional specimen examined
Nepal: Gandaki Pradesh: Kaski District, south of Ghandruk, 28.3680°N, 83.8098°E, 2009, Orange & Chhetri 18475 (KATH, NMW.C.2012.002.141).
Willeya cf. japonica (B. de Lesd.) Gueidan
In Gueidan et al., Lichenologist 46, 529 (2014).—Staurothele japonica B. de Lesd., Bull. Soc. Bot. Fr. 68, 494 (1921); type: Japan, Hokkaido, 1904, Faurie (KYO—lectotype [non vidi]).
Prothallus brown (but little thallus margin present in specimen). Thallus well developed, 130–290 μm thick, non-gelatinous, light greenish brown; initially of discrete patches, these soon coalescing; becoming strongly secondarily cracked, the cracks often delimiting discrete areoles; surface of areoles slightly uneven, more or less plane to slightly convex; sides of areoles black; thallus cells in weakly delimited vertical columns, with numerous air spaces between cells; upper surface of thallus a pseudocortex with dilute brown pigment; lower part of thallus with dark pigment, which ascends the sides of the areoles.
Perithecia almost completely immersed, not forming mounds, or these very low and indistinct; initially visible only by the pale brown ostiolar region (visible as a pale dot up to 60 μm wide), eventually apex exposed as a more or less plane, black disc up to 200 μm wide, not or scarcely projecting from the thallus. Involucrellum flanking the upper exciple, confluent with a dark basal layer of the thallus. Exciple dark brown. Centrum 190–273 μm diam. Periphyses short, c. 21 μm long. Hymenial algal cells broadly ellipsoid, 2.5–3.3 × 2.5–2.9 μm. Asci not seen when mature. Ascospores colourless, muriform, c. 21–24 × 9–14 μm (but only a small number of dead and immature spores seen).
Conidiomata not seen.
Ecology and distribution
A single collection on rock in forest in light shade.
Notes
Important characteristics include the well-developed thallus with dark-sided areoles, the immersed perithecia, a well-developed and more or less conical involucrellum, and ellipsoid hymenial algae. ITS sequences confirm that this species is distinct from W. honghensis, from which it differs in the thallus initially being composed of discrete areoles, the more uneven surface, and the absence of pycnidia. This species matches the published descriptions of Willeya japonica (Harada Reference Harada1992a; Gueidan et al. Reference Gueidan, Van Do and Lu2014: 529) in the brown, areolate thallus with more or less discrete marginal areoles, almost completely immersed perithecia and comparatively short hymenial algae. However, W. japonica, which is known only from the type collection, usually has an epinecral layer (Harada Reference Harada1992a). In the absence of molecular data, it is not possible to be certain that the Nepalese specimen is conspecific.
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, south-west of Chhomrong, 28.41066°N, 83.797°E, alt. 2200 m, on rock beside path in deciduous forest, in light shade, 2009, Orange & Chhetri 18510 (KATH, NMW.C.2012.002.139).
Willeya nepalensis Orange sp. nov.
MycoBank No.: MB 842617
Thallus cracked into dark-sided areoles; perithecia immersed, ascospores (19.5–)20–23.5(–25) μm long, hymenial algal cells broadly ellipsoid.
Type: Nepal, Gandaki Pradesh, Kaski District, c. 1 km north-west of Ghandruk, Chharnami, 28.3844°N, 83.7973°E, alt. 1990 m, on level surface of schist stones on and beside path in forest, in light shade, 4 October 2009, Orange & Chhetri 18480 (KATH—holotype; NMW.C.2012.002.136—isotype).
Prothallus inconspicuous, or dark brown and fimbriate. Thallus well developed, 80–180 μm thick, non-gelatinous, dull greyish brown or greenish brown; margin entire or of coalescing patches, soon secondarily cracked, cracks numerous, often enclosing discrete areoles, sides of cracks black; surface of areoles plane to slightly concave, smooth, matt; thallus cells in weak vertical columns, with numerous air spaces between cells; upper surface a pseudocortex with dull brown pigment, epinecral layer not seen.
Perithecia forming low, ill-defined mounds c. 300–340 μm diam.; apex of perithecium flattened, exposed only as a black disc, or as a low black mound 100–240 μm diam.; ostiolar region plane or slightly depressed, often rather conspicuous as a pale dot 20–80 μm diam. Involucrellum well developed, more or less conical, densely pigmented at the surface, more weakly pigmented below. Centrum 195–220 μm diam. Exciple externally brown. Periphyses c. 30–35 μm long. Hymenial algal cells broadly ellipsoid, 3.7–5.3 × 3.7–4 μm, solitary or divided by a transverse septum. Asci 8-spored, 55–65 × 29–36 μm. Ascospores colourless, muriform, (19.5–)20–21.6–23.5(–25) × (9–)10–10.9–12 μm, (1.7–)1.8–2.0–2.2(–2.3) times as long as wide [18/2], with 10–16 cells visible in optical section, 9–13 cells on margin in optical section.
