Introduction
Lichens have evolved similar morphologies often in rather distantly related lineages (Grube & Hawksworth Reference Grube and Hawksworth2007). Unsurprisingly, parallelism also occurs within more closely related groups, where the molecular results have changed the traditional views on relationships. For instance, molecular studies showed that the foliose genera of the Physciaceae are nested among the large crustose genus Rinodina (e.g. Grube & Arup Reference Grube and Arup2001; Helms et al. Reference Helms, Friedl and Rambold2003). Similarly, foliose and fruticose thallus shapes also arose within crustose Lecanora (e.g. Arup & Grube Reference Arup and Grube2000) and Caloplaca (Gaya et al. Reference Gaya, Navarro-Rosinés, Llimona, Hladun and Lutzoni2008).
Reduction of morphological complexity has also occurred in lichens. As we will show below, reduction of the thallus has occurred independently several times in the evolution of Teloschistaceae, resulting in several lineages that are difficult to separate on purely phenotypic grounds. An artificial unit, that we call the Caloplaca holocarpa group, contains many of these lineages, which are similar to C. holocarpa s. str. in external morphology and anatomy. Several lineages of the group will be characterized here and two new species will be described.
Materials and Methods
The material investigated has been collected by the authors mainly from Europe and Western Asia in 2000 and later (Table 1). Thirty phenotypic characters have been studied. The new species are fully described but only diagnostic characters are used for characterizing the other lineages. Fourteen selected characters are listed for investigated species in Table 2; primary data (measurements and measured samples) are available from the authors. Paraphyses tips were observed after pre-treatment with c. 10% KOH. Only those ascospores with well-developed septa were measured; in these ascospores loculi were connected by a thin cytoplasmatic channel, never disconnected. The measurements are given as (min.–) x ± SD (–max.), where x = mean value and SD = standard deviation; total numbers of measurements (n) are given. Morphological terminology follows Smith et al. (Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). Anthraquinone secondary metabolites were determined from their HPLC retention times and absorption spectra (see Søchting Reference Søchting1997 for details).
Table 1 Sample data and GenBank accession numbers of the ITS sequences used in the phylogenetic analysis; new sequences are highlighted in bold
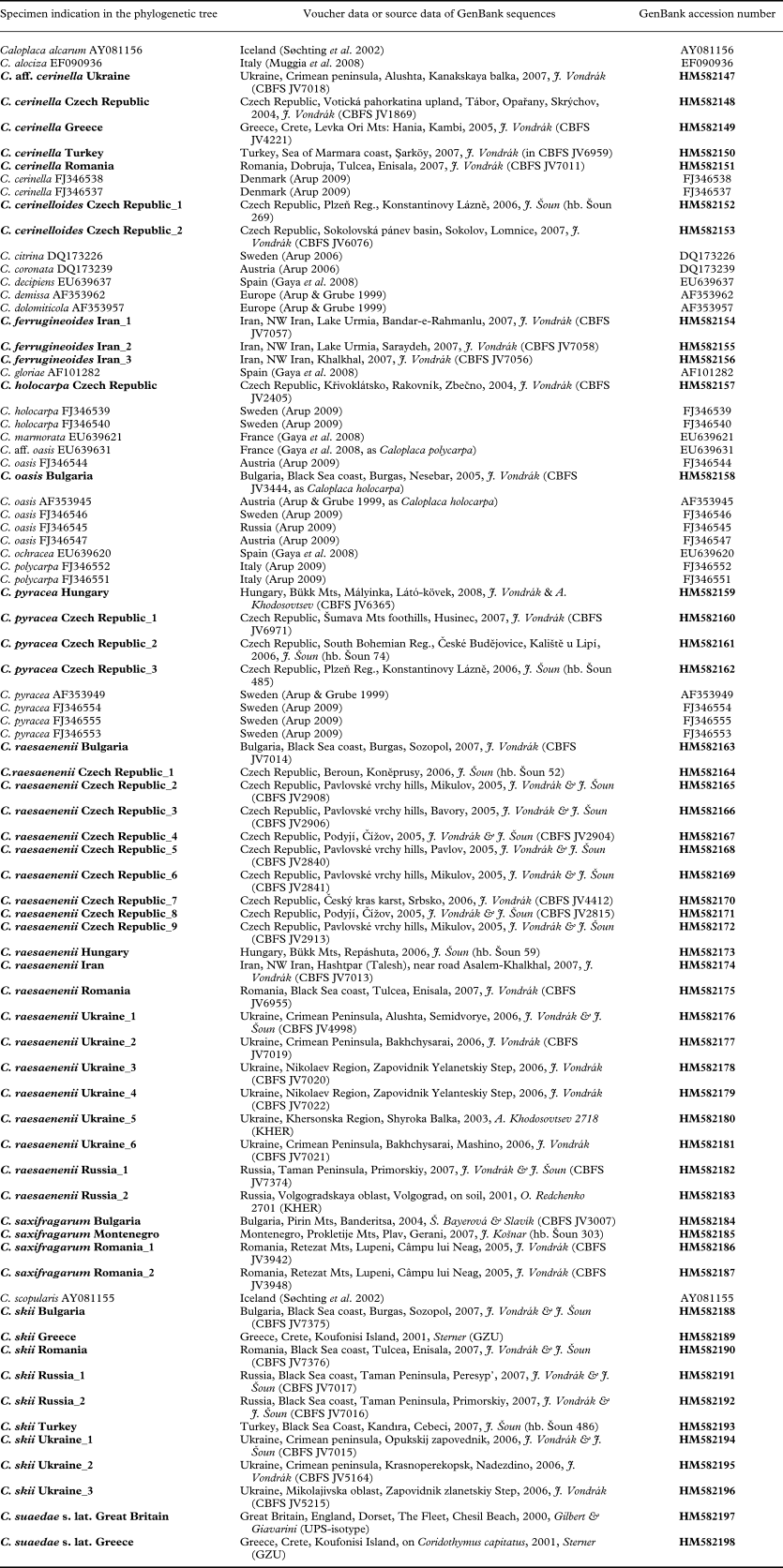
Table 2. Fifteen phenotypic characters for selected little-known epiphytic species of Caloplaca holocarpa-like Teloschistaceae including both new species. Numbers of measurements (n) are shown

DNA extraction, amplification and sequencing
Direct PCR was used for PCR-amplification of the ITS region including the 5.8S locus of the nuclear rDNA following Arup (Reference Arup2006). Primers used for amplification were ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990). PCR cycling parameters followed Ekman (Reference Ekman2001). Products were cleaned using JETquick PCR purification Spin Kit (Genomed). Both complementary strands were sequenced with BigDye (BigDye Terminator Cycle sequencing kit, version 3.1, Perkin-Elmer, Applied Biosystems, Foster City, CA) using the primers mentioned above, and run on an ABI 3130 × l Genetic Analyzer.
