Introduction
The lichen thallus is a collaborative microbial structure adapted to display photosynthetic symbionts effectively for light capture, protecting them while permitting sufficient periods of hydration and gas exchange. The great diversity of lichen thallus forms demonstrates that many different structural arrangements can satisfy these requirements (Henssen & Jahns Reference Henssen and Jahns1974; Sanders Reference Sanders2001; Honegger Reference Honegger2012). However, the range of suitable forms can be much more restricted for colonists of certain substrata and microhabitats. An interesting case is that of the living leaf, upon whose surface more than 800 species of specialized lichen fungi complete their life cycles, mainly in the humid tropics and subtropics (Lücking Reference Lücking2008). Oriented by the host plant for optimal light interception, the leaf offers the lichen colonist favourable exposure, but the leaf is an ephemeral structure typically shed after 2–3 years (Coley Reference Coley1988; Lücking Reference Lücking1998; Sanders Reference Sanders2014 b). Furthermore, the average leaf can bear little of the weight that most epiphytes, especially when hydrated, would burden them with. Much of the specialized biology of foliicolous (epiphyllous) lichens reflects adaptation to these and other conditions of their unique microhabitat (Lücking Reference Lücking2001).
Developmental studies have shown that foliicolous lichen fungi establish themselves, lichenize photobionts and progress towards reproduction with remarkable efficiency and limited investment in structure (Sanders Reference Sanders2002, Reference Sanders2014 a; Sanders & de los Ríos Reference Sanders and de los Ríos2015). However, details of their thallus organization are not well known. Foliicolous lichens are often inaccessible to workers in temperate climates, and their tiny thalli can be difficult to section effectively, a problem compounded by the withering of the substratum shortly after collection. Despite these challenges, some structural studies have been carried out successfully. Henssen & Lücking (Reference Henssen and Lücking2002) reported that thalli formed by fungi of the Asterothyriaceae consistently show a cortex composed of a single layer of radiating fungal filaments, an arrangement which may be unique to this group. Using fluorescence microscopy, Grube & Lücking (Reference Grube and Lücking2002) examined whole-mounted thalli of a variety of foliicolous lichens, describing net-like patterns of fungal hyphae below and above the photobionts. Where the photobiont is the multicellular Phycopeltis, this alga is the predominant structural component, and several distinct Phycopeltis thalli can often be distinguished within a single, mature lichen thallus (Sanders Reference Sanders2002). Where simple unicellular photobionts are involved, such as those partnering fungi of the Gomphillaceae and Pilocarpaceae, the fungus might be expected to take on a more prominent role in structuring the thallus. The present work focuses on the areolate thalli of diverse Gomphillaceae that colonize leaves in south-western Florida, using scanning and transmission electron microscopy to explore their thallus structure.
Materials and Methods
Lichens were collected from leaves of Sabal palmetto (Walt.) Lodd. ex Schultes & Schultes or Serenoa repens (Bartram) Small, in an oak hammock and transitional pine woodland habitat on the campus of Florida Gulf Coast University (Ft. Myers, Lee County, FL, USA). The leaves of these palms are very diversely oriented, permitting lichen colonization on either adaxial or abaxial surfaces, or both. Lichen specimens chosen for study included Aulaxina microphana (Vainio) R. Sant., Gyalectidium appendiculatum Lücking et al., G. floridense Safranek & Lücking, G. ulloae Herrera-Campos & Lücking, Gyalideopsis sessilis Sanders & Lücking, Tricharia duotela Sanders & Lücking and a sterile Tricharia sp. (probably T. vainioi R. Sant.), all members of the Gomphillaceae (Ostropales). Dissecting microscope images of these taxa may be found in Lücking (Reference Lücking2008) and Sanders & Lücking (Reference Sanders and Lücking2015). Leaf segments bearing the lichens were hand-sectioned c. 100–250 µm thick using a fragment of brittle razor blade held with a hemostat. Sections were placed immediately into tubes with 3% glutaraldehyde in phosphate buffer for c. 3 h, then washed three times in buffer, post-fixed with osmium tetroxide, washed again, and dehydrated in a graded ethanol series. Specimens were then infiltrated with either Spurr’s low viscosity resin (initially diluted with propylene oxide) or LR White, and polymerized. Specimen blocks were sectioned with an Ultracut ultramicrotome and the cut surfaces coated with carbon. The carbon-coated surfaces were imaged with a FEI INSPECT scanning electron microscope in backscattered electron detection mode. Some ultrathin sections were also obtained; these were stained with lead citrate and examined with a JEM 1010 transmission electron microscope.
Results
All species examined formed very thin crustose thalli positioned entirely above the leaf cuticle, with no components seen penetrating into or below this layer. Thallus construction was simple, and the same basic anatomical features were shared among the different species studied. Thalli sometimes covered the plant stomata, which occurred on both sides of the palm leaf (Fig. 1).
