Introduction
Despite being a group of relatively conspicuous macrolichens, the genus Leptogium (Ach.) S. F. Gray has recently proved to be more taxonomically difficult and complex than previously thought (e.g. Otálora et al. 2010 Reference Otálora, Aragón, Molina, Martínez and Lutzonia , Reference Otálora, Martínez, Aragón and Molinab , 2013, 2014). This is even the case in areas such as North America, where the genus was monographed historically (Sierk Reference Sierk1964) and subsequently treated in various floristic and taxonomic works (e.g. Wetmore Reference Wetmore1967; Dey Reference Dey1978; Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001; Jørgensen & Nash Reference Jørgensen and Nash III2004; Hinds & Hinds Reference Hinds and Hinds2007; McCune & Geiser Reference McCune and Geiser2009). As in other lichen groups, insights from the results of molecular phylogenetic analyses combined with new data from morphological and anatomical studies have led to revisions of the taxonomy and species delimitation of widely distributed Leptogium species long recognized to be morphologically variable or otherwise complex (e.g. Otálora et al. Reference Otálora, Martínez, Molina, Aragón and Lutzoni2008, 2010 Reference Otálora, Martínez, Aragón and Molinab , Reference Otálora, Jørgensen and Wedin2014). Here we present the results of a study aimed at resolving long-standing questions of species delimitation within North American populations belonging to isidiate members of Leptogium section Mallotium (hereafter referred to as the L. saturninum group), a group of taxa defined by the presence of long hairs on the lower surface, isidia, and an upper surface that is usually smooth (Sierk Reference Sierk1964; Jørgensen Reference Jørgensen1973; Otálora et al. Reference Otálora, Jørgensen and Wedin2014).
The genesis of this study stems from difficulty the authors encountered when attempting to identify material of isidiate Leptogium species that were clearly referable to the L. saturninum group. This difficulty stemmed from long standing issues surrounding the delimitation of species and the application of names in the L. saturninum group. For example, throughout North America the usage of the names L. burnetiae C. W. Dodge and L. hirsutum Sierk has long been inconsistent, and the distinctions between the two species unclear (e.g., Harris Reference Harris2004; Hinds & Hinds Reference Hinds and Hinds2007; McCune & Geiser Reference McCune and Geiser2009). Recognizing that a collaborative effort was necessary to address the taxonomic problems in this group we examined available North American material referable to the L. saturninum group, examined the type specimens of the names involved, and worked to resolve the application of names to North American populations using newly generated DNA sequence data. We developed working taxonomic hypotheses that attempted to correlate morphological characters with the results of analyses of the newly generated molecular data, and these hypotheses were further evaluated and refined with additional molecular data. The consensus from these efforts forms the taxonomic treatment presented here.
Materials and Methods
Fieldwork and herbarium materials
All four authors collected numerous long-haired Leptogium specimens in North America, with the best coverage in Nova Scotia, Ontario, Québec, northern New England, the southern Appalachians, the Ozarks, and the Pacific Northwest. These collections formed the core material for our study, and were supplemented in particular by types from FH, and specimens from NY. Smaller sets of specimens, including those identified as L. hirsutum and L. saturninum by Sierk and cited in his monograph (Sierk Reference Sierk1964), were borrowed on loan from the following herbaria: ASU; BG; CANL; LSU; MICH; MSC; NEB; US National Park Service, Alaska; OSC; QFA; SRP; Tongass National Forest; US; US Forest Service, Corvallis, Oregon; WIS; WTU. Additionally, fresh material of L. saturninum from the type region in Scotland was generously provided by Brian Coppins, and specimens were borrowed from the private collections of Derr, McCune, McMullin, Neily, Nelson, and Pepper.
Morphological and chemical analysis
Specimens were examined with dissecting and compound microscopes using standard techniques (Brodo et al. Reference Brodo, Sharnoff and Sharnoff2001), including hand-cut sections mounted in water for apothecial characters and hair structure. Cross-sections of thalli used for observations of internal anatomy were obtained by hand-sectioning with a high-quality, thin razor blade, cutting near and perpendicular to the lobe margin. Thin-layer chromatography was performed using Solvent C, following Culberson & Kristinsson (Reference Culberson and Kristinsson1970), on a small representative set of specimens to confirm that none of the species produced secondary metabolites that were not detected with standard spot tests.
Molecular data generation
DNA extractions were performed following Lendemer (Reference Lendemer2012). PCR amplification (nrITS primers: ITS4/5 of White et al. (Reference White, Bruns, Lee and Taylor1990); mtSSU primers: mtSSU1/3R of Zoller et al. (Reference Zoller, Scheidegger and Sperisen1999)), sequence generation and sequence assembly for the nrITS and mtSSU regions followed Hodkinson & Lendemer (Reference Hodkinson and Lendemer2012).
Taxon sampling and alignment assembly
Two alignments were prepared for this study: 1) an alignment of mtSSU sequences of ‘hairy’ Leptogium species from North America, designed to examine the monophyly of Leptogium section Mallotium; 2) an alignment of nrITS sequences designed to examine relationships among North American populations referred to L. burnetiae, L. hirsutum and L. saturninum.
The mtSSU alignment was prepared by downloading from GenBank, on 9 February 2014, the sequences (n=104) used by Otálora et al. (Reference Otálora, Martínez and Wedin2013, Reference Otálora, Jørgensen and Wedin2014) to examine generic boundaries in the Collemataceae. These sequences were pruned to include only members of Leptogium and Collema, the latter for use as an outgroup based on Otálora et al. (Reference Otálora, Jørgensen and Wedin2014). To enforce a basic identification criterion by monophyly, taxa represented by fewer than two sequences were also removed, with the exception of members of the L. saturninum clade as recovered by Otálora et al. (Reference Otálora, Jørgensen and Wedin2014). The newly generated sequences of ‘hairy’ Leptogium species were then added to the dataset (see Table 1). The sequences were aligned using the MAFFT online interface and manually adjusted in Mesquite 2.0 (Maddison & Maddison Reference Maddison and Maddison2015). GQ258990 was removed from the dataset because it could not be aligned with the remainder of the sequences.
Table 1 GenBank accession numbers and associated voucher information for sequences used in the molecular phylogenetic components of this study. New sequences generated in this study are in bold

The nrITS alignment was prepared by downloading from GenBank, on 20 October 2014, all nrITS sequences (n=10) identified as Leptogium burnetiae, L. hirsutum and L. saturninum. The newly generated sequences of populations referred to these taxa were then also added to the dataset (see Table 1). The sequences were then aligned in the same manner as for the mtSSU dataset. Several sequences were pruned from the dataset because they were missing large portions of nrITS (KJ409607) or contained numerous bases that differed from all other sequences included in the alignment (KJ409601, KJ409605, KJ409611).
GenBank accession numbers and associated voucher information for the specimens included in this study are found in Table 1. The datasets used in this study were archived in Dryad as doi: 10.5061/dryad.49f83.
Molecular phylogenetic analyses: mtSSU
The mtSSU alignment was prepared for maximum likelihood (ML) analyses by manually deleting the ambiguously aligned regions, transforming terminal gaps (-) to missing (?) and transforming uncertainties/polymorphisms to missing (?). It was then exported as a PHYLIP formatted file and subjected to a rapid ML topology search and bootstrap analysis with 500 replicates using the model GTRGAMMA implemented in RAxML 7.2.6 (Windows executable; Stamatakis Reference Stamatakis2006). The results of the ML analyses were visualized in FigTree 1.3.1 (Rambaut Reference Rambaut2009).
The mtSSU alignment, with ambiguously aligned regions and constant sites excluded, was then analyzed with Maximum Parsimony (MP) using PAUP* 4.0a130 (Swofford Reference Swofford2003). An initial search of 1000 random-addition-sequence (RAS) replicates was made with tree bisection and reconnection (TBR) branch swapping. The MulTrees option was in effect and zero-length branches were collapsed. A total of 816 equally most parsimonious trees were recovered, a strict consensus was calculated for reference, and the best tree island was hit in 80% of the replicates. Branch support was estimated with bootstrap analyses of 1000 bootstrap replicates with five RAS per bootstrap replicate, with all other settings as above. The results of these analyses were visualized in PAUP*. A majority-rule consensus tree was calculated using tree weights and visualized in PAUP*.
For Bayesian Inference (BI) analyses, the HKY+I+G model of nucleotide substitution was selected using the Akaike Information Criterion (AIC; Akaike Reference Akaike1973) as implemented in MrModeltest 2.3 (Nylander Reference Nylander2004). BI was performed with MrBayes 3.1.2 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001) on a NEXUS formatted version of the alignment. The model settings produced by MrModeltest were pasted directly into the MrBayes block. The Markov chain Monte Carlo (MCMC) parameters comprised 10000000 generations with four chains, and a tree sampled every 100 generations. The first 10000 trees were discarded as burn-in and the results were summarized as a 50% majority-rule consensus tree. Confirmation of MCMC convergence was assessed visually and via effective sample size values in Tracer v1.6 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2013). The results of the BI analyses were visualized in FigTree.
Molecular phylogenetic analyses: nrITS
The nrITS alignment was prepared for ML analyses in the same manner as the mtSSU alignment except that after being exported as a PHYLIP file it was parsed into three partitions corresponding to ITS1, 5.8s and ITS2. A separate text formatted partition file was then created wherein the three partitions were defined. ML analyses were carried out as described for the mtSSU dataset except with the additional implementation of partitioning via the ‘–q part’ command. The results were visualized in FigTree.
For BI analyses the alignment was again partitioned into ITS1, 5.8s and ITS2. Appropriate models of nucleotide substitution were selected using the AIC (ITS1=GTR, 5.8s=JC, ITS2=HKY+G) as implemented in MrModeltest and confirmed in PartitionFinder 1.1.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012). The model settings were applied to each partition and BI analyses were carried out and visualized as outlined above.
A Jukes Cantor distance matrix was calculated for the nrITS alignment in PAUP* in order to examine the average pairwise distances within and between sequence groups recovered in the ML and BI analyses. The distances were calculated using a version of the nrITS alignment where all sites were included (i.e. ambiguously aligned regions were not excluded).
Results and Discussion
For this study, a total of 249 specimens was examined and 66 new sequences were generated (31 nrITS, 35 mtSSU). The mtSSU alignment consisted of 857 characters, 251 of which were excluded and 606 were included. Of the 606 characters included, 459 were constant, 120 were parsimony-informative and 27 were parsimony-uninformative. The nrITS alignment consisted of 685 characters, 315 of which were excluded and 370 were included; of the 370 characters included, 272 were constant, 90 were parsimony-informative and 8 were parsimony-uninformative. The results include reference to the new and revised species delimitations described in the taxonomic treatment that follows the Results and Discussion section.
Delimitation of Leptogium section Mallotium and the L. saturninum group
In our analyses of mtSSU sequence data (Fig. 1), Leptogium section Mallotium was recovered as polyphyletic, albeit with poor support. This confirms the results of previous studies within the Collemataceae that employed broader taxon sampling and additional molecular markers (Otálora et al. Reference Otálora, Martínez and Wedin2013, Reference Otálora, Jørgensen and Wedin2014). As in previously published studies, and even with our more extensive geographical sampling of populations in North America, members of the section Mallotium were resolved largely in two different clades that correspond to the presence of long versus short or cobwebby hairs on the lower surface. With the exception of L. pseudofurfuraceum P. M. Jørg. & A. K. Wallace (see below), the species that produce long, multicellular hairs on the lower surface of the thallus were recovered as monophyletic, albeit with variable support (MP/ML/BI: 64/82/1·0), and we hereafter refer to the isidiate members of this clade as the L. saturninum group because many of the vouchers from this clade were previously identified as L. saturninum. Although several monophyletic groups were recovered within the L. saturninum group in our analyses of mtSSU sequence data, the relationships within the group were largely unsupported.