Alignment and phylogenetic analysis
Fifty-nine newly generated ITS sequences were aligned, along with 38 sequences from GenBank. Sequence fragments were subjected to the BLAST searches for a first verification of their identities. Sequences of species with known phylogenetic identity (Gaya et al. Reference Gaya, Navarro-Rosinés, Llimona, Hladun and Lutzoni2008), Caloplaca alociza (EF090936), C. demissa (AF353962), C. dolomiticola (AF353957), C. gloriae (AF101282) and Fulgensia fulgida (AY051359), were selected as an outgroup. Sequences were aligned using MAFFT 6 (on-line version in the Q-INS-i mode; see Katoh et al. Reference Katoh, Kuma, Toh and Miyata2002) and manually cut to eliminate the unalignable ends and ambiguously aligned regions of ITS1 and ITS2. The final alignment retained 510 positions. Bayesian phylogenetic analyses were carried out using the program MrBayes 3.1.1 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). The GTR+I+G model of molecular evolution was estimated with the program MrModeltest v2.3 (Nylander Reference Nylander2004) using the Akaike Information Criterion. The MCMC analyses were run for ten million generations, performed in two runs, each with four independent chains starting from a random tree. Trees were sampled every 100th generation, and the first 21 070 trees were discarded as burn-in, using standard deviation of splits between runs less than 0·01 as a convergence criterion. The remaining trees were used to calculate the posterior probabilities (PP) as a measure of support.
Results
The resulting dataset for molecular analysis contains 97 sequences of the ITS region. The final alignment has 510 positions, of which 298 are variable and 261 parsimony-informative. The resulting phylogenetic tree ( Fig. 1) revealed 14 well-resolved clades of highly similar Caloplaca holocarpa-like phenotypes in various positions and nested within lineages with more complex phenotypes. All basic thallus morphologies (fruticose, foliose, squamulose, lobate and crustose) appear within sister lineages to some simplified clades.

Fig. 1. Bayesian phylogeny of a section of the Teloschistaceae covering simplified Caloplaca holocarpa-like lineages (named on the right). Posterior probabilities are present at nodes.
The 14 Caloplaca holocarpa-like clades share the following characters.
1) Thallus poorly developed; endolithic or endophloeodal, or forming a thin yellowish or greyish crust on the substratum.
2) Yellow to orange apothecial discs and margins, both with a uniform anthraquinone content: parietin (main compound), ± emodin, fallacinal, parietinic acid and teloschistin.
3) Apothecial margin zeorine; composed of an inner purely fungal part (the true exciple) and an outer part (the thalline exciple) containing photobiont cells enclosed by a fungal tissue.
4) Ascospores c. 8–16 × 4–7 µm, with medium to thick septa (2·5–5·5 µm wide).
5) Pycnidia unknown in most of the species (and clades), but when present, producing ellipsoid conidia, c. 2·5–4·0 × 1·0–1·5 µm.
The species included here occur on a wide variety of substrata: tree bark, siliceous or calcareous rock, concrete, plant debris, mosses and loess. However, individual species are rather substratum specific, and this can be crucial for species identifications. The most favourable substrata for C. holocarpa-like species are twigs of shrubs in xeric conditions; five out of fourteen clades occur in this habitat.