Conidiomata not seen.
Etymology
Referring to the country of origin.
Ecology and distribution
Three specimens, from drystone retaining walls and flagstones in a path.
Notes
This species is characterized by the cracked thallus with dark-sided areoles, the weakly prominent perithecia, a well-developed involucrellum, ellipsoid hymenial algae, and the small ascospores. Amongst the species with short hymenial algae and relatively small ascospores, W. microlepis (Zahlbr.) Gueidan from southern China differs in the 2-spored asci (Gueidan et al. Reference Gueidan, Van Do and Lu2014). Willeya japonica differs in the involucrellum appressed to the upper exciple (Gueidan et al. Reference Gueidan, Van Do and Lu2014) and the frequent occurrence of an epinecral layer (Harada Reference Harada1992a), although these could prove to be unimportant features once a wider range of material is available for study. However, the two are not synonymized here as although Willeya nepalensis is very similar to W. cf. japonica (Orange & Chhetri 18510) in morphology, it is distinct in ITS sequence, highlighting the difficulty of identifying species by morphology alone in this genus. Willeya eminens (see above) differs in the more prominent perithecia and the oblong hymenial algae.
Additional specimens examined
Nepal: Gandaki Pradesh: Kaski District, south of Syauli Bajar, 28.3378°N, 83.8003°E, alt. 1135 m, on siliceous stone in drystone retaining wall by path, 2009, Orange & Chhetri 18472 (NMW.C.2012.002.137); Kaski District, NNW of Syauli Bajar in the direction of Kimche, 28°20.82ʹN, 83°48.21ʹE, alt. 1405 m, on rock beside path, 2009, Orange & Chhetri 18473 (NMW).
Willeya pallidipora (P. M. McCarthy) Gueidan
In Gueidan et al., Lichenologist 46, 530 (2014).—Staurothele pallidipora P. M. McCarthy, Muelleria 8, 275 (1995) as ‘pallidopora’; type: Australia, Queensland, P. M. McCarthy 768 (MEL—holotype; BRI—isotype [non vidi]).
Prothallus not seen. Thallus well developed, 50–195 μm thick, non-gelatinous, pale grey-green; cracks more or less numerous in mature thalli, but not delimiting discrete areoles; thallus cells in vertical columns, with numerous air spaces between cells; upper surface a pseudocortex, pigment absent; with a dark basal layer formed by confluent involucrella.
Perithecia almost completely immersed in thallus, not forming mounds, or the mounds very low and poorly delimited; at first only the pale brown ostiolar region visible, later apex visible as a more or less plane black disc up to 180 μm diam.; ostiolar region visible as a pale dot 20–40 μm diam. Involucrellum conical, densely pigmented at surface, less densely below; upper surface sometimes rough; cells in lower part of involucrellum with pigment confined to cell wall (cell outlines easily visible in thin section), containing large oil drops. Centrum c. 260 μm diam. (one measurement). Periphyses short, c. 20 μm. Hymenial algal cells rod-shaped, 3.7–6.6 × 2.1 μm, 1.8–3.1 times as long as wide. Asci 8-spored. Ascospores colourless, 20.5–21.6–22.5(–23) × 9.5–10.3–11 μm, 2.0–2.1–2.2(–2.3) times as long as wide [7/1], muriform, with c. 16–21 cells visible in optical section.
Pycnidia not seen.
Ecology and distribution
One collection from a rock beside a stream. Reported from Vietnam (Gueidan et al. Reference Gueidan, Van Do and Lu2014) and Australia (McCarthy Reference McCarthy1995a).
