Introduction
The lichen family Umbilicariaceae was long considered to be composed of two genera, Umbilicaria Hoffm. and Lasallia Mérat. Bendiksby & Timdal (Reference Bendiksby and Timdal2013) extended the family circumscription by including the two new genera Xylopsora Bendiksby & Timdal and Fulgidea Bendiksby & Timdal, and Davydov et al. (Reference Davydov, Peršoh and Rambold2017) reduced Lasallia to a subgenus of Umbilicaria and suggested seven additional subgenera. Umbilicariaceae belongs to Umbilicariales (Zhou & Wei Reference Zhou and Wei2007), within the recently established subclass Umbilicariomycetidae (Bendiksby & Timdal Reference Bendiksby and Timdal2013), which, according to a recent molecular clock analysis (Prieto & Wedin Reference Prieto and Wedin2013), branched off from the main lineages of Lecanoromycetes prior to the major diversification of the latter.
The Eastern Asiatic region is known as one of the diversity and endemism hotspots of the world and is the richest floristic region within the Holarctic (Takhtajan Reference Takhtajan and Crovello1986; Wu & Wu Reference Wu, Wu, Zhang and Wu1996; Kamelin Reference Kamelin2017), as well as one of the centres of species diversity and endemism of Umbilicaria (Wei & Jiang Reference Wei and Jiang1993).
Umbilicaria subg. Papillophora Davydov et al. is phylogenetically the most basal subgenus and includes species with a mostly Holarctic distribution and European, East Asian and South American endemic elements (Davydov et al. Reference Davydov, Peršoh and Rambold2017, Reference Davydov, Ahti and Sennikov2020). Four species of Umbilicaria subg. Papillophora are endemic to East Asia: U. koidzumii Yasuda ex Satô, U. loboperipherica J. C. Wei et al., U. pulvinaria (Savicz) Frey and U. squamosa J. C. Wei & Y. M. Jiang (Wei & Jiang Reference Wei and Jiang1993; Wei et al. Reference Wei, Jiang and Guo1996; Davydov et al. Reference Davydov, Tchabanenko, Makryi and Khanin2011b; Moon Reference Moon2013; Davydov Reference Davydov, Andreev and Himelbrant2017; Ohmura & Kashiwadani Reference Ohmura and Kashiwadani2018). However, distribution patterns of lichens are usually wider than those of vascular plants (see e.g. Feuerer & Hawksworth (Reference Feuerer and Hawksworth2007) and references in this paper; Feuerer & Höhne Reference Feuerer and Höhne2017), and many East Asian species also occur in the South Siberian mountains at the northern edges of their ranges (Urbanavichus Reference Urbanavichus2011) and Tibet in the south (Wei Reference Wei1991). The new species described here is represented by such a distribution pattern.
Ten species of the Umbilicaria vellea group are currently accepted for north-eastern Asia. Wei & Jiang (Reference Wei and Jiang1993) monographed Umbilicariaceae for East Asia and accepted nine species. One more species, U. loboperipherica, was described later (Wei et al. Reference Wei, Jiang and Guo1996). During herbarium work with material from East Asia, our attention was attracted to an Umbilicaria resembling U. vellea (L.) Ach. but with a deviating morphology. The population of this species was found during our fieldwork in 2014 in the Russian Far East. Both morphological and molecular studies confirmed its status as a new species, described here as Umbilicaria orientalis.
Materials and Methods
Sampling
Fresh material of the putative new species was collected by the authors and deposited in the herbaria ALTB, LE, UUH, and in the private herbarium of Davydov and Yakovchenko. Additionally, specimens were studied from the herbaria GZU and HMAS. Specimens of Umbilicaria americana Poelt & T. H. Nash, which has morphological similarities with U. orientalis, were obtained from the collection of B. McCune (Table 1).