Caloplaca holocarpa-like clades included in the molecular analysis
Caloplaca cerinella (Nyl.) Flagey
Thallus inconspicuous, mostly endophloeodal; apothecia small (0·25–0·4 mm diam.), yellow to orange; asci containing more than 8 spores; occurring on trees and shrubs throughout Europe (Arup Reference Arup2009).
Caloplaca cerinelloides (Erischsen) Poelt
Thallus inconspicuous, partly endophloeodal; apothecia small (0·2–0·4 mm diam.), yellow to orange; ascospores ellipsoid (length/breadth ratio c. 1·5); on nitrophilous bark throughout Europe, often together with C. pyracea.
Caloplaca ferrugineoides H. Magn
Thallus grey, partly endophloeodal, but often distinctly superficial; apothecia somewhat stipitate when mature, 0·4–1·0 mm diam.; thalline exciple covered by distinct paraplectenchymatous cortical tissue in lower part; on xerophilous shrubs in Central Asia, southern Russia, Turkey and Iran. The type material deposited in S (China: Kansu, Yü-ehr-hung, on dry twigs of a bush, 1932, B. Bohlin; nr L2612) is not well-developed but we consider it conspecific with our Central Asian material.
Caloplaca holocarpa (Hoffm.) A. E. Wade
Thallus inconspicuous, in shades of grey; apothecia yellow to orange, 0·3–0·7 mm diam.; a nitrophilous species usually on siliceous stones and rocks, but sometimes also on iron, wood and tree bases (Arup Reference Arup2009).
Caloplaca oasis (A. Massal.) Szatala
Thallus crustose, areolate or often inconspicuous; apothecia orange, small (0·2–0·5 mm diam.); occurring as a lichenicolous lichen on endolithic Verrucaria spp. on limestone rock, but also as a free-living pioneer on concrete and other lime-rich substrata in Europe (Arup Reference Arup2009).
Caloplaca polycarpa (A. Massal.) Zahlbr
Phenotypically and ecologically similar to C. oasis but with a more developed, yellow thallus (Arup Reference Arup2009).
Caloplaca pyracea (Ach.) Zwackh
Thallus grey to yellow-grey, endophloeodeal or superficial; apothecia 0·4–0·7 mm diam., orange, zeorine, usually with distinct white-grey or yellowish thalline exciple; thalline exciple covered by a distinct paraplectenchymatous cortical tissue in lower part; common species on nitrophilous bark throughout Europe (Arup Reference Arup2009).
Caloplaca raesaenenii Bredkina (syn. C. thuringiaca Søchting & Stordeur)
Thallus inconspicuous; apothecia orange, small (0·2–0·6 mm diam.), biatorine or zeorine, urceolate; on shrubs, plant debris, rabbit droppings, mosses and loess in arid regions of Europe and Asia.
Caloplaca saxifragarum Poelt
Thallus inconspicuous; apothecia orange, c. 0·2–0·4 mm diam., biatorine to zeorine; occurring on tufts of perennial plants and on twigs of shrubs in alpine habitats. Muscicolous species, Caloplaca chelyae I. Pérez-Vargas and C. schoeferi Poelt, have similar phenotypes (Pérez-Vargas & Pérez de Paz Reference Pérez-Vargas and Pérez de Paz2009) but their phylogenetic identities were not studied.
Caloplaca skii
(described below)
Caloplaca suaedae s. lat
Thallus grey, partly endophloeodal, but often distinctly superficial; apothecia orange, c. 0·3–0·5 mm diam.; on Mediterranean shrubs or lignicolous. This unit probably contains several species with yellow (anthraquinone-containing) pycnidia, a character which is absent in other clades. The maritime species C. suaedae O. L. Gilbert & Coppins (Gilbert Reference Gilbert2001) belongs here.
Caloplaca syvashica
(described below)
Caloplaca ulcerosa Coppins & P. James
Thallus inconspicuous, partly endophloeodal, whitish, with green-grey crater-like soralia; apothecia orange, c. 0·3–0·4 mm diam.; occurring on trees and shrubs around sea coasts of Europe (Vondrák et al. Reference Vondrák, Šoun, Arup, Aptroot and Redchenko2009b).
Caloplaca vitellinula (Nyl.) H. Olivier
Phenotypically and ecologically similar to C. holocarpa, to which it is closely related, but usually with a distinct, thin yellow thallus (Arup Reference Arup2009).
Caloplaca yarraensis S.Y. Kondr. & Kärnefelt
This recently described species from Australia (Kondratyuk et al. Reference Kondratyuk, Kärnefelt, Elix and Thell2009) is closely related to Caloplaca syvashica, but strongly differs in ascospore characters (see the description of C. syvashica).
The New Species
Caloplaca skii Khodosovtsev, Vondrák & Šoun sp. nov
MycoBank No.: MB 519676
A Caloplaca cerinelloides ascosporis angustioribus et minoribus (7·5–) 10·4 ± 1·06 (–12·8) × (3·5–) 5·2 ± 0·66 (–6·5) µm differt.