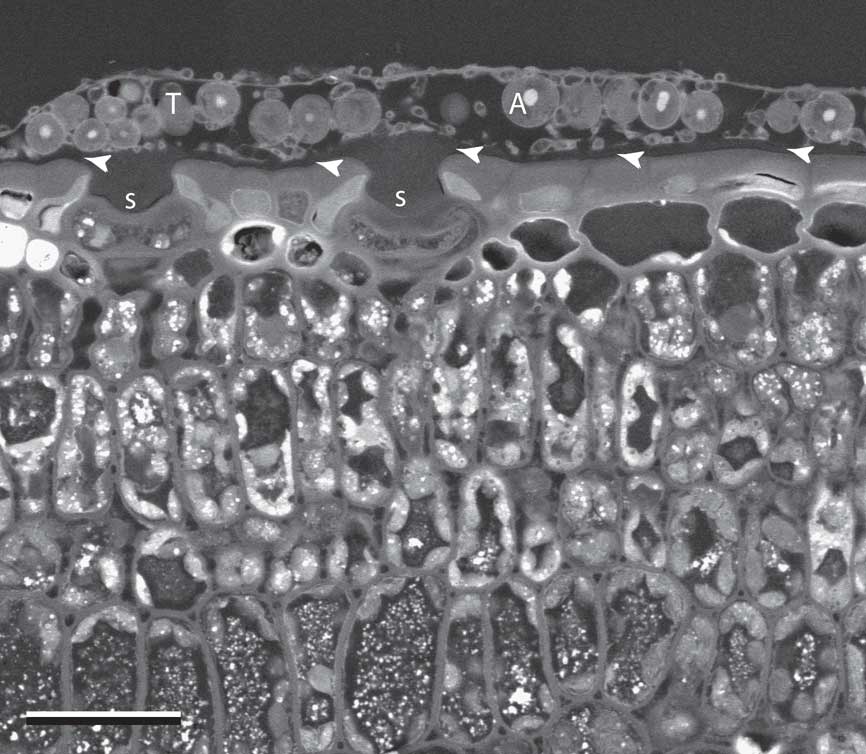
Fig. 1 SEM-BSE image of Gyalideopsis sessilis growing on the surface of a Sabal palmetto leaf, in transverse section. T=thallus; A=algal cell; S=sunken stomata covered by lichen thallus; arrowheads=location of leaf cuticle. Scale=25 µm.
Gyalideopsis sessilis
The thallus accommodated a photobiont layer mostly 1–2 algal cells thick. The upper surface of the lichen was composed of a distinct, continuous epilayer of fungal origin that consisted mainly of cell wall materials extending between relatively sparse individual cells, which were often of reduced diameter (Fig. 2A). Scattered fungal cells were also positioned on the upper and lower surfaces of this layer, as well as within it (Fig. 2A–C). Fungal cells were frequently in contact with algal cells. A loose assembly of fungal cells also occurred at the base of the thallus, but here no continuous layer was formed. Some algal cells were in direct contact with the substratum surface. Most fungal cells showed a horizontal orientation, although a few were seen oriented vertically between the upper and lower surfaces (Fig. 2A & B).
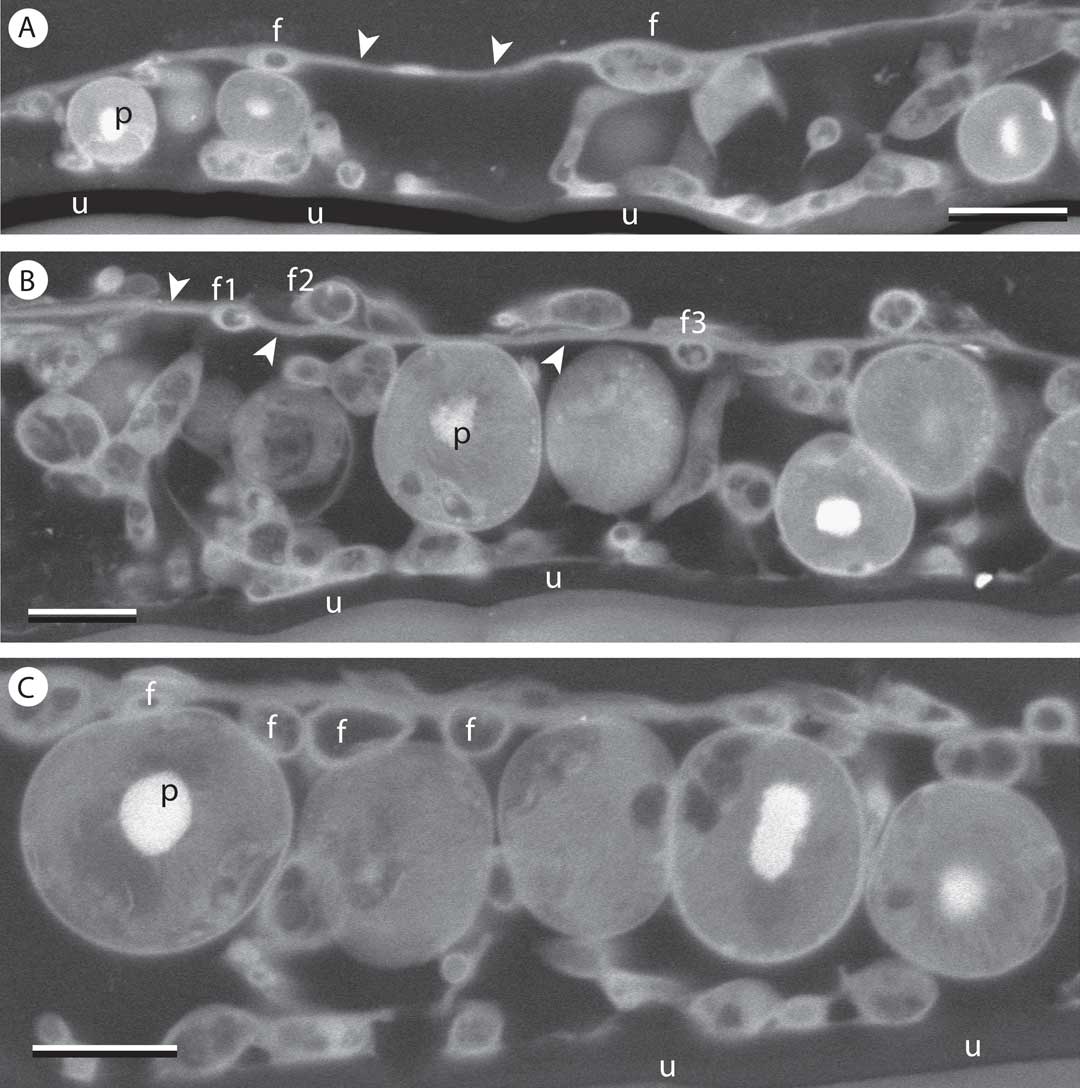
Fig. 2 SEM-BSE images of Gyalideopsis sessilis, in transverse section. A, near periphery of thallus areola. Fungal cells (f) in epilayer separated by extensive acellular wall material (arrowheads). B & C, more central portions of thallus; B, fungal cell lumina visible within (f1), upon (f2) and beneath (f3) continuous layer of wall material (arrowheads); C, some fungal cell lumina (f) in upper portion of thallus are adjacent, but do not constitute a continuous cortex. p=pyrenoid at centre of photobiont cells; u=leaf cuticle. Scales: A–C=5 µm.
Aulaxina microphana
The thallus epilayer was continuous, with the prothallus surrounding the lichenized thallus areolae (Fig. 3A). The epilayer included fungal cells and cell wall materials extending between them (Fig. 3B & C).

