Introduction
The lichen-forming ascomycetes of the genus Acarospora have for a long time been acknowledged as a true challenge for the taxonomist. There are more than 100 species recognized world-wide today, but the number of names in the literature exceeds 400 (CABI Bioscience et al. 2010). Most species form areolate to subsquamulose crusts on rocks in dry, exposed habitats. The apothecia are commonly immersed in the thallus and the photobiont is a chlorococcoid green alga. The most characteristic feature is the ascus which is polysporous and has a tholus that is not or only weakly amyloid and lacks amyloid structures. The species generally produce more than 100 spores per ascus by mitotic division prior to spore formation (true polyspory, Reeb et al. Reference Reeb, Lutzoni and Roux2004).
Acarospora and related genera in the Acarosporaceae were for a long time classified in the Lecanorales together with most lichenized fungi. Phylogenetic work, however, has shown that the Acarosporaceae is not closely related to the core-Lecanorales (Reeb et al. Reference Reeb, Lutzoni and Roux2004; Wedin et al. Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005) and the most recent classifications place the Acarosporaceae in the Acarosporomycetidae within the Lecanoromycetes, rather distantly from the Lecanorales (Hibbett et al. Reference Hibbett, Binder, Bischoff, Blackwell, Cannon, Eriksson, Huhndorf, James, Kirk and Lücking2007; Tehler & Wedin Reference Tehler, Wedin and Nash2008). Furthermore, several recent phylogenetic studies (Reeb et al. Reference Reeb, Lutzoni and Roux2004; Wedin et al. Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005; Crewe et al. Reference Crewe, Purvis and Wedin2006) have all suggested that Acarospora in its current delimitation is paraphyletic; with a well-supported, monophyletic Acarospora s. str. containing the type species A. schleicheri. The Acarospora smaragdula group, the topic of this paper, was the sister to a group consisting of Acarospora s. str., Sarcogyne, Pleopsidium, Timdalia and Polysporina in Wedin et al. (Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005) and Crewe et al. (Reference Crewe, Purvis and Wedin2006) indicating that this group deserves generic rank. Acarospora smaragdula was, together with A. rugulosa Körb. and A. sinopica (Wahlenb.) Körb., at one point included in Polysporinopsis Vězda, a segregate from Acarospora based on, for example, paraphysis morphology (Vězda Reference Vězda2002). Crewe et al. (Reference Crewe, Purvis and Wedin2006) showed that Polysporinopsis was not a natural group and found the type species of Polysporinopsis (Acarospora sinopica) to belong to Acarospora s. str.
The current species-level taxonomy in Acarospora s. lat. rests heavily on Magnusson's work (e.g., Magnusson Reference Magnusson1924, Reference Magnusson1929, Reference Magnusson1933, Reference Magnusson and Zahlbruckner1935, Reference Magnusson1937, Reference Magnusson1956) and few attempts have been made to revise the genus since then (but see Clauzade et al. Reference Clauzade, Roux and Rieux1982a). K. Knudsen began revising the group in North America some years ago (Knudsen Reference Roux2007b) and a new key to the European taxa was recently published (Roux Reference Roux2007). Within Acarospora there are several common and wide-spread species that are very variable and show a great morphological plasticity in thallus morphology (e.g., A. fuscata, A. glaucocarpa, A. nitrophila and A. smaragdula). The Acarospora smaragdula group is widely acknowledged as being a notoriously difficult complex with an unstable and poorly understood taxonomy at species level. The members of the complex are characterized by pale, whitish, greyish to brownish areoles or squamules, usually with several more or less punctiform, immersed apothecia, a tall hymenium, narrow paraphyses and an algal layer that is disrupted by bundles of medullary hyphae (Knudsen Reference Knudsen2005, Reference Purvis, Gilbert and James2007b). Chemically, the group is characterized by either the presence of norstictic acid causing a K+ red reaction of the thallus, or lacking secondary metabolites. The species are mostly found on rocks in open habitats, for example sea-shores, pastures and montane regions, and are also typically found on mineralized rocks and metalliferous spoil heaps (e.g. copper- or iron-rich rocks), church walls and fence posts. They are only occasionally found on wood or on soil. The morphological plasticity, a lack of consistency of the K-reaction, and a vast number of available names, many often treated as synonyms, are problems associated with the group. The various attempts to propose a classification usually include infraspecific taxa to account for the morphological variation (Table 1).
Table 1. Different taxonomies of the Acarospora smaragdula group in Europe. (Magnusson did not refer to an A. smaragdula group but often mentioned close relatives). Acarospora scabrida has usually not been considered as a member of the group

An intriguing taxonomic problem concerns the various colour morphs that occur in A. smaragdula as a result of metal fixation including copper and iron (Purvis et al. Reference Purvis, Gilbert and James1985, Reference Purvis, Elix, Broomhead and Jones1987, Reference Purvis, Clardige, Dawah and Wilson1997, Reference Purvis, Crewe, Kearsley, Cressey and Wedin2008a, Reference Purvis, Kearsley, Cressey, Batty, Jenkins, Crewe and Wedinb). Some representatives of the A. smaragdula complex are known to accumulate potentially toxic metals to several percent of their dry weight (Purvis Reference Purvis, Fletcher, Wolseley and Woods2001; Purvis et al. Reference Purvis, Gilbert and James1985, Reference Purvis, Fletcher, Wolseley and Woods2000, Reference Purvis, Gilbert and James2008b). Norstictic acid containing populations growing on copper rich rocks (or else influenced by Cu-rich run-off) typically have a yellow-green colour attributed to the formation of a Cu-norstictic acid complex in the cortex (Purvis et al. Reference Purvis, Gilbert and James1985). Such populations have been described as species on at least three occasions (A. isortoquensis, A. undata, and A. albertii), but elsewhere were considered as modifications of A. smaragdula (e.g., Purvis et al. Reference Purvis, Gilbert and James1985). Below, we show that the original material of Endocarpon smaragdulum (A. smaragdula) also belongs to the Cu-accumulating morph.
The distinctive rust-coloration is not simply due to hydrated iron oxides, as previously believed. Iron was found to be associated with sulphur and oxygen in samples of Acarospora sinopica and ‘A. smaragdula f. subochracea’ (= Silobia dilatata) from Sweden (Purvis et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2008a) and Acarospora sinopica from Parys Mountain (Purvis et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2008b). This suggests mixed sulphide and oxide phases with little crystallinity and other elements from clay minerals were present. These populations have often been treated as different taxa, even at species level, by taxonomists. We investigated the species delimitation in this group and the evolution of bioaccumulation of metals (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009), showing that green copper-rich forms were nested inside A. smaragdula s. str. (= Silobia smaragdula), confirming that these do not constitute a separate species. In contrast, iron-rich morphs were found to form two well-supported clades, and facultatively iron-accumulating populations occur in at least one further species. In addition we distinguished four more species belonging to the A. smaragdula group. The taxonomy we propose here is the formalization of the results from Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009), together with new morphological and anatomical investigations and analysis of secondary metabolites. The phylogenetic species found in Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) could also be characterized morphologically. Although the species are extremely variable, the majority of the collections in the herbaria could be identified with a high degree of confidence. Despite this, some problems remain including the clade called “Group F” by Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) that could not be described and named in the present revision. The material studied was poorly developed and originates from a mere two localities in Wales. As these collections are so meagre we feel that we cannot properly interpret and characterize this species.
In North America, at least five species apparently belonging to the A. smaragdula group have been recognized in recent literature (Knudsen Reference Knudsen2004, Reference Knudsen2005, Reference Wedin, Westberg, Crewe, Tehler and Purvis2007a, b), of which two have not been studied in the present revision (A. dispersa H. Magn. and A. hassei Herre). As commented on in relevant places, it is very unlikely that any of these names will refer to the new species described here.
The aim of this paper is to describe formally the species distinguished by Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) and to produce a taxonomic and nomenclatural revision of the A. smaragdula group in Sweden. We also propose the new genus Silobia for this phylogenetically distinct group.
