Introduction
Recent molecular studies have resolved the systematic position of sterile Arthoniales described from Great Britain. Enterographa sorediata Coppins & P. James was shown to be the sorediate morph of Syncesia myrticola (Fée) Tehler (Ertz et al. Reference Ertz, Coppins and Sanderson2018a), while Opegrapha multipuncta Coppins & P. James and Schismatomma quercicola Coppins & P. James were both reclassified in the genera Porina and Schizotrema respectively (Ertz et al. Reference Ertz, Sanderson, Coppins, Klepsland and Frisch2019). One remaining species is Opegrapha corticola Coppins & P. James, a corticolous crustose lichen characterized by a thick grey-green to dull brown thallus and pale greenish fawn to ochraceous soralia often becoming ±patchily continuous in irregular and erose groups 2–3 mm wide (Fig. 1). This sterile species was suspected to be a sorediate morph of Thelopsis rubella Nyl. because both taxa often grow together in Great Britain (Pentecost & James Reference Pentecost, James, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). Recent specimens collected by the second author (NS) produced typical Thelopsis perithecia. These were apparently in the same thallus of O. corticola and some were close to patches of soralia but without obvious separation between the areas of thalli with soralia and those with perithecia. These latter specimens were generally similar to Thelopsis rubella (Fig. 2), supporting the hypothesis that O. corticola might be the sorediate morph of T. rubella. The spores, however, were much smaller than those typical of T. rubella, suggesting that O. corticola might be a separate species, having a normally sterile sorediate thallus. In order to test these hypotheses, lichen fungal DNA of specimens was sequenced. The first sequences obtained from Opegrapha corticola placed the species surprisingly in the genus Gyalecta, as currently circumscribed by Baloch et al. (Reference Baloch, Lücking, Lumbsch and Wedin2010, Reference Baloch, Lumbsch, Lücking and Wedin2013b) and Lücking et al. (Reference Lücking, Moncada and Hawksworth2019). No sequences of Thelopsis were available from GenBank, therefore recently collected specimens of Thelopsis (including the generic type T. rubella) were used to generate fungal DNA sequences.

Fig. 1. Thelopsis corticola. A, thallus with perithecia (white arrows) and soralia (black arrows) (Sanderson 2053 & Cross). B, section through dry perithecium (Sanderson 1971). C, sterile sorediate thallus when ±fresh (Sanderson 2202). D, detail of the soralia when ±fresh (Sanderson 2202). E & F, ascospores in water (Sanderson 1971). Scales: A, C & D = 500 μm; B = 250 μm; E & F = 10 μm. In colour online.
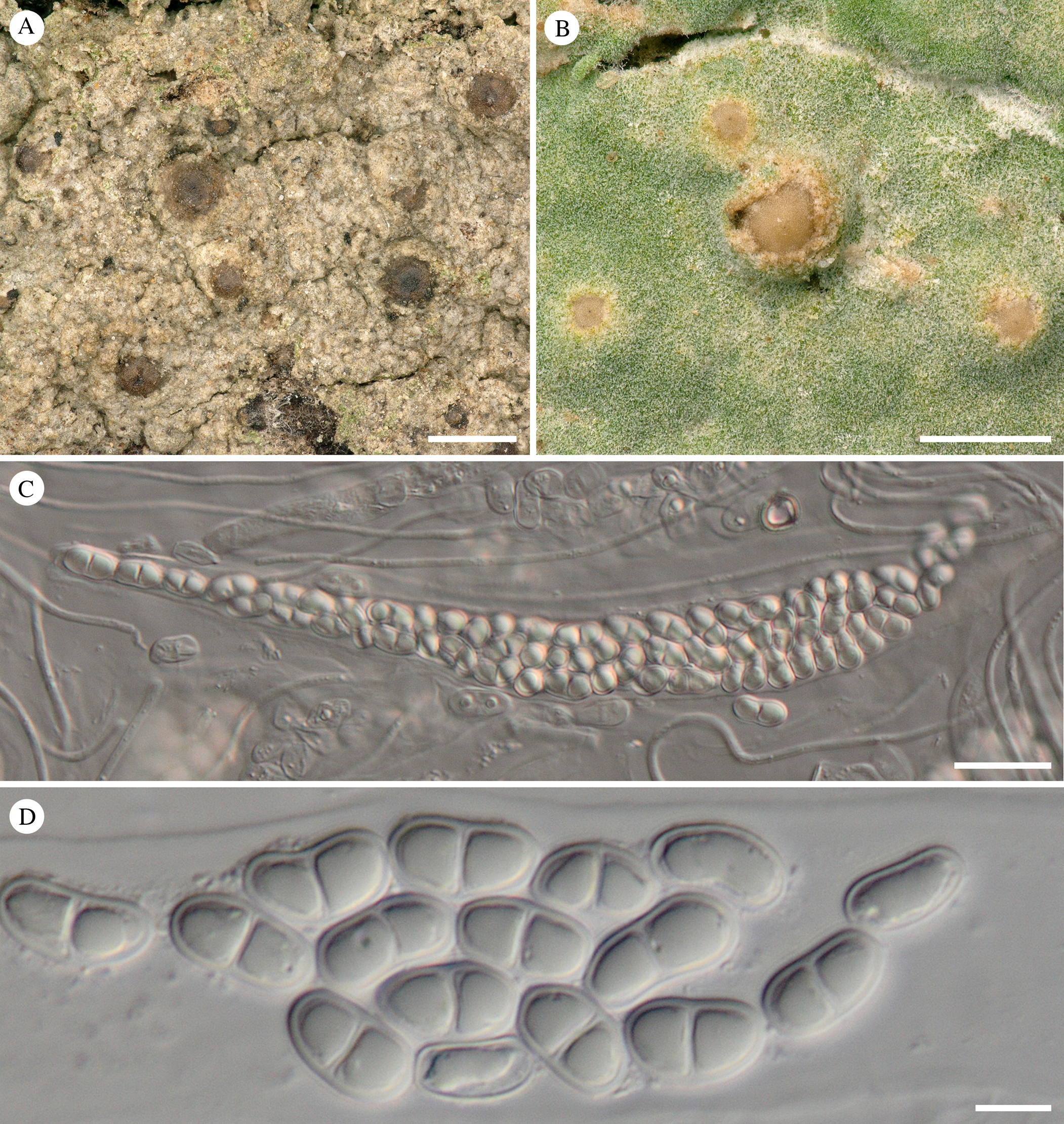
Fig. 2. Thelopsis rubella (A, Ertz 20377) and T. byssoidea (B–D, Ertz 17384). A, thallus with perithecia. B, byssoid thallus with four perithecia. C, ascus with mature ascospores, in water. D, ascospores, in water. Scales: A & B = 500 μm; C = 20 μm; D = 5 μm. In colour online.
