Introduction
Usnea is a hyperdiverse lichen-forming fungal genus, with more than 350 species distributed worldwide, that forms a strongly supported monophyletic lineage within the Parmeliaceae (Crespo et al. Reference Crespo, Lumbsch, Mattsson, Blanco, Divakar, Articus, Wiklund, Bawingan and Wedin2007; Divakar et al. Reference Divakar, Crespo, Wedin, Leavitt, Hawksworth, Myllys, McCune, Randlane, Bjerke and Ohmura2015). The combination of traditional characters (e.g. the shape of the branches, thickness of the cortex, medulla and central axis, presence/absence of pigments, chemistry) used in earlier taxonomic studies of the genus (Clerc Reference Clerc1998; Ohmura Reference Ohmura2001) proved to be a good predictor of species delimitation (Kelly et al. Reference Kelly, Hollingsworth, Coppins, Ellis, Harrold, Tosh and Yahr2011; Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ). However, due to the presence of homoplasious features, species with a similar morphology, anatomy or chemistry might not be closely related (Truong & Clerc Reference Truong and Clerc2016) and so traditional taxonomy seems to be unsuccessful in indicating species relationships within the genus. Thus, integrative taxonomy will prove to be a very important approach to circumscribe species and understand their relationships, helping to uncover the still poorly known diversity in tropical areas.
Recent investigations of Usnea in South America indicate that species diversity is high. New species have been described in several groups, as for instance in the saxicolous species (Rodriguez et al. Reference Rodriguez, Estrabou, Truong and Clerc2011), the pigmented species (Truong et al. Reference Truong, Bungartz and Clerc2011; Truong & Clerc Reference Truong and Clerc2012), the pendulous species (Truong et al. 2013 Reference Truong, Rodriguez and Clercb ), the eumitrioid species (with hollow axis) (Truong & Clerc Reference Truong and Clerc2013) and the shrubby sorediate species (Truong & Clerc Reference Truong and Clerc2016).
The species investigated herein share a short shrubby-erect thallus (i.e. the branches remain erect and divergent to the apices), usually numerous apothecia and an absence of vegetative propagules. Some of the species can occasionally exhibit a subpendulous thallus, especially under optimal conditions of humidity (Truong et al. 2013 Reference Truong, Rodriguez and Clercb ). They mostly grow on a variety of corticolous substrata (bark and the twigs of trees or bushes) or on fence posts and occasionally on rocks.
In the sexually reproducing Usnea species it is striking to see that, with a few exceptions such as the European-North American species of U. florida (L.) F. H. Wigg. and U. intermedia (A. Massal.) Jatta (Clerc 1984 Reference Clerca ; Halonen et al. Reference Halonen, Clerc, Goward, Brodo and Wulff1998), they tend to have a restricted geographical distribution range with most species occurring in subtropical and tropical areas (Swinscow & Krog Reference Swinscow and Krog1979; Awasthi Reference Awasthi1986; Ohmura Reference Ohmura2001; Stevens Reference Stevens2004; Clerc Reference Clerc2007). This observation agrees with the hypothesis that sorediate species have broader distribution ranges than most of the esorediate species (Hale Reference Hale1983; Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998). With more than 30 species recorded so far (Motyka Reference Motyka1936, Reference Motyka1938), South America holds the highest diversity of esorediate species. Despite this diversity there is no modern revision for this group within the Neotropics.
The aim of the present study, based on an integrative taxonomic approach and including morphological, anatomical, chemical, and ecological features, as well as molecular data, is to provide information on the 17 shrubby, esorediate species recognized from southern Brazil. It is the first step towards the taxonomic revision of the whole genus in Brazil.
Materials and Methods
Morphological, anatomical and chemical studies
The following account is based on field studies and on herbarium specimens deposited in the following herbaria: BHCB, BM, CESJ, CGMS, DUKE, FH, FI, G, H, HAS, ICN, JPB, LBL, M, MBM, PC, RB, S, SP, TUR, UFP, UPCB, W, WU and Z. Type material of all species discussed in this paper was studied. All voucher specimens collected during field trips are deposited in the Federal University of Rio Grande do Sul (ICN) and some duplicates in G. The morphology of specimens was examined using a Leica MS5 stereomicroscope, with measurements taken using a Leica DM2000 microscope. The species concept used in this study follows Clerc (Reference Clerc1998).
Density of fibrils is given as the number of fibrils mm–2 on a branch where the density was estimated to be the highest. For each specimen, three measurements were made. Microscopic examination of spores was carried out with a Leica DM2000 microscope at high magnification (×1000). The length and width of 10–30 mature ascospores per specimen were measured. Measurements for ascospores are given as mean
![]() $\left( {\bar{x}} \right)$
±1SD with extremes in parentheses. Normality of the data was tested with Shapiro tests in the software R 3.2.4 (R Development Core Team 2016) at the species level. To take into account non-normal distributions, Mann-Whitney-Wilcoxon with the Benjamini-Yekutieli correction (Benjamini & Yekutieli Reference Benjamini and Yekutieli2001) for non-parametric variables was carried out on groups of two species. Anatomical measurements of cortex, medulla and central axis were carried out in longitudinal sections of branches at ×40 magnification. The percentage thickness of cortex/medulla/axis of the total branch diameter (CMA) and the ratio of axis/medulla (A/M) of all the cited specimens were calculated according to Clerc (Reference Clerc1984
a, Reference Clerc1987). Measurements for CMA values are given as the mean
$\left( {\bar{x}} \right)$
±1SD with extremes in parentheses. Normality of the data was tested with Shapiro tests in the software R 3.2.4 (R Development Core Team 2016) at the species level. To take into account non-normal distributions, Mann-Whitney-Wilcoxon with the Benjamini-Yekutieli correction (Benjamini & Yekutieli Reference Benjamini and Yekutieli2001) for non-parametric variables was carried out on groups of two species. Anatomical measurements of cortex, medulla and central axis were carried out in longitudinal sections of branches at ×40 magnification. The percentage thickness of cortex/medulla/axis of the total branch diameter (CMA) and the ratio of axis/medulla (A/M) of all the cited specimens were calculated according to Clerc (Reference Clerc1984
a, Reference Clerc1987). Measurements for CMA values are given as the mean
![]() $\left( {\bar{x}} \right)$
±1SD with extremes in parentheses.and follow the categories described by Clerc (2011
Reference Clercb
).
$\left( {\bar{x}} \right)$
±1SD with extremes in parentheses.and follow the categories described by Clerc (2011
Reference Clercb
).
Analyses of the anatomical structure of the cortex were made according to Ohmura (Reference Ohmura2001), on thin hand-cut sections and observed at ×400 magnification with a Leica DM2000 microscope.
Chemical analyses were performed on all cited specimens by thin-layer chromatography (TLC) following Culberson & Ammann (Reference Culberson and Ammann1979), with solvent B modified according to Culberson & Johnson (Reference Culberson and Johnson1982). K, C and P spot tests, according to Hale (Reference Hale1979), were directly applied to the medulla in longitudinal sections of the branches.
Fieldwork was carried out between January 2013 and December 2014 in the states of Paraná (PR), Santa Catarina (SC) and Rio Grande do Sul (RS), between 22°30'–33°45'S and 48°02'–57°40'W. Approximately 800 specimens were collected. Southern Brazil comprises 573·41 km2 and the climate is humid subtropical with hot to temperate summers (Alvares et al. Reference Alvares, Stape, Sentelhas, de Moraes Gonçalves and Sparovek2013). Field trips were conducted in the Atlantic Forest and in the Pampa (also known as the Southern grasslands), the two main biomes of the southern Brazilian region (IBGE 2004). A relict of the Cerrado occurs in northern Paraná (2% of the area) and unfortunately this small area was not visited, but a few herbarium specimens previously collected in this biome were studied. A small number of specimens originating from other biomes in Brazil, such as the Caatinga, were also studied for comparative purposes. The Atlantic Forest is the second largest rainforest biome of South America, and corresponds to a complex mosaic of different vegetation types (see details in Iganci et al. Reference Iganci, Heiden, Miotto and Pennington2011; Oliveira-Filho et al. Reference Oliveira-Filho, Budke, Jarenkow, Eisenlohr and Neves2015). The following types of vegetation were visited: dense rainforest (including several hills and mountains up to 1887 m a.s.l. of the Serra do Mar), Araucaria forest (predominantly with Araucaria angustifolia (Bertol.) Kuntze), the high-altitude grasslands (also known as campos de altitude) and coastal areas known as restingas that are formed of sandstone. Localities of subtropical seasonal forests were also explored. The Pampa occurs in the southern half of Rio Grande do Sul and is a non-forest vegetation type, dominated by herbaceous, shrubby and treelet plants (Overbeck et al. Reference Overbeck, Müller, Fidelis, Pfadenhauer, Pillar, Blanco, Boldrini, Both and Forneck2007). In addition, urban parks and rural areas such as pastures with forest relicts, roadsides and deforested zones were visited. At least one specimen per locality is included in the list of selected specimens and the states are mentioned according to geographical order, from south to north and from east to west.
Phylogenetic analysis
DNA extraction, PCR amplification and sequencing
DNA was extracted following Truong et al. (2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) using the DNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions, with small modifications as in Crespo et al. (Reference Crespo, Blanco and Hawksworth2001). PCR amplifications of the ITS rDNA and fragments of the RPB1 and Mcm7 genes were performed. The following primers were used: USITS3-F and USITS4-R (Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) and four newly developed primers in this study: UsRPB1-R (5'-ACG GAT AAT ATC GCC AAG CT-3'), UsRPB1-F (5'-TGG AAA CAG TCT GCC ACA AC5-3'), UsMCM7-R (5'-TGC CCG TAT ATT TCT GGA GCG A-3') and UsMCM7-F (5'-ACA CCT GTG ATC GAT GTG GA-3'). For ITS, PCR reactions were performed with 5 μl of total genomic DNA, 2·5 μl ×10 buffer with 2 µM MgCl2, 0·5 μl dNTPs (10µM of each base), 1·25 μl of each primer at 10 μM, 0·625 μl of DNA polymerase (1Uμl−1) and sterile water to complete a reaction mixture of 25 μl. Thermal cycling parameters were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 54 °C for 1 min, 72 °C for 1·5 min and final elongation at 72 °C for 10 min. For Mcm7 and RPB1, PCR amplifications were conducted with the same proportions as with ITS, except increasing the concentration of the primers to 3 µl. For both genes, the thermal cycling parameters were as follows: initial denaturation at 94 °C for 10 min, followed by 6 cycles of 94 °C for 0·5 min, 56 °C for 0·5 min and 72 °C for 1 min, 30 cycles of 94 °C for 0·5 min, 52 °C for 0·5 min and 72 °C for 1 min, and a final elongation at 72 °C for 10 min. If the amplification failed, the PCR was repeated using PuReTaq Ready-To-Go PCR Beads (2·5 U of PuReTaqDNA Polymerase, 200 μM of each of dNTP, BSA, the buffers and stabilizers 10mM Tris-HCl pH 9·0, 50 mM KCl, 1·5 µM MgCl2; GE Healthcare, Little Chalfont, UK) adding to the lyophilized bead 1·5 μl of each primer at 10 μM, and increasing the DNA template to 7 μl when PCR products were too weak or absent, made up to 25µm with sterile water. Amplification products were viewed on a 1% agarose gel stained with SYBER, and purification was performed by adding 2 µl of illustraTM ExoProStar (GE Healthcare, Little Chalfont, UK) to 10 µl of PCR product, followed by a heat treatment of 15 min at 37 °C and 15 min at 80 °C. Sequencing was carried out with the same primers as for the PCR amplifications, using the sequencing kits ABI Prism™ Dye Terminator Cycle Sequencing Ready or BigDye™ (Applied Biosystems, Foster City, California, USA). Sequencing reactions underwent electrophoresis on a 3730 DNA Analyzer (Applied Biosystems) at the Unidad de Genómica (Parque Científico de Madrid).
Sequence alignment and phylogenetic reconstructions
The DNA sequences obtained were manually adjusted using SeqMan version 7.0 (DNAstar, Madison, WI, USA) and subjected to BLAST queries for an initial verification of their identities. To build the data matrix we chose specimens represented by more than one of the markers considered here (25 specimens), selecting a set of species from each clade determined by Truong et al. (2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) except clade 1. Then we added 30 specimens analyzed in this study representing 11 Usnea species from the group examined here. We were unable to obtain sequences from U. concinna, U. cristatula, U. kalbiana, U. lunaria, U. cf. moreliana and U. subelegans. Usnea densirostra Taylor, a saxicolous shrubby esorediate species from Brazil, and U. ghattensis G. Awasthi, a corticolous esorediate species from India with large spores, were included. The data matrix (Table 1) contains 55 specimens representing 25 Usnea species.
Table 1 Voucher infomation, major chemotypes and GenBank Accession numbers for the Usnea species referred to in this study. Newly described species and newly generated sequences are in bold

Key to Brazilian states: SC: Santa Catarina; MS: Mato Grosso do Sul; PR: Paraná, RS: Rio Grande do Sul.
Alignments for each locus were performed using MAFFT version 7 (Katoh & Standley Reference Katoh and Standley2013) with the G-INS-I alignment algorithm, a scoring matrix of 20 PAM/k=2, 0·1 as offset value and the remaining parameters set as default. The program Gblocks v0.91b (Talavera & Castresana Reference Talavera and Castresana2007) was used to delimit and remove ambiguous alignment nucleotide positions using the online web server (http://molevol.cmima.csic.es/castresana/Gblocks_server.html) and implementing the options for a less stringent selection of ambiguous nucleotide positions including the ‘Allow smaller final blocks’, ‘Allow gap positions within the final blocks’, and ‘Allow less strict flanking positions’ options. The alignments of each region and the concatenated one were analyzed using maximum likelihood (ML) and Bayesian (B/MCMC) approaches, with Usnea aurantiaco-atra (Neuropogon group) as outgroup to root the tree. Exploratory phylogenetic analyses of individual gene topologies showed no evidence of well-supported (≥70% bootstrap values) topological conflict and relationships were estimated from the concatenated, three-locus data matrices using a total-evidence approach (Wiens Reference Wiens1998; Divakar et al. Reference Divakar, Crespo, Wedin, Leavitt, Hawksworth, Myllys, McCune, Randlane, Bjerke and Ohmura2015). For the Bayesian analysis, MrBayes v3.2.1 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) was used. All loci were treated as separate partitions and for the protein-coding marker we used a three-partition approach using the first, second, and third codon positions as separate model partitions for the concatenated dataset. Models of DNA sequence evolution for each locus were selected with the program jModeltest2.0 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012), using the Akaike information criterion (Akaike Reference Akaike1974). The best-fit model of evolution was as follows: GTR+G for the ITS and RPB1 partitions and K80+G for the Mcm7 partition. We conducted two independent runs of 3 million generations, starting from a random tree and employing 12 simultaneous chains each, in which one in every 200 trees was sampled. Convergence among runs was visualized in Tracer v.1.5 (Rambaut & Drummond Reference Rambaut and Drummond2007) by plotting log likelihood per generation for each run and identifying the effective sample size (ESS>200). The 50% majority-rule consensus tree was constructed by pooling trees sampled from all runs and after discarding the first 25% as burn-in, with posterior probabilities (PP) as branch support. For maximum likelihood (ML) tree reconstruction, the program RAxML v7.2.8 (Stamatakis Reference Stamatakis2006) implemented in the Cipres Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) was used, with the GTRGAMMA model. The concatenated three-loci dataset was partitioned as described in the Bayesian analysis. Support values were assessed using the ‘rapid bootstrapping’ option with 1000 replicates. The final phylogenetic tree was drawn using the program FigTree v1.4 (Rambaut Reference Rambaut2009).
Results and Discussion
Morphology-anatomy
The habit of shrubby esorediate species depends mainly on characters that display broad phenotypic variability. This is the case for the density of ramification, the ramification type, the number of apothecia, the size of the apothecia, and the colour of the thallus. As a consequence, individuals within the same species might sometimes look very different in aspect. Clerc (Reference Clerc1998), Herrera-Campos et al. (Reference Herrera-Campos, Clerc and Nash1998), Ohmura (Reference Ohmura2001) and Truong et al. (Reference Truong, Bungartz and Clerc2011) discussed the characters that are diagnostic in delimiting Usnea species. Some important characters that were found here to be useful for delimiting shrubby apotheciate species are discussed below.
Fibrils
The shape, density and arrangement of these short branch-like appendages with a central axis that is not attached to the central axis of the mother branch (Clerc Reference Clerc1998) were found to be important in the systematics of this group of Usnea species. We define fibrils here as being spinulose when they are 2–5× taller than wide (Figs 3B, 6F, 9A & B), and slender when they are 6–15× longer than wide (Fig. 5F). Usnea aurantiaca-parvula, U. parvula, U. subelegans and U. subparvula are characterized by the presence of a majority of spinulose fibrils. Lageniform spinulose fibrils (swollen at the base, narrowed at the top) (Fig. 3C) are a special feature of U. aurantiaca-parvula. Fibril-like structures growing on the margin of apothecia usually share the same morphology as fibrils growing on branches.