Materials and Methods
Morphology and anatomy
The study is mainly based on our own samples from fieldwork in Sweden, and Swedish material from the herbaria in GB, LD, S and UPS. In addition some material from our fieldwork in Norway and the United Kingdom was included, as well as a few scattered collections from other European countries, and types and important material from several other herbaria including AMNH, BM, C, HBG, O, STU, STU-Wirth, and W have been consulted (abbreviations according to Thiers Reference Thiers2010). Hand-cut sections of thalli and apothecia mounted in water were studied by light microscopy. In the apothecial studies only the disc was measured and the margin (although distinct in some species) was excluded. Areole size is given for fertile areoles only; areole size and number of apothecia per areole are given as (min. value observed–) range including 85% of the variation (–max. value observed).The descriptions of the subhymenium includes the hypothecium.
Chemistry
A standard 10–15% water solution of potassium hydroxide (K) was used on squash preparations of the thallus cortex, and medulla, to test for presence of norstictic acid (K+ red). Selected samples were investigated with HPTLC according to the standard methods of Arup et al. (Reference Arup, Ekman, Lindblom and Mattsson1993).
Taxonomy
Silobia M. Westb. & Wedin, gen. nov
Similis Acarospora sed differt strato algarum hyphis medullaribus interrupto. Thallus saxicola vel raro muscicola, areolatus vel areolato-squamulosus, areolae et squamulae contiguae vel dispersae. Apothecia minuta vel dilatata, in areolis singula vel plura. Hymenium usque ad 250 µm altum, Asci plus 100-spori. Cortex acidum norsticticum continens vel thallo acido licheno destituta.
Type species: Silobia smaragdula (Wahlenb.) M. Westb. & Wedin.
Thallus epilithic, sometimes terricolous or muscicolous; areolate to subsquamulose, to squamulose; areoles/squamules dispersed or contiguous, rarely overlapping; upper surface creamy white, to pale brown or brown, sometimes yellow-green, or orange to rusty red, epruinose or rarely with a white pruina; upper cortex differentiated into an uppermost colourless epinecral layer that can be present or not, below a paraplechtenchymatous eucortex whose uppermost cells are often pigmented, the lower part intergrading into a prosoplechtenchyma that merges with the medulla, individual cells of the cortex ± isodiametric, distinct and easily visible or very difficult to distinguish, without crystals or with a scattered to continuous layer of crystals (interpreted as norstictic acid); photobiont a chlorococcoid green alga; algal layer uneven, interrupted by thick, anticlinal bundles of medullary hyphae; medulla white, reticulate; lower surface ecorticate, pale.
Apothecia 1–numerous per areole or squamule, immersed in the thallus or becoming somewhat elevated; disc round to oval, punctiform to dilated, sunken or level with the thallus or somewhat raised, reddish brown to brown to black, flat, smooth or rough, epruinose or rarely with a rusty red pruina; proper exciple colourless or yellow-brown, often expanding towards the surface and sometimes seen from the outside as an elevated, often blackening, more or less distinct margin around the disc; epihymenium brownish, sometimes with dark rusty red, small, granular crystals; hymenium colourless below, in the upper part usually with different brown colours, often very tall (up to 240 µm) but variable in height within the species; paraphyses septate, simple to sparingly branched and anastomosing, tips cylindrical to clavate; subhymenium (including hypothecium) colourless or greyish, yellowish or pale brown, always opaque with many oil-drops; asci narrowly clavate, 100+-spored; ascospores ellipsoid, narrowly ellipsoid to bacilliform.
Pycnidia rare, immersed in the thallus, visible as punctiform, brownish pits in the thallus, one-chambered; conidiophores correspond to type II–III sensu Vobis (Reference Vobis1980), conidiogenous cells elongated, tapering, flask-shaped; conidia subglobose to ellipsoid, colourless.
Chemistry. Norstictic acid (possibly with satellite substances in low concentration) in the cortex or without secondary metabolites detectable by HPTLC.
Etymology. The name Silobia is a combination of the Latin word silex (siliceous rock) and the Greek ending –bius (-living).
Substratum and ecology. The different species are mostly growing on exposed rocks, for example, coastal or montane rocks, and several species are found on metalliferous rocks. Rarely on calcareous schists, mosses or soil and very rarely found on wood.
Distribution. Europe, North and South America. The genus is possibly cosmopolitan and at least widely distributed in the Northern Hemisphere.
Notes. This species group is phylogenetically distinct and cannot be treated within Acarospora as the generally accepted Sarcogyne, Pleopsidium, Polysporina and Timdalia would then be nested within Acarospora (Wedin et al. Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005; Crewe et al. Reference Crewe, Purvis and Wedin2006). Although there are few morphological characters present in Silobia distinguishing the group from Acarospora s. str., they can usually be identified by their habitus with rather irregular areoles, squamules and rounded, brownish apothecia. The most distinct character we have found is the broken algal layer, which is disrupted at irregular intervals by bundles of medullary hyphae reaching the cortex above the algae (Fig. 1). Above the cortex there is often a colourless, gelatinous layer. Our interpretation is that it is an epinecral layer and not a syncortex as suggested by Knudsen (Reference Harris and Knudsen2007b) in, for example, S. scabrida. An epinecral layer is thought to be rare in Acarospora being known only in A. nodulosa (Harris & Knudsen Reference Harris and Knudsen2006) and a few other species. Other characteristic features include a tall hymenium (variable character), small, narrowly ellipsoid spores, narrow paraphyses, and a chemistry either without secondary metabolites that can be detected by HPTLC or with norstictic acid in the cortex.
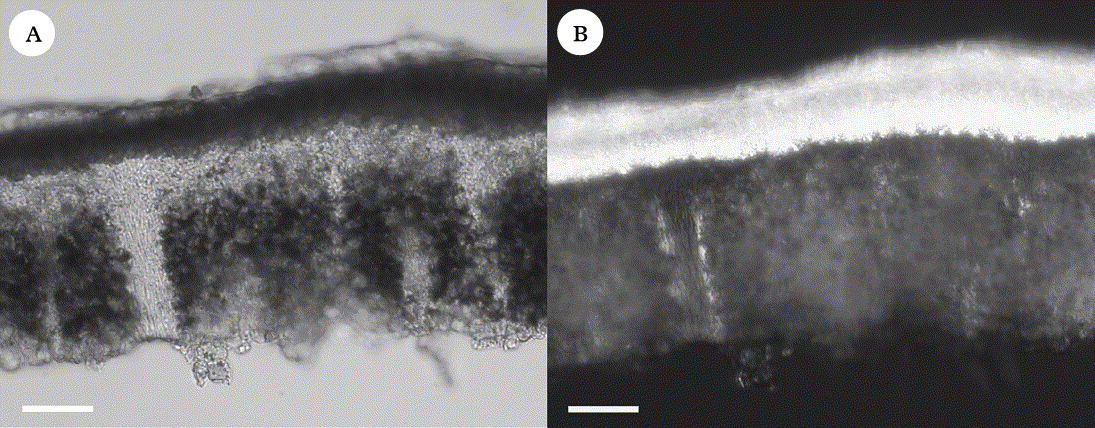
Fig. 1. Silobia, anatomy. A, thallus section showing algal layer broken by bundles of medullary hyphae; B, thallus section in polarized light showing crystals in the cortex (S. smaragdula, 7 viii 2003, UPS). Scales: A & B = 50 µm.
We investigated the possibility that the name Trimmatothelopsis Zschacke (Zschacke Reference Zschacke and Zahlbruckner1934) was available for this genus. Trimmatothelopsis was described to accommodate T. versipellis (Nyl.) Zschacke a species known only from the type material from France (H-Nyl! No. 4066—holotypus). The material was studied by Roux and Navarro-Rosines (Reference Roux and Navarro–Rosinés2002) who concluded that the name is a synonym of Acarospora scyphulifera and that it belongs to the A. smaragdula group. In his updated key to the European Acarospora, Roux (Reference Roux2007) accepted it as a distinct species. The name has otherwise not been used, as far as we can see. We have studied the type which is very small and in extremely poor condition. As stated by Roux and Navarro-Rosines (Reference Roux and Navarro–Rosinés2002) it is mostly overgrown by cyanobacteria. In addition we strongly suspect that it is heterogenous, as the few apothecia remaining appear to be carbonized, or in the process of becoming carbonized. These probably belong to a parasitic Polysporina. As the type is almost impossible to interpret and the name has hardly been used, we will formally propose in a separate paper that the name is rejected.