Thelopsis [nom cons.] is a species-poor but widespread genus occurring in temperate and tropical regions on bark and rocks. It is characterized by the combination of a crustose thallus with a trentepohlioid photobiont; globose semi-gelatinous perithecia; short, stiff periphyses; long, unbranched paraphyses; unitunicate, uniformly thin-walled, polysporous asci with an I+ usually blue wall; simple, transversely septate or (sub-)muriform, hyaline ascospores (Vězda Reference Vězda1968; Egea & Torrente Reference Egea and Torrente1996; Renobales et al. Reference Renobales, Barreno and Atienza1996; Aptroot et al. Reference Aptroot, Diederich, Sérusiaux and Sipman1997; Breuss & Schultz Reference Breuss and Schultz2007; Moon & Aptroot Reference Moon and Aptroot2009; Rose et al. Reference Rose, James, Orange, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). The genus is currently accepted in the family Stictidaceae (Eriksson Reference Eriksson1999; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017), but the systematic position has never been tested with molecular data. Vězda (Reference Vězda1968) treated six species in his revision of Thelopsis. He suggested a close relationship with the genus Ramonia within the family Thelotremataceae, but this genus is now accepted in Gyalectaceae (Lücking et al. Reference Lücking, Hodkinson and Leavitt2017). Ramonia differs from Thelopsis by having urceolate apothecia with the exciple splitting radially and exposing the sunken hymenial disc, while Thelopsis has perithecia with the ostiole-opening remaining punctiform. Jørgensen & Vězda (Reference Jørgensen and Vězda1984) suggested a close relationship between Thelopsis and Topelia. This latter genus differs from Thelopsis by having 8-spored asci and broadly ellipsoid muriform ascospores. Topelia and Thelopsis were placed in Thelotremataceae s. lat. by Vězda (Reference Vězda1968) and near Stictis, an ostropalean genus, by Sherwood (Reference Sherwood1977) and Eriksson & Hawksworth (Reference Eriksson and Hawksworth1986). Jørgensen & Vězda (Reference Jørgensen and Vězda1984) intimated possible placement in the Gyalectales because of similarities (ascus type, paraphyses, iodine reaction and excipulum) to Belonia. However, they retained these genera in the Ostropales because Belonia differs by the elongated, fusiform spores, no periphyses and the presence of small, yellowish oil droplets in the excipulum (characteristic of the Gyalectales according to Jørgensen et al. (Reference Jørgensen, Vězda and Botnen1983)). Breuss & Schultz (Reference Breuss and Schultz2007) published an identification key to all known species of Thelopsis and accepted 10 species. With the recent description of new species from Brazil (Aptroot et al. Reference Aptroot, Mendonça, Ferraro and Cáceres2014a), Cape Verde (van den Boom Reference van den Boom2012) and South Korea (Moon & Aptroot Reference Moon and Aptroot2009; Kondratyuk et al. Reference Kondratyuk, Lökös, Halda, Haji Moniri, Farkas, Park, Lee, Oh and Hur2016a, Reference Kondratyuk, Lökös, Halda, Upreti, Mishra, Haji Moniri, Farkas, Park, Lee and Liub, Reference Kondratyuk, Lökös, Halda, Farkas, Upreti, Thell, Woo, Oh and Hur2018), the genus now includes 16 accepted species. Thelopsis appears heterogeneous because it includes species having pale or entirely black perithecia, which are entirely (e.g. T. isiaca Stizenb.) or partially immersed in prominent thalline warts and possessing simple or septate ascospores. Thelopsis africana van den Boom was even described as having asci ‘with a small ocular chamber, I−’, while periphysoids were not mentioned.
This paper aims to: 1) determine the systematic position of the genus Thelopsis and of Opegrapha corticola; 2) test whether O. corticola is the sorediate morph of Thelopsis rubella or a different species; 3) test the monophyly of the genus Thelopsis; 4) describe new taxa of sterile sorediate lichens belonging to Gyalectaceae as a result of sequencing material from Amsterdam Island and Great Britain.
Materials and Methods
Voucher specimens are deposited in the herbaria BR and BM. The external morphology was studied and measured using an Olympus SZX12 stereomicroscope. Macroscopic images were captured with a Keyence VHX-5000 digital microscope and a VH-Z20R/W/T lens. Hand-cut sections and squash preparations of thalli were mounted in water, a 5% aqueous potassium hydroxide solution (K), or in Lugol's iodine solution (1% I2) without (I) or with K pretreatment (KI) and studied using an Olympus BX51 compound microscope.
Measurements refer to dimensions in water. Microscopic photographs were prepared using an Olympus BX51 compound microscope fitted with an Olympus SC50 digital camera. Colour reactions of the thallus were studied using K, household bleach (C), K followed by household bleach (KC), crystals of para-phenylenediamine dissolved in ethanol (PD) and long-wave UV (366 nm). Lichen secondary metabolites were investigated using thin-layer chromatography (TLC) in solvent C (Orange et al. Reference Orange, James and White2010).
Molecular techniques
Well-preserved specimens lacking any visible symptoms of fungal infection, either freshly collected (less than one month, except for T. melathelia Nyl. which was a four-year-old herbarium specimen) or kept in the freezer and frozen less than one month after collection, were used for DNA isolation. Hand-cut sections of the hymenium (Gyalecta carneola (Ach.) Hellb., G. farlowii Tuck., Petractis clausa (Hoffm.) Kremp., Porina leptalea (Durieu & Mont.) A. L. Sm., Thelopsis byssoidea Diederich, T. melathelia, T. rubella and a fertile specimen of Opegrapha corticola (Sanderson 2053)) or a small number of soredia (Gyalecta amsterdamensis Ertz, G. nidarosiensis (Kindt) Baloch & Lücking, sterile Opegrapha corticola) were used for direct PCR as described in Ertz et al. (Reference Ertz, Tehler, Irestedt, Frisch, Thor and van den Boom2015). The material was placed directly in microtubes with 20 μl H2O. Amplification reactions were prepared for a 50 μl final volume, as detailed in Ertz et al. (Reference Ertz, Sanderson, Łubek and Kukwa2018b). A targeted fragment of c. 0.8 kb of the mtSSU rDNA was amplified using primers mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999), a fragment of c. 1 kb of the RPB2 protein-coding gene was amplified using primers fRPB2-7cF and fRPB2-11aR (Liu et al. Reference Liu, Whelen and Hall1999), and a fragment of c. 1.1 kb at the 5’ end of the nuLSU rDNA was amplified using primers LIC15R (Miadlikowska et al. Reference Miadlikowska, McCune and Lutzoni2002) and LR6 (Vilgalys & Hester Reference Vilgalys and Hester1990). Both strands were sequenced by Macrogen® using the amplification primers, and with the additional primer LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990) for nuLSU. Sequence fragments were assembled with Sequencher v.5.4.6 (Gene Codes Corporation, Ann Arbor, Michigan). Sequences were subjected to ‘Megablast’ searches to verify their closest relatives and to detect potential contaminations.
Taxon selection and phylogenetic analyses
Two matrices using the same three loci (nuLSU, mtSSU and RPB2) were assembled: a first dataset for placing the newly sequenced taxa in a phylogeny of the order Ostropales s. lat. (now split into Graphidales, Gyalectales, Ostropales s. str., Thelenellales and Odontotrematales; Kraichak et al. Reference Kraichak, Huang, Nelsen, Leavitt and Lumbsch2018; Lücking Reference Lücking2019), and a second dataset for providing a detailed phylogeny of Gyalecta s. lat. (= sensu Baloch et al. (Reference Baloch, Lücking, Lumbsch and Wedin2010, Reference Baloch, Lumbsch, Lücking and Wedin2013b) and Lücking et al. (Reference Lücking, Moncada and Hawksworth2019)).
The closest relatives of the new sequences based on BLAST searches were retrieved from GenBank. Additional taxa were selected mainly from Baloch et al. (Reference Baloch, Lücking, Lumbsch and Wedin2010), with others from Aptroot et al. (Reference Aptroot, Parnmen, Lücking, Baloch, Jungbluth, Cáceres and Lumbsch2014b), Dou et al. (Reference Dou, Wu, Li, Zhao and Jia2018), Fernández-Brime et al. (Reference Fernández-Brime, Llimona, Molnar, Stenroos, Högnabba, Björk, Lutzoni and Gaya2011), Kauff & Lutzoni (Reference Kauff and Lutzoni2002), Lücking et al. (Reference Lücking, Moncada and Hawksworth2019), Lumbsch et al. (Reference Lumbsch, Schmitt, Palice, Wiklund, Ekman and Wedin2004), Lutzoni et al. (Reference Lutzoni, Pagel and Reeb2001), Miadlikowska et al. (Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014), Orange (2009), Pino-Bodas et al. (Reference Pino-Bodas, Zhurbenko and Stenroos2017) and Yang et al. (Reference Yang, Baral, Xu and Liu2019), in order to include an exhaustive list of taxa belonging to different families of the Ostropales s. lat., and a wide array of taxa belonging to the Gyalectaceae. One nuLSU sequence of Gyalecta leucaspis (AF465462) was not included owing to its poor quality (including 31 ‘N’ distributed throughout the sequence): the species groups with G. ulmi (Sw.) Zahlbr. in Kauff & Lutzoni (Reference Kauff and Lutzoni2002) and Orange (2009). The sequences of taxa listed in Table 1 were aligned using MAFFT v.7.402 (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) and manually corrected for errors using Mesquite 3.04 (Maddison & Maddison Reference Maddison and Maddison2015). Terminal ends of sequences, ambiguously aligned regions, and introns were delimited manually following Lutzoni et al. (Reference Lutzoni, Wagner, Reeb and Zoller2000) and excluded from the datasets.
Table 1. Species names, voucher specimens and GenBank Accession numbers of taxa belonging to different families of the Ostropales s. lat. Newly generated sequences are in bold. * = outgroup.