Cortex and CMA values
From a morphological point of view, on a longitudinal section, the cortex can be matt, shiny or vitreous like broken glass. Anatomical studies of the cortical tissue have been carried out by Awasthi (Reference Awasthi1986) and Ohmura (Reference Ohmura2001). These authors described different types of plectenchyma that were, however, rarely used as diagnostic characters to separate the species. Some of these types appear to us to be variable and we believe that further studies are necessary to establish their exact taxonomic value. Differences in the relative thickness of cortex, medulla and axis (%CMA) proved to be diagnostic characters in this group. Truong et al. (Reference Truong, Bungartz and Clerc2011) defined a CMA of the cornuta-type with a thin (5–8%) shiny cortex in cross-section, a moderately thick to thick medulla (28–36%), a thin axis (18–32%) and low A/M (0·5–1·3). We define here a brasiliensis-type CMA with a thinner shiny cortex (2–5%), a thicker medulla (35–45%), a much thinner axis (7–14%) and a very low A/M (0·2–0·4).
Apothecia and ascospores
Disposition of the apothecia on the branches was described by Herrera-Campos et al. (Reference Herrera-Campos, Clerc and Nash1998). This seems, however, to be a very variable character and only Usnea subelegans has a majority of lateral apothecia among the specimens studied. Apothecia might be scarce or even absent, and then pycnidia are usually present as small nodules on terminal branches. The two following characters are variable and thus not considered diagnostic for the Brazilian taxa: the shape of apothecia that varies from flat to mostly cup-shaped and the appearance of the disc which is usually pruinose and whitish, sometimes brownish when the pruina is absent. The density of marginal fibrils is variable (1–3 fibrils mm–1) in all species except in U. aurantiaca-parvula which has 8–12 fibrils mm–1. Ascospores are simple, ellipsoid to broadly ellipsoid, and hyaline. The size of ascospore in the genus Usnea has traditionally received little attention, as is the case for most of the Parmeliaceae (reviewed by Crespo et al. Reference Crespo, Divakar and Hawksworth2011). However, Clerc (1984 Reference Clerca ) found small but significant differences in the spore size of U. florida and U. intermedia, two European apotheciate taxa. Tavares & Sanders (Reference Tavares and Sanders1998) separated U. florida from other taxa mainly on the basis of spore size. Kirika et al. (Reference Kirika, Divakar, Crespo, Mugambi, Orock, Leavitt, Gatheri and Lumbsch2016) also found the spore size to be an important character for delimiting species in the genus Parmelinella Elix & Hale. Likewise, among the species studied here we found two species with distinctly larger spores: U. fleigiae and U. grandispora. In accordance with this we propose two classes of ascospore length: class I (spores<13 µm) and class II (spores≥13 µm) (Fig. 1). The depth of the hymenium seems to be proportional to the depth of the spores: 80–100 µm in U. fleigiae and U. grandispora, and 40–85 µm in all other species.

Fig. 1 Boxplots of spore length for each species of Usnea referred to in this study. Each boxplot shows the median (thick line) and standard deviation, and box width is proportional to the value of n. Dashed vertical lines correspond to the range. Outliers are represented by open circles.
Chemistry
Table 2 shows the main secondary metabolites for the 17 species treated in this study. All Usnea species contain usnic acid in the cortex. When correlated with other morphological or anatomical characters, secondary metabolites present in the medulla in Usnea have a strong taxonomic value (Clerc Reference Clerc1998). Variations in secondary metabolites without correlation with other characters are considered as chemotypes of the same species. Most of the species of the group studied here have two chemotypes. Three species (U. cladocarpa, U. kalbiana and U. lunaria) have only one chemotype and one species, U. erinacea, has five chemotypes. As already stated by Truong et al. (Reference Truong, Bungartz and Clerc2011, Reference Truong, Rodriguez and Clerc2013b ), the presence of triterpenoids is relatively common in Usnea in the neotropical region. For example, we found the same unidentified triterpenoids, UT6, referred to by Truong et al. (Reference Truong, Bungartz and Clerc2011) in U. erinacea and U. steineri. Barbatolic and alectorialic acids were found only in the apothecia of a small number of specimens of U. meridionalis. Unknown substances are relatively common in Usnea from Brazil. Some of them seem to be of special taxonomic importance: 1) an unknown yellow spot (Rf classes A/B/C: 6/1–2/5) found in U. parvula (Us1 in Table 2) and 2) an unknown substance with a blue-green fluorescence after charring (Rf class A: 4–5, B: 5–6) (Us2 in Table 2). Cortical, subcortical and medullary pigmentation is a significant character in the taxonomy of Usnea (Swinscow & Krog Reference Swinscow and Krog1979; Clerc Reference Clerc1984b , Reference Clerc2007; Ohmura Reference Ohmura2001, Reference Ohmura2012; Truong et al. Reference Truong, Bungartz and Clerc2011; Truong & Clerc Reference Truong and Clerc2012). It was observed in seven esorediate species from South America: U. aurantiaca-parvula, U. cristatula, U. erinacea, U. meridionalis, U. cf. moreliana, U. steineri and Usnea sp. 1.
Table 2 Major secondary metabolites and chemotypes of Brazilian Usnea species

Key to secondary metabolites: SAL=salazinic acid, STI=stictic acid, CST=constictic acid, CRY=cryptostictic acid, ME=menegazziaic acid, NOR=norstictic acid, GAL=galbinic acid, DIF=diffractaic acid, BAR=barbatic acid, PRO=protocetraric acid, FUM=fumarprotocetraric acid, PSO=psoromic acid, CAP=caperatic acid, TER=unidentified tri-terpenoids, FA=unidentified fatty acid, EU=eumitrin, Us1=unknown with yellow spot (Rf classes A/B/C=6/1-2/5), Us2=unknown with blue (Rf class A=4–5) and green (Rf class B=5–6), fluorescence after charring, Ch0=usnic acid alone; n=number of specimens studied; +=presence constant within species (highlighted in grey); ±=presence variable among specimens within species; tr=present in traces; rare=only in one/two specimens. Key to medulla colour test: y→r=yellow turning red; br. Y=bright yellow; y sl.→r=yellow slowly turning red; sl. dull y=slowly dull yellow; y=yellow; or.=orange. n=number of specimens examined for that chemotype
Phylogenetic studies
In the present study, we generated a total of 68 new sequences, including 29 nuclear ITS, 16 RPB1 and 23 Mcm7 from 20 samples of Usnea from Brazil, two from Costa Rica and one from India (Table 1). These were deposited in GenBank under Accession numbers KY021902–KY021930 and KY204412–KY204449. The ITS PCR product obtained ranged between 600 and 800 base pairs (bp). Differences in size were due to the presence or absence of insertions of c. 200 bp identified as group I introns (Ohmura Reference Ohmura2002; Gutierrez et al. Reference Gutierrez, Blanco, Divakar, Lumbsch and Crespo2007) at the 3' end of the SSU rDNA. Testing for topological incongruence showed no supported conflicts (results not shown here). The partitioned ML analysis of the concatenated data matrix yielded the optimal tree with Ln likelihood value=−5630·32. The effective sample sizes (ESS) of all estimated parameters were well above 200 in the Bayesian analysis, indicating that convergence among parallel runs was reached. The best ML tree inferred from the multi-locus dataset is illustrated in Fig. 2. It contains 26 highly supported nodes (bootstrap support BS≥70). The B/MCMC majority-rule consensus tree (LnL=−5713·45) with 30 highly supported nodes (PP≥0·95) was almost identical to the ML tree, except for the low resolution of some of its internal nodes. Therefore, only the ML tree is shown here with posterior probabilities added adjacent to BS values.

Fig. 2 Phylogenetic relationships among corticolous, shrubby and esorediate species of Usnea in Brazil based on maximun likelihood (ML) inference from the multi-locus dataset of ITS rDNA, Mcm7 and RPB1 gene markers. Bootstrap support (BS) followed by posterior probability (PP) from the Bayesian (B/MCMC) 50% majority-rule consensus tree are reported above branches. Thick branches indicate high support (black branches=BS≥70 and PP≥0·95; black grading into white branches=BS≥70 or PP≥0·95). Key to chemotypes: CAP=caperatic acid, NOR=norstictic acid, PRO=protocetraric acid, PSO=psoromic acid, SAL=salazinic acid, STI=stictic acid, TER=unidentified triterpenoid, THA=thamnolic acid. Newly described species are in bold. Neuropogon clade was used as outgroup.
Within the Usnea clade, four highly supported clades were recovered, named hereafter as Usnea 1 (Usnea-2 in Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ), Usnea 2 (Usnea-3 in Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ), Usnea 3 (Usnea-3 in Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) and Usnea 4 (Usnea-4 in Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) (Fig. 2), with a low degree of geographical structures. This is consistent with the results reported in Truong et al. (2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ). However, clade Usnea-3 of Truong et al. (2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) splits here into two clades (Usnea 2 and Usnea 3). The relationships among these clades remain unresolved. Specimens from Brazil included in this study were clustered in the clades Usnea 2, Usnea 3 and Usnea 4 respectively. While most of the traditionally circumscribed species in Usnea s. str. (Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca , Fig. 3) sampled for this study were found to be monophyletic, a few did not form monophyletic groups. This is not surprising as species-level polyphylies are commonly found in Parmeliaceae and in lichenized fungi in general (reviewed in Crespo & Lumbsch Reference Crespo and Lumbsch2010; Crespo et al. Reference Crespo, Divakar and Hawksworth2011; Lumbsch & Leavitt Reference Lumbsch and Leavitt2011). In the present study, U. cirrosa is shown to be polyphyletic for the first time. The clade Usnea 1 is formed by the species-pair U. florida-U. subfloridana from Europe clustered together with U. subrubicunda, a North American species. Clade 2 is composed only of neotropical species (U. cornuta s. lat., U. subglabrata) and included samples grouped in two strongly supported monophyletic clades referred to as U. fleigiae and U. grandispora. Samples clustered in the U. grandispora clade are morphologically similar to U. florida whereas U. florida belongs to the clade Usnea 1 (Fig. 2). Despite the morphological similarities found between U. grandispora and U. florida, our results clearly show that these are phylogenetically only distantly related. Corroborating morphological and molecular data, the clades U. fleigiae and U. grandispora are described below as two new species, respectively.
The clade Usnea 3 is composed of the European specimens U. glabrata and U. flavocardia, together with the Brazilian specimens of U. meridionalis and an undescribed species Usnea sp. 1, which corresponds to the possible fertile counterpart of U. flavocardia (see comments under Usnea sp. 1). Recent phylogenetic studies show that species differing only in the presence or absence of soralia (defined as “species-pairs” by Poelt Reference Poelt1970, Reference Poelt1972) usually correspond to the same lineage (Articus et al. Reference Articus, Mattsson, Tibell, Grube and Wedin2002; Truong & Clerc Reference Truong and Clerc2016). However, the opposite can also occur, as for example in the genera Letharia (Th. Fr.) Zahlbr. (Kroken & Taylor Reference Kroken and Taylor2001) and Heterodermia Trevis. (Lücking et al. Reference Lücking, Del-Prado, Lumbsch, Will-Wolf, Aptroot, Sipman, Umaña and Chaves2008). For instance, our results show that the apparent species-pair U. meridionalis and U. flavocardia (Truong et al. Reference Truong, Bungartz and Clerc2011) might belong to different lineages. In our study, U. meridionalis forms a well-supported sister group relationship with U. glabrata while U. flavocardia is grouped with Usnea sp. 1. Our results suggest that assumed species-pairs should be treated and tested individually. Furthermore, Truong & Clerc (Reference Truong and Clerc2016) stated that the evolutionary significance of reproductive traits should be corroborated with molecular data for each particular case before making any taxonomic conclusions.
Usnea clade 4 includes several species with a wide distributional range and two newly recovered clades referred to as U. subparvula and U. aurantiaca-parvula, related to U. parvula. Particular morphological and ecological features show that these two clades correspond to as yet undescribed taxa. Both U. cladocarpa and U. steineri appear monophyletic. The position of U. erinacea s. lat. in our phylogeny is unresolved but a previous phylogeny of the genus Usnea (Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ) clearly showed that this species is polyphyletic.
Usnea cirrosa appears to be paraphyletic (clade Usnea 4, Fig. 2). Our results indicate that these ‘morpho species’ include more than one undescribed taxon. Despite our intensive taxonomic analyses we were unable to draw any conclusions about them at this time. Species with a highly variable morphology, several chemotypes and/or a wide distributional range might include more than one taxon (as is the case for U. cornuta and U. erinacea, see Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca ). The use of molecular tools combined with a broader sampling over the whole geographical range of the species, in parallel with traditional methods, will facilitate the re-evaluation of phenotypic characters and the understanding of species boundaries in these groups.
Taxonomy
Usnea aurantiaca-parvula A. Gerlach & P. Clerc sp. nov.
MycoBank No.: MB 819420
Similar to U. parvula but differs by its smaller size, orange subcortical pigment that often spreads into the whole medulla, strongly irregular branches with sometimes±alate segments, numerous minute foveolae and±lageniform, simple to furcate, spinulose fibrils, and a compact medulla.
Type: Brazil, Pernambuco, Buíque, Serra do Catimbau, corticolous, 1970, L. Xavier Filho s. n. (JPB—holotype; ICN, G—isotypes). %C/M/A: 13.5/13.5/46. Ascospores: 8–9–10×5·0–5·5–6·0(–7·0) µm (n = 21). Chemistry: an unknown substance with a blue (Rf class A: 4–5) and a green (Rf class B: 5–6) fluorescence after charring.

Fig. 3 Usnea aurantiaca-parvula. A–C, holotype: A, thallus; B, irregular branches with lageniform fibrils; C, simple lageniform fibrils constricted at the base (arrows). D, several minute foveolae (arrows) (L. Krieger & M. Brügger 1407b); E, Section through thallus with strong orange pigmentation occurring in patches in medulla at arrows (M. Muryel s. n.); F, furcate lageniform fibrils (M. Muryel s. n.). Scales: A=1 cm; B=1 mm; C=200 µm; D=2 mm; E & F=500 µm. In colour online.
Thallus (n=10) erect-shrubby, yellow-green, small, up to 3 cm long, with isotomic-dichotomous ramifications; trunk often very short, concolorous with branches, not annulated; main branches 0·7–1·1 mm thick, irregular, distinctly segmented, with acute-angled to almost alate segments in cross-section, sometimes deformed by the presence of deep foveolae; lateral branches constricted or not at ramification point; foveolae usually numerous on the whole thallus; maculae, pseudocyphellae, papillae and tubercles absent; fibrils lageniform, short and spinulose (0·7–1·2(–5·0) mm), simple to sometimes bifurcate, numerous (10–15 mm–2), ±regularly distributed on the whole thallus; fibercles absent to rare; cortex±shiny, moderately thin to moderately thick, with ceratina-type plectenchyma; medulla dense to lax, moderately thin to thick, strongly orange pigmented, pigment at first subcortical, then spreading into the inner medulla, sometimes forming irregular patches; axis moderately thick to thick, remaining unpigmented. CMA (n=6): %C=(5·0–)6·0–8·0–10·5(–13·5);%M=(13·5–)19·5–24·5–29·5(–36·0); %A=(20·0–)25·5–35·0–44·5(–56·0). A/M=(0·4–)0·6–1·6–2·6(–3·3).
Apothecia numerous, lateral to terminal, often very small, 1 (-5)mm diam.; ascospores: length = (6·0−) 8·8 ± 1·0(−10·5) µm, width = (5·0−)5·6 ± 0·5(−7·0) µm, n = 4.
Pycnidia not seen.
Chemistry
Medulla: K−, P−. TLC: 1) unknown Us2 with blue-green fluorescence after charring (Rf class A=4–5, B=5–6), ±fatty acids (Rf classes A/B/C=2/3/4 and 3–4/4–5/5–6) (n=7); 2) usnic acid alone (n=5); 3) triterpenoid spot, grey-violet with orange fluorescence after charring (Rf classes A/B/C=4─5/4/4─5) (n=1).
Etymology
Named after the orange colour of the medulla and the resemblance to U. parvula.
Habitat and distribution
Corticolous or lignicolous, mainly in the Caatinga and Cerrado biomes in the north-eastern and south-eastern parts of Brazil. So far known only from Brazil (Mato Grosso do Sul, Minas Gerais, Bahia, Pernambuco and Ceará). It has not been found as yet in southern Brazil.