Key to the genus Silobia in Sweden
1 Thallus ochraceous or orange to rusty red ... 2
Thallus creamy white, grey to brown or green, never with red or reddish colours ... 4
2(1) Disc mostly punctiform, c. 0·1–0·2 mm diam.; apothecia c. 2–12 per areole/squamule, hymenium >180 µm tall ... S. tangerina
Disc mostly wider, c. 0·2–0·6 mm diam.; apothecia c. 1–3(–5) per areole/squamule, hymenium up to 180 µm tall ... 3
3(2) Thallus orange to pale brick-red and often in mixture with brownish areoles; disc (reddish when wet), epruinose; maritime, on sea-shore rocks in the spray-zone, southern Sweden ... S. rhagadiza
Thallus mostly dark rusty red; disc black or covered by a rust-red pruina; predominantly montane along rivers and lakes in northern Sweden ... S. dilatata
4(1) Thallus K+ red (norstictic acid) ... 5
Thallus K− ... 6
5(4) Areoles flattened; apothecia small, punctiform and with a smooth disc; thallus in section forming red needle-shaped crystals in K ... S. smaragdula
Areoles strongly convex; apothecia often dilated and with an uneven disc; thallus in section not forming needle-shaped crystals in K but usually a yellow mist and occasionally small, red, granular crystals ... S. myochroa
6(4) Crystals present in the cortex (norstictic acid), visible in polarized light/phase contrast (section) ... S. myochroa
Crystals not present in the cortex ... 7
7(6) Apothecium disc, when mature, distinctly raised above the thallus; on schistose rocks or on moss and soil, montane in northern Sweden ... S. scabrida
Apothecium disc remaining immersed (margin often somewhat raised); on sea-shore rocks or sandstone, southern Sweden ... 8
8(7) Thallus of irregular to angular areoles, fertile areoles 0·7–2·0 mm wide; apothecia 0·15–0·65 mm wide, hymenium 110–160 µm tall; maritime on sea-shore rocks in the spray-zone ... S. rhagadiza
Thallus of uniform, small, angular areoles, fertile areoles 0·4–1·5 mm wide; apothecia 0·07–0·40 mm wide, hymenium 100–125 µm tall; on sandstone; not maritime ... S. rufescens
Silobia dilatata M. Westb. & Wedin sp. nov
Similis Silobia smaragdula, sed differt a thallo ochraceus vel ferrugineus et acido licheno destituta et apothecia dilatata.
Typus: Sweden, Torne Lappmark, Jukkasjärvi par., Tornehamn, shore of lake Torneträsk, on silicate rock, 3 September 2006, E. Baloch SW116 (S F114109—holotype).
= p. max. p. Acarospora sinopica f. dilatata H. Magn. (Magnusson Reference Magnusson1929, p. 149) nomen nudum.
(Fig. 2C)

Fig. 2. Silobia species in Sweden, habitus. A, S. smaragdula, typical form (23 viii 2007, L & A. Stridvall, S); B, S. smaragdula, green Cu-form (Westberg 07-006, S); C, S. dilatata (holotype); D, S. rufescens (Turner 23, H-ACH 980—isolectotype); E, S. tangerina, orange form (Santesson 31292, UPS); F, S. tangerina, pale ochraceous form (Westberg 08 339, S). Scales: A–F = 1 mm.
Thallus epilithic, areolate; areoles rounded to angular, contiguous to dispersed, fertile areoles (0·4–)0·7–1·3(–1·6) mm wide; upper surface orange to dark rusty red occasionally pale rusty red in parts, smooth to somewhat roughened, flat to convex; epinecral layer absent; epicortical crystals golden red, granular, in a 5–15 µm thick layer; upper cortex without crystals, 15–35 µm thick, cells distinct, lumina 2–6 µm wide.
Apothecia 1–3(–5) per areole, immersed, round, often filling one areole and then appearing as a lecanorine apothecium, disc 0·2–0·4 mm diam., mostly black, sometimes rusty red pruinose and then of the same colour as the thallus, flat, smooth or somewhat rough, thallus often raised around the apothecium forming a distinct, sometimes finely striate margin; proper exciple colourless or somewhat yellowish in section, sometimes brownish in parts, widening to up to 125 µm across in the upper part; epihymenium red-brown to dark brown, sometimes with dark rusty red, granular crystals; hymenium 130–175 µm tall; paraphyses simple or sometimes branched near the tips, (1·0–)1·5–2·0 µm in midhymenium, tips clavate, to 4 µm wide; subhymenium colourless to pale brownish, with many oil-drops, up to 100 µm thick; asci up to at least 135 × 32 µm; ascospores narrowly ellipsoid, 3–4 × 1–1·5 µm.
Pycnidia not seen.
Chemistry. UV−, K−, KC−, C−; no substances detected by HPTLC.
Etymology. ‘Dilatata’ refers to the widened apothecium disc.
Substratum and ecology. This species grows on schistose, iron-rich, siliceous rocks or sometimes on somewhat calcareous rock. It is usually found rather close to water on river-banks or lake-shores.
Distribution. Silobia dilatata is known from several localities in northern Sweden and Norway along the Scandinavian mountain range, but is also known from scattered lowland localities and from the northern part of southern Sweden.
Notes. This species is easily recognized by the dark rusty colour of the thallus and the wide, usually black apothecia that typically occur singly, filling the thallus areoles. It can sometimes be difficult to separate S. dilatata from forms of Acarospora sinopica with widened apothecium discs but A. sinopica has a different inner structure, for example, a continuous algal layer, more narrow and branched paraphyses and ellipsoid to subglobose spores. Under the hand-lens A. sinopica can usually be recognized by the apothecia that have a thin furrow between the disc and the proper margin, whereas S. dilatata shows a continuous surface from the disc and outwards (see also fig. 1 in Purvis et al. Reference Purvis, Kearsley, Cressey, Batty, Jenkins, Crewe and Wedin2008). Compared to S. tangerina, S. dilatata has larger apothecia surrounded by a more prominent, often melanized margin, a lower hymenium and the thallus is more contiguous and usually has a darker rusty colour.
Forms morphologically similar to S. dilatata occur in the Alps in central Europe (often identified as Acarospora smaragdula var. subochracea) but preliminary DNA sequence data from the ITS-region of one specimen, indicate that these may represent a different, possibly undescribed species. We therefore avoid reporting the species from extra-Scandinavian localities in this study.
Material examined. Norway: Nordland: Bodö, Sommerfelt (UPS L-137965); Vega par., Igerøy, 1975, Degelius V-1470 (UPS). Troms: Storfjord, Wedin 7281, 7282, 7289, 7295, 7296 (UPS). Finnmark: Kaafjord, Alten, 21 v 1802, Wahlenberg (UPS).—Sweden: Härjedalen: Tännäs par., 1957, Sundell 1140 (UPS). Jämtland: Frostviken par., Thor 6910 (S); Undersåker par., 1917, Malme, Tibell 23546 (UPS). Lule Lappmark: 1871, Hellbom (GB). Lycksele Lappmark: Malå par., Crewe 66, 102 (UPS); Tärna par., Nordin 5507, Du Rietz 488, 508d, 887a, 900c, Magnusson 8561, 8810 (UPS). Pite Lappmark: Arjeplog, 22 viii 2006, Baloch (S). Torne Lappmark: Jukkasjärvi par., 9 vii 1936 Santesson (S); 24 vii 1936, Santesson (S); 18 viii 1943 Santesson (S); Magnusson 5672 (S, UPS), Magnusson 5455 (GB, UPS). Uppland: Dannemora par., Tibell 23666 (UPS); Västerbotten: Sörfors, Crewe 75, 118 (UPS).
Silobia myochroa M. Westb. sp. nov
Similis Silobia smaragdula sed differt a thallo crassior, saepe areolato-squamulosus et apothecia plano vel rugoso, saepe dilatata.
Typus: Sweden, Bohuslän, Orust, Morlanda par., near car park at Stocken. On the vertical surface of a SW-facing rock. Grid ref: (RT90) 6456258 1241653, alt. c. 0·1 m, 16 July 2003, Crewe & Purvis 719 (S—holotype).
(Fig. 3A & B)

Fig. 3. Silobia species in Sweden. A, S. myochroa, typical form (Källsten 1317, UPS); B, S. myochroa, large squamulose form (Westberg & Westberg 06-120, S); C, S. rhagadiza, pale reddish form (Westberg 06 040, S); D, S. rhagadiza, normal coloured form with unusually well-developed apothecial margins (9 vi 1926, Stenholm, S); E, S. scabrida, subsquamulose form (Santesson 31131, UPS); F, S. scabrida, areolate form (Westberg 07-011, S). Scales: A–F = 1 mm.