Fig. 1 Phylogenetic analysis of the genus Leptogium in this study inferred from mtSSU sequence data and displayed as the most likely tree with Collema selected as the outgroup. Support values are displayed in the following format: MP-BP (Maximum Parsimony - Bootstrap Percentages)/ML-BP (Maximum Likelihood – Bootstrap Percentages) /B-PP (Bayesian Posterior Probability) with all values ≥50 for MP and ML and ≥70 for BI (Bayesian Inference) included for completeness. Thickened branches are those that were recovered with MP-BP and ML-BP support ≥70% and B-PP ≥0·95. Clades shaded in grey are those comprised of species classified as Leptogium section Mallotium.
Leptogium pseudofurfuraceum, the only species with long hairs that was recovered outside of the L. saturninum group, occurs sporadically in mountainous regions of western North America and is particularly common in the Sonoran Desert Region (Jørgensen & Nash Reference Jørgensen and Nash III2004). It differs from members of the L. saturninum group in having a distinctly brown thallus similar to that of L. furfuraceum (Harm.) Sierk and was recovered as monophyletic in our mtSSU analyses. These two taxa (L. furfuraceum and L. pseudofurfuraceum) have been shown to be closely related (Otálora et al. 2010 Reference Otálora, Martínez, Aragón and Molinab ) and represent an additional clade within Leptogium comprised of taxa with long hairs.
The species with other types of hairs (short in Leptogium hibernicum M. E. Mitch. ex P. M. Jørg., L. laceroides B. de Lesd., and an apparently undescribed species; cobwebby in L. juressianum Tav.) were recovered as a separate monophyletic group (MP/ML/BI: 76/81/1·0). The relationships between the members of Leptogium section Mallotium with short or cobwebby hairs and the other more or less glabrous members of Leptogium were poorly supported in our analyses and require further study.
Species delimitation in the Leptogium saturninum group
As has been discussed above, our expanded taxon sampling within Leptogium section Mallotium recovered the L. saturninum group as a monophyletic group distinct from L. pseudofurfuraceum and distinct from a clade comprised of the taxa with short hairs. As such, we carried out analyses of nrITS sequence data from isidiate populations referable to members of the L. saturninum group to produce a phylogenetic framework to inform anatomical/morphological study and subsequent species delimitation via a process of reciprocal illumination (e.g. McDonald et al. Reference McDonald, Miadlikowska and Lutzoni2003; Lendemer Reference Lendemer2011; Leavitt et al. Reference Leavitt, Esslinger, Nelsen and Lumbsch2013; Medina et al. Reference Medina, Francisco, Goffinet, Garilleti and Mazimpaka2013). The nrITS region (comprising ITS1, 5.8s, and ITS2) was selected for this purpose because it has already proved useful in addressing questions of species delimitation in Leptogium (Otálora et al. Reference Otálora, Martínez, Molina, Aragón and Lutzoni2008, 2010 Reference Otálora, Martínez, Aragón and Molinab ), as well as many other groups of lichens (e.g. Elix et al. Reference Elix, Blanco and Crespo2010; Pérez-Ortega et al. Reference Pérez-Ortega, Spribille, Palice, Elix and Printzen2010; Vondrák et al. Reference Vondrák, Šoun, Søgaard, Søchting and Arup2010; Sohrabi et al. Reference Sohrabi, Stenroos, Högnabba, Nordin and Owe-Larsson2011; Hodkinson & Lendemer Reference Hodkinson and Lendemer2012; Leavitt et al. Reference Leavitt, Esslinger, Hansen, Divakar, Crespo, Loomis and Lumbsch2014; Moncada et al. Reference Moncada, Lücking and Suárez2014; Orange Reference Orange2014; Ahti et al. Reference Ahti, Pino-Bodas and Stenroos2015; Arup et al. Reference Arup, Vondrák and Halıcı2015; Boluda et al. Reference Boluda, Rico, Crespo, Divakar and Hawksworth2015; Divakar et al. Reference Divakar, Leavitt, Molina, Del-Prado, Lumbsch and Crespo2015; Gueidan & Lendemer Reference Gueidan and Lendemer2015; Magain & Sérusiaux Reference Magain and Sérusiaux2015).
Our analyses of nrITS sequence data from North American populations within this group recovered six clades and one isolated sequence (Fig. 2). Each clade comprised sequences that were <5% divergent from other members of the same group (Table 2). The average distances within these six groups are well below the threshold typically observed as within-species divergence in lichen-forming fungi, while the average distances between the seven groups for the most part fall within the range of distances observed between closely related species with nrITS sequence data (see e.g. Begerow et al. Reference Begerow, Nilsson, Unterseher and Maier2010; Del-Prado et al. Reference Del-Prado, Cubas, Lumbsch, Divakar, Blanco, Amo de Paz, Molina and Crespo2010, Reference Del-Prado, Divakar and Crespo2011; Divakar et al. Reference Divakar, Leavitt, Molina, Del-Prado, Lumbsch and Crespo2015).

Fig. 2 Phylogenetic analysisof the Leptogium saturninum group from this study inferred from nrITS sequence data and displayed as the most likely tree with midpoint rooting. Support values are displayed as ML-BP/B-PP with all values ≥50/0·5 included for completeness. Thickened branches are those that were recovered with ML-BP support ≥70% and B-PP ≥0·95. Sequence groups comprising morphologically defined entities herein recognized as species are denoted with black bars. Sequence groups (I – V) within morphologically defined entities are indicated by grey bars.
Table 2 Average Jukes-Cantor distances within and between nrITS sequence groups in the genus Leptogium recovered in molecular phylogenetic analyses (see Fig.2 for detail of sequence groups)

Subsequent detailed anatomical and morphological study of both the sequenced vouchers and additional herbarium specimens, representing populations from throughout North America and abroad, resulted in the recognition of four morphologically defined entities among the seven sequence groups (see tabular summary of morphological entities in Table 3 which also includes the fifth morphologically defined entity for which molecular data were unavailable). Two of these entities (Leptogium cookii and L. hirsutum, see below) correlated with clades in which sequence distances were within that typically observed as within-species variation. The remaining two entities (L. acadiense and L. saturninum), however, were correlated with multiple sequence groups whose average distances suggest that multiple species-level lineages are involved.
Table 3 Characters distinguishing the North American members of the Leptogium saturninum group

A fifth morphologically defined entity (Leptogium compactum) was found among existing herbarium specimens that did not correspond to any of the sequenced vouchers, and attempts to generate molecular data from the vouchers of this entity were unsuccessful. In addition to standard morphological characters already employed in Leptogium (e.g. thallus thickness, anatomy of the medulla, thallus coloration), our study revealed differences in the development of the isidia and in the morphology of the thalline hairs, characters not previously utilized in the delimitation of members of the L. saturninum group. These characters are discussed in detail below.
Three of the morphologically defined entities that we recognized are monophyletic (Fig. 2). The first of these corresponded to the type specimen of Leptogium hirsutum from Illinois, USA, which is morphologically congruent with sequenced vouchers from eastern North America. The second entity was composed of specimens representing populations scattered throughout north-western North America that are morphologically congruent with three sequenced vouchers from Juneau, Alaska. This entity did not correspond to the types of any Leptogium names that we have studied and is described here as L. cookii. The third entity comprised a large number of specimens from north-eastern Canada as well as some from the northern Great Lakes, New England, and southern Appalachian Mountain regions. This entity was morphologically congruent with two groups of sequences that were recovered as sister in our analyses and, like L. cookii, did not match the type specimens of names that we had studied. As such, this entity is formally described here as L. acadiense.
The fourth morphologically defined entity is problematic from a phylogenetic perspective because it is not monophyletic and comprises three distinct sequence groups, the largest of which (group III in Fig. 2) includes a voucher collected from near the type locality of Leptogium saturninum. Furthermore, two of the groups (group IV and V in Fig. 2) were recovered in a clade together with L. hirsutum, indicating a closer relationship to that taxon than to L. saturninum s. str. Despite extensive study we were unable to uncover any morphological or anatomical differences between the three sequence groups that comprise this morphological entity. Thus it is plausible that the lineages within this entity represent cryptic species, as has been posited for other lichen groups (e.g. An et al. Reference An, Degawa, Fujihara, Mikawa, Ohkuma and Okada2012; Altermann et al. Reference Altermann, Leavitt, Goward, Nelsen and Lumbsch2014; Muggia et al. Reference Muggia, Pérez-Ortega, Fryday, Spribille and Grube2014; Cornejo & Scheidegger Reference Cornejo and Scheidegger2015; Kraichak et al. Reference Kraichak, Lücking, Aptroot, Beck, Dornes, John, Lendemer, Nelsen, Neuwirth and Nutakki2015). Here we employ a pragmatic taxonomic approach and treat the three distinct sequence groups under the name L. saturninum with the recognition that further study of more populations using additional molecular markers is needed to fully resolve the circumscription of the taxon.
As noted above, a fifth morphologically defined entity was encountered in our examination of herbarium specimens belonging to the Leptogium saturninum group. Although not represented among the sequenced vouchers, this entity was highly distinctive on account of its compact medulla and is formally described as L. compactum herein.
Morphological characters: development of isidia
There are two major types of isidium development in the Leptogium saturninum group. In the first type (Fig. 3), fungal cells surrounding a small number of cyanobacterial cells push upwards into a tiny papilla while the upper cortex expands in a continuous layer upwards as the papilla elongates. Leptogium hirsutum and L. compactum form isidia of this type. The papillae are c. 20 µm in diameter, mostly darker than the lobe surface, and increase in height and diameter as they mature into isidia. In transverse sections of the thallus in the early stages of papilla development, the unbroken cortical layer can be seen clearly (Fig. 3C). We refer to this type of development as hirsutum-type isidium development. The young isidia are not constricted at the base, and when they break off, they leave a tiny light ring of cortical tissue on the lobe surface that is visible at ×20 (Fig. 3E). In L. compactum, the isidia become basally constricted with age, while those of L. hirsutum do not become constricted and instead remain cylindrical.

