Introduction
Diorygma is a common and sometimes abundant genus of the Graphidaceae, occurring chiefly in the tropics, often on the sheltered or overhanging side of trees. The genus is far more speciose in tropical Asia and Australia than in tropical America.
So far c. 50 species are known in the genus, half of which were treated in a preliminary monograph (Kalb et al. Reference Kalb, Staiger and Elix2004), the other half in subsequent papers (Cáceres Reference Cáceres2007; Archer Reference Archer2009; Makhija et al. Reference Makhija, Chitale and Sharma2009; Sharma & Makhija Reference Sharma and Makhija2009a , Reference Sharma and Makhija b ; Lumbsch et al. Reference Lumbsch, Ahti, Altermann, Amo de Paz, Aptroot, Arup, Bárcenas Peña, Bawingan, Benatti and Betancourt2011; Sharma & Khadilkar Reference Sharma and Khadilkar2012; Lima et al. Reference Lima, Maia, Aptroot and Cáceres2013).
During studies on Graphidaceae diversity by the first author in the states of Tocantins in the north and Paraná in the south of Brazil, three undescribed Diorygma species were encountered. They are described below, and a world key to all 52 currently known species of Diorygma is provided.
The study in Tocantins was carried out on the farm São Paulo, located 5 km from the town of Itaguatins on TO 126 between km 39 and 40, north-east of the Parrot's Beak region, at an altitude of 161 m above sea level. The farm covers 174 acres, divided into pasture and native cerrado area. The cerrado vegetation is composed of tortuous, small, isolated or clustered trees with thick leaves such as Aspidosperma macrocarpon Mart., Gochnatia polymorpha (Less.) Cabrera and Handroanthus impetiginosus (Mart. ex DC.) Mattos. The climate of the state is tropical, with average annual temperatures of 26°C during the rainy season (October to March), and 32°C in the dry season (April to September) (Nascimento Reference Nascimento2008).
The study in Paraná was conducted on the Ilha do Mel, located at the entrance of the Bay of Paranaguá, with an approximate area of 2760 acres. The island is composed of two well-defined areas in terms of geology and geomorphology, connected by a narrow sandy strip. The smaller southern area is formed of hills of crystalline rock and the northern area is a sedimentary plain, which originated mainly from marine deposits, with a small hill up to 80 m high (Silva et al. Reference Silva, Britez, Souza, Joly and Watanabe1994). The Paranaguá region is wet tropical, without a dry season. The vegetation of the island is pioneer Restinga with marine influence (on sandbanks), with mangrove and swamps, and the dense rainforest, as well as areas with secondary vegetation in various stages of regeneration. The Ilha do Mel has two protected areas: the State Park of Ilha do Mel, corresponding to c. 5% of the surface area of the island, and the Ecological Station of Ilha do Mel, covering c. 95% of the surface.
Material and Methods
In Tocantins, the collections were made by opportunistic sampling in the study area, during six trips between September 2008 and August 2009. In Ilha do Mel, Paraná, four field trips were carried out (of two to four days each), also by means of opportunistic sampling, two in winter (June and August 2012) and two in summer (February and April 2013). All vegetation types occurring on the island were sampled. Descriptive work was partly carried out in Imperatriz, at the Instituto de Ensino Superior do Sul do Maranhão, and partly in Curitiba, at the Universidade Federal do Paraná. Morphological and anatomical characters were studied by dissecting and compound microscopes respectively. Sections were mounted in tap water, in which all measurements were also taken. The specimens from this study are preserved in UPCB. The chemistry of the type specimens was investigated by thin-layer chromatography (TLC) using solvent C (Orange et al. Reference Orange, James and White2001).
The New Species
Diorygma incantatum Feuerstein & Eliasaro sp. nov.
MycoBank No.: MB808554
Diorygma with transversely 29–31-septate, filiform ascospores 105–108×6 µm, and thallus with unidentified substance at Rf 44 in solvent C.
Type: Brazil, Paraná: Paranaguá, Ilha do Mel, Encantadas, 25°32′57·7″S, 48°18′17·7″W, c. 8 m alt., on tree bark in Restinga forest, 24 August 2012, S. C. Feuerstein 1148 (UPCB—holotype).
