Introduction
Phyllopsora Müll. Arg. is a genus of crustose to squamulose lichens primarily inhabiting tree trunks and large branches in tropical and subtropical humid woodlands and rainforests. Members of this genus are mostly found on the bark of woody angiosperms but also on rock or bryophytes, rarely on leaves or dead wood (Brako Reference Brako1991). They occur on a wide range of tree species and do not show any particular host preference (Sequiera & Kumar Reference Sequiera and Kumar2008). Specimens of Phyllopsora have been collected at up to 3500 m above sea level but the genus seems to be most diverse in mountain forests at elevations of 500–2500 m (Brako Reference Brako1991). It is also found in lowland rainforests (Lakatos et al. Reference Lakatos, Rascher and Büdel2006) and even gallery forests in drier areas, but never exposed to direct sunlight (Brako Reference Brako1991). The genus is generally characterized by a growth form consisting of patches of small squamules or areoles developing on a basal white to reddish brown to dark brown prothallus (Fig. 1; Brako Reference Brako1991; Elix Reference Elix and McCarthy2009). Isidia, lacinules, phyllidia or soredia may be common in some species (Timdal Reference Timdal2008). Apothecia are biatorine with simple or 1-septate, hyaline, ellipsoid to fusiform ascospores (5–45 × 0·8–5·0 µm; Elix Reference Elix and McCarthy2009; Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a). However, morphological characters may vary considerably within a single species (Swinscow & Krog Reference Swinscow and Krog1981; Brako Reference Brako1991), making it difficult to tell the species of Phyllopsora apart.

Fig. 1. Phyllopsora breviuscula, the type species of Phyllopsora, illustrating the typical growth form with squamules growing on a thick prothallus (O L-207949). Scale = 2 mm. In colour online.
Throughout its taxonomic history, Phyllopsora has been placed in various families: initially placed in the Phyllopsoraceae Zahlbr. (Zahlbruckner Reference Zahlbruckner, Engler and Prantl1907), it was moved to the Lecideaceae Chevall. (Poelt Reference Poelt, Ahmadjian and Hale1973), then to the Cladoniaceae Zenker (Schneider Reference Schneider1980), back to the Phyllopsoraceae (Hafellner Reference Hafellner1984), into the Bacidiaceae Walt. Watson (Eriksson & Hawksworth Reference Eriksson and Hawksworth1986), and finally to the Ramalinaceae C. Agardh (Lumbsch & Huhndorf Reference Lumbsch and Huhndorf2007). Recently, a DNA-based phylogeny by Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) corroborated its affiliation with the family Ramalinaceae.
When Müller described the genus in 1894 from New Zealand, he included four species and one variety (Müller Reference Müller1894). Zahlbruckner (Reference Zahlbruckner, Engler and Prantl1907, Reference Zahlbruckner1921–1940) included additional species based on morphology. Clements & Shear (Reference Clements and Shear1931) designated P. breviuscula (Nyl.) Müll. Arg. (Fig. 1) as the lectotype of the genus. Later, several species were transferred to Phyllopsora or newly described, for example by Lamb (Reference Lamb1963), Riedl (Reference Riedl1973), Coppins & James (Reference Coppins and James1979) and Schneider (Reference Schneider1980). The last publication, however, provided provisional new species combinations only, pending a monographic treatment of the genus (“Eine formale Umkombination der hier neu zu Phyllopsora gestellten Taxa muß – wegen der ungeklärten Synonymie – dem Monographen vorbehalten bleiben”; Schneider Reference Schneider1980: 171). Hence, we treat Schneider's combinations as invalid, since he considered them provisional (ICN Art. 36.1). Most of the species later transferred to Phyllopsora were originally described in Lecidea Ach. Despite a constant increase in the number of Phyllopsora species described, a comprehensive monographic treatment of the genus has not been attempted for a long time, probably because species boundaries in Phyllopsora are difficult to establish by means of morphological and anatomical characters alone.
The advent of thin-layer chromatography (TLC) for the investigation of lichen secondary metabolites (i.e. lichen substances; Culberson & Kristinsson Reference Culberson and Kristinsson1970; Culberson Reference Culberson1972; Menlove Reference Menlove1974) provided new data for understanding the genus and disentangling its species. Swinscow & Krog (Reference Swinscow and Krog1981) provided the first general treatment of Phyllopsora, focusing on East African species. They investigated 90% of the types of all previously described species as well as newly collected material using a combination of morphology, anatomy and chemistry to delimit the genus and its species. However, formulating a clear and unambiguous generic delimitation of Phyllopsora proved difficult because of the highly diverse morphological characters. The authors regarded the inclusion of a species in Phyllopsora as being a question of probability: ‘The larger the number of the[se] characters that are combined in a species the more likely is it to be in Phyllopsora’ (Swinscow & Krog Reference Swinscow and Krog1981: 220) and made short morphological comparisons to similar genera, such as Bacidia De Not. Based on their investigations, Swinscow & Krog (Reference Swinscow and Krog1981) revised the species circumscriptions within the genus, accepting 11 species for East Africa, and provided guidelines for delimiting Phyllopsora species in general. At the same time, they emphasized the wide range of intraspecific variation observed in several Phyllopsora species and acknowledged that some accepted species may merely represent extreme forms or morphs of highly variable taxa.
The first monographic treatment with a focus on neotropical species was provided by Brako (Reference Brako1989, Reference Brako1991). Brako reassessed the species circumscriptions in Phyllopsora by investigating type specimens of nearly all published names (93 at the time), and by studying her own extensive collections (Brako Reference Brako1991). She compiled an updated genus description and accepted 18 species, including 11 varieties, based on detailed morphological, anatomical, ecological and chemical investigations. Furthermore, she delimited the genus from other similar genera, namely Bacidia, Bacidiopsora Kalb, Biatora Fr., Eschatogonia Trevis., Physcidia Tuck. and the newly described genus Squamacidia Brako.
Regional treatments of the genus followed: Timdal & Krog (Reference Timdal and Krog2001) studied freshly collected material from East Africa and the Mascarene Islands, accepting 20 species for that region. The Australian species were subsequently treated by Elix (Reference Elix2006a, Reference Elixb, Reference Elixc, Reference Elix and McCarthy2009), who described five new species, commented on the taxonomy of Phyllopsora and provided valuable chemical information for several other species and related genera. Timdal (Reference Timdal2008) studied material from Peru and accepted 20 species, eight of which were described as new. He also reduced the genera Squamacidia and Triclinum Fée into synonymy with Phyllopsora. By including both sorediate and long-spored species, he expanded the genus circumscription. In a study of the genus in the West Indies (Timdal Reference Timdal2011), 34 species were accepted, including four that were new to science. In addition, Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011) described two new species from India, while Kondratyuk et al. (Reference Kondratyuk, Lőkös, Halda, Upreti, Mishra, Haji Moniri, Farkas, Park, Lee and Liu2016) described a new species from South Korea. Thus, the number of accepted species in Phyllopsora increased from 18 (Brako Reference Brako1991) to over 70 extant species in only 25 years.
While chemical information proved useful for detecting species boundaries in Phyllopsora, it also raised new questions. Whether or not chemotypes are informative for delimiting species of Phyllopsora has remained uncertain. Chemotypes may indeed characterize distinct species, but they might also merely represent regional variation. Furthermore, several Phyllopsora specimens lack lichen substances, which is highly problematic in the case of sterile species. Thus, challenges remain in species delimitation and reliable identification despite the availability of chemical data. In our experience, c. 30% of all phyllopsoroid specimens that lack apothecia, vegetative dispersal units and lichen substances cannot be identified. In these cases, it is also difficult to discover potentially undescribed Phyllopsora species. With the rise in routine DNA sequence analysis, DNA sequence data now make it possible to test species hypotheses and investigate relationships using molecular phylogenies.
By the end of 2018, more than 130 Phyllopsora species names (including synonyms) existed in the literature. Lücking et al. (Reference Lücking, Hodkinson and Leavitt2017a, Reference Lücking, Hodkinson and Leavittb) accept 95 Phyllopsora species, while Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) later excluded seven species and included two more. In addition to the extant species, two fossil species enclosed in Dominican amber have been described (Rikkinen & Poinar Reference Rikkinen and Poinar2008; Kaasalainen et al. Reference Kaasalainen, Heinrichs, Renner, Hedenäs, Schäfer-Verwimp, Lee, Ignatov, Rikkinen and Schmidt2018), both estimated to be c. 15–20 million years old. Vegetative thalli reminiscent of those in Phyllopsora are also known in other, even unrelated, genera for example Cladonia P. Browne. This raises some doubt as to whether these fossils truly belong to the genus Phyllopsora. If indeed they do, these findings would give valuable insight into the evolutionary history of Phyllopsora, indicating that the genus had existed in its characteristic squamulose form for several million years.
Among the old named species found in the literature, several are known only from the type collection, for example P. bibula (Taylor) Swinscow & Krog and P. subcrustacea (Malme) Brako. Old type specimens are often small or in poor condition, prohibiting destructive sampling for morphological, chemical or molecular investigation. Clarifying the taxonomic status of such type names remains a challenge, particularly with respect to currently accepted species. In addition, DNA extraction and amplification has proved difficult from tropical lichen material after only a few years or even months of storage (Staiger et al. Reference Staiger, Kalb and Grube2006; Weerakoon et al. Reference Weerakoon, Aptroot, Lumbsch, Wolseley, Wijeyaratne and Gueidan2012; Gueidan et al. Reference Gueidan, Aptroot, Cáceres and Binh2015).
In a recent molecular phylogeny of the family Ramalinaceae, Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) included 16 Phyllopsora species and showed that the genus, as commonly understood, was polyphyletic. Three species seemingly belonged in the family Malmideaceae Kalb et al., two species belonged in Sporacestra A. Massal., one in Bacidia, one was transferred to Bacidina Vězda, and three were placed in the new genus Parallopsora Kistenich et al. Notably, it was mainly the long-spored and/or sorediate species that were excluded from Phyllopsora. The clade containing the type species P. breviuscula (i.e. the genus Phyllopsora) was resolved as the sister genus of Biatora. On the other hand, two Crocynia (Ach.) A. Massal. species (i.e. C. gossypina (Sw.) A. Massal. and C. pyxinoides Nyl.) as well as Lecidea thaleriza Stirt. were included in Phyllopsora based on their position in the molecular phylogeny. It appears that the typical growth form of Phyllopsora, being characterized by areoles or squamules overgrowing a well-developed prothallus (Fig. 1), originated through convergent evolution caused by ecophysiological advantages (Lakatos et al. Reference Lakatos, Rascher and Büdel2006) rather than representing a unique synapomorphy facilitating genus delimitation (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a). The overall results of the Ramalinaceae study show that additional revisionary work is urgently required for species classified in Phyllopsora (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
In this study, we use an integrative approach to test species hypotheses in Phyllopsora. We focus on the currently accepted species while excluding all fossil species as well as old types that cannot be linked to the current taxonomy (i.e. using 64 accepted species as a starting point; see Supplementary Material Table S1, available online). The study is based on morphological and chemical information as well as DNA sequence data from both herbarium specimens and freshly collected material. Our aim was to test correspondence between the traditional species boundaries and species delimitations supported by molecular phylogenies. We treat c. 85% of the currently accepted Phyllopsora species and discuss the degree of phylogenetic information provided from chemotypes. Based on the results of this integrative taxonomic study, we present an updated species taxonomy of the genus Phyllopsora.
Materials and Methods
Taxon sampling
We aimed to investigate specimens of all accepted non-fossil Phyllopsora species (see Supplementary Material Table S1, available online). One of the authors (ET) has been working on the genus Phyllopsora for more than 25 years. The present study is based on our own experience with identifying species. More than 2500 phyllopsoroid specimens, including all available type material (either seen the physical voucher or a digitized image), have been investigated within the last 25 years. We studied Phyllopsora material borrowed from the following herbaria: B, BG, BM, CANB, E, GZU, H, HUTPL, MPEG, PDA and TNS. We also received material from the private herbaria of P. Diederich, A. Frisch, D. Killmann, Z. Palice, S. Pérez-Ortega and P. van den Boom. In addition, we used our own collections in ISE, O, UPS and VEN. Fresh material was collected in Brazil, Venezuela and Sri Lanka. Author names for the species studied are provided in Table 1.
Table 1. Specimens used in this study with the revised taxonomy, voucher information and GenBank Accession numbers provided. New sequences are indicated in bold; accessions can be recognized by the extract number in Figs 2–4; * indicates types; N/A = not applicable; – indicates missing data; ch = chemotype.

Morphology and chemistry
Microscopic sections were cut on a freezing microtome and mounted in water, 10% KOH (K), lactophenol cotton blue, and a modified Lugol's solution in which water was replaced by 50% lactic acid. Amyloid reactions were observed in the modified Lugol's solution after pretreatment in K. The types of upper cortex referred to in this paper (types 1 and 2) are those described by Swinscow & Krog (Reference Swinscow and Krog1981). Crystals of lichen substances were observed using polarized light. Thin-layer chromatography was performed in accordance with the methods of Culberson (Reference Culberson1972), modified by Menlove (Reference Menlove1974) and Culberson & Johnson (Reference Culberson and Johnson1982). Examinations were made in the three standard solvent systems A, B′ and C; of these, solvent system B′ was preferred for initial analyses. The presence of fatty acids was generally not investigated. Two-dimensional chromatography (Culberson & Johnson Reference Culberson and Johnson1976) was performed in a small number of cases. Results from morphological and chemical investigations were used to assign specimens to morphospecies.
Molecular laboratory work
Methods for DNA extraction, PCR amplification and DNA sequencing of the mitochondrial ribosomal small subunit (mtSSU) and the entire nuclear ribosomal internal transcribed spacer region (ITS: ITS1, 5.8S, ITS2), as well as the procedures for sequence assembly, followed Kistenich et al. (Reference Kistenich, Rikkinen, Thüs, Vairappan, Wolseley and Timdal2018b). When PCR amplification or Sanger sequencing failed, we used a five-fold dilution of the DNA-extracts as template. We used a local BLAST search for all newly generated Phyllopsora sequences against our Ramalinaceae dataset (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a). We identified the phylogenetic clade (sensu Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a) for each sequence, and subsequently removed all sequences belonging to the Malmideaceae (clade A), the Bacidia-group (clade C) and the Parallopsora-group (in clade D). Only those sequences falling into the Phyllopsora s. str. group (in clade F) were used for the present study (Table 1).
Phylogenetic analyses
The mtSSU and ITS sequences were aligned separately using MAFFT v.7.408 (Katoh & Standley Reference Katoh and Standley2013) with the E-INS-i algorithm and the nucleotide scoring matrix set to 1PAM / κ=2. We trimmed the ends of the ITS alignment to comprise only the ITS region and deleted the residual 18S and 28S sequence information. Four Biatora species (B. beckhausii, B. rufidula, B. vacciniiciola and B. veteranorum) were included in the alignments and used for rooting in the subsequent phylogenetic analyses. For each dataset, IQ-TREE v.1.6.7 (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015) was used for finding the best-fitting nucleotide substitution model among those implemented in MrBayes (i.e. 1-, 2- and 6-rate models), for finding the best partitioning scheme (Chernomor et al. Reference Chernomor, von Haeseler and Minh2016; Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) and for constructing a maximum likelihood phylogeny with assessment of bootstrap branch support (BS) using 1000 standard non-parametric bootstrap replicates. The mtSSU data were not divided into subsets, whereas we proposed three subsets for the ITS data corresponding to the ITS1, 5.8S and ITS2 regions. We tested this partitioning scheme using the TESTMERGE function. We checked for incongruences between the gene trees generated by IQ-TREE using compat.py (Kauff & Lutzoni Reference Kauff and Lutzoni2002) with a 50% branch support cut-off. In addition, Bayesian phylogenetic inference was carried out separately on each dataset with MrBayes v.3.2.6 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003; Altekar et al. Reference Altekar, Dwarkadas, Huelsenbeck and Ronquist2004) as described in Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a). The temperature increment parameter was set to 0·01 and 0·04 for mtSSU and ITS, respectively. We projected the posterior probabilities (PP) from the MrBayes analysis onto each IQ-TREE consensus tree with BS values, and collapsed branches with BS < 50 and PP < 0·7. The resulting trees were edited in TreeGraph2 (Stöver & Müller Reference Stöver and Müller2010).
Relationships among Phyllopsora were investigated by inferring a species tree from the ITS and mtSSU gene trees using StarBEAST (*BEAST) v.2.0.3 (Heled & Drummond Reference Heled and Drummond2010) as implemented in the BEAST 2 package v.2.5.1 (Bouckaert et al. Reference Bouckaert, Heled, Kühnert, Vaughan, Wu, Xie, Suchard, Rambaut and Drummond2014). *BEAST estimates a species tree from the sequence data under the multi-species coalescent model and handles uncertainty associated with gene trees (Heled & Drummond Reference Heled and Drummond2010). Terminals were classified into 63 species approximately following our own revised taxonomy, except that the chemotypes of P. buettneri and P. porphyromelaena were treated as separate species. We used the best-fitting nucleotide substitution model as suggested by IQ-TREE for each gene with a fixed overall substitution rate. For the clock model, we chose a relaxed lognormal clock (Drummond et al. Reference Drummond, Ho, Phillips and Rambaut2006) for each partition. We assumed a linear species tree population size model with a constant root and estimated the population mean. Several operators were adjusted according to suggested output values after conducting a test run. Three Markov chain Monte Carlo (MCMC) runs were conducted with 400 × 106 generations each, sampling every 5000th generation. We assessed convergence of the three runs and the adequacy of sampling using Tracer v.1.7.1 (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018). The first 50% of the sampled trees from each run was discarded as burn-in when combining the tree-files using LogCombiner v.2.5.0 as implemented in the BEAST 2 package. We used TreeAnnotator v.2.5.0 (BEAST 2 package) to generate posterior probabilities of nodes from the remaining trees of the combined runs on the maximum clade credibility tree with mean node heights. The resulting tree was edited in TreeGraph2.
Species delimitation analyses
We subjected our datasets to species delimitation analyses to compare our morphological understanding of species with a delimitation based on DNA sequence data. We conducted a PTP (Poisson Tree Processes) analysis using mPTP v.0.2.4 (Zhang et al. Reference Zhang, Kapli, Pavlidis and Stamatakis2013; Kapli et al. Reference Kapli, Lutteropp, Zhang, Kobert, Pavlidis, Stamatakis and Flouri2017) on the mtSSU and ITS gene trees separately, as mPTP handles single-locus data only. This software models speciation by directly using the number of nucleotide substitutions and thus inferring borders of the coalescent process (Zhang et al. Reference Zhang, Kapli, Pavlidis and Stamatakis2013). We used as input the best tree for each alignment generated by IQ-TREE and conducted both MCMC and maximum likelihood (ML) analyses using both the single- and the multi-rate versions of mPTP. For each MCMC mPTP analysis, we conducted four MCMC runs with 100 × 106 generations, sampling every millionth generation and assessed convergence. The first 10% of the MCMC samples was discarded as burn-in. We compared the results of the single-rate and multi-rate versions using a simple hierarchical likelihood ratio test (hLRT) to examine for overparameterization.
Results
Morphology and secondary chemistry
Species delimitation based solely on morphology proved difficult. While some specimens could be unambiguously identified (e.g. P. cuyabensis, P. halei and P. parvifolia), others had to be re-identified after TLC analysis (e.g. P. buettneri, P. ochroxantha, P. porphyromelaena and P. swinscowii). To facilitate morphological species identification, we have provided a table summarizing the main morphological features of each species (see Supplementary Material Table S2, available online). In total, 29 known chemical compounds were identified in species of Phyllopsora, in addition to various unidentified terpenoids, xanthones, pigments and other substances (Table 2). Seven species showed intraspecific chemotypic variation, with two new chemotypes recorded for both P. africana and P. porphyromelaena (Table 2).
Table 2. Lichen substances detected in Phyllopsora species and their chemotypes.

NONE: no lichen substances, ATR: atranorin, BARB: barbatic acid, ARG: argopsin, NARG: norargopsin, PAN: pannarin, DPAN: dechloropannarin, VIC: vicanicin, NVIC: norvicanicin, PHY: phyllopsorin, CPHY: chlorophyllopsorin, MPS: methyl 2,7-dichloropsoromate, MNPS: methyl 2,7-dichloronorpsoromate, PARV: parvifoliellin, FUR: furfuraceic acid, MFUR: methyl furfuraceiate, MHFUR: methyl homofurfuraceiate, FPC: fumarprotocetraric acid, NOR: norstictic acid, STIC: stictic acid, LOB: lobaric acid, NLOB: norlobaric acid, PHYS: physodic acid, HSEK: homosekikaic acid, HHSEK: hyperhomosekikaic acid, DIV: divaricatic acid, SAL: salazinic acid, HMIC: hydroxymicareic acid, MMIC: methoxymicareic acid, SECA: secalonic acid A, ZEO : zeorin, TERP: terpenoids, XAN: xanthones, PIGM: pigments, UNKN: unknown compounds.
M = major; S = submajor; m = minor; t = trace; x = present; ± = present or absent
Based on our own experience with species identification of Phyllopsora using morphology and chemical data we grouped the specimens into 48 morphospecies. Approximately 25% of the total material investigated could not be assigned to any known species.
Molecular data
We selected up to 13 individuals per morphospecies and included five unidentified specimens for DNA extraction and sequencing. We obtained mtSSU and ITS sequences for most Phyllopsora species, but only rarely for old (>30 years old) and/or poor quality specimens. In general, specimens collected less than ten years ago performed the best for DNA work, although we also obtained sequences from a specimen collected in 1969 (P. confusa; Table 1). The sequencing success was higher for the mtSSU than for the ITS. We produced 153 new mtSSU and 134 new ITS sequences (Table 1). In total, we generated DNA sequence data from 48 out of 64 accepted species (Supplementary Material Table S1, available online), including sequences of 11 types. This study is published along with a revision of the genus Phyllopsora in South-East Asia (Kistenich et al. Reference Kistenich, Bendiksby, Vairappan, Weerakoon, Wijesundara, Wolseley and Timdal2019a), where the additional Asian material of Phyllopsora will be treated phylogenetically in detail.
Based on local BLAST searches, the following seven species were found to belong to different Phyllopsora-segregates, which were excluded from Phyllopsora in Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a): 1) Bacidia-clade: P. conwayensis Elix, and 2) Toninia-clade: P. cognata (Nyl.) Timdal, P. glaucescens Timdal, P. longispora Swinscow & Krog, P. pocsii Vězda, P. soralifera Timdal and P. tobagensis Timdal. These species were excluded from the subsequent phylogenetic analyses.
Alignment
The mtSSU alignment consisted of 195 accessions and was 854 bp long with 11·8% missing data. The ITS alignment consisted of 174 accessions and was 861 bp long with 10·9% missing data. Both alignments are available from TreeBase (study no. 23741).
Model selection
The software IQ-TREE reported the GTR+I+Γ model as the best-fitting substitution model for the mtSSU alignment. For ITS, the software reported the following models and partitioning schemes: GTR+I+Γ for ITS1 and ITS2 separately, SYM+I+Γ for 5.8S and GTR+I+Γ for the entire ITS region. For the *BEAST analysis, IQ-TREE reported the GTR+I+Γ model as the best-fitting substitution model for both the mtSSU and ITS alignments.
Phylogenetic analysis of separate markers
The software compat.py reported several incongruences between the two gene trees generated by IQ-TREE. Most of these incongruences involved subterminal branches within one species but no strongly supported topological differences in the backbone. We chose not to concatenate our datasets due to these incompatibilities.
The MrBayes analyses halted automatically, after 11 × 106 generations for the mtSSU alignment and after 12 × 106 generations for the ITS alignment, when the ASDSF in the last 50% of each run had fallen below 0·01. We used 22 004 trees from the mtSSU analysis and 24 004 trees from the ITS analysis for constructing each final majority-rule consensus tree. Overall, the mtSSU tree showed a better resolution than the ITS tree. In general, accessions belonging to the same predefined morphospecies grouped together in both gene trees but, when resolved, relationships between morphospecies were slightly different between gene trees. In total, five morphospecies (i.e. P. buettneri, P. byssiseda, P. chodatinica, P. furfuracea and P. parvifoliella) proved polyphyletic and fell into two different clades each in both trees (Figs 2 & 3). Two of the five unidentified specimens grouped closely together as sister to P. halei, while the remaining three only showed a weakly supported relationship with P. canoumbrina, P. isidiotyla and P. malcolmii, respectively, and sit on long branches (Figs 2 & 3). Both trees showed three occasions where accessions of different predefined morphospecies mixed with another: P. hispaniolae and P. rosei, P. homosekikaica and P. foliatella as well as P. buettneri, P. porphyromelaena and P. chodatinica (Figs 2 & 3). We indicate three groups of species complexes to facilitate discussion (Figs 2 & 3, groups A–C).
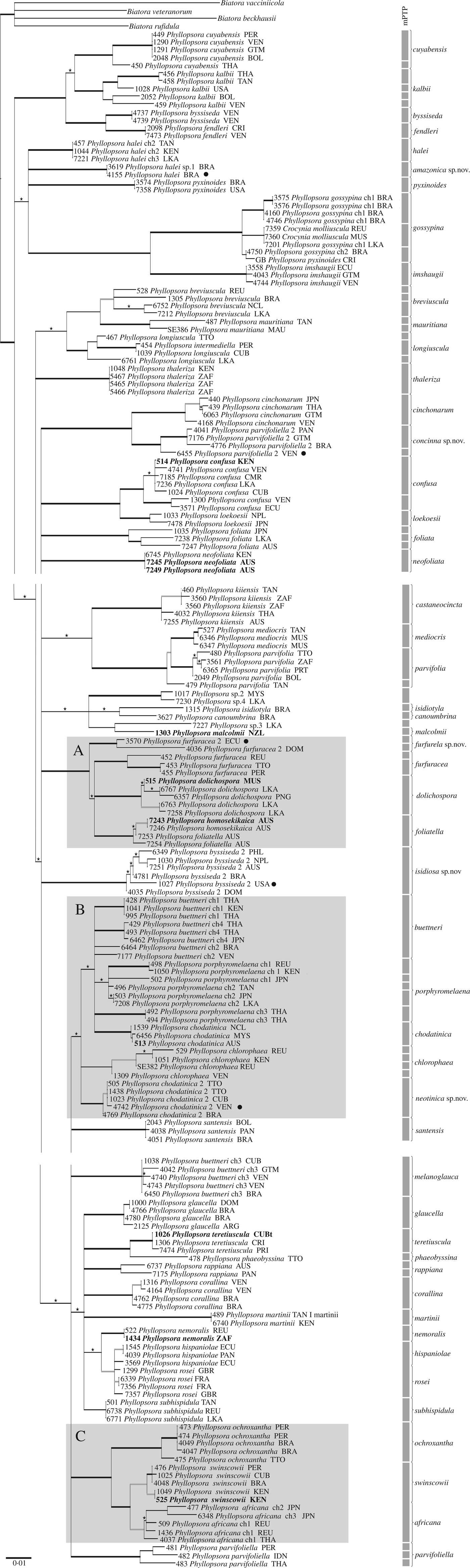
Fig. 2. mtSSU molecular phylogenetic tree. Extended majority-rule consensus tree resulting from the IQ-TREE analysis of the mtSSU alignment with Bayesian PP ≥ 0·7 and/or IQ-TREE maximum likelihood BS ≥ 50 and branch lengths. Strongly supported branches (PP ≥ 0·95 and BS ≥ 70) are marked in bold; branches with PP ≥ 0·95 and BS < 70 or PP < 0·95 and BS ≥ 70 are marked in bold grey; branches supported only with PP ≥ 0·7 or BS ≥ 50 are marked with an asterisk above the branch. Four species of Biatora were used for rooting. Accessions in bold indicate sequences of type specimens; black dots indicate sequences of type specimens for those species described here as new. All accession names include the official three-letter country codes according to ISO 3166-1 alpha-3. The species delimitation results of the mPTP analysis are indicated on the right, including the revised species understanding as of this study. Three major groups are distinguished to facilitate discussion (A, B, C). ch = chemotype. The numbers preceding the names are extract numbers for reference (Table 1).