Fig. 3 Hirsutum-type isidium development in Leptogium hirsutum (A & C) and L. compactum (B, D & E). A & B, surface of young lobes with developing isidia and papillae concolorous with the lobe surface (A, L. hirsutum, Goward & Miller 78-1302; B, L. compactum, McCune 35604); C, L. hirsutum, McCune 26588, transverse section showing papillae covered by continuous layer of cortical cells (black arrow); D, L. compactum, McCune 35605, transverse section showing formation of isidia at later stage than in C, taller, starting to branch, and with narrowing base; E, L. compactum, Derr 4Oct2001-C, surface of lobe showing tiny white rings of cortical tissue (black arrows) where the isidia have broken off. Scales: A & B=2 mm; C & D=100 µm; E=0·5 mm. In colour online.
In the second type of isidium formation (Fig. 4), pigmented bundles of several short fungal hyphal cells surrounding a small number of cyanobacterial cells form in the upper layer of the medulla, where they become separated from the surrounding tissue. Initially these developing isidia are c. 20 µm in diameter but they eventually grow to be c. 100 µm in diameter. As they enlarge, they break through the upper cortex of the thallus, causing a local disintegration of the cortex. Leptogium acadiense, L. cookii, and L. saturninum form isidia of this second type. We refer to this type of development as saturninum-type isidium development. In these three species, the lobe margins are darkened by the developing isidia that form beneath the surface. The pattern of this dark area differs slightly between the three species. In L. cookii the lobe margins darken almost evenly, in L. acadiense the dark area often appears evenly speckled, and in L. saturninum similar speckles appear but with thallus expansion these divide into defined angular patches with small light areas between the patches (Fig. 4). In all three species, as the mature dark granular isidia appear against the lighter cortical surface, the overall appearance of the lobes becomes markedly darker. In L. cookii most of the isidia become cylindrical, but in this species they are constricted at the base and do not have a continuous cortex covering them, so that when they break off, they do not leave a ring of cortical tissue visible under the dissecting microscope, as in L. compactum and L. hirsutum.

Fig. 4 Saturninum-type isidium development in Leptogium acadiense (A & D), L. cookii (B & E), and L. saturninum (C & F). A–C, isidia forming on surface of young lobes with dark isidia contrasting against the lighter lobe surface in A, L. acadiense, Harris 49924, B, L. cookii, McCune 22487, and C, L. saturninum Stone 3376 which in addition shows angular groups of speckles where isidia form beneath the cortex; D–F, transverse sections showing small dense aggregations of pigmented fungal hyphae and cyanobacteria below the upper surface that emerge to form granular isidia, the isidia then enlarging without any obvious cortical covering in D, L. acadiense, Hinds 4869, E, L. cookii, Stone 9067.16 and F, L. saturninum, McCune 35603. Scales: A–C=2 mm; D–F=50 µm. In colour online.
The two types of isidium development outlined above are often helpful in distinguishing sympatric or otherwise superficially similar species. For instance, Leptogium acadiense and L. hirsutum, which both occur in eastern North America, differ in the type of isidium development. Similarly, L. compactum and L. cookii, which are sympatric in western North America and may be difficult to tell apart without sectioning, also differ in the type of isidium development. While both species (L. acadiense and L. saturninum) treated in this study that have mostly granular isidia at maturity have saturninum-type development, so does L. cookii, which is one of the species with mostly cylindrical isidia at maturity.
The mature granular isidia of Leptogium acadiense and L. saturninum also aggregate in different ways. In L. saturninum these aggregations pile into dense mounds, either sessile or stalked (Fig. 5A & B, respectively), while in L. acadiense they concatenate in aggregations of up to ten granular isidia that line up to form branches of trees (Fig. 5C & D). The open branching tree-like aggregations of L. acadiense are quite distinctive and help to identify the species. Seen at low magnification or when densely clustered, however, they may resemble the branching cylindrical isidia of L. hirsutum, a feature that led some earlier workers to identify specimens of L. acadiense as L. hirsutum. In L. acadiense and L. saturninum, patches of granular isidia can also form in a single dense layer on the thallus surface or margin.

Fig. 5 Aggregations of granular isidia on mature lobes. A & B, Leptogium saturninum, Stone 5396.2. A, granular isidia forming pedicellate balls; B, granular isidia forming stalked balls. C & D, L. acadiense, looser, tree-like aggregations (C, Hinds 4869; D, Neily s. n.). Scales: A & B=1 mm; C & D=0·5 mm. In colour online.
Mature cylindrical isidia in Leptogium hirsutum, L. compactum, and L. cookii resemble each other but those of L. hirsutum are mostly smooth, (i.e. even in diameter) (Fig. 6B), while those of L. cookii are mostly uneven in diameter (Fig. 6A). In all three species, isidia may branch and often cover the thallus in dense patches. However, if isidia are broken off at the base, only those of L. hirsutum and L. compactum show a light-coloured ring of cortical tissue (Fig. 3E).

Fig. 6 Mature isidia. A, Leptogium cookii, Boyll NV190, lumpy cylindrical isidia resembling stacked granular isidia; B, L. hirsutum, Guccion s. n., smooth, cylindrical isidia with an even diameter; C, L. saturninum, Stone 7611.18, typical granular isidia. Scales: A–C=0·5 mm. In colour online.
Morphological characters: hairs
The five species recognized in this study belong to Leptogium section Mallotium (Jørgensen Reference Jørgensen1973), which is largely defined by having a mat of densely distributed hairs over most of the lower surface, referred to as a tomentum (Fig. 7). In the L. saturninum group, the tomentum is long and composed of cylindrical rather than spherical cells (Kitaura & Marcelli Reference Kitaura and Marcelli2013). In addition to tomentum on the lower surface, the five species reported in our study may also display hairs in various concentrations and configurations on the upper surfaces. In this paper we refer to the lower surface hairs as ‘tomentum’ (following Sierk Reference Sierk1964) and the upper surface hairs as ‘hairs’.

Fig. 7 Hairs on lower surface of thallus of North American members of the Leptogium saturninum group. A, L. acadiense, Anderson 13855, with hairs extending to the lobe edge; B, L. compactum, McCune 35605, with hairs usually extending to the lobe edge; C, L. cookii, Derr 380, with no hairs along most lobe edges; D, L. hirsutum, Hinds 5031, with long tangled hairs; E, L. saturninum, Stone 3376, with patches of long tangled hairs grading to short even-length hairs near lobe edge. Scales:=2 mm. In colour online.
The hairs often found on the upper lobe surface of these species have been a source of some confusion in the past. Upper surface hairs do not always occur, or may be very sparse and confined to one lobe. There appear to be two types of upper surface hairs (Fig. 8). The saturninum-type, found only in Leptogium saturninum and L. acadiense, mimics the short lower surface tomentum by being densely arrayed and more or less even in length, like bristles on a brush (Fig. 8A). Upper surface hairs in these two species rarely occur on lobe edges; more often they appear on the lobe surface where it is vertical or overhanging and the lobe is quite sheltered by or close to other lobes. The cylindrical cells comprising each hair vary in length irregularly over the length of the hair and are interspersed randomly with nearly isodiametric cells, and cell joints are slightly constricted.

Fig. 8 Hairs on upper surface of thallus. A, Leptogium acadiense, Neily, short, even-length hairs in a dense laminal patch; B, L. hirsutum, Guccion s. n, single and grouped, uneven-length hairs spread across the margin of the lobe. Scales=0·5 mm. In colour online.
The second type of upper surface hair is referred to as the hirsutum-type and is found in L. hirsutum, as well as occasionally in L. compactum and L. cookii. These hairs are typically of a wide range of lengths and appear singly or as tangled groups with visible thallus between groups. The tangled groups may be closely spaced (9–14 μm) as clusters of three or more. Long single hairs, or tangled groups of up to twice the length (to 150 μm) of shorter ones (to 75 μm), are interspersed across the upper surface of the lobes or along the edges of the lobes; frequently they are present only near the lobe margins (Fig. 8B). Cell lengths vary over the course of a single hair in all three species and are slightly constricted at the cell joints. Occasionally, some cells branch or bulge approaching ovoid in shape. The hirsutum-type upper surface hairs of L. hirsutum and L. compactum typically have wider and longer cells than those of L. cookii.
Lower surface tomentum length, density and distribution vary between species and among thalli within the five species (Fig. 7). All five species can have short or long, tangled and/or bundled lower surface hairs in differing quantities. This variability limits the use of tomentum morphology as a major diagnostic character for species separation. Nonetheless, some trends were observed during our study.
The tomentum in Leptogium hirsutum is relatively long (150–625 μm) across much of the lower surface; tangled, somewhat glossy hairs occasionally develop into longer, sinuous hapter-like bundles up to 1·5 mm long. Leptogium saturninum displays a tomentum of dense, short, nearly even-length hairs that resemble bristles on a brush, 50–110 µm long; the hairs become longer, somewhat glossy and slightly tangled in broad patches toward the centre of the thallus and often develop into longer tangled hapter-like bundles up to 1 mm long. The bundles and occasionally the patches with longer hairs in L. saturninum resemble the tomentum of L. hirsutum. The tomentum of L. acadiense resembles that of L. saturninum but the hairs typically are more uneven in length with more frequent long bundles. Long hapter-like bundles are less frequent and less distinct in L. cookii and L. compactum, where long entangled hairs occur in broad patches.
All five species display varying degrees of naked lower cortex along the undersurface lobe edges. Leptogium compactum is distinctly tomentose to the edge of many lobes, although some lobes are naked up to 0·3 mm from the margins. The tomentum extends to most lobe tips in L. acadiense, with a naked edge occurring on very small growing lobes or on the largest, old lobes. Leptogium cookii is naked along most undersurface lobe edges; L. hirsutum and L. saturninum vary considerably in the frequency and width of naked lower surface lobe edges.
Taxonomic Treatment
Leptogium acadiense J. W. Hinds, F. L. Anderson & Lendemer sp. nov.
MycoBank No.: 816807
Leptogium acadiense differs from L. saturninum in the usual presence of aggregated isidia that resemble open branching trees made up of concatenated granules, and in its medulla that is often composed of hyphae running in all directions with space between them, rather than hyphae arranged in an array parallel and perpendicular to the upper and lower cortices throughout the medulla, as in L. saturninum. However, in some specimens the medulla near the lobe edges may have many hyphae perpendicular to the cortex, making it look similar to L. saturninum.
Type: Canada, Nova Scotia, Shelburne Co., E side of Misery Lake, N of Hwy 103, 45·444°N, 63·595°W, mature Acer rubrum/Abies balsamea swamp with fern and grass understorey, on bark of Acer rubrum, 11 March 2013, F. Anderson 1590906 (NY!—holotype).