Thallus epilithic, areolate to subsquamulose; areoles scattered to contiguous through vegetative division, fertile areoles (0·6–)1·0–2·4 mm wide, sometimes becoming squamulose with large, up to 4·5 mm wide squamules; upper surface whitish to greyish or more often brown-grey to brown or sometimes even brownish black, flat to mostly uneven and strongly convex, dull to somewhat shiny, sometimes with concentric, wavy markings, epruinose; epinecral layer present, (2–)5–30(–45) µm thick, often very uneven in thickness; upper cortex 30–60 µm thick, with crystals, usually in large, scattered groups, sometimes rather sparse, uppermost pale brown, cells often obscured by crystals but otherwise distinct, 2–4(–5) µm wide.
Apothecia 1–4(–14) per areole, immersed; disc 0·15–0·50(–0·70) mm diam. often dilating, sunken or level with the thallus, brown to dark brown, smooth to very uneven, epruinose; proper exciple colourless, c. 30 µm below the hymenium, widening to up to 90 µm in the upper part; epihymenium reddish brown to golden brown to dark brown; hymenium 120–230 µm tall; paraphyses 1–1·5 µm in midhymenium, often branched near the tips, tips ±cylindrical or weakly clavate, to 2 µm wide; subhymenium colourless, to yellowish or greyish, with much oil, 70–90 µm tall; asci up to 120 × 22 µm; ascospores narrowly ellipsoid c. 3–5 × 1–1·5 µm.
Pycnidia rare; conidia ellipsoid c. 2 × 1 µm.
Chemistry. UV−, K− or (mostly in fresh specimens) K+ red (red needles not seen in microscope slides but the cortex often reacts with a K+ yellow mist). Sometimes small, red, granular crystals are formed in scattered groups in the lower part of the cortex, KC−, C−; norstictic acid present in small amounts and detectable by HPTLC in c. 50% of the samples.
Etymology. The name refers to the grey to brownish grey colour of this species.
Substratum and ecology. This species grows on acidic rock, mostly near the coast. It is frequently seen growing together with S. rhagadiza on sea-shore rocks in the spray-zone but can also be found in inland localities, on lake-shores and along rivers.
Distribution. This is a fairly common and widespread species in Fennoscandia. Its distribution in Europe is unknown but it should be searched for along the Atlantic coast.
Notes. This species partly corresponds to Acarospora murina sensu Magnusson (Reference Magnusson1924) and is represented in the phylogenetic investigation by the “murina” group (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009). The material obviously puzzled Magnusson and originally he treated A. murina as a distinct species but later changed his mind and included it as a variety of A. smaragdula (Magnusson Reference Magnusson1924, Reference Magnusson1929). It is common on the west coast of Sweden and the many specimens in the Swedish herbaria are usually identified as v. murina or v. lesdainii by Magnusson, apparently depending on the colour of the specimen. The epithet murina is not available for this species as the type belongs to S. smaragdula.
Silobia myochroa contains norstictic acid in the cortex, but seen under the dissecting microscope, the K+ red reaction is very inconsistent. It is more common in fresh material and older specimens in the herbaria usually react K− even though the collector sometimes has annotated that the specimen has a positive K-reaction. A chemical analysis by HPTLC found norstictic acid in small amounts in c. 50% of the specimens. Various quantities of crystals (which we interpret as norstictic acid) can always be found in the cortex when viewed in polarized light (Fig. 1D). We have, however, never seen the formation of the typical reddish needles when adding K to microscope slides. The presence of crystals in the cortex is a diagnostic character distinguishing this species from S. rhagadiza. These two species often grow together on sea-shore rocks in western Sweden. Typically the two species have a distinctly different appearance but as both species are highly variable it is sometimes not possible to recognize them using gross morphology only.
Compared to S. smaragdula, S. myochroa has larger, much thicker and strongly convex areoles or squamules. The apothecia are often dilated and then have a strongly uneven disc. From the descriptions of Acarospora dispersa and A. hassei, it is unlikely that either of these belong to this species. Both lack norstictic acid and also an epinecral layer (Knudsen Reference Knudsen2007b). Acarospora dispersa appears to have a similar ecology but has a corticate, dark lower surface whereas S. myochroa is pale and ecorticate.
Material examined. Finland: Lapponia Inarensis: Westberg LIN-159 (LD).—Norway: Nordland: Röst, Kaarö, 8 viii 1922, Du Rietz (UPS). Vest-Agder: Mandal, Eigebrekk, Westberg 08-127 (S).—Sweden: Bohuslän: Björlanda par., 11 x 1914, Magnusson (UPS); Dragsmark par., 11 viii 1915, Magnusson (UPS); Käringön par., Magnusson 23053 (UPS); Lycke par., Magnusson 7428 (UPS); Långelanda par., Magnusson 9979a, 12686 (UPS); Morlanda par. Tibell 22675, 23684 (UPS); Norum par., Westberg 06-051 (LD); Orust, Torp, Stenholm (GB), Skee par., Magnusson 13558 (UPS); Solberga par., Källsten 1317 (UPS); Spekeröd par., Magnusson 25146 (UPS); Stenkyrka par., Magnusson 7320, 7399, 7505, 17433 (UPS); Tanum par., 7 viii 1917, Magnusson (UPS); Tjärnö par., Arup L06040 (LD); Magnusson 22593 (UPS); Tånga par., Westberg & Westberg 06-120, 06-121a (LD); Öckerö par., 9 viii 1916, Magnusson (UPS); Ödsmål par., 1930, Magnusson [Magnusson, Lich. Sel. exs. No. 122] (GB); Magnusson 12689 (UPS). Gotland: Fårö par., Tibell 1340, 1223 (UPS). Halland: Ölmevalla, Arup L01786 (LD). Jämtland: Offerdal par., Westberg 08-382 (S). Torne Lappmark: Jukkasjärvi par., Magnusson 3311a (UPS). Västerbotten: Kvarken, Vallgrund, 1859, Malmgren (UPS). Västergötland: Göteborg, Magnusson 24363 (UPS); 11 vii 1951, Kjellmert (UPS); Landvetter, Magnusson 22, 7583 (UPS); Surte, Magnusson 2445 (UPS); Österplana par., Magnusson 17384 (UPS).
Silobia rhagadiza (Nyl.) M. Westb. comb. nov
Lecanora rhagadiza Nyl. Flora 64: 178 (1881); type: Great Britain, England, damp rocks, Barrow Mouth, Whitehaven, Cumberland, 1880, W. Johnson (H-NYL 24870—lectotype!, designated here; BM 000501100—isolectotype!).
Note. According to a letter attached to the isolectotype sheet written by Johnson in 1916, he divided the specimen in two and sent one half to Nylander from which the species was named. In the letter he also states that the type locality was destroyed by a quarry.
= Acarospora amphibola f. testacea H. Magn. Kgl. Svensk. Vetensk.-Akad. Handl., ser. 3, 7: 137 (1929); type: Sweden, Bohuslän, Ödsmål par., Hällesdalen, on stone by a brook, 1918, A. H. Magnusson 2001 (UPS—lectotype! designated here; UPS—isolectotype!).
= Acarospora amphibola sensu auct. (e.g. Magnusson Reference Magnusson1924, Reference Magnusson1929; Knudsen Reference Knudsen2005; Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009).
= Acarospora scyphulifera Vain. Ark. Bot. 8: 147 (1909); type: Russia, Chukotka, Siberia, Peninsula Jinretlen. Ad lapides et saxa gneissacea, parce etiam ad terram argillaceam. 1878–9, E. Almquist (TUR-Vainio 25481—neotype, selected here!).
Note. When Vainio described A. scyphulifera he distinguished three forms, f. impressa, f. pallescens and f. subdiscreta. He did not describe a f. scyphulifera and did not indicate any type material for A. scyphulifera. We here choose to neotypify A. scyphulifera on the best developed material of the syntypes of A. scyphulifera f. pallescens. The correct name for that form, as long as our choice of neotype is followed, is A. scyphulifera f. scyphulifera (see ICBN Art. 26.3 Ex. 7).