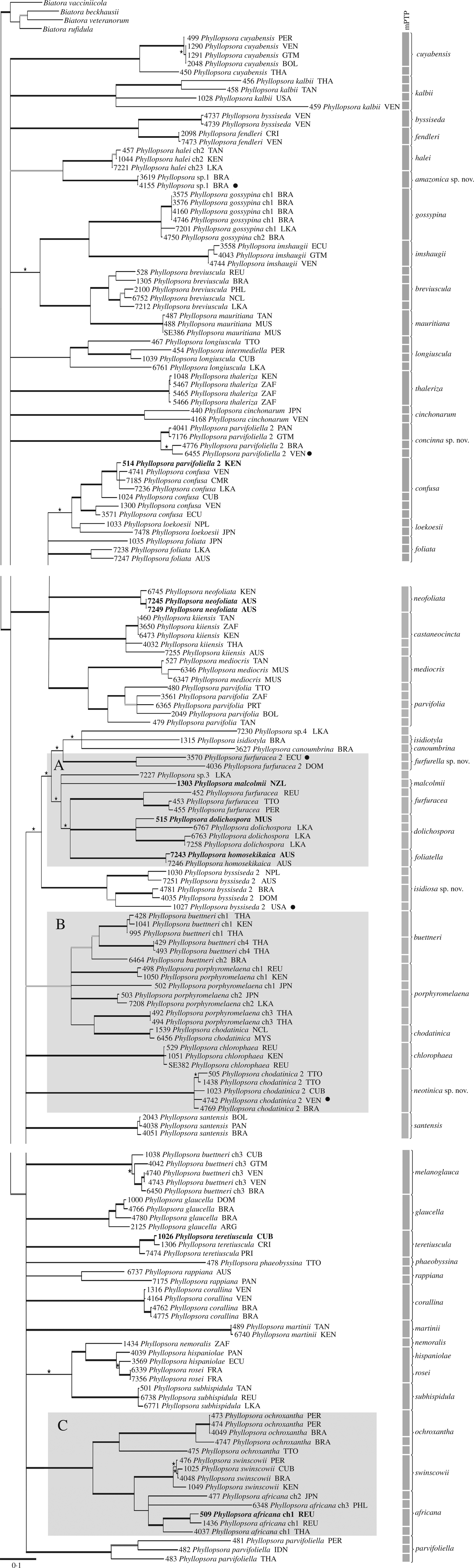
Fig. 3. ITS molecular phylogenetic tree. Extended majority-rule consensus tree resulting from the IQ-TREE analysis of the ITS alignment with Bayesian PP ≥ 0·7 and/or IQ-TREE maximum likelihood BS ≥ 50 and branch lengths. Strongly supported branches (PP ≥ 0·95 and BS ≥ 70) are marked in bold; branches with PP ≥ 0·95 and BS < 70 or PP < 0·95 and BS ≥ 70 are marked in bold grey; branches supported only with PP ≥ 0·7 or BS ≥ 50 are marked with an asterisk above the branch. Four species of Biatora were used for rooting. Accessions in bold indicate sequences of type specimens; black dots indicate sequences of type specimens for those species described here as new. All accession names include the official three-letter country codes according to ISO 3166-1 alpha-3. The species delimitation results of the mPTP analysis are indicated on the right, including the revised species understanding as of this study. Three major groups are distinguished to facilitate discussion (A, B, C). ch = chemotype. The numbers preceding the names are extract numbers for reference (Table 1).
Species delimitation analysis
According to the hLRT, the single-rate version of mPTP was preferred over the multi-rate version for each gene tree (P >0·01) and only the results of the single-rate version are presented here. The single-rate version of mPTP reported 79 delimited species for the mtSSU tree and 96 for the ITS tree. Results from the MCMC analyses were identical to the results from the ML analyses. In the single-rate analyses of each dataset, splitting morphological species was more common than lumping, and species were more often split in the ITS analysis (Figs 2 & 3). In general, long branches increased the frequency of inferring a species boundary in mPTP. Phyllopsora buettneri and P. porphyromelaena were divided into several species, partly according to chemotypes (Figs 2 & 3). The accessions of P. foliatella and P. homosekikaica as well as P. hispaniolae and P. rosei were delimited as only one species each (Figs 2 & 3).
Species tree reconstruction
For the species tree reconstruction with *BEAST, we used 160 004 trees to construct the maximum clade credibility tree (Fig. 4). The species tree does not show higher resolution than the gene trees (Figs 2 & 3) and is largely concordant with those. The phylogenetic placement of P. furfurella differs in the mtSSU and ITS trees (Figs 2 & 3), and the species is resolved here as sister to P. dolichospora, P. foliatella and P. furfuracea (Fig. 4). In the species tree, group B is resolved as a strongly supported clade (Fig. 4).
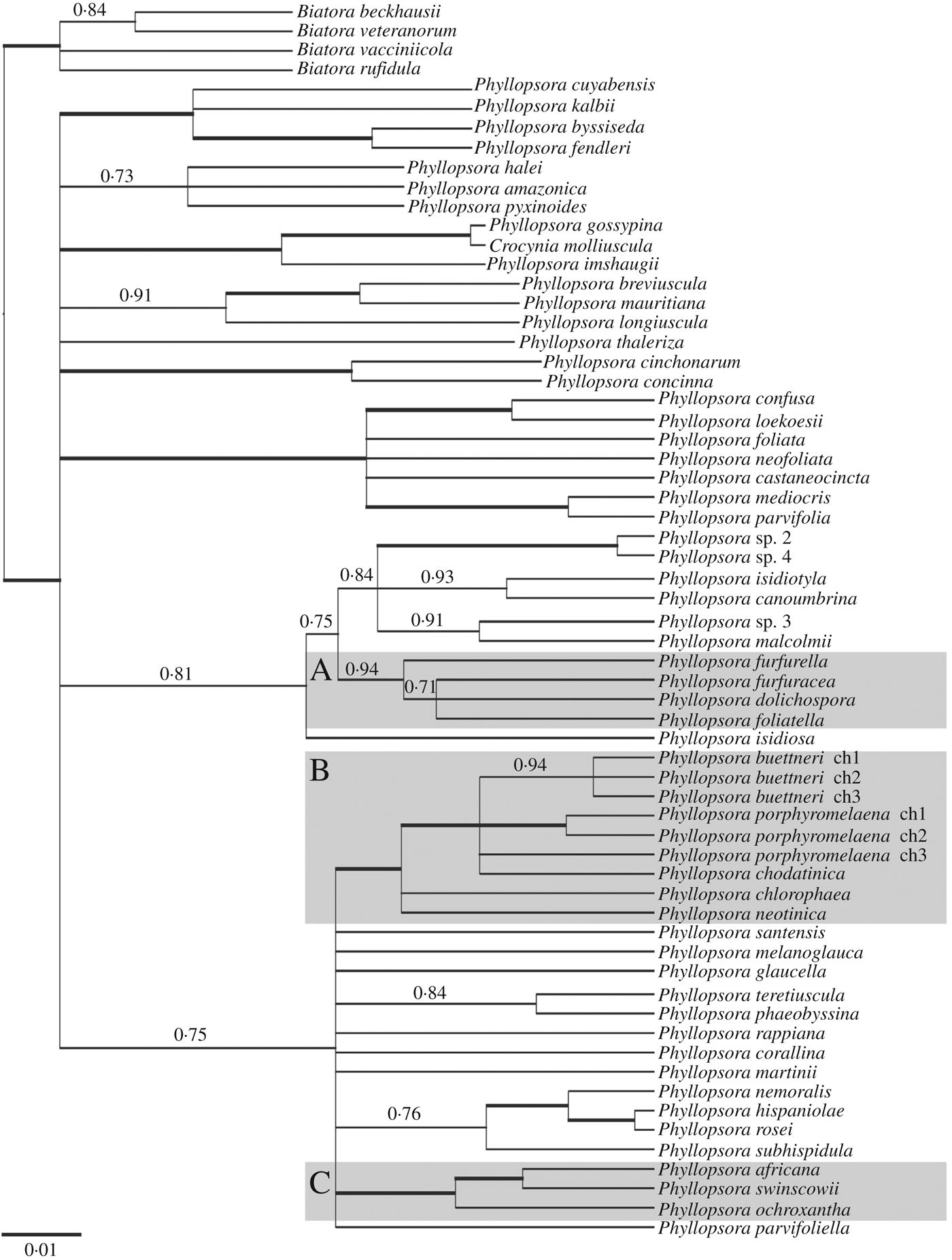
Fig. 4. Species tree reconstruction with a maximum clade credibility tree resulting from the *BEAST analysis of the combined mtSSU and ITS data with PP ≥ 0·7 and branch lengths. Strongly supported branches with PP ≥ 0·95 are marked in bold; PP values are given for PP ≤ 0·95. Four species of Biatora were used for rooting. Three major groups are distinguished to facilitate discussion (A, B, C). The classification is based on the revised taxonomy of accepted Phyllopsora species. ch = chemotype.
Taxonomic conclusions
As a result of the phylogenetic and species delimitation analyses, the species P. melanoglauca is resurrected for P. buettneri chemotype 3, and the species P. amazonica (‘Phyllopsora sp.1’; Fig. 5A), P. concinna (‘P. parvifoliella 2’; Fig. 5B), P. furfurella (‘P. furfuracea 2’; Fig. 5C), P. isidiosa (‘P. byssiseda 2’; Fig. 6A) and P. neotinica (‘P. chodatinica 2’; Fig. 6B) are described as new. Phyllopsora homosekikaica is synonymized with P. foliatella, and P. intermediella is synonymized with P. longiuscula.
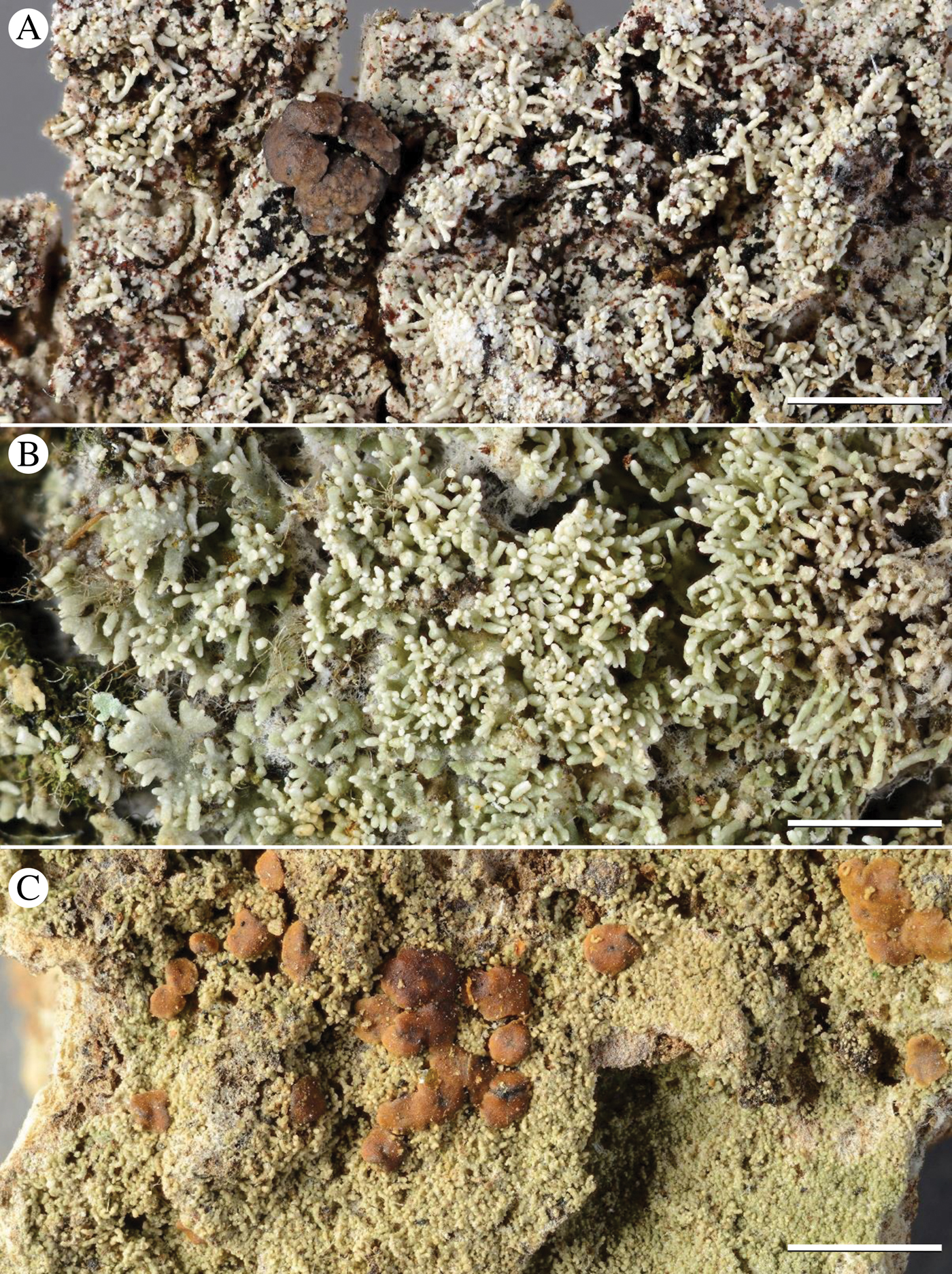
Fig. 5. Habit of Phyllopsora species described here as new: A, P. amazonica (O L-201094); B, P. concinna (O L-202505); C, P. furfurella (HUTPL, M. Prieto). Scales: A–C = 2 mm. In colour online.

Fig. 6. Habit of Phyllopsora species described here as new: A, P. isidiosa (BG L-93867); B, P. neotinica (O L-202526). Scales: A & B = 2 mm. In colour online.
Discussion
In this study, we provide a comprehensive contribution to the much needed revisionary work of the current Phyllopsora taxonomy. As species of Phyllopsora are generally difficult to identify based on morphology and chemistry only, molecular data give a new perspective on species circumscriptions in this mainly tropical genus. Based on our multiple sources of data, we describe five new species (i.e. P. amazonica, P. concinna, P. furfurella, P. isidiosa and P. neotinica), resurrect P. melanoglauca, and synonymize P. homosekikaica with P. foliatella and P. intermediella with P. longiuscula.
Species circumscriptions in Phyllopsora
In most cases accessions of the same species grouped together in well-supported clades on the molecular phylogenetic trees (Figs 2 & 3), supporting our traditional understanding of the species boundaries. This indicates that a detailed analysis of morphological characters in combination with patterns of lichen substances lay a useful foundation for species delimitation in Phyllopsora. Due to numerous incongruences in more ancestral and/or terminal nodes, we did not concatenate the mtSSU and ITS alignments, but decided to run a *BEAST analysis to obtain a species tree (Fig. 4). The gene trees taken together provide valuable information about species limits and indicate the extent of morphological and chemical character variation in each species. The species tree, in turn, informs us about relationships among Phyllopsora species. Detailed discussions of each accepted species are provided in the Taxonomy section, part A.
Morphological characters used to delimit Phyllopsora species mainly include thallus structure, texture and colour of the prothallus, presence or absence and type of vegetative dispersal units, as well as ascospore anatomy including spore dimensions (Timdal & Krog Reference Timdal and Krog2001). Even with experience in identifying Phyllopsora specimens, species identification using only morphological features is usually time-consuming and often unreliable. Many of the specimens investigated had to be renamed more than once after tentative identification by morphology, then chemistry and subsequently DNA sequence data. Not surprisingly, many herbarium specimens were incorrectly identified. This shows that new country reports for species of Phyllopsora cannot automatically be accepted, especially when TLC has not been performed. Herein, we therefore rely solely on our own species determinations and well-documented species records for mapping geographical species occurrences.
In general, we could observe that most morphological features used to characterize a species, such as vegetative dispersal units, may be found in a variety of not necessarily closely related species. Almost all Phyllopsora species seem to exhibit some form of vegetative dispersal structure. Isidia, lacinules and phyllidia seem to have evolved several times and have transformed frequently, rendering them of little use for predicting relationships among Phyllopsora species and evolutionary lineages therein: while some clades of sister species seem to be consistent in their means of vegetative dispersal, other clades seem to have switched the preferred dispersal units. Lacinules are found in a small number of closely related species (e.g. in the buettneri-chlorophaea-chodatinica-porphyromelaena group; Figs 2–4, group B), whereas isidia are the most common means of vegetative dispersal. They are observed in the dolichospora-foliatella-furfuracea group (Figs 2–4, group A), the africana-ochroxantha-swinscowii group (Figs 2–4, group C) as well as in numerous other species, such as P. cinchonarum, P. corallina, P. glaucella and P. rappiana. Within some clades (Figs 2–4), on the other hand, closely related species may form different vegetative dispersal propagules, for example, in the mediocris-parvifolia clade (lacinules and phyllidia) or the confusa-loekoesii clade (lacinules and isidia). We also found that different types of vegetative diaspores might be present on different specimens of the same species, such as in P. africana (isidiate and lacinulate morphs) and P. longiuscula (isidia or lacinules; the isidiate morph, previously named P. intermediella, being synonymized here). In other species previously not known to produce isidia, such as P. fendleri, we observed a few but distinct isidia. Previously Brako (Reference Brako1991: 7) suggested that the presence or absence of isidia was an unreliable character for identification of most species.
TLC analysis of lichen substances is often crucial for correct species identification in Phyllopsora. Some chemical compounds are not known to occur outside the genus, such as furfuraceic acid, parvifoliellin and phyllopsorin. In total, we identified 29 lichen compounds in addition to various pigments, terpenoids, xanthones and unidentified compounds throughout Phyllopsora, as circumscribed in this article (Table 2). About 30% of the species did not contain any lichen substances. We observed similar patterns in the distribution of lichen substances between species as in the distribution of vegetative dispersal units. Certain lichen substances are found both within and outside of groups of species complexes (Table 2). Furfuraceic acid, for example, is present in the species of the furfuracea-dolichospora group (Figs 2–4, group A), as well as in P. castaneocincta, P. chlorophaea and P. neofoliata; chlorophyllopsorin is present in the africana-ochroxantha group (Figs 2–4, group C) but also in P. hispaniolae, P. martinii and P. teretiuscula. Several Phyllopsora species are known to comprise different chemotypes, such as P. buettneri and P. porphyromelaena, including species with acid-deficient strains, such as P. foliatella (Table 2). In the latter, we found specimens with a rather complex chemistry (hyperhomosekikaic and homosekikaic acids) but also specimens lacking substances. We assume that the loss of chemical substances has been more common than switching to chemically unrelated substances, as previously suggested by Culberson & Culberson (Reference Culberson and Culberson2001). The presence of acid-deficient chemotypes is similarly found in P. castaneocincta but does not generally seem to be a common phenomenon in Phyllopsora species.
Species showing distinct morphological characters (e.g. P. cuyabensis) or a unique composition of lichen substances (e.g. P. dolichospora) are readily identifiable. Poorly developed morphotypes and/or acid-deficient strains, however, are far more challenging to identify. In these cases, DNA sequence data seem to be necessary to reliably identify the specimens. We found either genetic marker to be suitable for species identification, although the mtSSU tree was slightly more resolved than the ITS tree (Figs 2 & 3). Molecular species identification, however, may be ambiguous when no reference sequences exist or species clades are poorly resolved. The use of a fixed barcode gap has been suggested to facilitate species circumscription (Hebert et al. Reference Hebert, Cywinska, Ball and deWaard2003; Schoch et al. Reference Schoch, Seifert, Huhndorf, Robert, Spouge, Levesque and Chen2012). The gene trees showed that the molecular differences found within and between species based on branch lengths are highly variable (Figs 2 & 3). Many clades have only short intraspecific branches (e.g. P. corallina, P. glaucella and P. melanoglauca) while others are longer (e.g. P. kalbii and P. longiuscula; Figs 2 & 3). This indicates that a fixed barcode gap cannot be applied here, based on the genetic markers and Phyllopsora species circumscriptions used. Instead, each case has to be evaluated separately.
Unresolved species complexes
Most of our predefined morphospecies each grouped into a supported clade in the gene trees (Figs 2 & 3). However, some groups of species could not be fully resolved by mtSSU or ITS and require further attention in future studies. Sequencing additional markers, as well as increasing the sample size with specimens from additional geographical regions, will most likely provide improved resolution for delimiting the problematic species.
One of the species complexes that was not fully resolved is group B (Figs 2–4). We found several morphologically identical chemotypes (Table 2) in both P. buettneri and P. porphyromelaena (Timdal Reference Timdal2011). We were curious to investigate whether these represent species with chemical variation or include several distinct, yet morphologically inseparable taxa. In the case of P. buettneri, we sampled specimens from four out of five described chemotypes and recovered them according to chemotypes in the two gene trees (Figs 2 & 3). Chemotype 3, present in South America, was resolved as a separate species outside group B (Figs 2 & 3). This chemotype was originally described as a separate species, P. melanoglauca Zahbr., but was reduced into synonymy with P. buettneri in two steps; first by Brako (Reference Brako1991) who treated it as a variety of P. buettneri, and then by Timdal (Reference Timdal2008). As it is phylogenetically distinct from the morphologically identical P. buettneri, we resurrect the species P. melanoglauca (see also section on new species below). The other three chemical strains of P. buettneri grouped with varying support (Figs 2 & 3). Chemotypes 1 and 4 are currently known from the Palaeotropics, chemotype 2 from the Neotropics and chemotype 5 (not examined by us) from Australia (Elix Reference Elix2006b). The mPTP analysis resolved chemotypes 1, 2 and 4 as separate species on the ITS tree (Fig. 3), while they grouped into a single species on the mtSSU tree (Fig. 2), probably because the mtSSU is too conserved to distinguish among chemotypes. Our accessions of P. porphyromelaena were also resolved according to chemotype, albeit with less support than in P. buettneri. We also found two new chemical strains (chemotypes 3 and 4) in P. porphyromelaena. Chemotype 3 is present in Thailand, but its accessions cluster with P. chodatinica instead of the other P. porphyromelaena specimens and are resolved as a separate species in both mPTP analyses (Figs 2 & 3). Chemotype 4 of P. porphyromelaena occurs in the Neotropics. It is identical to chemotype 1 but additionally contains zeorin. Unfortunately, we were not able to obtain sequences of the investigated specimens of chemotype 4. The overall resolution of group B, containing P. buettneri, P. chodatinica and P. porphyromelaena among others, is poor (Figs 2–4). The three species exhibit slightly different thallus morphologies (mean squamule size and pruinosity), spore sizes and chemistry (Elix Reference Elix2006a, Reference Elixb, c; Table 2). Even though they are morphologically similar, they vary greatly in their chemical compositions. Brako (Reference Brako1991) described several chemical strains of the three varieties of P. buettneri, which Timdal (Reference Timdal2008, Reference Timdal2011) recognized as the chemotypes of three distinct species, P. buettneri, P. chodatinica and P. porphyromelaena. Even when using sequence data from two genetic markers, we were unable to resolve these species and chemotypes.
In contrast to the buettneri-chodatinica-porphyromelaena complex, the clade, consisting of P. africana, P. ochroxantha and P. swinscowii, is well delimited on our phylogenetic trees (Figs 2–4, group C) but proved to be more challenging with respect to species delimitation based on morphological and chemical characters. Prior to this study, the three species were regarded as morphologically similar (forming medium-sized, isodiametrical squamules with long, cylindrical isidia and growing on a well-developed reddish brown prothallus) but could be distinguished by chemical composition (argopsin and chlorophyllopsorin, phyllopsorin and chlorophyllopsorin, and methyl 2,7-dichloropsoromate and methyl 2,7-dichloronorpsoromate, respectively; Timdal & Krog Reference Timdal and Krog2001; Timdal Reference Timdal2008, Reference Timdal2011). They also exhibit different distribution ranges: Phyllopsora africana seems to be present in Asia and Africa, P. ochroxantha in South America, and P. swinscowii in Africa and South America. Our phylogenies show that the species indeed form a monophyletic group (Figs 2–4, group C). However, relying on chemical patterns for species delimitation has now become more difficult with additional chemotypes described for P. africana and sequencing seems to be necessary to assign problematic specimens correctly to either P. africana or P. swinscowii. However, P. ochroxantha may still be distinguished from the other two species by its unique chemistry (Table 2). On the ITS tree, the accession of P. ochroxantha from Trinidad and Tobago is separated from the remaining P. ochroxantha accessions from Brazil (Fig. 3) by a long branch. This accession might represent a new species but more sequence data from different genetic markers are necessary to determine its status. Phyllopsora africana and P. swinscowii are more closely related to each other than either is to P. ochroxantha (Figs 2–4, group C) and were resolved as a single species in the mtSSU mPTP analysis (Fig. 2). The two psoromate lichen substances, previously characteristic for P. swinscowii, were also found in some specimens of P. africana. Here we show that P. africana forms three different chemotypes: chemotype 1 is found in the holotype of P. africana; chemotype 2 is identical to the chemical pattern found in P. swinscowii; chemotype 3 represents a combination of 1 and 2 (Table 2). Moreover, the specimens with chemotypes 1 and 3 may also form lacinules instead of isidia. The P. africana specimens of chemotype 2 are morphologically identical to P. swinscowii and thus the two currently represent a closely related pair of cryptic species (Struck et al. Reference Struck, Feder, Bendiksby, Birkeland, Cerca, Gusarov, Kistenich, Larsson, Liow and Nowak2018). Therefore, P. africana seems to be a heterogeneous assemblage of specimens with regard to chemistry and morphology, and difficult to delimit from P. swinscowii. More sequence data from different markers and from additional specimens are necessary to provide more robust information about whether the new circumscription of P. africana (with different chemo- and morphotypes) comprises a good species, or whether it should be synonymized with P. swinscowii, or split into several species. Detailed population genetic studies from different parts of the Palaeotropics might improve our knowledge about its taxonomic status.
Another unresolved species complex is the P. hispaniolae-rosei complex (Figs 2 & 3). The two species are morphologically different: P. rosei forms a granulose thallus on a white prothallus and has 1–3-septate ascospores, while P. hispaniolae forms coralloid squamules on a reddish brown prothallus and has simple ascospores. They also differ in their lichen substances (Table 2) and have different distribution ranges, with P. rosei being a temperate and P. hispaniolae a tropical species. Hence, we suggest keeping the two species separate until further specimens and genetic markers have been examined.