Fig. 9 Morphology of Leptogium acadiense. A, thallus, with distinctly dark isidia against a lighter grey cortex (Anderson 15074); B, cracks where the cortex has pulled apart (Anderson 14967); C, transverse section of lobe showing medullary hyphae loosely interwoven and curving in all directions (Hinds 4869); D, transverse section of lobe showing some medullary hyphae nearly straight and arranged distinctly perpendicular to upper and lower cortices (Anderson 1590754). Scales: A=5 mm; B=1 mm; C & D=50 µm. In colour online.

Fig. 10 Line drawing of the proper exciple of Leptogium acadiense, based on Hinds 1253, illustrating how it is wide at the surface and narrows quickly to one cell thick at the base of the hymenium. Scale=50 µm.
Thallus foliose, 2–9 cm diam., loosely adnate; lobes flat to concave, undulating, often with downturned margins, resembling rose petals, rounded, 3–12 mm wide, separated, contiguous or overlapping, 80–200 µm thick near the margins in wet mount, margins entire to isidiate, occasionally lobulate, darkened in a speckled pattern where isidia form beneath the surface; upper surface usually dull, occasionally shiny in places; colour ranging from light bluish grey to dark brownish grey, often with olivaceous overtones, margins with evenly spread speckles, smooth or with low bumps, often with low, poorly defined wrinkles; in many lobes, the cortex pulls apart leaving light tan cracks into the medulla (Fig. 9B); hairs rarely sparse on exposed surfaces, more commonly on parts of the upper surface that curve down towards the substratum or when underneath another lobe, forming a dense thicket of even-length hairs 50–125 µm long×5 µm wide of cylindrical to isodiametric cells up to 10 µm long; isidia development saturninum-type; mature isidia granular, usually distinctly dark grey to blackish and contrasting against the lobe surface, scattered singly or in small groups to more densely arrayed and covering parts of the lobe both laminally and marginally; granular isidia aggregate into open, upright trees with branches composed of up to c. 10 concatenated granules, often attached to a ‘trunk’ either of cortical tissue or of medullary hyphae erupting through the cortex; lower surface medium grey, densely covered to the lobe margins with short, white to tan tomentum, hairs even length within a patch, 110–270 µm long×5–6 µm wide, of cylindrical to isodiametric cells up to 14 µm long, except for a small naked zone near the margins of some young growing or very mature lobes, and interspersed with smaller patches of longer, tangled, sometimes branched white to tan hairs up to 1 mm long.
Internal anatomy: loosely interwoven hyphae 1·3–2·6 µm wide, of uneven thickness over their length and only slightly constricted where cells join, cell junctions mostly indistinct; hyphae usually curving in all directions, but near the lobe margins in some thalli many nearly straight and arranged distinctly perpendicular to the cortices; long chains of Nostoc between hyphae; upper and lower cortices consisting of a single (rarely double) layer of rounded-rectangular cells, with upper cortex cells 5–13 µm wide ×4–8 µm high, lower cortex cells 7–9 µm wide×5–8 µm high; upper cortex soon interrupted by isidia formation and disintegrating; lower cortex usually remaining intact and apparent.
Apothecia rare, laminal, usually stipitate, sometimes sessile, 0·2–1·2 mm diam.; disc reddish brown, usually plane but sometimes slightly concave or convex; apothecial margin thalline, concolorous to lighter than upper surface of thallus, sometimes covered with variable amounts of granular isidia; proper exciple euparaplectenchymatous at upper margin c. 25 µm wide, narrowing to one cell wide (c. 4 µm) and subparaplenctenchymatous below (Fig. 10); thalline exciple with tightly to loosely intertwined, ±straight hyphae arranged perpendicular to each other with chains of cyanobacteria woven between them; subhymenium pale yellowish; hymenium hyaline, c. 100 µm tall, with pale tan epihymenium; paraphyses unbranched, slightly inflated apically; asci cylindrical to clavate, 75–90×13–16 µm, 8-spored; ascospores not observed in mature state; immature ascospores in asci hyaline and 7–8 µm wide.
Pycnidia unknown.
Chemistry. No substances detected. Spot tests: K−, KC−, C−, P− and UV−.
Etymology. The epithet derives from where the species seems most common, the Acadian region of North America (i.e. eastern Maine in the United States, combined with the Maritime Provinces of Canada).
Ecology and distribution. Leptogium acadiense is widely distributed in boreal and northern temperate regions of eastern North America, with populations ranging from the tundra and taiga of northern Québec, south into the Canadian Atlantic Provinces and northern Great Lakes region (Fig. 11). It occurs primarily on the bark of deciduous trees (e.g. Acer, Aesculus, Fraxinus, Populus) in mixed hardwood-conifer forests; however, it has also been found on the bark of conifers (Thuja) and on mossy logs or rocks. Disjunct populations also occur in middle to high elevation northern hardwood forests of the southern Appalachian Mountains, habitats that have long been recognized as sharing lichen floristic elements with the boreal forests (Dey Reference Dey1976, Reference Dey1978; Lendemer et al. Reference Lendemer, Harris and Tripp2013). The species appears to be endemic to eastern North America.

Fig. 11 Map of north-eastern North America showing known distribution of Leptogium acadiense based on specimens examined for this study.
Conservation status. There are older collections from Michigan, Massachusetts, and Vermont, but no collections are known from there in the last 65 years. We suspect that this reflects a genuine decline within this part of the species’ range, perhaps because of land use changes and legacy effects from air pollution. In the northern and eastern portions of its distribution there is less conservation concern as a number of extant subpopulations are known.
Discussion. Leptogium acadiense can be difficult to distinguish from L. saturninum, which is what most specimens were identified as prior to our study. In the small area of geographical overlap (to date only in the eastern Arctic and the northern shore of Lake Superior), it may be necessary to take sections near the lobe tips and compare the arrangement of hyphae, noting either a less organized pattern of hyphae or hyphae distinctly perpendicular to, but not parallel to, the cortex in L. acadiense, versus hyphae perpendicular to and parallel to the cortex in L. saturninum (compare Fig. 9 with Fig. 19).
The presence in L. acadiense of aggregations of granular isidia that form elongated branching structures (Fig. 5C & D), hairs on the upper surface and its sometimes bluish grey colour have also caused much confusion of this species with L. hirsutum. The mode of isidium development in L. acadiense (Fig. 4) is strikingly different to that in L. compactum and L. hirsutum (Fig. 3) and is the most reliable characteristic upon which to base its identification in this case. It differs from L. cookii and L. saturninum by usually having open tree-like aggregations of granular isidia. Additionally, the geographical distribution of L. cookii and L. acadiense do not currently overlap.
Although our molecular analyses of nrITS sequence data recovered sequences of Leptogium acadiense in two well-supported clades, we were unable to observe any morphological differences between the sequenced vouchers in these clades. Further study with detailed population level sampling, and additional molecular markers, is needed to determine whether two cryptic species are involved, if there are subtle morphological differences that we did not observe, or if the molecular divergence simply reflects genetic variation among subpopulations of the species.
Selected additional specimens examined. Canada: New Brunswick: Albert Co., Fundy National Park, 1980, S. Gowan & I. M. Brodo 4427 (CANL). Newfoundland and Labrador: Newfoundland, Upper Sandy Point, on Populus, 28 iv 1894, A. C. Waghorne s. n. (NY). Nova Scotia: Annapolis Co., Lily Lake Road, on Acer, 2006, T. Neily 285 (hb. Anderson); Colchester Co., Economy River Wilderness Area, vicinity of Economy Falls, on Acer, 2004, J. W. Hinds 4869 (NY); Cumberland Co., Folly Lake Brook, on Acer, 2011, F. Anderson 15113 (NY); Guysborough Co., Canso, on Acer, 26 vi 2013, T. Neily s. n. (hb. Anderson); Halifax Co., Echo Lake, on Fagus, xi 2013, C. Pepper s. n. (NY); Hants Co., St. Croix, on Fagus, xi 2013, C. Pepper s. n. (NY); Kings Co., near Dodge Brook, on Populus, 2005, T. Neily 244 (hb. Anderson); Lunenburg Co., Beech Hill, on Acer, 2009, F. Anderson 14967 (NY); Shelburne Co., E side of Clyde River Rd, on Acer, 2008, F. Anderson 1590804 (hb. Anderson); Victoria Co., near North River, on road to quarry, on hardwood bark, 2009, F. Anderson 150431 (hb. Anderson). Ontario: Heighington Township, Iroquois Falls Forest, on Populus, 2008, R. T. McMullin 10141a (NY); Thunder Bay Distr., 14 mi E of Nipigon, on rock cliff, 1957, R. Cain 26442 (NY). Québec: MRC Beauce-Sartigan, Armstrong, on Populus, 1990, D. Bastien 534 (QFA); Nunavik, Caniapiscau River, on Salix, 2012, J. Gagnon 50-8a (QFA); Lac St.-Jean-Ouest, île de la Traverse, on Fraxinus, 1984, F. Lutzoni & N. Dignard L168 (QFA); Rimouski, Parc du Bic, Anse à Wilson, on conglomerate, 2014, F. Anderson 1622330 (QFA).—USA: Georgia: Towns/Rabun Co., Chattahoochee National Forest, on Acer, 1992, R. C. Harris 28086 (NY). Maine: Aroostook Co., Crystal, on Populus, 1991, J. W. Hinds 2539 (MAINE); Penobscot Co., Alton, Hirundo Wildlife Refuge, on fallen tree, 1991, J. W. Hinds 2554.2 (MAINE); Piscataquis Co., T2 R9 WELS, Lower Togue Pond, on Acer, 1984, J. W. Hinds 1271 (MAINE); Somerset Co., Moxie Gore, Moxie Falls, on Acer?, 1992, J. W. Hinds 2758 (MAINE); Washington Co., Edmunds Township, Cobscook Bay State Park, on Fraxinus, 1986, J. W. Hinds 997 (MAINE). Massachusetts: Bristol Co., New Bedford, 1900, H. Willey s. n. (NY). Michigan: Marquette Co., W end Huron Mountains, 1949, H. A. Imshaug 4573 (NY). Minnesota: Itasca Co., location not known, on Fraxinus, 1996, B. McCune 23348 (hb. McCune); St. Louis Co., Voyageur National Park, N side of Mica Bay, on Fraxinus, 1978, C. M. Wetmore 33016 (NY). New Hampshire: Coos Co., Dixville Notch State Park, on old tree, 1997, J. W. Hinds 3977 (MAINE). North Carolina: Clay Co., Nantahala National Forest, Buck Creek Rd, on Quercus, 1998, J. W. Hinds 4220 (MAINE); Haywood Co., Great Smoky Mountains National Park, Rough Fork Creek trailhead, on deciduous tree, 2000, J. P. Dey 29698 (NY); Swain Co., Great Smoky Mountains National Park, trail to Mingus Creek, on Aesculus, 2000, J. P. Dey 29325 (NY). Tennessee: Carter Co., Jane Gap near Rogan Mountain, on Aesculus, 1972, J. P. Dey 2299 (NY); Sevier Co., Great Smoky Mountains National Park, near Newfound Gap, vi 1935, A. J. Sharp s. n. (NY). Vermont: Chittenden Co., Charlotte, 1874, C. G. Pringle s. n. (NY).
Leptogium compactum D. F. Stone, F. L. Anderson & J. W. Hinds sp. nov.
MycoBank No.: 816808
Leptogium compactum differs from all other members of the L. saturninum group by the tightly packed hyphae in the medulla.
Type: USA, Alaska, Kenai Peninsula Borough, Lake Clark National Park, near mouth of Horn Creek, 59·874°N, 152·071°W, riparian Picea-Populus balsamifera forest, on Alnus, 18 July 2014, B. McCune 35605 (NY!—holotype).