Typus: Russia, Black Sea coast, Taman Peninsula, sand dunes E of Peresip', 45°20′17·60″N, 37°11′04·59″E, on stems of Artemisia, 19 May 2007, J. Vondrák & J. Šoun (CBFS JV7017—holotypus; LD, GZU—isotypi). ITS sequence of the holotypus: HM582191.
(Fig. 2A)

Fig. 2. The new species. A, Caloplaca skii, holotypus; B, C. skii, distribution; C, Caloplaca syvashica, holotypus; D, C. syvashica, distribution. Scales: A, C = 1 mm.
Thallus thin, film-like, inconspicuous, visible only around apothecia, whitish, light grey or pale yellowish, (20–)35 ± 13(–60) µm high (n = 10). Prothallus not developed. Cortex not developed. Alveolate cortex (sensu Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009a) thin, (5·0–)6·8 ± 1·6(–10·0) µm wide (n = 10), formed of loose paraplectenchyma with cells of lumina c. 3·5–5 µm wide. Algal layer (10–)20 ± 6(–30) µm high (n = 10); algal cells globose, (11·3–)14·3 ± 2·26(–18·0) µm diam. (n = 10). Medulla not developed.
Apothecia zeorine to biatorine, dispersed, small, (0·2–)0·3 ± 0·08(–0·5) mm wide (n = 45); apothecial primordia and young apothecia immersed in thallus, yellowish to white yellowish; mature apothecia sessile to weakly constricted at the base; disc flat to weakly convex in mature ascomata, yellow-orange, non-pruinose; true exciple well defined, persistent, yellow, non-pruinose, always paler than disk, (25–)38 ± 7(–50) µm wide (n = 10) in upper part, consisting of radiating cells; marginal cells ± isodiametric with lumina (2·5–) 3·7 ± 0·7 (–5·0) µm (n = 10); thalline exciple visible only in young apothecia, white greyish to yellow-white, later inconspicuous but persistent on lower side of apothecial margin, (10–)40 ± 14(–60) µm wide (n = 10). Alveolate cortex of thalline exciple thin, (5·0–)7·4 ± 2·45(–12·0) µm wide (n = 10) formed of loose paraplectenchyma, with cells (2·5–)3·5 ± 0·62(–4·5) µm diam. (n = 10). Hypothecium colourless, (30–)38 ± 6(–50) µm high (n = 15), formed of variously shaped cells. Hymenium hyaline (50–)60 ± 7(–70) µm high (n = 15), with oil drops. Paraphyses of thin-walled cells, 1·5–2·0 µm thick in lower part, sometimes branched; apical cells swollen, sometimes with oil drops, (3·5–)4·3 ± 0·6(–5·5) µm wide (n = 35) covered by external anthraquinone pigments. Asci clavate, 8–spored, Teloschistes-type, (35·0–)42·8 ± 5·5 (–55·0) × (11·0–)13·1 ± 1·5(–16·0) µm (n = 15). Ascospores polarilocular, ellipsoid, (7·5–) 10·4 ±1·06(–12·8) × (3·5–)5·2 ± 0·66(–6·5) µm (n = 76); length/breadth ratio (1·55–)2·0 ± 0·35(–3·2); ascospore septa (3·5–)5·0 ± 0·69(–6·5) µm thick (n = 76); ratio of septum width/ascospore length (0·33–)0·5 ± 0·06(–0·61); ascospore wall thin (up to 0·5 µm).
Conidiomata not seen.
Chemistry. Thallus C−, K− (pale yellow thalli K+ purple); apothecia K+ (purple), C− or rarely C+ reddish, P−, UV+ orange-red. Compounds: parietin (major), ± traces of emodin, parietinic acid, fallacinal and teloschistin.
Etymology. The name honours the Ukrainian lichenologist Sergiy Kondratyuk for his significant contribution to the taxonomy of Teloschistaceae.
Phylogeny. Nine ITS sequences of Caloplaca skii obtained from a large territory are almost identical with only two variable nucleotide positions. They form a well supported monophyletic group (PP = 1·00) related to C. saxifragarum, but not closely. The noteworthy characters shared by sequences of C. skii are synapomorphies causing the long branch of the group in Fig. 1.
Ecology and distribution. Caloplaca skii prefers branches of shrubs in lowlands, especially close to sea shores. It has been found on Artemisia arenaria, A. lerchiana, A. santonica, Corydothymus capitatus, Ephedra distachya, Halocnemum strobilaceum, Helichrysum stoechas, Jasminum sp., Kochia prostrata, Lycium sp., Paliurus spina-christi, Pinus halepensis, Thymus dimorphus, and T. cretaceus in arid and semi-arid regions. Sometimes it grows over old bones or plant debris. The accompanying species are, for example, Caloplaca haematites (Chaub.) Zwackh, C. lobulata (Flörke) Hellbom, C. phlogina (Ach.) Flagey, C. sterilis Šoun, Khodosovtsev & Vondrák, C. raesaenenii, Lecanora hagenii (Ach.) Ach., Physcia adscendens H. Olivier, Rinodina pyrina (Ach.) Arnold, and some species of Xanthoria. It has a southern distribution in Europe (Bulgaria, Greece, Romania, Spain, southern Russia and Ukraine); it is also known from Turkey (Fig. 2B).