Notes
Important characters in the specimen from Nepal include the pale thallus (pigment apparently absent even in unshaded situation) which shows few cracks, the immersed perithecia and rod-shaped hymenial algae. The single specimen forms a well-supported clade with three specimens of W. pallidipora from Vietnam and two from Australia (Fig. 1). Three additional sequences from Vietnam were named W. pallidipora s. lat. by Gueidan et al. (Reference Gueidan, Van Do and Lu2014), and these appear to represent a distinct species. The single specimen from Nepal agrees well with the protologue of W. pallidipora, including the cracked and pale but not strongly areolate thallus; the absence of pigment on the sides of the thallus cracks is not mentioned by McCarthy (Reference McCarthy1995a) but is suggested by his fig. 2B. Willeya malayensis (Zahlbr.) Gueidan, Staurothele paraguayensis Malme, Willeya japonica and W. microlepis differ in the globose hymenial algae; W. rimosa Müll. Arg. in the projecting perithecia; W. laevigata Gueidan and W. protrudens differ in ITS sequence (Gueidan et al. Reference Gueidan, Van Do and Lu2014). Willeya australis (Groenh.) Gueidan from Java appears to be very similar in morphology (Gueidan et al. Reference Gueidan, Van Do and Lu2014).
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, c. 1 km north-west of Ghandruk, Chharnami stream, 28°22.92ʹN, 83°47.93ʹE, alt. 1975 m, on rock by stream, unshaded, 2009, Orange & Chhetri 18478 (NMW.C.2012.002.138).
Willeya Species A
Prothallus not seen (no free thallus margin present). Thallus well developed, 100–260 μm thick, non-gelatinous, very pale grey-brown, cracks numerous and extensive but not delimiting discrete areoles; sides of areoles not pigmented except adjacent to the dark basal layer of thallus, which is absent in young areas of thallus, becoming visible in older thallus areas as a result of confluent involucrella; thallus cells in weakly defined vertical columns, with numerous air spaces between cells; upper surface a non-pigmented pseudocortex; lower parts of thallus usually with a dark basal layer.
Perithecia completely immersed in thallus, not forming mounds, at first visible only as a pale brown disc c. 100 μm diam., later the apex sometimes exposed as a plane black disc up to 180 μm diam.; ostiolar region comprising a small pale depression c. 40 μm diam. surrounded by a pale brown circle to 100 μm wide. Involucrellum scarcely developed in the upper half of the exciple, hardly spreading, merging with the dark basal layer when present, densely pigmented at the surface, weakly and patchily pigmented below. Centrum 180–230 μm diam. Exciple colourless or brown on the outside. Periphyses c. 18–33 μm long, enclosed in a common gel. Hymenial algal cells oblong, 5–9 × 2.5 μm, 2–3.7 times as long as wide, often dividing by a transverse septum, or forming short chains of several cells. Asci 8-spored, c. 74 × 26 μm. Ascospores immature.
Conidiomata not seen.
Ecology and distribution
A single collection from shaded siliceous rock.
Notes
Important features include the well-developed, pale, extensively cracked but non- areolate thallus, the completely immersed perithecia and the oblong hymenial algae. Unfortunately, the ascospores are immature. It is not possible to identify this with any previously described species; amongst those with oblong to elongate hymenial algae, Staurothele fauriei B. de Lesd., S. paraguayensis, Willeya malayensis and W. rimosa differ in the more or less emergent perithecia (Gueidan et al. Reference Gueidan, Van Do and Lu2014). Willeya iwatsukii (H. Harada) Gueidan agrees in the immersed perithecia and the in-part bacilliform hymenial algae (Harada Reference Harada1992a), but these features are not distinctive enough to allow an identification in the absence of sequence data. Willeya fusca Gueidan, W. laevigata and W. pallidipora differ in ITS sequence (Fig. 1). The type specimen of W. australis is similar in the immersed perithecia and elongate hymenial algae but differs in the more strongly areolate, grey-brown thallus and the pigmented sides to the areoles.
Specimen examined
Nepal: Gandaki Pradesh: Kaski District, south-east of Tolka, Bhedi Kharka, 28.33616°N, 83.8302°E, alt. 1890 m, on small boulder on slope by platform, by path in forest, shaded, 2009, Orange & Chhetri 18554 (NMW.C.2012.002.140).
Key to Verrucariaceae reported from Nepal
The records of Verrucaria acrotella Ach. and V. margacea (Wahlenb.) Wahlenb. published by Rai et al. (Reference Rai, Nag, Khare, Upreti and Gupta2016) are not included in the key because the descriptions provided are insufficient to characterize these collections.
1 Thallus foliose or squamulose ……… 2
Thallus crustose ……… 11
2(1) Thallus foliose, attached by usually one central holdfast, underside smooth or with rhizinomorphs ……… 3
Thallus squamulose, underside attached by its underside or by rhizohyphae ……… 5
3(2) Lower surface smooth ……… Dermatocarpon miniatum
Lower surface with rhizinomorphs ……… 4
4(3) Rhizinomorphs coralloid, with dense, congested branches ……… Dermatocarpon vellereum
Rhizinomorphs simple to weakly branched, pointed or thickened at apex ……… Dermatocarpon moulinsii (Mont.) Zahlbr.