Fig. 12 Morphology of Leptogium compactum. A, thallus, showing lobes that retain the same colour throughout (McCune 23258); B, transverse section of lobe showing tightly packed medullary hyphae coursing mostly parallel to surface but with some hyphae also oriented perpendicular to surface; lower cortical cells taller than typical (Geiser 5678); C, transverse section of lobe with equally tightly packed medullary hyphae but with almost all coursing parallel to the surface (Goward & Rutley s.n.). Scales: A=5 mm; B & C=50 µm. In colour online.
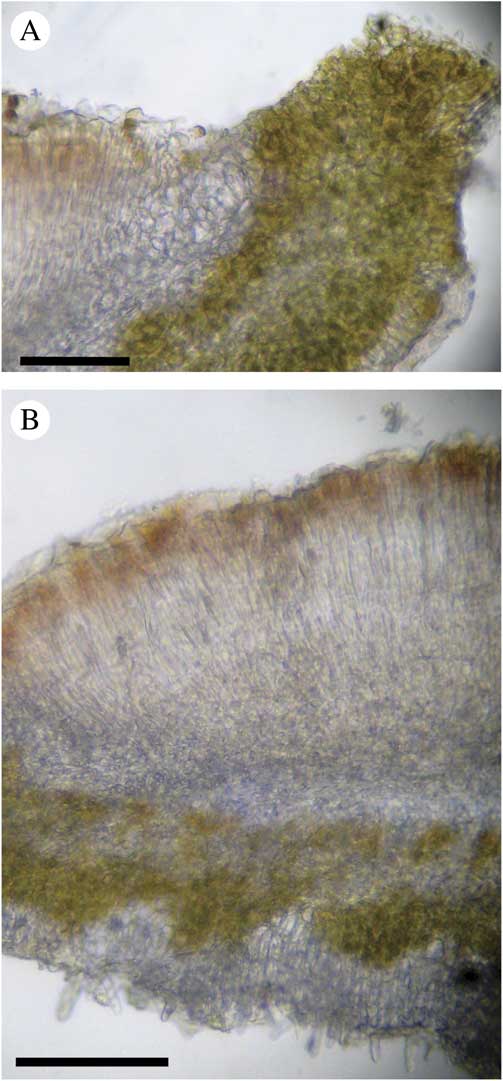
Fig. 13 Morphology of apothecium of Leptogium compactum (from McCune 35605). A, transverse section showing thalline and proper exciple; B, transverse section showing irregular algal layer beneath apothecium. Scales: A & B=100 µm. In colour online.
Thallus foliose, 3–6 cm diam., loosely adnate; lobes rounded, slightly concave, margin involute, resembling rose petals, 4–20 mm wide, separated, contiguous or overlapping, 70–100 µm thick near margins in wet mount; upper surface usually dull, shiny in some young lobes, blue-grey, colour of margins similar to older parts of the lobes, smooth, becoming rough and brown as isidia emerge on the surface, with unorganized, low wrinkles or otherwise unevenly textured; in some lobes, the cortex pulls apart leaving shiny, light tan cracks into the medulla; sometimes with white hairs of somewhat uneven length (60–135 µm long), with cells up to 20 µm long on very sheltered surfaces; isidia development hirsutum-type, emerging as cortex-covered papillae c. 20 µm wide that are not constricted at the base when young, but become constricted with age and grow into tall isidia that are irregular in width but fairly cylindrical and often branched, up to 0·5 mm tall, darker or browner than the lobe surface, sometimes the cortex of isidia visible as light-coloured rings on the lobe surface where isidia have broken; lower surface grey, densely covered with patches of somewhat even-length white to tan tomentum; hairs 200–600 µm long, with cells up to 20 µm long, tomentose to the margins or with a small naked zone near the margins and interspersed with smaller patches of longer, tangled white to tan hair bundles up to c. 1·4 mm long.
Internal anatomy: thallus extremely tightly packed, interwoven, long, undulating hyphae running more or less parallel to cortices; hyphae 2·5–5·0 µm wide and even in diameter, not constricted at cell junctions; some approximately perpendicular (columnar) hyphae made of several short cells 3–4:1 that are pinched at the joint with next cell; Nostoc appears as single cells or short chains between the compactly packed hyphae; upper and lower cortices consisting of a single (rarely double) layer of ±isodiametric cells, upper cortex cells 5–11 µm wide × 5·5–10·0(–17·5) µm high, lower cortex cells (5·0–)7·5–10·0 µm wide×6–11 µm high.
Apothecia rare, 1–3 mm diam., stipitate; disc reddish brown, flat; apothecial margin outer part concolorous with the thallus and becoming covered with isidia, 0·1–0·2 mm wide; proper exciple visible as a distinct narrow, yellowish tan line around the inside edge, euparaplectenchymatous, 55 µm wide at margin narrowing to 25 µm at base of hymenium, with cells up to 15 µm tall, grading into subparaplectenchymatous beneath the hymenium; thalline exciple with layer of cyanobacteria 75 µm thick at the upper surface that continues under the apothecium and extends down to a 3–5 cell thick layer of hyaline, rectangular cells above the lower cortex (of the amphithecium); cyanobacterial layer even on upper side and even to undulating on the lower side; subhymenium pale yellowish tan; hymenium 110–137 µm tall, hyaline, with pinkish brown epihymenium; paraphyses unbranched, c. 2 µm wide; asci c. 50–75 µm tall, clavate with a thick-walled tip, 15 µm wide near tip, 2–7 ascospores discernible per ascus; ascospores hyaline, ellipsoid, 4–5×0–1 septate, 22·5–27·5×12·5–13·0 µm.
Pycnidia unknown.
Chemistry. No substances detected. Spot tests: K−, KC−, C−, P− and UV−.
Etymology. The epithet ‘compactum’ refers to the distinctive internal anatomy of the medulla in this species, which is composed of dense, compactly arranged hyphae.
Ecology and distribution. This species appears to be endemic to north-western North America where it is widely distributed at both coastal and inland sites from western Oregon north through British Columbia and into Alaska (Fig. 14). It is primarily found in humid habitats associated with riparian corridors or bodies of water such as lakes, and occurs on rock and on the bark of both hardwoods (Populus trichocarpa, Alnus viridis) and conifers (Abies sp.).

Fig. 14 Map of north-western North America showing known distribution of Leptogium compactum based on specimens examined for this study.
Conservation status. The status of this species is not possible to determine with certainty because of previous confusion with other species. Detailed field and herbarium studies of this and other sympatric members of the Leptogium saturninum group are required in western North America to determine the status of extant populations and whether there has been a historical decline in portions of its range. The species may be rare in the southern part of its range as our study only uncovered three vouchers from the whole of the United States south of Canada.
Discussion. The most distinctive feature of Leptogium compactum that separates it from all other members of the L. saturninum group is the medulla that is composed entirely of densely packed rather than the loosely interwoven hyphae with large intracellular spaces of other species. It resembles L. cookii, a sympatric species, in its external morphology, but has pale blue-grey rather than distinctly darkened lobe margins. It also closely resembles L. hirsutum in its external morphology; however, L. hirsutum has isidia which are smooth and cylindrical compared to elongate isidia that have constrictions at the base and along the shaft. It differs from L. saturninum and most specimens of L. acadiense in its bluish grey colour, from L. acadiense and L. saturninum in lacking patches of single-layered, densely packed granular isidia, and from L. acadiense, L. cookii and L. saturninum in its type of isidia formation in which cortex covers the isidia.
Selected additional specimens examined. Canada: British Columbia: Cariboo Co., Wells Gray Provincial Park, near Dawson Falls, on conifer twigs, 1994, B. McCune 21871 (hb. McCune).—USA: Alaska: Denali Borough, Denali National Park, N side of Savage River on foot slopes of Mt. Margaret, on moss over schist, 1997, A. Rosso NV-03536 (USFS-CORVALLIS); Borough of Juneau, Juneau, Mendenhall Glacier Visitor Center, on Alnus, 2001, C. Derr 4-Oct-01-A (hb. Stone); Haines Borough, Mosquito Lake Campground, 2 mi off Haines Hwy, 1967, J. Thomson & T. Ahti 22348 (WIS); Kenai Peninsula Borough, Kenai Borough, epiphytic, 2009, H. Root 1974 (OSC); Kodiak Island Borough, on Populus, 1991, T. Tønsberg 15403 (BG); Matanuska-Susitna Borough, Eklutna, epiphytic, 2009, H. Root 1977 (OSC); North Slope Borough, Gates of the Arctic National Park and Preserve, mountains above SE corner of Narvak Lake, 2012, P. Nelson 12-658 (NY); Borough of Yakutat, ESE of village of Yakutat, end of Dangerous River Rd, near bridge, on Populus, 2002, T. Tønsberg 29930 (BG). Minnesota: Itasca Co., Highland Trail above Highland Lake, 13 km E of Marcell, 1996, B. McCune 23258 (hb, McCune). Oregon: Benton Co., by Jackson Creek, on Acer, 1995, B. McCune 22209 (hb. McCune). Washington: Skamania Co., Carson Depot Rd, T3N R8W Sec 29, on oak, 1990, J. R. Davis 2538 (hb. McCune).
Leptogium cookii D. F. Stone & Lendemer sp. nov.
MycoBank No.: 816809
Leptogium cookii is similar to L. hirsutum in the blue-grey colour of its lobes, but differs in its darkened lobe margins (vs. margins concolorous with the lobe surface), isidia that erupt through and disrupt the cortex (vs. emerge with intact cortex), and the medulla composed of hyphae that form angles but are not perpendicular to or parallel to the cortices vs. hyphae that are more perpendicularly arranged).
Type: USA, Alaska, Kenai Peninsula Co., Lake Clark National Park, near mouth of Horn Creek, 59·87423°N, 153·07143°W, in riparian Picea-Populus balsamifera forest, on Alnus, 18 July 2014, B. McCune 35603 (NY!—holotype).
(Fig. 15)