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) included two species of Crocynia in Phyllopsora. Previously Crocynia was accepted as a distinct genus based on its characteristic cobwebby, byssoid thallus lacking an upper cortex (e.g. Hue Reference Hue1909, Reference Hue1924). Many species have been assigned to this genus, most of which are expected to be reassigned to other genera, such as Lepraria Ach. The present study corroborates the findings of Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) that Crocynia gossypina and C. pyxinoides indeed belong to Phyllopsora (Figs 2–4). Although their clade is not fully resolved, the two species do not group together (Figs 2 & 4), indicating that they are not sister species. Unfortunately, we were only able to generate mtSSU sequences of P. pyxinoides. The accession of P. pyxinoides downloaded from GenBank seems to be misidentified, as it groups together with the various chemotypes of P. gossypina and not with the other two P. pyxinoides accessions in the mtSSU tree (Fig. 2). The accessions of P. gossypina also group together with a third species of Crocynia, C. molliuscula, in the mtSSU tree (Fig. 2). The latter differs clearly from P. gossypina in forming bright brown, convex, non-marginate apothecia instead of dark brown apothecia with a lighter margin. Both species overlap in their chemistry by containing norstictic acid, as found in P. gossypina chemotype 2 (Table 2). Surprisingly, these species group into one clade with rather short branches (Fig. 2) but a possible synonymy of the two species is difficult to comprehend based on morphology. As we only generated short mtSSU sequences of two C. molliuscula specimens, we recommend sequencing additional specimens and providing ITS sequences before drawing taxonomic conclusions. From a morphological point of view, one would have expected species of Crocynia to group with P. cuyabensis, a species also lacking an upper cortex, but neither species did (Figs 2–4). Our results indicate that the upper cortex has been lost more than once within Phyllopsora and is not a reliable criterion for distinguishing Crocynia. As Crocynia (priority 1860) is an older name, Phyllopsora is proposed for conservation (Kistenich et al. Reference Kistenich, Ekman, Bendiksby and Timdal2019b).
Species delimitation with mPTP
When comparing results generated by the single- and multi-rate models of mPTP, we found that the single-rate model split species more often (Figs 2 & 3), while the multi-rate model lumped several morphologically well-distinguished species into one entity (data not shown). Kapli et al. (Reference Kapli, Lutteropp, Zhang, Kobert, Pavlidis, Stamatakis and Flouri2017) found the multi-rate model to outperform the single-rate model on a variety of different datasets. In our datasets, however, the hLRT preferred the single-rate model, indicating that the multi-rate model constituted an overparameterization. The single-rate model delimited 3–4 times more entities than the multi-rate version, which is indeed a huge difference and shows the necessity for conducting an hLRT. However, the results generated by both multi-rate and single-rate models seemed to under- and overestimate the correct number of species, respectively. The most reasonable number of species probably lies somewhere in between the two models. Due to this huge difference in delimited entities, we set out to perform a second kind of species delimitation analysis using the software BPP v.4.0 (Bayesian Phylogenetics and Phylogeography; Yang Reference Yang2015; Flouri et al. Reference Flouri, Jiao, Rannala and Yang2018) for a combined species tree investigation. This method uses the multispecies coalescent (MSC) model to compare different models of species delimitation (Yang & Rannala Reference Yang and Rannala2010; Rannala & Yang Reference Rannala and Yang2013) and species phylogeny (Yang & Rannala Reference Yang and Rannala2014; Rannala & Yang Reference Rannala and Yang2017) in a Bayesian framework, accounting for incomplete lineage sorting due to ancestral polymorphism and gene tree-species tree discordance as implemented in analysis type A11. As our data consisted of only two loci but a large number of tentative species (c. 40–45) with few sequences per species, we encountered severe mixing and convergence problems in the MCMC runs in our analyses. Despite several attempts to adjust our priors and MCMC fine-tune values, we were unable to resolve these issues. Additional loci and/or more sequences per species might lead to better mixing and convergence (Yang & Rannala Reference Yang and Rannala2014). Carstens et al. (Reference Carstens, Pelletier, Reid and Satler2013) recommend testing several different species delimitation programs and trust only those delimitations that are congruent across methods.
In mPTP, speciation is modelled by using the number of substitutions, that is branch lengths are compared between and among tentative species (Zhang et al. Reference Zhang, Kapli, Pavlidis and Stamatakis2013). This means that long branches usually indicate the presence of a separate species. In several cases, mPTP split off one or two accessions of a morphospecies as a separately delimited species, usually placed on a longer branch and collected from a different continent than the remainder of the accessions (Figs 2 & 3). In P. breviuscula, our accessions from South-East Asia (i.e. New Caledonia, Philippines and Sri Lanka) were resolved as one species, while the accessions from La Réunion and Brazil were each delimited as a separate species (Figs 2 & 3). Also, in P. cuyabensis the Asian accession was delimited as a separate species from the South American accessions (Figs 2 & 3). In P. isidiosa, the case is slightly different: accessions from North America were separated from those from South America and Asia/Australia only in the ITS tree (Fig. 3). In other cases, mPTP split almost all accessions belonging to one morphospecies into different species, as for instance in P. kalbii (Figs 2 & 3). The species is pantropical and seemingly genetically highly variable. For the time being, we consider that the accessions belong to only one species in the instances mentioned above, because of the shared morphological characters and lack of lichen substances (or traces of atranorin). Hence, we recommend treating species delimitations inferred by statistical programs, such as mPTP, with caution.
In general, uneven sampling of a species is known to decrease mPTP accuracy (Zhang et al. Reference Zhang, Kapli, Pavlidis and Stamatakis2013; Kapli et al. Reference Kapli, Lutteropp, Zhang, Kobert, Pavlidis, Stamatakis and Flouri2017). In our data, sampling additional specimens to improve the geographical coverage might adjust the delimitation results. On the other hand, mPTP results may be correct in recognizing populations on different continents as separate species if the extent of intercontinental genetic exchange has been severely restricted for a long time. Where there is no morphology or chemistry to support separate species, we have conservatively chosen to treat them as a single species.
Taxonomic conclusions
New species
In this study, we found several clades that seem to represent undescribed species. Based on our phylogenetic trees, we resurrect P. melanoglauca for chemotype 3 of P. buettneri and describe five new species: P. amazonica (‘Phyllopsora sp.1’; Fig. 5A), P. concinna (‘P. parvifoliella 2’; Fig. 5B), P. furfurella (‘P. furfuracea 2’; Fig. 5C), P. isidiosa (‘P. byssiseda 2’; Fig. 6A) and P. neotinica (‘P. chodatinica 2’; Fig. 6B). Before describing the new species, we considered the possibility that they could belong to poorly understood or currently synonymized species. Based on the characteristic morphology and/or chemistry of the new species, however, we could not find any congruent specimens among the old types (‘P. furfuracea 2’ is excluded from that statement but see discussion below). Hence, we describe them here as new species.
The sequences of the newly described species grouped into distinct, strongly supported clades, indicating that they comprise entities to be recognized at species level (Figs 2 & 3). While we considered P. amazonica (Fig. 5A) to be a new species from first sight, the other four species were discovered only after phylogenetic analyses. These four species are morphologically and/or chemically similar or identical to well-known Phyllopsora species. Phyllopsora neotinica (Fig. 6B), for example, was at first regarded as a chemical strain of P. chodatinica due to its morphological similarity, although it lacks the name-giving xanthone chodatin. Phyllopsora neotinica occurs in the Neotropics only, while P. chodatinica occurs in the Palaeotropics. In P. concinna (Fig. 5B), we encountered a mixture of the morphology of P. cinchonarum and the chemistry of P. parvifoliella. Thus, specimens of P. concinna may be distinguished from each of the two aforementioned species by chemistry and morphology, respectively. Also P. furfurella (Fig. 5C) was initially assumed to belong to P. furfuracea because of the presence of furfuraceic acid. The former differs, however, in forming a white prothallus and only small and sparse isidia. Most of our P. furfuracea accessions (in addition to further unpublished sequences) clustered into a clade sister to P. dolichospora and P. foliatella (Figs 2 & 3, group A) and are morphologically closer to the type of P. furfuracea than the P. furfurella specimens. The type of P. furfuracea is old and was described from the Mariana Islands and, unfortunately, we could not obtain any fresh specimens from Micronesia or South-East Asia for sequencing. Some of the specimens of P. isidiosa (Fig. 6A) initially showed some similarity to P. byssiseda, while others rather resembled P. isidiotyla. Specimens of P. isidiosa form more delicate isidia than those found in P. byssiseda but are coarser than those in P. isidiotyla. Hence, P. isidiosa seems to be morphologically intermediate between these two species and single specimens of all three species may be challenging to correctly identify without DNA sequence data.
Three unidentified specimens (i.e. extract numbers 1017, 7227 and 7230), that are typically sterile and not containing lichen substances, resolved on long branches in close phylogenetic proximity to P. canoumbrina, P. isidiotyla and P. malcolmii (Figs 2–4). The identification of the specimens of P. canoumbrina and P. isidiotyla, however, is based only on morphological comparisons to the type material and is ambiguous as these two species are rather poorly understood and rarely collected. We regard the three unidentified specimens as morphologically different from their identified sister species. However, it is possible that some of them are conspecific with one or more of the species that we could not investigate molecularly and are generally poorly understood, for example P. minor. As all of the specimens show considerable sequence variation as well as minor morphological differences, it is possible that they represent one or more new species. However, we consider it premature to describe them now as we do not know the full extent of morphological, chemical and molecular variation in these groups. As the unidentified specimens seem to be closely related to group A (Figs 2–4), which contains many morphologically similar species, it is uncertain whether the minor morphological differences are diagnostic characters. Chemistry is also variable inside group A (Figs 2–4) since specimens of P. foliatella and P. furfuracea may also be acid deficient. Therefore, additional collections and/or more sequence data should be studied before new species are described.
Species not sequenced
In this study we accept 54 Phyllopsora species (including four new and one resurrected species) which we consider well understood (Taxonomy, part A). We generated sequences from 51 of the species listed in part A, but could not obtain sequences from P. himalayensis, P. methoxymicareica and P. microdactyla due to lack of fresh material. We still consider those to be well delimited by morphology and/or chemistry.
In addition, we have listed 19 species names which we consider poorly understood or doubtful, as well as fossil species (Taxonomy, part B). None of these could be sequenced. Many of the species are known only from collections made more than 30 years ago, rendering PCR amplification and Sanger sequencing of their DNA extracts a challenging task with a high risk of failure. In addition, many specimens are small and in poor condition so that destructive sampling for DNA sequencing is only acceptable when positive results are highly likely. So far, however, no such methods have been developed to routinely and successfully sequence old lichen material.
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) excluded from the genus all studied species formerly assigned to Phyllopsora producing long, acicular ascospores and/or soredia. By extension, those characters provide a basis for suggesting that some of the species listed in part B may have to be excluded from Phyllopsora, such as P. microphyllina and P. catervisorediata. The former species forms acicular ascospores (Timdal Reference Timdal2011), while the latter forms soredia (Mishra et al. Reference Mishra, Upreti, Nayaka and Haridas2011). Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011) suggest a close relationship between P. catervisorediata and P. soralifera; the latter is a species we find not to belong in Phyllopsora based on unpublished sequence data (see section on excluded species below and Taxonomy, part B). However, molecular data are needed before conclusions on species boundaries and generic affiliations are drawn for P. catervisorediata and P. microphyllina.
There are several species names in the genus Phyllopsora which are based solely on old and often poor quality types, for example P. griseocastanea, P. manipurensis and P. subhyalina. Thus, morphological characters of those specimens are difficult to interpret. Considering the high range of morphological (and chemical) variation exhibited in some species, it is currently impossible to ascertain whether some of our unidentified sequences belong to those species. It is also likely that additional 19th century names exist in Phyllopsora, originally described in the genera Bacidia or Lecidea, which we did not study.
Excluded species
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) found the genus Phyllopsora to be polyphyletic. In addition to Phyllopsora s. str. in the Ramalinaceae, two species groups occurred in other clades of the same family, whereas P. atrocarpa, P. lividocarpa and P. nigrocincta belonged in the family Malmideaceae. Among the sequenced Phyllopsora specimens that did not belong to Phyllopsora s. str., three species grouped into the Bacidia clade: P. sorediata belongs in Bacidia, while P. pertexta and P. borbonica represent the resurrected genus Sporacestra. Based on a local BLAST search of unpublished sequences produced in the present study, we propose to exclude an additional seven species from Phyllopsora: P. conwayensis, P. cognata, P. glaucescens, P. longispora, P. pocsii, P. soralifera and P. tobagensis. To determine the respective generic placements of these species prior to making formal recombinations, detailed phylogenetic studies are necessary, including more representatives of each species and a broader taxonomic and distributional sampling of their close relatives. See also the Taxonomy section, part C, for a brief discussion of these species. Phyllopsora pyrrhomelaena is excluded from the genus Phyllopsora even though we were not able to produce sequences. This species appears to be a close relative of P. atrocarpa, P. lividocarpa and P. nigrocincta because of their shared apothecial anatomy, pigmentation, and chemistry (Timdal Reference Timdal2008, Reference Timdal2011). Hence, it is considered better accommodated in another genus in the Malmideaceae.
Our sequences of the isotype of P. conwayensis were found to be associated with the Bacidia clade. Both P. conwayensis and P. sorediata produce acicular ascospores, c. 25–30 × 0·8–1·2 µm in size, and have similar apothecial and thallus morphologies (see Elix Reference Elix2006c; Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007), but P. conwayensis differs from P. sorediata in lacking soralia and having a more complex chemistry (Elix Reference Elix2006c; Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007). Despite these differences, we do not discount the possibility that P. conwayensis might merely be a chemical strain of P. sorediata. However, before making formal combinations, further molecular studies including additional specimens of these two species are needed to clarify their status.
Four Phyllopsora species (i.e. P. brakoae, P. lacerata, P. labriformis and P. leucophyllina) occur in the Toninia-clade in Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a). While P. lacerata was transferred to Bacidina, the new genus Parallopsora was described to accommodate the other three species. In the present study we also found unpublished sequences of P. cognata, P. glaucescens, P. longispora, P. pocsii, P. soralifera and P. tobagensis to group into this clade (data not shown). All species contain acicular ascospores (Swinscow & Krog Reference Swinscow and Krog1985; Vězda Reference Vězda2003; Timdal Reference Timdal2008, Reference Timdal2011), indicating that they do not belong to Phyllopsora (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a). Our unpublished accessions of P. longispora clustered together with Aciculopsora salmonea Aptroot & Trest, the type of a recently described genus containing two species (Aptroot et al. Reference Aptroot, Umana, Chaves and Trest2006; Cáceres Reference Cáceres2007). Aciculopsora salmonea differs from P. longispora in having a typical salmon-coloured hymenium, 7–9-septate ascospores and lacking both lichen substances and isidia (Swinscow & Krog Reference Swinscow and Krog1985; Aptroot et al. Reference Aptroot, Umana, Chaves and Trest2006). In addition, A. salmonea is known from dry forests, while P. longispora prefers humid moist forests which are also typical habitats of species of Phyllopsora (Swinscow & Krog Reference Swinscow and Krog1985; Aptroot et al. Reference Aptroot, Umana, Chaves and Trest2006). Further morphological and molecular studies are currently being prepared to investigate whether these two species are conspecific or represent different species in the same genus (S. Kistenich, G. Weerakoon & E. Timdal, unpublished data). The remaining Phyllopsora species share common features with each other and the three Parallopsora species, such as ascospore size and chemistry, but are variable in thallus morphology. We suggest transferring them to the new genus Parallopsora pending further molecular investigations.
Outlook
In this study, we have attempted to construct an initial baseline taxonomy of the tropical genus Phyllopsora by integrating phenotypic and genetic information to better understand species circumscriptions. Much remains to be done, however, to understand species delimitation in the genus. As PCR amplification and subsequent Sanger sequencing of many samples with some highly degraded DNA have proved to be challenging and time-consuming, the applicability of high throughput sequencing (HTS) platforms should be explored, for example using genome-skimming approaches. Thus, time and costs could be substantially reduced while gaining multiple phylogenetically relevant markers of many specimens simultaneously. Since the DNA of tropical Phyllopsora species seems to degrade rapidly after only a few years of storage, type material can rarely be sequenced. Here, HTS approaches might also be ideal for retrieving sequence data from such highly fragmented DNA, including old types (Prosser et al. Reference Prosser, deWaard, Miller and Hebert2016).
Phyllopsora is still poorly known in many parts of the world, such as the inner part of the Amazon, West and Central Africa, and South-East Asia. Generally, old-growth forests in tropical regions are becoming rare due to increased deforestation worldwide and are usually difficult to access. Obtaining formal sampling and export permissions poses an additional challenge. We discovered several new species from South America by exploring easily accessible secondary rainforests. However, little is known about the diversity of Phyllopsora in primeval tropical forests. As some Phyllopsora species are rarely collected or known from old type material only, more collections of Phyllopsora are needed to fully explore the diversity of the genus and the geographical distribution of the species.
Taxonomy
Some of the type specimens cited as ‘holotype’ by Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991) are merely part of a gathering of a given species. In these cases, we have corrected the authors’ use of ‘holotype’ to ‘lectotype designated by’ (Art. 9.10; see also McNeill Reference McNeill2014). In some cases, it is unclear whether the author(s) saw one or more specimens of the same gathering or perhaps even multiple gatherings. In these cases, we have kept the assignments favoured by those authors but note that some types of names listed by them as holotypes might be lectotypes.
The Taxonomy section is divided into three parts: part A comprises the well-understood, extant species as accepted in this study; part B contains those species, which are poorly understood or doubtful, as well as the two fossil species; part C lists excluded species. As species identification in Phyllopsora is difficult, we recommend consulting the morphological characteristics in Table S2 (see Supplementary Material, available online) in combination with chemistry in Table 2 for a first identification, and subsequently referring to the original species description. The Phyllopsora website can also be visited for additional pictures and information about the species: http://nhm2.uio.no/lichens/Phyllopsora.
A. Accepted, extant species
Phyllopsora africana Timdal & Krog
Mycotaxon 77: 64 (2001); type: La Réunion, along road to Plaine d'Affouches, above Bras Citron, at point where road meets track, 20°57′S, 55°25′E, alt. 1220 m, 26-09-1996, H. Krog & E. Timdal RE8/13 (O L-798!—holotype; UPS!—isotype) (TLC: chlorophyllopsorin (major), argopsin (minor); DNA: MK352138 (mtSSU), MK352317 (ITS)).
Description
Timdal & Krog (Reference Timdal and Krog2001), Elix (Reference Elix and McCarthy2009).
Chemistry
Chemotype 1: chlorophyllopsorin (major), argopsin (minor to trace); chemotype 2: methyl 2,7-dichloropsoromate (major), methyl 2,7-dichloronorpsoromate (submajor); chemotype 3: chlorophyllopsorin (major), methyl 2,7-dichloropsoromate (submajor), methyl 2,7-dichloronorpsoromate (submajor to trace), argopsin (minor to trace).
Distribution
Africa, Asia, Australia.
Discussion
Phyllopsora africana shows large morphological and chemical diversity. Some specimens (e.g. the holotype (509), 477, 1436 and 4037) form well-developed, cylindrical isidia, while others (e.g. 6348) form lacinules. The latter morphotype is reported here for the first time and observed in both chemotypes 1 and 3. The isidiate morphotype of P. africana is apparently morphologically identical to P. ochroxantha and P. swinscowii. Chemotype 1 represents the chemistry of the holotype; chemotype 2 is identical to the chemistry found in P. swinscowii; chemotype 3 represents a mixture of chemotypes 1 and 2. Our specimen of chemotype 2 (477) was initially identified as P. swinscowii due to its identical chemistry and morphology, but its sequences associate with those of P. africana. We have further sequences of P. africana chemotype 2 from Asia (Kistenich et al. Reference Kistenich, Bendiksby, Vairappan, Weerakoon, Wijesundara, Wolseley and Timdal2019a) which confirm its nested position among the other P. africana chemotypes.
The five specimens of P. africana form a supported clade in our phylogenies (Figs 2 & 3). Most branches are long in the ITS tree and the mPTP analysis suggests splitting them into four species (Fig. 3), while the mtSSU tree resolves them as belonging to one species together with P. swinscowii (Fig. 2). Phyllopsora africana is sister to P. swinscowii in our phylogeny and is also closely related to P. ochroxantha (Figs 2–4, group C).
Phyllopsora africana and P. swinscowii overlap in their distribution range and are morphologically similar. They also overlap in their chemistry and our phylogenetic trees confirm that the two species are more closely related to each other than either is to P. ochroxantha. The current taxonomy seems unsatisfactory but it is questionable if all specimens assigned to P. africana comprise just one, highly variable species or a complex of species. As we cannot distinguish chemotype 2 of P. africana from P. swinscowii by either morphology or chemistry, they currently have to be considered a cryptic taxon pair. See Discussion for further comments.
Phyllopsora amazonica Kistenich & Timdal sp. nov.
MycoBank No.: MB 829272
Differs from P. halei in forming an irregular, effuse thallus with smaller areoles on a thin, white prothallus and in having more persistently marginate and less convex apothecia.
Type: Brazil, Pará, Melgaço, Floresta Nacional de Caxiuanã, Estação Científica Ferreira Penna, at the research station, 1°44·22′S, 51°27·32′W, 30 m alt., on tree trunk in tropical rainforest, 0·7 m above ground, trunk diam. 50 cm, 13 March 2015, S. Kistenich & E. Timdal 85 (MPEG!—holotype; O L-201094!—isotype) (TLC: atranorin and a series of terpenoids; DNA: MK352208 (mtSSU), MK352379 (ITS)).
(Fig. 5A)
Thallus effuse, crustose; areoles small, up to 0·4 diam., adnate, isodiametric, scattered when young, later often contiguous, plane to weakly convex, pale green to white, glabrous, not pubescent along the margin; isidia common, cylindrical to lageniform, simple, medium thick, up to 0·12 × 0·70 mm; upper cortex of type 1, 10–20 µm thick, containing crystals dissolving in K; medulla containing scattered crystals dissolving in K; prothallus thin, white.
Apothecia common, up to 1·0 mm diam., rounded, simple, plane to weakly convex, medium brown to dark brown, with a rather thick, dark brown to black, glabrous margin which may become more or less excluded when old; excipulum dark olivaceous brown in inner part, paler at the rim, containing some crystals dissolving in K (K−); hypothecium dark olivaceous brown, not containing crystals; epithecium colourless, K−; ascospores narrowly ellipsoid, simple, 7–10 × 2–3 µm (n = 20, from the holotype).
Conidiomata not seen.
Chemistry
Atranorin (major) and a series of terpenoids, the main one in R f classes A: 6–7, B′: 8, C: 6–7 (chemistry identical to that of P. halei chemotype 1).
Etymology
The species is described from the Amazonian rainforest.
Distribution
Brazil (Pará).
Discussion
The species is resolved as a separate species in the mPTP analyses (Figs 2 & 3). It is sister to P. halei (Figs 3 & 4) and P. pyxinoides (Fig. 4). The species resembles P. halei in forming adnate areoles with thick, partly lageniform isidia and it contains the same lichen substances as P. halei chemotype 1. It differs, however, in forming a less prominent, thinner, white (not reddish brown) prothallus and in having isidia growing sometimes directly out of the prothallus. While P. halei forms rosette-like thalli, P. amazonica produces irregular, effuse thalli. In addition, the apothecia of P. amazonica are more persistently marginate and less convex than those in P. halei.
Additional specimen examined
Brazil: Pará: Paragominas, Hydro mining area, collecting site 2, 3°14·82′S, 47°40·99′W, 150 m alt., on tree trunk in tropical rainforest, in the canopy of a felled tree, 2014, R. S. Barbosa, R. Haugan & E. Timdal 90 (MPEG, O L-193960) [DNA: MK352194 (mtSSU), MK352365 (ITS)].
Phyllopsora breviuscula (Nyl.) Müll. Arg.
Bull. Herb. Boissier 2(App. 1): 45 (1894).—Lecidea breviuscula Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 339 (1863); type: Cuba, s. loc., C. Wright (H-NYL 20557!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 225; B 60-35829!, UPS L-74707!—probably isolectotypes, issued as Tuckerman, Wright Lich. Cub. No. 181, more isolectotypes listed by Brako (Reference Brako1991): 56) (TLC: no lichen substances).
Lecidea subbreviuscula Nyl., Sert. Lich. Trop.: 40 (1891).—Phyllopsora subbreviuscula (Nyl.) Zahlbr., Cat. Lich. Univ. 4(3): 401 (1926); type: Cuba, s. loc., C. Wright (H-NYL 20524!—holotype; FH-TUCK 2922, isotype, not seen, issued as Tuckerman, Wright Lich. Cub., ser. 2, No. 120).
Phyllopsora brachyspora Müll. Arg., Bot. Jahrb. Syst. 20: 264 (1895); type: Tanzania, Usambara, Hochwald ob Kwa Mstufa, Holst 9181 pr. p. (G 00066323, upper right specimen—lectotype, designated here, MycoBank typification MBT 387683, image seen; M 0024443—isolectotype, image seen; BM, W—isolectotypes, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): no lichen substances. Synonymy according to Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991)).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001), Elix (Reference Elix and McCarthy2009).
Chemistry
No lichen substances.
Distribution
Pantropical.
Discussion
All accessions (four in the mtSSU and five in the ITS tree) form a well-supported clade sister to P. mauritiana in our phylogenies (Figs 2–4). The mPTP delimitation analyses in both trees split the accessions into three and four entities, respectively (Figs 2 & 3). Our first impression when studying the Asian specimens (Fig. 1) morphologically was that they represented an unknown species. These specimens exhibit strongly ascending squamules that are both narrower and longer than those known from neotropical P. breviuscula, which has more adnate and procumbent squamules. When comparing all five specimens, we found that the specimen from La Réunion showed a transient morphology between the two extremes in forming medium-long but adnate squamules. We therefore consider these accessions to belong to the same species, P. breviuscula, developing different morphologies depending on the geographical region.
Phyllopsora buettneri (Müll. Arg.) Zahlbr.
Cat. Lich. Univ. 4(3): 396 (1926).—Psora buettneri Müll. Arg., Bot. Jahrb. Syst. 15: 506 (1893); type: Togo, Bismarksburg, Büttner L. Afr. 7 (G 00066290—holotype, image seen; BM!—isotype) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): pannarin, zeorin, fatty acids).
Lecidea munda Malme, Ark. Bot. 28A(7): 49 (1936).—Phyllopsora munda (Malme) Zahlbr., Cat. Lich. Univ. 10(24): 377 (1939); type: Brazil, Rio Grande do Sul, Hamburgerberg pr. São Leopold, 18-10-1892, G. A. Malme Lich. Regnell. 617B (S!—holotype) (TLC (Brako Reference Brako1991): pannarin, phyllopsorin, zeorin).
Lecidea schizophylloides Malme, Ark. Bot. 28A(7): 45 (1936); type: Brazil, Rio Grande do Sul, Silveira Martins, 07-03-1893, G. A. Malme Lich. Regnell. 1227B [sic!, in protologue: ‘1251B’] (S!—holotype) (TLC (Brako Reference Brako1991): pannarin, phyllopsorin, zeorin).
Descriptions
Swinscow & Krog (Reference Swinscow and Krog1981), Timdal & Krog (Reference Timdal and Krog2001), Timdal (Reference Timdal2008, as P. buettneri chemotypes 1 and 2), Elix (Reference Elix and McCarthy2009).
Chemistry
Chemotype 1: pannarin, zeorin; chemotype 2: pannarin, phyllopsorin, zeorin; chemotype 3: dechloropannarin, zeorin. An additional chemotype (4) is reported from Norfolk Island (Elix Reference Elix2006b), containing argopsin, norargopsin and zeorin. According to Elix (Reference Elix and McCarthy2009), chemotypes containing pannarin as a major compound may also contain dechloropannarin as minor or trace compound, and vice versa.