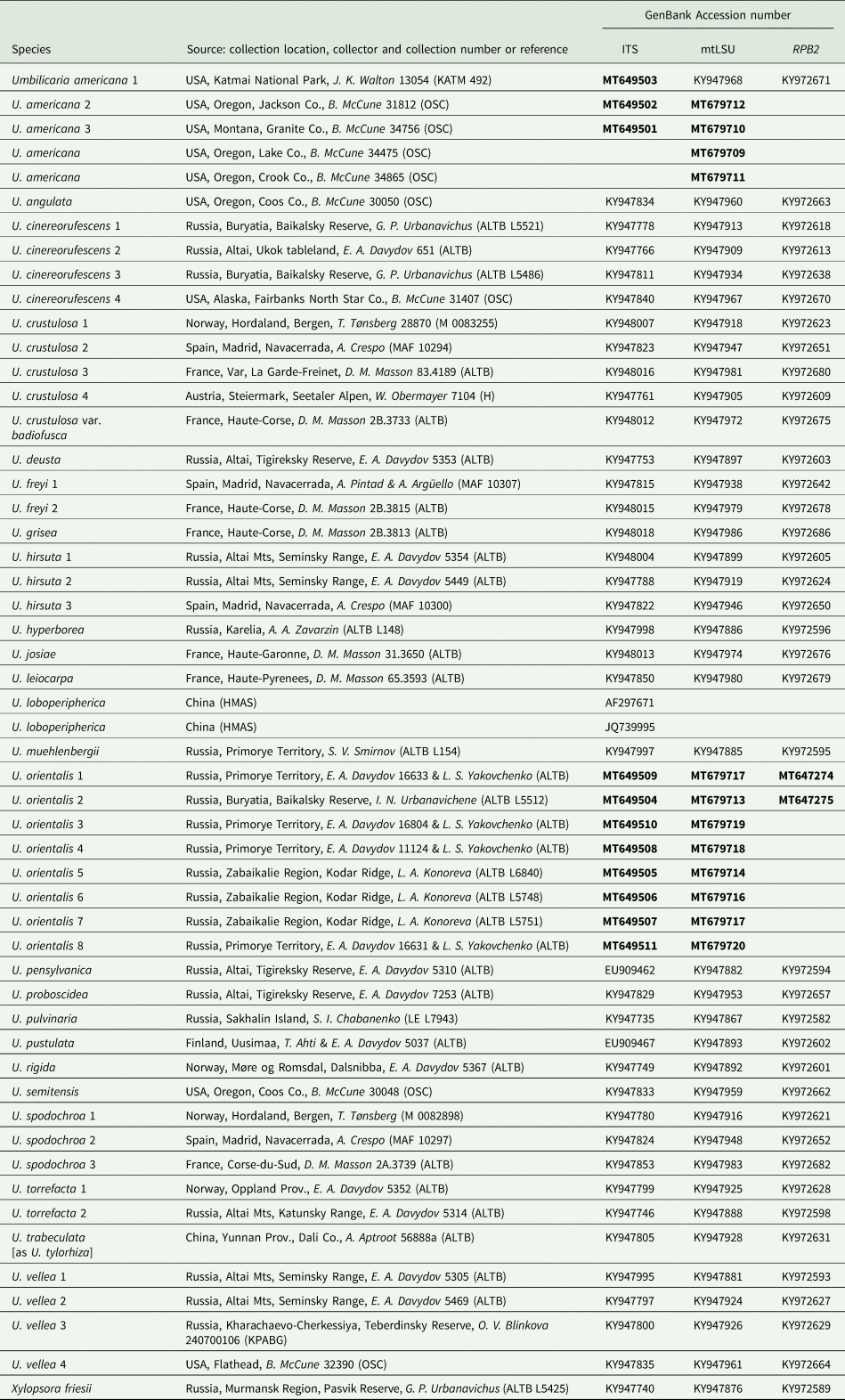
Table 1. Umbilicaria species, sample information and GenBank Accession numbers for the phylogenetic analyses (Figs 1 & 2). Sequences newly generated for this study are indicated in bold and the remaining sequences are from Davydov et al. (Reference Davydov, Peršoh and Rambold2017) and GenBank. Xylopsora friesii is included as the outgroup.
Morphology and anatomy
Morphological observations were made using a dissecting microscope. Cross-sections were cut by hand with a razor blade and observed in water mounts. Measurements are presented as follows: (smallest value recorded–) (x̅ – SD) − x̅ − (x̅ + SD) (–largest value recorded), where x̅ is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values are given to the nearest 0.5 μm and the sample mean to the nearest 0.1 μm.
Chemical analyses
Secondary products were analyzed by applying standard thin-layer chromatography techniques (Culberson & Kristinsson Reference Culberson and Kristinsson1970) using solvents A, B and C (Culberson & Johnson Reference Culberson and Johnson1982). The high-performance liquid chromatography (HPLC) analysis was performed as described in Davydov et al. (Reference Davydov, Blum, Kashevarov and Grakhov2019a).
DNA extraction, amplification and sequencing
Single thallus parts (100–200 mg) or 3–4 apothecia were carefully checked for fungal infections and thoroughly cleaned of extraneous matter. DNA extraction, amplification and sequencing followed the methods of Davydov & Yakovchenko (Reference Davydov and Yakovchenko2017) and those outlined in Table 2. The program Geneious 6.0 (Biomatters Ltd., New Zealand) was used for assembling partial and complementary sequences. Consensus sequences were exclusively compiled from double-stranded parts of the sequences.
Table 2. Summary statistics, PCR settings and substitution models used for the different datasets. Analyses shown were performed with RAxML (Stamatakis Reference Stamatakis2014) and MrBayes (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). ASD = average standard deviation.