The resulting matrix of Ostropales s. lat. consisted of 77 terminals, while the matrix of Gyalecta s. lat. consisted of 31 terminals. Orceolina kerguelensis (Tuck.) Hertel was used as the rooting taxon in the Ostropales s. lat. dataset, with Coenogonium leprieurii (Mont) Nyl., C. luteum (Dicks.) Kalb & Lücking and C. pineti (Ach.) Lücking & Lumbsch selected in the Gyalecta s. lat. dataset. The datasets of Ostropales s. lat. and Gyalecta s. lat. consisted of 2342 (860 for nuLSU, 597 for mtSSU and 885 for RPB2) and 2361 (851 for nuLSU, 646 for mtSSU, 864 for RPB2) unambiguously aligned sites, respectively. The datasets were deposited in TreeBASE as submissions 26711 and 26712, respectively.
Best-fit evolutionary models were estimated using the Akaike Information Criterion (AIC) as implemented in jModelTest v.2.1.6 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). For Ostropales s. lat., the GTR + I + G model was selected for the nuLSU, RPB2/1st position and RPB2/3rd position datasets, and the TVM + I + G model was selected for the mtSSU and the RPB2/2nd position datasets. For Gyalecta s. lat., the GTR + I + G model was selected for the nuLSU and mtSSU datasets, the TIM1 + I + G model was selected for the RPB2/1st position, the TVM + G model for the RPB2/2nd position and the TrN + G model for the RPB2/3rd position datasets.
Analyses for topological incongruence among loci were carried out for both the three-locus dataset of the Ostropales s. lat. and the three-locus dataset of Gyalecta s. lat. The single locus datasets were analyzed with a maximum likelihood (ML) approach using the program RAxML v.8.2.12 (Stamatakis Reference Stamatakis2014) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) with 1000 ML bootstrap iterations (ML-BS). The GTRGAMMA model was used and node support was assessed running 1000 bootstrap replicates. We analyzed the three single locus datasets for their topological incongruence by assuming a conflict significant when two different relationships (one being monophyletic and the other being non-monophyletic) for the same set of taxa were both supported with bootstrap values ≥ 70% (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996; Reeb et al. Reference Reeb, Lutzoni and Roux2004). Based on this criterion, we detected partial conflict among the nuLSU and RPB2 datasets for Gyalecta s. lat. In the nuLSU tree, Gyalecta ulmi was basal to the rest of Gyalecta s. lat. with a bootstrap support of 80%, while in the RPB2 tree G. ulmi was nested in Gyalecta s. lat. with a bootstrap support of 88%, taxa of Thelopsis and the clade with Gyalecta flotowii + G. geoica + G. truncigena being at the base of the tree. Including or removing the RPB2 of G. ulmi from both the single locus and the combined analyses had no impact on the topology of the trees, therefore the nuLSU, mtSSU and RPB2 datasets were concatenated.
Bayesian analyses were carried out on the three-locus datasets under the selected models for five partitions (nuLSU, mtSSU, RPB2/1st, RPB2/2nd, RPB2/3rd positions), using the Metropolis-coupled Markov chain Monte Carlo method (MCMCMC) in MrBayes v.3.2.7a (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). For the Ostropales s. lat. dataset, two parallel MCMCMC runs were performed each using four independent chains and 80 million generations, sampling trees every 1000th generation. Posterior probabilities (PP) were determined by calculating a majority-rule consensus tree generated from the 120 002 post burn-in trees of the 160 002 trees sampled by the two MCMCMC runs using the sumt option of MrBayes. Similarly, for the Gyalecta s. lat. dataset, two parallel MCMCMC runs were performed each using four independent chains and 40 million generations, sampling trees every 1000th generation. Posterior probabilities (PP) were determined by calculating a majority-rule consensus tree generated from the 60 002 post burn-in trees of the 80 002 trees sampled by the two MCMCMC runs using the sumt option of MrBayes. For both Bayesian analyses, Tracer v.1.7.1 (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018) was used to ensure that stationarity was reached by plotting the log-likelihood values of the sample points against generation time, making sure that the ESS values were much higher than 200. Convergence between runs was also verified using the PSRF (Potential Scale Reduction Factor), where values were all equal or close to 1.000.
In addition, a maximum likelihood analysis was performed using RAxML v.8.2.12 (Stamatakis Reference Stamatakis2014) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) with 1000 ML bootstrap iterations (ML-BS). The two three-locus datasets were divided into five partitions (nuLSU, mtSSU, RPB2/1st, RPB2/2nd, RPB2/3rd positions) with the GTRGAMMA model.
The ML trees did not contradict the Bayesian tree topologies for the strongly supported branches. Therefore, only the ML trees are shown with the ML-BS values added above the internal branches and the PP values added below (Figs 3 & 4). Internodes with ML-BS ≥ 70 and PP ≥ 0.95 were considered to be significant. Phylogenetic trees were visualized using FigTree v.1.4.2 (Rambaut Reference Rambaut2012).

Fig. 3. Phylogeny of Ostropales s. lat. (with Orceolina kerguelensis as outgroup) based on a dataset of nuLSU, mtSSU and RPB2 sequences that resulted from the RAxML analysis. Maximum likelihood bootstrap values are shown above internal branches and posterior probabilities obtained from a Bayesian analysis are shown below. Internal branches, considered strongly supported by both analyses, are represented by thicker lines. The newly sequenced samples are in bold and their names are followed by collection numbers of authors, which act as specimen and sequence identifiers. Lineages corresponding to the genera Francisrosea, Neopetractis, Ramonia and Thelopsis are highlighted. In colour online.

Fig. 4. Phylogeny of Gyalectaceae (with three species of Coenogonium as outgroup) based on a dataset of nuLSU, mtSSU and RPB2 sequences that resulted from the RAxML analysis. Maximum likelihood bootstrap values are shown above internal branches and posterior probabilities obtained from a Bayesian analysis are shown below. Internal branches, considered strongly supported by both analyses, are represented by thicker lines. The newly sequenced samples are in bold and their names are followed by collection numbers of authors, which act as specimen and sequence identifiers. The lineage corresponding to the genus Thelopsis is highlighted, showing the genus nested in Gyalecta s. lat. Generic names in use before Baloch et al. (Reference Baloch, Lumbsch, Lücking and Wedin2013b) and Lücking et al. (Reference Lücking, Moncada and Hawksworth2019), together with Petractis (for P. farlowii), are shown in brackets. ![]() indicates the type of the earlier genus name. In colour online.
indicates the type of the earlier genus name. In colour online.
Results
Phylogenetic analysis
Forty-four new sequences (17 nuLSU, 13 mtSSU, 14 RPB2) were obtained for this study and 144 additional sequences (54 nuLSU, 52 mtSSU, 38 RPB2) were retrieved from GenBank (Table 1). The RAxML tree obtained from the combined three-locus analysis of the Ostropales s. lat. dataset is shown in Fig. 3. The main well-supported lineages are in accordance with the results obtained by Baloch et al. (Reference Baloch, Lücking, Lumbsch and Wedin2010) and Spribille et al. (Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020: fig. 7). The Ostropales s. lat. are now split into Graphidales, Gyalectales, Ostropales s. str., Thelenellales and Odontotrematales (Kraichak et al. Reference Kraichak, Huang, Nelsen, Leavitt and Lumbsch2018; Lücking Reference Lücking2019), but Spribille et al. (Reference Spribille, Fryday, Pérez-Ortega, Svensson, Tønsberg, Ekman, Holien, Resl, Schneider and Stabentheiner2020: fig. 7) includes Graphidales and Odontotrematales in Gyalectales. We prefer to use Ostropales s. lat. which is more appropriate for the topology of our tree, the backbone of which is poorly resolved. The genus Thelopsis is recovered as polyphyletic. The type of the genus, T. rubella, forms a well-supported lineage with T. byssoidea and Opegrapha corticola within the genus Gyalecta sensu Baloch et al. (Reference Baloch, Lücking, Lumbsch and Wedin2010) and Lücking et al. (Reference Lücking, Moncada and Hawksworth2019). Thelopsis melathelia is the sister species to Ramonia valenzueliana (Mont.) Stizenb., a relationship supported only by the RAxML analysis.