Remarks. Caloplaca cerinelloides is similar, but C. skii has shorter and narrower ascospores and prefers the bark of shrubs in arid regions of S/SE Europe. In steppes, C. skii sometimes grows together with C. raesaenenii, but both species are usually distinguishable in the field by the colour of their apothecia; dark orange in C. raesaenenii and yellow-orange in C. skii. Aberrant forms of C. skii with more orange apothecia can be distinguished by the wider septa. The boreal species C. sibirica is similar but has a strongly reduced thalline exciple and the true exciple is darkened by an olive pigment (at least in the lower part).
Paratypes. Bulgaria: Black Sea coast: Burgas, Sozopol, sand dunes near seashore c. 5·5 km S of town, 42°21′59·76″N, 27°42′31·11″E, on coastal shrubs, 9 iv 2007, J. Vondrák & J. Šoun (CBFS JV7375, will be distributed in Vondrák: Sel. Exs. of Caloplaca, fasc. 3; JV7626; JV8321).—Greece: Crete: Koufonisi islands, auf Corydothymus capitatus, 2001, Sterner (GZU).—Romania: Dobruja: Tulcea, Enisala, limestone outcrops 250 m SE of Enisala castle ruin, alt. c. 70 m, 44°52′56·03″N, 28°50′12·41″E, on Thymus, 3 iv 2007, J. Vondrák & J. Šoun (CBFS JV7376).—Russia: Black Sea coast: Novorossiysk, coastal rocks near Yuzhnaya Ozereevka, 44°40′13·38″N, 37°37′57·04″E, on twigs of Paliurus spina-christi, 18 v 2007, J. Vondrák (CBFS JV7508); ibid.: on Jasminum, 18 v 2007, J. Vondrák (CBFS JV7537); Taman Peninsula, loess steppe near road E of Primorskiy, 45°16′24·05″N, 36°57′12·40″E, on dead shrub twigs, 19 v 2007, J. Vondrák & J. Šoun (CBFS JV7016); Taman Peninsula, salt marsh SW of Primorskiy near Taman' Bay coast, 45°15′16·05″N, 36°53′40·46″E, on Halocnemum strobilaceum, 20 v 2007, J. Vondrák & J. Šoun (CBFS JV7377).—Spain: Andalucia: Alora, El Chorro, at village, alt. c. 250 m, 36°54′N, 4°45′W, on bark of Pinus halepensis, 24 ii 2008, J. Vondrák (CBFS JV6263); Antequera, Torcal de Antequera, c. 7 km S of town, alt. c. 1200 m, 36°57′21·41″N, 4°32′23·30″W, on bark of small shrubs in shrub vegetation, 5 iii 2008, J. Vondrák (CBFS JV8320); Tarifa, El Santiscal, coastal cliffs 1·5 km W of village, 36°5′9·38″N, 5°47′4·37″W, on bark of shrub close to sea, 29 ii 2008, J. Vondrák (CBFS JV6312). Basque Country: on bark of shrub Helichrysum stoechas at Atlantic coast, 30 vi 2002, J. Vondrák (CBFS JV7816). Catalonia: Lleida, Balaguer, wasteland c. 4 km NEN of town, alt 250 m, 41°48′6·51″N, 0°51′7·13″E, on small shrub in garrigue, 7 iii 2008, J. Vondrák (CBFS JV6283).—Turkey: Black Sea coast: Kandıra, sand dunes and coastal limestone rocks 6 km E of Cebeci, 41°12′00·35″N, 30°19′47·18″E, on coastal shrubs, 15 iv 2007, J. Vondrák & J. Šoun (CBFS JV7378, hb. Šoun 486).—Ukraine: Crimean Peninsula: Leninsky district, Kerch Peninsula, Opukskiy zapovednik, coastal cliffs, alt. c. 100 m, 45°01′53·00″N, 036°12′47·94″E, on Thymus, 14 vi 2006, J. Vondrák & J. Šoun (CBFS JV7015); Krasnoperekopsky district, vill. Nadezdino, at small shallow gulf S of village, alt. 0 m, 46°01′48·09″N, 033°59′42·57″E, on Artemisia twigs, 8 vi 2006, J. Vondrák (CBFS JV5164); Chornomorskiy district, vil. Olenevka, cape Tarchankut, coast of Black Sea, on twigs of Artemisia lerchiana, N 45·20617°, E 32·30476°, alt. 15 m, 4 v 2010, A. Khodosovtsev (KHER, KW); Sudak, Meganom cape, on Ephedra distachya, 4 v 2004, A. Khodosovtsev (KHER 2933). Kherson region: Goloprystansky district, Black Sea biosphere reserve, Tendrovs′ka kosa island, on bones, 6 vii 1992, A. Khodosovtsev (KHER); ibid.: on Artemisia arenaria, 1 v 2009, A. Khodosovtsev (KHER, CBFS JV7381, KW); Skadovsky district, Dzharylgach island, on Artemisia arenaria, 28 vii 2009, A. Khodosovtsev (KHER); Genichesky district, Biryuchy island, on plant debris, 1995, A. Redchenko 2931 (KHER). Kharkiv region: Vivchansky district, vill. Mala Vovcha, right bank of river Vovcha, chalk outcrops, on plant debris, 9 ix 2005, A. Gromakova (KHER). Lugansk region: Starobilsky district, vill. Starobilska, cretaceous outcrops, on Thymus cretaceus, 28 ix 1953, A. Oxner (KW 3600). Nikolaev region: nature reserve ′Yelanetskiy Step′, c. 60 km N of town, alt. c. 50 m, 47°32′40·54″N, 032°04′54·27″E, 7 vi 2006, J. Vondrák (CBFS JV5215).