Fig. 15 Morphology of Leptogium cookii. A, thallus, showing even darkening near lobe edges (Derr 4Oct2001-B); B, transverse section of lobe showing moderately densely interwoven hyphae making an angled pattern with few hyphae perpendicular to or parallel to the cortices (McCune 35603). Scales: A=5 mm; B=50 µm. In colour online.
Thallus foliose, 2–5 cm diam., loosely adnate; lobes rounded, slightly concave with some margins involute, resembling rose petals, separated, contiguous or overlapping, 4–20 mm wide, 80–140 µm thick near margins in wet mount, margins minutely rough and evenly dark grey to black where isidia originate below the surface; upper surface shiny to matt, blue-grey when young, becoming yellowish brown-grey to brownish grey with age, smooth sometimes unevenly textured; white hairs occasionally sparse, uneven in length (60–140 µm) with cells up to 20 µm long on sheltered or flat lobe surfaces; isidia development saturninum-type; mature isidia c. 10 µm diam., granular and cylindrical of uneven diam., constricted at base even early in development, often abundantly branched with age, and covering patches of the thallus surface, darker and browner than thallus surface; lower surface grey, densely covered with white to tan tomentum of even length (65–500 µm, with cells up to 25 µm long) except for a small bare zone near the margins and interspersed with small patches of longer, tangled white to tan hairs 60–550 µm long, in bundles up to 1·2 mm long.
Internal anatomy: moderately densely interwoven hyphae making an angled pattern with few hyphae perpendicular to or parallel to the cortices, hyphae 1·5–3·0 µm wide, uneven and narrowed where hyphal cells join; long chains of Nostoc between hyphae; upper and lower cortices consisting of a single (rarely double) layer of ±isodiametric cells, upper cortex cells 5–10 µm wide×4·0–7·5 µm high, lower cortex cells 7·5–12·5 µm wide × 6–10 µm high.
Apothecia rare, barely raised above thallus when young, later becoming stipitate; disc reddish brown, flat to slightly concave; apothecial margin thalline, 0·1 mm wide, concolorous with the thallus and becoming covered with isidia; filled with cyanobacteria that extend to lower cortex below apothecium; proper exciple subparaplectenchymatous at margin, 50 µm wide, soon narrowing to 25 µm wide and euthyparaplectenchymatous just beneath surface, cells c. 5 µm wide, not aligned into distinct columns; further reduced to one cell wide at base of hymenium and euthyplectenchymatous there; continuing beneath the subhymenium and becoming thicker with more layers of cells; thalline exciple of long, loosely arranged, ±straight hyphal strands going in all directions with cyanobacterial chains woven between them; subhymenium hyaline; hymenium 100–110 µm tall, hyaline with yellowish epihymenium; paraphyses unbranched, 1·5–2·0 µm wide, slightly wider at apices; asci c. 75 µm tall, clavate with a thick-walled tip; ascospores not observed.
Pycnidia unknown.
Chemistry. No substances detected. Spot tests: K−, KC−, C−, P− and UV−.
Etymology. The new species honours Dr Stanton Cook, Professor Emeritus of Ecology, Evolution, and Geography at the University of Oregon, and doctoral dissertation advisor of one of the authors (DS).
Ecology and distribution. Leptogium cookii appears to be endemic to north-western North America where it is widely distributed at coastal and inland sites from Washington and Idaho north through British Columbia and into Alaska (Fig. 16). It is primarily found in humid habitats associated with riparian corridors or bodies of water such as lakes, and occurs on the bark of both hardwood trees (Fraxinus latifolia and Populus trichocarpa) and shrubs (Alnus sp. and Salix sp.). One specimen was found growing directly on rock along a lake shore.

Fig. 16 Map of north-western North America showing known distribution of Leptogium cookii based on specimens examined for this study.
Conservation status. As is the case for Leptogium compactum, further study is needed to determine the conservation status of L. cookii. Nonetheless, based on the specimens examined it may be rare in the southern portions of its range.
Discussion. Thalli of Leptogium cookii can be very similar to the blue-grey thalli of L. hirsutum and L. compactum, but it differs clearly from these taxa in its saturninum-type isidium development. The darkened margins where isidia form beneath the surface distinguish it from L. compactum and L. hirsutum, and from the latter in the uneven diameter of its elongate isidia that resemble an array of stacked granules (Fig. 6A). It differs from L. saturninum in its elongate isidia and from L. acadiense by the absence of single-layered, densely packed, granular isidia in patches. The known geographical range of L. cookii (strictly north-western) does not overlap with that of L. hirsutum (known to date from temperate eastern North America, extending only as far west as South Dakota).
Selected additional specimens examined. Canada: British Columbia: East Kootenay Co., Yahk Provincial Park, along Moyie River near town of Yahk, on Populus, 1995, B. McCune 22487 (hb. McCune).—USA: Alaska: Borough of Juneau, Mendenhall Glacier Visitor Center, on Alnus, 2001, C. Derr 4-Oct-01-A (hb. Stone); Borough of Nome, Seward Peninsula, S side of Bear River, 4 km S of Council on Council Rd, on Salix, 1997, K. Dillman & L. Geiser NV-03586 (USFS-Corvallis); Haines Borough, Misty Fjords, Unuk River, on Populus, 1991, C. Derr 3539 (OSC); Kodiak Island Borough, on Populus, 1991, T. Tønsberg 15403 (BG); Lake and Peninsula Borough, Lake Clark National Park, on SW shore of Portage Lake, on lakeshore rock, 2014, B. McCune 35145 (hb. McCune); Northwest Arctic Borough, Noatak National Preserve, 2004, E. Holt 21841 (OSC). Washington: Skamania Co., Columbia River Gorge National Scenic Area, Carson Depot Rd “Camas Patch”, on Quercus, 1994, M. Boyle NV-190 (OSC).
Leptogium hirsutum Sierk
Bryologist 67: 267 (1964); type: USA, Illinois, county and specific location unknown, 1877, E. Hall s. n. (FH!—holotype). ≡ Leptogium burnetiae var. hirsutum (Sierk) P. M. Jørg., Herzogia 2(4): 457 (1973).
(Fig. 17)

Fig. 17 Morphology of Leptogium hirsutum. A, thallus, showing uniformly light blue-grey colour (Hinds 2554.1); B, transverse section of lobe showing relatively densely interwoven medullary hyphae, some arranged perpendicular to and parallel to the cortices, making rectangular spaces in the medulla (Buck 46536); C, transverse section of lobe showing some hyphae distinctly wider near their junctions, perpendicular pattern not clearly evident (Buck 48748); D, apothecia showing numerous hairs on the thalline exciple (Hall s. n.). Scales: A=5 mm; B & C=50 µm; D=1 mm. In colour online.
(Apothecial characters adopted from Sierk Reference Sierk1964). Thallus foliose, 2–11(–15) cm diam., loosely adnate; lobes undulating, often involute, resembling rose petals, rounded, 3–10 mm wide; contiguous or overlapping, 60–110 µm thick near margins in wet mount, the margins entire to isidiate; upper surface usually dull, light to medium bluish grey to olivaceous grey, colour of margins similar to older parts of the lobes, smooth to slightly textured; hairs common, often in variable aggregations, c. 8 µm wide and 40–150 µm long, made up of cylindrical (occasionally ovoid) cells up to c. 15 µm long, on exposed surfaces, especially near the lobe edges; isidia development hirsutum-type, emerging as cortex-covered papillae c. 20 μm wide and elongating apically to become cylindrical and eventually branched, up to 0·5 mm tall, not constricted at base, with the cortex visible as light-coloured rings on the lobe surface where isidia have broken, concolorous with or slightly darker than the thallus surface, scattered singly or more commonly densely covering parts of the lobe; lower surface medium grey, with dense tomentum of whitish hairs except for a small naked zone near the margins, hairs 4–6 µm wide and 150–625 µm long, composed of cylindrical cells up to 20 µm long, interspersed with smaller patches of longer, tangled, sometimes branched hairs up to 1·5 mm long.
Internal anatomy: moderately densely interwoven hyphae 2·5–4·0 µm wide, of slightly uneven width; some hyphae perpendicular to and parallel to the cortices, forming rectangular spaces in the medulla, with some wide (>4 µm), short hyphae near the cortices that are slightly narrowed at the points of attachment; chains of Nostoc curving in all directions in the medulla; upper and lower cortices consisting of a single (rarely double) layer of irregular cells, upper cortex cells 6·5–8·0 µm wide×5·0–6·5 µm high, lower cortex cells 6·5–9·5 µm wide×5·5–8·0 µm high; these cortices remaining intact and distinct even on older parts of the thallus.
Apothecia rare, sessile to short-stipitate on the upper surface, 0·5–1·5 mm diam.; disc concave to plane, dark reddish brown to black; apothecial margin thalline, entire, concolorous with the thallus, 90–125 µm thick, often bearing numerous white hairs; proper exciple euparaplectenchymatous, 10–25 µm near the margin, widening to 35–80 µm below; thalline exciple present; subhymenium 30–45 µm thick, yellowish to brownish; hymenium 110–150 µm thick, hyaline, with a thin yellow to brown epihymenium above; paraphyses unbranched, c. 1·5 µm diam., slightly expanded at the apices; asci cylindrical-clavate, 90–115×16–21 µm, 8-spored; ascospores hyaline, ellipsoid, 3–4×0–1 septate, 21–35×14 µm.
Pycnidia unknown.
Chemistry. No substances detected. Spot tests: K−, KC−, C−, P− and UV−.
Etymology. The epithet refers to the frequent and conspicuous presence of hairs on the upper surface of the thallus.
Ecology and distribution. Leptogium hirsutum occurs on the bark of deciduous trees, as well as on mossy logs and limestone rocks, in humid mixed hardwood forests. This species is endemic to, and widely distributed in, temperate eastern North America with the majority of extant populations concentrated in the Appalachian Mountains and Ozark Highlands. Scattered collections have also been made from Minnesota, Wisconsin, New Jersey (on limestone) and central Maine since 1990, but historically the species was found in the region from Iowa to Massachusetts (Fig. 18). The absence of records since 1990 in that region may in some cases reflect a lack of modern collections, but in most instances it probably represents a genuine decline.