Distribution
Chemotype 1: Africa, Asia; chemotype 2: Central and South America; chemotype 3: Asia; chemotype 4: Australia.
Discussion
The chemotypes of P. buettneri were discussed by Brako (Reference Brako1991) and Timdal (Reference Timdal2008, Reference Timdal2011). The chemotype containing vicanicin, norvicanicin and zeorin (referred to as chemotype 3 in Timdal (Reference Timdal2011)) is accepted here as a distinct species, P. melanoglauca, falling outside group B (Figs 2 & 3). Hence, P. buettneri now consists of three chemotypes (chemotype 1 from Africa and Asia, chemotype 2 from Central and South America, and chemotype 3 from Asia), all containing pannarin and/or dechloropannarin, with a possible fourth Australasian chemotype (argopsin; see also discussion for P. subhispidula). However, we were not able to examine the last chemotype. The accessions of chemotypes 1, 2 and 3 group into a well-supported clade (Figs 3 & 4). In the ITS tree, mPTP splits all chemotypes into separate species (Fig. 3), whereas the mPTP analysis on the mtSSU tree delimits one species for all accessions (Fig. 2). All chemotypes are apparently morphologically identical and group together in the ITS and *BEAST trees (Figs 3 & 4); thus, we assume they belong to the same species. Phyllopsora buettneri is morphologically rather similar to P. chodatinica and P. porphyromelaena. These species differ mainly in their chemistries and exhibit slightly different spore sizes, squamule forms and presence of pruina. See Discussion for more detail on this species complex.
Phyllopsora byssiseda (Nyl.) Zahlbr.
Cat. Lich. Univ. 4(3): 396 (1926).—Lecidea byssiseda Nyl. in Hue, Nouv. Arch. Mus. Hist. Nat., Sér. 3 3: 103 (1891); type: Mexico, s. loc., Fr. Müller s. n. (H-NYL 20517!—holotype) (TLC: no lichen substances).
Description
Timdal (Reference Timdal2011).
Chemistry
No lichen substances or atranorin (minor to trace).
Distribution
Central and South America.
Discussion
In this study, we originally included eight specimens believed to represent P. byssiseda. However, they resolved into two separate clades (Figs 2 & 3) and six of the specimens correspond to the new species P. isidiosa (Fig. 6A).
Our two remaining accessions of P. byssiseda form a strongly supported clade sister to P. fendleri in the phylogenetic trees (Figs 2–4). mPTP resolves them as a distinct species in both analyses (Figs 2 & 3). Phyllopsora byssiseda is morphologically similar to its sister species in forming a dense white prothallus with large, lobate squamules with a pubescent margin. While P. fendleri tends to be richly fertile, P. byssiseda forms numerous isidia. The two species have also been reported to differ slightly in chemistry, P. fendleri being acid deficient (Brako Reference Brako1991) and P. byssiseda containing traces of atranorin (Timdal Reference Timdal2011). In our sequenced specimens of P. byssiseda, we found one (4739) without lichen substances, while the other (4737) contained not only atranorin but also two additional unknown compounds (possibly contaminants). The two sequenced specimens of P. fendleri contained atranorin (2098) or no lichen substances (7473). Apothecia were found in one of the P. byssiseda specimens and a few, small isidia in the richly fertile P. fendleri specimens. Based on these discoveries, the morphological and chemical differences between the two species become small. Even though they are resolved as two distinct species in both analyses, we find a similar variation of branch lengths in other species, for example in P. kalbii, and regard it as not unlikely that they belong to the same species despite the mPTP results.
Phyllopsora canoumbrina (Vain.) Brako
Mycotaxon 35: 12 (1989).—Lecidea canoumbrina Vain., Proc. Amer. Acad. Arts. Sci. 58: 135 (1923); type: Trinidad and Tobago, Trinidad, Maraval Valley, ad corticem arboris, R. Thaxter 19 (FH—lectotype, designated by Brako (Reference Brako1991): 33 (as ‘holotype’, Art. 9.10), not seen; TUR-V 23680!—isolectotype) (TLC: no lichen substances).
Lecidea granulifera Fink in Hedrick, Mycologia 22: 252 (1930); type: Puerto Rico, Rio de Maricao, on rock, 14-02-1915, N. L. Britton & J. F. Cowell 4235 (MICH—holotype, not seen; NY!—isotype).
Description
Brako (Reference Brako1991).
Chemistry
No lichen substances.
Distribution
Central and South America.
Discussion
We know of no reliably identified and recently collected material from the geographical region from which this poorly understood species was described (the West Indies). The isotype is in a poor condition and was not used for DNA extraction. The sequenced specimen is from Brazil and identified as P. canoumbrina as it is richly fertile, has an almost crustose thallus on a white prothallus, forms minute squamules, small cylindrical isidia, lacks lichen substances, and the ascospores (5·0–7·5 × 2–3 µm) are largely congruent with those measured from the isotype (6·5–9·5 × 2·5–3·0 µm; Brako Reference Brako1991). Our accession of P. canoumbrina is supported as sister to P. isidiotyla (Figs 2–4) but sits on a long branch (Figs 2 & 3) and is morphologically clearly distinct from that species. The mPTP analyses resolve the accession as a separate entity (Figs 2 & 3).
Phyllopsora castaneocincta (Hue) Kistenich & Timdal comb. nov.
MycoBank No.: MB 82927
Pannaria castaneocincta Hue, Nouv. Arch. Mus. Hist. Nat., Sér. 4 8: 262 (1906); type: Japan, Kin. Kuwasan, 1902, s. coll. 5183 (PC 0012756!—holotype) (TLC: furfuraceic acid).
Lecidea kiiensis Vain., Bot. Mag. (Tokyo) 35: 67 (1921).—Phyllopsora kiiensis (Vain.) Elix, Fl. Australia 57: 52 (2009); type: Japan, Prov. Kii, 30-12-1918, Yasuda 268 (TUR-V 22631—holotype, not seen; TNS!—isotype) (TLC: furfuraceic acid).
Phyllopsora phaeoglauca (Vain.) Zahlbr., Cat. Lich. Univ. 4(3): 400 (1926).—Lecidea phaeoglauca Vain., Ann. Acad. Sci. Fenn., Ser. A 15(6): 112 (1921); type: Philippines, Luzon, Prov. Bataan, Limay, 31-12-1909, C. B. Robinson 9631 (TUR-V 22617!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 244) (TLC: no lichen substances).
Description
Timdal & Krog (Reference Timdal and Krog2001) and Elix (Reference Elix and McCarthy2009), both as P. kiiensis.
Chemistry
Furfuraceic acid (major) or rarely no lichen substances.
Distribution
Africa, Asia, Australia.
Discussion
The name Phyllopsora kiiensis is antedated by P. castaneocincta and has been mistakenly used for this species so far. Phyllopsora phaeoglauca is also synonymized here as its lectotype apparently represents the acid-deficient chemotype of P. castaneocincta, which Kistenich et al. (Reference Kistenich, Bendiksby, Vairappan, Weerakoon, Wijesundara, Wolseley and Timdal2019a) show is nested within furfuraceic acid-containing specimens of that species. The five accessions of P. castaneocincta in this paper all contain furfuraceic acid and group together in a strongly supported clade, where the African specimens form a group distinct from the Asian and Australian ones (Figs 2 & 3). All specimens are resolved as one species in the mtSSU tree (Fig. 2), while the mPTP analysis of the ITS tree separates them into three entities according to continent (Fig. 3). We still consider them to belong to the same species as they all share a characteristic morphology with a thick brownish prothallus, adnate squamules and cylindrical isidia, as well as the presence of furfuraceic acid. Phyllopsora castaneocincta is weakly resolved as sister to P. mediocris and P. parvifolia in the mtSSU tree (Fig. 2), whereas it is found in a strongly supported clade with P. confusa, P. foliata, P. loekoesii, P. mediocris, P. neofoliata and P. parvifolia in the ITS and the *BEAST trees (Figs 3 & 4). It is distinguished from phylogenetically related species by morphology and chemistry.
Phyllopsora chlorophaea (Müll. Arg.) Müll. Arg.
Bull. Soc. Roy. Bot. Belgique 32: 132 (1893 [1894?]).—Psora chlorophaea Müll. Arg., Flora 70: 320 (1887); type: Brazil, São Paulo, Apiahy, 06-1881, Puiggari 1721 (G 00293365—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 228, image seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): no lichen substances).
Lecidea haemophaea var. subparvifolia Müll. Arg., Flora 60: 473 (1877).—Phyllopsora subparvifolia (Müll. Arg.) Müll. Arg., Hedwigia 34: 141 (1895); type: Venezuela, Caracas, Ernst 114 (G 00293364—holotype, image seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): no lichen substances). Synonymy according to Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991).
Lecidea furfuracea f. schizophylla Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 47 (1890).—Lecidea schizophylla (Vain.) Malme, Ark. Bot. 28A(7): 43 (1936).—Phyllopsora schizophylla (Vain.) Gotth. Schneid., Biblioth. Lichenol. 13: 172 (1980), nom. inval., Art. 36.1 (a); type: Brazil, Minas Lafayette, E. A. Vainio (TUR-V 22641—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 228, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): triterpenoid, trace). Synonymy according to Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001), Timdal (Reference Timdal2008).
Chemistry
Chemotype 1: no lichen substances or atranorin (trace to minor); chemotype 2: furfuraceic acid and sometimes atranorin (trace).
Distribution
Central and South America, Africa.
Discussion
All accessions of P. chlorophaea group together in a supported clade in the phylogenetic trees (Figs 2 & 3, group B). They are resolved as one species in the ITS tree (Fig. 3), while mPTP suggested four delimited species in the mtSSU tree, which has long branches (Fig. 2). All specimens are recognized by the same morphological features: ascending, lacinulate squamules attached to a well-developed, reddish brown prothallus, dark brown apothecia and narrowly ellipsoid to fusiform ascospores. Hence, we assume they all belong to the same species. Phyllopsora chlorophaea is resolved in a clade together with the new species P. neotinica and the buettneri-chodatinica-porphyromelaena complex (Figs 2 & 4, group B), with which it shares the presence of lacinules. It is, however, readily distinguished from those species by forming smaller squamules and by containing either no lichen substances or furfuraceic acid, often with small amounts of atranorin.
Phyllopsora chodatinica Elix
Australas. Lichenol. 59: 23 (2006); type: Australia, Queensland, Blencoe Creek, Cardwell Range, 48 km NW of Cardwell, 18°03′S, 145°39′E, 740 m alt., on mossy trunk in Lauraceae-Syzygium-Prunus-dominated forest, 17-06-1986, J. Elix & H. Streimann 20109 (BRI—holotype, not seen; CANB—isotype, not seen).
Descriptions
Elix (Reference Elix2006c, Reference Elix and McCarthy2009).
Chemistry
A chemosyndrome of xanthones based on chodatin (Elix Reference Elix2006c).
Distribution
Australasia and Oceania.
Discussion
We included seven specimens in the phylogenetic analyses, originally identified as P. chodatinica based on morphology and chemistry, as well as a paratype. The species splits into two different, strongly supported clades, one containing the palaeotropical specimens including the paratype, and the other comprising only neotropical specimens (Figs 2 & 3). The mPTP analyses delimits each clade as a separate species (Figs 2 & 3). The two clades are rather closely related to each other and are found as sister to P. buettneri, P. chlorophaea and P. porphyromelaena (Figs 2–4, group B). All species within group B are morphologically very similar but can be separated by their chemical compounds. We describe here the neotropical clade of P. chodatinica as the new species P. neotinica (Fig. 6B); see that species for further discussion. Phyllopsora chodatinica can be separated from P. neotinica by chemistry: both species contain various xanthones but chodatin is found only in P. chodatinica, while P. neotinica usually also contains argopsin and zeorin.
Phyllopsora cinchonarum (Fée) Timdal
Lichenologist 40: 346 (2008).—Triclinum cinchonarum Fée, Essai Crypt. Écorc.: 148 (1825); type: Fée, Essai Crypt. Écorc.: Tab. 33, Fig. 4 (1825) (lectotype, designated by Jørgensen (Reference Jørgensen2003): 76, with epitype: “the type specimen of Physcidia endococcinea Zahlbr. (W!)”).—Physcidia endococcinea Zahlbr., Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 83: 159 (1909).—Squamacidia janeirensis var. endococcinea (Zahlbr.) Brako, Mycotaxon 35: 10 (1989); type: Brazil, São Paulo, prope Barra Mansa in districtu urbis Itapecirica, in silvaticis, c. 1000 m alt., 06-1901, V. Schiffner s. n. (W 8343!—holotype) (TLC: atranorin, lobaric acid, scarlet pigment in R f classes 1–2:1:1).
Thalloidima janeirense Müll. Arg., Hedwigia 31: 280 (1892).—Phyllopsora janeirensis (Müll. Arg.) Swinscow & Krog, Lichenologist 13: 242 (1981).—Squamacidia janeirensis (Müll. Arg.) Brako, Mycotaxon 35: 8 (1989); type: Brazil, Rio de Janeiro, s. loc., Portella s. n. (BM!—holotype; G 00294395—isotype, image seen) (TLC: fumarprotocetraric acid, lobaric acid).
Phyllopsora stenosperma Zahlbr., Repert. Spec. Nov. Regni Veg. 33: 44 (1933); type: Taiwan, Chiayi Prov., Mt. Arisan, Toroyen, 24-12-1925, Y. Asahina F-170 (W—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 245 (as ‘holotype’, Art. 9.10), not seen; TNS!—isolectotype; NY—isolectotype, not seen) (TLC: atranorin, lobaric acid).
Descriptions
Brako (Reference Brako1989, as Squamacidia janeirensis), Timdal (Reference Timdal2008) and Elix (Reference Elix and McCarthy2009, as Triclinum cinchonarum).
Chemistry
Lobaric acid (major) and often atranorin, fumarprotocetraric acid, an unknown substance, and/or a scarlet pigment. Additional compounds are reported by Aptroot et al. (Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007) and Elix (Reference Elix2007).
Distribution
Central and South America, Asia, Australia.
Discussion
We included four accessions of P. cinchonarum in our study. All of them clustered together in a strongly supported clade in both phylogenetic trees (Figs 2 & 3) and are resolved as phylogenetic sister to P. concinna (Figs 2 & 4). The mPTP analysis of the mtSSU tree resolves P. cinchonarum as one species (Fig. 2), while the ITS mPTP analysis separates the two included accessions (Fig. 3). All four specimens agree in morphology and chemistry (lobaric acid in all, atranorin and fumarprotocetraric acid being variable) and we therefore assume they belong to one species. Phyllopsora cinchonarum is morphologically similar to its sister species P. concinna in forming long, simple isidia and adnate to ascending, medium-sized squamules on a white prothallus. It is readily distinguished, however, by its chemical composition as P. concinna contains parvifoliellin instead of lobaric acid.
The species was first described as Triclinum cinchonarum and is the type species of the genus Triclinum Fée. As the name Triclinum antedates Phyllopsora, we propose the latter for conservation (Kistenich et al. Reference Kistenich, Ekman, Bendiksby and Timdal2019b). Unfortunately, the specimens sequenced here lack the characteristic scarlet pigment present in the epitype but, based on general morphology and chemistry (lobaric acid), we believe that the presence of the pigment merely represents a minor chemical variation in some specimens within the species.
Phyllopsora concinna Kistenich & Timdal sp. nov.
MycoBank No.: MB 829273
Differs from the chemically similar species P. parvifoliella and P. rappiana in forming larger isidia, having a white prothallus, an apothecial margin paler than the disc, and longer and broader ascospores; differs from the morphologically similar species P. cinchonarum in containing parvifoliellin, not lobaric acid.
Type: Venezuela, Capital District, Parque Nacional Macarao, 1·5 km E of El Junquito, 10°27·60′N, 67°04·45′W, 1920 m alt., on tree trunk by visitor's centre, 0·4–1·2 m above ground, trunk diam. 60 cm, 12 November 2015, M. S. Dahl, J. E. Hernández M., S. Kistenich, E. Timdal & A. K. Toreskaas SK1-225 (O L-202505!—holotype; VEN!—isotype) (TLC: atranorin (major), parvifoliellin (major); DNA: MK352236 (mtSSU), MK352404 (ITS)).
(Fig. 5B)
Thallus effuse, squamulose; squamules medium sized, adnate, isodiametrical or rarely somewhat elongated at the thallus margin, entire to crenulate or incised, plane to weakly convex; upper side pale green, glabrous, epruinose; margin concolorous with upper side, sometimes finely pubescent; isidia numerous, both marginal and laminal on the squamules, cylindrical, simple, up to 0·2 × 1·5 mm; upper cortex of type 1, 35–60 µm thick, containing crystals dissolving in K (K−); medulla containing a few scattered crystals dissolving in K (K−); prothallus usually well developed, white.
Apothecia rare, up to 1 mm diam., irregular, conglomerate, weakly convex, medium brown, with an indistinct, paler margin; ascospores narrowly ellipsoid to fusiform, simple, 12·5–16·0 × 3·5–4·0 µm (n = 20).
Conidiomata not seen.
Chemistry
Atranorin (major), parvifoliellin (major).
Etymology
The epithet refers to the species being beautiful.
Distribution
Central and South America.
Discussion
The four accessions of this species used here were originally identified as P. parvifoliella on the basis that the specimens contained parvifoliellin. However, they are resolved in a separate, strongly supported clade (Figs 2 & 3), being clearly distinct from P. parvifoliella and sister to P. cinchonarum (Figs 2 & 4). Upon closer morphological investigation, we found the specimens to resemble P. cinchonarum more than P. parvifoliella. mPTP resolves the accessions of P. concinna as representing two species in the mtSSU tree (Fig. 2) and three in the ITS tree (Fig. 3). We anyway treat them as a single species based on morphology and chemistry, and attribute the mPTP results to regional variation among populations. The species is separated from the two other species that contain parvifoliellin (P. parvifoliella and P. rappiana) mainly by forming larger isidia, having a white prothallus and larger ascospores. It is distinguished from the morphologically very similar P. cinchonarum mainly by the presence of parvifoliellin rather than lobaric acid.
Additional specimens examined. Brazil: Rio de Janeiro: Parque Nacional do Itatiaia, surroundings of Lago Azul, 22°27·10′S, 44°36·92′W, 830 m alt., on tree trunk in Atlantic rainforest, 2015, M. S. Dahl, S. Kistenich, E. Timdal & A. K. Toreskaas SK1-359 (O L-202639); surroundings of Abrigo Lamego, 22°25·66′S, 44°37·19′W, 1140 m alt., on tree trunk in Atlantic rainforest, 2015, M. S. Dahl, S. Kistenich, E. Timdal & A. K. Toreskaas SK1-405 (O L-202685); along trail to Três Picos, 22°26·04′S, 44°36·82′W, 1090 m alt., on Arecaceae trunk in Atlantic rainforest, 2015, M. S. Dahl, S. Kistenich, E. Timdal & A. K. Toreskaas SK1-445 (O L-202725) [DNA: MK352224 (mtSSU), MK352395 (ITS)].—Ecuador: Pastaza: Mera, 1100 m alt., roadside, epiphyte, 1972, L. Arvidsson & D. Nilson 206 (GB).—Guatemala: Alta Verapaz: Parque Nacional Las Victorias, Cobán (tierra templada), 1100–1300 m alt., Pinus-dominated forest, on Liquidambar styraciflua, 13 viii 2002, C. Andersohn s. n. (B! 60 127220) [DNA: MK352251 (mtSSU), MK352418 (ITS)].—Panama: Coclé: SW of Panama City, NW of small village El Valle, in old crater of extinct volcano, trail in tropical forest, from El Valle up to La India Dormida, 8°36·9′N, 80°08·27′W, 585 m alt., edge forest/field, 2010, P. van den Boom 43947 (hb. v. d. Boom) [DNA: MK352202 (mtSSU), MK352373 (ITS)].
Phyllopsora confusa Swinscow & Krog
Lichenologist 13: 229 (1981); type: Kenya, Central Province, Kirinyaga District, Mt. Kenya, 2 km NW of Irangi Forest Station in damp deciduous forest near River Ena, 0°20′S, 37°28′E, 2000 m alt., 02-1972, H. Krog & T. D. V. Swinscow K48/177 (O L-1145!—holotype) (TLC: no lichen substances; DNA: MK352140 (mtSSU), MK352318 (ITS)).
Descriptions
Swinscow & Krog (Reference Swinscow and Krog1981), Timdal & Krog (Reference Timdal and Krog2001), Elix (Reference Elix and McCarthy2009).
Chemistry
No lichen substances.
Distribution
Pantropical.
Discussion
The seven accessions of P. confusa, including that from the holotype, group together in a strongly supported clade in both phylogenies (Figs 2 & 3). All specimens are resolved here as sister to P. loekoesii (Figs 2–4) from which they are distinguished by forming more distinct lacinules and shorter ascospores. In the species delimitation analyses, mPTP splits the accessions into two and four species in both trees (Figs 2 & 3), respectively. While the holotype groups together with three/four other specimens, the specimen from Ecuador and one from Venezuela are resolved as a different species in both mPTP analyses (Figs 2 & 3). It is interesting to note, however, that the two specimens from Venezuela end up in two different clades. These two specimens do not show any striking morphological or chemical differences to the other P. confusa specimens. Therefore, we assume that all specimens belong to the same species. To investigate whether the separated specimens form a different (cryptic) species or whether they merely reflect intraspecific genetic variation, more specimens of P. confusa should be collected and analyzed genetically.
The species is difficult to understand morphologically, having a thallus forming minute squamules that effectively turn into lacinules (fragmenting into diaspores). Swinscow & Krog (Reference Swinscow and Krog1981), in the protologue, were unsure about the extent of morphological variation present in this species. In our experience, identification of this species is often based on a process of elimination: when no significant morphological characteristics are present in a sterile, lacinulate specimen and TLC results are negative, we assume the specimen to be P. confusa until contradicted by DNA sequence data.
Phyllopsora corallina (Eschw.) Müll. Arg.
Bot. Jahrb. Syst. 20: 264 (1895).—Lecidea corallina Eschw. in Martius, Fl. Bras. Enum. Pl. 1(1): 256 (1833); type: Brazil, Bahia, Martius s. n. (M 0024451—holotype, image seen; G 00293368, H-NYL 20483—isotypes, images seen) (TLC (Brako Reference Brako1991): no lichen substances).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001), Elix (Reference Elix and McCarthy2009).
Chemistry
No lichen substances or small amounts of argopsin or atranorin.
Distribution
Neotropical; palaeotropical records need confirmation.
Discussion
The interpretation of P. corallina remains difficult. According to Brako (Reference Brako1991), the holotype does not contain lichen substances, although the species (as P. corallina var. corallina) may contain atranorin. In this study, we use four neotropical specimens which conform morphologically to our understanding of the species (i.e. to that of Timdal & Krog Reference Timdal and Krog2001), but some contain minor amounts or traces of what appears to be argopsin. The four specimens form a strongly supported clade and are resolved as a single species in the mPTP analyses (Figs 2 & 3). The palaeotropical species P. martinii is morphologically similar to P. corallina but differs in forming shorter ascospores and in containing argopsin, norargopsin and chlorophyllopsorin (see Timdal & Krog Reference Timdal and Krog2001). The other species in this clade can be distinguished from P. corallina mainly by forming more distinct morphological characters or different lichen substances.
Phyllopsora cuyabensis (Malme) Zahlbr.
Cat. Lich. Univ. 10(24): 377 (1939).—Lecidea cuyabensis Malme, Ark. Bot. 28A(7): 48 (1936); type: Brazil, Mato Grosso, Serra da Chapada, Buritis, in silva umbrosa, 26-06-1894, G. O. A. Malme s. n. (S!—lectotype, designated by Brako (Reference Brako1991): 44 (as ‘holotype’, Art. 9.10); UPS L-010377!—isolectotype) (TLC (Brako Reference Brako1991): no lichen substances).
Description
Timdal (Reference Timdal2008).
Chemistry
No lichen substances.
Distribution
Central and South America, Asia.
Discussion
The five accessions of P. cuyabensis group into a strongly supported clade in both phylogenetic trees (Figs 2 & 3). The specimen from Thailand is separated from the four neotropical specimens in both mPTP analyses (Figs 2 & 3). As all five specimens share the same morphology, we assume they represent the same species and the long branches result from the geographical distance between the populations. The species is weakly resolved as sister to P. kalbii (Figs 2 & 4) and forms a larger clade with P. byssiseda and P. fendleri (Figs 2 & 4). The species forms a thallus reminiscent of that of the former genus Crocynia (i.e. non-corticate and more or less rosette-forming), which readily distinguishes it from P. kalbii. However, it is not closely related to the two species of Crocynia in our phylogeny (P. gossypina and P. pyxinoides) (Figs 2 & 3). Hence, we assume that the reduction of the upper cortex has occurred independently in P. cuyabensis and the former species of Crocynia.
Phyllopsora dolichospora Timdal & Krog
Mycotaxon 77: 76 (2001); type: Mauritius, Plaine Wilhems, Macchabee Forest, 0·5–1 km ESE of Macchabee kiosk, 20°24′S, 57°26′E, 600 m alt., 21-11-1991, H. Krog & E. Timdal MAU65/22 (O L-22197!—holotype) (TLC: furfuraceic acid, methyl furfuraceiate, methyl homofurfuraceiate; DNA: MK352141 (mtSSU), MK352319 (ITS)).
Description
Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Furfuraceic acid (major), methyl furfuraceiate (major or minor) and methyl homofurfuraceiate (major or minor).
Distribution
Africa, Asia.
Discussion
All accessions of P. dolichospora, including the holotype, form a strongly supported clade in our phylogenetic analyses (Figs 2 & 3). They are resolved as one species in the mtSSU mPTP analysis (Fig. 2) but are divided into three species in the ITS mPTP analysis (Fig. 3), where the accessions appear on long branches. We also observed large introns in the residual 18S region, sequenced as part of the primer ITS-1F but trimmed for the phylogenetic analyses, that were not found in other Phyllopsora species. The specimens showed some morphological variation regarding the quantity of isidia and colour of the prothallus corresponding to the different lineages in Fig. 2. We still consider them to belong to the same species as they all share the unique chemistry consisting of furfuraceic acid, methyl furfuraceiate and methyl homofurfuraceiate. The species groups into a weakly supported clade with P. furfuracea and P. foliatella (Figs 2–4). It resembles both species morphologically by forming an areolate thallus, which often only consists of the prothallus and long isidia. This makes it hard to distinguish between them based on morphology, and many of our specimens had been identified as P. furfuracea after an initial morphological investigation. Phyllopsora dolichospora is distinguished from the other species by its long ascospores and its distinct chemistry (Table 2).
Phyllopsora fendleri (Tuck. & Mont.) Müll. Arg.
Bot. Jahrb. Syst. 20: 264 (1895).—Biatora fendleri Tuck. & Mont. in Montagne, Ann. Sci. Nat., Bot., Sér. 4 8: 296 (1857); type: Venezuela, Fendler (FH-TUCK 2923—lectotype, designated by Brako (Reference Brako1991): 44 (as ‘holotype’, Art. 9.10), not seen; H-NYL 20523—isolectotype, image seen) (TLC (Brako Reference Brako1991): no lichen substances).
Description
Brako (Reference Brako1991).
Chemistry
No lichen substances or atranorin (minor).
Distribution
Central and South America.