Taxonomic remarks
The subcortical orange pigmentation, the irregular branches with numerous foveolae and±alate segments, the numerous lageniform spinulous fibrils and the K−, P− medulla are the main characteristics of this taxon. Sometimes the pigmentation is very weak (as observed in old herbarium specimens) and the typical fibrils might be present only on some parts of the branches. Usnea steineri is another fertile species with a K−, pigmented medulla. It differs from U. aurantiaca-parvula by its slenderer, not spinulose and lageniform fibrils. Furthermore, the pigment in U. steineri is reddish, forming a usually thin subcortical layer, often spreading into the cortex but not into the medulla. Bayesian qanalysis (Fig. 2) shows that U. aurantiaca-parvula constitutes a distinct lineage related to U. parvula.
Specimens examined
Brazil: Mato Grosso do Sul: Porto Murtinho, Fazenda São Fernando, 21°34'26·57''S, 57°45'04·81''W, 94 m, pasture field near edge of deciduous forest, 2015, V. Pott 11873 (CGMS). Minas Gerais: Diamantina, Cerrado, 1976, L. Krieger 14076 (JPB); Entre Rios, Fazenda da Pedra Branca, 1977, L. Krieger 14430 (CESJ). Bahia: Morro do Chapéu, proche du centre ville (1–2 km) sur une route de terre vers des affleurements rocheux, 11°33'S, 41°09'W, 1000 m, 1989, S. Vermont-Grundlehner s. n. (G). Pernambuco: Buíque, Parque Nacional do Catimbau, Trilha das Pinturas, 2013, E. L. Nascimento 1801, 1804 (URM); Serra do Bituri, 1968, E. Carrazzani s. n. (JPB). Ceará: Crato, Chapada do Araripe, Malhada Bonita, 2013, M. Alves s. n. (ICN).
Usnea cirrosa Motyka s. lat.
Lich. Gen. Usnea Stud. Monogr., Pars Syst. 2: 526 (1937); type: Mexico, Morelia, Corrindapaz, alt. 2200 m, 1909, Brouard s. n. (LBL—holotype; G!—isotype). %C/M/A: 3/39/16 (isotype, specimen 57), 2/40.5/15 (isotype, specimen 58). Ascospores: 8·5–9·5–10·5×5·0–5·5–6·3(–7·0) µm (n = 20). Chemistry: usnic, salazinic and norstictic acids (Herrera-Campos et al. Reference Herrera-Campos, Nash and Garcia2001).

Fig. 4 A–D, Usnea cirrosa: A, branches constricted and inflated at ramification and foveolae (E. Gumboski 5020); B, branches slightly constricted and inflated at ramification and fibercles (L. Canêz 480); C, section through branch (S. Grundlehner s. n.); D, verrucose papillae (A. Gerlach 1510). E & F, Usnea cladocarpa: E, branches strongly constricted and inflated at ramification (Schäfer–Verwimp L9580); F, section through branch (B. Canestraro 485). Scales: A & E=2 mm; B, C & F=1 mm; D=500 µm. In colour online.
Thallus and apothecia (n=97). For a detailed description, see Herrera-Campos et al. (Reference Herrera-Campos, Nash and Garcia2001) and Clerc (Reference Clerc2007). However, we were not able to see the reddish pigment on the apothecial margin in our specimens mentioned by Herrera-Campos et al. (Reference Herrera-Campos, Nash and Garcia2001), neither was norstictic acid present. CMA (n=17): %C=(3·0–)4·5–6·5–8·5(–11·0); %M=(22·5–)27·5–32·5–37·5(–40·5); %A=(11–)22–22–30(–40); A/M=0·3–0·7–1·3(–1·8). Cortex with plectenchyma intermediate between ceratina and merrillii-type. Ascospores: length = (7·0−)9·0 ± 0·9(−12·0) µm, width = (4·8−)6·0 ± 0·5(−8·0) µm, n = 12.
Chemistry.K+ yellow→red. TLC: salazinic and ±protocetraric (trace) acids.
Habitat and distribution
USA (Tavares & Sanders Reference Tavares and Sanders1998; Clerc Reference Clerc2007), Colombia (Motyka Reference Motyka1938) and Mexico (Herrera-Campos et al. Reference Herrera-Campos, Nash and Garcia2001). In southern Brazil, Usnea cirrosa is frequent in montane areas, and less abundant in coastal areas. Specimens from coastal areas seem to be smaller, more compact and have more fibercles than specimens from montane areas where they are often well developed with larger thalli (≥8 cm). Usnea cirrosa occurs on a variety of corticolous (twigs and trunk) or lignicolous substrata. This species is recorded here for the first time in Brazil.
Taxonomic remarks
As circumscribed here, this taxon can be identified easily by the distinctly to slightly constricted lateral branches at attachment points, the swollen branch segments, the usually thin and glossy cortex and the medulla reacting K+ yellow→red due to the presence of salazinic acid as the major chemical substance. However, the CMA varies from the cornuta- to the brasiliensis-type. Detailed molecular studies might show that there could be more than one species here. Usnea cirrosa is paraphyletic with European samples of U. cornuta and additional study is needed in order to critically examine species boundaries. Usnea cirrosa and U. cladocarpa are morphologically closely related but they are readily separated by their secondary metabolites: U. cirrosa with salazinic acid (K+ yellow→red, P+ yellow) and U. cladocarpa with protocetraric acid (K−, P+ orange). Clerc (Reference Clerc2007) disagreed with Herrera-Campos et al. (Reference Herrera-Campos, Nash and Garcia2001) and considered U. cirrosa and U. cladocarpa (as U. ramillosa) to belong to the same species. Our study (Fig. 2) shows, however, that both species are distinct at the molecular level and hence should not be considered as one species. Usnea subelegans has numerous spinulous fibrils and a different chemistry. Usnea meridionalis is another species with a cornuta-type CMA and salazinic or norstictic acid chemotypes. However, this species always has minute red dots on the cortex surface, especially on terminal branches.
Selected specimens examined
Brazil: Rio Grande do Sul: Cambará do Sul, Parque Nacional dos Aparados da Serra, Cânion Itaimbezinho, 2014, A. Gerlach 1416 (ICN); Esmeralda, Estação Ecológica Aracuri, 1984, M. Fleig 2453 (ICN); São Francisco de Paula, Floresta Nacional, 2014, A. Gerlach 1509 (ICN); ibid., Lago São Bernardo, 29°27'34''S, 50°34'16''W, 1000 m, 1989, S. Grundlehner s. n. (G); Vacaria, Localidade de Fazenda da Estrela, campo com Araucaria angustifolia, 28°04'56''S, 50°58'32·6''W, 980 m, 2003, L. Canêz 518 (CGMS). Santa Catarina: Campo Alegre, Serra do Quiriri, on twigs, 2012, A. Charnei 562 (ICN); Florianópolis, Parque Municipal da Lagoa do Peri, 2014, A. Gerlach 1214 (ICN); Garuva, rural area, 2013, A. Gerlach 1159 (ICN); São Francisco do Sul, Capri, on Syagrus romanzoffiana, 2013, A. Gerlach 980 (ICN); Urubici, Parque Nacional de São Joaquim, 2014, A. Gerlach 1318 (ICN). Paraná: Balsa Nova, Serra S’Ana, cloud forest, 1969, G. Hatschbach 21365 (MBM); Campina Grande do Sul, Serra Ibitiraquire, Morro Tucum, saxicolous, 1739 m, J. Cordeiro 1784 (MBM); Guaraqueçaba, Ilha de Superagui, 1988, S. Eliasaro 605 (BHCB); Guaratuba, Morro dos Perdidos, A. Gerlach 1032 (ICN); Lapa, Gruta do Monge, on twigs, 1996, S. Eliasaro s. n. (UPCB); Paranaguá, Ilha do Mel, 2012, A. Gerlach 785 (ICN). São Paulo: São Luis do Paraitinga, Parque Estadual da Serra do Mar, 23°18'48''S, 45°07'13·7''W, 930 m, 2007, L. Canêz 2233 (CGMS); Serra da Bocaina, 22°47'S, 44°38''W, 1550 m, 1988, Schäfer-Verwimp & Verwimp L-9580 (G). Minas Gerais: Catas Altas, Parque Natural do Caraça, 20°06'S, 43°29'W, 1275 m, 2006, M. Benatti 1923 (SP); Lima Duarte, Parque Estadual do Ibitipoca, 1994, C. H. Ribeiro 221 (CESJ). Rio de Janeiro: Parque Nacional do Itatiaia, 1750 m, 1966, G. Eiten & L. Eiten 7443 (G); ibid., estrada para o Pico das Agulhas Negras, 1900 m, 2010, A. Cervi 9627 (MBM); Marica, restinga, on twigs of Erythroxylum ovalifolium, 1985, M. A. A. Santos s. n. (RB).
Usnea cladocarpa Fée
Essai Crypt. Ecorc. Officin. 1: 101 (1824); type: Brazil, ad arborum truncos et ramos, misit D. de Gestas s. n. (G!—holotype). %C/M/A: 4.5/39/13 (thallus 12), 5.5/41/7 (thallus 13). Ascospores (apothecia absent). Chemistry: usnic and protocetraric acids (TLC by Clerc in 2008).
Usnea ramillosa Motyka syn. nov. Lich. Gen. Usnea Stud. Monogr. Pars Syst. 2: 527 (1938); type: Insula Cuba, Wright s. n. (H-NYL!—holotype). %C/M/A: 4/40/12. Ascospores: (8·8–)9·1–9·6–10·0×(6·4–)6·7–7·0–7·2 µm (n = 10). Chemistry: usnic and protocetraric acids (%CMA, ascospores and chemistry by Clerc in 1995).
Thallus and apothecia (n=20). For a detailed description, see Herrera-Campos et al. (Reference Herrera-Campos, Nash and Garcia2001). CMA (n=9): %C=2–3–4(–5); %M=(36–)38–40–42(–43); %A=(8·0–)8·5–13·0–18·0(–23·0). A/M=0·2–0·3–0·4(–0·6). Cortex with ceratina-type plectenchyma. Ascospores: length = (7·0–)9·0 ± 1·1(–12·5) µm, width = (5·0–)6·0 ± 0·6(–7·5) µm, n = 7.
Distribution and habitat
Commonly found in Cuba, rarely in Jamaica and Texas (Motyka Reference Motyka1938, as U. ramillosa). This species also occurs in Ecuador (Nöske & Sipman Reference Nöske and Sipman2004) and Mexico (Herrera-Campos et al. Reference Herrera-Campos, Nash and Garcia2001). Its presence in Chile is doubtful (Motyka Reference Motyka1938). For Brazil, it has been reported from Santa Catarina (Motyka Reference Motyka1938), Rio de Janeiro (Motyka Reference Motyka1938; Rizzini Reference Rizzini1952), Minas Gerais and São Paulo (Motyka Reference Motyka1938). Usnea cladocarpa is less frequent in southern Brazil compared to U. cirrosa, a closely related species. Based on unpublished observations of Usnea material from Costa Rica by the second author, the opposite situation pertains in Costa Rica, where U. cladocarpa is more common than U. cirrosa. Moreover, U. cladocarpa has not, so far, been found in coastal areas.
Taxonomic remarks
Usnea cladocarpa is recognized by its fusiform branches that are constricted at the attachment point, conspicuous foveolae, brasiliensis-type CMA, the A/M ratio ≤0·6 and the occurrence of protocetraric acid as the main secondary medullary substance. For differences with U. cirrosa, see under this latter taxon. With Usnea meridionalis it shares the constricted and swollen branches with the brasiliensis-type CMA, but differs in its chemistry (see under U. meridionalis for more details).
Usnea cladocarpa and U. ramillosa share the same swollen branches that are constricted at the attachment points, the brasiliensis-type CMA, as well as protocetraric acid in the medulla. Therefore they are considered here to belong to the same species and have been newly placed in synonymy.
Selected specimens examined
Brazil: Paraná: Campina Grande do Sul, 2012, V. Ariati 295 (ICN); Curitiba, en allant vers Vila Velha, 25°21'S, 49°34'W, 1989, S. Grundlehner s. n. (G); Piraí do Sul, 2012, B. Canestraro 485 (ICN); Tijucas do Sul, Ambrósios, on Araucaria angustifolia, 1991, R. Kumrow 3262 (MBM). São Paulo: Campos do Jordão, Parque Estadual de Campos de Jordão, 1996, C. Ribeiro 1003 (CESJ); Mogi-Guaçu, interior do Cerrado, próximo ao riacho, 22°15'20·8''S, 47°09'56''W, 650 m, 2007, A. Spielmann 7088 (CGMS); ibid., Martinho Prado Jr., Reserva Biológica e estação experimental, Cerrado e mata ciliar do córrego, 22°16'S, 47°09'W, 630 m, M. Benatti 2782 (SP); Serra da Bocaina bei Sao José do Barreiro, an Sträuchern in einer Weide bei “Shangrila”, 22°47'S, 44°38'W, 1550 m, 1988, Schäfer-Verwimp & Verwimp L 9580 (G). Rio de Janeiro: Rio de Janeiro, 1878, Glaziou s. n. (G); Tijucas, 1983, Schwacke 4825 (RB). Minas Gerais: Catas Altas, Parque Natural do Caraça, 20°06'S, 43°29'W, 1275 m, M. Benatti 1923 (SP); Serra da Mantiqueira, Fazenda São Mateus, östlich von Camanducaia, 1800 m, 1980, K. Kalb s. n. (G).
Usnea concinna Stirt.
Scott
Naturalist (Perth) 6: 103 (1881); type: Brazil, s. loc., Mr. Weir s. n. (BM 97192!—lectotype designated here; BM 97193!—isolectotype). %C/M/A: 9/19.5/43. Ascospores (lectotype): 8·0–10·5(–12·5)×5·0–7·5(–8·0) µm (n = 20). Chemistry (lectotype): usnic, stictic, constictic, menegazziaic, cryptostictic and (trace) norstictic acids (TLC by Clerc in 1996).
Usnea radiata Stirt. syn. nov., Scott. Naturalist (Perth) 6: 103 (1881); type: Brazil, statione exactius non-indicata, Mr. Weir s. n. (BM 97191!—lectotype designated here; BM 97190!—isolectotype). %C/M/A: 8/27/30 (lectotype). Ascospores (lectotype: 10·0–11·0(–12·5)×7·5–8·0 µm (n = 6). Chemistry (lectotype): usnic, stictic, constictic, menegazziaic, cryptostictic, norstictic acids and an unknown with Rf classes A/B/C 5/3/5 and green fluorescence after charring.
Usnea florida var. scabrosa Zahlbr. syn. nov., Expedition der kaiserlichen Akademie der Wissenschaften nach Südbrasilien 83: 103 (1909); type: Brazil, São Paulo, in silvaticis prope urbem Iguape, 20–100 m, 1901, V. Schiffner s. n. (BM 733848!—holotype). %C/M/A: 11.5/26.5/24. Ascospores: 10·0–10·2–10·5(–11·0)×(5·5–)6·5–7·2–8·0 µm (n=10). Chemistry: stictic, constictic, menegazziaic, cryptostictic and norstictic acids and an unknown substance with green fluorescence after charring and Rf classes: A/B/C: 5/3/5.