Fig. 3 SEM-BSE images of Aulaxina microphana on palm leaf, in transverse section. A, edge of thallus areola showing continuity of epilayer (arrowheads) with prothallus at periphery; B, detail of 3A showing close contacts between fungal and photobiont cells, altering shape of hypha (f1) and adjacent algal cells. Another contact appears to penetrate algal cell (f2), but may be merely positioned over it. Penetration of unicellular photobiont cells by fungal haustoria, if they occur at all, are not typical of any of the foliicolous lichens examined so far; C, detail of epilayer showing extensive fungal wall material (arrowhead) between cell lumina (f). Scales: A=10 µm; B=5 µm; C=3 µm.
Tricharia sp. (lacking apothecia but probably T. vainioi)
Perhaps the thinnest crust of any of the species examined, much of the thallus was only slightly thicker than the diameter of mature photobiont cells contained within (Fig. 4A). The fungal epilayer was closer in thickness to that of a thallus fungal cell, but only limited portions of the epilayer appeared cellular (Fig. 4B). Broadening of a fungal hypha into an appressoria-like structure contacting a photobiont cell is seen in Fig. 4C.

Fig. 4 SEM-BSE images of Tricharia sp. (sterile but most likely T. vainioi) on palm leaf, in transverse section. A, thallus areola, epilayer indicated by arrowheads; B, detail of fungal cells (f) on lower surface of epilayer; C, fungal cell (f) broadening into appressoria-like contact with adjacent photobiont cell. Scales: A–C=5 µm.
Tricharia duotela
The fungal epilayer was again closer in thickness to regular fungal hyphae, but mainly stretched remnants of fungal cell lumina could be distinguished within this layer, while intact fungal cells occurred scattered in various orientations on its upper and lower surfaces (Fig. 5A). Formation of setae or hyphophores (the conidiomata characteristic of Gomphillaceae) involved convergence and upward growth of thick-walled fungal cells above and below the epilayer (Fig. 5B & C). Beneath the base of the emergent structure, fungal cells were more densely concentrated and photobionts absent.