Fig. 18 Map of temperate eastern North America showing known distribution of Leptogium hirsutum based on specimens examined for this study.
Conservation status. The absence of records since 1990 in states where the species formerly occurred (Iowa, Illinois, Michigan, Ohio, Kentucky, southern Ontario, New York, and Massachusetts) is of conservation concern and suggests that there is now a broad section of eastern temperate North America that no longer has a suitable habitat for L. hirsutum. We suspect that this decline and regional extirpation resulted from past large-scale conversion of natural habitat to agriculture and development as well as air pollution. The presence of the species on limestone in New Jersey differs from its substratum in the region historically, and may reflect the buffering effect of limestone on acid precipitation; other normally corticolous lichens recently found on limestone in areas of significant air pollution include Lobaria pulmonaria (western Connecticut) and Coccocarpia palmicola (western Massachusetts) (J. Hinds, unpublished data).
Discussion. One of the major sources of confusion within the Leptogium saturninum group in the North American literature is the usage of the names L. hirsutum and L. burnetiae. Remarkably both names were introduced in the same year; L. hirsutum was described based on material from Illinois (Sierk Reference Sierk1964) and L. burnetiae was described from East Africa (Dodge Reference Dodge1964). Subsequently these names were recognized as applying to similar material differing primarily in the hairiness of the upper surface (see e.g., Jørgensen Reference Jørgensen1973). The distinction between the two taxa was not clear-cut, as is indicated by their treatment as infraspecific taxa (Jørgensen Reference Jørgensen1973) and by the use of one name or the other in recent literature (Harris Reference Harris2004; Hinds & Hinds Reference Hinds and Hinds2007; McCune & Geiser Reference McCune and Geiser2009). Field observations by several of the authors of the current study further suggested that only one species was present in eastern North America; however, it was unclear which name should be applied to the taxon.
In this study, eastern North American populations referable to this taxon were recovered as a strongly supported monophyletic entity (Fig. 2). A study of the types of both names found that the type of Leptogium burnetiae differs from that of L. hirsutum in having a much thicker thallus (182 µm vs. 60–110 µm) and a medulla with different internal anatomy. The medullary hyphae of L. burnetiae curve in all directions without perpendicular and parallel orientation, while those of L. hirsutum are more closely interwoven with most perpendicular or parallel to the cortices and short, wide cells near the cortices with constrictions at cell joints. These differences as well as the different geographical distributions, L. burnetiae from East Africa and L. hirsutum from North America, permit the two names to be treated as applying to independent species and consequently L. burnetiae is excluded from the North American lichen checklist (Esslinger Reference Esslinger2015). Further study of the L. saturninum group in Africa and other regions of the world is required to understand the circumscription and distribution of L. burnetiae.
Examination of North American material referred to as either Leptogium burnetiae or L. hirsutum, revealed western North American populations belonged to other morphologically distinct species L. compactum and L. cookii (described here) and all eastern North American populations were referable to a single species that usually produces conspicuously cylindrical isidia. It should be noted that some North American populations with granular isidia that may have been referred to L. burnetiae previously, actually belong to L. acadiense, which is a species that typically produces granular isidia. However, in our experience the majority of specimens of L. acadiense were originally classified as L. saturninum based on the dark colour of the thallus.
Leptogium hirsutum is best distinguished from L. acadiense, L. cookii and L. saturninum by its isidium development in which cortex-covered papillae elongate into cylindrical isidia. Its mature isidia resemble those of L. compactum, but the isidia of the latter species are not as smooth, and are constricted at the base. In addition, L. compactum has a very different, extremely compact medulla that is readily visible in section.
Selected additional specimens examined. Canada: New Brunswick: Charlotte Co., Pomeroy Ridge, on Populus, 2008, S. Clayden 18418 (NBM); York Co., SW of Browns Mountain, on dead Thuja, 2006, S. Clayden 14526 (NBM). Ontario: Northumberland Co., Seymour West, on old trunks, 1893, J. Macoun 200 (NY).—USA: Alabama: Clay Co., Horn Mountain, SE of Talladega, on Quercus, 1974, J. P. Dey 8091 (NY). Arkansas: Searcy Co., Buffalo National River, Tyler Bend, on dolomite bluffs, 2005, W. R. Buck 48748 (NY); Cleburne Co., N portion of North Unit of Big Creek Natural Area, on Quercus, 2010, J. C. Lendemer 26368 & D. Ladd (NY); Pope Co., Ozark National Forest, Kings Bluff, on Quercus, 2005, J. W. Hinds 5029 (NY). Georgia: Murray Co., Chattahoochee National Forest, Cohutta Wilderness, on Quercus, 1992, R. C. Harris 28171 (NY); Towns Co., Southern Nantahala Wilderness, Chattahoochee National Forest, Hightower Gap to Rich Knob, 2007, J. C. Lendemer et al. 10919 (NY). Kentucky: Harlan Co., top of Big Black Mountain, on trees, 1981, C. F. Reed 113419 (NY). Maine: Penobscot Co., Milford, on Acer, 1984, J. W. Hinds 1250 (MAINE); Alton, Hirundo Wildlife Refuge, on bark of fallen log, 1991, J. W. Hinds 2554.1 (MAINE). Maryland: Washington Co., C&O Canal along Potomac River, on mosses over limestone, 1966, C. F. Reed 73710 (NY). Massachusetts: Middlesex Co., Blue Hill, sine date, E. Tuckerman s. n. (NY). Michigan: Gogebic Co., Ottawa National Forest, SW of Marenisco, on Fraxinus, 1975, W. R. Buck B-216B (NY). Minnesota: Cass Co., Ottertail Peninsula in Leech Lake, on fallen Thuja, 1975, W. R. Buck B-508 (NY). New Jersey: Warren Co., Johnsonburg Presbyterian Camp, on limestone, 1992, R. C. Harris 27982 (NY). New York: Albany Co., sine loc., 1917, C. H. Peck 14A (NY). North Carolina: Avery Co., Linville, on Ulmus, 1936, E. B. Harger 30 (NY); Cleveland Co., N of King’s Mountain, Cleveland-Gaston County Line, on Quercus, 1990(?), W. L. Culberson 5506 (NY); Jackson Co., Cedar Cliff Mountain, 3·5 mi E of Tuckasegee (NC 107) on NC 281, on Juniperus, 1994, R. C. Harris 33063 (NY); Macon Co., Mt. Satulah, 12 v 1934, G. P. Anderson s. n. (NY); Polk Co., Tryon, on tree base, 1928, A. W. Evans 4 (NY); Swain Co., Great Smoky Mountains National Park, Double Springs [sic=Spring] Gap, along the Appalachian Trail, on Fagus, 1972, J. P. Dey 2395 (NY). Oklahoma: Cherokee Co., Cookson Wildlife Management Area, Bolin Hollow, on limestone, 2004, W. R Buck 46536 (NY). South Carolina: Chester Co., sine loc., on Juniperus, 12 iii 1884, L. M. Loomis s. n. (NY). Tennessee: Hickman Co., Middle Tennessee State University Wildlife Management Area, directly (c. 10 mi) WNW of Centerville and c. 2 mi S of the Duck River, on Carya base, 1997, L. R. Phillippe 29564 (NY). Virginia: Page Co., Shenandoah National Park, along Jeremy Run Trail, from Elk Wallow to Jeremy Run fire foot trail, on boulder, 1972, J. G. Guccion 1067 (NY). Wisconsin: Douglas Co., Brule River State Forest, Divide Swamp NW of trail bridge (14·5 mi SW of Brule), 2009, C. M. Wetmore 98761 (NY).
Leptogium saturninum (Dicks.) Nyl.
Act. Soc. Linn. Bordeaux ser. 3, 21: 272 (1856); type: Fasc. Pl. Crypt. Brit. 22: 22, tab. 6, Figure 8 (1790) (icon!—holotype); United Kingdom, Scotland, Perthshire, Glen Lochay, J. M. Crombie s. n.≡Lich. Brit. Exs. No. 5 (BM[n.v.]—epitype). ≡ Lichen saturninus Dicks., Fasc. Pl. Crypt. Brit. 2: 21 (1790); ≡ Parmelia saturnina (Dicks.) Ach., Method. Lich. 221 (1803); ≡ Collema saturninum (Dicks.) DC. & Lam. in Lamarck & de Candolle, Fl. Franc., ed. 3, 2: 385 (1805); ≡ Mallotium saturninum (Dicks.) Gray, Nat. Arr. Brit. Pl. 1: 399 (1821).
(Fig. 19)