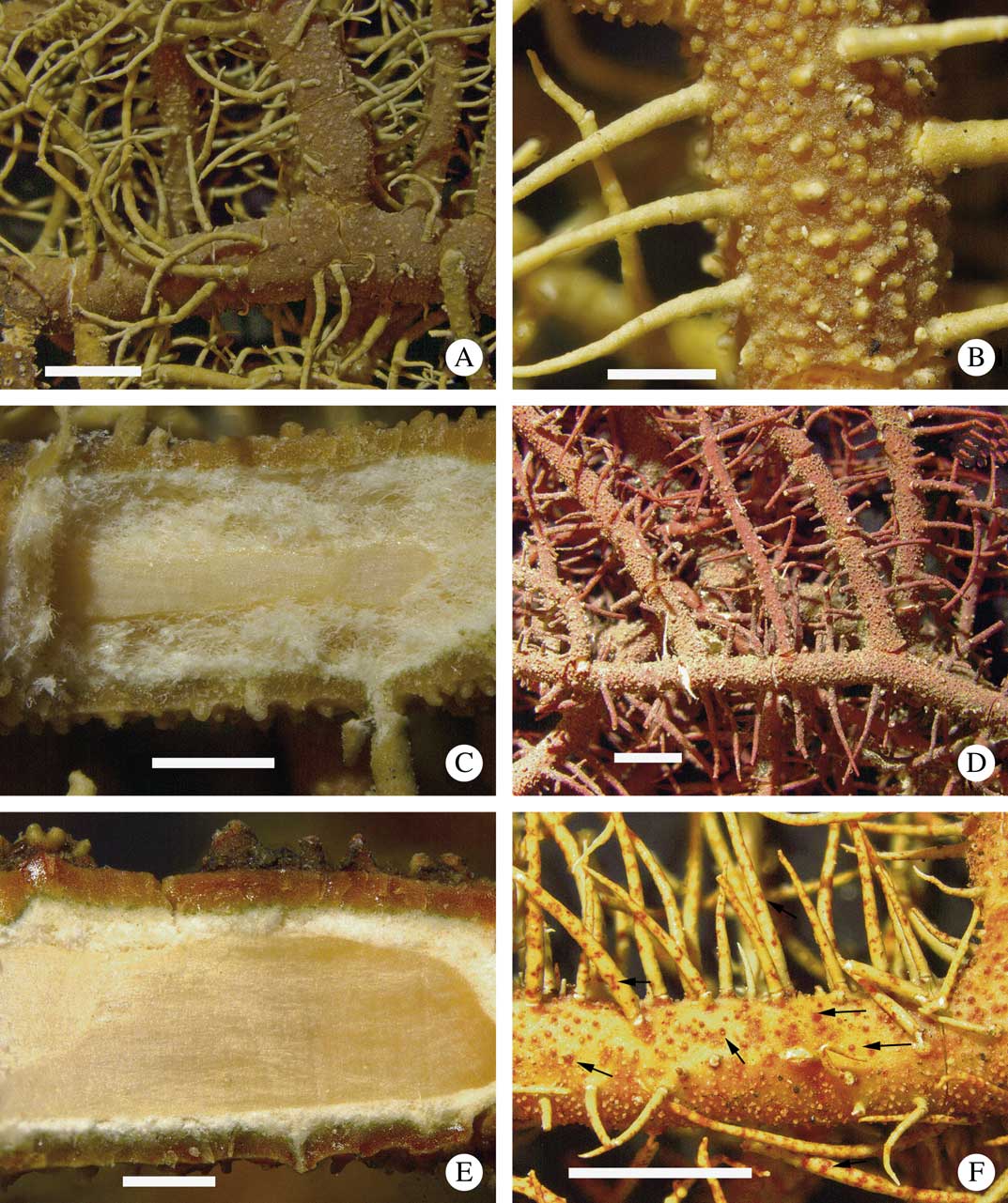
Fig. 5 A–C, Usnea concinna (K. Kalb s. n.): A, branches slightly constricted and inflated at ramification; B, verrucose tubercles; C, section through branch. D–F, Usnea erinacea: D, terete and tapering branches, the cortex is diffusely pigmented red on whole branches (A. Gerlach 1112); E, section through branch (Schäfer–Verwimp L9118); F, detail of thallus surface with darker spots containing red cortical pigmentation (arrows) (A. Gerlach 1211). Scales: A=2 mm; B & E=500 µm; C=1 mm; D & F=2 mm. In colour online.
Thallus (n=20) erect-shrubby, yellowish green, up to 8 cm long; trunk often short, 0·2–1·0 cm, rarely up to 2 cm long, concolorous with branches, always with thin annulations; ramifications mostly isotomic- to rarely anisotomic-dichotomous; main branches 0·9–2·4 mm thick, often slightly irregular, cylindrical, little segmented towards the terminal branches (1 annular crack/0·5 cm) to more segmented towards the base (3–6 annular cracks/0·5 cm) usually exposing the medulla, often with slightly swollen segments; lateral branches not to usually slightly constricted at the ramification point, distinctly segmented; foveolae, maculae and pseudocyphellae absent; papillae absent to rare; tubercles numerous, small (0·7 mm), verrucose to cylindrical, often with paler apices and sometimes eroded, regularly distributed on the whole thallus; fibrils present, usually numerous, slender (1–7 mm long), regularly distributed; fibercles often present mostly in the basal main branches, scarce to numerous; cortex matt to rarely ±shiny, never vitreous, moderately thick to thick, often with many irregular cracks, with merrillii-type plectenchyma; medulla white, often pale orange periaxially pigmented (probably due to the oxidation of secondary compounds), dense to compact, thin to moderately thick; axis±thin to moderately thick. CMA (n=11): %C=(8·0–)8·5–10·3–12·0; %M=(14·0–)18·5–22·5–27·0(–28·0); %A=(30·0–)27·0–35·0–42·5(–48·0). A/M=1·0–1·5–2·5(–3·5).
Apothecia numerous, often terminal, up to 10 mm diam.; ascospores: length = (7·0–)10·0 ± 1·1(–12·5) µm, width = (5·0–)6·0 ± 0·8(–8·5) µm, n = 9.
Chemistry
Medulla: 1) K+ bright yellow, TLC=stictic, constictic, cryptostictic, ±menegazziaic and ±norstictic (trace) acids (n=12); 2) K+ yellow slowly→red, TLC= cryptostictic, norstictic, ±constictic, ±menegazziaic and protocetraric (trace) acids (n=2).
Habitat and distribution
Usnea concinna is known only from Central and South America where it seems to be widespread and found in Argentina, Bolivia, Cuba, Mexico, Paraguay, Peru and Venezuela (Motyka Reference Motyka1938). In Brazil, it has been recorded from Rio Grande do Sul (Fleig & Grüninger Reference Fleig and Grüninger2008), Santa Catarina, Minas Gerais and Rio de Janeiro (Motyka Reference Motyka1938). This species usually occurs in mountainous areas, above 900 m, mainly in the states of São Paulo and Minas Gerais.
Taxonomic remarks
Usnea concinna can be identified by the very slightly constricted and swollen branches covered with minute whitish verrucose to cylindrical tubercles, the matt and thick cortex (8·5–10·3–12·0%) and the dense to compact medulla, reacting K+ yellow (stictic acids group). Although the majority of specimens have a matt cortex, sometimes it can be somewhat shiny. The density of fibrils, fibercles and tubercles as well as the degree of constriction of the branches are also variable.
For differences from U. kalbiana see under the latter species. Usnea cirrosa differs from U. concinna by its branches that are distinctly constricted at the attachment point, the swollen branch segments, the cornuta-type CMA and the K+ yellow→red medulla (salazinic acid). We were unable to obtain freshly collected material for sequencing and hence the phylogenetic position of U. concinna remains unclear.
Usnea radiata corresponds to a smaller and more branched form of U. concinna that otherwise shares all the characteristics of the latter species, and the holotype of Usnea florida var. scabrosa is similar morphologically, anatomically and chemically to the original material of U. concinna. Therefore, both Usnea radiata and U. florida var. scabrosa are considered as synonyms of U. concinna.
Selected specimens examined
Brazil: Rio Grande do Sul: São Francisco de Paula, Centro de Pesquisa e Conservação da Natureza, Pró-Mata, on bark of Araucaria angustifolia, 918 m, 1998, M. Fleig & Grüninger 983136 (ICN). São Paulo: Campos do Jordão, 1991, M. Fleig 4455 (ICN); ibid., Serra da Mantiqueira, Nebelwald am Pico do Itapeva, 2000 m, 1987, Schäfer-Verwimp L/8493 (G); ibid., 150 km nordöstlich von São Paulo in einem hellen, feuchten Urwald, 1700 m, K. Kalb & G. Plöbst s. n. (G-260927). Rio de Janeiro: Itatiaia, Regenwald oberhalb des Museums, an Ästen auf dem Weg zum Fernsehturm, 1350 m, 1987, Schäfer-Verwimp L/9264 (G). Minas Gerais: Fazenda São Mateus, östlich von Camanducaia, 1800 m, 1980, K. Kalb s. n. (G-260940); Serra de Ibitipoca, 1400 m, 1975, L. Krieger 13464 (CESJ).
Usnea cristatula Motyka
Lich. Gen. Usnea Stud. Monogr. Pars Syst. 2(2): 641 (1938); type: Mexico, Michoacan, Morelia, Cerro Azul, Brouard s. n. (LBL—holotype; LBL, S, G!—isotypes). %C/M/A: 11/17.5/42.5. Ascospores (isotype): (7·5–)8·0–9·0–9·5(–10·0)×5·0–5·5–6·0 µm (n = 22). Chemistry: usnic, diffractaic and squamatic (trace) acids (Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998).
Thallus and apothecia (n=15). For a detailed description and illustrations see Herrera-Campos et al. (Reference Herrera-Campos, Clerc and Nash1998), Clerc (Reference Clerc2007) and Truong & Clerc (Reference Truong and Clerc2012). CMA (n=14): %C=8·5–11·0–13·5(–18·0); %M=(19·0–)20·0–23·0–26·60(–27·5); %A=(25·0–)26·5–32·0–37·5(–43·0). A/M=1·0–1·4–1·8(–2·2). Cortex with baileyi-type plectenchyma. Ascospores: length = (6·0–)8·3 ± 0·8(–10·0) µm, width = (4·5–)5·4 ± 0·4(–6·0) µm, n = 10.
Chemistry
Medulla C+ yellow. TLC: 1) diffractaic, ±barbatic acids (n=13); 2) barbatic acid (n=2).
Habitat and distribution
Previously reported for the USA (Knudsen & Lendemer Reference Knudsen and Lendemer2006), Mexico (Herrera-Campos et al. Reference Herrera-Campos, Clerc and Nash1998), Bolivia, Colombia, Peru and Venezuela (Truong & Clerc Reference Truong and Clerc2012). Also known in Europe from Portugal (Clerc 2011 Reference Clerca ). Newly reported here for Brazil. Despite extensive sampling conducted in southern Brazil, U. cristatula could not be found and only herbarium specimens were examined.
Taxonomic remarks
Usnea cristatula is characterized by its pink/reddish medulla containing diffractaic and/or barbatic acids, the presence of numerous fibercles and±slender fibrils as well as a thick and glossy cortex. The localization of the pigment can vary from subcortical to almost subaxial, rarely over the whole width of the medulla, sometimes with a periaxial yellow pigment. Usnea strigosa (Ach.) Eaton is a North American species with a pigmented medulla and diffractaic acid, amongst other chemotypes (Hale Reference Hale1979), but the pigment is dusky red and usually fills the whole medulla, fibercles are lacking and numerous spinulose fibrils are present (Clerc Reference Clerc2007). For differences between this species and Usnea flavorubescens Truong & P. Clerc, see Truong & Clerc (Reference Truong and Clerc2012). We were unable to acquire freshly collected material to obtain good quality DNA, hence the phylogenetic position of U. cristatula remains unclear.
Selected specimens examined: Brazil: Rio Grande do Sul: Santa Maria, 150 m, 1980, M. Fleig 1207 (ICN); Novo Cabrais, near Santa Maria, 1999, A. Spielmann 11884 (CGMS). Santa Catarina: Nova Teutonia, 1944, F. Plaumann s. n. (RB). Paraná: Vila Velha, 25°21'S, 49°34'W, 1989, S. Grundlehner s. n. (G); Pinhão, on fences of Phoebe porosa, 1975, L. Krieger s. n. (JPB); Ponta Grossa, Uvaia, 1976, L. Krieger 15374 (CESJ). Minas Gerais: Grão Mogol, Trilha dos garimpeiros, campo rupestre dos afloramentos rochosos, 1100 m, 1991, M. Hatschbach 55090 (MBM). Distrito Federal: Brasilia, Fazenda Água Limpa, on trunk of embaúba Cecropia sp., mata ciliar, 1980, E. Sato 3 (JPB). Bahia : Carrentina, 1967, D. Vital s. n. (JPB).
Usnea erinacea Vain. s. lat.
Dansk Botan. Arkiv. 4: 3 (1926); type: Mexico, Chimantla, 1841, Liebmann s. n. (TUR-V!—holotype). %C/M/A: 7.5/17.5/50 (Clerc 2011 Reference Clerca ). Ascospores: (7·5–)8·0–8·5–9·0(–10·0)×5·0–5·5–6·0(–7·0) µm. Chemistry: usnic, salazinic and norstictic acids (Clerc 2011 Reference Clerca ).
Thallus (n=130). For a detailed description, see Clerc (Reference Clerc2004, Reference Clerc2007). CMA (n=20): %C=(4·5–)6·5–10·0–14·0(–16·0); %M=(7·0–)15·5–24·5–33·5(–36·0); %A=(14–)18–31–44(–60). A/M=0·4–1·5–3·0(–6·5). Cortex with baileyi-type plectenchyma.
Apothecia numerous, lateral, terminal to subterminal, up to 25 mm diam.; ascospores: length = (7·0−)9·2 ± 1·2(−13·0) µm, width = (5·0−)5·7 ± 0·5(−7·0) µm, n = 7.
Chemistry
Medulla: 1) K−, P+ orange, TLC=protocetraric acid, ±undetermined triterpenoids (n=17); 2) K−, P−, TLC=undetermined triterpenoids (n=10); 3) K+ yellow slowly→red, TLC=norstictic, ±undetermined triterpenoids (n=7); 4) K+ yellow→red, TLC= salazinic, ±norstictic, ±protocetraric (trace) acids (n=5); 5) K+ bright yellow, TLC= stictic, constictic, cryptostictic, menegazziaic, norstictic (trace), undetermined triterpenoids (n=4).
Habitat and distribution
Usnea erinacea has a wide ecological range, from sea level to 1800 m elevation. This species is frequently found growing on the bark of Araucaria angustifolia in mountainous areas and on fences in rural areas. It is known from North and South America, Europe and Africa (Clerc Reference Clerc2004, Reference Clerc2007, 2011 Reference Clerca ). In South America, this species is so far known from Bolivia, Colombia, Equador, Peru and Venezuela (Truong et al. Reference Truong, Bungartz and Clerc2011). Usnea erinacea is probably the most abundant fertile species in Brazil but interestingly it has not been cited previously for this country. It is newly recorded here for Brazil.
Taxonomic remarks
The reddish orange pigmentation of the cortex, the tapering and terete branches that are not constricted at the attachment point, the thick (≥10%) and vitreous cortex, the compact medulla and the ratio A/M≥1·5 are diagnostic for U. erinacea s. str. In Brazil, however, we consider U. erinacea s. lat. to be a very polymorphic species that shows a high level of variability in the following important characters: 1) the pattern of cortical pigmentation, 2) the shape of branches, 3) the CMA values, and 4) the chemistry (see Table 2).
Three main patterns of cortical pigmentation were found among the specimens studied: a diffuse pigmentation throughout the whole cortex (Usnea erinacea s. str.) (Fig. 5D); a superficial pigmentation in the upper part of the cortex; and a spot-like, irregular or punctiform cortical pigmentation that often also coloured the papillae (Fig. 5F). The branches may vary from tapering to irregular in longitudinal section and terete to obtuse-angled in cross-section. The A/M ratio may vary from ≤1 to ≥2. Intermediate forms were common and the pattern of pigmentation could not be clearly correlated with any other morphological or chemical characters. This group is weakly supported and unresolved (Truong et al. 2013 Reference Truong, Divakar, Yahr, Crespo and Clerca , Fig. 4) and a large-scale morphological and molecular study is needed.
Selected specimens examined
Brazil: Rio Grande do Sul: Caxias do Sul, Distrito de Santa Lucia do Piai, 29°11'48·6''S, 50°59'21·6''W, 735 m, 2010, A. Spielmann 8641 (CGMS); Gramado, surroundings of Lago Negro, Araucaria moist forest, 29°22'44''S, 50°52'26''W, 800 m, 2013, M. Dal Forno 2108 (ICN); Mariana Pimentel, beira de estrada, em poste, 1989, S. Grundlehner s. n. (ICN); São Francisco de Paula, Centro de Pesquisa e Conservação da natureza Pró-Mata, 1998, M. Fleig 983010 (ICN); Vacaria, Fazenda da Estrela, 28°01'58''S, 50°58'17·5''W, 900 m, 2003, L. Canêz 442 (CGMS). Santa Catarina: Alfredo Wagner, RPPN Rio das Furnas, 2014, A. Gerlach 1228 (ICN); Florianópolis, Parque Municipal da Lagoa do Peri, 2013, A. Gerlach 1211 (ICN); Bergland bei Fraiburgo, Regenwald im Park des Hotels Renar, 1070 m, 1987, Schäfer-Verwimp L9118 (G); Joinville, rural area, on fences, 2013, A. Gerlach 1112 (ICN); Rio Negrinho, Fazenda Velha, 2007, E. Gumboski 1020 (ICN); São Joaquim, Fazenda Santa Rita, campo de pastagem, 1400 m, 1992, M. Fleig 4705 (ICN); Urubici, Parque Nacional de São Joaquim, A. Gerlach 1363 (ICN). Paraná: Carambeí, Catanduva de Fora, 2013, M. Engels s. n. (ICN); Castro, Cânion Guartela, 2013, L. Rocha s. n. (ICN); Curitiba, en allant vers Vila Velha, 25°21'S, 49°34'W, saxicolous, 1989, S. Grundlehner s. n. (G); Guarapuava, 2013, M. Engels s. n. (ICN); Tijucas do Sul, Vossoroca, on Arecastrum sp., 1973, R. Kumrow 150 (MBM); estrada antiga da Graciosa, on fences, 1999, W. Sanders 99801.1 (UFP). São Paulo: Campos de Jordão, Sekundärwald bei Minalba, epiphytisch, 1420 m, 1989, Schäfer-Verwimp L/11022 (G); Piquete, Pico dos Marins, 22°30'30·8''S, 45°07'46·4''W, 1900 m, 2007, L. Canêz 2438 (CGMS). Rio de Janeiro: Itatiaia, Parque Nacional do Itatiaia, em direçao ao Pico das Agulhas Negras, 22°23'07·5''S, 44°40'48·1''W, 2355 m, 2012, A. Spielmann 10153 (CGMS). Minas Gerais: Catas Altas, Parque Natural do Caraça, 20°06'52·7''S, 43°29'29·4''W, 1265 m, 2006, L. Canêz 1789 (CGMS); Itamonte, Parque Nacional do Itatiaia, Estrada das Prateleiras, 22°21'41·8''S, 44°44'08·3''W, 2134 m, 2009, A. Spielmann 7641 (CGMS); Lima Duarte, Parque Estadual do Ibitipoca, 1993, C. Ribeiro 134 (CESJ); National Park Serra de Caparo, Regenwald, epiphytisch am Rande der Erdstraβe bei 1870 m, 1987, Schäfer-Verwimp L8908 (G).