Sequence alignment and phylogenetic analyses
All obtained sequences of the putative new species were supplemented with sequences obtained during a comprehensive study of Umbilicariaceae phylogeny (Davydov et al. Reference Davydov, Peršoh and Rambold2017), representing different subgenera with an emphasis on Umbilicaria subg. Papillophora; Xylopsora friesii (Ach.) Bendiksby & Timdal was used as the outgroup. This selection is based on the studies of Wedin et al. (Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005), Bendiksby & Timdal (Reference Bendiksby and Timdal2013) and Davydov et al. (Reference Davydov, Peršoh and Rambold2017), in which Xylopsora forms the sister clade to Umbilicaria. GenBank Accession numbers are provided in Table 1. The sequences were aligned in Geneious 6.0 (Biomatters Ltd., New Zealand) using the MUSCLE algorithm (Edgar Reference Edgar2004) and visible deviations in position homology were then manually optimized.
Three single-gene datasets were assembled for this study: the internal transcribed spacer region of nuclear ribosomal DNA (ITS), the large subunit of the mitochondrial ribosomal DNA (mtLSU), and RNA polymerase II between six and seven conserved parts (RPB2). Phylogenetic analyses were performed as described by Davydov et al. (Reference Davydov, Peršoh and Rambold2017). Summary statistics, PCR settings and substitution models used for the different datasets are summarized in Table 2. The most likely tree and 1000 rapid bootstrap replicates were calculated using RAxML 8.0.26 (Stamatakis Reference Stamatakis2014) implemented in raxmlGUI software v.1.3.1 (Silvestro & Michalak Reference Silvestro and Michalak2012). Bayesian inference with the Markov chain Monte Carlo (BMCMC) method (Larget & Shimon Reference Larget and Shimon1999) was performed using MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). Three parallel analyses, each with six incrementally heated chains using the default heating factor of 0.2, were run for 40 million generations and every 200th generation was sampled until the average standard deviation (ASD) of split frequencies had dropped to 0.001. Initially we set ASD at 0.01 but the calculation stopped after c. 0.2–0.8 million generations; therefore, the number of sampled trees after burn-in was not enough to calculate the relevant consensus tree. The first 40% of trees was discarded as burn-in and a 50% majority-rule consensus tree was calculated from the remaining trees of the three runs, with the sumt command implemented in MrBayes 3.2.3.
For combining the nrITS, mtLSU and RPB2 datasets, alignments were trimmed to include only those specimens for which we had information on all markers. We tested trimmed ITS, mtLSU and RPB2 datasets for topological incongruence by studying single-gene maximum likelihood consensus trees (not shown) from separate RAxML analyses. There were no well-supported (bootstrap (BS) ≥ 70%) incongruences and so we concatenated the datasets into a combined dataset. As phylograms were similar regarding well-supported clades and lacking conflicts, all sequences were combined into 2-marker and 3-marker matrices and used for RAxML and Bayesian analyses. The optimal substitution model was inferred initially assuming seven independent subsets (ITS1, 5.8S, ITS2, mtLSU and RPB2 1st, 2nd and 3rd codon positions) using PartitionFinder v.1.1.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012). Phylogenetic trees were visualized in FigTree v.1.4.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Results
The phylogenetic study
For the phylogenetic analyses, we used original sequences obtained during this study, as well as those obtained in earlier investigations (Davydov et al. Reference Davydov, Peršoh and Rambold2017) and deposited in GenBank (Table 1). To test the monophyly and phylogenetic relationships of species, three markers were used as single-gene matrices and in combined datasets. Summary statistics are provided in Table 2. ITS and mtLSU sequences were successfully obtained from six specimens and ITS, mtLSU and RPB2 for two specimens of the putative new species, described below as Umbilicaria orientalis. The set of sequences of U. orientalis and U. americana were incomplete regarding the phylogenetic markers (Table 1), therefore we present two phylograms: one for ITS + mtLSU and one for ITS + mtLSU + RPB2 datasets. Since the Bayesian 50% majority-rule consensus tree had the same topology as that of RAxML, both phylograms are combined in Figs 1 and 2.

Fig. 1. Phylogenetic relationships amongst Umbilicaria species, based on a maximum likelihood analysis (RaxML; Stamatakis Reference Stamatakis2014) of ITS1-5.8S-ITS2, mtLSU, and RPB2. The reliability of each branch was tested by maximum likelihood (ML) and Bayesian methods. Numbers at tree nodes indicate bootstrap values of ML (left) and Bayesian inference with the Markov chain Monte Carlo (BMCMC) posterior probabilities (right). Thicker branches indicate when both the bootstrap value of ML is ≥ 70% and the BMCMC posterior probability is ≥ 0.95. GenBank Accession numbers are given in Table 1. Branch lengths represent the estimated number of substitutions per site assuming the respective models of substitution. An exception is the branch with a black dot, which was shortened to reduce the overall figure size. In colour online.

Fig. 2. Phylogenetic relationships amongst Umbilicaria species, based on a maximum likelihood analysis (RAxML; Stamatakis Reference Stamatakis2014) of ITS1-5.8S-ITS2 and mtLSU. The topology results from the RAxML analysis. The reliability of each branch was tested by maximum likelihood (ML) and Bayesian methods. Numbers at tree nodes indicate bootstrap values of ML (left) and Bayesian inference with the Markov chain Monte Carlo (BMCMC) posterior probabilities (right). Thicker branches indicate when both the bootstrap value of ML is ≥ 70% and the BMCMC posterior probability is ≥ 0.95. GenBank Accession numbers are given in Table 1. Branch lengths represent the estimated number of substitutions per site assuming the respective models of substitution. An exception is the branch with a black dot, which was shortened to reduce the overall figure size. In colour online.