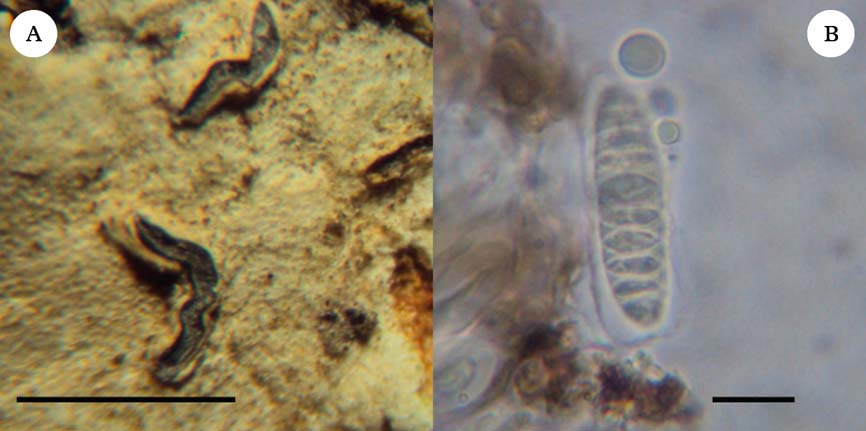
(Fig. 1)

Fig. 1. Diorygma incantatum, holotype. A, habitus; B, ascospores. Scales: A=1 mm; B=10 µm. In colour online.
Thallus corticolous, continuous, without crystals, soredia and isidia absent; surface whitish grey, opaque, irregular, without cortex.
Ascomata circular to elongated, isolated, simple, with rounded ends, immersed to erumpent, 0·6–1·6×0·3–0·6 mm, white; disc exposed, pinkish, white pruinose; margins entire, not corticate, divergent; excipulum hyaline. Hymenium not inspersed 150–175 µm, I−; epithecium not differentiated; hypothecium hyaline; paraphyses simple, filiform, 1·0–1·5 µm thick, periphysoids absent. Ascospores 8 per ascus, hyaline, transversely 29–31-septate, filiform, I−, 105–108×6 µm, surrounded by a c. 1 µm wide gelatinous sheath.
Chemistry
Thallus and ascomata K+ pale yellow. TLC: an unidentified compound forming a spot of purple colour, in UV fluorescent orange, at approximately Rf 44 in solvent system C.
Ecology and distribution
Known only from coastal Restinga forest in Brazil.
Notes
The species is named after the type locality Encantadas, meaning enchanted, which is an area located on the Ilha do Mel, Paraná. Only four other Diorygma species are known to have transversely septate ascospores, viz. D. circumfusum (Stirt.) Kalb, Staiger & Elix, D. minisporum Kalb et al., D. wallamanense A.W. Archer & Elix, and D. wilsonianum (Müll. Arg.) A.W. Archer (Kalb et al. Reference Kalb, Staiger and Elix2004; Archer Reference Archer2009; Makhija et al. Reference Makhija, Chitale and Sharma2009; Sharma & Makhija Reference Sharma and Makhija2009a , Reference Sharma and Makhija b ; Sharma & Khadilkar Reference Sharma and Khadilkar2012). Diorygma circumfusum is known only from Australia and can be distinguished by wider ascospores (8·5–16·0 µm) with up to 22 septa, and by producing norstictic acid as the main secondary compound, while D. minisporum is distinguished by very small ascospores, not exceeding 20 µm in length, and the presence of constictic, hypostictic, hypoconstictic and stictic acids (Kalb et al. Reference Kalb, Staiger and Elix2004). Diorygma wallamanense and D. wilsonianum are differentiated mainly by smaller ascospores, which do not exceed 55 µm in length and with a maximum of 15 septa, and in addition, the chemical compounds differ: the first has perstictic and stictic acids and the second contains norstictic acid (Archer Reference Archer2009). The new species is somewhat reminiscent of Chapsa indica A. Massal., which differs by the presence of periphysoids and the absence of lichen substances (Cáceres Reference Cáceres2007).