Usnea fleigiae A. Gerlach & P. Clerc sp. nov.
MycoBank No.: MB 819421
Similar to Usnea florida but differs in its concolorous with branches or paler basal part, the lax medulla, and the presence of norstictic and/or salazinic acids.
Type: Brazil, Rio Grande do Sul, Cambará do Sul, Parque Nacional da Serra Geral, Cânion Fortaleza, on Drimys winteri, 16 December 1986, M. Fleig 2877 (ICN—holotype; G—isotype). %C/M/A: 9.5/13/55 (holotype), 14/5/62 (isotype). Ascospores: (9·0–)9·5–10·5–11·5(–13·0)×(5·0–)6·0–7·3–8·0 µm (n=20). Chemistry: usnic, salazinic and norstictic acids.
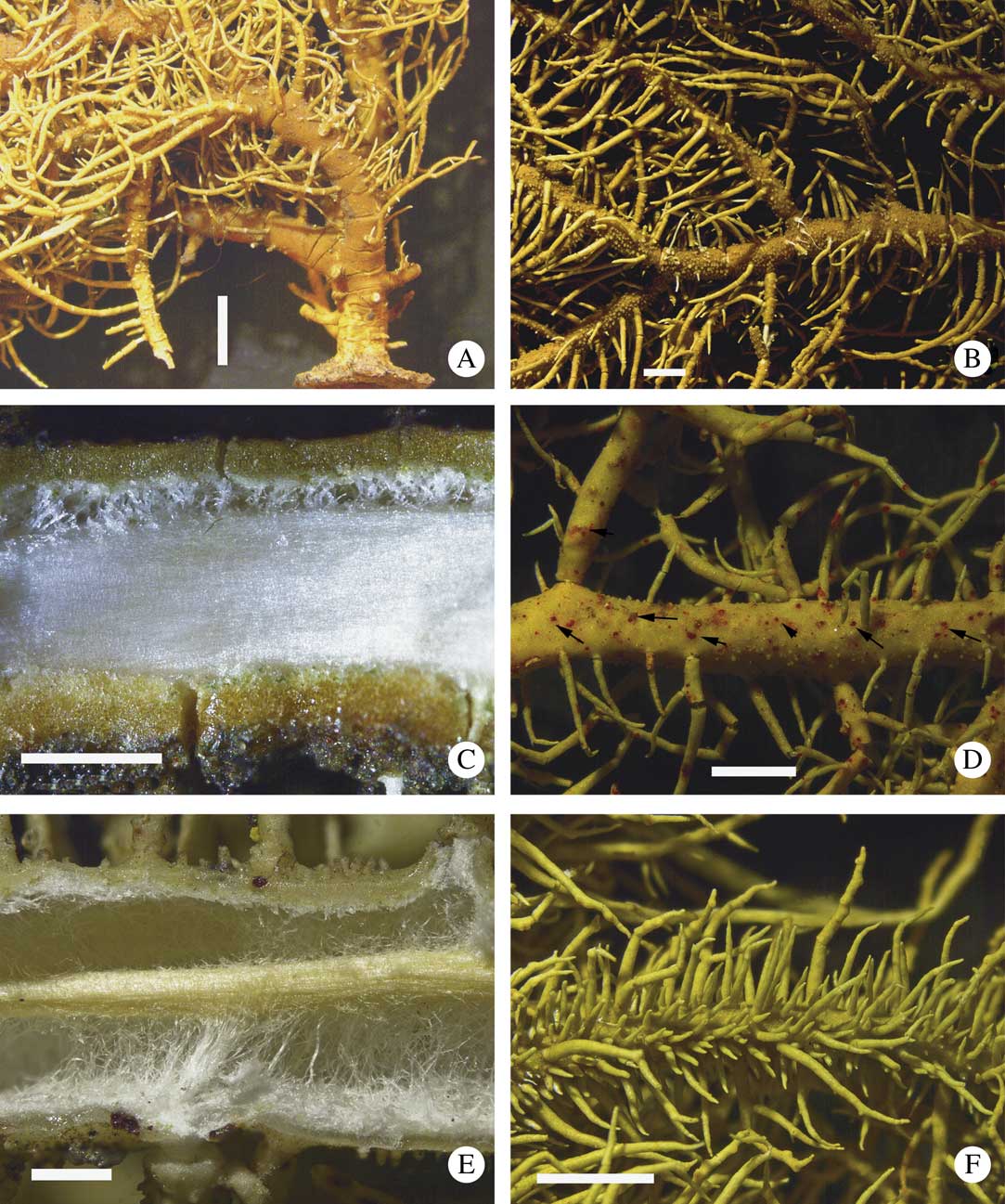
Fig. 6 A–C, Usnea fleigiae: A, trunk annulated, concolorous (holotype); B, branches annulated, slightly constricted and inflated at ramification (holotype); C, section through branch (isotype). D & E, Usnea meridionalis: D, fusiform branches with dark, red-pigmented dots on the cortex surface (arrows) (M. Engels s.n.); E, section through branch, the periaxial tissue is pigmented yellow (E. Fazolino s.n.); F, Usnea subelegans, branches densely covered with spinulose fibrils (E. Fazolino s. n.). Scales: A & B=2 mm; C & E=500 µm; D & F=1 mm. In colour online.
Thallus (n=16) erect-shrubby to rarely almost subpendulous, yellow-green, up to 8 cm long, with anisotomic-dichotomous, often very dense ramifications; trunk usually short, up to 3 mm long, usually concolorous, with branches rarely black pigmented, with thin annulation; main branches tapering, terete in cross-section, distinctly segmented with c. 7 annular cracks/0·5 cm, with cylindrical to somewhat swollen segments; lateral branches not to slightly constricted at the ramification point; foveolae, maculae and pseudocyphellae absent; papillae and tubercles often numerous (>10 mm–2), ±verrucose, ±regularly and densely distributed on the whole thallus, except sometimes close to the basal part; fibrils often numerous (>20/3 mm–2), slender (1–7 mm long), ±regularly distributed on the whole thallus; fibercles few to absent; cortex shiny, moderately thin to thick, with plectenchyma intermediate between ceratina- and florida-type; medulla white, dense (near the base) to lax (in lateral branches), thin; axis thick. CMA (n=15): %C=(6·0–)7·5–10–12·5(–14·5); %M=5·0–9·0–13·0(–17·5); %A=47–62–70(–76). A/M=(3·0–)4·5–8·0–11·5(–15·0).
Apothecia numerous, mainly terminal, up to 8 mm diam.; ascospores: length = (9·0–)13·9 ± 1·8(–18·0) µm, width = (5·0–)9·5 ± 1·1(–12·0) µm, n = 14.
Chemistry
Medulla: 1) K+ yellow slowly→red, TLC=norstictic acid and ±undetermined triterpenoid (n=9); 2) K+ yellow→red, TLC=salazinic and±norstictic acids (n=5).
Etymology
This species is named in honour of the Brazilian lichenologist Mariana Fleig. Her rich Usnea collections that are housed in the ICN herbarium allowed the first author to begin her studies on Usnea in Brazil.
Habitat and distribution
Usnea fleigiae is known only from southern Brazil where it occurs in mountainous areas (above 900 m) in the Serra Geral and Serra do Mar, in three types of vegetation: dense rainforest, Araucaria forest and high elevation grasslands. It is found mainly on twigs of shrubby trees and is quite rare.
Taxonomic remarks
The blackish pigmentation that is sometimes seen in the basal part of the thallus might be owing to the presence of a lichenicolous fungus. Among the shrubby and esorediate Usnea species known from Brazil, U. fleigiae might be confused with U. grandispora. See under this taxon for differences between the two.
Selected specimens examined
Brazil: Rio Grande do Sul: Cambará do Sul, Parque Nacional da Serra Geral, Cânion Fortaleza, 1983, M. Fleig 2197 (ICN); ibid., on Drimys winteri, 1986, M. Fleig 2878 (ICN); ibid., 1030 m, 2012, A. Spielmann 10200, 10208 (CGMS); São Francisco de Paula, Área de Preservaçao Ambiental Rota do Sol, 2002, S. Martins s. n. (HAS). Santa Catarina: Campo Alegre, Serra do Quiriri, 1200 m, 2012, A. Charnei 562, 563, 566 (ICN); Urubici, 1650 m, 2004, A. Cervi 8712 (UPCB). Paraná: Campina Grande do Sul, Serra do Ibitiraquire, cume do Morro Itapiroca, 1800 m, 2014, M. Engels s. n. (ICN).
Usnea grandispora A. Gerlach & P. Clerc sp. nov.
MycoBank No.: MB 819422
Similar to U. florida but differs in its production of protocetraric or salazinic acids in the medulla and the larger spore size.
Type: Brazil, Rio Grande do Sul, São Francisco de Paula, Floresta Nacional de São Francisco de Paula, on bark of Araucaria angustifolia, near the lodging, 29 November 2014, A. Magnago 1114 (ICN—holotype; G—isotype). %C/M/A: 14/14/44 (holotype); 12/19.5/37 (isotype). Ascospores (holotype): (13–)14–15·5–17(–18)×9–10–11(–12) µm (n = 12). Chemistry: usnic, protocetraric and fumarprotocetraric (trace) acids (holotype).

Fig. 7 Usnea grandispora. A, thallus (holotype); B, thallus with dense ramifications (A. Gerlach 1009); C, section through branch (holotype); D, trunk annulated, jet black (isotype); E, cylindrical papillae (holotype); F, verrucose tubercles, often not eroded at the apex. Scales: A & B=1 cm; C & D=1 mm; E & F=500 µm. In colour online.
Thallus (n=25) erect-shrubby, up to 8 cm long, yellow-green, isotomic- to anisotomic-dichotomously branched; trunk often short, up to 1 cm long, pigmented jet black at least for the first 1 mm, always with thin annulations; main branches 0·7–1·8 mm thick, tapering to slightly irregular, terete in cross-section, distinctly segmented (3–10 annular cracks/0·5 cm) often exposing the medulla, with cylindrical to somewhat swollen segments; lateral branches not to rarely slightly constricted at the ramification point; foveolae, maculae and pseudocyphellae absent; papillae and tubercles numerous (10–30 mm–2), thin and±cylindrical to thick and±verrucose,±regularly distributed on the whole thallus, except sometimes close to the basal part; fibrils present, usually numerous, slender (1–7 mm long) to spinulous (1–2 mm long), irregularly to regularly and then densely distributed; fibercles absent (or rare); cortex matt, thick with few irregular cracks, with plectenchyma intermediate between ceratina- and florida-type; medulla white, dense to compact, thin; axis moderately thick to thick. CMA (n=20): %C=(11·0–)13·0–14·5–16·0(–19·0); %M=(5·0–)9·0–13·0–17·0(–19·5); %A=(33·0–)37·5–45·5–53·5(–59·0). A/M=1·5–4·0–6·5(–11·0).
Apothecia numerous, lateral, terminal to subterminal, up to 10 mm diam.; ascospores: length = (11·0–)14·8 ± 1·3(–18·0) µm, width = (6·0–)9·9 ± 0·9(–13·0) µm, n = 18.
Chemistry
Medulla: 1) K+ yellow→red, TLC=salazinic acid (n=15); 2) K−, P+ orange, TLC=protocetraric and fumarprotocetraric acids (n=8).
Etymology
Named after the notably large spore size.
Habitat and distribution
Usnea grandispora has the same ecological range as U. fleigiae, occurring in montane areas. This is a corticolous species, occasionally saxicolous (only two specimens). It has been found only in the southern part of Brazil.
Taxonomic remarks
Two chemotypes were found: 1) salazinic acid chemotype, usually associated with large and conspicuous tubercles/papillae and a more branched thallus (Figs 7B & F) and 2) protocetraric acid chemotype, usually associated with smaller and thinner tubercles/papillae and a less branched thallus (Fig. 7A & E). These chemotypes seem to have a distinct geographical distribution. However, they belong to the same clade (Fig. 2). Further collecting and subsequent studies are needed to evaluate both chemotypes. Usnea grandispora is morphologically very similar to U. florida. The latter species has smaller spores (8·5–11·0 µm) and a different chemistry (Clerc 1984 Reference Clerca ). In addition, our molecular phylogenetic analyses show that the species are not conspecific. Usnea fleigiae shares its annulated branches, the large spores and the salazinic acid chemotype with U. grandispora, but differs from the latter species mainly by the distinctly lax medulla and the CMA values (the cortex and medulla are thinner and the axis thicker in U. fleigiae). Moreover, the basal part of U. fleigiae is often concolorous with the branches and protocetraric acid is absent. These two species are only distantly related (Fig. 2). Usnea subfusca Stirt. is a similar north-eastern American species (Clerc & Herrera-Campos Reference Clerc and Herrera-Campos1997) but with smaller ascospores (<10 µm long) and never with protocetraric acid in the medulla. Three Indian apotheciate and esorediate species, U. ghattensis, U. norkettii G. Awasthi (BM!—holotype) and U. spinosula Stirt. (BM!—type), also have large ascospores (≥10 µm). Usnea ghattensis has a very stiff thallus without identified medullary substances, a thinner cortex and axis as well as a larger medulla. Furthermore, U. ghattensis is grouped in the Usnea 4 clade (Fig. 2). Usnea norkettii and U. spinulosa have strongly constricted lateral branches, a CMA of the brasiliensis-type and different medullary substances.
Selected specimens examined. Brazil: Rio Grande do Sul: Cambará do Sul, Parque Nacional dos Aparados da Serra, 1000 m, 1986, M. Fleig 2837 (ICN); ibid., Cânion Itaimbezinho, on Araucaria angustifolia, 2014, A. Gerlach 1406 (ICN); São Francisco de Paula, Paulinas de São Francisco, 29°27'S, 50°34'W, 900–1000 m, 1989, S. Grundlehner s. n. (G). Santa Catarina: Serra Geral, in silva Araucariarum, 1891, E. Ule 120 (G); Campo Alegre, Serra do Quiriri, 1200 m, 2012, A. Charnei 562 (ICN); Urubici, Parque Nacional de São Joaquim, 2014, A. Gerlach 1354, 1360 (ICN). Paraná: Campina Grande do Sul, Serra do Ibitiraquire, cume do Morro Itapiroca, c. 1800 m, 2014, M. Engels s. n. (ICN); Guaratuba, Morro dos Perdidos, 2011, S. Eliasaro 5019 (UPCB); ibid., 2014, B. Canestraro 691 (ICN); ibid., 1260 m, 2013, A. Gerlach 1015 (ICN); ibid., saxicolous, 2013, E. Gumboski 4489 (ICN).
Usnea kalbiana P. Clerc & A. Gerlach sp. nov.
MycoBank No.: MB 819423
Similar to U. lunaria but differs in its matt instead of vitreous cortex and in the presence of annular instead of irregular cracks in the basal part of the thallus.
Type: Brazil, Minas Gerais, Serra da Mantiqueira, Fazenda São Mateus, östlich von Camanducaia, 1800 m, 30 November 1980, K. Kalb s. n. (G—holotype; ICN, UPS, TNS—isotypes). %C/M/A: 13.5/11.5/50 (holotype). Ascospores (holotype): (7·5–)8·0–8·5–9·0 µm (n = 11). Chemistry: usnic and protocetraric acids (holotype).