Both Umbilicaria orientalis and U. americana clustered within Umbilicaria subg. Papillophora (RAxML: 100%; BS MrBayes: 1.0 posterior probability (PP)). Sequences of U. orientalis combined in a well-supported clade (100% BS; 1.0 PP) in all single marker (not shown) and combined phylograms (Figs 1 & 2). Within this clade, sequences from the Russian Far East separated from sequences from South Siberia with high support (87% BS; 0.98 PP; Fig. 2). In the three-marker phylogeny, sequences of Umbilicaria orientalis clustered with the sequence of U. trabeculata Frey & Poelt in a supported clade (88% BS; 1.00 PP; Fig. 1).
Sequences of Umbilicaria americana clustered in a well-supported clade (100% BS; 1.0 PP; Fig. 2) within the Umbilicaria subg. Papillophora clade. Umbilicaria americana clustered as the sister clade (Fig. 2) or sister branch (Fig. 1) to U. torrefacta (Lightf.) Schrad., but without statistical support. Two ITS sequences of U. loboperipherica clustered as the sister clade to U. crustulosa in an ITS phylogram (not shown).
Taxonomy
Umbilicaria orientalis Davydov sp. nov.
MycoBank No.: MB821426
Umbilicaria orientalis Davydov, in Andreev and Himelbrant (eds), Fl. Lich. Ross., 78 (2017), nom. nud.
Umbilicaria orientalis is similar to U. vellea but differs in the morphology of the rhizinomorphs and thalloconidia. Its rhizinomorphs are simple, cylindrical or strap-like. Its thalloconidia develop both on the lower surface and on the rhizinomorphs, and are 1–2 (rarely 4)-celled or in aggregates of up to 5–6 cells.
Type: Russia, Primorye Territory, Lazovsky District, 21.5 km NE of Lazo, Zov Tigra National Park, at ‘Zuby drakona’, Betula ermanii forest, 43°30′46″N, 134°05′27″E, elev. 1400 m, on siliceous rock outcrops, 20 May 2014, E. A. Davydov (11124) and L. S. Yakovchenko (LE-L13181—holotype; ALTB, GZU, TNS—isotypes). GenBank Accession nos: MT649508, MT679718.

Fig. 3. A–G, Umbilicaria orientalis. A, holotype (LE-L13181), upper surface. B, holotype, lower surface. C, juvenile specimen (Davydov 16804 & Yakovchenko), upper surface. D, lower surface without thalloconidia. E, holotype, gyrose apothecia. F, holotype, cylindrical and strap-like rhizinomorphs at the periphery of the thallus. G, holotype, small thalli developing on the periphery of the upper surface. Scales: A & B = 1 cm; C & D = 5 mm; E & G = 1 mm; F = 2 mm. In colour online.

Fig. 4. A–C, Umbilicaria orientalis, holotype (LE-L13181). A, thallus cross-section. B, lower cortex producing thalloconidia. C, mature thalloconidia. Scales: A = 50 μm; B & C = 10 μm. In colour online.
Thallus of medium size, 3–6(–12) cm diam. and 0.2–0.3 mm thick, umbilicate, monophyllous, rigid, undulating with broad folds, the margins first entire, later incised; upper surface dull, minutely rimose to areolate; pale to dark grey, sometimes with a brown or violet tinge, often pruinose; lower surface of juvenile thalli light brown, later black, lighter towards the margins, smooth to slightly rimose with abundant rhizinomorphs; trabeculae emerging from the umbilicus often present in radius c. 1 cm; rhizinomorphs cylindrical unbranched or once branched, sabre-shaped; also strap-like rhizinomorphs often developing both at the central part of the thallus, derived from the trabeculae, often long and attached to the lower surface in several places and bearing secondary cylindrical rhizinomorphs, and at the periphery where they are perpendicular to the lower surface and similar in size to cylindrical ones; rhizinomorphs sometimes on the upper surface along holes, cracks or around apothecia; thalloconidia usually present and developed on the lower surface and at the base of rhizinomorphs, making them appear thicker and entirely black up to 1/3–2/3 of their length; sometimes also developing on strap-like rhizinomorphs; 1–2 (rarely 4)-celled or in aggregates of up to 5–6 cells, with a single cell (5.0–)6.0–6.5(–8.0) × (5.0–)6.0–6.5 (–7.5) μm (Fig. 4). Upper cortex paraplectenchymatous, brownish at the outer part and hyaline in the inner part, 10–14 μm thick, epicortex 5–10 μm thick, algal layer continuous, 38–65 μm thick, algal cells trebouxioid; medulla colourless, more or less dense, 45–75 μm thick; lower cortex colourless, scleroplectenchymatous, 31–45 μm thick, including a colourless inner layer and a brown outer layer transforming into thalloconidia (Fig. 4A & B).