Caloplaca syvashica Khodosovtsev, Vondrák & Šoun sp. nov
MycoBank No.: MB519677
A Caloplaca suaedae ascosporis latioribus, (10·5–) 12·87 (–16·0) × (4·75–) 5·91 (–7·25) µm, et apotheciis pruinosis differt.
Typus: Ukraine, Crimean Peninsula, Sivash lake, Krasnoperekopsk, Nadezhdino, salt marshes at small shallow sea-gulf S of village, alt. 0 m, 46°01′48·09″N, 33°59′42·57″E, on wooden stems of Limonium suffruticosum, 8 June 2006, J. Vondrák & J. Šoun (CBFS JV4996—holotypus; CBFS JV7012—isotypus; isotypi will be also distributed in Vondrák: Sel. Exs. of Caloplaca, fasc. 3). ITS sequence of the isotypus: HM582201.
(Fig. 2C)
Thallus crustose, rimose areolate, sometimes inconspicuous; (50–)145 ± 40(–200) µm thick (n = 11). Areoles flat to weakly convex, (0·10–)0·3 ± 0·1(–0·5) mm wide (n = 15), greenish, grey greenish or greenish white; ± covered by white crystals; sometimes indistinctly granular in shaded conditions (e.g. on lower sides of twigs). Prothallus dirty whitish, usually not developed. Cortex not developed, but indistinct alveolate cortex (sensu Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009a) present, (7–)12 ± 3(–18) µm high (n = 10), composed of loose paraplectenchyma of rounded cells with lumina c. 3·5–5·0 µm wide. Epinecral layer present, partly formed by colourless crystals, not dissolving in K and N. Algal layer (40–)76 ± 23(–120) µm high (n = 10) with algonecral medulla (sensu Vondrák et al. Reference Vondrák, Šoun, Hrouzek, Říha, Kubásek, Palice and Søchting2008) below; algal cells globose, (11·3–) 14·1 ± 3·8(–22·0) µm diam. (n = 10). Medulla inconspicuous, white, formed by loose prosoplectenchyma with 2·5–3·5 µm thick hyphae.
Apothecia zeorine (0·2–)0·5 ± 0·19(–1·0) mm wide (n = 35), associated in small groups, rounded or angular, yellow-orange to orange-red, sessile to constricted at the base. Disc flat or slightly convex; orange-red, often with white pruina. True exciple in upper part (20–)51 ± 13 (–65) µm wide (n = 15), orange, consisting of gelatinous radiating cells; marginal cells with ± rounded lumina (2·0–)3·0 ± 0·52(–3·8) µm diam. (n = 10). Thalline exciple (50–)73 ± 14(–100) µm wide (n = 10), yellow to greyish green, distinct in mature apothecia; cortical tissue of the thalline exciple (5·0–)7·4 ± 1·8(–10·0) µm wide (n = 10), formed of paraplectenchymatous cells (2·0–)3·0 ± 0·61(4·0) µm diam. (n = 10), usually covered by white pruina. Hypothecium colourless, (35–)53 ± 15(–80) µm high (n = 11), formed of variously shaped cells. Hymenium hyaline, (60–)73 ± 8(–85) µm high (n = 10), rarely with some oil drops. Paraphyses of thin-walled cells, 1·5–2·0 µm thick, sometimes branched; apical cells swollen, (3·5–)4·8 ± 0·7(–6·0) µm wide (n = 15). Epihymenium covered by orange granules of anthraquinones dissolving in K; colourless crystalline pruina ± present, insoluble in K and N. Asci clavate, 8-spored, Teloschistes-type (50·0–)56·7 ± 4·3(–65·0) × (12·0–)14·5 ± 1·6(–17·0) µm (n = 10). Ascospores polarilocular, narrowly ellipsoid, (10·5–)12·9 ± 1·59(–16·5) × (4·8–)5·9 ± 0·81(–7·5)µm (n = 34); length/breadth ratio (1·67–)2·2 ± 0·32(–3·05); ascospore septa (2·5–)3·7 ± 0·62(–5·0) µm thick (n = 34); ratio of septum width/ascospore length (0·20–)0·3 ± 0·05(–0·41); ascospore wall thin (up to c. 0·5 µm).