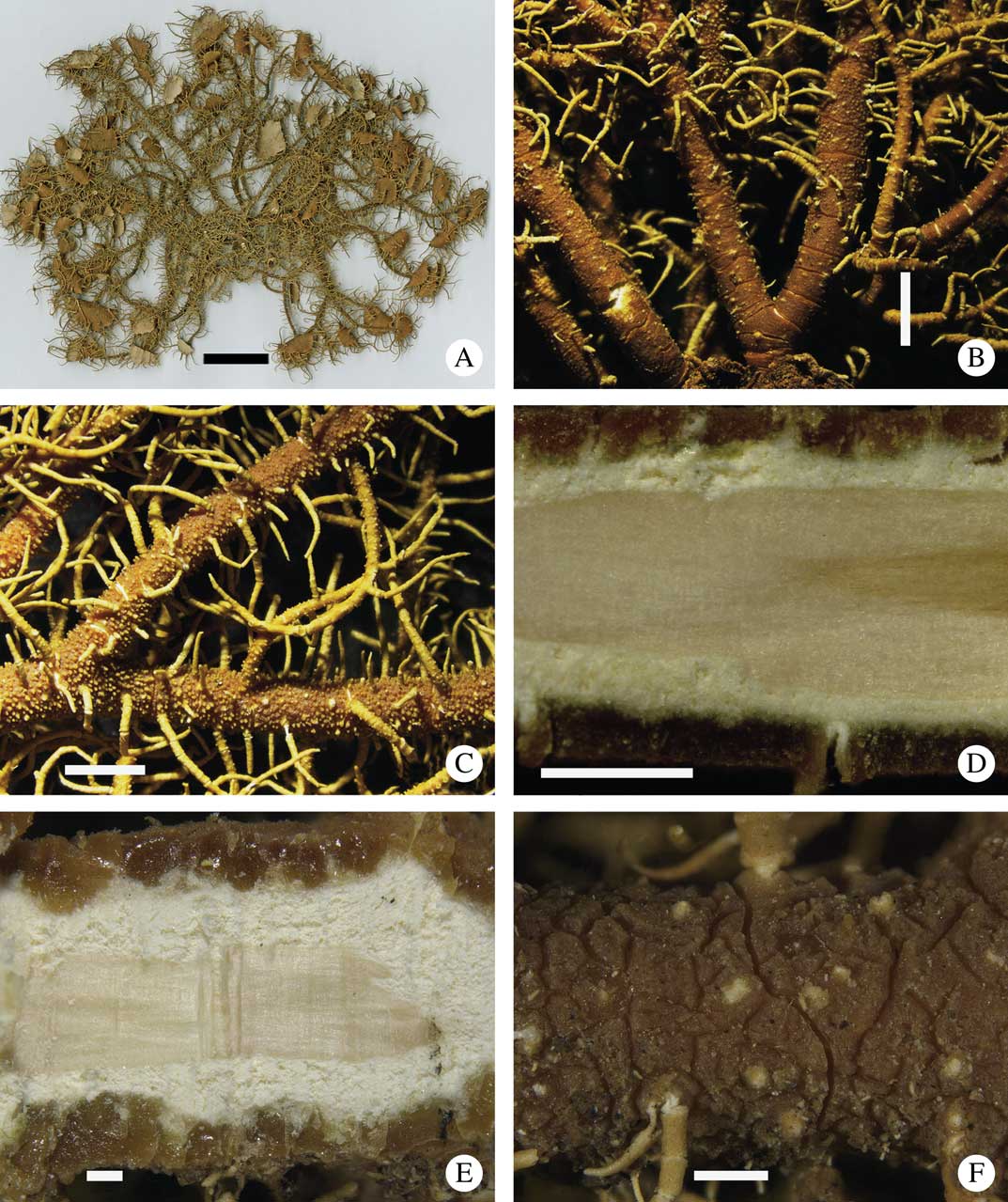
Fig. 8 A–D, Usnea kalbiana (holotype): A, thallus; B, trunk concolorous with branches with conspicuous annular cracks; C, branches annulated, terete and cylindrical, tubercles cone-shaped; D, section through branch with matt cortex. E & F, Usnea lunaria (holotype): E, section through branch with vitreous cortex; F, irregular cracks on the cortex surface. Scales: A=4 cm; B & C=2 mm; D & E=500 µm; F=1 mm. In colour online.
Thallus (n=33) erect-shrubby, yellowish green, up to 12 cm long, mostly isotomic- dichotomously branched; trunk often short, up to 3 mm long, concolorous with main branches, with annular cracks; main branches up to 1·5 mm thick, tapering, terete in cross-section, distinctly segmented; segments cylindrical and terete; lateral branches not constricted at the ramification point; foveolae, maculae and pseudocyphellae unknown; papillae scarce to none; tubercles (young fibrils?) often numerous, evenly distributed, cone-shaped, often eroded and whitish at summit; fibrils slender, up to 4 mm, few and unevenly distributed to numerous and in fishbone-like pattern; fibercles few to none; cortex matt in cross-section, sometimes slightly shiny, rarely with irregular cracks, moderately to usually thick, with florida-type plectenchyma; medulla white, dense to compact, thin; axis moderately thick to thick. CMA (n=15): %C=(8·5–)10·0–12·5–15·0(–16·0); %M=(8·0–)10·5–14·0–17·5(–18·0); %A=(33–)37–47–57(–67). A/M=2–4–6(–8).
Apothecia numerous, terminal and lateral, up to 10 mm diam.; ascospores: length = (7·0–)8·8 ± 9·7(–10·0) µm, width = (5·5–)6·0 ± 0·4(–7·0) µm, n = 10.
Chemistry
Medulla K−, P+ orange. TLC: protocetraric acid (n=25).
Etymology
Named after the distinguished lichenologist Klaus Kalb who has contributed so much to the current knowledge of the South American lichen flora, including numerous collections of Usnea from Brazil.
Habitat and distribution
Usnea kalbiana is a corticolous and lignicolous species. It is known only from Brazil, mainly in mountainous areas (above 1200 m) in the Serra da Mantiqueira of Minas Gerais.
Taxonomic remarks
Usnea kalbiana ressembles U. lunaria and both taxa are characterized by the presence of protocetraric acid in the medulla. However, the cortex in cross-section is matt in U. kalbiana (Fig. 8D) and vitreous in U. lunaria (Fig. 8E). Furthermore, U. lunaria has conspicuous irregular cortical cracks (Fig. 8F) whereas U. kalbiana produces annular cracks (Fig. 8B). Usnea subparvula is another species of the group with protocetraric acid. However, it differs from U. kalbiana by the absence of annulation in the basal thallus, the absence of tubercles and the presence of numerous spinulous fibrils evenly and densely distributed on the branches, a thinner cortex, a thicker medulla and a thinner axis. Usnea concinna has slightly constricted lateral branches, a thinner cortex and axis and a wider medulla, as well as a different chemistry (stictic acid group) to U. kalbiana. We were unable to get freshly collected material to obtain good quality DNA and hence the phylogenetic position of U. kalbiana remains unclear.
Specimens examined
Brazil: Paraná: Balsa Nova, Serra S’Ana, matinha nebular, epifita, 1969, G. Hatschbach 21365 (MBM). Santa Catarina: Caçador, Rodovia SC 451, on fences, 2013, E. Gumboski 4718, 4719 (ICN). São Paulo: Serra da Mantiqueira, Campos do Jordão, 150 km Nordöstlich von São Paulo in einem hellen, feuchten Urwald, 1700 m, 1978, K. Kalb & G. Plöbst s. n. (G-260935); Piquete, próximo ao Pico dos Marins, corticicola, 1200 m, 2012, A. Spielmann 10023 (CGMS). Minas Gerais: Serra da Mantiqueira, Vila Monte Verde, etwa 30 km östlich von Camanducaia, 1978, K. Kalb & G. Plöbst s. n. (G─260938). Rio de Janeiro: Itatiaia, zwischen Registro do Picú und Agulhas Negras, 1978, K. Kalb & G. Plöbst s. n. (G-260936).
Usnea lunaria Motyka
Lich. Gen. Usnea Stud. Monogr. Pars Syst. 2: 328 (1938); type: Brazil, Minas Gerais, Plateau d’Itacolumi, ad saxa, Damazio s. n. (W!—holotype). %C/M/A: 13.5/18/36. Ascospores: (7·0–)8·0–8·8–9·5(–10·0)×6·0–6·5–7·0 µm (n = 20). Chemistry: usnic and protocetraric acids.
Thallus (n=2) erect-shrubby, up to 9 cm long, mostly anisotomic-dichotomously branched; trunk up to 7 mm long, concolorous with main branches, with annular cracks; main branches up to 1·9 mm thick, tapering, terete in cross-section, distinctly segmented; segments cylindrical and terete; lateral branches not constricted at the ramification point; foveolae, maculae and pseudocyphellae unknown; papillae numerous, cylindrical, ±evenly distributed; tubercles (young fibrils?) often numerous, ±evenly distributed, cylindrical, rarely eroded; fibrils slender, up to 3 mm, few and unevenly distributed; fibercles scattered; cortex vitreous in cross-section, with many irregular cracks on main branches, thick, with plectenchyma intermediate between ceratina- and merrillii- type; medulla white, compact, thin; axis moderately thick to thick. CMA (n=2): %C=12·5–14·5–16·5; %M=12·5–14·5–18; %A=30·0–40·5–51·0. A/M=2–3–4.
Apothecia numerous, terminal and lateral, up to 18 mm diam.; ascospores: length = (7·0−)8·7 ± 1·1(−12·0) µm, width = (5·0−)5·7 ± 0·5(−7·5) µm, n = 2.
Chemistry
Medulla K−, P+ orange. TLC: protocetraric acid (n=2).
Habitat and distribution
The holotype was collected on rocks (Motyka Reference Motyka1938) but the specimen collected by Schenck that was seen for this study grew on trees. Thus U. lunaria is both saxicolous and corticolous. In Brazil, it is known from Mato Grosso (Motyka Reference Motyka1938), Minas Gerais and Rio de de Janeiro.
Taxonomic remarks
Usnea lunaria is characterized by its thick tapering and terete branches that have a thick, vitreous and irregularly cracked cortex (Fig. 8E & F), the numerous apothecia and the presence of protocetraric acid in the medulla. For differences with U. kalbiana, see under this species. We were unable to get freshly collected material to obtain good quality DNA and hence the phylogenetic position of U. lunaria remains unclear.
Specimen examined
Brazil: Rio de Janeiro: Corcovado, an Bäumen, 1887, H. Schenck 4458 (G—260937).
Usnea meridionalis Zahlbr.
Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl., 83: 187 (1909); type: Brazil, Rio Grande do Sul, Neu-Württemberg, prope Elsenau, ad ramos Accaciarum, A. Bornmüller s. n. (W!—holotype; FH!—isotype). %C/M/A: 2/44/8 (measurements by Clerc in 1999). Ascospores (28 spores measured): (9·0–)10·0–10·5–11·0×(6·0–)6·6–7·0–7·5(–8·0) µm (measurements by Herrera-Campos in 1997). Chemistry: usnic acid and an unknown substance with white fluorescence after charring with Rf classes A/B/C: 1–2/2/2.
Usnea michauxii I. I. Tav., syn. nov., Mycotaxon 30: 54 (1987); type: USA, Carolina (PC!—lectotype). %C/M/A: 6/34.5/19. Ascospores: (7·5–)8·0–9·0–10·0×5·0–5·5–6·0 µm (n = 7). Chemistry: usnic acid and an unknown substance with blue fluorescence after charring with Rf classes A/B/C=2–3/4/4.
Thallus and apothecia (n=55). For a detailed description, see Truong et al. (Reference Truong, Bungartz and Clerc2011). CMA (n=23): %C=(2–)3–4–5(–8); %M=(24·5–)31·0–35·0–39·0(–44·0); %A=(8–)15–21–27(–41). A/M=(0·3–)0·4–0·6– 0·8(–1·2). Cortex with ceratina-type plectenchyma. Ascospores: length = (7·0–)9·6 ± 1·0(–13·0) µm, width = (4·5–)5·9 ± 0·4(–7·5) µm, n = 12.
Chemistry
Medulla: 1) K−, P−, TLC=undetermined triterpenoids, ±fatty acids (n=8); 2) K+ yellow→red, TLC=salazinic acid (n=5); 3) K+ yellow slowly→red, TLC=norstictic, ±salazinic, ±stictic (trace) acids (n=5); 4) K−, P−, TLC=no medullary substances detected (n=2).
Habitat and distribution
Usnea meridionalis is a species that is frequent in southern Brazil, occurring in a wide range of habitats: Araucaria forest, Pampa, high elevation tropical grasslands, restinga, open pastures and urban parks. It seems to be frequent in humid areas along river banks. This species occurs on a variety of corticolous (twigs and trunk) or lignicolous substrata and often grows together with U. steineri and/or U. erinacea.
Taxonomic remarks
Usnea meridionalis is characterized by the minute, ±numerous red dots (sometimes appearing as black dots on old herbarium specimens) on the cortex surface, the fusiform branches that are constricted at the attachment points and a cornuta- or brasiliensis-type of CMA with a lax medulla. The holotype of U. meridionalis represents an extremely well-developed thallus with main branches that are distinctly swollen, numerous foveoles and a brasiliensis-type of CMA. All transitional forms seem to exist between this form and the lectotype of the North American U. michauxii that is characterized by less swollen branches and a cornuta-type of CMA. The occasional presence of a yellow medullary periaxial pigment and the red dots on the cortex surface indicate that this species might be closely related to U. flavocardia Räsänen. Both species would benefit from revision, including molecular phylogenetic analysis. For differences between U. meridionalis, U. cirrosa and U. cladocarpa, see under these taxa. In our phylogenetic analysis, U. meridionalis was closely related to U. glabrata (Fig. 2).
Selected specimens examined
Brazil: Rio Grande do Sul: Bagé, Casa de Pedra, on shrubby tree, near river, 1989, M. Fleig 4082 (ICN); Caçapava do Sul, on shrubby tree, riverside of Rio Camacuã, 1988, M. Fleig 3349 (ICN); Cachoeira do Sul, on twigs, riverside Capanezinho, 1993, M. Fleig 5600 (ICN); Cambará do Sul, Parque Nacional da Serra Geral, Cânion Itaimbezinho, 2014, A. Gerlach 1410 (ICN); Caxias do Sul, Santa Lucia do Piai, Água Azul, 735 m, 2010, A. Spielmann 8666 (CGMS); Esmeralda, Estação Ecológica de Aracuri, 1982, M. Fleig 1469 (ICN); Mariana Pimentel, 100 m, 1989, S. Grundlehner s. n. (G); Lagoão, on shrubby tree, borda de mata, 2000, A. Spielmann 11902 (CGMS); Piratini, Pampa, 2015, R. Jeeval s. n. (ICN); Rio Grande, Estação Ecológica do Taim, on twigs, 2015, E. Fazolino s. n. (ICN); São Francisco de Paula, Colinas de São Francisco, on Araucaria angustifolia, 1000 m, 1989, S. Grundlehner 4273 (ICN); Triunfo, Copesul, beira de rio, 1992, N. Cardoso s. n. (HAS). Santa Catarina: Bom Jardim da Serra, near Parque Nacional de São Joaquim, on trunk of Mimosa scabrela, 1994, M. Fleig 6569 (ICN); Urubici, Parque Nacional de São Joaquim, on twigs, 2014, A. Gerlach 1348 (ICN); São Joaquim, Serra do Rio do Rastro, an alten Araukarien in einer Weide am Ortsrand, 1420 m, 1988, Schäfer-Verwimp L/10566 (G). Paraná: Carambeí, Catanduva de Fora, Rio Jotuba, 2013, M. Engels s. n. (ICN); Curitiba, Parque Tanguá, 2012, A. Gerlach 838 (ICN); ibid., Universidade Federal do Paraná, Centro Politécnico, 1993, S. Eliasaro 1064 (UPCB); Palmeira, margens Rio Cariú, 2013, M. Engels s. n. (ICN); Ponta Grossa, Pinhão, on fences of Phoebe porosa, 1975, L. Krieger 13824 (JPB); Quatro Barras, Parque Estadual da Serra do Baitaca, Morro do Anhangava, 1200 m, 2014, E. Santos 101 (UPCB). São Paulo: Serra do Mar bei Paranapiacaba an der Eisenbahnlinie zwischen SP und Santos, Regenwald, 1000 m, 1986, Schäfer-Verwimp L7616 (G). Minas Gerais: Serra de Ibitipoca, 1975, L. Krieger s. n. (JPB). Rio de Janeiro: Itatiaia, Brejo da Lapa, 2160 m, 1984, M. Guerra s. n. (RB); Serra do Picu, Schenck 4448 (G).
Usnea cf. moreliana Motyka
Lich. Gen. Usnea Stud. Monogr. Pars Syst. 2: 584 (1938); type: Mexico, Morelia, Cerro San Miguel, 1910, Brouard 137 (LBL!—neotype, isoneotype). %C/M/A: 6/32/24.5. Chemistry: usnic acid, unidentified triterpenoids UT6 (Truong & Clerc Reference Truong and Clerc2016).
Thallus (n=10). For a detailed description and illustrations see Truong et al. (Reference Truong, Bungartz and Clerc2011) (as U. rubricornuta) and Truong & Clerc (Reference Truong and Clerc2016). Cortex with ceratina-type plectenchyma.
Chemistry
Medulla K−, P−. TLC: 1) triterpenoid UT6 (n=4); 2) no medullary substances detected (n=3).
Habitat and distribution
Usnea cf. moreliana is an uncommon species occurring in southern Brazil in São Paulo and Rio de Janeiro. This taxon sometimes grows together with U. erinacea.