= Acarospora scyphulifera f. subdiscreta Vain. Ark. Bot. 8: 148 (1909); type: Russia, Chukotka, Siberia, Peninsula Jinretlen. Ad lapides graniticos et gneissaceos. 1878-9, E. Almquist (TUR-Vainio 25483—lectotype!, selected here!).
Note. Three syntypes were found (TUR-Vainio 25483, 25484, 25485). Of these we found the chosen lectotype to be the best developed specimen.
? Acarospora scyphulifera f. impressa Vain. Ark. Bot. 8: 148 (1909); type: Russia, Chukotka, Siberia, Peninsula Jinretlen. Ad lapides et saxa granitica et gneissacea locis numerosis.. 1878, E. Almquist (TUR-Vainio 25477—syntype!).
Note. This syntype is rather meagre and as the other syntype was not available to us in this investigation we avoid lectotypifying this name here.
(Fig. 3C & D)
Thallus epilithic, areolate; areoles rounded or more often irregular to angular, contiguous through vegetative division or sometimes dispersed, separated by broad cracks, fertile areoles 0·7–1·5(–2·0) mm wide; upper surface dirty greyish brown, to dark brown or pale rusty red, dull, epruinose, usually flat or with margins somewhat upturned or somewhat convex; epinecral layer absent or more often present but thin, (0–)3–20 µm thick; upper cortex 15–35 µm thick, uppermost cells with red-brown to dark brown caps, cells distinct, 2–5 µm wide.
Apothecia 1–4(–6) per areole, immersed; disc 0·15–0·55(–0·65) mm, level with the thallus, reddish brown to brown, reddish when wet, smooth, epruinose; proper exciple colourless, thin and indistinct below the hymenium, widening to up to 90 µm in the upper part, uppermost cells with dark brown caps, from the outside seen as a distinct, somewhat raised, pale brown or often blackened margin; epihymenium yellow-brown to dark red-brown; hymenium 110–160 µm tall; paraphyses sparsely branched, 1·0–2·0 µm in midhymenium, tips cylindrical to clavate, up to 2·5 µm wide; subhymenium colourless to greyish or yellowish, with many oil-drops, 50–75 µm tall; asci 105 × 21 µm; ascospores ellipsoid, c. 2·5–3 × 1·0–1·5 µm.
Pycnidia not seen.
Chemistry. UV−, K−, KC−, C−; no secondary metabolites detected by HPTLC.
Substratum and ecology. In Sweden and Norway S. rhagadiza has a very distinct ecology, always growing in the spray-zone on sea-shore rocks. Further south in Europe this ecology becomes less distinct but it still has a pronounced maritime ecology always occurring near the sea, although not necessarily on the shore.
Distribution. Silobia rhagadiza has an Atlantic distribution. In Scandinavia it is found along the west coast of Sweden and Norway with scattered localities on the Swedish east coast. It is also known from the United Kingdom, Iceland and Italy. In North America it has been reported (as A. amphibola) from the east coast (Magnusson Reference Magnusson1956; Knudsen Reference Knudsen2005).
Notes. This species was represented in our earlier phylogenetic analysis as the “amphibola” group (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009). It is a morphologically extremely variable species. When characteristic it is recognized by the rather large apothecia with a distinct and raised margin, the low hymenium, the absence of crystals in the cortex and by its ecology. It is sometimes found together with S. myochroa and there are forms that cannot be distinguished by gross morphology alone. The latter species normally has a distinct layer of crystals in the cortex (norstictic acid, visible in polarized light) and a thicker epinecral layer.
The Swedish specimens were identified by Magnusson (Reference Magnusson1924) as A. amphibola, although he had not had the opportunity to study the type material, which contains norstictic acid and belongs to A. smaragdula.
This species is the only facultatively iron-accumulating species in the A. smaragdula group known to us so far. In populations from the Swedish west coast, it is not unusual to find rusty red thalli or areoles (= A. amphibola f. testacea H. Magn.) on iron-containing sea shore rocks, often in a mosaic mixture with normally coloured thalli. Such material was included in the phylogeny in Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) where it was nested within other S. rhagadiza samples (referred to as Acarospora ‘amphibola’ in that paper).
Acarospora scyphulifera was considered by Magnusson as a close relative to A. smaragdula and A. amphibola (Magnusson Reference Magnusson1929). The type material of A. scyphulifera and A. scyphulifera f. subdiscreta is similar to S. rhagadiza in all characters except that the apothecia have unusually thick and distinct margins round the discs. The syntype cited of A. scyphulifera f. impressa is more similar to S. smaragdula with flattened areoles and a thick epinecral layer above a cortex with small cells, but the cortex lacks crystals and does not contain norstictic acid.
A possible synonym to S. rhagadiza is Acarospora benedarensis (Knowles Reference Knowles1913, p. 131; Fletcher et al. Reference Fletcher, James, Purvis, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). This is a rare species on hard-packed soil on coastal cliffs in Great Britain and Ireland (Fletcher et al. Reference Fletcher, James, Purvis, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009). It differs from S. rhagadiza mainly in its choice of substratum and possibly in a lower hymenium height. As we have no field experience of A. benedarensis and the sparse material we have seen is in rather poor condition, we leave this case to future studies.
Selected material examined. Denmark: Bornholm: Polsker, 1888, Hellbom (GB).—Iceland: ISu, Vestm.: Surtsey, Kristinsson L-28811, L-28868, L-28886, L-28889, L-28903, Heidmarsson 2074 (AMNH); IVe, Snæfellsnes: Hellisandur, Kristinsson 7109 (AMNH, filed under S. smaragdula).—Norway: Vest-Agder: Farsund, v 1871, Fries (UPS). Nordland: Vega par., Degelius V-425 (UPS); Du Rietz (UPS).—Sweden: Bohuslän: Dragsmark par., 30 vi 1915, Magnusson (UPS); Tånga par., Westberg & Westberg 06-121d (LD, filed under S. myochroa); Jörlanda par., 19 vi 1926, Stenholm (GB, UPS); Strömstad, Magnusson 10098a (UPS); Lycke par., 28 vi 1956, Sundell (UPS); Långelanda par., Magnusson 9473, 11584 (UPS); Lycke par., Magnusson 7418, 11332 (UPS); Marstrand par., Magnusson 6560 (UPS); Norum par., Magnusson 10012, 19936 (UPS); Skaftö par., 4 viii 1928, Degelius (UPS); Stenkyrka par., Magnusson 7402, 7506 (UPS); Tjärnö par., Magnusson 20028 (UPS); Tjörn, Westberg 06-034 (LD); Norum par., Magnusson 7493, 10012, 19936, 20035a, 21208 (UPS); Westberg 06-040 (LD); Torsby par., Magnusson 7254 (UPS); Öckerö par., Magnusson 22293 (UPS); Ödsmål par., Magnusson 5356, 11850 (GB); 1777, 1995, 2061, 5356, 5356a, 12227 (UPS). Halland: Eftra, Arup L01814 (LD); Onsala par., 15 viii 1962, Degelius (UPS). Medelpad: Njurunda par., 5 ix 1926, Eriksson (UPS). Södermanland: Ornö par., 11 vi 1941, Degelius (UPS). Västergötland: Styrsö par., Magnusson 24097, 24679 (UPS). Öland: Högsrum par., 31 vii 1914, Du Rietz (UPS).—Italy: Mt Vesuvius, 1999, Purvis (BM).—Great Britain: Wales: Rug, Crewe A84, A65, A86, A90 (UPS). England: VC 1, West Cornwall: Pendeen, Wedin 7309, 7311 (UPS).
Silobia rufescens (Ach.) M. Westb. & Wedin comb. nov
Sagedia rufescens Ach. Lichenogr. Univ.: 329 (1810); type: Anglia, Turner 23 (H-ACH No 980—lectotype!, selected here!).
= Acarospora fusca auct. Suec. (non B. de Lesd.?)
(Fig 2D)
Thallus epilithic, areolate, areoles rounded to angular, distinctly separated by cracks but forming a uniform, contiguous crust, becoming thinner and somewhat scattered in the periphery, fertile areoles 0·4–0·9(–1·5) mm wide, thallus consistently raised around the apothecia forming a distinct, usually blackened margin; upper surface dark grey to dark greyish or reddish brown, smooth to somewhat verruculose, flat to somewhat convex; epinecral layer lacking; upper cortex 40–60 µm thick, paraplechtenchymatous but individual cells difficult to distinguish, up to 5 µm wide.