Discussion
Our two accessions of P. fendleri cluster together in a strongly supported clade as sister to P. byssiseda (Figs 2–4) and are resolved as one species in the mPTP analyses (Figs 2 & 3). Phyllopsora fendleri is morphologically almost identical to the isidiate P. byssiseda but differs in typically being richly fertile and forming no or few isidia. Both may contain (traces of) atranorin. It is possible that they are conspecific but the few available specimens of both species make evaluation of the morphological variation difficult. See also the discussion under P. byssiseda.
Phyllopsora foliata (Stirt.) Zahlbr.
Cat. Lich. Univ. 4(3): 397 (1926).—Lecidea foliata Stirt., Trans. & Proc. Roy. Soc. Victoria 17: 71 (1881); type: Australia, Queensland, Brisbane, F. M. Bailey 156 (GLAM—lectotype, designated by Rogers (1982): 504, not seen; BRI—isolectotype, not seen).
Description
Elix (Reference Elix and McCarthy2009).
Chemistry
No lichen substances.
Distribution
Asia, Australia.
Discussion
Our three accessions of P. foliata group together in a strongly supported clade in the mtSSU tree (Fig. 2). However, only two accessions group together with strong support, without the Japanese accession, in the ITS tree (Fig. 3). As all three accessions appear on long branches, they are delimited as three separate species in both mPTP analyses (Figs 2 & 3). We still regard them as belonging to the same species, since all specimens are morphologically and chemically congruent: they form densely proliferating and imbricate lacinules on adnate squamules with a white prothallus and lack lichen substances. As the species is collected only rarely, we assume that sequencing additional specimens might lead to a better understanding of the possible genetic variation in the species. We were not able to determine the species’ closest relative due to poor resolution in the trees, but the ITS and *BEAST trees resolve the species in a clade together with P. confusa, P. mediocris, P. neofoliata and P. parvifolia, among others (Figs 3 & 4).
Phyllopsora foliatella Elix
Australas. Lichenol. 58: 11 (2006, January).—Psora foliata var. subcorallina Müll. Arg., Flora 65: 483 (1882); type: Australia, Queensland, Toowoomba, C. H. Hartmann s. n. (G 00052927—lectotype, designated by Elix (Reference Elix and McCarthy2009): 50, image seen).
Phyllopsora homosekikaica Elix, Australas. Lichenol. 59: 25 (2006, July); type: Australia, Queensland, Mt. Spec State Forest, Paluma Range, 6 km W of Paluma, 19°01′S, 146°09′E, 920 m alt., on sapling in Lauraceae-Syzygium-dominated forest, 18-06-1986, J. A. Elix & H. Streimann 20241 (BRI—holotype, not seen: CANB!, O L-1135!—isotypes) (TLC (Elix, on label): homosekikaic acid (submajor), hyperhomosekikaic acid (major); DNA: MK352262 (mtSSU), MK352428 (ITS)).
Descriptions
Elix (Reference Elix2006c, as P. homosekikaica; Reference Elix and McCarthy2009, as both P. foliatella and P. homosekikaica).
Chemistry
Chemotype 1: no lichen substances; chemotype 2: homosekikaic acid (major or submajor), hyperhomosekikaic acid (major).
Distribution
Australia.
Discussion
Our study contains two accessions of P. foliatella and two of P. homosekikaica, including an isotype of the latter. All four accessions group together in a strongly supported clade in the mtSSU tree and are resolved as a single species by mPTP (Fig. 2). As we were unable to generate ITS sequences of P. foliatella, the ITS tree contains only the two accessions of P. homosekikaica, which also group together with strong support and are resolved as one species (Fig. 3). The two species are morphologically identical, with the isidia developing directly from the prothallus, but differ in their chemistry: P. foliatella is acid deficient, while P. homosekikaica contains homosekikaic and hyperhomosekikaic acids. Based on the phylogenetic results and the lack of morphological differentiation, we conclude that the species are conspecific and they are synonymized here.
All accessions group into a weakly supported clade with P. dolichospora and P. furfuracea (Figs 2–4). The three species are characterized by having a light to dark brown prothallus, minute squamules or areoles, and by forming isidia. Their close relationship is therefore quite understandable from a morphological point of view. The two species are readily distinguished from P. foliatella by having slightly different spore sizes and by their chemistries: P. dolichospora contains furfuraceic acid and a series of related compounds, and P. furfuracea contains furfuraceic acid only.
Phyllopsora furfuracea (Pers.) Zahlbr. in Engler
Nat. Pflanzenfam. 1, 1*(225): 138 (1906).—Lecidea furfuracea Pers. in Gaudichaud, Voy. Uranie: 192 (1827); type: Mariana Islands, Gaudichaud s. n. (PC—lectotype, designated by Brako (Reference Brako1991): 46, not seen; H-NYL 20507—isolectotype, not seen).
Lecidea haemophaea Nyl., Flora 52: 122 (1869).—Phyllopsora haemophaea (Nyl.) Müll. Arg., Hedwigia 34: 141 (1895); type: Peru, Yurimaguas, Spruce Lich. Amaz. 185 (H-NYL 20520—holotype, image seen; BM—isotype, not seen; G 00293371, 00293372—isotypes, images seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): furfuraceic acid (as haemophaea unknown). Synonymy according to Brako (Reference Brako1991)).
Lecidea rhypoderma C. Knight, Trans. & Proc. New Zealand Inst. 12: 375 (1880).—type: New Zealand (not seen) (synonymy according to Zahlbruckner (1925): 761).
Lecidea hypochrysea Vain., Ann. Acad. Sci. Fenn., Ser. A 15(6): 114 (1921).—Phyllopsora hypochrysea (Vain.) Swinscow & Krog, Lichenologist 13: 241 (1981); type: Philippines, Mindanao, subprov. Butuan, 320 m, 1911, Weber 1393 (TUR-V 22622—holotype, not seen) (TLC (Brako Reference Brako1991): furfuraceic acid (as furfuracein). Synonymy according to Brako (Reference Brako1991)).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001), Timdal (Reference Timdal2008), Elix (Reference Elix and McCarthy2009).
Chemistry
Furfuraceic acid (major).
Distribution
Pantropical.
Discussion
We include five specimens originally identified as P. furfuracea in this study. Surprisingly, they group into two separate, strongly supported clades: three accessions form a clade with P. dolichospora and P. foliatella, while two accessions are resolved as sister to this clade (Figs 2–4, group A). mPTP resolves the five accessions as belonging to four different species in both analyses (Figs 2 & 3), thus separating all of them except for the ones from Peru and Trinidad and Tobago. All specimens contain furfuraceic acid as chemical compound. Upon closer morphological examination, we found the clade with the accessions from La Réunion, Peru, and Trinidad and Tobago to conform most closely to the current concept of P. furfuracea, while the specimens from Ecuador and the Dominican Republic are described as the new species P. furfurella (Fig. 5C). See the discussion of P. furfurella for further details.
In addition to the five specimens discussed above, we investigated some specimens of the P. furfuracea chemotype 2 of Timdal & Krog (Reference Timdal and Krog2001) and Timdal (Reference Timdal2008) (i.e. the acid-deficient strain), but all were resolved to belong in other species, mainly P. longiuscula. It is therefore unclear whether an acid-deficient chemical strain of P. furfuracea exists. Phyllopsora furfuracea is distinguished from the related species P. dolichospora, P. foliatella and P. furfurella either by chemistry (Table 2) and spore size or by having a reddish to dark brown prothallus.
Phyllopsora furfurella Kistenich & Timdal sp. nov.
MycoBank No.: MB 829274
Differs from P. furfuracea in having a white, not reddish brown, prothallus, an orange brown, K+ purple hypothecium containing skyrin, and in details of the mtSSU and ITS sequences.
Type: Ecuador, Loja, Espíndola, buffer zone of Colambo-Yacuri National Park, 4°33′35″S, 79°23′21″W, 2211–2537 m alt., secondary managed forest, regrown after selective or total logging events on primary montane forest, 10 May 2011, G. Aragón, Y. González, A. Benítez & M. Prieto (HUTPL!—holotype) (TLC: furfuraceic acid (major), skyrin (in the hypothecium); DNA: MK352189 (mtSSU), MK352361 (ITS)).
(Fig. 5C)
Thallus effuse, crustose; areoles minute, granular, up to 0·1 mm diam., scattered or contiguous, pale to medium green, dull, glabrous or slightly pubescent; isidia c. 0·1 mm thick, up to 0·4 mm long, simple, more or less straight, pale to medium green, glabrous, adnate to ascending; upper cortex poorly defined, formed by 1–2 layers of thin-walled hyphae with rounded lumina, not containing crystals; medulla containing crystals dissolving in K; prothallus poorly to partly well developed, white.
Apothecia common, up to 1·5 mm diam., round or slightly irregular, sometimes conglomerate, weakly to moderately convex, orange-brown to medium brown, with an indistinct, slightly paler or slightly darker, glabrous margin; excipulum orange-brown in inner part, paler at the rim, K+ purple; hypothecium orange-brown, K+ purple; epithecium colourless; no crystals or granules in the apothecium; ascospores narrowly ellipsoid to fusiform, simple, 6·5–9·5 × 2·0–2·5 µm (n = 30).
Conidiomata not seen.
Chemistry
Furfuraceic acid (major), skyrin (in the hypothecium).
Etymology
The epithet indicates the morphological resemblance to P. furfuracea.
Distribution
Central and South America.
Discussion
The two accessions of P. furfurella included in this study were originally named P. furfuracea based on the presence of furfuraceic acid, as well as having minute areoles and isidia. The phylogenetic trees, however, reveal the two accessions as a strongly supported group separate from the remaining three accessions of P. furfuracea (Figs 2 & 3). Even though the mPTP analyses delimited the two P. furfurella accessions as two separate species due to the long branches (Figs 2 & 3), we treat them as one species since both are morphologically similar. They are resolved as sister to the clade consisting of P. dolichospora, P. foliatella and P. furfuracea in the mtSSU and *BEAST trees (Figs 2 & 4), while they are weakly resolved as sister to P. canoumbrina, P. isidiotyla and one unidentified specimen in the ITS tree (Fig. 3).
Upon closer morphological examination, we found the specimens of P. furfurella to have a pure white prothallus, a K+ purple hypothecium due to the presence of skyrin, and slightly smaller ascospores than those of P. furfuracea. Two of the three specimens of P. furfuracea in our phylogeny did not contain skyrin (hypothecium K−); the third was sterile and hence not examined. We were able to recognize the skyrin-containing taxon after re-examining our material in three further collections of specimens. These were originally identified as P. furfuracea from Brazil, Ecuador and Jamaica, although these were not sequenced. Other fertile specimens from the Neotropics, for example those reported from Peru by Timdal (Reference Timdal2008), did not contain skyrin, and nor did all examined fertile specimens from the Palaeotropics reported by Timdal & Krog (Reference Timdal and Krog2001). Assuming that the presence of skyrin in the hypothecium is a diagnostic character for the distinction of the two species, and that the skyrin-containing species is restricted to the Neotropics, we choose to retain the name P. furfuracea for the pantropical species.
Additional specimens examined. Brazil: Rio de Janeiro: Serra da Mantiqueira, Parque National do Itatiaia, 850 m alt., in einem feuchten, dunklen Primärregenwald, 22 vii 1978, K. Kalb & G. Plöbst, Kalb, Lich. Neotropici No 341 (O L-150058).—Dominican Republic: Puerto Plata: S of Puerto Plata, Parc National Isabel de Torres, Pico Isabel de Torre, 19°45·73′N, 70°42·68′W, 770 m alt., botanical garden with damp and open forest with mixed trees and shrubs, on palm, 2008, P. van den Boom 39069 (hb. v. d. Boom) [DNA: MK352198 (mtSSU), MK352369 (ITS)].—Ecuador: Loja: Espíndola, upper part of buffer zone of Colambo-Yacuri National Park, 4°33′27″S, 79°22′09″W, 2700–2882 m alt., very dense primary montane forest, evergreen, unmanaged and characterized by a dense canopy layer, 10 v 2011, G. Aragón, Y. González, A. Benítez & M. Prieto (HUTPL).—Jamaica: ‘Island of Jamaica’, on bark and vegetable debris, 3 iii 1905, C. E. Cummings, Merrill, Lich. Exsicc. No. 37 (O L-146420).
Phyllopsora glaucella (Vain.) Timdal
Lichenologist 40: 349 (2008).—Lecidea breviuscula var. glaucella Vain., Dansk Bot. Ark. 4(11): 21 (1926); type: Mexico, Veracruz, Mirador, 08-1841, Liebmann 7381a (TUR-V 34026!—holotype) (TLC: vicanicin, norvicanicin).
Description
Timdal (Reference Timdal2008).
Chemistry
Vicanicin, norvicanicin.
Distribution
Central and South America.
Discussion
The four accessions of P. glaucella form a strongly supported clade in both phylogenetic trees (Figs 2 & 3) and are resolved as one species in both mPTP analyses (Figs 2 & 3). The species is mainly characterized by the squamulose thallus on a well-developed, reddish brown prothallus, the long, marginal isidia and the chemistry (vicanicin and norvicanicin; Table 2). Based on this combination, it is readily distinguished from other species. The combination of vicanicin and norvicanicin (in addition to zeorin) is also found in the phyllidiate P. melanoglauca, which is found in the same large, unresolved clade (Figs 2 & 4).
Phyllopsora gossypina (Sw.) Kistenich et al.
Taxon 67: 894 (2018).—Lichen gossypinus Sw., Prodr.: 146 (1788).—Symplocia gossypina (Sw.) A. Massal., Neagen. Lich.: 4 (1854).—Crocynia gossypina (Sw.) A. Massal., Atti Reale Ist. Veneto Sci. Lett. Arti, Ser. 3 5: 252 (1860); type: Jamaica, 1784–1786, O. Swartz s. n. (UPS L-000259! & L-134473!—syntypes).
Phyllopsora leprosa Riedl, Oesterr. Bot. Z. 121: 145 (1973); type: Surinam, 1827, Weigel s. n. (W—holotype, not seen) (synonymy according to Brako (Reference Brako1989)).
Chemistry
Chemotype 1: barbatic acid, divaricatic acid (submajor), two unknown terpenoids (minor); chemotype 2: norstictic acid (major), salazinic acid (major, sometimes absent), unknown compound (minor to trace or absent, R f classes A:4, B′:6, C:6).
Description
Hue (Reference Hue1909).
Distribution
Pantropical.
Discussion
To our knowledge, only Sipman (Reference Sipman2018) has described the chemistry of P. gossypina prior to this study. Whereas Sipman (Reference Sipman2018) merely lists the main compounds, here we describe two pantropical chemotypes identified in our material. The major compound of chemotype 1 was identified as barbatic acid with divaricatic acid as submajor compound and two unknown terpenoids (minor). The unknown compound of chemotype 2 resembles divaricatic acid in colour and fluorescence and has similar R f values in solvent systems A and C, but lower R f value in B′ (moves just below 3-chlorodivaricatic acid).
The six accessions of P. gossypina group together in a strongly supported clade and are sister to P. imshaugii (Figs 2–4). We were surprised to find these specimens mixed with our accessions of Crocynia molliuscula, as well as with the P. pyxinoides sequence from GenBank (Fig. 2). All of the chosen P. gossypina specimens exhibit an unambiguous gossypina-like morphology with a bluish white, felt-like thallus and dark brown apothecia with a lighter margin. As the P. pyxinoides sequence from GenBank groups together with a Brazilian specimen of P. gossypina chemotype 2 and not with the P. pyxinoides specimens identified by us (Fig. 2), we assume that the GenBank specimen is misidentified. See also the discussion for P. pyxinoides. Crocynia mollis (Nyl.) Nyl. has been regarded as a K+ red variety of P. gossypina (Hue Reference Hue1909; Zahlbruckner 1923), and it is possible that P. gossypina chemotype 2 represents that taxon. However, more material of typical C. mollis has to be investigated before conclusions can be made.
The two accessions of C. molliuscula from La Réunion and Mauritius group together with the Sri Lankan specimen of P. gossypina chemotype 1 (Fig. 2). Crocynia molliuscula is morphologically distinct from P. gossypina in forming small light brown, non-marginate apothecia. While the specimen from La Réunion contains diffractaic acid just as the holotype of C. molliuscula (TLC by Kalb, on label attached to H-NYL 22052), the specimen from Mauritius contains norstictic acid. Since both specimens of C. molliuscula and that of P. gossypina from Sri Lanka are from the Palaeotropics in contrast to the other P. gossypina accessions in our tree, which are from the Neotropics, it seems as if the topology was resolved according to geography. Still, the apparent morphological differences prevent us from accepting the synonymy of C. molliuscula with P. gossypina without further investigation. We were able to generate only short sequences of those two specimens and more individuals with a typical C. molliuscula morphology and chemistry should be sampled to find out whether C. molliuscula is a distinct species or merely a morphologically and chemically deviating form of P. gossypina.
mPTP resolves P. gossypina as three different species in the mtSSU tree (Fig. 2), while it is delimited as one species in the ITS tree (Fig. 3). This clearly indicates that species of the former genus Crocynia need to be investigated more closely. There are no recent taxonomic studies on the former species of Crocynia, except for the description of three new species (Lumbsch et al. Reference Lumbsch, Ahti, Altermann, Amo de Paz, Aptroot, Arup, Bárcenas Peña, Bawingan, Benatti and Betancourt2011; Aptroot & Cáceres Reference Aptroot and Cáceres2014; Sipman Reference Sipman2018) albeit without providing DNA sequences. Crocynia is poorly understood and comprises an unnatural assembly of species. The typical felt-like thallus morphology has been shown not to be a taxonomically relevant character at either genus or family level, and it is probable that additional Crocynia species belong in Phyllopsora.
Phyllopsora halei (Tuck.) Zahlbr.
Cat. Lich. Univ. 4(3): 398 (1926).—Pannaria halei Tuck., Amer. J. Sci. Arts, Ser. 2 25: 424 (1858); type: USA, Louisiana, 1853, Hale (FH-TUCK 2828—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 241 (as ‘holotype’, Art. 9.10), not seen; H-NYL 20521!—isolectotype; H-NYL 20522!—isolectotype) (TLC (Timdal & Krog Reference Timdal and Krog2001): atranorin, terpenoid T3).
Phyllopsora pannosa Müll. Arg., Bot. Jahrb. Syst. 20: 265 (1895); type: Tanzania, Tanga Prov., Usambara, Kwambugu-Hochwälder, 1894, C. Holst 1432 (G—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 235, image seen; BM—isolectotype, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): atranorin, fatty acids, triterpenoids).
Descriptions
Swinscow & Krog (Reference Swinscow and Krog1981, as P. pannosa), Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Atranorin (major) and unknown compounds, partly terpenoids (see Timdal & Krog (Reference Timdal and Krog2001) for characterization of three chemotypes).
Distribution
North America (chemotype 1), Africa (chemotypes 1, 2 and 3), Asia (chemotype 3).
Discussion
Our three accessions of P. halei (chemotypes 2 and 3) from the Palaeotropics form a strongly supported clade and are resolved as one species in both mPTP analyses (Figs 2 & 3). They are resolved as sister to the new species P. amazonica (Figs 3 & 4) and P. pyxinoides (Fig. 4). Phyllopsora halei has a characteristic morphology with a thick, reddish brown prothallus, pale green squamules originating from small areoles at the margin of the prothallus, and thick isidia. In combination with chemistry, it is thus readily distinguished from all other known Phyllopsora species. The new species P. amazonica resembles P. halei in forming pale green squamules, isidia and brown-black apothecia, as well as by the presence of atranorin and a series of terpenoids (chemically identical to P. halei chemotype 1), but it forms a thinner and less distinct prothallus (see P. amazonica for further discussion).
Phyllopsora halei was described from Louisiana and the only published North American collection known to us is the type material. African material of this species was known as P. pannosa (e.g. by Swinscow & Krog Reference Swinscow and Krog1981) until the two species were synonymized by Brako (Reference Brako1991). Whereas the African material is richly isidiate, the American specimens lack isidia (Swinscow & Krog Reference Swinscow and Krog1981). Unfortunately, we were not able to sequence an American specimen but we agree with Brako (Reference Brako1991) that the species are synonyms because of their otherwise identical morphology, as well as the presence of atranorin and terpenoids.
Phyllopsora himalayensis G. K. Mishra et al.
Mycotaxon 115: 38 (2011): type: India, Himachal Pradesh, Kullu District, Great Himalayan National Park, Shilt, 2800 m alt., on bark, 04-11-2002, S. Nayaka & R. Srivastava 02-001037 (LWG—holotype, not seen).
Description
Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011).
Chemistry
Atranorin.
Distribution
Asia.
Discussion
The species was not studied by us due to the lack of response from LWG to our repeated loan requests. Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011) assumed that the species was close to P. kalbii in having globular isidia and a dark brown prothallus. We find several long branches for our P. kalbii specimens (Figs 2 & 3), indicating the presence of several (cryptic) species. It would therefore be interesting to generate sequences of P. himalayensis and check whether they associate with some of our P. kalbii sequences. Some of our unidentified specimens partly fit the description of P. himalayensis, but detailed morphological comparisons with the type specimen or sequences are necessary to gain more information about conspecificity.
Phyllopsora hispaniolae Timdal
Biblioth. Lichenol. 106: 333 (2011); type: Dominican Republic, Prov. Independencia, Sierra de Baoruco, Charco de la Paloma, 48·4 km S of Puerto Escondito, c. 18°15′N, 71°36′W, 1800 m alt., humid hardwoods around waterhole, 25-01-1987, R. C. Harris 20672 (NY!—holotype) (TLC: argopsin, chlorophyllopsorin).
Description
Timdal (Reference Timdal2011).
Chemistry
Argopsin, chlorophyllopsorin.
Distribution
Central and South America.
Discussion
In this study, we include three accessions of P. hispaniolae, which form a well-supported clade together with P. rosei as sister to P. nemoralis (Figs 2–4). Both mPTP analyses resolve P. hispaniolae and P. rosei to form one entity only (Figs 2 & 3). Phyllopsora hispaniolae differs from P. rosei in morphology, chemistry and distribution range so we regard it as premature to synonymize these two species. More specimens should be investigated to see whether a morphological and chemical overlap might be observed. See also the discussion under P. rosei.
Phyllopsora imshaugii Timdal
Biblioth. Lichenol. 106: 334 (2011); type: Jamaica, Parish of Portland or St. Thomas, summit of Blue Mt. Peak, 7400 ft alt., 08-10-1952, H. A. Imshaug 13037 (MSC 25550!—holotype) (TLC: norstictic acid).
Description
Timdal (Reference Timdal2011).
Chemistry
Norstictic acid (major).
Distribution
Central and South America.
Discussion
The three accessions of P. imshaugii group together in a strongly supported clade and are resolved as one species in both mPTP analyses (Figs 2 & 3). The specimens are strongly resolved as sister to the byssoid P. gossypina (Figs 2–4) but both sit on long and distinct branches (Figs 2 & 3). Phyllopsora imshaugii is not byssoid, as it has a distinct upper cortex and forms isidia. The P. imshaugii specimen from Ecuador, however, shows a smooth white prothallus with finely pubescent squamules, which may resemble a byssoid thallus on first sight. In addition, P. imshaugii forms distinctly marginate apothecia similar to P. gossypina and both share the presence of norstictic acid. Thus, the phylogenetic relationship is reflected at least partly in morphology and chemistry.
Phyllopsora isidiosa Kistenich & Timdal sp. nov.
MycoBank No.: MB 829275
Differs from P. byssiseda in forming a crustose, areolate thallus and more delicate and branched isidia, and from P. isidiotyla in forming less branched, thicker isidia and having a more indistinct and non-pubescent apothecial margin.
Type: USA, North Carolina, Jackson Co., Nantahala National Forest, Chattooga Wild and Scenic River/Ellicot Rock Wilderness, above Fowler Creek, just S of Bull Pen Road, 35°01′08″N, 83°06′12″W, 3000 ft alt., granitic bald on SE-facing slope and adjacent mixed hardwood forest, on Quercus, 18 September 2006, J. C. Lendemer, S. Beeching & A. Moroz 7765 dupl. (BG L-93867!—holotype) (TLC: no lichen substances; DNA: MK352153 (mtSSU), MK352328 (ITS)).
(Fig. 6A)
Thallus effuse, crustose; areoles minute, granular, up to 0·1 mm diam., more or less scattered, pale to medium green, dull, glabrous or slightly pubescent; isidia c. 0·1 mm thick, up to 0·8 mm long, simple or branched, more or less straight, pale to medium green, glabrous, adnate to ascending; upper cortex poorly defined, up to 15 µm thick, formed by a few layers of thin-walled hyphae with rounded lumina (type 2), not containing crystals; medulla not containing crystals; prothallus usually well developed, white.
Apothecia not common, up to 1 mm diam., round or slightly irregular, mostly simple, weakly to moderately convex, orange-brown to medium brown, when young with an indistinct, slightly paler, glabrous margin; excipulum yellowish brown in inner part, paler at the rim, K−; hypothecium yellowish brown, K−; epithecium colourless; no crystals or granules in the apothecium; ascospores narrowly ellipsoid to fusiform, simple, 7·5–11·5 × 2·5–3·0 µm (n = 20).
Conidiomata not seen.
Chemistry
No lichen substances.
Etymology
The epithet indicates that the species is richly isidiate.
Distribution
Pantropical; also occurring in temperate Asia and North America.
Discussion
Initially, specimens of P. isidiosa were identified as P. byssiseda, albeit being more filigree, but the phylogenetic analyses revealed them to form a separate, strongly supported clade (Figs 2 & 3), which is weakly resolved as sister to the clade containing group A and several other species (Figs 3 & 4, group A). mPTP delimits the accessions as a single species in the mtSSU tree (Fig. 2), while it splits them into four species corresponding to geography in the ITS tree (Fig. 3). The species seems to be widespread, occurring both in tropical and subtropical regions. It is morphologically intermediate between P. byssiseda and P. isidiotyla, differing from the first in forming a more crustose thallus with more delicate isidia, and from the second in forming somewhat coarser, less branched isidia. It also resembles the new species P. furfurella (Fig. 5C) in forming a white prothallus with crustose areoles and isidia. However, P. furfurella is readily distinguished by its lichen substances (containing furfuraceic acid in the thallus and skyrin in the hypothecium).