Taxonomic remarks
Usnea moreliana s. str. is a reddish-pigmented, sorediate taxon characterized by distinctly constricted branches at the attachment point, a cornuta-type CMA and a K−, P− medulla (triterpenoids UT6) (Truong & Clerc Reference Truong and Clerc2016). The specimens studied here most probably correspond to the fertile counterpart of U. moreliana s. str. and to Usnea sp. 2 mentioned by Truong et al. (Reference Truong, Bungartz and Clerc2011: 65). More material and molecular assessment are necessary before any taxonomic decisions can be taken.
Selected specimens examined
Brazil: Rio Grande do Sul: Esmeralda, Estação Ecológica de Aracuri, on fences, M. Fleig 1815a (ICN). Paraná: Ponta Grossa, Parque Estadual de Vila Velha, em ramos, Sanders s. n. (UFP). Santa Catarina: Campo Alegre, Campos do Quiriri, campo de altitude, 2012, E. Gumboski 3584 (ICN); Prudentópolis, rural area, 2012, A. Charnei 551 (ICN); Rio Negrinho, Fazenda Velha, Araucaria forest, 2007, E. Gumboski 1020 (ICN). São Paulo: Mogi-Guaçu, Martinho Prado Jr., Reserva Biológica e Estação Experimental de Mogi Guaçu, 2007, 22°16'S, 47°09'W, c. 630 m, M. Benatti et al. s. n. (SP); Pindamonhangaba, Pico de Itapeva, 1966, D. Vital s. n. (JPB). Rio de Janeiro: Santa Maria Madalena, Parque Estadual do Desengano, Pedra do Desengano, 1500 m, campo de altitude, 1986, G. Martinelli et al. 11995 (RB).
Usnea parvula Motyka
Lich. Gen. Usnea Stud. Monogr., Pars Syst. 2: 599 (1938); type: Argentina, Cordoba, Sierra Achala, 1876, Hieronymus s. n. (G!—isotype). %C/M/A: 6/25/38. Chemistry: usnic acid, an unknown yellow spot with Rf classes A/B/C=6/2/5–6 and a fatty acid with Rf classes=4/2/5–6. Ascospores: 8·0–8·5–9·0(–9·5)×4·5– 5·0–5·5(–6·0) µm (n = 20) (TLC and measurements by Clerc in 1995).
(Fig. 9A)

Fig. 9 A, Usnea parvula. A, branches densely covered with fibrils (A. Gerlach 870). B–D, Usnea subparvula (holotype): B, branches wider at ramification point, covered with spinulose fibrils; C, section through branch; D, details of conical spinulose fibrils. E & F, Usnea sp. 1 (A. Gerlach 1321): E, fusiform branches, constricted at attachment point; F, section through branch, the periaxial tissue is strongly pigmented yellow (area indicated by arrows). Scales: A & E=2 mm; B & F=1 mm; C=500 µm; D=200 µm. In colour online.
Thallus and apothecia (n=82). For a detailed description, see Clerc (Reference Clerc2007). CMA (n=20): %C=(3·0–)4·5–6·0–7·5(–8·5); %M=(11·0–)21·0–27·0–33·0(–37·5); %A=(19–)24–34–44(–62). A/M=0·5–1·5–2·5(–5·5). Cortex with ceratina-type plectenchyma. Ascospores: length = (6·0−)7·8 ± 0·8(−11·0) µm, width = (4·0−)5·2 ± 0·5(−6·0) µm, n = 17.
Chemistry
Medulla K−, P−. TLC: 1) unknown yellow spot with Rf classes A/B/C=6/1-2/5 (Us1), ±caperatic acid, ±triterpenoid (rare), and ±eumitrin (rare) (n=25); 2) caperatic acid, ±Us1, and ±triterpenoid (n=6); 3) triterpenoid and fatty acids (n=5); 4) no medullary substances detected (n=2).
Habitat and distribution
Usnea parvula is known only from the American continent: USA, Mexico (Clerc Reference Clerc2007), Argentina, Colombia, Paraguay, Uruguay and Brazil (where it was previously mentioned only from Minas Gerais) (Motyka Reference Motyka1938) and Venezuela (Vareschi Reference Vareschi2001). In southern Brazil, U. parvula occurs mainly in coastal habitats close to the seashore where it grows on shrubby trees on sandbanks or at the edge of lagoons. It also occurs in rural areas on trees in pastures.
Taxonomic remarks
Usnea parvula is characterized by the numerous spinulose fibrils that densely cover the branches, the irregular branches that are±obtuse- to acute-angled andwith ±swollen segments with foveoles and transverse furrows, the shiny cortex, the dense medulla and the presence of fatty acids (K−, P−) in the medulla. Unlike Clerc (Reference Clerc2007), we found that the lateral branches might be slightly to distinctly constricted, reflecting the variability in the shape of the branches in this species (Fig. 9A). For differences between U. parvula and U. subparvula or U. complanata (Müll. Arg.) Motyka, see under U. subparvula. Usnea subelegans shares the numerous spinulose fibrils with U. parvula, but the former species has galbinic acid in the medulla (K+ yellow→red) as well as less irregular and more cylindrical branches that usually have terete segments in cross-section. Usnea steineri also has a K−, P− medulla but its fibrils are usually not spinulose and it has a thin red subcortical pigmentation. The three specimens included in the molecular study form a strongly supported group in Fig. 2 but the phylogenetic affinities of this group remain unresolved.
Selected specimens examined
Brazil: Rio Grande do Sul: Caraá, Área de Preservação Ambiental, 2014, A. Gerlach 1501 (ICN); Mariana Pimentel, 100 m, 1989, S. Grundlehner s. n. (G); Pelotas, in kleiner Baumpflanzung, 100 m, 1986, Schäfer-Verwimp L/7884 (G); Porto Alegre, Morro Santana, 2014, A. Gerlach 1085 (ICN); ibid., Botanical Garden, 2009, F. Lucheta s. n. (HAS); Rio Grande, Estação Ecológica do Taim, on twigs, 2015, E. Fazolino s. n. (ICN); Rondinha, Arroio do Sal, on twigs in sandbank, 2014, E. Fazolino s. n. (ICN); Triunfo, Fazenda Santa Maria, 2013, F. Lucheta s. n. (ICN); Vale do Sol, 15 de Novembro, on Arecastrum sp., 1993, M. Fleig 5285 (ICN); Uruguaina, Parque Estadual do Espinilho, 1991, T. Burdulis s. n. (ICN); Viamão, Morro da Grota, on Dodonea viscosa, 1980, L. Aguiar 490 (HAS); ibid., Itapuã, 200 m, 1989, T. Ahti 21 (ICN). Santa Catarina : Concórdia, Presidente Kennedy, 1986, C. Grabauska 430 (ICN); Florianópolis, Parque Municipal da Lagoa do Peri, on Schizolobium parahyba, 2013, A. Gerlach 1203 (ICN); praia da Armação, em galhos, restinga, 2013, A. Gerlach 871 (ICN); Joaquina beach, on shrub on sandstone, 1988, S. Eliasaro 632 (BHCB). Paraná : Curitiba, Universidade Federal do Paraná, Centro Politécnico, 1994, C. Morales 12 (UPCB); Ponta Grossa, riverside, 1978, L. Krieger 15798 (CESJ). São Paulo: Pardinho, Fazenda Águas de Janeiro, 800 m, 2011, P. Jungbluth 2860 (ICN); São Bento do Sapucaí, Serra da Mantiqueira, Westanstieg zum Pedra do Baú, epiphytisch in einer Weide bei 1350 m, 1989, Schäfer-Verwimp L/11816 (G).
Usnea steineri Zahlbr.
Denkschrift. Math. Naturw. Classe Kais. Akad. Wiss. Wien 83: 183, 186 (1909); type: Brazil, São Paulo, ad Sta. Anna propre Lapa in distr. urbis S. Paulo, 1901, Schiffner s. n. (W!—holotype; G!—isotype). %C/M/A: 7.5/35/15. Ascospores: 7·5–10·0×6–7 µm. Chemistry: unidentified triterpenoids UT6 (CMA, chemistry, ascospores fide Truong et al. Reference Truong, Bungartz and Clerc2011).
(see Fig. 8 in Truong et al. (Reference Truong, Bungartz and Clerc2011: 497))
Thallus (n=73): for a detailed description, illustrations and synonyms see Truong et al. (Reference Truong, Bungartz and Clerc2011). CMA (n=4): %C=(7·0–)7·5–8·0–9·5; %M=(21–)22–28–34(–35); %A=(15–)17–29–41(–44). A/M=0·5–1·5–2·5. Cortex with baileyi-type plectenchyma.
Apothecia numerous, lateral, terminal to subterminal, up to 25 mm diam.; ascospores: length = (6·0−)8·3 ± 0·8(−11·0) µm, width = (4·0−)5·4 ± 0·5(−6·0) µm, n = 11.
Chemistry
Medulla K−, P−. TLC: 1) unidentified triterpenoids (n=14); 2) fatty acids (n=5); 3) no medullary substances detected (n=7).
Habitat and distribution
Argentina, Bolivia, Peru (Truong et al. Reference Truong, Bungartz and Clerc2011), Brazil, Colombia, Uruguay (Motyka Reference Motyka1938) and Venezuela (Vareschi Reference Vareschi2001). Also known from tropical Africa (Swinscow & Krog Reference Swinscow and Krog1979). For Brazil, this species has been recorded in Rio Grande do Sul, Santa Catarina, Paraná, São Paulo and Minas Gerais (Motyka Reference Motyka1938). It is rare in the neotropical Andes (Truong et al. Reference Truong, Bungartz and Clerc2011). Usnea steineri is common in southern Brazil, where it can grow side by side with U. erinacea on a variety of corticolous or lignicolous substrata.
Taxonomic remarks
Usnea steineri can be recognized by its red subcortical pigmentation that is found just below the cortex (the pigmentation might also spread into the lower cortex) and by the K−, P− medulla. However, U. steineri is a morphologically polymorphic species. According to Truong et al. (Reference Truong, Bungartz and Clerc2011) there are three morphotypes differing in the shape of branches, the axis/medulla ratio and the type of fibrils. Most of the Brazilian specimens belong to the steineri-morphotype with inflated and constricted branches, a dense to often lax medulla and long fibrils scattered all along the thallus. Specimens of the subdasaea-morphotype (with short spinulose fibrils) and of the krempelhuberi-morphotype (with non-inflated, non-constricted branches) are less frequent in southern Brazil. In the phylogenetic analyses, U. steineri is a sister group to U. erinacea (Fig. 2). In Fig. 2, both specimens from Peru appear to belong to the subdasaea-morphotype whereas the two specimens from Brazil belong to the steineri-morphotype. These results, combined with the discovery in Brazil (outside the southern area) of several specimens with a different chemistry (galbinic, salazinic or stictic acids), indicate that this species might form a complex of several so far undescribed species. Usnea aurantiaca-parvula, U. erinacea and U. meridionalis also have an orange-reddish pigmentation. In U. aurantiaca-parvula the pigmentation is diffuse in the whole medulla, there are numerous foveoles and the spinules are constricted at the base. The pigmentation of Usnea erinacea and U. meridionalis is exclusively cortical.
Selected specimens examined
Brazil: Rio Grande do Sul: Caçapava do Sul, arroio Seival, mata de galeria junto a campo de pastagem, 1993, M. Fleig 5681 (ICN); Cachoeira do Sul, arroio Capanezinho, riparian forest, 1993, M. Fleig 5599 (ICN); Camaquã, margens do Arroio Velhaco, 1985, C. Grabauska 8 (ICN); Esmeralda, Estação Ecológica de Aracuri, on Araucaria angustifolia, 1984, M. Fleig 2441 (ICN); Itaqui, Fazenda Bola de Ouro, on twigs of shrub, mata de galeria, 1994, M. Fleig 6546 (ICN); Mariana Pimentel, près de Barra do Ribeiro, 30°21'S, 51°35'W, 100 m, 1989, S. Grundlehner s. n. (G); Passo dos Freire, São Sepé, on shrubby tree, 1985, M. Fleig 2533 (ICN); Rio Grande, Estação Ecológica do Taim, 2014, E. Fazolino s. n. (ICN); Santana do Livramento, APA do Ibirapuitã, Fazenda Lolita, 2012, M. Käffer 867 (HAS); São Gabriel, mata de galeria junto a campo de pastagem, 1993, M. Fleig 5455 (ICN). Santa Catarina: Alfredo Wagner, RPPN Rio das Furnas, 2013, A. Gerlach 1263 (ICN); São Bento do Sul, APA do Rio Vermelho, 2012, E. Gumboski 3822 (ICN); São Joaquim, Serra do Rio do Rastro, an alten Araukarien in einer Weide am Ortsrand, 1420 m, 1988, Schäfer-Verwimp L/10567 (G); Urubici, Morro da Igreja, 1650 m, 2004, A. Cervi 8715 (UPCB). Paraná: Campo Largo, on fences, 2012, A. Gerlach 771 (ICN); Carambeí, Catanduva de Fora, on fences, 2013, M. Engels s. n. (ICN); Castro, Cânion Guartelá, 2013, L. Rocha s. n. (ICN); Curitiba, margem do rio Iguaçú, on Sebastiana commersoniana, 1985, M. Fleig 2639 (ICN); Guarapuava, 2013, M. Engels s. n. (ICN); Piraí do Sul, Fazenda Nova Era, 2012, B. Canestraro 483 (ICN); Ponta Grossa, Pinhão, on fences of Phoebe porosa, L. Krieger 13831 (JPB); Prudentópolis, 2012, A. Charnei 547 (ICN); São José dos Pinhais, Rio Iguaçú, on Prunus sellowii, 1985, M. Fleig 2651 (ICN). São Paulo: Mogi-Guaçu, Reserva Biológica de Mogi-Guaçu, 22°15'06·1''S, 47°09·28'6''W, 620 m, A. Spielmann 7145 (CGMS); Piquete, 22°31'30·1''S, 45°08'59''W, 1200 m, 2012, A. Spielmann 10023 (CGMS). Rio de Janeiro: Serra dos Órgãos, Paβstraβe zwischen Teresopolis und Petropolis, in Bergregenwald epiphytisch bei 1330 m, 1986, Schäfer-Verwimp L/7401 (G).
Usnea subelegans (Vain.) B. de Lesd.
Ann. Cryptog. Exot. 6: 112 (1933).—Usnea barbata var. subelegans Vain., Étud. Lich. Brésil 1: 6 (1890); type: Brazil, Minas Gerais, Sitio, 1885, Vainio (TUR-V 639!—lectotype designated here). %C/M/A: 6.5/26.5/34. Ascospores: 9–9·5–10×6–6·5–7(–8) µm (measurements by Herrera-Campos in 1997). Chemistry: usnic, galbinic, norstictic and salazinic acids (TLC by Clerc in 1996).
(Fig. 6F)
Nomenclatural note
Five syntypes of U. subelegans were found in TUR-V. TUR-V 638 corresponds to a spinulose sorediate thallus with galbinic acid (=U. dasaea Stirt.). The four remaining packets contain specimens with apothecia but without soralia: TUR-V 753 and 754 with salazinic acid, strongly inflated and irregular primary branches and a brasiliensis-type of %CMA (2·5–4/40–45/6–13); TUR-V 639 and 661 with galbinic, norstictic and salazinic acids, and with±cylindrical, not inflated primary branches and a cornuta-type %CMA (5–6/28–35/20–32). It is possible that two different species might be present here (see under taxonomic remarks below). In the protologue, the taxon was described as reacting K+ yellow, then orange-red. Only the galbinic acid specimens were found to show such a reaction (the specimens with only salazinic acid showed almost no reaction to K). We thus decided to lectotypify this name using one of the galbinic acid-containing specimens.
Thallus (n=82). For a detailed description see Clerc (Reference Clerc2007). C/M/A (n=18): %C=(3·5–)4·5–6·0–7·5(–9·5); %M=(24·0–)25·5–29·5–33·5(–35·0); %A=(20–)22–28–32(–39). A/M=(0·6–)0·7–1·0–1·3(–1·6). Cortex with merrillii-type plectenchyma.
Apothecia mainly lateral, up to 10 mm diam.; ascospores: length = (7·0−)8·9 ± 0·8(−11·0) µm, width = (4·0−)5·7 ± 0·7(−8·0) µm, n = 10.
Chemistry
Medulla: 1) K+ yellow→red, TLC=salazinic, norstictic, galbinic and±constictic acids (n=48); 2) K+ yellow →slowly red, TLC=stictic, constictic, cryptostictic, menegazziaic and norstictic acids (n=1).