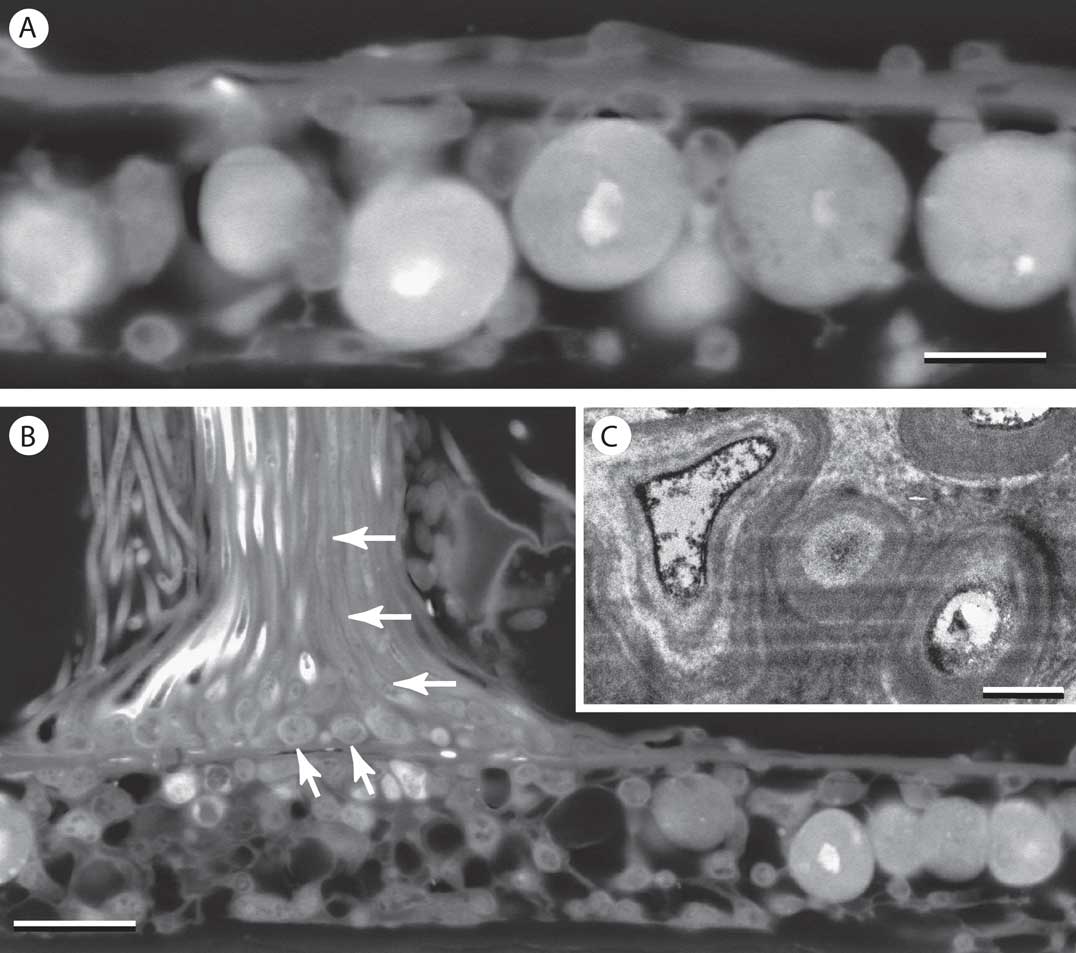
Fig. 5 Transverse sections of Tricharia duotela on palm leaf. A & B, SEM-BSE images. A, thallus showing epilayer of thickness similar to that of fungal hyphae beneath; B, base of hyphophore arising from thallus areola. Hyphophore cells are thickened fungal hyphae shown in transverse view at base (oblique arrows) and reoriented vertically within shaft (horizontal arrows); C, TEM image of section showing highly thickened walls of hyphophore cells. Scales: A=5 µm; B=10 µm; C=1 µm.
Gyalectidium ulloae
Nearly the entire thallus was encrusted with whitish crystals. They were not preserved in specimen processing, but large spaces noted between the algal cells and the covering epilayer were presumed to represent the previous locations of the crystalline deposits (asterisks in Fig. 6A–C). The epilayer is quite thin and includes only sparse fungal cells of much reduced diameter (Fig. 6C–E). The epilayer is continuous with the prothallus at the periphery of the alga-containing areolae (Fig. 6C).