Additional specimen seen. Brazil: Paraná: Paranaguá, Ilha do Mel, Charmed, S. C. Feuerstein 1164, 1165 (UPCB).
Diorygma pauciseptatum Feuerstein, I. P. R. Cunha-Dias, Aptroot & M. Cáceres sp. nov.
MycoBank No.: MB808555
Diorygma with norstictic and connorstictic acids and ascospores transversely 7–9-septate, I+ blue-violet, 28–32×7 µm, surrounded by a c. 2 µm wide gelatinous sheath that is often somewhat expanded at one end.
Type: Brazil, Tocantins, Itaguatins, Fazenda São Paulo, 5°44′56·90″S, 47°32′25·00″W, c. 152 m alt., on bark of tree, 1 February 2009, S. C. Feuerstein 123 (UPCB—holotype).
(Fig. 2)

Fig. 2. Diorygma pauciseptatum, holotype. A, habitus; B, ascospore. Scales: A=1 mm; B=10 µm. In colour online.
Thallus corticolous, continuous, with crystals, soredia and isidia absent; surface yellowish cream, opaque, irregular, without cortex.
Ascomata elongated, isolated, winding, simple to rarely branched, with pointed ends, immersed to erumpent, 0·3–1·1×0·1 mm, of thallus colour; disc exposed, dark grey, thinly white pruinose; margins entire, not corticate, divergent; excipulum hyaline. Hymenium not inspersed 75–100 µm, I−; epithecium consisting of brown and branched paraphysis apices; hypothecium hyaline; paraphyses branched at the tips, filiform, 1·5–2·0 µm thick, periphysoids absent. Ascospores 8 per ascus, hyaline, transversely 7–9-septate, fusiform, I+ blue-violet, 28–32×7 µm, surrounded by a c. 2 µm wide gelatinous sheath that is often somewhat expanded at one end.
Chemistry
Thallus: K+ yellow, releasing orange-red, needle-shaped crystals in microscopic section. TLC: norstictic and connorstictic acids.
Notes
Within the genus Diorygma, only four species are known with transversely septate spores (see above). Among these species, Diorygma wallamanense and D. circumfusum also contain norstictic acid, but are differentiated by the longer ascospores, which always exceed 40 µm in length. The new species has ascospores with the fewest septa in the genus. The dark grey apothecium discs are unusual in the genus, and are more common in Graphidaceae genera with carbonized apothecium margins. The new species is therefore at first glance more reminiscent of, for example, a Platygramme or even a Graphis.
Diorygma tocantinense Feuerstein, I. P. R. Cunha-Dias & Aptroot sp. nov.
MycoBank No.: MB808556
Diorygma with protocetraric acid and ascospores that are muriform, broadly fusiform with rounded ends, 24–40×10–15 µm, surrounded by a gelatinous sheath up to c. 5 µm wide that is somewhat expanded at central parts of the ascospore.
Type: Brazil, Tocantins: Itaguatins, Fazenda São Paulo, 5°44′56·90″S, 47°32′25·00″W, c. 152 m alt., on bark of tree, 9 August 2009, S. C. Feuerstein 447 (UPCB—holotype).
(Fig. 3)

Fig. 3. Diorygma tocantinense, holotype. A, habitus; B, ascospore. Scales: A=1 mm; B=10 µm. In colour online.
Thallus corticolous, continuous, with crystals, soredia and isidia absent; surface whitish cream, opaque, irregular, without cortex.
Ascomata elongated, isolated, winding, simple to rarely branched, with pointed ends, immersed to erumpent, 0·5–6·0×1·0 mm, of thallus colour; disc narrowly exposed, dark grey, cream pruinose; margins entire, not corticate, divergent, with dark grey line; excipulum hyaline. Hymenium not inspersed 85–100 µm, I−; epithecium brown, 15–40 µm; hypothecium hyaline; paraphyses branched at the tips, filiform, c. 1·0 µm thick, periphysoids absent. Ascospores 8 per ascus, hyaline, muriform, broadly fusiform with rounded ends, I+ faintly blue, 24–40×10–15 µm, surrounded by up to c. 5 µm wide gelatinous sheath that is somewhat expanded at central parts of the ascospore.