Apothecia 1–3(–4) per areole, immersed; disc rounded, 0·07–0·25(–0·40) mm diam., reddish brown to dark reddish brown or blackening, flat, smooth, epruinose; proper exciple colourless in section, c. 15 µm below the hymenium, expanding to up to 60 µm at the surface, uppermost cells with dark brown caps, from the outside forming an elevated and indistinct margin; epihymenium brown, reddish brown to dark brown; hymenium 100–160 µm tall; paraphyses c. 1·5 µm in midhymenium, sparsely branched, tips cylindrical to clavate, to 2·5 µm wide; subhymenium opaque, 40–55 µm tall; asci up to 150 × 27 µm; ascospores narrowly ellipsoid to bacilliform, 3–6 × 1·5 µm.
Pycnidia not seen.
Chemistry. UV−, K−, KC−, C−; no substances detected by HPTLC.
Ecology. Most specimens were collected on sandstone and appear to be from smooth man-made habitats.
Distribution. Apparently a rare species. We have only seen a few, old, specimens from Great Britain, Germany and Sweden, with the exception of the material discussed below.
Notes. This species was not explicitly treated in our earlier study (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) although two samples discussed below were included in that analysis. From the disrupted algal layer and the general appearance S. rufescens clearly belongs in Silobia. The type is similar to S. rhagadiza and we first suspected that it perhaps would be conspecific with this species, in which case the name rufescens has precedence. However, the smaller apothecia and the consistently uniform thallus of small, angular areoles indicate that it could be a separate species and we prefer to keep it as such. We are here tentatively identifying two similar Swedish specimens from Härjedalen as S. rufescens. These were included by Wedin et al. (Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) and grouped as the sister to the rest of the smaragdula clade (A. smaragdula s. str.) on a long branch. These specimens are also characterized by lacking norstictic acid, a fairly low hymenium (c. 160 µm), lack of an epinecral layer and having small apothecia with a somewhat raised and blackened thallus margin. In addition, the thallus forms a rather continuous and partly cracked, areolate thallus. The identity of these two specimens, that is if they actually are conspecific with the type of S. rufescens, or if S. rufescens and S. rhagadiza are conspecific, is a problem that requires more material to be solved with certainty. Fletcher et al. (Reference Fletcher, James, Purvis, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) report a hymenium height of only 60–80 µm in S. rufescens which differs considerably from our observations. Hopefully recent material and DNA sequence data can shed more light on this taxon.
A specimen from Göteborg was reported as Acarospora fusca B. de Lesd. (Degelius Reference Degelius1961). The identity of A. fusca is unclear and this problem may never be solved if the type was destroyed in the Second World War, which is probably the case. Magnusson (Reference Magnusson1929), however, did study original material and concluded that it was a close relative to S. rufescens differing in smaller apothecia, a higher hymenium and more distinct cortical cells. He also confirmed Degelius' identification of the specimen from Göteborg (in hb.). In our opinion, however, after comparing it with the type material of S. rufescens, we cannot separate this specimen from S. rufescens.
Material examined. Germany: Anhalt, Bernburg, 25 x 1907, Zschacke (S, 4 specimens).—Sweden: Västergötland: Göteborg Botanical Garden, 27 iv 1956, Degelius (GB); Härjedalen, Mt Gruvvålen, Wedin 6615, 6616 (UPS). Without locality [United Kingdom? see Magnusson Reference Magnusson1929, p. 134] (S).
Silobia scabrida (H. Magn.) M. Westb. comb. nov
Acarospora scabrida H. Magn. Götebergs Kgl. Vet. och Vitterh. Samh. Handl. 28: 55 (1924); type: Norway, Sör-Tröndelag, Röros. På jord å vägkant, 15 July 1895, E. Vrang & J. P. Gustafsson (UPS—lectotype!, selected by Knudsen Reference Knudsen, Nash, Gries and Bungartz2007b).
= Acarospora verruciformis H. Magn. Göteborgs Kgl. Vet. och Vitterh. Samh. Handl. 28:. 58 (1924); type: Norway, Sör-Tröndelag, Rörås, alt. 600 m, on stone, 24 July 1919, A. H. Magnusson 3688 (UPS—lectotype!, selected by Jørgensen & Nordin Reference Jørgensen and Nordin2009; S—isolectotype!).
(Fig. 3E & F)
Thallus epilithic or muscicolous or rarely terricolous, sometimes areolate, with round, smooth, scattered to crowded, bullate areoles, but mostly squamulose; squamules rounded, incised to irregularly lobate, often imbricate, fertile areoles/squamules 0·5–3·5(–7) mm wide; upper surface pale grey, grey, brownish grey to brown, occasionally partly white pruinose, flat to somewhat convex; epinecral layer present, 10–23 µm thick, colourless; upper cortex without crystals, 30–60 µm thick, uppermost 10–15 µm reddish brown to brown, lower part colourless; cells distinct, lumina, 2–6 µm wide.
Apothecia 1–7(–12) per areole/squamule, immersed or becoming somewhat elevated; disc rounded, 0·2–1·0(–1·8) mm diam., red-brown to almost black, flat, smooth or more often scabrid, epruinose; proper exciple colourless to yellow-brown, c. 30 µm thick below the hymenium, expanding to up to 120 µm in the upper part, uppermost cells with golden brown to dark brown caps, from the outside seen as a distinct, somewhat raised, pale brown or often blackened margin; epihymenium dark golden brown to dark red-brown; hymenium 100–170 µm tall; paraphyses 1·5–2·0 µm in midhymenium, tips clavate, up to 4 µm wide; subhymenium colourless to yellowish to pale brown, with much oil, up to 70 µm thick; asci up to 145 × 20 µm; ascospores narrowly ellipsoid–ellipsoid, c. 3–5 × 1·5–2 µm.
Pycnidia rare; conidia ellipsoid to subglobose, c. 1·5–2·5 × 1 µm.
Chemistry. UV−, K−, KC−, C−; no substances detected by HPTLC.
Ecology. This species grows on acidic often somewhat schistose rocks. It is also found on calcareous ground on soil and over bryophytes.
Distribution. Northern Europe, Germany, France, North & South America (Knudsen Reference Knudsen, Nash, Gries and Bungartz2007b; Magnusson Reference Magnusson1929).
Notes. Like the other species in this group, A. scabrida is very variable. When well-developed it is easily recognized by the large, pale grey to pale brown, often imbricate squamules and the large apothecia with a dark brown colour, typically somewhat raised and with a distinct margin formed by the proper exciple. Sometimes, however, it grows as scattered, bullate areoles but can then usually be recognized by the appearance of the apothecia. In young stages, when the apothecia are still punctiform and immersed, it is very similar to A. smaragdula. Terricolous specimens can be confused with A. rhizobola (Nyl.) Alstrup, but the thallus of this species has a reddish brown colour and the squamules are attached to the substratum by a thick rhizoid strand. Furthermore, A. rhizobola has unusual, long conidia, c. 5 ×1 µm (Alstrup Reference Alstrup1981) and has never been found fertile outside Greenland.
The type specimens of A. verruciformis represent in our opinion, abberant and poorly developed specimens of S. scabrida.
Material examined. Finland: Lapponia Inarensis: Westberg LIN-272 (LD).—Norway: Hordaland: Granvin, Havaas 2, 9 (UPS); 29 ix 1938, Havaas (UPS). Møre og Romsdal: Kristiansund, 1902, Havaas (UPS). Nordland: Narvik, 7 viii 1924, Magnusson (UPS). Oppland: Sel, 1924, Magnusson 9150, 9163, 15 vii 1932, Degelius (UPS); Lom, Bøvertun, Westberg 08-188 (S). Sør-Trøndelag: Röros, vii 1926, Vrang (S, UPS); 6 viii 1912, Vrang (UPS); Rörås, 1919, Magnusson 3747 (UPS); Oppdal, 10 viii 1864, Fries (UPS). Troms: Storfjord, Westberg 2824 (LD); Tromsöe, Flöjfjeldet, 27 vi 1864, Fries (UPS). Finnmark: Mortensnes, 16 ix 1864, Fries (UPS); Tanen, Guldholmen, 23 vi 1857, Fries (UPS).—Sweden: Dalarna: Falun, 1867, de Laval (UPS); vi 1862, Cleve (UPS). Härjedalen: Ljusnedal par., Westberg 07-008, 07-009, 07-010, 07-011 (S); Moberg 9350 (UPS); Santesson 22538b, 23933, 23934, 30945, 31131, 31144c, 32961, 33638 (UPS). Jämtland: Kall par., Westberg 08-345 (S); Offerdal par., Westberg 08-379 (S). Lule Lappmark: Gällivare par., 15 vii 1922, Du Rietz (UPS). Lycksele Lappmark: Tärna par., 29 vii 1924, Stenholm (GB); Magnusson 8039, 8040, 8461, 8480, 8556, 8964a (UPS). Torne Lappmark: Jukkasjärvi par., 20 viii 1924, Du Rietz (UPS); Magnusson 5492a, 5544, 5896 (UPS). Åsele Lappmark: Vilhelmina par., Marsfjäll, 5 viii 1930, Larsson (S).