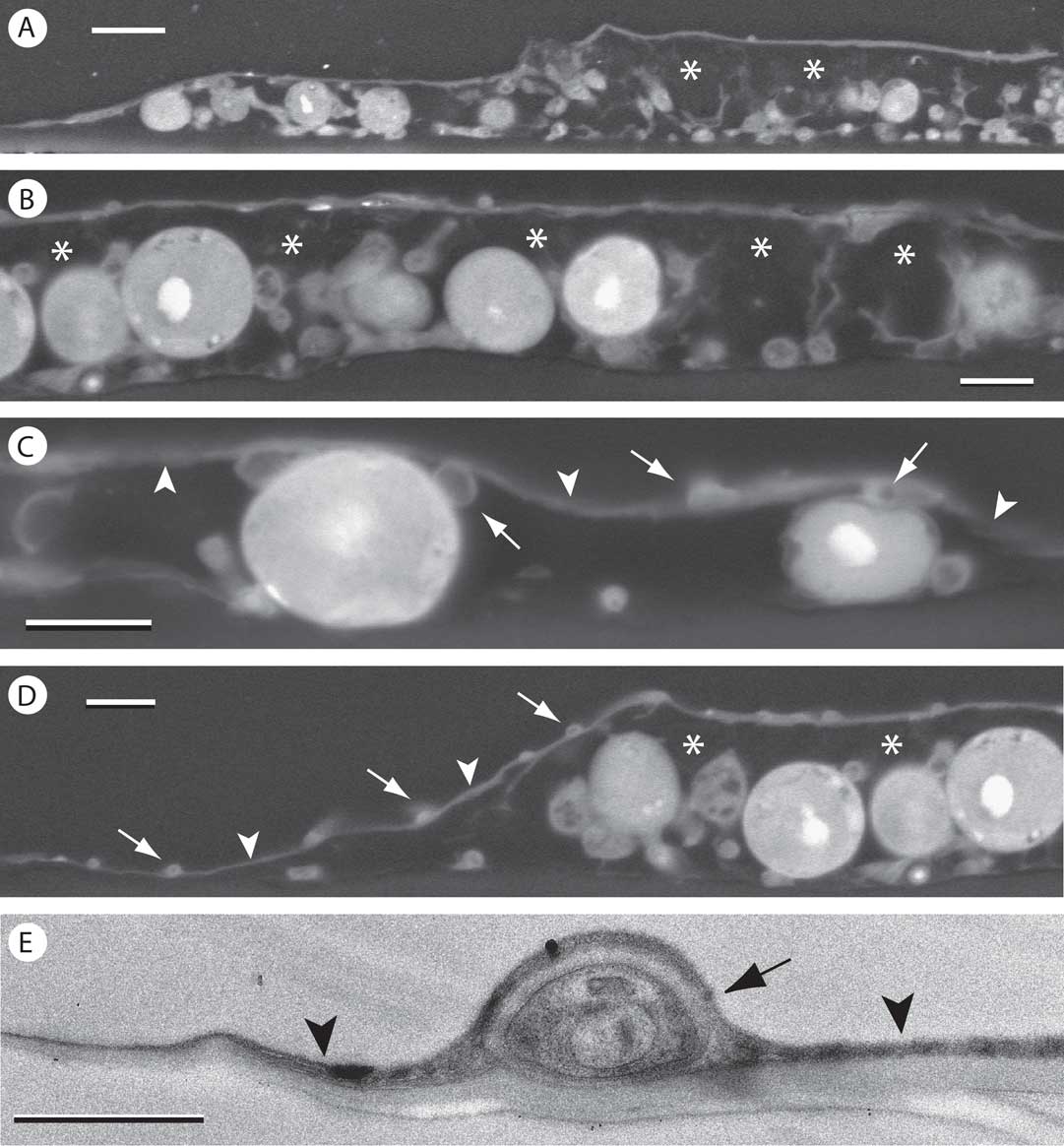
Fig. 6 Transverse sections of Gyalectidium ulloae on palm leaf. A-D, SEM-BSE images. A & B, thallus areola with substantial spaces (asterisks) between thin epilayer and underlying cells, presumably occupied by abundant crystalline material present before processing; C, near edge of areola; epilayer (arrowheads) with fungal cells (arrows) associated with its upper and lower surface; D, edge of areola showing continuity of epilayer (arrowheads) with peripheral prothallus. Small fungal cell lumina (arrows) are present within epilayer; E, TEM image of ultrathin section showing fungal cell (arrow) within epilayer composed mainly of wall material (arrowheads). Scales: A=10 µm; B–D=5 µm; E=1 µm.
Gyalectidium floridense
The thallus epilayer showed clear continuity with the prothallus (Fig. 7A–C). Spaces probably occupied by crystalline deposits were frequently visible between the epilayer and the remaining part of the thallus below (asterisks in Fig. 7D). In some places, the epilayer showed discontinuities (Fig. 7D), particularly where elevated by the putative crystal deposits below. Previously known only from a single locality on the east coast of Florida (Safranek & Lücking Reference Safranek and Lücking2005), this taxon is not uncommon in Lee County but is easily overlooked.