Chemistry
Thallus K+ deep yellow, P+ orange. TLC: protocetraric acid.
Notes
This new species of Diorygma is characterized by small muriform ascospores only 24–40 µm long. The vast majority of Diorygma species have much larger ascospores. The species that most resemble it are D. erythrellum (Mont. & Bosch) Kalb et al. and D. nothofagi (A.W. Archer) A.W. Archer. Diorygma erythrellum differs by the corticate thallus and the norstictic acid chemistry, and D. nothofagi differs by having even shorter ascospores (18–23 µm) and also by the norstictic acid chemistry.
Additional specimen seen. Brazil: Tocantins: Itaguatins, Fazenda São Paulo, S. C. Feuerstein 412 (UPCB).
World key to the species of Diorygma
-
1 Thallus with isidia; ascomata as yet unknown ... 2
Thallus without isidia; ascomata present ... 3
-
2(1) Protocetraric acid present as major substance, norstictic and salazinic acids minor or absent; no black hypothallus present ... D. australasicum (Elix) Lücking et al.
Norstictic and salazinic acids present as major substances, protocetraric acid minor or absent; with a black, brittle hypothallus ... D. antillarum (Vain.) Nelsen et al.
-
3(1) Ascospores only transversely septate ... 4
Ascospores muriform or submuriform ... 9
-
4(3) Ascospores more than 60 µm long ... 5
Ascospores less than 55 µm long ... 6
-
5(4) Ascospores 60–100×8·5–16·0 µm, 12–22-septate; norstictic acid present; thallus K+ yellow>red (crystals formed in microscopic section) ... D. circumfusum (Stirt.) Kalb, et al.
Ascospores 105–108×6 µm, 29–31-septate; norstictic acid absent; unknown substance present; thallus K+ pale yellow ... D. incantatum Feuerstein & Eliasaro
-
6(4) Ascospores up to 35 µm long ... 7
Ascospores 40–55 µm long ... 8
-
7(6) Hypostictic, hypoconstictic, stictic and constictic acids present; ascospores 17–20×5·0–6·5 µm ... D. minisporum Kalb et al.
Norstictic acid present; ascospores 28–32×7 µm ... D. pauciseptatum Feuerstein et al.
-
8(6) Perstictic and stictic acids present; ascospores 40–50×8–10 µm ... D. wallamanense A. W. Archer & Elix
Norstictic acid present; ascospores 45–50(–54)×(6–)8–9 µm ... D. wilsonianum (Müll. Arg.) A. W. Archer
-
9(3) Protocetraric acid present (thallus P+ orange-red; no crystals formed with K) ... 10
Protocetraric acid absent (thallus not P+ red, if P+ yellow-orange, then K+ yellow>red, with crystals formed which are visible in microscopic section) ... 16
-
10(9) Asci 2–8-spored ... 11
Asci 1(–2)-spored ... 13
-
11(10) Stictic acid present (in addition to protostictic and cryptostictic acids); thallus K+ yellow in section; ascospores 87–125×20–37 µm ... D. agumbense B.O. Sharma & Khadilkar
Stictic acid absent (only protocetraric acid present); thallus not clearly K+ yellow in section ... 12
-
12(11) Ascospores 24–40×10–15 µm ... D. tocantinense Feuerstein et al.
Ascospores 125(–250)×30–40(–50) µm ... D. hololeucum (Mont.) Kalb et al.
-
13(10) Norstictic acid present in addition to protocetraric acid; thallus K+ yellow → red, with crystals formed which are visible in microscopic section ... 14
Norstictic acid absent; only protocetraric acid present; thallus K− ... 15
-
14(13) Exciple partly carbonized; ascospores 110–230×35–80 µm ... D. reniforme (Fée) Kalb et al.
Exciple not carbonized; ascospores 120(–215)×30–45(–63) µm ... D. rufopruinosum (A. W. Archer) Kalb, et al.
-
15(13) Ascospores up to 150 µm long (or very rarely to 170 µm long), 95–150(–170)×19–50 µm; peripheral and central ascospore locules of more or less equal size ... D. pruinosum (Eschw.) Kalb et al.