Apothecia sometimes occurring at the periphery of thalli, (0.8–)1.1–1.3–1.4(–1.8) mm diam., sessile, gyrose; epihymenium brown, 12.5–15.0 μm thick; hymenium hyaline 45–55 μm thick, hypothecium light brown, 35–45 μm thick; excipulum in the inner part yellowish, in the outer part brown; paraphyses septate, branched, 1.8 μm wide, sometimes slightly thickened at the tips, up to 1.8–2.0 μm wide; asci 40–45 × 11–13 μm, 8-spored; ascospores hyaline, simple, only immature spores seen.
Pycnidia common at the periphery of the thalli, 120–150 μm diam., with black prominent ostiole; wall brown, 10 μm thick; pycnoconidia bacilliform, 3.5–4.5 × 1 μm.
Chemistry
Thallus K−, C+ red, KC+ red, Pd−, UV−; gyrophoric and lecanoric acids, and sometimes also crustinic acid detectable by TLC. All specimens examined have been shown to contain gyrophoric acid as major and lecanoric acid as minor lichen compounds. The holotype contains a trace of crustinic acid detected only by HPLC, while one specimen from Tibet (W. Obermayer 8353, GZU) contained crustinic acid in a higher concentration than lecanoric acid and was detected both by TLC and HPLC.
Etymology
The name refers to the geographical distribution of the species (i.e. East Asia).
Ecology
Umbilicaria orientalis grows mostly on steep siliceous rocks of the upper mountain belt in an elevation range of 1000–1900 m in South Siberia, 500–1700 m in Far East Russia and 2000–4650 m in southern China. It occurs in wet conditions but sheltered from direct precipitation. In Siberia, U. orientalis was also collected mostly on wet rock: within stonefields over subsurface rivers, on mossy boulders at the lakeshore, and on rocks by flowing water.
Distribution
Umbilicaria orientalis is currently known from a wide range of localities in East Asia from the Far East to South Siberia and Mongolia in the north, to Hebei and Tibet in the south (Fig. 5).

Fig. 5. Known distribution of Umbilicaria orientalis. Large red circles = sampling locations; small black circles = major cities. In colour online.
Additional material examined
Russia: Primorye Territory: Zov Tigra National Park, 43°30′39″N, 134°05′16″E, elev. 1300 m, 2014, E. A. Davydov 16222 & L. S. Yakovchenko (hb. Davydov & Yakovchenko); Oblachnaya Mt, 43°40′35″N, 134°11′59″E, elev. 1620–1650 m, 2014, E. A. Davydov 14361 & L. S. Yakovchenko (hb. Davydov & Yakovchenko); 50 km WNW of Amgu settlement, 46°01′54″N, 137°06′58″E, elev. 495 m, 2014, E. A. Davydov 16737 & L. S. Yakovchenko (hb. Davydov & Yakovchenko); ibid., 46°02′00″N,137°06′44″E, elev. 510 m, 2014, E. A. Davydov 16732 & L. S. Yakovchenko (hb. Davydov & Yakovchenko). Republic of Buryatia: Kurumkansky District, Dzherginsky Nature Reserve, Yuzhno-Muisky Range, Lake Balan-Tamur, 55°13′42″N, 111°41′57.1″E, elev. 1308 m, W slope, 2013, T. M. Kharpukhaeva (UUH L1243, ALTB L5480); Kabansky District, headwaters of Pereemnaya River near Lake Chernoye, 51°22′35″N, 105°13′30″E, elev. 1086 m, 2014, I. N. Urbanavichene s. n. (LE, ALTB L5512). Zabaykalsky Krai: Kodar Ridge, 56°54′55.6″N, 117°37′40.7″E, elev. 1667 m, 2013, L. A. Konoreva (LE, ALTB L5748); ibid., 56°54′49.7″N, 117°36′51.4″E, alt. 1877 m, 2014, L. A. Konoreva (LE, ALTB L5751); ibid., 57°04′47.9″N, 118°01′59.1″E, elev. 1521 m, 2016, L. A. Konoreva (LE, ALTB 6782).—Mongolia: NW, basin of Dzhargalanta River [Dzhargalant-Gol], Uber-Dzhargalanta River [Uver-Dzhargalant-Gol] between headwaters and Agit Mt., 47°N,104–105°E, 1925, I. M Krascheninnikov & B. Zamatkinov (LE L6618, dupl.: ALTB L5581).—China: Sichuan: Tibet, 29°33′05″N, 100°17′25″E, elev. 4330 m, 2000, W. Obermayer 8353 (GZU); ibid., 29°31′02″N, 100°16′21″E, elev. 4650 m, 2000, W. Obermayer 9721 (GZU). Hebei: Xinglong County, 40.36°N, 117.29°E, elev. 2000 m, 1998, J. C. Wei 84297 & Y. M. Jiang (HMAS L84294, L84297).