Additional specimens examined. Australia: Queensland: Girringun National Park, Yamanie Section, 14 km WNW of Abergowrie, remnant rainforest along Herbert River, 18°24′49″S, 145°46′18″E, 55 m alt., on trunk of treelet, 2006, J. A. Elix 38478 (CANB 798838) [DNA: MK352267 (mtSSU), MK352433 (ITS)].—Brazil: Mato Grosso do Sul: etwa 30 km südlich von Campo Grande, 550 m, in einem dichten cerrado, 14 xi 1979, K. Kalb & G. Plöbst, Kalb, Lich. Neotrop. Exsicc. 343 (B 60-156328). São Paulo: Município de Mogi-Gauçu, Distrito de Martinho Prado Jt., Reserva Ecológica de Mogi-Guaçu, cerrado between gravel road and ‘pau brasil’ plantation, 2007, R. Lücking & E. Rivas Plata 23302 (SP 393465) [DNA: MG925907 (mtSSU), MG926004 (ITS)].—Dominican Republic: Puerto Plata: S of Puerto Plata, Parc National Isabel de Torres, Pico Isabel de Torre, 19°45·73′N, 70°42·68′W, 770 m alt., botanical garden with damp and open forest with mixed trees and shrubs, on Spathodea campanulata, 2008, P. van den Boom 39012 (hb. v. d. Boom) [DNA: MK352197 (mtSSU), MK352368 (ITS)]; ibid., on big tree, P. van den Boom 39074 (hb. v. d. Boom).—Indonesia: West Java: Cibodas, Botanical Garden, c. 1400 m alt., on tree, 2003, L. Sudirman & H. Sipman 51474 (B 60-168671).—Malaysia: Sabah: Malaysian Borneo, SAFE-Project area, mostly Macaranga-dominated secondary forest, 2012, P. Wolseley, H. Thüs & C. Vairappan S.P.5 (BORH).—Nepal: from Thulo Syabru to Bamboo, Machilus, 1800 m alt., 2007, L. R. Sharma et al. M16 (E 305556) [DNA: MK352155 (mtSSU), MK352330 (ITS)]; from Thulo Syabru to Bamboo, river/suspension bridge, 28°08′34″N, 85°22′11″E, 2000 m alt., on Castanopsis tree trunk, low temperate mixed broad-leaved forest, 2007, L. R. Sharma et al. L25-2 (E 305558).—Philippines: Laguna Province: Luzon, Los Baños, Mount Makiling Forest Reserve, 14°08′N, 121°14′E, 370 m alt., parkland close to the university, corticolous, 1994, P. Diederich 13210 (hb. Diederich) [DNA: MK352232 (mtSSU)].—Thailand: Chiang Mai: Doi Suthep, King's Palace, 18°49′N, 99°53′E, 1550 m alt., oak/chestnut forest, 1991, P. A. Wolseley & B. Aguirre-Hudson 5552 (BM 749822).—USA: South Carolina: Darlington Co., S edge of Louthers Lake (oxbow lake W of Great Pee Dee River), 34°18′05″N, 79°42′42″W, c. 30 m alt., large Stream Swamp (cypress forest) on lake shore, partly shaded, on Taxodium distichens trunk, 2008, G. B. Perlmutter, S. Q. Beeching & M. F. Hodges 1598 (NY); Macon Co., Bank of Chattooga River, near the 3-state corner, 35°00′N, 83°06′W, 630 m alt., on trunk of Magnolia fraseri in thick Rhododendron thickets, 1995, A. Nordin 4187 (UPS L-71532).
Phyllopsora isidiotyla (Vain.) Riddle
Mycologia 15: 81 (1923).—Lecidea isidiotyla Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 49 (1890); type: Brazil, Minas Gerais, Lafayette, 1885, E. A. Wainio, Lich. Bras. Exs. 222 (TUR-V 22634!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 242 (as ‘holotype’, Art. 9.10); BM, M, UPS, ZT—isolectotypes, not seen) (TLC: atranorin (major), zeorin (major)).
Descriptions
Brako (Reference Brako1991), Elix (Reference Elix and McCarthy2009).
Chemistry
Atranorin, zeorin; possibly also acid deficient (see below).
Distribution
Brazil; reports from elsewhere require confirmation.
Discussion
We were able to sequence only one specimen considered to be P. isidiotyla in this study. The accession is resolved as sister to P. canoumbrina (Figs 2–4), from which it differs by forming small, branched isidia. It is delimited as a single species in both mPTP analyses (Figs 2 & 3). Even though P. isidiotyla is supposedly widespread (e.g. Brako Reference Brako1991; Elix 2009; Mishra et al. Reference Mishra, Upreti, Nayaka and Haridas2011), it proved difficult to obtain material that could be unambiguously identified as P. isidiotyla. The holotype contains major amounts of zeorin (and atranorin) but we have found zeorin in Phyllopsora only in P. buettneri, P. melanoglauca, P. neotinica, P. porphyromelaena and P. subhispidula, species that differ markedly from P. isidiotyla in morphology. Our specimen representing P. isidiotyla in the phylogenetic analyses is from Brazil and resembles the holotype in morphology but lacks the lichen substances. We regard all published reports of P. isidiotyla as doubtful and recommend sequencing more specimens to investigate the full morphological and geographical extent of this species.
Phyllopsora kalbii Brako
Fl. Neotrop. Monogr. 55: 51 (1991); type: Brazil, Mato Grosso do Sul, Estrada do Pantanal, some kms E of Coxim, 270 m alt., 29-06-1980, K. Kalb 250 p.p. (NY—holotype, not seen).
Descriptions
Brako (Reference Brako1991), Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Atranorin (minor to trace) or no lichen substances.
Distribution
North, Central and South America, Africa, Asia.
Discussion
All accessions of P. kalbii form a strongly supported clade in both phylogenies but are delimited as several species in the mPTP analyses (Figs 2 & 3). All specimens appear on very long branches, particularly in the ITS tree (Fig. 3), most likely because of highly variable ITS1 sequences in all specimens. The palaeotropical and South American specimens group together respectively, while the position of the North American specimen varies (Figs 2 & 3). However, all specimens are morphologically similar, having small, pale green squamules growing on a thin white prothallus and short globular isidia, and they lack lichen substances (or contain small amounts of atranorin). Hence, we consider them to belong to the same species, although more specimens from additional geographical regions are likely to provide better resolution. Phyllopsora kalbii is resolved as sister to P. cuyabensis in a clade with P. byssiseda and P. fendleri (Figs 2 & 4). It differs from P. cuyabensis in, for example, forming an upper cortex and from P. byssiseda and P. fendleri in forming smaller squamules and a thinner prothallus. Phyllopsora kalbii might also be confused with P. corallina based on morphology, but the latter differs in having long and cylindrical isidia. Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011) considered P. himalayensis to be a close relative of P. kalbii; unfortunately, we were not able to sequence that species.
Phyllopsora loekoesii S. Y. Kondr. et al.
Acta Bot. Hung. 58: 349 (2016); type: Korea, Gyeongsangbuk-do, Ulleung-gun, Ulleung-eup, between Naesujeon and Soekpo waterfall, 37°31′19·51″N, 130°54′16·03″E, 415 m alt., at a rock wall, on siliceous rocks, 09-07-2016, S. Y. Kondratyuk & L. Lökös 161759 (Korean Lichen Research Institute 39977!—holotype).
Description
Kondratyuk et al. (Reference Kondratyuk, Lőkös, Halda, Upreti, Mishra, Haji Moniri, Farkas, Park, Lee and Liu2016).
Chemistry
No lichen substances.
Distribution
Asia.
Discussion
The two accessions of P. loekoesii group together in a supported clade in both analyses and are revealed as sister to P. confusa (Figs 2–4). The two specimens are recovered as two separate species in the mPTP analyses (Figs 2 & 3) but cluster together with unpublished sequences by Kondratyuk of the holo- and isotype in a separate phylogenetic analysis (data not shown). The two specimens are morphologically similar and therefore we choose to treat them as the same species despite the mPTP results. In morphology, P. loekoesii is highly similar to its sister species P. confusa. Both have small squamules and do not contain lichen substances, but P. loekoesii differs from P. confusa in forming isidia (vs. lacinules) and having longer ascospores.
The species is new to Japan and Nepal.
Phyllopsora longiuscula (Nyl.) Zahlbr.
Cat. Lich. Univ. 4(3): 398 (1926).—Lecidea longiuscula Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 339 (1863); type: Cuba, s. loc., C. Wright s. n. (H-NYL 20537!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 242; BM!, UPS L-108157!—isolectotypes, issued as Tuckerman, Wright Lich. Cub. No. 179) (TLC: no lichen substances).
Lecidea intermediella Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 339 (1863).—Phyllopsora intermediella (Nyl.) Zahlbr., Cat. Lich. Univ. 4(3): 398 (1926); type: Cuba, s. loc., C. Wright s. n. (H-NYL 20558!—lectotype, designated by Brako (Reference Brako1991): 49 (as ‘holotype’, Art. 9.10); BM!, UPS L-108152!—isolectotypes, issued as Tuckerman, Wright Lich. Cub. No. 183) (TLC (Brako Reference Brako1991): no lichen substances).
Description
Brako (Reference Brako1991).
Chemistry
No lichen substances.
Distribution
Central and South America, Asia, Australia.
Discussion
When selecting specimens for this study, we struggled to find correctly identified specimens of P. intermediella, some being misidentified. When investigating the holotypes of both species, we found them to be strikingly similar in morphology. The main difference is that P. intermediella forms isidia while P. longiuscula forms lacinules, and also the ascospores are reported to be shorter in the former species. Many specimens of P. intermediella were collected from rocks, which is highly unusual in Phyllopsora. We have only once encountered a saxicolous, typical (i.e. lacinulate) P. longiuscula specimen, sequenced here as specimen 1039. In our phylogeny (Figs 2 & 3) the sequence of an isidiate specimen (454) is nested within a clade of lacinulate specimens (467, 1039, 6761).
Isidia are generally common in Phyllopsora species and Brako (Reference Brako1991) found the presence or absence of isidia to be an unreliable taxonomic character. It is possible that the presence of isidia or lacinules depends on ecological factors. Other species, for example P. breviuscula, also show a generally wide morphological variability. Even though ascospores are reported to be longer in P. longiuscula, we suspect that this character is unreliable in this case, as only a small number of spores have been measured.
As all of the four accessions used in this study group together in a supported clade (Figs 2 & 3), we consider them to belong to the same species and synonymize P. intermediella with P. longiuscula. Additional, unpublished but incomplete sequences of P. intermediella specimens support this decision. However, mPTP suggests that the specimens belong to several species due to the long branches (Figs 2 & 3). The closest relatives of P. longiuscula seem to be P. breviuscula and P. mauritiana (Figs 2 & 4), from which it differs by forming smaller squamules and vegetative propagules (lacinules or isidia).
The species is new to Australia (New South Wales, Elix 42451, CANB).
Phyllopsora malcolmii Vězda & Kalb
In Vězda, Lich. Rar. Exsicc. 20: 4 (1995); type: New Zealand, South Island, Nelson, loco ‘Brook Stream track’ dicto, ad corticem arborum, 120 m alt., 23-05-1994, W. Malcolm s. n., Vězda, Lich. Rar. Exs. 200 (CHR—holotype, not seen; BM!, GZU!—isotypes) (TLC: no lichen substances; DNA: MK352170 (mtSSU), MK352344 (ITS)).
Description
Galloway (Reference Galloway2007).
Chemistry
No lichen substances.
Distribution
New Zealand.
Discussion
The species is known only from the type collection and we were able to generate sequences from an isotype. The accession is resolved differently in the two trees: in the mtSSU and *BEAST trees (Figs 2 & 4) it is the sister to the unidentified specimen 7227 from Sri Lanka in a clade with P. canoumbrina, P. isidiotyla and additional unidentified specimens. In the ITS tree (Fig. 3), in contrast, it falls into group A as sister to P. dolichospora, P. foliatella and P. furfuracea. Phyllopsora malcolmii seems to be closely associated to the unidentified specimen Phyllopsora sp. (7227) from Sri Lanka but is resolved as a distinct species in the mPTP analyses (Figs 2 & 3). The two specimens differ morphologically, since P. malcolmii has a marked white prothallus with arachnoid hyphae whereas the Sri Lankan specimen has flat adnate squamules when young, growing into small coralloid squamules when older. Argopsin (reported in the protologue) was not detected by us in the isotype and specimen 7227 is also acid deficient.
Phyllopsora martinii Swinscow & Krog
Lichenologist 13: 232 (1981); type: Kenya, Coast Province, Kwale District, Shimba Hills, 25 km SW of Mombasa, Kivumoni Forest, tree trunk in shady forest, rather dry, 02-1972, T. D. V. Swinscow & H. Krog K42/3 (BM—holotype, not seen; O L-1144!—isotype) (TLC: argopsin, chlorophyllopsorin, norargopsin).
Descriptions
Swinscow & Krog (Reference Swinscow and Krog1981), Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Argopsin (major), chlorophyllopsorin (major), norargopsin (minor).
Distribution
Africa.
Discussion
The two accessions of P. martinii cluster together with strong support and are resolved as a single species in both mPTP analyses (Figs 2 & 3). Phyllopsora martinii is morphologically similar to P. corallina with its medium-sized squamules and isidia, but can be distinguished by the shorter ascospores and the chemistry (argopsin, chlorophyllopsorin and norargopsin in P. martinii vs. no lichen substances in P. corallina).
Phyllopsora mauritiana (Taylor) Swinscow & Krog
Lichenologist 13: 242 (1981).—Lecidea mauritiana Taylor, London J. Bot. 6: 151 (1847); type: Mauritius, s. loc. (FH—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 242, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): no lichen substances).
Description
Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
No lichen substances.
Distribution
Africa.
Discussion
The three accessions of P. mauritiana group into a strongly supported clade in both phylogenetic trees (Figs 2 & 3) and as sister to P. breviuscula (Figs 2–4). They are delimited as a single species in the ITS mPTP analysis (Fig. 3). In the mtSSU tree, mPTP splits the accessions into two species (Fig. 2), most likely due to long branches. The species is characterized by the crustose thallus, which is formed by discrete to adjoining areoles on a thick, reddish brown prothallus, the absence of vegetative dispersal units and lack of lichen substances. Thus, its phylogenetic sister-relationship to P. breviuscula (Figs 2–4) is also reflected in morphology and chemistry: both lack lichen substances and vegetative dispersal units. In addition, it resembles the neotropical morphotype of P. breviuscula in forming a dense prothallus with flat, pubescent squamules, but is distinguished by its squamules being more adnate, isodiametric and more crust-like than those of P. breviuscula.
Phyllopsora mediocris Swinscow & Krog
Lichenologist 13: 234 (1981); type: Tanzania, Tanga Province, Usambara Mountains, Amani, near Forestry House, alt. c. 900 m, 5°07′S, 38°38′E, 09-01-1971, R. Moberg 1481a-1 (UPS L-10381!—holotype) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): no lichen substances).
Descriptions
Swinscow & Krog (Reference Swinscow and Krog1981), Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
No lichen substances.
Distribution
Africa, Asia.
Discussion
The three accessions of P. mediocris are resolved in a strongly supported clade as sister to P. parvifolia (Figs 2–4) and delimited as one species in both mPTP analyses (Figs 2 & 3). The species is readily distinguished from other species of Phyllopsora by the medium-sized, soon ascending squamules on a medium thick, reddish brown prothallus, simple lacinules and the lack of lichen substances. The sister species, P. parvifolia, also lacks lichen substances but forms a more rosulate thallus and phyllidia.
Phyllopsora melanoglauca Zahlbr.
Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 83: 133 (1909); type: Brazil, São Paulo, in silvaticis prope urbem Iguape, 20–100 m alt., 09-1901, V. Schiffner s. n. (W—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 242 (as ‘holotype’, Art. 9.10), not seen; BM!—isolectotype) (TLC: vicanicin, zeorin).
Descriptions
Brako (Reference Brako1991, as P. buettneri var. glauca chemotype I) and Timdal (Reference Timdal2008, as P. buettneri chemotype 3).
Chemistry
Vicanicin (major), norvicanicin (minor, trace, or absent), zeorin (major).
Distribution
Neotropical; palaeotropical records need confirmation.
Discussion
We include five specimens of P. buettneri chemotype 3 in this study. They group together in a strongly supported clade and are resolved as a separate species not closely related to the remaining chemotypes of P. buettneri (Figs 2 & 3). We therefore conclude that they comprise a distinct species and resurrect the old name P. melanoglauca for this taxon. Unfortunately, we were not able to resolve the closest sister to P. melanoglauca in either tree (Figs 2–4). The species is morphologically identical to P. buettneri but can be readily distinguished by its chemistry, containing vicanicin, zeorin, and often norvicanicin. Vicanicin and norvicanicin are also found in P. glaucella, which might be a close relative and occurs in the same larger clade. All specimens we have examined of P. melanoglauca are from the Neotropics. See also P. buettneri and the Discussion for more information.
Phyllopsora methoxymicareica Elix
Australas. Lichenol. 59: 25 (2006); type: Australia, New South Wales, Clyde Mountain, below the road, 20 km SE of Braidwood, 35°35′S, 149°57′E, 700 m alt., in wet sclerophyll forest on base of Eucalyptus vimialis, 14-02-1989, J. A. Elix 22773 (CANB 743017—holotype, fragment seen).
Descriptions
Elix (Reference Elix2006c, Reference Elix and McCarthy2009).
Chemistry
Methoxymicareic acid (major), hydromicareic acid (trace) (Elix 2009).
Distribution
Australia.
Discussion
We were unable to generate sequences from a fragment of the holotype sent to us, despite it being only 29 years old. The species resembles P. furfuracea and P. foliatella as all three species have a crustose, areolate thallus and form numerous isidia, but they differ in spore size and chemistry (Table 2). Sequencing fresh specimens is necessary in order to draw further conclusions. Phyllopsora methoxymicareica is best identified by its characteristic chemistry.
Phyllopsora microdactyla (C. Knight) D. J. Galloway
New Zealand J. Bot. 21: 196 (1983).—Lecidea microdactyla C. Knight, Trans. & Proc. New Zealand Inst. 12: 375 (1880); type: New Zealand, s. loc., C. Knight (BM—lectotype, designated by Galloway (Reference Galloway1983): 196, not seen; H!—three probable isolectotypes) [TLC: no lichen substances].
Lecidea carpodeti Zahlbr., Denkschr. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 104: 308 (1941); type: New Zealand, Otago, Dunedin, Boyd's Bush, J. S. Thomson T 492 (ZA 566) (CHR 347017—lectotype, designated by Galloway (Reference Galloway1983): 196, not seen; BM!—isolectotype).
Parmeliella mucorina Zahlbr., Denkschr. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 104: 272 (1941); type: New Zealand, Wellington, Greatford, on Melicytus ramiflorus, 07-1933, H. H Allan 138 (W 2304—holotype, not seen) (synonymy based on Galloway (Reference Galloway1985) and Jørgensen (Reference Jørgensen2003)).
Description
Galloway (Reference Galloway1985).
Chemistry
No lichen substances.
Distribution
New Zealand.
Discussion
We know of no reliably identified, recently collected material of P. microdactyla, and did not attempt to extract DNA from the old, probable isolectotypes in H. The species is characterized by coralloid, granular to microphylline squamules on a pale prothallus, cylindrical isidia, large ascospores and the absence of lichen substances. Some of the unidentified Phyllopsora specimens from Malaysia and Sri Lanka resemble this species but differ, for example, in having dark brown, more distinctly marginate apothecia. As we have no information regarding the extent of the morphological variability in P. microdactyla, sequences of the type material or of freshly collected material from the type locality are essential for gaining information about the species’ phylogenetic relationships.
Phyllopsora nemoralis Timdal & Krog
Mycotaxon 77: 85 (2001); type: La Réunion, Forêt de Bélouve, track from Gite de Bélouve to viewpoint, 21°03′S, 55°32′E, 1500–1550 m alt., 30-09-1996, H. Krog & E. Timdal RE25/32 (O L-867!—holotype) (TLC: argopsin, atranorin; DNA: MK352142 (mtSSU)).
Description
Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Argopsin (major) and atranorin (minor).
Distribution
Africa.
Discussion
The two accessions of P. nemoralis, including the holotype, cluster together in a strongly supported clade in the mtSSU tree (Fig. 2). Both mPTP analyses delimit P. nemoralis as a separate species, which is sister to the hispaniolae-rosei complex (Figs 2–4). Several of our specimens, which were identified as P. nemoralis, were found to belong to other species by molecular data, such as P. confusa, while the specimen from South Africa was initially named P. hispaniolae. This indicates the morphological similarity of P. nemoralis with its sister clade. Indeed, all three species, P. hispaniolae, P. nemoralis and P. rosei, share the presence of argopsin and form ascospores of a similar size. However, thallus morphology, vegetative dispersal units, colour of the prothallus, and additional minor compounds are slightly different between the species. Phyllopsora nemoralis is the only species forming isidia and containing atranorin in addition to argopsin.
Phyllopsora neofoliata Elix
Australas. Lichenol. 59: 26 (2006); type: Australia, New South Wales, Lord Howe Island, Max Nicholls Track, 31°31′08″S, 159°03′03″E, 5 m alt., on tree in lowland forest, 20-06-1992, J. A. Elix 32714 (CANB 740185—holotype, not seen; O L-1319!—isotype, fragment) (DNA: MK352263 (mtSSU), MK352429 (ITS)).
Descriptions
Elix (Reference Elix2006c, Reference Elix and McCarthy2009).
Chemistry
Furfuraceic acid (major), ± physodic acid (minor or trace) (Elix Reference Elix2006c, Reference Elix and McCarthy2009).
Distribution
Africa, Australia.
Discussion
The three accessions of P. neofoliata group together in a strongly supported clade and are resolved as a single species in both mPTP analyses (Figs 2 & 3). Its sister species could not be resolved in either phylogenetic tree, but P. neofoliata is found in a larger clade with P. castaneocincta, P. confusa, P. mediocris and P. parvifolia among others (Figs 2–4). The chemistry can be similar to P. castaneocincta (furfuraceic acid) but may also contain physodic acid as minor to trace (Elix Reference Elix2006c). The specimen from Kenya, however, seems to represent an acid-deficient strain, since it did not contain any lichen substances when investigated by TLC. That specimen also differs slightly in morphology from the Australian specimens by forming narrower squamules and a brownish prothallus. We assume this to be due to geographical variation within the species. It was named neofoliata because of its similarity to P. foliata (Elix Reference Elix2006c). That species occurs in the same larger clade (Figs 3 & 4) although it is uncertain to what degree the species are related.
The species is new to Africa (Kenya).
Phyllopsora neotinica Kistenich & Timdal sp. nov.
MycoBank No.: MB 829276
Differs from P. chodatinica in containing argopsin and often zeorin, and apparently lacking chodatin.
Type: Venezuela, Capital District, Parque Nacional Macarao, 1·5 km E of El Junquito, 10°27·50′N, 67°04·52′W, 1880 m alt., tree trunk in tropical moist forest, 0–3 m above ground, trunk diam. 20 cm, 12 November 2015, M. S. Dahl, J. E. Hernández M., S. Kistenich, E. Timdal & A. K. Toreskaas SK1-246 (O L-202526!—holotype; VEN!—isotype) (TLC: argopsin (major), unknown xanthone (major), zeorin (trace); DNA: MK352215 (mtSSU), MK352386 (ITS)).
(Fig. 6B)
Thallus effuse, squamulose; squamules medium-sized to large, ascending, elongated, often imbricate, incised to deeply divided, plane to weakly convex; upper side yellowish green, glabrous, epruinose; margin concolorous with upper side or somewhat paler, finely pubescent; lacinules numerous, developing from lobe-tips; upper cortex of type 1, 25–40 µm thick, containing a few crystals dissolving in K (PD−, K−); medulla containing crystals dissolving in K (PD+ orange or PD−, K−); prothallus usually well developed, reddish brown.
Apothecia seen in one collection, up to 1·2 mm diam., rounded, simple or slightly conglomerate, weakly to moderately convex, reddish brown, with an indistinct and often darker margin; excipulum reddish brown (K+ faintly purple), darkest near the rim; hypothecium pale brown; epithecium colourless; apothecium containing scattered groups of orange crystals dissolving in K (K+ yellow); ascospores narrowly ellipsoid to fusiform, simple, 5–8 × 2·0–2·5 µm (n = 20, from a single apothecium).
Conidiomata not seen.
Chemistry
Argopsin (major, rarely absent), unknown xanthone (major) and zeorin (minor to trace, or rarely absent).
Etymology
The epithet is a contraction of ‘the neotropical Phyllopsora chodatinica’.
Distribution
North, Central and South America.
Discussion
The five accessions of P. neotinica were initially named P. chodatinica. They are resolved with strong support within the clade P. buettneri-chodatinica-porphyromelaena and P. chlorophaea (Fig. 4). The mPTP analyses resolve the accessions as a species distinct from P. chodatinica (Figs 2 & 3). Phyllopsora neotinica was first thought to be a chemical variety of P. chodatinica occurring in the Neotropics. It is morphologically identical to P. chodatinica but differs in its chemical compounds: Phyllopsora neotinica usually contains argopsin and zeorin in addition to an unknown xanthone, although apparently not chodatin, whereas P. chodatinica contains only xanthones, including chodatin. Sequences from the paratype of P. chodatinica turned out to be invaluable for fixing the name chodatinica to the correct clade. The possible substitution of chodatin by a xanthone with very similar R f values in the neotropical ‘P. chodatinica’ was discussed by Timdal (Reference Timdal2008). We assume that most or all of the species records of P. buettneri var. glauca chemotype II in Brako (Reference Brako1991), as well as all neotropical P. chodatinica specimens in Timdal (Reference Timdal2008, Reference Timdal2011), belong to P. neotinica. See also discussion under P. chodatinica for more details.
Selected specimens examined. Brazil: Rio de Janeiro: Parque Nacional do Itatiaia, surroundings of Abrigo Lamego, 22°25·63′S, 44°37·23′W, 1150 m alt., on tree trunk in Atlantic rainforest, 2015, M. S. Dahl, S. Kistenich, E. Timdal & A. K. Toreskaas SK1-402 (O L-202682) [DNA: MK352222 (mtSSU), MK352393 (ITS)].—Costa Rica: Puntarenas Prov.: Esquinas rainforest area SW of the village La Gamba (c. 8 km NNW of Golfito), ridge S above the field station ‘Tropenstation La Gamba’, along the trail from the field station into the Valle Bonito tropical lowland rainforest, 8°42′10″N, 83°12′30″W, 200 m alt., on rough bark of evergreen trees, 2003, J. Hafellner & B. Emmerer 1247 (GZU).—Cuba: Pinar del Rio: Reserva de la Biosfera Sierra del Rosario, S side of “Loma el Salón”, 22°49·74′N, 82°57·89′W, 500–510 m alt., corticolous on trunk of unidentified tree in mixed hardwood forest on N-facing slope near crest, 2007, T. Tønsberg 37923 (BG L-89975) [DNA: MK352149 (mtSSU), MK352324 (ITS)].—Dominica: St. David: Parish of St. David, L'Or, 1000 ft alt., rainforest, 1963, F. H. Imshaug & H. A. Imshaug 33186 (MSC 25592).—Dominican Republic: La Vega: La Sal, 13·3 km N of El Río, then 10 km E of Paso Bajito, on road to Casabito, 3500–3600 m alt., humid hardwoods along stream, 1982, R. C. Harris 15005 (NY).—Guatemala: Baja Verapaz: SSE of Coban, SE of Purulhá, Biotopo Mario Dary Rivera (Biotopo del Quetzal), ‘Fern Trail’, 15°13·5′N, 90°13·6′W, 1700 m alt., NE exposed slope with tropical rainforest, 2004, P. van den Boom 33395 (immixture) (hb. v. d. Boom).—Jamaica: Portland: Parish of Portland, Moodies Gap Trail near Hardwar Gap, Blue Mountains, 3800 ft alt., 1952, H. A. Imshaug 13101 (MSC 25514).—Peru: San Martin: Cerro Escalera (c. 20 km, road distance, NE of Tarapoto), 6°27′S, 76°15′W, 900–1100 m alt., 1981, R. Santesson & G. Thor P72:20 (S).—Puerto Rico: Humacao: Caribbean National Forest, Luquillo Division, Mt. El Toro, trail from El Verde side on Hwy 186, 850 m alt., 1988, R. C. Harris 22248 (NY).—St. Lucia: Mt. Casteau, Quarter of Soufriére, 2000–2000 ft alt., 1963, F. H. Imshaug & H. A. Imshaug 29810 (MSC 25633).—St. Vincent and the Grenadines: St. Vincent: Bow Woods, 800 ft alt., on trees, 1896, W. R. Elliot 135 (BM).—Trinidad and Tobago: Tobago: Parish of St. Paul, along Roxborough Parlatuvier Road, 11°16·81′N, 60°36·64′W, 500–520 m alt., on tree trunk in rainforest, 2008, S. Rui & E. Timdal 10763 (O L-152060) [DNA: MK352176 (mtSSU), MK352349 (ITS)]; same site, 11°17·04′N, 60°35·69′W, 400–450 m alt., on tree trunk in rainforest, 2008, S. Rui & E. Timdal 10774 (O L-152071) [DNA: MK352137 (mtSSU), MK352316 (ITS)].—USA: Florida: Wakulla County Apalachicola National Forest, along Forest Serv. Rd 309 at Lost Creek just S of Leon Co. line, 5·6 mi W of Florida Hwy 267, swamp forest, on Fraxinus, 1988, R. C. Harris 23375 (NY).