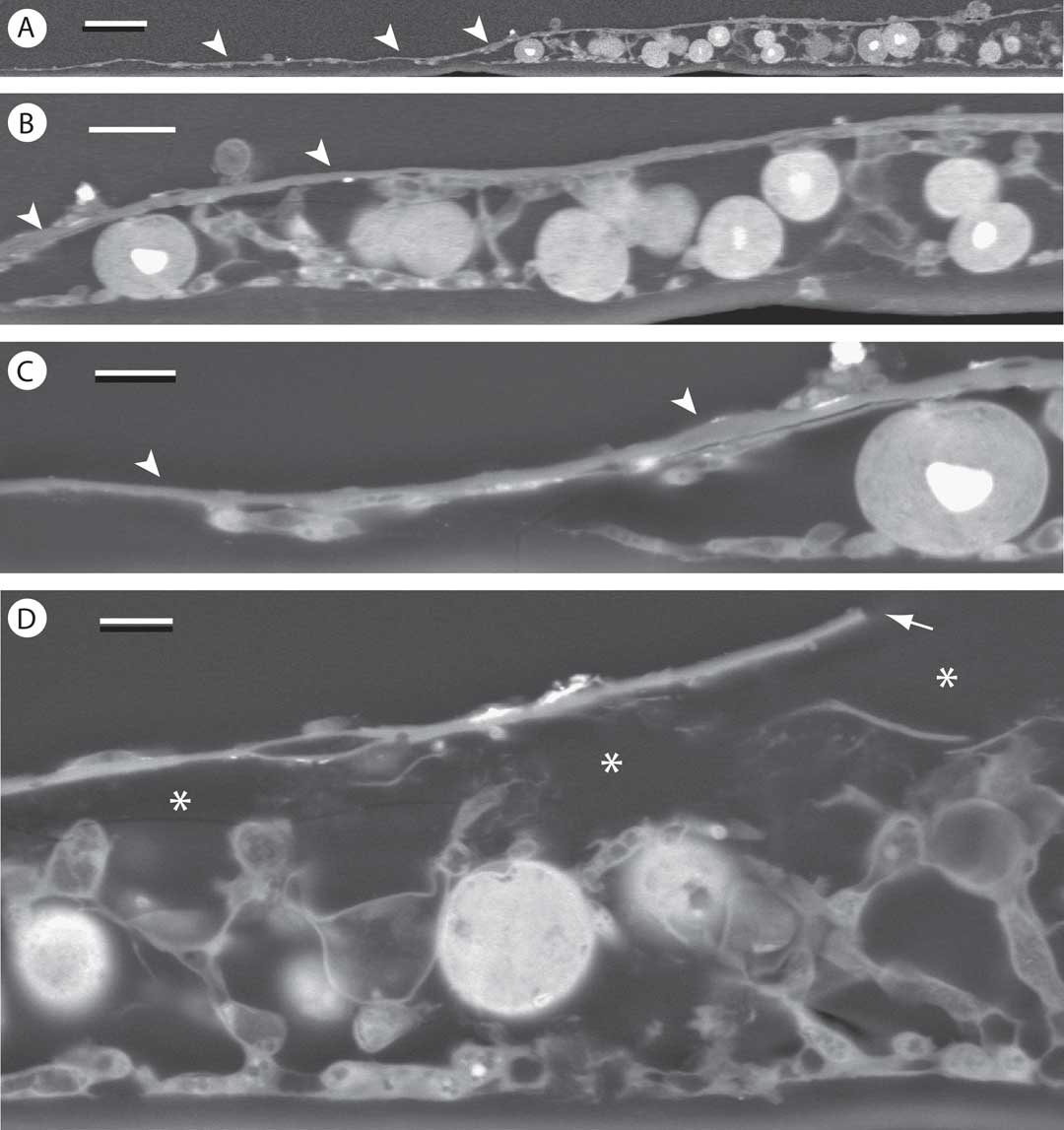
Fig. 7 SEM-BSE images of Gyalectidium floridense on palm leaf, in transverse section. A–C, periphery of thallus areola with detail (B & C) showing continuity of epilayer and prothallus (arrowheads); D, central portion of thallus with spaces (asterisks) presumably occupied by crystals in fresh material, and discontinuity in epilayer (arrow). Scales: A=25 µm; B=10 µm; C & D=5 µm.
Gyalectidium appendiculatum
The thallus showed relatively greater thickness in some places, but development of a cellular cortex was not seen here either. The crystalline deposits seen in fresh material again appeared to correspond to large spaces visible immediately below the epilayer in SEM (Fig. 8A & B). The well-developed prothallus observed at the periphery of the thallus areolae was again covered by an epilayer continuous with that of the areolae (Fig. 8C).