Fig. 19 Morphology of Leptogium saturninum. A, thallus, showing margins darkened by isidia on upper left (McCune 21731); B, transverse section of lobe showing relatively loosely interwoven hyphae, many of which course either parallel or perpendicular to surface creating a cross-hatched look; hyphae composed of long, even cells (Stone 5396.2). Scales: A=2 mm; B=50 µm. In colour online.
(Apothecial characters adopted from Jørgensen Reference Jørgensen2007). Thallus foliose, (1·5–)2·0–8·0 cm wide, loosely adnate; lobes rounded, slightly concave with some margins involute, resembling rose petals, separated, contiguous or overlapping, 3–10(–15) mm wide, 85–170 µm thick near margins in wet mount; upper surface matt to rough, medium to dark brown-grey to blackish; near margins of lobes where isidia form beneath the cortex, a dark pattern of speckles appears that breaks into angular groups as the lobes expand before the isidia emerge through the cortex (Fig. 4C); rarely sparse white hairs, 4–5 µm wide×40–65 µm long occur on exposed surfaces, more commonly, on parts of the upper surface that curve down toward the substratum or when underneath another lobe, forming a dense thicket of even-length hairs 4–5 µm wide ×35–90 µm long made up of cylindrical cells up to 12·5 µm long; isidia development saturninum-type; mature isidia usually granular but occasionally cylindrical, evenly spread across the lobes or forming mounds or spheres of granules; lower surface grey, densely covered with tomentum to lobe edges or to a small naked margin, hairs white to tan, 50–140 µm long ×4–5 µm wide, made up of cylindrical to isodiametric cells up to 12 µm long, occurring in patches of even lengths and interspersed with smaller patches of longer, tangled white to tan hairs up to 1·0 mm long.
Internal anatomy: moderately densely woven hyphae with many perpendicular and parallel to the cortices; hyphae 2–4 µm thick, of slightly uneven width over their length, some cells narrowing abruptly at the joints making cell junctions distinct; chains of Nostoc curving in all directions in the medulla; upper and lower cortices consisting of a single (rarely double) layer of ±isodiametric cells, upper cortex cells 6·5–9·5 µm wide×5–6 µm high, lower cortex cells 6·5–9·5 µm wide×4·0–5·5 µm high, upper cortex soon interrupted by isidia formation and disintegrating; lower cortex usually remaining intact.
Apothecia very rare, up to 2 mm wide; disc brownish; apothecial margin thalline, isidiate; exciple characteristics unknown; subhymenium characteristics unknown; hymenium colour and thickness unknown; paraphyses characteristics unknown; asci shape and size unknown; ascospores hyaline, submuriform, ellipsoid, 20–25 × 8–10 µm.
Pycnidia unknown.
Chemistry. No substances detected. Spot tests: K−, KC−, C−, P− and UV−.
Etymology. The epithet refers to the dark coloration of the thallus.
Ecology and distribution. Leptogium saturninum is widespread throughout western North America, particularly in montane habitats, from Alaska and British Columbia south to the Coastal Ranges of the Pacific Northwest and the high elevations of the Rocky Mountains, from Idaho to Colorado, Arizona and New Mexico (Fig. 20). Truly disjunct populations likely occur north of Lake Superior as mapped herein. Nonetheless we suspect that the apparent disjunction in the tundra of northern Québec probably reflects collection bias and that the species is continuously distributed in the arctic regions of North America. The species occurs in a wide variety of habitats typically associated with boreal and arctic ecosystems and is found on the bark of a wide variety of trees, as well as on mossy rocks. It should be noted that most of the specimens identified as L. saturninum from Mexico and south-western USA (e.g. Jørgensen & Nash Reference Jørgensen and Nash III2004) were not examined in this study, and thus are not treated here.

Fig. 20 Map of North America showing known distribution of Leptogium saturninum based on specimens examined for this study.
Conservation status. Because of its wide distribution throughout western North America, largely in undisturbed montane, boreal, and arctic habitats, Leptogium saturninum does not appear to be of conservation concern at the present time. However, petroleum exploration, excavation of tar sands and pipeline construction in the north has wide-reaching disruptive ecological implications and could diminish habitat significantly in the future.
Discussion. Leptogium saturninum, as defined here on morphological grounds, corresponds with three clades inferred from sequence data using nrITS (Fig. 2). Clades III and IV appear to be more closely related to L. hirsutum than to clade V, the latter of which includes a specimen collected near the type locality in Scotland. Thus L. saturninum as defined here is not monophyletic. It is likely that three distinct species are involved; however, additional sequence data and detailed field and herbarium studies are needed to determine whether these are cryptic taxa or taxa that can be recognized by subtle morphological differences that we were unable to detect.
Leptogium saturninum can be distinguished from L. hirsutum and L. compactum by its predominantly granular rather than cylindrical mature isidia, and mode of isidium development in which small groups of hyphae with Nostoc form beneath the cortex then break through the surface and grow larger as opposed to papillae with an intact cortex elongating into cylindrical isidia. Leptogium saturninum differs from L. cookii in its lack of elongate isidia, which are very rare in L. saturninum and common in L. cookii, and by the often blue-grey colour of L. cookii. L.satuninum possesses concatenated granular isidia that resemble open branching trees which L. acadiense lacks, although it should be noted that these structures can be absent from immature thalli of L. acadiense. In such instances the two species can usually be separated by their geographical distribution, since they are largely allopatric. In the relatively small area of sympatry, the two species can be distinguished by the pattern of medullary hyphae as seen in transverse sections near the lobe tip; many hyphae parallel and perpendicular to the lobe surface in L. saturninum, and either curving in all directions or ±perpendicular to the cortices in L. acadiense.
Selected additional specimens examined. Canada: Alberta: Clearwater Co., Alhambra, E of Hinton, near Crimson Lake Provincial Park, on bark, 2012, D. Haughland 2012-351 (PMAE); Yellowhead Co., William A. Switzer Provincial Park, NW of Hinton, 2012, D. Haughland 2012-149 (PMAE). British Columbia: Cariboo Co., Wells Gray Provincial Park, near Dawson Falls, on bark, 1989, R. Rosentreter 6332 (SRP). Québec: Nunavik, Rivière Koksoak, 28 vii 2013, J. Gagnon s. n. (QFA). Yukon: W slope of Grey Mt., 9 mi SE of Whitehorse, on humus on calcareous cliff, 2012, J. C. Lendemer 28520 (NY).—Norway: Hordaland: Etne, NW of Tungesvilestranda, on Fraxinus, 1 viii 2006, T. Myhre s. n. (hb. Stone).—USA: Alaska: Borough of Nome, Seward Peninsula, on saddle above Norton Bay, on Ledum twig, 2005, E. Holt 22643 (OSC); Fairbanks North Star Borough, UAK Fairbanks campus along ski trail NW of the science buildings, on Populus, 2014, D. Stone 9067.16 (hb. Stone); Kenai Peninsula Borough, Primrose Creek, S end of Kenai Lake, on Populus, 2010, B. McCune 32876 (hb. McCune); Lake and Peninsula Borough, Katmai National Park, shore of Naknek Lake, on Populus, 2013, B. McCune 32876 (hb. McCune); North Slope Arctic Borough, Gates of the Arctic NP, W end of limestone ridge S of Kurupa Lake, 2012, P. Nelson 12-1044 (NY); Northwest Arctic Borough, Noatak National Preserve, on rock/soil, 2005, E. Holt 23328a (OSC). Arizona: Navajo Co., Mount Baldy Wilderness, on shaded rock, 1994, T. H. Nash 34885 (MSC, WIS). California: Humboldt Co., E of Arcata, on Quercus, 2001, D. Stone 4919.3 (hb. Stone). Colorado: Boulder Co., Castle Rock in Middle Boulder Creek, on moss, 3 vii 1961, R. Anderson & S. Shushan s. n.=Lich. West. N. Amer. Exs. 46 (ASU, SRP, BRY, DUKE, LSU, MONT, TLE, US, UC, OMA, WIS); La Plata Co., near Durango, Junction Creek at trailhead of CO Trail, on Quercus, 2008, D. Stone & M. Sweeney 7611.18 (hb. Stone); Mineral Co., W of South Fork, on conifer, 23 vii 2004, K. Boscheinen s. n. (hb. Stone). Idaho: Clearwater Co., Little Canyon Creek, 4·5 mi SW of Orofino, on Abies, 10 x 1994, S. Walker & K. Gray s. n. (CANL); Nemhi Co., 80 mi downriver from Salmon, Barth Hot Springs along the Main Salmon River, on bark, 1988, R. Rosentreter 4961a (SRP); Shoshone Co., along Saint Maries River, 1999, J. Hutchinson ID-412-08 (OSC). Minnesota: Cook Co., Grand Portage Island, on trees, 1897, B. Fink 24 (NY). Montana: Flathead Co., Glacier National Park, head of Kintla Lake, on Picea bark, 1984, B. McCune 14239 (hb. McCune); Glacier Co., Glacier National Park, Virginia Falls, on cliff, 1950, H. A. Imshaug 6409 (MSU, WIS); Missoula Co., McNamara bridge on MT 200, on moss over rock, 1979, B. McCune 10332 (hb. McCune); Ravalli Co., Bitterroot Range on granite boulder, 1981, B. McCune 11933 (hb. McCune). Nevada: Clark Co., Hermit’s Peak, on rock, 1952, H. A. Imshaug 9866 (MSU, WIS). Oregon: Benton Co., Finley National Wildlife Refuge, on tree, 1987, B. McCune 17142 (hb. McCune); Douglas Co., Irwin Rocks ACEC, W of Roseburg, on Quercus, 1997, D. Stone 3376 (hb. Anderson); Jackson Co., E of Trail off Crowfoot Rd, on Quercus, 2014, D. Stone 9060.3 (hb. Stone); Jefferson Co., Deschutes National Forest, near FSR-2076, on Populus, 1996, B. McCune 22946 (hb. McCune); Lane Co., 7 mi W of Eugene, on Quercus, 1940, M. Doty s. n. (NY); Linn Co., Horse Rock Ridge Research Natural Area, on Acer, 1998, B. McCune 24315 (hb. McCune); Multnomah Co., Benson State Park, on Acer, 2004, M. Boyll NV-04157 (USFS-Corvallis); Wallowa Co., floodplain of Lostine River, near Pole Bridge Picnic Area, on Populus, 1994, B. McCune 21731 (hb. McCune). Washington: Lewis Co., Camp Creek Cliffs, on Quercus branch, 1999, B. McCune 24442 (hb. McCune); Skamania Co., Columbia River Gorge National Scenic Area, W side of Drano Lake, on Quercus, 1993, J. Riley NV-0080 (USFS-Corvallis).

J. Lendemer was supported by NSF-DEB 1145511 awarded to J. Lendemer and R. C. Harris. Two joint meetings of the authors at NYBG were supported in part by NSF-DEB 1145511, and in part by research funds obtained by J. Lendemer. We thank Brian Coppins for providing fresh material of Leptogium saturninum from Scotland for molecular work. We are also grateful to Bruce McCune, Peter Nelson, Chiska Derr, Chris Pepper, Troy McMullin and Tom Neily for lending specimens used in this study. The curators of the following herbaria are also thanked for providing loans of specimens: ASU, BG, CANL, LSU, MICH, MSC, NEB, US National Park Service (AK), OSC, QFA, SRP, Tongass National Forest, US, US Forest Service (Corvallis, OR), WIS, WTU.

