Habitat and distribution
Usnea subelegans usually grows in the same habitat as U. parvula, mainly in coastal and rural areas. It occurs in Mexico (Clerc Reference Clerc2007), Panama (Motyka Reference Motyka1938) and in South America where it is widespread: Argentina, Colombia, Paraguay, Peru, Uruguay (Motyka Reference Motyka1938) and Venezuela (Marcano et al. Reference Marcano, Morales Méndez, Sipman and Calderon1996). In Brazil, it was previously recorded from Rio Grande do Sul, Santa Catarina and Paraná (Motyka Reference Motyka1938), São Paulo (Zahlbruckner Reference Zahlbruckner1909), Minas Gerais (Vainio Reference Vainio1890), Rio de Janeiro (Rizzini Reference Rizzini1952), Mato Grosso do Sul (Osório Reference Osório1992) and Mato Grosso (Motyka Reference Motyka1938).
Taxonomic remarks
Usnea subelegans is the only erect-shrubby apotheciate species with galbinic acid found in southern Brazil. The densely spinulose branches (Fig. 6F), the mainly lateral apothecia, the thin and ± shiny cortex, the dense to somewhat lax medulla and the usually orange medullary pigmentation (probably due to the oxidation of secondary metabolites) around the axis make this species easy to recognize. This taxon seems to be quite variable with ± swollen main branches (diameter varying from 0·8 to 1·8 mm), ±numerous spinulose fibrils (20 to 40 fibrils mm–2) and with a variable %CMA (see above). A few specimens were found to be subpendulous to pendulous. The chemistry (salazinic, norstictic and galbinic acids) seems to be constant, although we found one specimen with only stictic acid. The syntypes containing only salazinic acid (TUR753 and TUR754), with irregular and very swollen main branches and an extremely low A/M (0·1–0·4), correspond well to U. tincta (Zahlbr.) Motyka (W!—holotype (chemistry: norstictic, salazinic and galbinic acids; %CMA=4/39.5/14, A/M=0·3; ascospores: (8·5–)9·0–9·5–10·0×(5·5–)6·0–6·5–7·0 µm); BM!—isotype (chemistry: salazinic acid; %CMA=2.5/41.5/12, A/M=0·3)). More material and molecular studies are needed in order to decide whether these taxa are conspecific. The specimens mentioned by Motyka (Reference Motyka1938: 522) for Paraná and Rio Grande do Sul under U. tincta correspond to the U. subelegans morphotype. Usnea leioclada (Zalhbr.) Motyka is another esorediate, apotheciate species with galbinic acid described from Brazil, but not found as yet in the south. It differs from U. subelegans mainly by the absence of spinulose fibrils and by the CMA values (7/17.5/51, BM!—holotype). For differences between U. subelegans, U. parvula and U. subparvula see under the latter two species. We were unable to obtain good quality DNA and hence the phylogenetic position of U. subelegans remains unclear.
Selected specimens examined
Brazil: Rio Grande do Sul: Arambaré, restinga, 2014, F. Lucheta s. n. (ICN); Camaquã, riverside, 1985, C. Grabauska 5 (ICN); Cachoeira do Sul, on twigs of Sebastiana commersoniana, riparian forest, 50 m, A. Spielmann 6387 (CGMS); Caraá, 2013, N. Koch s. n. (ICN); Caxias do Sul, 735 m, 2010, A. Spielmann 8666 (CGMS); Mariana Pimentel, 1989, S. Grundlehner (G); Passo dos Freire, São Sepé, 1985, M. Fleig 2603 (ICN); Porto Alegre, Morro Santana, 2014, A. Gerlach 1084 (ICN); Rio Grande, Estação Ecológica do Taim, 2014, E. Fazolino s. n. (ICN); Rondinha, Arroio do Sal, on sandstone, 2014, E. Fazolino s. n. (ICN); Santa Maria, on shrub along the road, 150 m, 1980, M. Fleig 1208 (ICN); Santana do Livramento, Fazenda Lolita, 2011, M. Käffer 445 (HAS); São Francisco de Paula, Parque Nacional da Ronda, N. Koch 65R (ICN); Torres, on fences, 2012, E. Gumboski 4063 (ICN); Viamão, Parque St. Hilaire, 1989, S. Grundlehner 363 (ICN). Santa Catarina: Fraiburgo, 2013, E. Gumboski 4744 (ICN); Joinville, Alto da Serra Dona Francisca, on fences, 2013, E. Gumboski 4664 (ICN); Major Vieira, 2012, E. Gumboski 4040 (ICN); Rio Negrinho, Fazenda Velha, 2009, E. Gumboski 937 (ICN); São Francisco do Sul, Capri, on Syagrus romanzoffiana, 2014, A. Gerlach 972 (ICN). Paraná: Campina Grande do Sul, Sitio do Belizario, 1000 m, 1967, G. Hatschbach 16437 (MBM); Carambeí, Catanduva de Fora, 2013, M. Engels (ICN); Guarapuava, Colônia São judas Tadeu, on shrub, 850 m, 1991, G. Hatschbach 55407 (MBM); Paranaguá, Ilha do Mel, 2012, A. Gerlach 784 (ICN); Paula Freitas, riparian forest, 2013, M. Engels s. n. (ICN); Piraquara, Mananciais da Serra, 2004, R. Reis 112 (UPCB); Ponta Grossa, 1978, L. Krieger 15805 (CESJ); Pontal do Paraná, Pontal do Sul, restinga, 2006, anon. (UPCB). Minas Gerais: Serra da Mantiqueira, Fazenda São Mateus, östlich von Camanducaia, 1800 m, 1980, K. Kalb s. n. (G); Antonio Carlos, L. Krieger 15942 (CESJ). Mato Grosso do Sul: Bonito, Fazenda América, deciduous forest, 2009, V. Pott 10682 (CGMS).
Usnea subparvula A. Gerlach & P. Clerc sp. nov.
MycoBank No.: MB 819424
Similar to U. parvula, but differs in the less numerous spinulose fibrils, with lateral branches that are often somewhat wider at the ramification point, a thicker cortex (8–10%), and the production of protocetraric acid in the medulla.
Type: Brazil, Mato Grosso do Sul, Porto Murtinho, Fazenda Sao Fernando, 21°34'26·57''S, 57°45'04·81''W, 94 m, pasture field near edge of deciduous forest, on fence posts, 13 September 2015, V. J. Pott & A. Pott 11873 (CGMS—holotype; G, ICN, UPS—isotypes). %C/M/A: 10/24.5/31. Ascospores: (6·0–)6·5–7·0–7·5(–8·0)×4·5–5·0 µm (n=22). Chemistry: usnic and protocetraric acids, an unknown protocetraric acid group with grey spot (Rf classes A/B/C=4/4–5/5–6) and an unknown triterpenoid (?) with yellow fluorescence after charring (Rf class A=6).
Thallus (n=64) erect-shrubby, up to 8 cm long, yellow-green, with anisotomic-dichotomous ramifications; trunk usually short, concolorous or paler than branches, rarely reddish, not annulated, smooth but occasionally wrinkled; main branches 1·0–1·8 mm thick, irregular to cylindrical, terete to flattened or often obtuse- to acute-angled in cross-section; lateral branches not constricted (rarely slightly constricted), often wider at the ramification point; foveolae usually present on main branches, not abundant; maculae and pseudocyphellae absent; papillae and tubercles absent; fibrils numerous (c. 12/mm2), spinulose, short and thick, 0·5–1·4(–3·0)×0·1–0·3 mm, regularly distributed on the whole thallus; fibercles present; cortex shiny, moderately thin to moderately thick, with ceratina-type plectenchyma; medulla white, compact, moderately thin to moderately thick; axis moderately thin to moderately thick. CMA (n=20): %C=(5·0–)6·0–8·0–10·0(–11·5); %M=(11·5–)15·0–20·5–26·0(–29·0); %A=(30·0–)34·5–43·0–51·5(–58·0). A/M=(1·0–)1·3–2·4–3·5(–5·0).
Apothecia numerous, mainly terminal, up to 5 mm diam.; ascospores: length = (5·5–)7·8 ± 0·8(–10·0) µm, width = (4·0–)5·2 ± 0·5(–6·0) µm, n = 6.
Chemistry
Medulla: 1) K−, P+ orange, TLC=protocetraric acid, ±an unknown acid with grey spot (Rf classes A/B/C=4/4–5/5–6) (n=16); 2) K+ slowly dull yellow, P+yellow, TLC=psoromic and conpsoromic acids (n=4).
Etymology
Named after the strong morphological similarity to U. parvula.
Habitat and distribution
Corticolous on twigs of shrubby trees or lignicolous on fence posts. It occurs in relatively open sites around farms, along roads, in Cerrado, Chaco, Pampa, riparian forest and occasionally in subtropical seasonal forest. This species is so far known from Argentina, Brazil and Paraguay. There are only two herbarium specimens of U. subparvula from southern Brazil. Usnea subparvula seems to occur inland whereas U. parvula is more of a coastal species occuring in the Atlantic forest.
Taxonomic remarks
Usnea parvula has a similar morphology to this new species, with its numerous spinulose fibrils and irregular branches in cross-section that have obtuse- to acute-angled segments. It differs mainly by the K−, P− reacting medulla and the density of spinulose fibrils (U. parvula: 16–24 mm–2, U. subparvula: 10–15 mm–2). Both taxa seem to belong to distinct lineages within the Usnea clade 4; however, their phylogenetic relationship lacks support (Fig. 2). Usnea subelegans has a K+ reacting medulla, a higher density of fibrils (18–30 mm–2), less irregular and more cylindrical branches usually with terete segments in cross-section and a much lower A/M. Usnea complanata is a small apotheciate African species (Swinscow & Krog Reference Swinscow and Krog1979) which also has spinulose fibrils and psoromic acid in the medulla. However, it has a brasiliensis-type CMA with a sinuose axis and more lageniform fibrils.
Selected specimens examined
Argentina: Cordoba, Cerro Colorado, bosque serrano, on Acacia praecox, 2004, J. Rodríguez 1788 (G).—Brazil: Rio Grande do Sul: Uruguaiana, Parque Espinilho, 1991, T. Burdulis s. n. (ICN). Paraná: Guaíra, Regenwald am Rio Paraná, 200 m, 1980, K. Kalb s. n. (G). São Paulo: Pindamonhangaba, Reserva Ecologica Municipal do Trabiju, 22°48'S, 44°32'W, 1100 m, 2010, M. Benatti 3193 (SP). Mato Grosso do Sul: Aquidauana, Piraputanga, Cerrado and Caatinga, 1987, I. Riquelme s. n. (ICN); Bodoquena, Fazenda Marambaia, campo rodeado por capim-navalha, 669 m, 2012, E. Souza 121 (CGMS); Bonito, Fazenda América, cerradão com afloramento rochoso, 21°10'12·90''S, 56°35'59·40''W, 414 m, 2010, V. Pott 11321 (CGMS); Campo Grande, on fences, 1989, Helio s. n. (ICN); Corguinho, Distrito de Taboco, 19°44'37·27''S, 55°15'52·86''W, 400 m, Cerrado, 2013, T. Sinani 18 (CGMS); Corumbá, sub-região Pantanal do Paraguai, margen da baia do Taquaral, 18°02'42·3''S, 57°30'15·2''W, 83 m, 2010, A. Spielmann 8784 (CGMS); Jaraguari, Furnas do Dionisio, 20°08'34·9''S, 54°34'21·2''W, 450 m, 2015, A. Spielmann 11885 (CGMS); Nova Andradina, Fazenda Laranjal, RPPN Cachoeira do Mimoso, cerradão, 22°2'44·8''S, 53°23'66·5''W, 359 m, 2014, A. L. Simal 245 (CGMS); Poconé, 36 km ao sul, pantanal, on fence beira estrada, Cerrado inundado, 100 m, 1989, M. Marcelli 4444 (ICN); Porto Murtinho, Fazenda Retiro Conceição, on fences on chaco vegetation, 21°40'57·60''S, 57°45'43·70''W, 91 m, 2010, L. Canêz 3689 (CGMS); Rio Negro, pantanal da Nhecolândia, on fences, Cerrado, 19°17'55·83''S, 55°06'1·04''W, 165 m, 2013, A. P. de Souza 51 (CGMS); Terenos, Fazenda Modelo da Embrapa, on Heteropterys coriacea, campo úmido de Cerrado, 20°33'33·8''S, 54°47'33·6''W, 2010, A. Spielmann 8103 (CGMS). Goiás: Água Fria, Estação Repetidora da Telebrasilia de Roncador, on twigs of Clusia sp., campo rupestre, 1992, G. Hatschbach 58931 (MBM).—Paraguay: Gran Chaco, zwischen B. Aceval und Algarrobo, on Eucalyptus sp., 150 m, 1980, K. Kalb s. n. (G).
Usnea sp. 1
This species is characterized by the fusiform branches that are constricted at the point of attachment, the brasiliensis-type CMA (%C=4·5–6·0, %M=35–40, %A=11–18, A/M=0·4, n=2) and the strongly yellow-pigmented medulla. Cortex with ceratina-type plectenchyma. The ascospores belong to class 2: (8–)9–10–11(–13)×(5·0–)5.5–6.5–7·0(–8·0) µm (n=2). The medulla reacts K+ yellow→slowly red (norstictic acid, n=1).
Taxonomic remarks
This morphologically distinctive species clusters together with a specimen of U. flavocardia Räsänen from Europe with psoromic acid in the medulla (Fig. 2). Norstictic acid is another chemotype of U. flavocardia in Europe (Clerc 1984 Reference Clercb , as U. wirthii). Furthermore, in the phylogenetic analyses Usnea sp. 1 forms a strongly supported group with U. flavocardia from Ireland (Fig. 2) and it could be the fertile counterpart of U. flavocardia. However, since what is called U. flavocardia in Europe might not be the same species as the South American U. flavocardia and as we found only one specimen corresponding to Usnea sp. 1, more material is needed before any taxonomic decisions can be taken.
Specimen examined
Brazil: Santa Catarina: Urubici, Parque Nacional de São Joaquim, on Araucaria angustifolia, near the lodging, 2014, A. Gerlach 1321 (ICN).
Uncertain or excluded species
Usnea comosa (Ach.) Vain., nom. invalid.
This species is a synonym of U. subfloridana Stirt. (Laundon Reference Laundon1965), the sorediate form of U. florida. The specimens from Brazil that were previously identified as U. barbata var. comosa Ach. belong in fact to several different species, such as U. cirrosa, U. erinacea or U. subelegans, and might even include one as yet undescribed species from Minas Gerais.
Usnea florida (L.) Wigg.
Usnea florida is the type species of the genus. It is a European shrubby apotheciate species with thamnolic acid that does not occur in Brazil. The apotheciate specimens from Brazil that were previously identified as U. florida or U. barbata var. florida Fr. belong to U. erinacea, U. cladocarpa, U. meridionalis, Usnea cf. moreliana or U. subelegans.
Usnea ludicra Rizz.
Usnea ludicra is an apotheciate species described by Rizzini (Reference Rizzini1952) from material collected around Rio de Janeiro. Unfortunately the type specimen(s) could not be found in Jardim Botânico do Rio de Janeiro (RB), the Museu Nacional (R), or in the Universidade Federal do Rio de Janeiro (RFA).
Usnea strigosa (Ach.) A. Eaton
Usnea strigosa is a North American shrubby apotheciate species that does not occur in Brazil. The specimens collected in Brazil that were named U. barbata var. strigosa Ach. belong to U. cirrosa.

We gratefully acknowledge Pradeep K. Divakar for his invaluable support in the molecular studies and A. Crespo for hosting the first author in her laboratory and for discussing the phylogenetic results. This study was supported by a grant to A. Crespo and P. K. Divakar from the Spanish Ministerio de Economía y Competitividad (Spain) projects CGL2013-42498-P. The first author thanks her colleagues for their help in fieldwork, as well as those from the Conservatoire et Jardin Botaniques de la Ville de Genève and the Systemol-lab of the Departamento de Biología Vegetal II, Facultad de Farmacia, Universidad Complutense de Madrid. We also thankDavid Alors for help in designing specific primers for Mcm7 and RPB1 markers. We thank E. L. de L. Nascimento (CNPq—Projeto Catimbau No. 552.083/2011-9) and M. Muryel for sending specimens vital to this study, amongst which was material of the new species U. aurantiaca-parvula. We are grateful to Y. Ohmura (TNS) for helping with the identification of cortical tissue types. We also acknowledge the directors of the collection sites for granting collecting permits and the curators of the herbaria cited above for the loan of specimens. Special thanks are due to L. Canêz and A. Spielmann for sending specimens from their Usnea collection that is deposited in CGMS. We thank Jefferson Prado (Instituto de Botânica de São Paulo, Brazil) for nomenclatural advice and Pierre-Emmanuel Du Pasquier for help with the R Core Team program. We would like to thank two anonymous reviewers for their insightful and helpful comments on an earlier version of this manuscript. We also thank Michelle Price for her review of the English. The first author acknowledges financial support from CAPES (process 99999.010114/2014-09) and CNPq (Brazil) that supported her year-long stay in Switzerland.