Phyllopsora ochroxantha (Nyl.) Zahlbr.
Cat. Lich. Univ. 10 (24): 377 (1939).—Lecidea ochroxantha Nyl., Ann. Sci. Nat., Bot., Sér. 4 11: 223 (1859); type: Bolivia, Campolican, Weddell s. n. (H-NYL 20489!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 243; H 9504194—isolectotype, image seen; PC—isolectotype, not seen) (TLC: phyllopsorin, chlorophyllopsorin).
Lecidea subvirescens Nyl., Ann. Sci. Nat., Bot., Sér. 5 7: 321 (1867).—Phyllopsora subvirescens (Nyl.) Swinscow & Krog, Lichenologist 13: 240 (1981); type: Colombia, Nova Granata, Rio Negro, 1200 m alt., 1863, Lindig s. n. (H-NYL 20492—holotype, image seen) (TLC (Brako Reference Brako1991): phyllopsorin, chlorophyllopsorin; synonymy according to Brako (Reference Brako1989, Reference Brako1991)).
Lecidea ernstiana Müll. Arg., Flora 60: 473 (1877).—Phyllopsora ernstiana (Müll. Arg.) Müll. Arg., Bot. Jahrb. Syst. 20: 265 (1895); type: Venezuela, Caracas, Ernst 190 (G 00293369—holotype, image seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): phyllopsorin, chlorophyllopsorin (as ochroxantha unknowns 1 and 2). Synonymy according to Swinsow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1989, Reference Brako1991)).
Psora polydactyla Müll. Arg., Flora 70: 320 (1887).—Phyllopsora polydactyla (Müll. Arg.) Zahlbr., Cat. Lich. Univ. 4(3): 400 (1926); type: Brazil, São Paulo, Apiahy, 04-1882, Puiggari 2156 (G 00293370—holotype, image seen) (TLC (Brako Reference Brako1991): argopsin, phyllopsorin, chlorophyllopsorin. Synonymy according to Brako (Reference Brako1989, Reference Brako1991)).
Lecidea spinulosa Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 46 (1890).—Phyllopsora spinulosa (Vain.) Zahlbr., Cat. Lich. Univ. 4(3): 401 (1926); type: Brazil, Minas Geraës, Sitio, 1885, E. A. Wainio, Lich. Brasil. Exsicc. 993 (TUR-V 22627—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 245 (as ‘holotype’, Art. 9.10), not seen; BM!—isolectotype, issued as Vainio, Lich. Brasil. Exs. No. 993) (TLC: phyllopsorin, chlorophyllopsorin and two unknown compounds).
Lecidea glabriuscula Nyl., Sert. Lich. Trop.: 40 (1891).—Phyllopsora glabriuscula (Nyl.) Swinscow & Krog, Lichenologist 13: 241 (1981); type: Cuba, s. loc., C. Wright Lich. Cub. ser. 2, 105 (H-NYL 20534!—holotype; FH-TUCK 2922—isotype, not seen, issued as Tuckerman, Wright Lich. Cub., ser. 2, 105) (TLC: phyllopsorin, chlorophyllopsorin).
Descriptions
Timdal (Reference Timdal2008), Elix (Reference Elix and McCarthy2009).
Chemistry
Phyllopsorin (major), chlorophyllopsorin (major to minor), argopsin (occasional trace), norargopsin (occasional trace) and unknown compounds (occasional traces).
Distribution
Neotropical; palaeotropical records require confirmation.
Discussion
The five accessions of P. ochroxantha cluster together in a strongly supported clade (Figs 2 & 3). The mtSSU mPTP analysis resolves all accessions as a single species (Fig. 2) while the ITS analysis splits the accessions from Brazil as well as Trinidad and Tobago as separate species (Fig. 3). The Caribbean specimen appears on a long branch in the ITS tree (Fig. 3) whereas the branch is considerably shorter in the mtSSU tree (Fig. 2). As this specimen agrees with the remaining specimens in morphology and chemistry, we consider that all of them belong to P. ochroxantha. The species is sister to the africana-swinscowii clade (Figs 2–4, group C). Phyllopsora ochroxantha is distinguished from its two morphological and phylogenetic sister species only by its main chemical compounds (chlorophyllopsorin and phyllopsorin in P. ochroxantha vs. various combinations of argopsin, chlorophyllopsorin, methyl 2,7-dichloropsoromate and methyl 2,7-dichloronorpsoromate in the two other species). See P. africana and Discussion for further details.
Phyllopsora parvifolia (Pers.) Müll. Arg.
Bull. Herb. Boissier 2(App. 1): 45 (1894).—Lecidea parvifolia Pers. in Gaudichaud, Voy. Uranie: 192 (1827); type: Brazil, Rio de Janeiro, Gaudichaud s. n. (PC—holotype, not seen; G 00293379—isotype, image seen).
Phyllopsora weberi L. I. Ferraro, Bol. Soc. Argent. Bot. 24: 179 (1985); type: Argentina, Misiones, Dept. San Ignacio, 08-12-1981, L. I. Ferraro et al. 2231 (CTES—holotype, not seen; UPS L-55195!—isotype) (TLC (Brako (Reference Brako1991): no lichen substances. Synonymy according to Brako (Reference Brako1991)).
Description
Elix (Reference Elix and McCarthy2009).
Chemistry
No lichen substances.
Distribution
North, Central and South America, Europe, Africa, Australia, Oceania.
Discussion
The five accessions of P. parvifolia cluster together in a strongly supported clade as sister to P. mediocris in the ITS tree (Fig. 3). In the mtSSU tree, the specimen from Tanzania groups as sister to a clade consisting of P. mediocris and the remaining specimens of P. parvifolia and is delimited as a separate species (Fig. 2). In the ITS tree, the accessions are delimited as five separate species (Fig. 3). Also in the ITS tree, the specimen from Tanzania is resolved as sister to the other specimens of P. parvifolia, which form a strongly supported clade (Fig. 3). The Tanzanian specimen shows more sequence divergence than the other specimens but is morphologically similar in forming a rosulate thallus with numerous phyllidia. Hence, we consider all five specimens belong to the same species for now, although it is possible that the population in Tanzania is genetically isolated from other populations. The European specimen has an overall less developed and smaller thallus than the other specimens, perhaps caused by environmental influences. The sequences of the European specimen, however, do not differ markedly from the others. Phyllopsora parvifolia is readily distinguished from other species by its thallus morphology and from its sister P. mediocris, which has a squamulose thallus and forms lacinules.
The species is reported here as new to Europe (Portugal, specimen 6365). We have also examined, but not sequenced, a specimen from the Azores: Terceira, Canada do Celis, 15-01-2004, A. F. Rodrigues TCCE-46 (B 60-173086).
Phyllopsora parvifoliella (Nyl.) Müll. Arg.
Bull. Soc. Roy. Bot. Belgique 32: 131 (1893 [1894?]).—Lecidea parvifoliella Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 339 (1863); type: Cuba, s. loc., C. Wright s. n., Tuckerman, Wright Lich. Cub. No. 182 (BM!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 244; H-NYL 20545!, UPS L-108289!—isolectotypes) (TLC: atranorin, parvifoliellin).
Description
Timdal (Reference Timdal2008).
Chemistry
Parvifoliellin (major) and often atranorin (minor to trace).
Distribution
Central and South America, Asia.
Discussion
In this study, we included seven specimens originally identified as P. parvifoliella based on the presence of isidia and the detection of parvifoliellin. Surprisingly, they are resolved as two distantly related clades: one clade is left unresolved in a large clade with P. hispaniolae and P. rappiana among many others (Fig. 4); the other clade is sister to P. cinchonarum (Figs 2–4) and described here as the new species P. concinna (Fig. 5B). Upon closer examination, we also found several morphological differences, including the isidia and their placement on the squamules: the three specimens from Peru, Indonesia and Thailand agree with the type material of P. parvifoliella and form isidia growing from the tip of the squamule lobes, forming an extension of the squamules while the four neotropical specimens of the other clade form cylindrical isidia growing from the squamule surface. We therefore consider the former pantropical clade to represent the true P. parvifoliella. See also P. concinna for further information.
The three specimens of P. parvifoliella are resolved as a supported group in both trees (Figs 2 & 3). mPTP resolves them as representing three separate species due to the long branches (Figs 2 & 3). As they are morphologically congruent, we assume that they comprise one species only. Unfortunately, we could not resolve their closest relatives. Parvifoliellin is a rare compound, known only from P. concinna, P. parvifoliella and P. rappiana; all three also contain atranorin.
The species is new to Asia.
Phyllopsora phaeobyssina (Vain.) Timdal
Biblioth. Lichenol. 106: 342 (2011).—Lecidea breviuscula var. phaeobyssina Vain., Ann. Acad. Sci. Fenn., Ser. A 6(7): 127 (1915); type: Guadeloupe, Houelmont, sur un Coffea arabica, P. Duss 481 (TUR-V 22602!—holotype; NY—isotype, not seen) (TLC: argopsin).
Description
Timdal (Reference Timdal2011).
Chemistry
Argopsin (major), norargopsin (absent to minor).
Distribution
Neotropical.
Discussion
In this study, we were able to include only one specimen of P. phaeobyssina. The specimen is resolved as a distinct species in both mPTP analyses (Figs 2 & 3) and groups together with P. teretiuscula (Figs 2 & 4). The two species are similar in morphology and chemistry but P. phaeobyssina forms broader, more flattened squamules and never contains chlorophyllopsorin. See also discussion under P. teretiuscula.
Phyllopsora porphyromelaena (Vain.) Zahlbr.
Cat. Lich. Univ. 4(3): 401 (1926).—Lecidea porphyromelaena Vain., Ann. Acad. Sci. Fenn., Ser. A 15(6): 113 (1921); type: Philippines, Luzon, Bataan Prov., Mount Mariveles, ad truncos arborum, 12-1908, E. D. Merrill 6273 (TUR-V 22619!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 224; BM, TUR-V 22620, US—isolectotypes, not seen) (TLC: argopsin (major), norargopsin (major)).
Phyllopsora formosana Zahlbr., Repert. Spec. Nov. Regni Veg. 33: 43 (1933); type: Taiwan, Prov. Taitung, Raisha, 05-01-1926, Asahina s. n. (W—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 224 (as ‘holotype’, Art. 9.10), not seen; TNS!—isolectotype) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): argopsin, norargopsin (as albicans unknowns 1 and 2)).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001) and Elix (Reference Elix and McCarthy2009), both as P. albicans.
Chemistry
Chemotype 1: argopsin (major), norargopsin (major to trace); chemotype 2: argopsin (major), pannarin (major); chemotype 3: unknown compound (major, R f classes A:4, B′:4–5, C:5), zeorin (major); chemotype 4: argopsin (major), norargopsin (minor to trace), zeorin (minor to rarely trace).
Distribution
Chemotype 1: palaeotropical; chemotype 2: palaeotropical; chemotype 3: Thailand; chemotype 4: neotropical.
Discussion
The species was named P. albicans by Swinscow & Krog (Reference Swinscow and Krog1981), Timdal & Krog (Reference Timdal and Krog2001) and Elix (Reference Elix2006a, Reference Elix and McCarthy2009); the current name P. porphyromelaena was established by Timdal (Reference Timdal2011) since P. albicans is regarded as a synonym of P. santensis (Brako Reference Brako1991). Chemotypes 1 and 2 were recognized by Timdal & Krog (Reference Timdal and Krog2001), and chemotypes 3 and 4 are recognized in this paper. Brako (Reference Brako1991) treated neotropical material of this species as P. buettneri var. glauca chemotypes 1 and 3, a distinction of chemotypes that we do not recognize. Hence, here we call those chemotype 4, while var. glauca chemotype 2 is treated here as P. neotinica.
In total, we include eight accessions of P. porphyromelaena in our phylogenetic study: three specimens of chemotype 1, three of chemotype 2, and two of chemotype 3. We had no fresh material of the fourth chemotype for sequencing. The eight accessions are not resolved as a monophyletic clade in either tree (Figs 2–4). In the mtSSU tree, P. porphyromelaena chemotypes 1 and 2 cluster weakly together, while those of chemotype 3 cluster with P. chodatinica in the ITS tree (Fig. 3). mPTP delimits several different entities corresponding to chemotype and geographical region (Figs 2 & 3). Chemotype 3 might form a separate species but more data are necessary to obtain sufficient phylogenetic support for species description. All accessions form a clade with P. buettneri and P. chodatinica (Figs 2–4, group B) as well as a larger clade with P. chlorophaea and P. neotinica (Figs 2 & 4, group B). These species are morphologically similar. Phyllopsora buettneri might be confused with P. porphyromelaena in particular but forms pruinose and slightly larger lobes than P. porphyromelaena. All five species can be distinguished mainly by their differing chemistries (Table 2). The relationships between these species have long been unclear, and the phylogenies show that the currently available molecular data are unable to resolve species delimitations. More in-depth analyses with additional data from all chemical strains of all included species are necessary to understand the limits and relationships of the species involved. See Discussion for further comments.
Phyllopsora pyxinoides (Nyl.) Kistenich et al.
Taxon 67: 894 (2018).—Crocynia pyxinoides Nyl., Sert. Lich. Trop.: 37 (1891); type: Cuba, ‘in ins. Cuba’, C. Wright, Tuckerman, Wright Lich. Cub. Ser. 2, No. 145 (H-NYL 22059—holotype, image seen) (TLC (Harris, on label): atranorin).
Crocynia biatorina (Mont.) Hue, Mém. Soc. Sci. Nat. Math. Cherbourg 37: 231 (1909).—Parmelia gossypina var. biatorina Mont., Ann. Sci. Nat., Bot., Sér. 2 16: 116 (1841); type: French Guiana, ‘ad cortices arborum in insulâ Cayennâ’, Leprieur 512 (PC—holotype, not seen).
Description
Hue (Reference Hue1909, as Crocynia biatorina).
Chemistry
Atranorin (major), stictic acid (major), terpenoids (minor to traces).
Distribution
Pantropical.
Discussion
Crocynia pyxinoides was transferred to Phyllopsora based on the phylogenetic position of a GenBank accession (Lücking 16052) in a molecular tree of the Ramalinaceae by Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a). In this study, we include three mtSSU accessions of P. pyxinoides, the one from GenBank and two new specimens (Table 1). Here we found that the GenBank accession clustered among our P. gossypina specimens and not with the two other P. pyxinoides accessions. Therefore the GenBank sequence seems to be a misidentified P. gossypina chemotype 2, the norstictic acid strain. The other two accessions grouped into a strongly supported clade and were resolved as a single species in a clade with P. amazonica, P. gossypina, P. halei and P. imshaugii (Figs 2 & 4). Longer sequences, as well as sequences of additional specimens (including ITS), might provide better resolution. It seems, however, that P. gossypina is not the closest relative of P. pyxinoides. This indicates that the former genus Crocynia was not monophyletic and corroborates the decision to synonymize it with Phyllopsora in Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a). Sequences of further Crocynia species, such as C. microphyllina, C. minutiloba, C. mollis and C. molliuscula, are needed to draw further conclusions about the former Crocynia species’ phylogenetic relationships.
Phyllopsora rappiana (Brako) Elix
Australas. Lichenol. 58: 6 (2006).—Phyllopsora corallina var. rappiana Brako, Fl. Neotrop. Monogr. 55: 42 (1991); type: USA, Florida, Sarasota Co., Myakka River State Park, along Myakka River, moist and shady oak wood and scrub, 16-08-1985, L. Brako 8229 (NY!—holotype) (TLC: atranorin, parvifoliellin).
Descriptions
Brako (Reference Brako1991), Elix (Reference Elix and McCarthy2009).
Chemistry
Parvifoliellin (major), atranorin (major).
Distribution
North, Central and South America, Australia.
Discussion
The two accessions of P. rappiana cluster together in a supported clade (Figs 2 & 3). mPTP resolves them as separate species in both analyses due to the long branches (Figs 2 & 3). Based on morphology and chemistry, we still regard them as one species. In the mtSSU tree they are resolved as sister, among others, to P. glaucella (Fig. 2) from which they differ in morphology and chemistry. The species may be confused with P. parvifoliella and P. concinna because of the presence of isidia, parvifoliellin and atranorin (the latter compound not always present). The phylogenies show, however, that the species are not closely related and that the occurrence of the rare lichen substance parvifoliellin has evolved independently in those species (Figs 2–4). Phyllopsora rappiana has a more reduced thallus and shorter, thinner isidia than P. parvifoliella and P. concinna, and generally a higher concentration of atranorin.
Phyllopsora rosei Coppins & P. James
Lichenologist 11: 166 (1979); type: UK, Wales, Merioneth, Dolgellau, vallis Nant Gwynant, in cortice umbroso Fraxini, cum Catillaria pulverea, alt. c. 30 m, 04-1960, P. W. James (BM—holotype, not seen).
Description
Coppins & James (Reference Coppins and James1979), Rose et al. (Reference Rose, Purvis, Coppins, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009).
Chemistry
Argopsin (major), norargopsin (minor or absent).
Distribution
Europe.
Discussion
In this study, we include four specimens of P. rosei, two from France and two from the UK. We found our accessions to form a well-supported clade together with accessions of P. hispaniolae in the mtSSU tree (Fig. 2), while they are nested in P. hispaniolae in the ITS tree (Fig. 3). Both mPTP analyses resolve P. rosei and P. hispaniolae to form one species only. We were surprised by these results as the species are morphologically and chemically different: P. rosei forms a minutely granulose thallus on a white prothallus, thinly 1–3-septate ascospores, and contains argopsin and often norargopsin, while P. hispaniolae forms deeply divided, coralloid squamules on a reddish brown prothallus, simple ascospores and contains argopsin and chlorophyllopsorin. Hence, we suggest keeping the two species separate until further specimens are examined.
Phyllopsora santensis (Tuck.) Swinscow & Krog
Lichenologist 13: 236 (1981).—Lecidea santensis Tuck., Amer. J. Sci. Arts, Ser. 2 25: 428 (1858); type: USA, South Carolina, Santee Canal, 1849, H. W. Ravenel 182 (FH-TUCK 2822—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 236 (as ‘holotype’, Art. 9.10), not seen; B 35832!, BG L-4032!, O L-150045!—isolectotypes, issued as Reliq. Tuck. No. 15) (TLC: argopsin, norargopsin).
Phyllopsora albicans Müll. Arg., Bull. Soc. Roy. Bot. Belgique 32: 132 (1893 [1894?]); type: Costa Rica, Terraba, Tonduz, 1893, ex hb. Müll. Arg. (G 110889!—holotype; US—isotypes, not seen) (TLC: argopsin, norargopsin).
Lecidea miradorensis Vain., Dansk Bot. Ark. 4(11): 22 (1926).—Phyllopsora miradorensis (Vain.) Gotth. Schneid., Biblioth. Lichenol. 13: (1980), nom. inval., Art. 36.1 (a); type: Mexico, Veracruz, ad Mirador, 18-03-1842, Liebmann 7373 (TUR-V 34034—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 236, not seen; FH, TUR-V 34035—isolectotypes, not seen) (TLC: (Swinscow & Krog Reference Swinscow and Krog1981): argopsin, norargopsin (as albicans unknowns 1 and 2). Synonymy according to Brako (Reference Brako1989, Reference Brako1991)).
Descriptions
Timdal (Reference Timdal2008), Elix (Reference Elix and McCarthy2009).
Chemistry
Argopsin (major), norargopsin (submajor to minor).
Distribution
North, Central and South America, Asia, Australia.
Discussion
The three accessions of P. santensis form a strongly supported cluster in an otherwise unresolved clade (Figs 2 & 3). They are delimited as one entity in both mPTP analyses (Figs 2 & 3). The species resembles P. phaeobyssina morphologically and chemically but differs, for example, in forming longer ascospores. Both species cluster in the same higher clade in the trees (Figs 2–4), indicating that a relationship is possible.
Phyllopsora subhispidula (Nyl.) Kalb & Elix
Biblioth. Lichenol. 57: 293 (1995).—Psoroma subhispidulum Nyl., Ann. Sci. Nat., Bot., Sér. 4 11: 256 (1859); type: La Réunion, ‘Ins. Borbonia’, Lepervanche–Mézières 73 (H-NYL 30812!—holotype) (TLC (Kalb & Elix 1995): argopsin, norargopsin, zeorin).
Description
Timdal & Krog (Reference Timdal and Krog2001).
Chemistry
Argopsin (major), norargopsin (minor), zeorin (major), atranorin (trace).
Distribution
Africa, Asia.
Discussion
The three accessions of P. subhispidula group together in a supported clade in the phylogenies and are resolved as one species in both mPTP analyses (Figs 2 & 3). It is weakly resolved as sister to the hispaniolae-nemoralis-rosei clade (Figs 3 & 4), from which it differs greatly in morphology.
Phyllopsora subhispidula is morphologically highly similar to P. buettneri but differs in forming long, cylindrical isidia, not phyllidia. Chemically, it conforms to P. buettneri chemotype 4 (argopsin, norargopsin and zeorin) which we have not seen nor sequenced. Kalb & Elix (Reference Kalb and Elix1995) erroneously synonymized Brako's P. buettneri var. glauca with P. subhispidula, which reflects the morphological similarity between the two species. Indeed, P. subhispidula is found in the same larger clade in the trees as P. melanoglauca (Figs 2–4), the former chemotype 3 of P. buettneri, indicating a possible relationship.
Phyllopsora swinscowii Timdal & Krog
Mycotaxon 77: 88 (2001); type: Mauritius, Black River, along the path from Plaine Champagne towards Piton de la Petite Rivière Noire, 20°25′S, 57°25′E, 600 m alt., 05-11-1991, Krog & Timdal MAU9/50 (O L-21220!—holotype) (TLC: methyl 2,7-dichloropsoromate, methyl 2,7-dichloronorpsoromate; DNA: MK352143 (mtSSU)).
Descriptions
Timdal & Krog (Reference Timdal and Krog2001), Timdal (Reference Timdal2008), Elix (Reference Elix and McCarthy2009).
Chemistry
Methyl 2,7-dichloronorpsoromate (major), methyl 2,7-dichloropsoromate (major to minor).
Distribution
Central and South America, Africa. Asian and Australian records need confirmation.
Discussion
The five accessions of P. swinscowii, including the holotype, form a well-supported clade (Figs 2 & 3) and are sister to P. africana (Figs 2–4). The two species also form a complex with P. ochroxantha (Figs 2 & 3, group C). The ITS mPTP analysis resolves all specimens of P. swinscowii as belonging to a single entity (Fig. 3), while the mtSSU mPTP analysis suggests a single species for all accessions of P. swinscowii and P. africana (Fig. 2).
The three species in clade C are morphologically nearly identical. Phyllopsora swinscowii differs from P. ochroxantha in its chemistry (methyl 2,7-dichloropsoromate and methyl 2,7-dichloronorpsoromate in P. swinscowii vs. chlorophyllopsorin and phyllopsorin in P. ochroxantha). The delimitation from P. africana, on the other hand, is more difficult. Chemotype 2 of P. africana is identical to the chemistry of P. swinscowii, but chemotypes 1 and 3 differ in containing chlorophyllopsorin. As P. swinscowii is morphologically and chemically identical to P. africana chemotype 2, they should be regarded as a cryptic taxon pair. However, it is questionable whether P. swinscowii and P. africana should be synonymized (see discussion under P. africana and the general Discussion) and we suggest investigating this complex with additional material before making a conclusion.
Phyllopsora teretiuscula Timdal
Biblioth. Lichenol. 106: 346 (2011); type: Cuba, Pinar del Río, Reserva de la Biosfera Sierra del Rosario, N of and near lake ‘La Palma’, near river, downstream from the path/road, 22°51·31′N, 82°56·25′W, 140–145 m alt., over mosses on trunk of Roystonea regia in mixed hardwood forest, 21-03-2007, T. Tønsberg 37814 (BG L-87831!—holotype) (TLC: argopsin, norargopsin; DNA: MK352152 (mtSSU), MK352327 (ITS)).
Description
Timdal (Reference Timdal2011).
Chemistry
Argopsin (major), norargopsin (minor to absent), chlorophyllopsorin (minor to absent).
Distribution
The West Indies.
Discussion
In our study we use three accessions of P. teretiuscula, including the holotype. In both trees, all three accessions form a well-supported clade and are delimited as one species by mPTP (Figs 2 & 3). Phyllopsora teretiuscula is resolved as sister to P. phaeobyssina (Figs 2 & 4). The two species are morphologically and chemically quite similar. Phyllopsora teretiuscula differs, however, in forming narrower, more terete lobes and in sometimes containing chlorophyllopsorin, while P. phaeobyssina forms broader lobes and never contains chlorophyllopsorin. More specimens of P. phaeobyssina and sequences of additional genetic markers of both species are necessary to investigate their possible synonymy.
The species is new to Costa Rica and Puerto Rico.
Phyllopsora thaleriza (Stirt.) Swinscow & Krog
Lichenologist 13: 238 (1981).—Lecidea thaleriza Stirt., Rep. Trans. Glasgow Soc. Fld Nat. 5: 217 (1877); type: South Africa, Eastern Cape, Somerset East, Boschberg, 1874, McOwan (BM—holotype, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): atranorin, trace).
Psora compaginata Müll. Arg., Rev. Mycol. (Toulouse) 10: 60 (1888).—Phyllopsora compaginata (Müll. Arg.) Swinscow & Krog, Lichenologist 13: 240 (1981); type: Paraguay, Cerro San Thomas, 06-1881, Balansa 4134 (G 00292483—holotype, image seen) (synonymy according to Brako (Reference Brako1989)).
Description
Swinscow & Krog (Reference Swinscow and Krog1981).
Chemistry
Atranorin (minor to trace).
Distribution
South America, Africa.
Discussion
Swinscow & Krog (Reference Swinscow and Krog1981) considered P. thaleriza to be intermediate between a Phyllopsora and a Bacidia because of its nearly crustose thallus as well as dense white prothallus. Brako (Reference Brako1989) excluded the species from Phyllopsora because of differences in the hypothecium, thallus structure and algal symbiont. Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) resolved it to cluster, unrelated to Bacidia, among other Phyllopsora species in a molecular phylogeny of the family. Here we corroborate these results: all four accessions of P. thaleriza form a strongly supported clade in both phylogenetic trees and both mPTP analyses delimit them as one species (Figs 2 & 3). Due to poor resolution of the trees, we could not identify their closest relative. The species is readily distinguished by its areolate-crustose thallus, lack of vegetative dispersal units and the presence of atranorin.