The RAxML tree obtained from the combined three-locus analysis of the Gyalecta s. lat. dataset is shown in Fig. 4. The generic names in use before Baloch et al. (Reference Baloch, Lumbsch, Lücking and Wedin2013b) and Lücking et al. (Reference Lücking, Moncada and Hawksworth2019) are shown in brackets. The topology of this tree is in accordance with the results obtained by Lücking et al. (Reference Lücking, Moncada and Hawksworth2019). Relationships within Gyalecta s. lat. are generally well supported. The analysis of this reduced dataset of Gyalectaceae resulted in 19 more unambiguously aligned sites than in the Ostropales s. lat. dataset, and in a slightly different placement of Thelopsis as sister to the clade from Gyalecta carneolutea to G. amsterdamensis (Fig. 4). In addition to the genus Thelopsis and Opegrapha corticola, other taxa are newly included in the phylogeny. Gyalecta carneola is the sister species of Gyalecta fagicola (Hepp ex Arnold) Kremp. Gyalecta nidarosiensis is sister to G. herrei Vězda. Gyalecta farlowii and the new species G. amsterdamensis cluster close to G. herculina (Rehm) Baloch et al.
The family Gyalectaceae is not monophyletic in our tree (Fig. 3). The genera Francisrosea, Ramonia, Neopetractis and ‘Gyalidea’ praetermissa form a different lineage sister to the families Sagiolechiaceae + Coenogoniaceae + Porinaceae. However, this relationship is not supported. Miadlikowska et al. (Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014) wrote that ‘Petractis nodispora and P. luetkemuelleri (Stictidaceae), Gyalidea praetermissa (Graphidaceae) and Ramonia sp. (Gyalectaceae) should be accommodated in different genera outside of their respective families’. Further studies using more loci and more taxa are needed to investigate whether this lineage might represent a different family or not. The topology is unresolved, and it is unclear what might be observed when more markers are added, and the amount of missing data is reduced. Merging the families Coenogoniaceae, Gyalectaceae, Porinaceae and Sagiolechiaceae into a single family is also possible, particularly as these families include few genera, a big contrast to the family Graphidaceae. In this context, the lineage with Petractis clausa is also orphaned and needs further studies. We could confirm the published nuLSU sequence of P. clausa by sequencing a second specimen (Fig. 3), but we were unsuccessful obtaining mtSSU and RPB2 sequences.
Taxonomy
Francisrosea Ertz & Sanderson gen. nov.
MycoBank No.: MB 836494
A new genus in the family Gyalectaceae, distinguished by having an isolated phylogenetic position as sister to a clade including Gyalidea praetermissa and the genera Neopetractis and Ramonia, and characterized by an inconspicuous thallus with small discrete erumpent soralia lacking acetone-soluble secondary metabolites detectable by TLC.
Type species: Francisrosea bicolor Ertz & Sanderson.
Etymology
Named after Francis Rose for his outstanding contribution to the protection and study of the lichen flora of forests with a long historical continuity, in Great Britain.
Description
See specific description below.
Francisrosea bicolor Ertz & Sanderson sp. nov.
MycoBank No.: MB 836495
Distinguished from all known Gyalectaceae by a unique phylogenetic position as sister to a clade including Gyalidea praetermissa and the genera Neopetractis and Ramonia, and characterized by an inconspicuous thallus with small discrete erumpent soralia, pale greenish inside, orange-ochre at the surface, and by lacking acetone-soluble secondary metabolites detectable by TLC.
Type: Great Britain, V.C.11, New Forest, Busketts Wood, Little Stubby Hat, Grid Ref. SU30532 10627, Quercus-Fagus-Ilex pasture woodland, wound track on ancient Fagus sylvatica, 27 September 2016, Sanderson 2200 (BR—holotype!).

Fig. 5. Francisrosea bicolor (A & B, Sanderson 2200: specimens kept frozen since fieldwork) and Ramonia melathelia (C–G, Ertz 20503). A, inconspicuous thallus with discrete soralia. B, soralia. C, thallus with black perithecia. D, close-up of a perithecium showing the wrinkled surface. E–G, ascospores showing the thick gelatinous sheath, in water. Scales: A & C = 1 mm; B = 500 μm; D = 250 μm; E–G = 10 μm. In colour online.
Thallus inconspicuous, immersed in the bark, only visible by the soralia. Soralia erumpent, first punctiform, later becoming ±rounded to ellipsoid, erose, slightly convex and elevated above the substratum, 0.2–0.8(–1) mm diam., pale greenish inside, orange-ochre at the surface and mainly at the margins, discrete, scarcely distributed, rarely 1–4 becoming confluent and forming patches up to c. 1.5 mm across. Soredia without projecting hyphae, (25–)30–50(–70) μm diam., composed of hyaline smooth hyphae 4–6(–7) μm diam., I−, KI− and trentepohlioid cells 6–13 μm diam. in short chains of 2–6(–8) cells. Crystals absent (polarized light).
Apothecia and pycnidia unknown.
Chemistry
Soralia C−, K−, KC−, PD−, UV−. TLC: nil (small amount of lichen material used).
Etymology
The epithet refers to the two colours of the soralia.
Distribution and ecology
In the New Forest, this species has been recognized as distinct from Thelopsis corticola for some time, although the separation from Porina multipuncta (Coppins & P. James) Ertz et al. was not fully understood. However, beyond this area it continues to be confused with T. corticola. As such, the national distribution is not yet clearly known but it is widespread in old-growth Fagus-Quercus-Ilex pasture woodlands in the New Forest, Hampshire, where it has been recorded from 26 woods since 1992. Here it is most frequently found in wound tracks on senescent Fagus sylvatica, but has also been found in wound tracks on Quercus robur. There are often no associated lichens in the habitat but usually only algae crusts and a scattering of bryophytes such as Metzgeria furcata (L.) Dumort. and Zygodon rupestris Schimp. ex Lorentz. It has been noted as occasionally growing with typical wound track colonist lichens such as Alyxoria varia (Pers.) Ertz & Tehler, Caloplaca ulcerosa Coppins & P. James, Opegrapha vulgata (Ach.) Ach., Porina aenea (Körb.) Zahlbr. and Strigula taylorii (Carroll ex Nyl.) R. C. Harris, along with outlying thalli of species from adjacent stable communities such as Agonimia octospora Coppins & P. James, Enterographa crassa (DC.) Fée, E. elaborata (Lyell ex Leight.) Coppins & P. James, Pyrenula chlorospila Arnold and Porina rosei Sérus. s. str. The distribution of Francisrosea bicolor outside of the New Forest is not known with any certainty but the species has been noted as causing confusion in the recording of Thelopsis corticola in Exmoor and North Wales and was recently definitively recorded in a wound track on an ancient Quercus at Rydal Park, Lake District, England. The latter indicates that it occurs much further north than most Thelopsis corticola records and may account for at least some of the outlying records of T. corticola north of its main southern English distribution. Examination of herbarium collections of T. corticola and potentially Porina multipuncta is likely to produce further records.
Discussion
Thelopsis corticola (Gyalectaceae) is similar to the new species in the ochre-coloured soralia, but having a more even colour with the deeper orange tints mostly absent and the internal green colouring paler and less often visible, and a thallus that is always visible at least near the soralia. The soralia are more often confluent, forming larger patches of 2–3 mm diam., and more dense with smaller soredia (up to 25 μm in Sanderson 2202); the difference between soralia size is easily apparent in the field. Porina multipuncta, in the Porinaceae, differs in having a superficial thallus with numerous minute (0.1–0.3 mm) soralia that have a uniformly bright orange colour when fresh. Zwackhia sorediifera (P. James) Ertz has C+ pink-red soralia and belongs to the Arthoniales. Caloplaca lucifuga G. Thor (Teloschistales) has pale yellow to dirty yellow-orange-brown soralia that are K+ purple.
Additional specimens examined
Great Britain: England: V.C.11, South Hampshire, New Forest, Busketts Wood, The Ridge, Grid Ref. SU31128 10988, 2016, Sanderson 2183 (BM); ibid., New Forest, Gritnam Wood, Grid Refs SU282 067 & SU284 064, 1992, Sanderson 2745 (BM); ibid., New Forest, Eyeworth Wood, Grid Ref. SU22517 15574, 2020, Sanderson 2746 (BM); ibid., New Forest, Allum Green, Bramble Hill, Grid Ref. SU2775 0676, 2020, Sanderson 2747 (BM).
Gyalecta amsterdamensis Ertz sp. nov.
MycoBank No.: MB 836496
A species of Gyalecta characterized within the genus by a smooth, rimose, saxicolous thallus with discrete soralia.