Silobia smaragdula (Wahlenb.) M. Westb. & Wedin. comb. nov
Endocarpon smaragdulum Wahlenb. In Acharius, Methodus Suppl.: 29 (1803); type: Finmarkiae Norvegicae in petris insula sinus Altensis, 10 May 1802, G. Wahlenberg (UPS L-137960—lectotype!, selected here!).
Note: The lectotype chosen is a rich specimen collected by Wahlenberg in the Altenfjord. It has apparently been overlooked that this material belongs to the greenish Cu-accumulating morph (see below) which would explain the epithet smaragdula (“emerald-like”). The sample has become somewhat brownish with age, but it still has a clear greenish tinge.
= Acarospora alberti Samp. Liquenes Inéditos: 5 (1920), (as “Alberti”); type: Portugal, Minho, Ponte de Lima, Sá; nas paredes (muito rara), 28 September 1920, G. Sampaio [PO—lectotype!; BM (Sampaio, Lich. Portugal No. 115) —syntype!, published as A. smaragdula var. alberti].
= Acarospora amphibola Wedd. Mém. Soc. Nat. Sc. Nat. Cherb. 19: 279 (1875); type: France, Ile d'Yeu (Vendee). Rochers á fleur de terre de la Pointe des Corbeaux, 28 Mai 1875, Weddell (PC 0113610—lectotype!, selected here!),
Note. The lectotype was chosen among the three specimens at PC collected by Weddell in May 1875 on Ile d'Yeu.
= Acarospora isortoqensis Alstrup Nordic J. Bot. 1: 120 (1981); type: Greenland, Søndre Isortoq, Ivnarssuaq, on N-facing vertical rock, 1977, Alstrup 77899 (C—holotype!).
= Acarospora lesdainii A. L. Sm. A monograph of British Lichens: 334 (1918); type: Great Britain, England, Yorkshire, near Keighley, Harden Moor, road to Ryecroft from guide post at c. 1000 ft. alt. [undated] T. Hebden (BM 000501103—lectotype!, selected here!).
= Acarospora murina Sandst. Abhandl. Heraus. Naturw. Ver. 21: 141 (1912); type: Germany, Oldenburg, Edewecht, An Dachziegeln, 1891, Sandstede (HBG—lectotype!, selected here!).
= Acarospora undata Clauz., Cl. Roux & V. Wirth Bull. Mus. d'Hist. Natur. Marseille 41: 39 (1982); type: Germany, Baden, Südschwarzwald, Präg (Kr. Lörrach), auf abgesprengten Felsen an der Strasse kurz oberhalb des Ortes, 4 Sept. 1971, V. Wirth (STU-Wirth—holotype!).
Thallus epilithic, areolate; areoles dispersed or contiguous through vegetative division, sometimes growing along fissures in the rock, rounded to irregular, margin not raised from the substratum, sometimes with a blackened margin, fertile areoles (0·4–)0·6–1·7(–3·2) mm wide; upper surface white, to greyish white, to pale brown or yellow–green (Cu-rich forms), flat to slightly convex, dull to somewhat shiny, sometimes with concentric, wavy markings, epruinose; epinecral layer present, 5–20 µm thick; upper cortex 30–50 µm thick, upper part opaque-yellowish, with a dense layer of crystals (norstictic acid) evenly dispersed in the cortex, also visible in a light microscope using polarized light (Fig. 1B), individual cells small and difficult to distinguish, rounded, to 4 µm wide.
Apothecia (1–)2–7(–8) per areole, immersed; disc rounded, 0·07–0·20(–0·35) mm diam., usually sunken, sometimes level with the thallus, brown to dark brown, smooth, epruinose, thallus sometimes blackening around the apothecia and occasionally somewhat raised forming a thin margin; proper exciple colourless, thin, sometimes expanding towards the surface, to 35 µm across; epihymenium reddish brown; hymenium 140–240 µm tall, paraphyses 1–1·5 µm in midhymenium, tips cylindrical to clavate, to 3·5 µm wide; subhymenium colourless to greyish; asci up to 150 × 31 µm; ascospores narrowly ellipsoid to bacilliform, (2·5–)3·5–4·5 × 1–1·2 µm.
Pycnidia not seen.
Chemistry. UV−, K+ red (red needles visible in microscope slides), KC−, C−, norstictic acid detected by HPTLC.
Substratum and ecology. This species grows on acidic rocks in open to semi-open habitats. It sometimes grows on Cu-rich rocks but to our knowledge it avoids Fe-rich rocks.
Distribution. Silobia smaragdula occurs in Europe and North America. In Sweden it is widespread but appears to be more frequent in the north.
Notes. Silobia smaragdula is a morphologically characteristic species usually easily recognized in the field. It forms small rounded, flat and pale coloured areoles with a shiny surface, often with concentric markings and with several small, punctiform apothecia. It is always recognized by the presence of norstictic acid which is easily confirmed by a spot test (K+ red, forming needle-shaped crystals in sections). It should be noted that using TLC, norstictic acid is not detected in the green Cu-rich forms. The reason for this is the complex formed between Cu and norstictic acid preventing detection of norstictic acid (see Purvis et al. Reference Purvis, Elix, Broomhead and Jones1987). Our molecular phylogeny (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009) clearly showed that the green forms were nested within a clade containing forms with a more common, pale brownish colour and should not be recognized as a species. It has apparently been overlooked that the name smaragdula itself implies a greenish colour (from the Greek “smaragdos” meaning “emerald”), as noted in the diagnosis (“…integra viridi-lutea…”) and the original material, including the lectotype designated here, clearly belongs to the copper-rich form. The copper-rich forms have been described several times as individual species, that is, A. alberti, A. undata and A. isortoqensis (see also Alstrup Reference Alstrup1984; Purvis et al. Reference Purvis, Gilbert and James1985), but no-one seems to have interpreted the epithet correctly in modern times.
The only other species in Silobia containing norstictic acid is S. myochroa. This species has larger and more convex areoles and the disc is often dilated. In addition we have not seen the conspicuous red-needles being formed in this species when treated with K, but occasionally small, granular crystals were formed in the lower part of the cortex in parts of the thallus.
The type specimen of A. murina has a strong K+ red reaction forming red needles in microscope slides, and in our opinion it belongs to S. smaragdula. It is unusual in its dark brown colour, probably caused by the substratum (a roofing tile). Likewise, the type of A. amphibola is an unusual brownish to red-brown form of S. smaragdula. The types of both these species contain norstictic acid forming needle-shaped crystals in section.