Discussion
Phylogenetic position
The phylogenetic position of Umbilicaria orientalis within Umbilicaria subg. Papillophora was first assumed a priori based on morphological data, and then confirmed by molecular phylogenetic analyses; subsequent selection of sequences therefore emphasized this subgenus. Umbilicaria orientalis occurs in the U. vellea group and fully corresponds to this group phenotypically by having a medium-sized to large, rigid grey thallus with a smooth to areolate, never pustulate or reticulate upper thallus surface, and a lower surface with trabeculae emerging from the umbilicus, the lower surface and rhizinomorphs being characteristically papillose or areolate, and rhizinomorphs bearing thalloconidia.
Umbilicaria orientalis clustered as sister to U. trabeculata. The number of different nucleotides in the ITS region is the smallest in our analysis (17 mismatches, 2 gaps). Umbilicaria trabeculata has capitate rhizinomorphs and multicellular thalloconidia. The two species overlap in distribution in South-East Asia (Poelt Reference Poelt1977; Wei & Jiang Reference Wei and Jiang1993). Umbilicaria loboperipherica, another East Asian species, clustered within the U. vellea group but apart from U. orientalis and U. trabeculata, and seems to be only distantly related.
Umbilicaria americana clustered apart from U. orientalis and the U. vellea group; however, it is morphologically and ecologically similar to the species of the U. vellea group and has (like U. orientalis) thalloconidia on the lower surface and rhizinomorphs. Umbilicaria americana is distributed in North America (Poelt & Nash Reference Poelt and Nash1993) and represents an independent phylogenetic lineage.
Diagnostic traits and variability
Poelt & Nash (Reference Poelt and Nash1993: 422) emphasized that the Umbilicariaceae mostly ‘consists of individuals that exhibit a series of developmental stages’. As in vascular plants, these ontogenetic stages can be roughly divided into juvenile, mature and senescent stages. Juvenile stages were examined at the locus classicus and presented thalli up to 3 cm in diameter with entire margins, a light grey smooth upper surface, and a light brown lower surface with simple, same-coloured rhizinomorphs lacking thalloconidia and apothecia. It was unclear if these specimens really belonged to U. orientalis, but this was confirmed by DNA evidence. Juvenile specimens might be confused with U. dendrophora (Poelt) Hestmark, but that species has thalloconidia at the tips of rhizinomorphs. Mature specimens resembled U. vellea but differed by lacking peg-like thalloconidial rhizinomorphs and multicellular thalloconidia. Umbilicaria orientalis usually lacks vegetative propagules; however, some specimens in the locus classicus bore small thalli developing on the periphery of the upper surface (Fig. 3G). We have not observed such miniature thalli in other parts of its distribution area; they could be non-specific regeneration lobes on deteriorating parts of the thallus. Peripheral vegetative propagules characterize many species in the Umbilicaria vellea group, including East Asian species such as U. hirsuta (Sw.) Ach., U. loboperipherica and U. squamosa. Umbilicaria squamosa has squamules developing into miniature thalli on the upper surface but, unlike U. orientalis, such squamules develop permanently; moreover, U. squamosa lacks thalloconidia and is usually sterile. Senescent specimens have an areolate-papillate black lower surface with a mess of different types of rhizinomorphs: unbranched, branched, flattened, ligulate or in various ways deformed, and thalloconidia are often not recognizable. Such thalli may resemble U. cinereorufescens (Schaer.) Frey or U. trabeculata. The latter species, however, is characterized by balls of multicellular thalloconidia on its rhizinomorphs, clearly differing from the thalloconidia of U. orientalis; furthermore, rhizinomorphs of U. orientalis are never capitate.
Umbilicaria orientalis is often fertile; the majority of specimens from the Russian Far East and Tibet possess apothecia, while specimens from South Siberia and Hebei were sterile. Fertility is a relatively rare trait in species of the Umbilicaria vellea group that produce thalloconidia (Davydov et al. Reference Davydov, Peršoh and Rambold2017). Although apothecia were found, mature ascospores were not observed in U. orientalis. Infertility could be explained by functional coupling of traits (for species having thalloconidia or lichenized propagules, the significance of propagation by ascospores is decreased). Another possible explanation is that spores are produced during a short season or sporadically as conditions allow (McCune et al. Reference McCune, Curtis and Meglio2017). Wei & Jiang (Reference Wei and Jiang1993: 182) similarly recognized only immature ascospores in fertile collections of the most closely related species, U. trabeculata from Yunnan (H-NYL 31523! as ‘U. tylorhiza’).