Type: Terres Australes et Antarctiques Françaises (TAAF), Île Amsterdam, Base Martin de Viviés, Jardin Météo, 37°47′57.2″S, 77°34′10.8″E, 50 m elev., paroi de roche volcanique ±abritée, 19 December 2016, Ertz 21404 (BR—holotype!; PC—isotype!).

Fig. 6. Gyalecta amsterdamensis (B & C) and G. farlowii (D). A, crater of Île Saint-Paul, one of the localities of G. amsterdamensis. B, three-year-old herbarium specimen (Ertz 21404) showing thallus with soralia that have turned pale cream. C, specimen kept frozen since fieldwork (Ertz 21355) showing thallus with soralia having a pink-orange tinge. D, thallus with ascomata (Ertz 18328). Scales: B–D = 1 mm. In colour online.
Thallus saxicolous, crustose, forming patches of c. 0.5–5 cm diam., ±neatly delimited, continuous, distinctly rimose, smooth, matt, ecorticate, sorediate, c. 100–300 μm thick, pale greenish grey or pale greyish cream; calcium oxalate crystals absent; thallus in section not distinctly layered, mainly composed of more or less loosely interwoven fungal hyphae, with photobiont cells scattered irregularly. Prothallus absent. Soralia discrete, not or rarely confluent, flat to slightly convex, with a pinkish orange tinge when fresh, fading to pale cream, often paler than the thallus, 0.2–0.8(–1) mm diam.; soredia (17–)22–38 μm diam., formed of individual or short chains of photobiont cells surrounded by hyaline hyphae of (2.5–)3–4 μm diam., without projecting hyphae. Photobiont trentepohlioid, visible as individual globose cells, 9–15(–20) μm diam. or in short chains of c. 2–4 cells, with individual cells elliptical to rectangular, 9–20 × 7–13 μm.
Ascomata and conidiomata unknown.
Chemistry
Thallus and soralia K−, C−, PD−, UV−; hyphae I+ pale orange, KI−. TLC: traces of two UV+ red spots of high R f (specimen Ertz 21404 tested in solvent C).
Etymology
The specific epithet refers to Amsterdam Island, the type locality.
Distribution and ecology
So far known only from the islands of Amsterdam and Saint-Paul (Fig. 6A), where it inhabits volcanic rock in rather open and ±sheltered conditions near the sea.
Discussion
The generic placement in Gyalecta s. lat. of this sterile species is confirmed by our phylogenetic analysis (Fig. 4). Gyalecta amsterdamensis is at present the only known member of the genus having a rimose thallus with discrete soralia. The saxicolous Gyalecta nidarosiensis differs by having a thallus that is finely powdery-granular, without well-defined, discrete soralia. Among species from the Antarctic, G. pezizoides Vězda et al. has a leprose-granulose, yellowish brown thallus and grows on moss and soil (Vězda et al. Reference Vězda, Øvstedal and Smith1992). Despite the rather rich material collected on the islands of Amsterdam and Saint-Paul, ascomata could not be found, nor observed in the field where the species was easily recognized by its thallus with discrete soralia having a pink-orange tinge. This colour fades to pale greyish cream in the herbarium, as known in other members of the genus. No other species of Gyalectaceae is known from Amsterdam and Saint-Paul Islands (Aptroot et al. Reference Aptroot, Van de Vijver, Lebouvier and Ertz2011).
Additional specimens examined
Terres Australes et Antarctiques Françaises (TAAF): Île Amsterdam: Del Cano, 37°52′S, 77°32′E, 170 m elev., 2016, Ertz 21359 (BR); ibid., 37°52′07.7"S, 77°32′30.4″E, 172 m elev., 2016, Ertz 21355 (BR). Île Saint-Paul: versant extérieur du cratère, crète de la Novara, non loin des terres chaudes à Sphagnum, 38°42′57.7″S, 77°31′07.1″E, 226 m elev., 2016, Ertz 21051 (BR).
Neopetractis Ertz gen. nov.
MycoBank No.: MB 836497
Similar to Petractis but differing from the type of that genus (P. clausa, which associates with a cyanobacterial photobiont) in associating with a trentepohlioid photobiont.
Type species: Neopetractis luetkemuelleri (Zahlbr.) Ertz.
Description (based mainly on the descriptions in Orange (2009) and Vězda (1965))
Thallus crustose, endolithic or semi-epilithic, continuous, rarely with fine cracks, smooth to minutely rugose, whitish grey or pale pink, ecorticate. Photobiont trentepohlioid.
Apothecia immersed, at first perithecioid, finally with a slightly to rather widely expanded disc; margin slightly raised, of the same colour as the thallus or slightly paler, with or without radial cracks, up to 0.5 mm diam.; disc beige-pink to pale brown, smooth, concave or flat, sunken below level of margin. Exciple thin, colourless or yellowish; cells angular, isodiametric to oblong. Hymenium colourless, I− or I+ faint blue, KI+ blue. Hypothecium thin, colourless. Paraphyses simple, apex not or slightly widened. Asci narrowly clavate, thin-walled, 8-spored, KI+ blue. Ascospores hyaline, ellipsoid, 3–5-transversally septate to submuriform (with 1–2 additional longitudinal septa), medium-sized (c. 16–25 × 5.5–10 μm), with a distinct gelatinous sheath, c. 2–4 μm thick.
Conidiomata pycnidia, immersed in the thallus; conidiogenous cells holoblastic, not proliferating; conidia colourless, simple or formed of irregular multicellular clusters.
Chemistry
No lichen substances detected by TLC.
Etymology
The name reflects its morphological similarity to the cyanolichen genus Petractis.
Discussion
Neopetractis differs from Petractis in having a trentepohlioid photobiont and from Gyalecta s. lat. in having ascospores with a thick gelatinous sheath. Orange (2009) described P. nodispora, which is the sister species of P. luetkemuelleri in his molecular study. In our phylogenetic tree, these two Petractis species form a lineage close to the genus Ramonia and are distantly related to Petractis clausa. Because of the different photobiont and the distinct phylogenetic position, both species are transferred to the new genus Neopetractis (see also general discussion below). Petractis crozalsii (B. de Lesd.) Clauzade & Cl. Roux is a species with non-halonate ascospores and is now considered to be a species of Gyalecta closely related to Gyalecta hypoleuca (Ach.) Zahlbr. (Roux et al. Reference Roux, Bauvet, Bricaud and Coste2008), thus leaving Petractis as a monotypic genus. Both species of Neopetractis grow on calcareous rocks.
Neopetractis luetkemuelleri (Zahlbr.) Ertz comb. nov.
MycoBank No.: MB 836498
Gyalecta luetkemuelleri Zahlbr. [as ‘lütkemülleri’], Österreichische Botanische Zeitschrift 53, 178 (1903).—Petractis luetkemuelleri (Zahlbr.) Vězda, Preslia 37, 137 (1965); type: Jugoslawien, Insel Hvar (Lesina), auf Kalkfelsen am Wege von Lesina nach Citavecchia, 1902, Lütkemüller (W—holotype, not seen).
Neopetractis nodispora (Orange) Ertz comb. nov.
MycoBank No.: MB 836499
Petractis nodispora Orange, Lichenologist 41, 217 (2009); type: Great Britain, Wales, Glamorgan, Southerndown, Dunraven Park, Pant y Slade, national grid reference 21/8872.7330, 51°26′50″N, 3°36′05″W, 30 August 2008, on vertical side of unshaded, north-west-facing limestone wall, Orange 17573 (NMW [C.2007.001.284]—holotype; AIX—isotype, not seen).
Ramonia melathelia (Nyl.) Ertz comb. nov.
MycoBank No.: MB 836500
Thelopsis melathelia Nyl., Flora, Regensburg 47, 358 (1864).—Verrucaria melathelia (Nyl.) Leight., Lich.-Fl. Great Brit., 447 (1871).—Sagedia melathelia (Nyl.) Jatta, Syll. Lich. Ital., 553 (1900); type: [United Kingdom: Scotland], Ben Lawers, Jones s. n. (H-NYL 1427, lectotype fide Vězda (Reference Vězda1968: 380); see https://plants.jstor.org/specimen/h9504760).