B. Poorly understood, doubtful and fossil species
Phyllopsora bibula (Taylor) Swinscow & Krog
Lichenologist 13: 239 (1981).—Lecanora bibula Taylor, London J. Bot. 6: 160 (1847); type: Chile, ins. Juan Fernandez, in cortice arbor., locis umbrosis, 04-1830, Bertero 1648 (FH—lectotype, designated by Brako (Reference Brako1991): 29 (as ‘holotype’, Art. 9.10), not seen; BM!, H-NYL 20540!, H-NYL PM4109!—isolectotypes) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): fatty acid).
This poorly understood species is known only from the type collection. No attempt was made to extract DNA from the examined isotypes, which are in poor condition. Zahlbruckner (1921–1940) lists this species as a synonym of P. parvifolia which, however, generally forms larger squamules. Further collections of similar specimens from the type locality are necessary to gain more knowledge regarding the correct taxonomic affiliation of P. bibula.
Phyllopsora catervisorediata G. K. Mishra et al.
Mycotaxon 115: 33 (2011); type: India, Uttarakhand, Bageshwar Distr., en route to Pindari glacier, from Dwali to Khati, 2734–3210 m alt., on bark, 13-05-2007, S. Joshi & Y. Joshi 07-008932 (LWG—holotype, not seen) (TLC (Mishra et al. Reference Mishra, Upreti, Nayaka and Haridas2011): atranorin).
This species is known only from the type material. It was not studied by us due to the lack of response from LWG to our repeated loan requests. The presence of soredia indicates that it might not belong in Phyllopsora, as does the statement in the protologue that it is close to P. soralifera, a species that is excluded from the genus here. Sequences are needed to understand the correct taxonomic affiliation of this species.
Phyllopsora cinerella Zahlbr.
Ark. Bot. 31A(6): 18 (1944); type: USA, Hawaii, Iles Sandwich, Robinson Summer House Kauai, 02-1910, Faurie 308 (PC—lectotype, designated by Brako (Reference Brako1991): 40, not seen), Faurie 307 (BM!—syntype) (TLC (Brako Reference Brako1991): phyllopsorin, chlorophyllopsorin).
Although treated as a synonym of P. ochroxantha by Brako (Reference Brako1991), we found the isotype in BM indeterminable.
Phyllopsora densiflorae (Vain.) Swinscow & Krog
Lichenologist 13: 241 (1981).—Lecidea densiflorae Vain., Bot. Mag. (Tokyo) 35: 67 (1921); type: Japan, Prov. Kozuke, on Pinus densiflora, 25-02-1918, A. Yasuda 350 (TUR-V 22632!—holotype) (TLC: unidentified fatty acid in R f class B:6).
This poorly understood species is known only from the type collection and no attempt was made to extract DNA from it. According to Brako (Reference Brako1991), it is a synonym of P. corallina, while Swinscow & Krog (Reference Swinscow and Krog1981) considered a possible synonymy with P. confusa. We regard P. densiflorae as being crustose, consisting of areoles up to 0·2 mm diam., and not synonymous with either of the other two. Rather it should be considered for inclusion in Biatora. Whereas Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991) reported no lichen substances from the holotype, our TLC examination of the specimen revealed an unidentified fatty acid. The extent of morphological variation in this species cannot be assessed without further specimens and thus DNA sequences will have to be obtained to determine its status.
Phyllopsora dominicana Rikkinen
J. Exp. Bot. 59: 1008 (2008); type: Poinar B 1–23 (Oregon State University—holotype, not seen).
This species is known only as a fossil from Dominican amber.
Phyllopsora griseocastanea (Vain.) Swinscow & Krog
Lichenologist 13: 241 (1981).—Lecidea griseocastananea Vain., Ann. Acad. Sci. Fenn., Ser. A 15(6): 114 (1921); type: Philippines, Luzon, Benguet Prov., Pauai, ad corticem arboris, 1909, E. D. Merrill 6651 (TUR-V 22625!—holotype) (TLC: no lichen substances).
This poorly understood species is known only from the type collection and no attempt was made to extract DNA from it. Swinscow & Krog (Reference Swinscow and Krog1981) mention a similarity with P. manipurensis in the coloration of the hypothecium, but DNA sequence data are necessary to investigate the taxonomic affinity of the type.
Phyllopsora magna Kaasalainen et al.
Earth Environm. Sci. Trans. Roy. Soc. Edinburgh 107: 322 (2017); type: AMNH DR-15-3 (American Museum of Natural History, New York—holotype, not seen).
This species is known only as a fossil from Dominican amber.
Phyllopsora manipurensis (Müll. Arg.) Müll. Arg.
Bull. Soc. Roy. Bot. Belgique 32: 132 (1893 [1894?]).—Psora manipurensis Müll. Arg., J. Linn. Soc., Bot. 29: 219 (1893); type: India, Manipoor, G. Watt (G—holotype, image seen; BM—isotype, not seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): atranorin, trace).
The species is known only from the type material. Mishra et al. (Reference Mishra, Upreti, Nayaka and Haridas2011) suggest a close relationship to P. subcrustacea, another poorly known species. Sequence data might clarify its taxonomic affiliation.
Phyllopsora microphyllina (Nyl.) Swinscow & Krog
Lichenologist 13: 243 (1981).—Lecidea microphyllina Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 347 (1863); type: Cuba, s. loc., C. Wright s. n. (H-NYL 17345a!—holotype; BM!, UPS L-135785!—isotypes, issued as Tuckerman, Wright Lich. Cub. No. 211) (TLC: no lichen substances).
We know of no reliably identified, recently collected material of this poorly understood species and have not attempted DNA extraction of the old type material. It is characterized by having a squamulose thallus without vegetative dispersal units, acicular ascospores, and by the lack of lichen substances. It is morphologically similar to P. neofoliata but differs in chemistry and ascospore size. Due to the acicular ascospores, it is doubtful whether this species really belongs in Phyllopsora. It is possible that it should be excluded like many other former Phyllopsora species having acicular ascospores, such as Bacidina lacerata or Parallopsora leucophyllina.
Phyllopsora microsperma Müll. Arg.
Bull. Herb. Boissier 2: 89 (1894); type: Mexico, Jalisco, 1890, J. W. Eckfeldt 190 (G 00293373—holotype, image seen) (TLC (Swinscow & Krog Reference Swinscow and Krog1981): traces of atranorin(?) and triterpenoid).
Lecidea subglabella Malme, Ark. Bot. 28A(7): 41 (1936).—Phyllopsora subglabella (Malme) Swinscow & Krog, Lichenologist 13: 245 (1981); type: Brazil, Mato Grosso, Guia pr. Cuyabá, in silva ripæ fluvii, 14-05-1894, G. O. A. Malme, Lich. Regnell. 2547 (S!—lectotype, designated by Brako (Reference Brako1991): 48 (as ‘holotype’, Art. 9.10); UPS L-10379!—isolectotype) (TLC (Brako Reference Brako1991): no lichen substances. Synonymy according to Brako (Reference Brako1991)).
Lecidea glabella Nyl., Sert. Lich. Trop.: 37 (1891), nom. illeg. (non Kremp. 1876).—Phyllopsora glabella Swinscow & Krog, Lichenologist 13: 241 (1981); type: Cuba, s. loc., ad palmas, C. Wright s. n., Tuckerman, Wright Lich. Cub. Ser. 2, 142 (H-NYL 20518!— holotype) (TLC (Brako Reference Brako1991): no lichen substances. Synonymy according to Brako (Reference Brako1991)).
We know only a small number of collections of this species and all were made before the 1960s. As we have been able to generate sequences of specimens from the late 1960s, it might be possible to generate sequences from the Haitian specimen of P. microsperma collected in 1958, when taking special measures to avoid contamination. However, we decided not to attempt DNA extraction from those specimens, anticipating that better methods for extracting and sequencing old material will be developed. The species is characterized by adnate, rather thick, shiny squamules growing on a reddish brown prothallus, short ellipsoid ascospores as well as a lack of vegetative dispersal units and lichen substances. It may be similar to P. breviuscula and P. mauritiana but both species form larger ascospores.
Phyllopsora minor Brako
Mycotaxon 35: 15 (1989).—Lecidea corallina var. schizophylloides Vain., J. Bot. 34: 106 (1896); type: St. Vincent and the Grenadines, St. Vincent, Richmont Peak, ad corticem arboris, 1000–2000 ft alt., W. R. Elliot 261[a] (TUR-V 22612!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 240; BM!—isolectotype) (TLC: no lichen substances).
Phyllopsora minor is known only from the old type material and we have not attempted to extract DNA. The species is generally characterized by an effuse thallus consisting of irregularly oriented, narrow squamules which are closely adnate to well developed, growing on a reddish brown prothallus, medium to dark brown apothecia with ellipsoid ascospores, and the lack of lichen substances. Sequences are necessary to determine the phylogenetic placement of this species.
Phyllopsora purpurescens (Vain.) Zahlbr.
Cat. Lich. Univ. 4(3): 401 (1926).—Lecidea purpurescens Vain., Univ. Calif. Publ. Bot. 12: 10 (1924); type: Tahiti, in valle Punaruu, W. A. Setchell & H. E. Parks 5380 p.p. (TUR-V 22618—holotype, not seen; BM 001048828, US 00433394—isotypes, images seen).
The species is known only from the old type collection and we did not attempt to extract DNA. Swinscow & Krog (Reference Swinscow and Krog1981) found the species to be morphologically similar to P. societatis and to contain the same fatty acids; the two species are only distinguished by the colour of their prothallus. Sequence data should be obtained from both species to investigate their potential synonymy and their phylogenetic placement in Phyllopsora.
Phyllopsora societatis (Vain.) Zahlbr.
Cat. Lich. Univ. 4(3): 401 (1926).—Lecidea societatis Vain., Univ. Calif. Publ. Bot. 12: 10 (1924); type: Tahiti, Papehue River, 07-06-1922, W. A. Setchell & H. E. Parks 5349 (TUR-V 22614!—holotype; BM—isotypes, not seen) (TLC: no lichen substances).
The species is known only from the old type collection and we did not attempt to extract DNA. It might be conspecific with P. purpurescens (Swinscow & Krog Reference Swinscow and Krog1981); see discussion under that species. We did not detect the fatty acids in the holotype that were reported from the isotype in BM by Swinscow & Krog (Reference Swinscow and Krog1981).
Phyllopsora subcrustacea (Malme) Brako
Mycotaxon 35: 15 (1989).—Lecidea corallina var. subcrustacea Malme, Ark. Bot. 28A(7): 47 (1936); type: Paraguay, Asuncion, 18-08-1893, G. O. A. Malme Lich. Regnell. 1612B (S!—lectotype, designated by Brako (Reference Brako1991): 57 (as ‘holotype’, Art. 9.10); UPS L-010380!—isolectotype, not seen) (TLC (Brako Reference Brako1991): no lichen substances).
Phyllopsora subscrustacea is another species known only from the type collection. We were not able to locate any reliably identified, recently collected material from the geographical region where this poorly understood species was described (Paraguay), and did not extract DNA from the old type material. The species is characterized by closely adjoined, adnate to ascending squamules, which form an almost continuous crust, cylindrical isidia and bright orange-red, marginate apothecia. The species might be similar to P. loekoesii but sequences of the type material are essential for determining its correct phylogenetic position.
Phyllopsora subhyalina (Stirt.) Zahlbr.
Cat. Lich. Univ. 4(3): 401 (1926).—Lecidea subhyalina Stirt., Trans. & Proc. Roy. Soc. Victoria 17: 77 (1881); type: Australia, Victoria, Gippsland, Waterloo, Stirton 8662 (BM—holotype, not seen).
The type material was studied by Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991) but left uninterpreted due to its poor condition. Swinscow & Krog (Reference Swinscow and Krog1981) noticed the absence of a prothallus and Brako (Reference Brako1991) noted the gelatinized apothecia, characters that are not typical of Phyllopsora species. It is therefore unclear whether the species belongs in Phyllopsora and sequence data are necessary for clarification.
Lecidea thysaniza Nyl.
Lich. Nov. Zel.: 82 (1888); type: ‘Nova Zelandia’, 1867, Knight 117 (H-NYL 20481!—holotype) (TLC: terpenoids).
The species is known only from the old type collection and we did not attempt DNA extraction. The type material might represent a Phyllopsora species based on its thallus morphology but sequences are necessary for clarification.
Phyllopsora viridis Paulson
J. Siam Soc., Nat. Hist. Suppl. 2: 101 (1930); type: Thailand, Kaw Tao, c. 100 m alt., 22-09-1918, Paulson 29 (BM—holotype, not seen).
This species is known from the type collection only. The type material was studied by Swinscow & Krog (Reference Swinscow and Krog1981) and Brako (Reference Brako1991); the former found no Phyllopsora in the collection and the latter found the material too small for comprehensive examination.
C. Excluded species
Phyllopsora aleuroides (Stirt.) Müll. Arg.
Bull. Herb. Boissier 2(App. 1): 45 (1894).—Lecidea aleuroides Stirt., J. Linn. Soc., Bot. 14: 469 (1875); type: not seen (see Galloway & James Reference Galloway and James1985).
This species belongs in Psoromidium Stirton (Galloway & James Reference Galloway and James1985; Brako Reference Brako1989; Jørgensen & Andersen Reference Jørgensen and Andersen2015).
Phyllopsora atrocarpa Timdal
Lichenologist 40: 341 (2008); type: Peru, Loreto, Jenaro Herrera, within a 3·6 km distance from the Research Centre, N of the road, site 116, 4°53·87′S, 73°38·85′W, 120–150 m alt., tree trunk in rainforest, 28-09-2006, E. Timdal 10425 (O L-144795!—holotype) (TLC: fumarprotocetraric acid, 2'-O-methylhyperlatolic acid).
This species probably belongs to an undescribed genus in the family Malmideaceae, together with P. lividocarpa and P. nigrocincta (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora borbonica Timdal & Krog
Mycotaxon 77: 68 (2001); type: La Réunion, along road towards Plaine d'Affoches, above Bras Citron, at point where road meets track, 20°57′S, 55°25′E, 1220 m alt., 1996, H. Krog & E. Timdal RE8/12 (O L-797!—holotype) (TLC: no lichen substances).
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) showed that this species belongs in the resurrected genus Sporacestra.
Phyllopsora brakoae Timdal
Lichenologist 40: 343 (2008); type: Peru, Loreto, Reserva Nacional Allpahuayo Mishana, within a 2·3 km distance from Centro de Investigaciones Allpahuayo, N of the road, site 43, 3°58·48′S, 73°25·86′W, 120–150 m alt., tree trunk in rainforest, “bosque de varillal seco”, 22-09-2006, E. Timdal 10253 (O L-144623!—holotype) (TLC: no lichen substances).
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) transferred this species to the new genus Parallopsora based on DNA sequence data.
Phyllopsora cognata (Nyl.) Timdal
Biblioth. Lichenol. 106: 331 (2011).—Lecidea cognata Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 347 (1863); type: Cuba, s. loc., C. Wright, Tuckerman, Wright Lich. Cub. 218 (BM!—lectotype, designated by Timdal (Reference Timdal2011): 331; UPS L-135790!—isolectotype) (TLC: atranorin).
Unpublished sequences of this species have shown that it does not belong in Phyllopsora.
Phyllopsora congregans (Zahlbr.) D. J. Galloway
New Zealand J. Bot. 21: 196 (1983).—Lecidea congregans Zahlbr., Denkschr. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 104: 305 (1941); type: not seen (see Brako Reference Brako1989).
This species belongs in Trapeliopsis Hertel & Gotth. Schneid. (Brako Reference Brako1989).
Phyllopsora conwayensis Elix
Australas. Lichenol. 59: 24 (2006); type: Australia, Queensland, Conway State Forest, 18 km E of Prosperpine, 20°21′S, 148°45′E, 180 m alt., in lowland rainforest, on tree trunk, J. A. Elix & H. Streimann 20190 (BRI—holotype, fragment seen; B 125907!—isotype).
Unpublished sequences of the isotype have shown that the species does not belong in Phyllopsora.
Phyllopsora coroniformis (Kremp.) Zahlbr. in Engler
Nat. Pflanzenfam. 1, 1*(225): 138 (1906).—Lecidea coroniformis Kremp., Verh. K. K. Zool.-Bot. Ges. Wien. 18: 326 (1868); type: USA, Texas, s. loc., s. coll., ex hb. Krempelhuber October 1883 (M!—holotype) (TLC: norstictic acid).
This species belongs in Psora Hoffm. and is a synonym of Psora crenata (Taylor) Reinke (Timdal Reference Timdal1986).
Phyllopsora cryptocarpa Riddle
Mycologia 15: 80 (1923); type: not seen (see Brako Reference Brako1989).
This species belongs in Fellhanera Vězda (Brako Reference Brako1989).
Phyllopsora curatellae (Malme) Swinscow & Krog
Lichenologist 13: 240 (1981).—Lecidea curatellae Malme, Ark. Bot. 28A(7): 42 (1936); type: Brazil, Mato Grosso, Cuyabá, in “cerrado”, 27-11-1893, G. A. O. Malme 2038 (S!—lectotype, designated by Swinscow & Krog (Reference Swinscow and Krog1981): 240).
According to Brako (Reference Brako1989, Reference Brako1991), this species belongs in an undescribed genus in the Lecanoraceae Körb.
Phyllopsora glaucescens Timdal
Lichenologist 40: 349 (2008); type: Peru, Loreto, Jenaro Herrera, within a 3·6 km distance from the Research Center, N of the road, site 111, 4°53·88′S, 73°38·90′W, 120–150 m alt., tree trunk in rainforest, 28-09-2006, E. Timdal 10418 (O L-144788!—holotype) (TLC: methyl barbatate).
Unpublished sequences of several specimens, including the holotype, have shown that this species does not belong in Phyllopsora.
Phyllopsora labriformis Timdal
Lichenologist 40: 350 (2008); type: Peru, Loreto, Jenaro Herrera, within a 3·6 km distance from the Research Center, N of the road, site 112, 4°53·93′S, 73°83·91′W, 120–150 m alt., tree trunk in rainforest, 28-09-2006, E. Timdal 10419 (O L-144789!—holotype) (TLC: methyl barbatate).
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) placed this species in the new genus Parallopsora based on DNA sequence data.
Phyllopsora lacerata Timdal
Lichenologist 40: 352 (2008); type: Peru, Loreto, Reserva Nacional Allpahuayo Mishana, within a 2·3 km distance from Centro de Investigaciones Allpahuayo, N of the road, site 19, 3°57·31′S, 73°25·46′W, 120–150 m alt., tree trunk in rainforest, 21-09-2006, E. Timdal 10213 (O L-144583!—holotype) (TLC: no lichen substances).
This species was shown to belong to Bacidina (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora leucophyllina (Nyl.) Timdal
Lichenologist 40: 352 (2008).—Lecidea leucophyllina Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 347 (1863); type: Cuba, ‘in ins. Cuba’, C. Wright s. n. (H-NYL 17345e!—lectotype, designated here, MycoBank typification MBT 387680; BM!, H-NYL 17345c!, UPS L-108156!—isolectotypes) (TLC: homosekikaic acid, sekikaic acid).
The new genus Parallopsora was established to accommodate this species based on DNA sequence data (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora lividocarpa Timdal
Lichenologist 40: 353 (2008); type: Peru, Loreto, Jenaro Herrera, within a 3·6 km distance from the Research Center, N of the road, site 126, 4°53·66′S, 73°38·56′W, 120–150 m alt., tree trunk in rainforest, 30-09-2006, E. Timdal 10447 (O L-144817!—holotype) (TLC: 2'-O-methylhyperlatolic, an unknown fatty acid).
This species probably belongs to an undescribed genus in the family Malmideaceae, together with P. atrocarpa and P. nigrocincta (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora longispora Swinscow & Krog
Nordic J. Bot. 5: 493 (1985); type: Kenya, Western Province, Kakamega District, Kakamega Forest, near Forest Station (c. 13 km ESE of Kakamega). Alt. c. 1700 m, 0°15′N, 34°52′E, on the trunk of a tree in dense rainforest, 20-01-1970, R. Santesson 21698a (UPS—holotype!) (TLC (Swinscow & Krog Reference Swinscow and Krog1985): small amounts of triterpenoids).
We have unpublished sequences of this species which suggest a close relationship to the genus Aciculopsora Aptroot & Trest (Ramalinaceae).
Phyllopsora melanocarpa Müll. Arg.
Hedwigia 34: 28 (1895); type: not seen (see Brako Reference Brako1989).
This species belongs in Neophyllis F. Wilson and is a synonym of N. pachyphylla (Müll. Arg.) Gotth. Schneid. (Swinscow & Krog Reference Swinscow and Krog1981; Brako Reference Brako1989).
Phyllopsora nigrocincta Timdal
Lichenologist 40: 354 (2008); type: Peru, Loreto, Jenaro Herrera, within a 3·6 km distance from the Research Center, N of the road, site 124, 4°53·44′S, 73°37·39′W, 120–150 m alt., tree trunk in rainforest, 29-09-2006, E. Timdal 10443 (O L-144813!—holotype) (TLC: fumarprotocetraric acid, norsolorininc acid).
This species probably belongs to an undescribed genus in the family Malmideaceae, together with P. atrocarpa and P. lividocarpa (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora pertexta (Nyl.) Swinscow & Krog
Lichenologist 13: 244 (1981).—Lecidea pertexta Nyl., Ann. Sci. Nat., Bot., Sér. 4 19: 347 (1863); type: Cuba, ‘in ins. Cuba’, C. Wright s. n. (H-NYL 17344, left specimen!—lectotype, designated here, MycoBank typification MBT 387681) (TLC: no lichen substances).
The genus Sporacestra has been resurrected to accommodate this species (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018a).
Phyllopsora pocsii Vězda
Lich. Rar. Exsicc. 49: 2 (Reference Vězda2003); type: Tanzania, montes Kiboriani, prope Mpwapwa, ad latera montis prope Kikombo, 1200 m alt., ad corticem arborum, 11-05-1972, T. Pócs & L. Mezösi 6564/C, Vězda, Lich. Rar. Exsicc. No 484 (BM!, GZU!—isotypes) (TLC: no lichen substances).
Our unpublished sequences of the isotype in GZU have shown that the species does not belong in Phyllopsora.
Phyllopsora pyrrhomelaena (Tuck.) Swinscow & Krog
Lichenologist 13: 244 (1981).—Biatora pyrrhomelaena Tuck., Amer. J. Sci. Arts, Ser. 2 28: 205 (1859); type: Cuba, Monte Verde Woods, on trunks of trees near the ground, C. Wright s. n., Tuckerman, Wright Lich. Cub. No. 178 (FH 286104!—lectotype, designated here, MycoBank typification MBT 387682; FH 197468!, UPS L-74560!—isolectotypes) (TLC: norsolorinic acid and at least three additional pink pigments).
This species is morphologically and chemically similar to P. atrocarpa, P. lividocarpa and P. nigrocincta. Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) have shown that the three latter species belong to an unknown genus in the family Malmideaceae.
Phyllopsora soralifera Timdal
Lichenologist 40: 358 (2008); type: Peru, Loreto, Reserva Nacional Allpahuayo Mishana, within a 2·3 km distance from Centro de Investigaciones Allpahuayo, N of the road, site 78, 3°57·80′S, 73°25·59′W, 120–150 m alt., tree trunk in rainforest, 24-09-2006, E. Timdal 10342 (O L-144712!—holotype) (TLC: no lichen substances).
Unpublished sequences of several specimens have shown that the species does not belong in Phyllopsora.
Phyllopsora sorediata (Aptroot & Sparrius) Timdal
Lichenologist 39: 341 (2008).—Triclinum sorediatum Aptroot & Sparrius in Aptroot et al., Fungal Diversity 24: 130 (2007); type: Thailand, Uthai Thani Prov., Huay Kha Khaeng Wildlife Sanctuary, Kapou Kapiang, 15°29′N, 99°18′E, 500 m alt., on bark, 14-02-1993, B. Aguirre-Hudson, P. W. James & P. A. Wolseley 2817 (BM—holotype, not seen; ABL—isotype, not seen).
Kistenich et al. (Reference Kistenich, Timdal, Bendiksby and Ekman2018a) have shown that this species belongs in Bacidia.
Phyllopsora stylophora (Malme) Swinscow & Krog
Lichenologist 13: 245 (1981).—Lecidea stylophora Malme, Ark. Bot. 28A(7): 40 (1936); type: Brazil, Mato Grosso, Serra da Chapada, Buriti, in silvula, 27-06-1894, G. A. O. Malme s. n. (S!—lectotype, designated by Brako (Reference Brako1991): 58 (as ‘holotype’, Art. 9.10); G 00293002—isolectotype, image seen; H, US—isolectotypes, not seen) (TLC (Brako Reference Brako1991): atranorin, terpenoids).
According to Brako (Reference Brako1989, Reference Brako1991), this species belongs in an undescribed genus in the Lecanoraceae.
Phyllopsora subcorallina Zahlbr.
Ann. Mycol. 33: 43 (1935); type: not seen (see Brako Reference Brako1989).
This species belongs in Catinaria Vain. (Brako Reference Brako1989).
Phyllopsora subfilamentosa Zahlbr.
Ann. Mycol. 33: 44 (1935); type: not seen (see Brako Reference Brako1989).
This species belongs in Fuscidea V. Wirth & Vězda (Brako Reference Brako1989).
Phyllopsora tobagensis Timdal
Biblioth. Lichenol. 106: 346 (2011); type: Trinidad & Tobago, Tobago, Parish of St. Paul, along Roxborough–Parlatuvier Road, 11°16·80′N, 60°36·66′W, 500–520 m alt., on tree trunk in rainforest, 12-03-2008, S. Rui & E. Timdal 10764 (O L152061!—holotype; CANB!—isotype) (TLC: perlatolic acid, hyperlatolic acid, superlatolic acid).
We have unpublished sequences of the holotype which show that this species does not belong in Phyllopsora.
Phyllopsora wellingtonii (Stirt.) Müll. Arg.
Bull. Herb. Boissier 2(App. 1): 45 (1894).—Psoromidium wellingtonii Stirt., Proc. Roy. Philos. Soc. Glasgow 10: 304 (1877); type: not seen (see Galloway & James Reference Galloway and James1985).
This species belongs in Psoromidium and is a synonym of Psoromidium aleuroides (Stirt.) D.J. Galloway (Galloway & James Reference Galloway and James1985; Brako Reference Brako1989).
We acknowledge the following herbaria for kindly providing loans of material for our study: B, BG, BM, CANB, E, GZU, H, HUTPL, MPEG, PDA, TNS. Furthermore, we gratefully received specimens from the private herbaria of P. Diederich, A. Frisch, D. Killmann, Z. Palice, S. Pérez–Ortega, M. Prieto and P. van den Boom. S. Y. Kondratyuk provided us with additional, unpublished sequences for comparison with our own sequence data. We thank Siri Rui for curatorial assistance as well as the staff of the DNA laboratory at the Natural History Museum, University of Oslo, for help and support. Special thanks are due to everyone who accompanied us in the field in Brazil, Sri Lanka and Venezuela. The Natural History Museum, University of Oslo, is acknowledged for financial support.
Authors’ Contributions
ET and MB planned the study. ET, JH, MC and SK planned and conducted the fieldwork. MC and JH provided collection and export permits for Brazil and Venezuela, respectively. MB and SK generated DNA sequences. SK conducted the phylogenetic analyses under the guidance of SE. SK wrote the first draft and ET wrote the Taxonomy section. All authors corrected and completed the manuscript.
Supplementary Material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0024282919000252