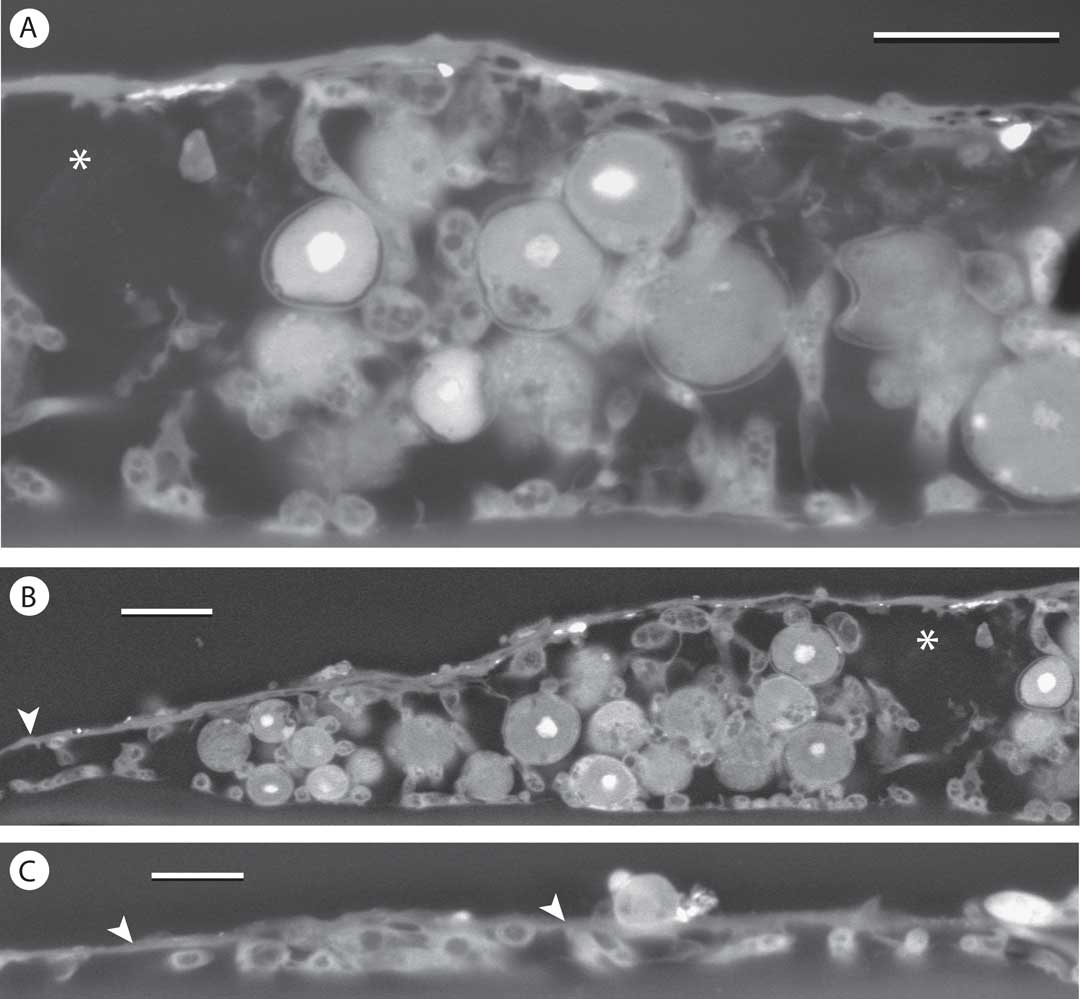
Fig. 8 SEM-BSE images of Gyalectidium appendiculatum on palm leaf, in transverse section. A–C, centre, periphery and prothallus, respectively, of thallus areola, showing spaces presumably occupied by crystalline material (asterisks) and continuity of epilayer with prothallus (arrowheads). Scales: A & B=10 µm; C=5 µm.
Discussion
Thallus areolae of all seven species of Gomphillaceae examined were similar in basic organization. A characteristic feature was the presence of a mycobiont-derived epilayer, which included sparse fungal cell lumina of reduced diameter within a continuous layer of cell wall material. To varying degrees among the species examined, scattered fungal cells were also found adhered to the lower and upper surfaces of the epilayer. However, no continuously cellular covering was observed in any of the lichens studied, including three species of the genus Gyalectidium, for which a true cortex is said to be characteristic (Santesson Reference Santesson1952; Ferraro et al. Reference Ferraro, Lücking and Sérusiaux2001). Perhaps such a layer occurs irregularly in portions of the thalli, as observed previously in G. paolae (Sanders & de los Ríos Reference Sanders and de los Ríos2015), but not sampled in our sections. The consistent presence of the thin fungal epilayer in the lichens studied is the only feature we found that might justify viewing their thallus organization as stratified. In all other parts of the lichen, except the prothallus, the cells of the two symbionts appeared to be essentially intermixed. By contrast, Grube & Lücking (Reference Grube and Lücking2002) examined Echinoplaca pellicula as a representative of this family and recognized an algal layer “enclosed by a thick hyphal layer above and below”. In the species we studied, fungal cells were seen to be perhaps slightly more numerous at the base of the thallus, but algal cells often rested directly upon the substratum. There was no indication of any layer remotely resembling the fungal medulla that is present in most stratified lichens. Symbiont contacts involved wall-to-wall apposition, with haustorial penetration of photobionts occurring only rarely if at all.