5(2) Thallus of small grey-green squamules up to 1 mm long, lacking rhizohyphae; perithecia amongst the squamules, not immersed in thallus; hymenial algae absent; ascospores muriform, (42–)57–120(–150) μm long ……… Agonimia tristicula
Thallus of squamules that are sometimes attached by rhizohyphae; perithecia immersed in the thallus ……… 6
6(5) Ascospores muriform, hymenial algae present ……… 7
Ascospores simple or 1-septate, hymenial algae absent ……… 8
7(6) Ascospores 45–59 μm long, (4?–)8 per ascus ……… Nesothele globosa
Ascospores 24–30 μm long, 2 per ascus ……… Endocarpon spp.
8(6) Ascospores 1-septate ……… Placidiopsis pseudocinerea Breuss
Ascospores simple ……… 9
9(8) Rhizohyphae colourless ……… Placidium squamulosum (Ach.) Breuss
Rhizohyphae dark ……… 10
10(9) Lower cortex present, paraplectenchymatous, blackish; with blackish rhizohyphae; squamules usually white-pruinose……… ……… Catapyrenium cinereum (Pers.) Körb.
Lower cortex absent, medulla merging into dark rhizohyphae; squamules not or weakly pruinose ……… Catapyrenium daedaleum (Kremp.) Stein.
11(1) Ascospores 9.5–11.5 μm long, simple; thallus with embedded black punctae that can be visible at the surface ……… Hydropunctaria rheitrophila
Ascospores longer; thallus without punctae ……… 12
12(11) Ascospores simple; hymenial algae absent ……… 13
Ascospores septate or muriform (or ascospores apparently simple, but hymenial algae present) ……… 21
13(12) Thallus with small soralia; thallus thin (to 105 μm) with few or no cracks; perithecia forming moderately projecting mounds 200–300 μm diam., mostly without a thalline covering; ascospores 18.5–24.5 μm long ……… Verrucaria craterigera
Thallus without soralia ……… 14
14(13) Thallus of areoles arising singly on a dark prothallus, later discrete or contiguous, narrowed at base; perithecia forming convex mounds without a thalline covering, but sometimes surrounded by areoles ……… Verrucaria parvipeltata
Thallus continuous or cracked, areoles not narrowed at base ……… 15
15(14) Thallus brown, divided into areoles with an uneven surface; perithecia forming moderately projecting mounds 240–500 μm diam., at most with thin patches of thallus in lower half, mostly naked ……… Verrucaria senta
Thallus various; perithecia immersed, or forming projecting mounds with a thalline covering to near apex ……… 16
16(15) Thallus very thin, to 30 μm thick, sometimes translucent when fresh and wet, uncracked; perithecia forming low to rather strong projections, at first with a thin thalline covering; involucrellum conical ……… Verrucaria elaeomelaena, V. hydrophila, Verrucaria Species A
Note: these three taxa are not closely related, but they can look very similar. Verrucaria elaeomelaena tends to have larger perithecia and ascospores than V. hydrophila but material is too sparse and ascospores often too poorly developed for a meaningful comparison.