Discussion
Sequences obtained from a specimen surprisingly place Thelopsis melathelia as sister species to a specimen of Ramonia valenzueliana. This latter specimen was published by Lumbsch et al. (Reference Lumbsch, Schmitt, Palice, Wiklund, Ekman and Wedin2004) as Xerotrema sp. and was later included as Ramonia valenzueliana, the type species of the genus, in the phylogeny of Rivas Plata et al. (Reference Rivas, Parnmen, Staiger, Mangold, Frisch, Weerakoon, Hernández, Cáceres, Kalb and Sipman2013). Ramonia valenzueliana shares with Thelopsis, the presence of periphysoids, multispored asci and small few-septate ascospores. Thelopsis melathelia differs from the type species of Thelopsis in having ascomata with a wrinkled surface, an excipulum with a darker outer layer all around and ascospores with a rather thick gelatinous sheath. These features support a closer relationship with the type species of Ramonia rather than with Thelopsis s. str. and Gyalecta sensu Lücking et al. (Reference Lücking, Moncada and Hawksworth2019). The wrinkled ascomatal surface, dark excipulum and shape of ascospores also fits with Ramonia s. str. (= section Ramonia sensu Vězda (Reference Vězda1966)). Therefore, the species is transferred to the genus Ramonia.
Specimen used for fungal DNA sequencing
Austria: Karnten, National Park Hohe Tauern, Glockner-Gruppe, above Hochtor, 47°05′04″N, 12°50′10″E, c. 2600 m, on soil-mosses in alpine vegetation, 2015, Ertz 20503 (BR).
Thelopsis corticola (Coppins & P. James) Sanderson & Ertz comb. nov.
MycoBank No.: MB 836501
Opegrapha corticola Coppins & P. James, Lichenologist 11, 162 (1979); type: Ireland, V.C. H3, West Cork, 4 miles east of Baltimore, on poplar, 27 February 1965, P. W. James (BM—holotype; see https://plants.jstor.org/specimen/BM000501110).
(Fig. 1)
Description (of thallus partly from Coppins & James (1979))
Thallus continuous, thin, smooth, matt, grey-green in shaded situations, becoming grey-brown in more exposed situations; soralia initially punctiform, scattered, becoming patchily contiguous in irregular and erose groups 2–3 mm wide, greenish fawn, pale grey-brown or ochraceous, fading to whitish grey in the herbarium.
Perithecia scattered, discrete, immersed in the thallus, sometimes visible as verrucae covered laterally by the thallus, with usually only the upper part of the perithecia visible, rarely the upper 1/3 emerging from the thallus, 0.4–0.6 mm diam., pale brownish to reddish brown or dark brown, often darker around the ostiole. Excipulum hyaline to pale yellowish, c. 30–40 μm thick laterally, becoming thicker around the ostioles, c. 60–70 μm, composed of hyphae with thick gelatinized walls, I−, K/I−. Hymenium hyaline, not inspersed, I+ orange-reddish, K/I+ blue (mainly the ascus walls). Paraphyses unbranched, (1.5–)2 μm, apex not widened, hyaline. Periphysoids simple or with short lateral branches, 20–40(–50) μm long. Asci over 100-spored, c. 150–180 × 12.5–20 μm; wall I+ reddish, K/I+ blue. Ascospores hyaline, ellipsoid-oblong, ends rounded, (2–)3-septate, 7.5–10.3–13 × 3–4.1–5 μm (n = 40), without a gelatinous sheath.
Discussion
Typical Thelopsis perithecia are described for the first time for Opegrapha corticola, a species previously known only as a sterile crustose sorediate lichen. DNA sequences obtained independently from both, the soralia of three specimens and the hymenium of one fertile specimen, clearly place the species in Thelopsis as defined here (Figs 3 & 4). Opegrapha corticola is similar to Thelopsis rubella but differs by having a sorediate thallus and distinctly smaller ascospores ((10–)12–16(–18) × 4–8 µm in T. rubella (Rose et al. Reference Rose, James, Orange, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009)). Perithecia of O. corticola are generally also duller and less reddish when wet than those of T. rubella, and when dry, are generally a bit more sunken into the thallus than is typical for T. rubella. Our morphological observations along with our phylogenetic results leave no doubt that O. corticola is a normally sorediate Thelopsis and that it is a separate taxon from T. rubella. Therefore, a new combination is made in Thelopsis.
Specimens used for fungal DNA sequencing (Sanderson 2053 is fertile, the others sterile; all on trunks of Quercus)
France: Brittany: Concoret, château de Comper, 48°04′18″N, 2°10′23″W, 109 m elev., 2012, Ertz 17602 (BR).—Great Britain: Wales: V.C.48, Merionethshire, Nannau, The Deer Park, Tree NN274, Grid Ref. SH74931 19550, 2015, Sanderson 2053 & Cross (BM). England: V.C.11, South Hampshire, New Forest, Sunny Bushes, Grid Ref. SU25949 14155, 2016, Sanderson 2188 (BM); ibid., Matley Wood, Grid Ref. SU33395 07824, 2016, Sanderson 2202 (BM).
Additional fertile specimens examined (all on trunks of Quercus)
Great Britain: England: V.C.11, South Hampshire, New Forest, Frame Wood, Grid Ref. SU35970 03286, 2013, Sanderson 1971 & Wessex Lichen Group (BM); V.C.8, South Wiltshire, Longleat Park, The Rookery, Grid Ref. ST80704 43776, 2015, Sanderson 2120 (BM).
Other specimens used for fungal DNA sequencing
Gyalecta (Pachyphiale) carneola. Norway: Hordaland: Tysnes, Hovdanes, Beltestadknappen, 59°59′43″N, 5°27′14″E, 2018, Ertz 22499 (BR).
Gyalecta farlowii. Netherlands Antilles: Curaçao: Westpunt, Playa Piskadó (Grandi), 12°22′12″N, 69°09′11″W, c. 12 m, limestone rocks, 2013, Ertz 18328 (BR).
Gyalecta nidarosiensis. Belgium: Yvoir, Champalle, grand affleurement rocheux au sud du village d'Yvoir, 50°19′00″N, 4°52′59″E, 167 m elev., limestone rocks, 2019, Ertz 23169 (BR).
Petractis clausa. Belgium: commune d'Anhée, à 500 m au nord-est du village de Foy, Bois de la Saute, sur le versant droit de la Molignée, juste en aval du confluent Molignée-Flavion, 50°17′46″N, 4°48′59″E, 150 m elev., paroi de calcaire compact, 2019, Ertz 23174 (BR).
Porina leptalea. Belgium: commune d'Anhée, à 500 m au nord-est du village de Foy, Bois de la Saute, sur le versant droit de la Molignée, juste en aval du confluent Molignée-Flavion, 50°17′46″N, 4°48′59″E, 140 m elev., trunk of Carpinus, 2019, Ertz 23175 (BR).
Thelopsis byssoidea. Thailand: Trat Prov.: Sapan Hin Waterfall, 12°06′09″N, 102°42′44″E, c. 30 m elev., tropical rainforest along a river, base of a big tree, 2012, Ertz 17384 (BR).
Thelopsis rubella. Belgium: Rochefort, grotte de Lorette, 50°09′17″N, 5°13′40″E, 220 m, on Tilia, 2013, Ertz 18094 (BR).—Great Britain: England: V.C.11, South Hampshire, New Forest, Sunny Bushes, Grid Ref. SU26175 14316, Quercus-Fagus-Ilex pasture woodland, base rich bark on old Quercus petraea, 2016, Sanderson 2186 (BM).—Italy: Genoa Prov.: Genoa, Pegli, Villa Doria, 44°25′47″N, 8°48′53″E, c. 55 m elev., park, on big trunk of Quercus, 2015, Ertz 20377 (BR).
Discussion
Should Thelopsis be merged with Gyalecta?