Pycnidia small, c. 20–35 µm diam., inconspicuous, sometimes several per areole, deeply immersed in thallus; ostioles often do not emerge at the surface. Pycnoconidia bacilliform, (2·5–)3·0 ± 0·61(–4·0) × (0·9–)1·1 ± 0·15(–1·3) µm (n = 5).
Chemistry. Thallus K−, C−, apothecia K+ (purple), C−, P−, UV+ orange-red. Compounds: parietin (major), ± traces of emodin, parietinic acid, fallacinal and teloschistin.
Etymology. The species is named after the Sivash salt lake, a big lagoon of the Azov Sea situated between the Ukrainian mainland and the Crimean Peninsula.
Phylogeny. Three ITS sequences of Caloplaca syvashica from different localities are identical and form the monophyletic group (PP = 0·93). The Australian C. yarraensis is closely related since its two identical sequences generated from isotype samples only differ from those of C. syvashica in three nucleotide positions. The grouping of both species is in a sister relationship to C. suaedae s. lat. clade (Fig. 1), but both clades differ considerably in the characters of their ITS sequences.
Ecology and distribution. The species grows on small halophilous shrubs (Halocnemum strobilaceum and Limonium suffruticosum) in salt marshes, especially at the Sivash Lake in the northern Black Sea region (Fig. 2D). It prefers shrubs in the driest parts of salt marshes, which are not periodically inundated by water. The associated species are, for example, Arthonia apatetica (A. Massal.) Th. Fr., Caloplaca phlogina, Candelariella boikoi Khodosovtsev & S. Kondr., Lecania inundata (Hepp ex Körb.) M. Mayrhofer and Lecanora hagenii. We have searched for, but not found, C. syvashica in similar habitats with Halocnemum strobilaceum on the northern Caspian Sea coast, at the salt lake Baskunchak (Russia, Astrkhan region) and at salt lakes Inder and Cholcar in Kazakhstan.
Remarks. Morphologically and ecologically, C. suaedae s. str. (Gilbert Reference Gilbert2001) is a similar species, but it has non-pruinose apothecia, narrower ascospores and is known only from England, where it occurs in maritime habitats. The widespread C. pyracea has thick ascospore septa and a thick cortex in the lower part of a grey yellowish thalline exciple. Caloplaca raesaenenii differs in its smaller, non-pruinose apothecia with reduced thalline margin and in having a less conspicuous, film-like thallus.
The Australian C. yarraensis is surprisingly closely related to the new species. It also occurs in salt marshes and shares some morphological characters with C. syvashica (Kondratyuk et al. Reference Kondratyuk, Kärnefelt, Elix and Thell2009). We have investigated two isotype samples of C. yarraensis in detail and found some considerable differences from the new species: 1) ascospores small, (9·0–) 11·0 ± 1·1(–13·75) × (4·5–)5·0 ± 0·25(–5·75) µm (n = 20); 2) ascospore septa very thin (1·0–) 1·5 ± 0·25(–2·0) µm (n = 20); 3) paraphyses tips strongly swollen, (4·0–)4·9 ± 0·7(–6·25) µm (n = 21). Among these characters, ascospore septa differ the most; their ranges in these two species do not overlap.
As the new species has a close relative in Australia and their clade is very isolated from the other European clades investigated, we suggest that C. syvashica may be a recent colonizer of a restricted area in the Black Sea region, and that it may belong to some Southern Hemisphere group of Teloschistaceae.
Paratypes. Ukraine: Crimean Peninsula: Dzhankoys'ky district, vill. Predmostnoye, Sivash lake coast, on Halocnemum strobilaceum, 4 iv 2007, A. Yena (KHER); Nizhnegirsky district, vill. Dmytrivka, on Halocnemum strobilaceum, 8 vi 2003, A. Khodosovtsev (KHER 2734). Kherson region: Chaplynsky district, SE from vill. Pershokonstantinovka, coast of Sivash Laguna, on Halochemum strobilaceum, 12 vi 2008, A. Khodosovtsev & J. Vondrak (KHER, CBFS JV7208, KW); Genichesky district, peninsula Chongar, Sivash coast, on Halocnemum strobilaceum, 3 v 1996, R. Mishustin (KHER 2725; 2724, 2721); Station Sivash, Sivash coast, 20 ix 1995, A. Khodosovtsev (KHER 2719); Island Kuyk-Tyk, on Halocnemum strobilaceum, 18 ix 1994, A. Khodosovtsev (KHER 2720); Golopristanskiy district, vill. Sadove, Nikolaivka, section of protected area Black Sea biosphere reserve, on Halocnemum strobilaceum in salt marsh, with Caloplaca phlogina, 26 vi 2009, J. Vondrák (CBFS JV7142). Nikolaev region: Ochakov district, regional nature reserve “Kinburnskaya kosa”, on Halocnemum strobilaceum, with Caloplaca phlogina, 26 vi 2009, J. Vondrák (CBFS JV7131).