Thallus thicker, not translucent when fresh and wet, cracks sparse to numerous ……… 17
17(16) Perithecia forming low to moderately projecting mounds more or less covered to the apex by the thallus; ascospores (23.5–)27–43 μm long; pycnidia numerous ……… Verrucaria bella
Perithecia immersed in the thallus, forming at most very low and indistinct projections ……… 18
18(17) Thallus extensively cracked, often into discrete areoles with pigmented sides; ascospores small, 13.5–16.5 μm long; pycnidia numerous ……… Verrucaria antepotens
Thallus extensively cracked or cracks sparse, sides of areoles pigmented or not; ascospores 18–33 μm long; pycnidia not seen ……… 19
19(18) Involucrellum irregular in shape; exciple well pigmented when mature, often darker than adjacent involucrellum; ascospores with perispore, dilute brown when overmature ……… Verrucaria luchunensis
Involucrellum more or less conical, typically dark at surface and paler below, often confluent with other involucrella; exciple colourless or only lightly pigmented; ascospores with or without perispore, not becoming brown with age ……… 20
20(19) Ascospores c. 18–20 μm long ((16.0–)18.0–25.0(–28.0) × (6.5–)7.0–10.0(–10.5) μm in material from Europe); perispore absent ……… Verrucaria praetermissa
Ascospores (23.5–)26.5–32(–33) μm long; perispore present ……… Verrucaria lactea
21(12) Ascospores 1- or 3-septate ……… 22
Ascospores muriform ……… 24
22(21) Ascospores 3-septate, 36–43 μm long; perithecial mounds 530–670 μm diam.; involucrellum thick, c. 75–100 μm in upper part ……… Thelidium papulare
Ascospores 1-septate, not exceeding 25 μm long ……… 23
23(22) Involucrellum appressed to exciple or slightly diverging at the base, sometimes the two scarcely discernible as separate structures; ascospores 21.5–25 μm long ……… Thelidium aff. minutulum
Involucrellum distinct, spreading from the exciple in lower part; ascospores 15.5–19.5 μm long ……… Thelidium uvidulum
24(21) Thallus comprising closely adnate squamules with rhizohyphae below; perithecia immersed in large thalline mounds 380–670 μm diam.; ascospores 45–59.5 μm long ……… Nesothele globosa
Thallus of discrete areoles or extensively cracked, never squamulose; perithecia variously immersed or forming projections; ascospores smaller ……… 25
25(24) Thallus of discrete convex areoles; perithecia projections without a thalline covering or with a few areoles below; ascospores 29.5–41 μm long ……… Nesothele glebulosa
Thallus a cracked crust but without discrete primary areoles; perithecia immersed or forming projection with a partial thalline covering below; ascospores often smaller ……… 26
26(25) Hymenial algal cells oblong to cylindrical, some at least 3 times as long as wide ……… 27
Hymenial algal cells broadly ellipsoid ……… 29
27(26) Ascospores (28.5–)29.5–36(–40) μm long (but measured in poor condition); thallus cracks with black sides; pycnidia present ……… Willeya irrigata
Ascospores 19–29 μm long; pycnidia not seen ……… 28
28(27) Perithecia forming conical-hemispherical projections; black basal thallus layer absent ……… Willeya eminens
Perithecia immersed in thallus, not forming projections ……… Willeya pallidipora and Willeya Species A Note: these two species are similar in the pale, weakly cracked thallus.
29(26) Ascospores c. 32–38 μm long; pycnidia present ……… Willeya honghensis Ascospores 19.5–25 μm long; pycnidia not seen ……… Willeya cf. japonica and W. nepalensis
Note: these two species are distinct in ITS sequence but no consistent morphological differences can be found in the sparse material studied.
Discussion
Although most of the material documented in this paper has come from a single collecting trip, the results are a significant advance in our knowledge of the family Verrucariaceae in Nepal. However, there is little doubt that the taxa described here are only a fraction of the diversity present in Nepal, especially since collecting for the present study was confined to moderate altitudes due to unseasonably wet weather experienced during the expedition. Unfortunately, habitats above the forest zone were not sampled.
The small number of collections available for each taxon means that morphological and genetic variability cannot be fully determined. Species of Verrucariaceae are often difficult to distinguish, being separated by subtle and overlapping characters. This makes it particularly difficult to match Nepalese collections with previously published species, as there is little sequenced material available from the region. In this study, some collections have been identified as previously known taxa because of either ITS sequences or clear-cut morphological features. In some instances, although morphology might suggest an identification, ITS sequences show differences from previously published sequences, indicating that more than one taxon may be present (for example with Hydropunctaria rheitrophila). Reassessment of species complexes, including Verrucaria elaeomelaena and V. hydrophila, would certainly benefit from a geographically broader study incorporating additional gene regions. Where it has not been possible to identify a collection with total confidence, such taxa have mostly been described here as new, since the only alternative would have been an unhelpful list of unidentified collections. All newly published taxa are associated with an ITS sequence which provides a reliable, though not infallible, way of fixing the application of a name in the future. Although it is likely that most of the species can be reliably identified by morphological characters, the limited material available means that the extent of variation in each species is unknown. Due to the frequently subtle differences between species, DNA sequencing is recommended in floristic surveys of Verrucariaceae in other underexplored regions. Indeed, if sequencing is currently impractical, freezing of some material for future study might be helpful.
Acknowledgements
We would like to gratefully acknowledge permission to collect specimens, granted by the Department of National Parks and Wildlife Conservation (DNPWC), Ministry of Forests and Environment, Government of Nepal. Hem Sagar Baral is warmly thanked for his assistance in obtaining an export licence and other matters.
Author ORCID
Alan Orange, 0000-0002-3181-5435.
Competing interests
The authors declare none.


