The placement of Thelopsis in the genus Gyalecta is surprising because Thelopsis is well recognized by the combination of the following morphological characters: perithecioid ascomata, well-developed periphysoids, polysporous asci ((30–)40–150(–300) spores), and small few-septate ellipsoid-oblong ascospores. Thelopsis is a further remarkable example of parallel evolution of perithecioid ascomata within Gyalectaceae, in addition to Belonia. No previous studies have mentioned the possibility that Thelopsis should be merged into Gyalecta and the genus was even considered to belong to the family Stictidaceae (Lücking et al. Reference Lücking, Hodkinson and Leavitt2017). Only Jørgensen & Vězda (Reference Jørgensen and Vězda1984) intimated placement in the Gyalectales but they retained Thelopsis in the Ostropales (see Introduction). While the combination of morphological characters makes Thelopsis unique within Gyalectaceae, none of the morphological features taken alone supports Thelopsis as being distinct from Gyalecta. Perithecioid ascomata are known in Gyalecta species formerly included in Belonia (e.g. the sequenced G. herculina, G. nidarosiensis and G. russula), but these taxa lack periphysoids (Jørgensen et al. Reference Jørgensen, Vězda and Botnen1983; Navarro-Rosinés & Llimona Reference Navarro-Rosinés and Llimona1997). Henssen (Reference Henssen, Brown, Hawksworth and Beiley1976) proved that periphysoids are present during ascomal ontogeny of several species of Gyalectaceae. In mature apothecia of Gyalecta ulmi, periphysoids are still present but restricted to the outermost margin, while in Gyalecta jenensis (Batsch) Zahlbr. the periphysoids remain short and imbedded in mucilage forming a rim along the inner boundary of the excipulum against the hymenium (Henssen Reference Henssen, Brown, Hawksworth and Beiley1976). In Belonia, Cryptolechia and Pachyphiale, these structures are reduced and generally visible only in young apothecia (Henssen Reference Henssen, Brown, Hawksworth and Beiley1976; Kauff & Büdel Reference Kauff and Büdel2005, as ‘lateral paraphyses’). However, the periphysoids if present are never as well developed as in Thelopsis, where they occupy a broad zone around the ostiole in mature ascomata. In the Stictidaceae, the genera Carestiella and Schizoxylon lack periphysoids but they have been recorded within the genus Stictis. Species of Stictis have periphysoids (Wedin et al. Reference Wedin, Döring, Könberg and Gilenstam2005, Reference Wedin, Döring and Gilenstam2006), suggesting that the importance of this character (=presence vs absence of periphysoids) might have been overestimated for generic delimitation. These different genera were maintained until now (Lumbsch & Papong Reference Lumbsch and Papong2009; Fernandéz-Brime et al. Reference Fernández-Brime, Llimona, Molnar, Stenroos, Högnabba, Björk, Lutzoni and Gaya2011, Reference Fernández-Brime, Olariaga, Baral, Friebes, Jaklitsch, Senn-Irlet and Wedin2018), however, and the generic delimitations in the Stictidaceae need further investigation. Because Stictis is a large and poorly known group with many species that are mainly tropical, short-lived and growing on debris of various sorts, it is much more likely that the genus will eventually ‘fall to pieces’ (Mats Wedin, personal communication), as suggested by the results of recent studies in the family (e.g. Fernandéz-Brime et al. Reference Fernández-Brime, Olariaga, Baral, Friebes, Jaklitsch, Senn-Irlet and Wedin2018; Phukhamsakda et al. Reference Phukhamsakda, McKenzie, Phillips, Jones, Bhat, Marc, Bhunjun, Wanasinghe, Thongbai and Camporesi2020).
Polyspory originated many times during the evolution of lichenized fungi (Reeb et al. Reference Reeb, Lutzoni and Roux2004; Aptroot & Schumm Reference Aptroot and Schumm2012). It is usually not considered as a character deserving genus recognition in the Gyalectaceae (e.g. Vězda (Reference Vězda1967) for Ramonia sect. Ramonia) or in the Stictidaceae (e.g. Baloch et al. (Reference Baloch, Gilenstam and Wedin2013a) for Sphaeropezia). The inclusion of the genera Pachyphiale and Cryptolechia in Gyalecta renders polyspory a character important only at the species level in the Gyalectaceae (Baloch et al. Reference Baloch, Lücking, Lumbsch and Wedin2010, Reference Baloch, Lumbsch, Lücking and Wedin2013b; Lücking et al. Reference Lücking, Moncada and Hawksworth2019). Thelopsis shares the polysporous asci characteristic with Cryptolechia and Pachyphiale but does not group with them in our phylogenetic analyses. It differs from Cryptolechia and Pachyphiale in having perithecioid ascomata with periphysoids and generally more spores per ascus (e.g. 100–150 in T. rubella). Regarding ascospore shape and size, a large variation is observed in Gyalecta, from small few-septate ellipsoid spores to long, many septate and needle-shaped or muriform spores.
Therefore, individual morphological characters might not appear to prevent the separation of Thelopsis from Gyalecta. Yet we refrain from merging Thelopsis with Gyalecta for several reasons. The genus is well recognized by the combination of morphological characters (see above) and a wider combination of morphological characters has proved useful in refining genera more accurately in other groups such as the Graphidaceae (e.g. Frisch et al. Reference Frisch, Kalb and Grube2006; Parnmen et al. Reference Parnmen, Cáceres, Lücking and Lumbsch2013). Furthermore, the three species of Thelopsis (viz. T. byssoidea, T. corticola and T. rubella) included in our phylogeny of Gyalecta s. lat. (Fig. 4) form a well-supported monophyletic lineage lower down the tree. Gyalecta friesii Flot. ex Körb. and G. ulmi, which form a lineage outside Gyalecta + Thelopsis, might be transferred to another genus. They differ from the other Gyalecta species included in the phylogeny by the larger ascomata with a distinctly constricted base and generally with a widely exposed hymenium at maturity, giving them some similarities with Lecanora species in the field. Moreover, in the framework of phylogenies of the Ostropales s. lat., the branch lengths also support the recognition of more than one genus within the broadly defined Gyalecta lineage, as can be seen for instance in Fig. 3 or in other published phylogenies (e.g. fig. 1 in Aptroot et al. (Reference Aptroot, Parnmen, Lücking, Baloch, Jungbluth, Cáceres and Lumbsch2014b)). Transferring Thelopsis to Gyalecta would also necessitate the introduction of new names for the well-established epithets of T. rubella and T. corticola because these epithets are already in use for other species in Gyalecta. This is a minor practical point but would not be welcomed by field lichenologists. For all these reasons, we see no gain in transferring Thelopsis species to Gyalecta. Instead, we suggest keeping Thelopsis as distinct pending further studies with a more exhaustive sampling of Gyalectaceae. The genera Myeloconis and Trichothelium are also maintained within a paraphyletic Porina for similar reasons.
Towards a refined generic concept of Gyalecta?
A wider combination of characters applied to a more exhaustive molecular analysis might lead to a revision of the generic classification in the Gyalectaceae, as pointed out by Lücking et al. (Reference Lücking, Moncada and Hawksworth2019) who have already listed some promising morphological features (e.g. the nature of the paraphyses). In this context, the sequencing of the genus Topelia is of great interest because of its supposed close relationship with Thelopsis (Vězda Reference Vězda1968; Jørgensen & Vězda Reference Jørgensen and Vězda1984). Topelia differs from Thelopsis by having eight muriform ascospores per ascus. However, Moon & Aptroot (Reference Moon and Aptroot2009) and Aptroot et al. (Reference Aptroot, Mendonça, Ferraro and Cáceres2014a) highlighted the existence of intermediate species between the genera Thelopsis and Topelia for the ascospore types: for example, Thelopsis muriformis Aptroot & K. H. Moon with truly muriform ascospores (Moon & Aptroot Reference Moon and Aptroot2009), and Thelopsis cruciata Aptroot & M. Cáceres with cruciate septate ascospores (Aptroot et al. Reference Aptroot, Mendonça, Ferraro and Cáceres2014a). They suggested that both genera are indistinguishable and should probably be merged.
Pachyphiale fagicola (Arnold) Zwackh is considered by Lücking et al. (Reference Lücking, Moncada and Hawksworth2019) as the most crucial taxon regarding the generic concept in Gyalectaceae because it forms the longest branch in the tree, has the most deviating feature in the family besides Belonia and would involve splitting Gyalecta s. lat. into six different genera if Pachyphiale is maintained. The placement of G. carneola as the sister species of G. fagicola, both forming a well-supported lineage, suggests that the genus Pachyphiale could be resurrected from the synonymy of Gyalecta if a refined generic concept of Gyalecta s. lat. is justified. In that case, several other generic names need to be considered and are available for all the lineages of Gyalecta s. lat. (Fig. 4). According to Lücking et al. (Reference Lücking, Moncada and Hawksworth2019: 292), the type species of Gyalecta is G. geoica, but a typification does not seem to have been published. The genus Gyalecta could thus possibly be restricted to the G. truncigena-G. geoica clade (Fig. 4). The genus Secoliga Norman (typification missing too?) appears to be available for the basal lineage formed by G. friesii and G. ulmi, Cryptolechia A. Massal. for its type G. carneolutea, Belonia Körb. for its type G. russula, and Clathroporinopsis M. Choisy (lectotype G. nidarosiensis fide Lücking et al. (Reference Lücking, Hodkinson and Leavitt2017)) and Protoschistes M. Choisy (lectotype G. herculana fide Lücking et al. (Reference Lücking, Hodkinson and Leavitt2017)) for the clade G. caudiospora-G.amsterdamensis (Fig. 4); a small number of other generic names are listed in MycoBank and need to be evaluated too, but this is beyond the scope of the present study.