Key to the corticolous Caloplaca holocarpa-like species in Europe
1 Thallus with soredia ... 2
Thallus without soredia ... 3
2(1) Apothecia yellowish; soralia small and often sparse, 80–120 µm wide, dark grey (soredia K+ violet in section); in boreal zone of Europe ... ... Caloplaca ahtii Søchting
Apothecia orange; soralia larger and frequent, 200–400 µm wide, green-grey (K− in section); Mediterranean-Atlantic ... Caloplaca ulcerosa
3(1) Asci (8–)12–16-spored ... Caloplaca cerinella
Asci 8-spored ... 4
4(3) Septa wide – mean in representative sample of ascospores (n >10) 4–5 µm thick; septum width/spore length ratio mostly >0·35 ... 5
Septa thinner – mean in representative sample of ascospores (n >10) 3–4 µm thick; septum width/spore length ratio mostly <0·35 ... 11
5(4) Thallus, apothecial margin, or disc with olive-black to olive-grey pigment (K− or K+ sordid violet, but its reaction is overshadowed by K+ purple anthraquinones) ... 6
Thallus, apothecial margin and disc without olive-black or olive-grey pigmentation ... 7
6(5) Outer apothecial margin usually with olive pigmentation (young apothecia often lack pigmentation); disc yellow to pale orange without olive pigmentation; corticolous in boreal zone of Europe ... Caloplaca borealis (Vain.) Poelt
Apothecial margin usually without olive pigmentation; disc of mature apothecia with olive pigment (at least in parts); on arctic-alpine shrubs, plant debris or bryophytes ... Caloplaca tiroliensis Zahlbr.
7(5) Thalline exciple clearly visible, 50–80 µm thick, yellow greyish to pale grey, with distinct cortex in lower part, up to 20 µm wide; disc orange to dark orange contrasting with greyish outer margin ... Caloplaca pyracea
Thalline exciple usually not apparent, 0–50 µm thick, without a cortex or cortex poorly developed, up to 10 µm wide; disc yellow to orange ... 8
8(7) Ascospores narrowly ellipsoid, c. 4·0–6·0 µm wide; length/breadth ratio often >2·0; on shrubs; S-SE Europe ... Caloplaca skii
Ascospores ellipsoid, c. 6·0–8·0 µm wide; length/breadth ratio usually <2·0 ... 9
9(8) Mature apothecia 0·3–0·7 mm diam., sometimes with yellow or greyish areoles around apothecia; usually saxicolous but rarely on dusty bark and lignum ... ... Caloplaca holocarpa
Mature apothecia c. 0·2–0·4 mm diam.; thallus greyish, inconspicuous; not saxicolous ... 10
10(9) Apothecia yellowish, on bark of deciduous trees, temperate-boreal ... ... Caloplaca cerinelloides
Apothecia orange to dark orange; on dead tufts of Saxifraga and on small shrubs and woody plants, alpine ... Caloplaca saxifragarum
11(4) Mature apothecia stipitate, cortex of thalline exciple 15–40 µm thick with large isodiametric to oval cells, 5–10 µm wide; on steppe shrubs, in Europe restricted to southern Russia ... Caloplaca ferrugineoides
Mature apothecia sessile or weakly constricted at base, cortex of thalline exciple not developed, but an indistinct alveolate cortex (sensu Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009a), 0–15 µm wide, sometimes present ... 12
12(11) Pycnidia present, with yellow (K+ purple) anthraquinone pigments around ostioles ... Caloplaca suaedae s. lat.
(Caloplaca suaedae O.L. Gilbert & Coppins s. str. is characterized by narrowly, ellipsoid ascospores, c. 3–5 µm thick; length/breadth ratio c. 3·0; known from Suaeda vera shrubs in England)
Pycnidia absent or present but without yellow (K+ purple) anthraquinones; ascospores ellipsoid, c. 5–7 µm thick; length/breadth ratio c. 2·0 ... 13
13(12) Apothecia 0·2–1·0 mm diam., orange-red, ± white pruinose, usually zeorine with paler yellowish-orange thalline exciple; thallus green-grey, rimose areolate covered by crystals, known only from Halocnemum strobilaceum and rarely from Limonium suffruticosum in salt marshes in southern Ukraine ... Caloplaca syvashica
Apothecia 0·2–0·6 mm diam., bright orange to dark orange, not pruinose, usually biatorine without visible thalline margin; thallus usually film-like, greyish, without crystals, on shrubs (not on Halocnemum strobilaceum and rarely on Limonium suffruticosum), soil, plant debris, wood, rarely on bases of trees in arid regions of Eurasia ... Caloplaca raesaenenii
We are grateful to Pavel Hrouzek for performing HPLC chromatography and identifying secondary metabolites. Ulf Arup and Ulrik Søchting commented on the manuscript and Ulf Arup also provided the ITS sequences from types of Caloplaca suaedae and C. yarraensis. Walter Obermayer arranged loans of valuable material from GZU. Andriy Yena and Alla Gromakova made some collections. Linda in Arcadia revised the English and Latin and made useful comments on the manuscript. Our study has been supported by the International Visegrad Fund (project 51000067) and the whole institute grant (AVOZ60050516).