Material examined. Austria: Tirol: Innsbruck, 1988, Wirth 17299, 17332 (STU); Vorarlberg, Verwall-Gruppe: S von Klösterle, 1986, Wirth 15025 (STU).—Denmark: Anholt: Degelius Anholt-378, Anholt-474 (UPS).—Faroe Islands: Bordö, 4 ix 1956, Degelius (UPS).—Germany: Niedersachsen, Krypt. exs. No. 4856 (UPS).—Greenland: Tugtugtooq, Crewe 104 (UPS).—Iceland: ISu, Vestm.: Surtsey Kristinsson LA-25343 (AMNH). IVe, Snæfellsnes: Hellisandur, Kristinsson 7109 (AMNH); Olavsvik Westberg 09-532 (S).—Norway: Finnmark: Magerö, Havaas 26 (UPS); Maasöe, 16 vii 1864, Fries (UPS); Alten, 19 v 1802, Wahlenberg (UPS); Altenfjord, 1802, Wahlenberg (BM). Hordaland: Bergen, Westberg 08-145, 08-146 (S); Havaas, Lich. Norv. Occ. exs. No. 215 (UPS). Møre og Romsdal: Sundalen, 4 viii 1902, Havaas (UPS). Nordland: Akenes par., Moberg 7022 (UPS); Varö, 6 viii 1922, Du Rietz (UPS); Röst, Kaasö, 8 viii 1922, Du Rietz (GB). Rogaland: Randelberg, Magnusson 16857 (UPS). Troms: Storfjord, 7 viii 2003, Lättman (UPS).—Portugal: Tavares, Lich. Lusit. Sel. exs. No. 14 (UPS);—Svalbard: sin. loc. Malmgren (UPS).—Sweden: Bohuslän: Narestad par., Magnusson 7543 (UPS); Stenkyrka par., Magnusson 7501a (UPS). Gotland: Fårö par., Tibell 1216 (UPS). Gästrikland: Gävle par., Nordin 1105 (UPS). Halland: Askome, Ätrafors, Magnusson 13712a (UPS). Hälsingland: Kårböle par., Nordin 5772 (UPS); Söderhamn, Ågren 384 (UPS). Härjedalen: Ljusnedal par., Wedin 6618, 6619, 6620 (UPS); Tännäs par., 5 iv 1947, B. Magnusson (UPS); Lilla Mittåkläppen, 1867, Hellbom (GB). Jämtland: Åre, Handöl, Nordin & Owe-Larsson 139 (UPS); Frostviken, Gäddede, Magnusson 14492 (UPS); Kall par., 13 vii 1914, Magnusson (UPS); Westberg 08-346, 08-372 (S); Snasahögen, 23 vii 1913, Du Rietz (UPS). Lycksele Lappmark: Tärna par., Magnusson 8557, 8924, Du Rietz 552, 574b, 936c (UPS). Medelpad: Njurunda par., 13 viii 1926, Eriksson (UPS). Torne Lappmark: Jukkasjärvi par., 16 vii 1936, Santesson (S). Värmland: Brunskog par., Sundell 12583 (UPS); Trankil par., Sundell 2112 (GB, UPS), Sundell 15231 (GB); Norra Finnskoga par., Sundell 12521 (UPS). Västergötland: Askim par., 25 ii 1961, Tibell (UPS); Göteborg, 10 ix 1916, Magnusson (UPS), Magnusson 10198 (UPS); Medelplana par., Magnusson 17099 (UPS). Östergötland: Sandviken i Krokek, 1909, Hulting (GB).—Great Britain: England: V. C. 1, West Cornwall: Pendeen, Wedin 7310 (UPS).
Silobia tangerina M. Westb. & Wedin sp. nov
Similis Silobia smaragdula, sed differt a thallo pallide ochraceus vel tangerinus et acido licheno destituta.
Typus: Sweden, Härjedalen, Tännäs par., [Ljusnedal par.,] Mt Gruvvålen, just N of Glimsjön (c. 2 km NE of Ramundbergets fjällgård), alt. 920 m. An old copper mine, on asbestos, 17 August 1983, R. Santesson 30943 (UPS—holotype).
= Acarospora lesdainii var. subochracea H. Magn. Rep. Scient. Res. Norw. Exp. N. Zemlya 1921 34: 5 (1926) (non Acarospora subochracea H. Magn., Magnusson Reference Magnusson1933, p. 39); typus: Russia, Novaya Zemlya, Mashigin Fjord, outside Nausenfjell, 10 August 1921, Lynge (O—lectotype!, selected here!).
Note. Of the two syntypes cited by Magnusson this specimen is the better developed while the other mainly contains S. smaragdula.
(Fig. 2E & F)
Thallus epilithic, areolate to subsquamulose; areoles dispersed or somewhat contiguous through vegetative division, rounded to very irregular, margin not raised from the substratum, fertile areoles (0·65–)1·1–2·6(–5·8) mm wide; upper surface pale rusty red to bright orange, often paler towards the margin, areoles without rusty colour occur fairly frequently mixed with areoles with distinct reddish colours, smooth to more often uneven, sometimes flattened but often strongly convex, dull to somewhat shiny, sometimes with concentric, wavy markings; epinecral layer present or not, 0–17µm thick; upper cortex 60–95 µm thick, uppermost usually with small orange crystals, cells very difficult to distinguish, rounded, to 5 µm wide, becoming elongated in the lower part. Apothecia (1–)2–10(–62) per areole (in a few specimens the largest squamules have a very high number of apothecia), immersed; disc rounded (0·07–)0·10–0·25(–0·40) mm, brown, sometimes blackening, sunken or level with the thallus, smooth to very uneven, sometimes with a conical tip in the centre, epruinose, thallus not forming a raised rim around the disc; proper exciple colourless, c. 15–20 µm below the hymenium, sometimes widening to up to 80 µm at the surface, but indistinctly delimited from the cortex; epihymenium yellow brown to golden red; hymenium 180–250 µm tall; paraphyses sparsely branched and anastomosing, 0·8–1·0, tips ±cylindrical; subhymenium colourless to greyish, with much oil, to c. 50 µm thick; asci up to 150 × 30 µm; ascospores narrowly ellipsoid to bacilliform 3·0–4·0 × 1–1·5 µm.
Pycnidia not seen.
Chemistry. UV− or UV+ yellow-green, K−, KC−, C−; no substances detected by HPTLC.
Etymology. The name refers to the orange colour of the thallus.
Substratum and ecology. This species grows on iron containing rocks, mostly in open subalpine to alpine habitats. It is usually growing exposed on top of boulders or on vertical rocks, but is sometimes found in more sheltered places, for example under overhangs.
Distribution. This species is known from Sweden, Norway, the Czech Republic and Russia (Novaya Zemlja). In Scandinavia it has a distinctly northern distribution.
Notes. Silobia tangerina is usually easily recognized by its pale, orange-ochraceous squamules that are larger than, for example, S. smaragdula and S. dilatata, and the small punctiform apothecia. The colour of the thallus is rather variable, however, and often a few pale areoles without or with only a faint rusty colour can be found. It is separated from S. smaragdula not just by the colour but also in always lacking norstictic acid (no crystals in the cortex). The two species may grow in similar habitats and have been found at the same localities. Compared to S. dilatata it has a taller hymenium, mostly a paler rusty colour of the thallus, smaller and more numerous apothecia and smaller and less distinct cells in the cortex. In the phylogenetic analysis this group is represented by the “subochracea” group (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009).
Material examined. Czech Republic: Bohemia: Krkonose Mtns, Halda & Palice 1382 (hb. Palice).—Norway: Oppland: Ringebu, Magnusson 11438 (UPS). Sogn og Fjordane: Laerdal Santesson 31292 (UPS). Troms: Storfjord, Wedin 7287 (UPS).—Russia: Novaya Zemlya, Mashigin Fjord, Mt Dietrichson. 30 vii 1921, Lynge (O).—Sweden: Härjedalen: Ljusnedal par., Westberg 07-005 (S). Jämtland: Undersåker par., Tibell 23548 (UPS). Lycksele Lappmark: Malå par., Crewe, 86, 88, Wedin 6873 (UPS); Tärna par., Mjölkbäcken, 1924, Magnusson 8811 (UPS); Stensele, Kyrkberget 1924, Magnusson 7747 (UPS). Torne Lappmark: Jukkasjärvi par., 1986, Tibell 16327 (UPS). Västergötland: Undenäs par., 15 vii 2007, Berglund (hb. Toni Berglund). Östergötland: Värna par., Thor 936 (UPS).
This study was supported by The Swedish Research Council (VR 621-2006-3760, VR 621-2009-537) to Mats Wedin, and by ‘Gunnar och Ruth Björkmans fond för norrländsk botanisk forskning’ to Anna Crewe. The study was finalized thanks to support to Martin Westberg from The Swedish Taxonomy Initiative (Svenska Artprojektet), administered by Swedish Species Information Centre (ArtDatabanken). We are grateful to the Directors and Curators of cited herbaria for the loan of specimens. Ulf Arup is thanked for loan of material and assistance during field work in 2008, and Teuvo Ahti is thanked for his kind help and advice. Finally we thank our two reviewers for valuable comments on the manuscript.