The continuity observed between the thallus epilayer and the fungal prothallus surrounding the thallus areolae was unexpected. Many crustose lichens are fringed by an alga-free mycelium that appears to play a developmental role, forming a foundation over which the alga-containing thallus areolae advance, or upon which new areolae develop following capture of new photobiont cells (Galløe Reference Galløe1927, 1932, 1954; Létrouit-Galinou & Asta Reference Létrouit-Galinou and Asta1994; Sanders Reference Sanders2002, Reference Sanders2014 a; Sanders & Lücking Reference Sanders and Lücking2002). This prothallus is sometimes also termed a hypothallus in recognition of its presumably basal position, but its observed continuity with the upper surface of the thalli examined here calls that assumption into question. As the prothallus must precede the epilayer in development, the continuity between these structures is likely established at a later stage. However, developmental observations will be needed to clarify how the prothallus and epilayer are related ontogenetically.
Particularly notable in the Gyalectidium thalli was the abundant presence of crystalline deposits in living material that give them a shiny, whitish appearance. Although not preserved in the embedded material, the deposits appeared to be largely concentrated between the epilayer and the underlying algal cells, as suggested by the large spaces observed there (Figs 6–8); occasionally the epilayer may be disrupted (Fig. 7D). Such spaces and disruptions were not observed in Aulaxina microphana or Tricharia vainioi, taxa which are not known to produce crystals (Lücking Reference Lücking2008). The crystalline compounds were not studied chemically, but most likely correspond to calcium oxalates, which are frequently reported in the genus Gyalectidium (Ferraro et al. Reference Ferraro, Lücking and Sérusiaux2001), in other genera of the Gomphillaceae (de Oliveira et al. Reference de Oliveira, Edwards, Feo-Manga, Seaward and Lücking2002), and in a number of other lichens (Giordani et al. Reference Giordani, Modenesi and Tretiach2003). The significance of these mineral deposits is unknown; suggested biological roles include concentrating suboptimal light (Modenesi et al. Reference Modenesi, Piana, Giordani, Tafanelli and Bartoli2000), photoprotective reflection of excessive radiation (Lücking Reference Lücking1999), and even storage of water in the form of chemical hydrates (Wadsten & Moberg Reference Wadsten and Moberg1985). They do not appear to significantly deter invertebrate browsers of foliicolous lichens (Lücking & Bernecker-Lücking Reference Lücking and Bernecker-Lücking2000).
The minimal investment in thallus structure observed is consistent with adaptation to a short-lived substratum with limited ability to bear weight, but the significant reduction in the fungal component might also represent increased selection pressures to streamline the thallus for metabolic efficiency. By replacing living fungal cells in the protective layer with non-living wall materials and crystalline deposits, the lichen can minimize the maintenance expenditures associated with fungal respiration, which may be particularly costly in humid tropical and subtropical lowlands where foliicolous lichens thrive. The elevated nightly temperatures in these climates substantially raise fungal respiration rates, and have been implicated in significant carbon losses in lichens; the paucity of foliose and fruticose forms among lichens in these habitats has been explained on this basis (Zotz & Winter Reference Zotz and Winter1994; Lange et al. Reference Lange, Büdel, Meyer, Zellner and Zotz2000).
All thalli studied were epicuticular; no evidence of penetration into the cuticle or underlying plant cells was observed. This is consistent with previous studies of other foliicolous lichens, with the exception of Strigula and its subcuticular photobiont Cephaleuros (Chapman Reference Chapman1976; Margot Reference Margot1977). However, there are other means by which epicuticular foliicolous lichens might negatively impact host leaves. Anthony et al. (Reference Anthony, Holtum and Jackes2002) considered their light-blocking effect on the underlying leaf, but found that the plant responds simply by adjusting leaf chlorophyll levels, in the same way that it reacts to shading by other sources. As shown in Fig. 1, another potential impact on the host leaf may need to be considered: the covering of stomata by foliicolous thalli and its possible effect on host gas exchange. This may not be an issue with most dicotyledonous leaves, but on palm leaves such as Sabal and Serenoa, lichens and stomata often coincide; stomata are formed on both adaxial and abaxial surfaces, either of which may be colonized by lichens due to the varied spatial orientation of the complex leaf.
Funding was provided by Spanish Ministry of Economy and Competitiveness grant CTM2012-38222-C02-02 to A. de los Ríos. Sections were prepared by Dr Virginia Souza-Egipsy (ICA-CSIC). The manuscript benefitted from critical review by anonymous referees. We also thank the microscopy service personnel at the Museo Nacional de Ciencias Naturales (CSIC, Madrid) for technical support.