Many species of the Umbilicariaceae develop thalloconidia, which have been shown to be highly species-specific (Hasenhüttl & Poelt Reference Hasenhüttl and Poelt1978; Hestmark Reference Hestmark1990). Umbilicaria orientalis has 1–2 (rarely 4)-celled thalloconidia, but sometimes thalloconidia aggregate into clusters of up to 5–6 cells. In the U. vellea group, such thalloconidia may develop on the lower surface of U. vellea, especially in its juvenile stage, but multicellular thalloconidia soon develop on the bases of long rhizinomorphs as well as on short thalloconidial rhizinomorphs. Thalloconidia of U. orientalis develop on the lower surface and on rhizinomorphs. This pattern is also characteristic for U. americana. However, that species has multicellular thalloconidia similar to those in U. vellea. Moreover, rhizinomorphs of U. americana are covered by thalloconidia entirely, while those of U. orientalis are covered by thalloconidia up to 1/3–2/3 of their length, and the tips look smooth and pale (Fig. 3B, D & F).
Gyrophoric acid is the major and lecanoric acid is the minor secondary compound detected by TLC and HPLC in the majority of Umbilicaria species (Narui et al. Reference Narui, Culberson, Culberson, Johnson and Shibata1996). However, crustinic acid is characteristic for the U. vellea group. It can occur in major or minor quantities, or in traces within the same species, for example in Umbilicaria crustulosa (Ach.) Lamy (Posner et al. Reference Posner, Feige and Huneck1992; Huneck et al. Reference Huneck, Porzel, Schmidt, Feige and Posner1993; Seriña et al. Reference Seriña, Arroyo, Manrique and Sancho1996).
East Asian distribution pattern
Umbilicaria orientalis is distributed in mountain regions with relatively high precipitation, mostly in East Asia. The Eastern Asian region, in the sense of Takhtajan (Reference Takhtajan and Crovello1986), seems to be the main area of the species' distribution. In isolated localities in South Siberia and Mongolia, the species occurs in the subalpine belt of mountain ranges situated in the wettest climates, and under special historic-geographical conditions, namely the Kodar Range (Stanovoye Nagor'e Highlands), the Yuzhno-Muisky Range (north-eastern Baikal region) and the northern macroslope of the Khamar-Daban Range (south Baikal area). Recently we reported two East Asian species, Umbilicaria formosana Frey and U. kisovana (Zahlbr. ex Asahina) Zahlbr., from the Stanovoye Nagor'e Highlands (Davydov et al. Reference Davydov, Chesnokov, Konoreva and Andreev2019b); they were previously known exclusively from East Asia. There are only a small number of saxicolous lichen species with Far East Asian-East Siberian distributions, for example Boreoplaca ultrafrigida Timdal (Davydov & Wei Reference Davydov and Wei2009; Kharpukhaeva Reference Kharpukhaeva2010), Fuscidea submollis Mas. Inoue (Chesnokov et al. Reference Chesnokov, Konoreva and Andreev2017; Yakovchenko et al. Reference Yakovchenko, Davydov, Paukov and Ohmura2019) and Parmelia shinanoana Zahlbr. (Lishtva Reference Lishtva1998; Lishtva et al. Reference Lishtva, Himelbrant and Stepanchikova2013).
Lichens with an East Asian distribution range (Siberian-East Asian sensu Golubkova (Reference Golubkova1983)) comprise nemoral and boreal-montane species associated with floodplain and upland fringe biotopes. In the Baikal region those species are at the north-western boundaries of their distributions (Urbanavichus Reference Urbanavichus1997). Epiphytes predominate among lichens with Siberian-East Asian distributions: Hypogymnia bullata Rass., H. submundata (Oxner) Rass., Lobaria orientalis (Asahina) Yoshim. and Parmelia asiatica A. Crespo & Divakar (Urbanavichus Reference Urbanavichus1997; Lishtva et al. Reference Lishtva, Himelbrant and Stepanchikova2013). The Baikal region is also the north-western boundary for distributions of East Asian species of mosses and vascular plants (Bardunov Reference Bardunov1990). Plagiomnium acutum (Lindb.) T. J. Kop. is a species of moss which seems very close in its distribution and ecological preferences to Umbilicaria orientalis (Bardunov Reference Bardunov1990). At present the known East Asian distribution range of P. acutum has been confirmed by additional records and occurs in the north-east of the Baikal region, south of the Russian Far East, Mongolia and China (Koponen & Ignatova Reference Koponen, Ignatova and Ignatov2018). For such species as Cladonia kanewskii Oxner, Coccocarpia erythroxyli (Spreng.) Swinscow & Krog and Fuscopannaria ahlneri (P. M. Jørg.) P. M. Jørg., floodplain and lakeland-montane biotopes serve as a ‘migration path’ for these temperate oceanic lichen species (Ahti & Brodo Reference Ahti and Brodo1981; Urbanavichus Reference Urbanavichus1997; Urbanavichene & Urbanavichus Reference Urbanavichene and Urbanavichus1999, Reference Urbanavichene and Urbanavichus2009). Alternatively, one can treat the distribution of those species as remnants of previously more continuous hygro-mesophytic vegetation existing today in mountain regions of Eurasia within local refuges, distant from the ocean sectors.