Ascospores mostly over 150 µm long, 140–200×40–65 µm; peripheral ascospore locules distinctly smaller than central ones ... D. africanum Kalb et al.
-
16(9) Lichexanthone present (thallus UV+ yellow) ... 17
Lichexanthone absent (thallus not UV+ yellow) ... 19
-
17(16) Asci 6–8-spored; ascospores 40–60×10–14 µm; apothecia short, often aggregate; without black hypothallus ... D. alagoense M. Cáceres & Lücking
Asci 1(–2)-spored; apothecia longer, distinctly lirelliform and never aggregate; thallus with brittle black hypothallus ... 18
-
18(17) Ascospores up to 135 µm long (or very rarely to 145 µm long), 80–135(–145)×25–45(–50) µm; peripheral and central ascospore locules of more or less equal size ... D. confluens (Fée) Kalb et al.
Ascospores mostly >135 µm long, (120–)135–203×35–70 µm; peripheral ascospore locules distinctly smaller than central ones ... D. epiglaucum (Müll. Arg.) Kalb et al.
-
19(16) Norstictic acid present as major substance ... 20
Norstictic acid absent or only present as trace accompanying other major substances ... 42
-
20(19) Asci (4–)6–8-spored ... 21
Asci 1–2(–4)-spored ... 24
-
21(20) Ascospores up to 65 µm long ... 22
Ascospores over 75 µm long ... 23
-
22(21) Ascospores 18–23(–25)×6–8 µm; thallus without cortex ... D. nothofagi (A. W. Archer) A. W. Archer
Ascospores 30–65×12–20 µm; thallus with smooth cortex ... D. erythrellum (Mont. & Bosch) Kalb et al.
-
23(21) Ascospores up to 20 µm wide, long ellipsoid, 100–115×15–18(–20) µm ... D. longisporum E. L. Lima et al.
Ascospores over 24 µm wide, broad ellipsoid, 75–145×24–34 µm ... D. subalbatum (Patw. & Makhija) B. O. Sharma & Makhija
-
24(20) Salazinic acid present ... 25
Salazinic acid absent or only present as trace accompanying other major substances ... 34
-
25(24) Asci always only 1-spored ... 26
Asci variably 1–2(–4)-spored ... 30
-
26(25) Ascospores 79–96×29·5–33·5 µm ... D. inaequale B. O. Sharma & Makhija
Ascospores over 96 µm long ... 27
-
27(26) Ascospores >50 µm wide, 150–200(–230)×50–75 µm ... D. salvadoriense Kalb et al.
Ascospores up to 40 µm wide ... 28
-
28(27) Exciple distinctly convergent, disc mostly concealed; ascospores 125–175×27–35 µm ... D. dandeliense B. O. Sharma & Khadilkar
Exciple divergent, disc wide open ... 29
-
29(28) Thallus verrucose; ascospores 97–126×25–33 µm ... D. verrucirimosum B. O. Sharma & Makhija
Thallus smooth; ascospores 105–147×33–37 µm ... D. dealbatum B. O. Sharma & Makhija
-
30(25) Ascospores up to 100 µm long ... 31
Ascospores >130 µm long ... 32
-
31(30) Methylstictic acid present in addition to salazinic and norstictic acids; ascospores 75–99×24–30 µm ... D. panchganiense Makhija et al.
Methylstictic acid absent, only salazinic and norstictic acids present; ascospores 76–92×21–25 µm ... D. manipurense B. O. Sharma & Makhija
-
32(30) Methylstictic and cryptostictic acids (and sometimes also constictic acid) present in addition to salazinic and norstictic acids ... 33
Methylstictic acid absent, only salazinic and norstictic acids present; ascospores 130–200×32–42 µm ... D. karnatakense B. O. Sharma & Khadilkar
-
33(32) Ascospores 135–150×18–27 µm ... D. albocinerascens Makhija et al.
Ascospores 147–273×34–67 µm ... D. excipuloconvergentum Makhija et al.
-
34(24) Asci 2–8-spored ... 35
Asci 1-spored ... 36
-
35(34) Asci 8-spored; ascospores submuriform, 12–15×6–7 µm ... D. microsporum M. Cáceres & Lücking
Asci 2–4-spored; ascospores muriform, 151–294×38–63(–84) µm ... D. megistosporum Makhija et al.