Thelopsis byssoidea deviates from all known Thelopsis species by the distinct byssoid thallus (Fig. 2B–D), but our phylogenetic results confirm its placement within the Thelopsis lineage (Figs 3 & 4). The species is a nice example of parallel evolution of the byssoid thallus within genera known otherwise to have a more compact thallus, in addition to, for example, Crocynia in the Phyllopsora clade (Kistenich et al. Reference Kistenich, Timdal, Bendiksby and Ekman2018) and Sagenidium in the Roccellaceae (Ertz et al. Reference Ertz, Tehler, Irestedt, Frisch, Thor and van den Boom2015). Furthermore, Gyalecta amsterdamensis and Thelopsis corticola are the first examples of sorediate lichens confirmed in the Gyalecta s. lat. clade (Figs 3 & 4). It is evident that thallus morphology does not provide useful taxonomic information at the genus level in this group, at least for the byssoid and sorediate character states.
The genus Ramonia and polyphyly of Thelopsis
The placement of Thelopsis melathelia as sister species to Ramonia valenzueliana (Fig. 3) is interesting for our understanding of character evolution in the Gyalectaceae. Vězda (Reference Vězda1966) emended Ramonia but recognized three groups within his enlarged concept of the genus. He admitted that these groups could be recognized as distinct genera because of a combination of important morphological differences. Therefore, the genus Ramonia appears clearly heterogeneous. The type species of Ramonia, R. valenzueliana, shares several important morphological similarities with Thelopsis, such as the ascomatal anatomy including the presence of periphysoids and multispored asci containing small ellipsoid ascospores, and Vězda (Reference Vězda1968) has already suggested a close relationship between the genera. Ramonia valenzueliana differs from Thelopsis mainly by the type of ascomata that slightly widen in a late stage, while in Thelopsis the ascomata remain closed (Vězda Reference Vězda1968). However, the degree of opening of the ascomata is variable within genera of Gyalectaceae, as illustrated for example by species of Gyalecta with perithecioid ascomata (G. herculina and G. nidarosiensis; Jørgensen et al. Reference Jørgensen, Vězda and Botnen1983) that cluster with other Gyalecta species having a narrow ascomatal opening (e.g. G. farlowii, G. herrei, G. hypoleuca and G. thelotremella) (Fig. 4). The placement of Thelopsis melathelia as sister species to Ramonia valenzueliana suggests that other phenotypic characters might be used to predict phylogenetic relationships, such as the wrinkled ascomatal surface (smooth in Thelopsis s. str.), a darker excipulum all around the ascomata and ascospores with a thick gelatinous sheath. The wrinkled ascomatal surface, dark excipulum and shape of ascospores also fits with Ramonia s. str. (= section Ramonia sensu Vězda (Reference Vězda1966)), which led us to combine Thelopsis melathelia in Ramonia (see Taxonomy section). In this context, further molecular data are needed to investigate whether these morphological characters might predict closer affinities of other species of Thelopsis with Ramonia. Thelopsis lojkana Nyl. and Topelia heterospora (Zahlbr.) P. M. Jørg. & Vězda are two species that deviate from the core of their genus in having distinctly halonate ascospores. Further studies might prove Thelopsis to be more heterogeneous: T. flaveola Arnold deviates by its simple ascospores and T. isiaca by perithecia remaining entirely immersed in prominent thalline warts. Ramonia also needs to be investigated further, in particular regarding the three sections distinguished by Vězda (Reference Vězda1966).
The genus Petractis
In his revision of Petractis, Vězda (Reference Vězda1965) accepted five species, four of which he newly transferred from Gyalecta because these taxa share the same structure and ontogeny of ascomata. However, as stated by Orange (2009), the distinction of the genus from other genera of gyalectoid lichens is unclear at present owing to uncertainties in the circumscription of the genus. The clade from G. hypoleuca to G. amsterdamensis (Fig. 4) includes three members that were treated as Petractis species by Vězda (Reference Vězda1965): P. hypoleuca (Ach.) Vězda and P. thelotremella (Bagl.) Vězda were shown to be phylogenetically related to Gyalecta by Kauff & Lutzoni (Reference Kauff and Lutzoni2002), while our study shows that P. farlowii (Tuck.) Vězda also belongs here (Fig. 4). These three species share with Gyalecta the non-halonate ascospores and the trentepohlioid photobiont. The type species of Petractis (P. clausa) is not phylogenetically related to these Gyalecta species (Fig. 3). It differs morphologically by having a cyanobacterium (Scytonema) as photobiont, a fully endolithic thallus (vs ‘pseudoepilithic’ in the other species treated by Vězda (Reference Vězda1965)), ascospores having a distinct gelatinous sheath and by a more fissured apothecial margin. It is therefore surprising that Vězda (Reference Vězda1965) enlarged the concept of Petractis by transferring species from Gyalecta. However, he distinguished two groups within Petractis: 1) P. clausa and P. luetkemuelleri, with a similar ascomatal type (= in young stage, always covered by a radially fissured thallus) and ascospores having a notably thick (2–4 μm) gelatinous sheath; 2) P. farlowii, P. hypoleuca and P. thelotremella where a fissured ascomatal thallus cover is only occasionally observed and this only in specimens having a thin epilithic thallus with more protruding ascomata, and the ascospores lacking a gelatinous sheath. The separation of these two groups is now supported by phylogenetic results. However, the first group has not been recovered as monophyletic because P. luetkemuelleri did not cluster with P. clausa in various phylogenetic studies (e.g. Kauff & Lutzoni Reference Kauff and Lutzoni2002; Orange 2009; Miadlikowska et al. Reference Miadlikowska, Kauff, Högnabba, Oliver, Molnár, Fraker, Gaya, Hafellner, Hofstetter and Gueidan2014; this study, Fig. 3). Both species differ, however, in their photobionts and the endolithic (P. clausa) versus epilithic or ‘pseudoepilithic’ (P. luetkemuelleri) thallus. As already shown by Orange (2009), Petractis nodispora is the sister species of P. luetkemuelleri (Fig. 3). In our phylogenetic tree, these two Petractis species cluster in a strongly supported lineage close to the genus Ramonia and are distantly related to Petractis clausa. Ramonia differs from Petractis notably in having periphysoids and polysporous asci. Since Petractis luetkemuelleri and P. nodispora differ morphologically and phylogenetically from P. clausa and Ramonia, they are transferred to the new genus Neopetractis (see Taxonomy section).
Conclusion
Our phylogenetic results shed light on the taxonomic significance of some morphological features in the family Gyalectaceae (e.g. degree of opening of the ascomata, carbonization of ascomatal wall, byssoid/sorediate thallus, multispory, periphysoids, gelatinous sheath around the ascospores) and the placement of Thelopsis in Gyalecta challenges the generic circumscription in this genus. Fieldwork and sequencing also revealed a hidden diversity for the group among sterile sorediate specimens, resulting in the discovery of two new taxa: Francisrosea bicolor and Gyalecta amsterdamensis. Much remains to be done to improve our understanding of evolution within the Gyalectaceae and relatives, since the molecular data available at present are still limited.
Acknowledgements
We wish to warmly thank Lynn Delgat and Wim Baert (Meise Botanic Garden) for their help with the molecular work. We are grateful to Cyrille Gerstmans for his help with the figures. Fieldwork by DE and ML on the islands of Amsterdam and Saint-Paul was organised as part of the 1167 BIODIV_AMS programme supported by the French Polar Institute (IPEV). Finally, we thank Toby Spribille and the referees for their critical and helpful comments and suggestions.
Author ORCIDs
Damien Ertz, 0000-0001-8746-3187; Neil Sanderson, 0000-0002-3719-3104; Marc Lebouvier, 0000-0002-0852-789X.