Key to the Umbilicaria vellea group in north-eastern Asia
We propose this provisional key for identification of species of the Umbilicaria vellea group in north-eastern Asia, although some species require additional investigation to clarify their circumscription, and some to verify their occurrence in the region. Of these, Umbilicaria spodochroa Hoffm. is known by the single verified collection from Sichuan (China) distributed as exsiccatae more than a century ago (Elenkin Reference Elenkin1904; LE-L6063!) and not confirmed by more recent collections; other reports for China and Russia were re-identified (Wei & Jiang Reference Wei and Jiang1993; Davydov et al. Reference Davydov, Himelbrant and Stepanchikova2011a). However, U. crustulosa (Ach.) Lamy and U. grisea Hoffm. are also correctly reported from north-eastern Asia only once from Khangai (Mongolia), and occur here as a significant disjunct (Byazrov Reference Byazrov1986). We treat U. cirrosa Hoffm. as a synonym of U. vellea (Davydov et al. Reference Davydov, Ahti and Sennikov2020). Umbilicaria trabeculata, described from Himalaya (Poelt Reference Poelt1977), was proposed to be probably identical with U. cinereorufescens based on characteristics of the thalloconidia (Hestmark Reference Hestmark1990), but accepted as a separate species by Poelt & Nash (Reference Poelt and Nash1993) and synonymized with U. tylorhiza (Nyl.) Nyl. from the Kola Peninsula (Nylander Reference Nylander1866) by Wei & Jiang (Reference Wei and Jiang1993). Here we use the name Umbilicaria trabeculata since our sequence data indicates that specimens from Yunnan and the Kola Peninsula do not belong to U. cinereorufescens and are significantly different from each other. Therefore, it is more plausible that the specimen from Yunnan belongs to a species described from the Himalaya, namely U. trabeculata. Umbilicaria trabeculata differs from U. cinereorufescens mostly in the characteristics of the rhizinomorphs. However, this trait is variable and for clarification, more specimens with a range of morphologies are required for study. Molecular phylogenetic evidence is also required for Umbilicaria koidzumii Yasuda ex Satô, known from the type locality in Japan (Satô Reference Satô1935).
1 Lichenized propagula on upper surface present 2
Lichenized propagula on upper surface absent (thallyles on rhizinomorphs may develop) 5
2(1) Schizidia present 3
Parasoredia present 4
3(2) Schizidia long associated with the thallus, soon transforming into abundant squamules (i.e. miniature thalli with lower cortex and rhizinomorphs), over time covering all of the upper surface U. squamosa
Schizidia soon detached, developing mostly on periphery of thallusU. loboperipherica
4(2) Rhizinomorphs present, lower surface smooth to areolate, parasoredia clumping U. hirsuta
Rhizinomorphs absent or scarce, lower surface areolate to papillate, parasoredia not clumping, becoming shield-shaped U. grisea
5(1) Thalloconidia present 6
Thalloconidia absent 10
6(5) 1–2(–6)-celled thalloconidia developing on lower surface and rhizinomorphs U. orientalis
6–10 to 30–50-celled thalloconidia developing on rhizinomorphs 7
7(6) Lower surface of mature specimens possessing two different classes of rhizinomorphs: longer, pale, simple to branched and shorter, black, covered by thalloconidia 8
Rhizinomorphs only thalloconidial 9
8(7) Ascospores simple and hyaline. Longer rhizinomorphs simple to branched, pale, without thalloconidia; shorter rhizinomorphs tuberose to peg-like, black, covered by thalloconidia U. vellea
Ascospores muriform and brown. Longer rhizinomorphs branched, dark, often with balls of thalloconidia on tips; shorter rhizinomorphs branched to peg-like, black, covered by thalloconidia U. koidzumii
9(7) Rhizinomorphs very irregular, tuberose to peg-like, often flattened U. cinereorufescens
Rhizinomorphs scarce, separate, short and knob-like U. trabeculata
10(5) Lower surface and rhizinomorphs black to brown, areolate to papillate U. spodochroa
Lower surface white to dirty grey-brown, smooth to areolate U. crustulosa
Acknowledgements
We are grateful to Dr Christian Printzen for the opportunity to sequence a part of the material in his laboratory, to Prof. J. C. Wei (HMAS) and Prof. H. Mayrhofer (GZU) for their hospitality during the visit of ED to Beijing and Graz respectively, and to Prof. B. McCune (Oregon) for providing specimens of Umbilicaria americana, valuable comments and suggestions, and improvements to the text. The studies of LY and IU were carried out within the framework of the institutional research projects AAAA-A17-117062710098-4 and AAAA-A19-119020690077-4, respectively.
Author ORCIDs
Evgeny A. Davydov, 0002-2316-8506; Lidia S. Yakovchenko, 0000-0002-4342-7771; Irina Urbanavichene, 0000-0002-5492-5215; Liudmila Konoreva, 0000-0002-4487-5154; Sergey Chesnokov, 0000-0001-9466-4534; Tatiana Kharpukhaeva, 0000-0003-2213-3202; Walter Obermayer, 0000-0003-1674-1463.