-
36(34) Stictic acid present in addition to norstictic acid; ascospores 95–150(–170)×30–45 µm ... D. hieroglyphicum (Pers.) Staiger & Kalb
Stictic acid absent, only norstictic and often connorstictic acids present ... 37
-
37(36) Lirellae long and curved; exciple distinctly convergent; ascospores 105–113×33–42 µm ... D. longilirellatum B. O. Sharma & Makhija
Lirellae shortened; exciple divergent ... 38
-
38(37) Thallus with carbonaceous basal layer; ascospores 90–115×30–40 µm ... D. tinctorium Eschw.
Thallus without carbonaceous layer ... 39
-
39(38) Ascospores up to 145 µm long ... 40
Ascospores 170–250×42–58 µm ... D. pachygraphum (Nyl.) Kalb et al.
-
40(39) Peripheral and central ascospore locules of more or less equal size ... 41
Peripheral ascospore locules distinctly smaller than central ones; ascospores 100–130×44–48 µm ... D. tuberculosum (Stirt.) Kalb et al.
-
41(40) Hymenium completely I+ blue-violet; ascospores (60–)80–125×21–42 µm ... D. junghuhnii (Mont. & Bosch) Kalb et al.
Hymenium weakly I+ blue-violet (mostly laterally); ascospores 110–145×36–45 µm ... D. soozanum (Zahlbr.) Nakan. & Kashiw.
-
42(19) Asci (2–)4–8-spored ... 43
Asci 1(–2)-spored ... 48
-
43(42) Hypostictic and hypoconstictic acids present, stictic acid absent or minor ... 44
Hypostictic and hypoconstictic acids absent, stictic acid present (sometimes with other major substances) ... 46
-
44(43) Hymenium completely I+ blue-violet ... 45
Hymenium I− or weakly I+ blue-violet; ascospores 45–100×14–30(–40) µm ... D. sipmanii Kalb et al.
-
45(44) Ascospores 40–65×10–18 µm ... D. poitaei (Fée) Kalb et al.
Ascospores (60–)70–115×16–22 µm ... D. intermedium Kalb et al.
-
46(43) Ascospores submuriform, 10–13×6–8 µm ... D. sticticum Sutjaritturakan et al.
Ascospores muriform, over 65 µm long ... 47
-
47(46) Ascospores 66–99×12–36 µm; thallus corticolous ... D. albovirescens Makhija et al.
Ascospores 143–172×29–34 µm; thallus saxicolous ... D. saxicola B. O. Sharma & Makhija
-
48(42) Hypostictic and hypoconstictic acids present, stictic acid absent; ascospores 105–165×35–60 µm ... D. monophorum (Nyl.) Kalb et al.
Hypostictic and hypoconstictic acids absent, stictic acid present (sometimes with several other major substances) ... 49
-
49(48) Ascospores up to 205 µm long ... 50
Ascospores 231–244×59–76 µm ... D. megaspermum Makhija et al.
-
50(49) Ascospores up to 145 µm long ... 51
Ascospores usually >145 µm long, (135–)168–205×54–96 µm ... D. rufosporum (Patw. & C. R. Kulk.) B. O. Sharma & Makhija
-
51(50) Stictic acid present alone; thallus corticolous; ascospores 90–136×30–44 µm ... D. talisense (A.W. Archer) A.W. Archer
Constictic and cryptostictic acids present in addition to stictic acid, also sometimes traces of norstictic acid; thallus saxicolous; ascospores 88–147×25–42 µm ... D. rupicola B. O. Sharma & Makhija
SCF thanks Jacyane Ramos de Sousa for assistance in the field and Neusa Araújo Oliveira for allowing her to collect on the private farm in Tocantins. Robert Lücking is thanked for supervising the first author. We are grateful to IAP (Instituto Ambiental do Paraná) for the authorization to collect in Paraná (authorization number 402/12), and to CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior) for awarding a masters scholarship to the first author. MESC thanks CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a research grant (Processo 311706/2012-6).