Introduction
Ramonia is a widespread genus of the Gyalectaceae, species of which occur equally in tropical and temperate regions, often on soft bark or decaying wood, rarely on rock or terricolous (Coppins et al. Reference Coppins, Thor and Nordin1994; Lendemer et al. Reference Lendemer, Knudsen and Coppins2009). Species of this genus have tiny, round apothecia with concave discs, and a hymenium with paraphyses and periphyses.
So far 27 species are known in Ramonia, only eight of which were treated in the revision of the genus (Vězda Reference Vězda1966), the others being described in subsequent papers (Vězda Reference Vězda1967, Reference Vězda1973; Sherwood Reference Sherwood1977; Thor & Vězda Reference Thor and Vězda1984; Coppins Reference Coppins1987; Canals & Gómez-Bolea Reference Canals and Gómez-Bolea1992; Coppins et al. Reference Coppins, Thor and Nordin1994; Øvstedal & Gremmen Reference Øvstedal and Gremmen2001; Lendemer & Knudsen Reference Lendemer and Knudsen2008; Lendemer et al. Reference Lendemer, Knudsen and Coppins2009; Gagarina & Stepanchikora Reference Gagarina and Stepanchikova2013).
During studies by the authors on lichen ecology and diversity in mountain forests in the state of Pernambuco in the north-eastern region of Brazil, an undescribed species was encountered, which is described below. An updated artificial world key to all currently known species of Ramonia is given, with their currently known distribution ranges. The key is based on Lendemer & Knudsen (Reference Lendemer and Knudsen2008), but includes the additional species R. subantarctica Øvstedal, which was already described but not included in that key. The key leaves out the species that have been transferred to the Graphidaceae [in the genera Pseudoramonia and Topeliopsis; see Rivas Plata et al. (Reference Rivas Plata, Lücking, Sipman, Mangold, Kalb and Lumbsch2010) for references and keys to these species], as well as Ramonia athallina Sherwood, which was referred to Odontura rhaphidospora (Rehm) Clem. by Coppins (Reference Coppins1987). Vězda (Reference Vězda1966) recognized three different formal sections within the genus Ramonia. The type species and the other tropical species are all corticolous, not especially short-lived, and have a relatively well-developed thallus and polysporous asci. Examples are Ramonia valenzueliana (Mont.) Stizenb. (Fig. 3B) with a verruculose thallus, and R. malmei Vězda (Fig. 3C) and R. kandleri Kalb (Fig. 3D) with a granulose thallus. The new species is close to the type species. The genus also, however, contains many temperate (and even one subantarctic) species which are generally short-lived, occur on various substrata including wood, soil and rock, all with a much less developed thallus or are even not lichenized at all, as is the case in many specimens of R. interjecta Coppins (Fig. 3E, which illustrates a non-lichenized specimen with an apothecium protruding through bark covered with free-living algae), and the asci are less often polysporous. The species now united in Ramonia may even ultimately prove to belong to different genera, but separating them falls beyond the scope of this study.
The new species was found in Triunfo, one of the few places with mountain forest in the lowland state of Pernambuco. The lichens of these mountain forests, called Brejo de Altitude and part of the former more extensive Atlantic Rainforest biome (Thomas & Barbosa Reference Thomas and Barbosa2008) are not yet well studied. The only lichen records from Brejo de Altitude are those by Cáceres (Reference Cáceres2007).
Material and Methods
Identification and descriptive work was carried out in Itabaiana, Universidade Federal de Sergipe, using a Leica EZ4 stereomicroscope and a Leica DM500 compound microscope, and also in Soest using an Olympus SZX7 stereomicroscope and an Olympus BX50 compound microscope with interference contrast, connected to a Nikon Coolpix digital camera. Sections were mounted in tap water, in which all measurements were also taken. Ascospore measurements are given as average (in italics) +/− the standard deviation, with extremes between brackets. The chemistry of the type specimen was investigated by thin-layer chromatography (TLC) using solvent C (Orange et al. Reference Orange, James and White2001).
The following is a selection of specimens studied.
Ramonia interjecta: The Netherlands: Gelderland: Ermelo, Groevenbeekse heide, 1999, A. Aptroot (BR).
Ramonia kandleri: Brazil: Minas Geraes: Catas Altas, Parque Natural do Caraça, 1997, A. Aptroot 40827 (ABL, SP).
Ramonia malmei: Argentina: Misiones: Puerto Iguazú, near Hotel Selvático Don Horacio, 2013, L. I. Ferraro, A. Aptroot & M. E. S. Cáceres 10526 (ABL, CTES).
Ramonia micrococca: Kenya: Nyanza: Kisumi-Londiani, Tinderet Forest Reserve, 1949, R. A. Maas Geesteranus 5135 (BR, L).
Ramonia microspora: Indonesia: Java: Jogyakarta, University Garden, 14 vi 1995, T. Gandjar (BR).
Ramonia valenzueliana: Argentina: Misiones: Puerto Iguazú, near Hotel Selvático Don Horacio, 2013, L. I. Ferraro, A. Aptroot & M. E. S. Cáceres 10626 (ABL, CTES).
Ramonia variospora: type (URM).
The New Species
Ramonia variospora Sobreira, Aptroot & M. Cáceres sp. nov.
MycoBank No.: MB 809917
Similar to Ramonia valenzueliana but with a verrucose thallus, concave, initially almost closed apothecia and variably 8 or c. 32–64, 1-septate ascospores per ascus; when 8 per ascus ellipsoid and 16–22×6–10 µm; when c. 32–64 per ascus broadly ellipsoid and 10–14×6–8 µm.
Type: Brazil, Pernambuco, Triunfo, Carro Quebrado trail, 7°52′S, 38°06′W, on bark of tree, c. 877 m alt., 1 August 2013, P. N. B. Sobreira 46 (URM—holotype).

Fig. 1. Ramonia variospora, holotype. A, habitus; B, ascoma margin showing periphyses, a polysporus ascus and the tip of an 8-spored ascus (at white arrow), this is a different ascoma than D and E; C, section through ascoma; D–F, parts of the hamathecium of three different ascomata; D, section through part of young ascoma showing octosporous ascus (lower left, at white arrow) and polysporous ascus (right) with young, still aseptate ascospores; E, section through part of old ascoma showing octosporous ascus (right, at white arrow) and polysporous ascus (left); F, section through part of ascoma showing young polysporous ascus (left) and mature polysporous ascus (right), this is the same ascoma as B which also shows octosporous asci; G, ascospores with gelatinous sheaths; H, released ascospores of varying size and shape. Scales: A=0·1 mm; B & E=25 μm; C=50 μm; D, F, G & H=10 μm. In colour online.
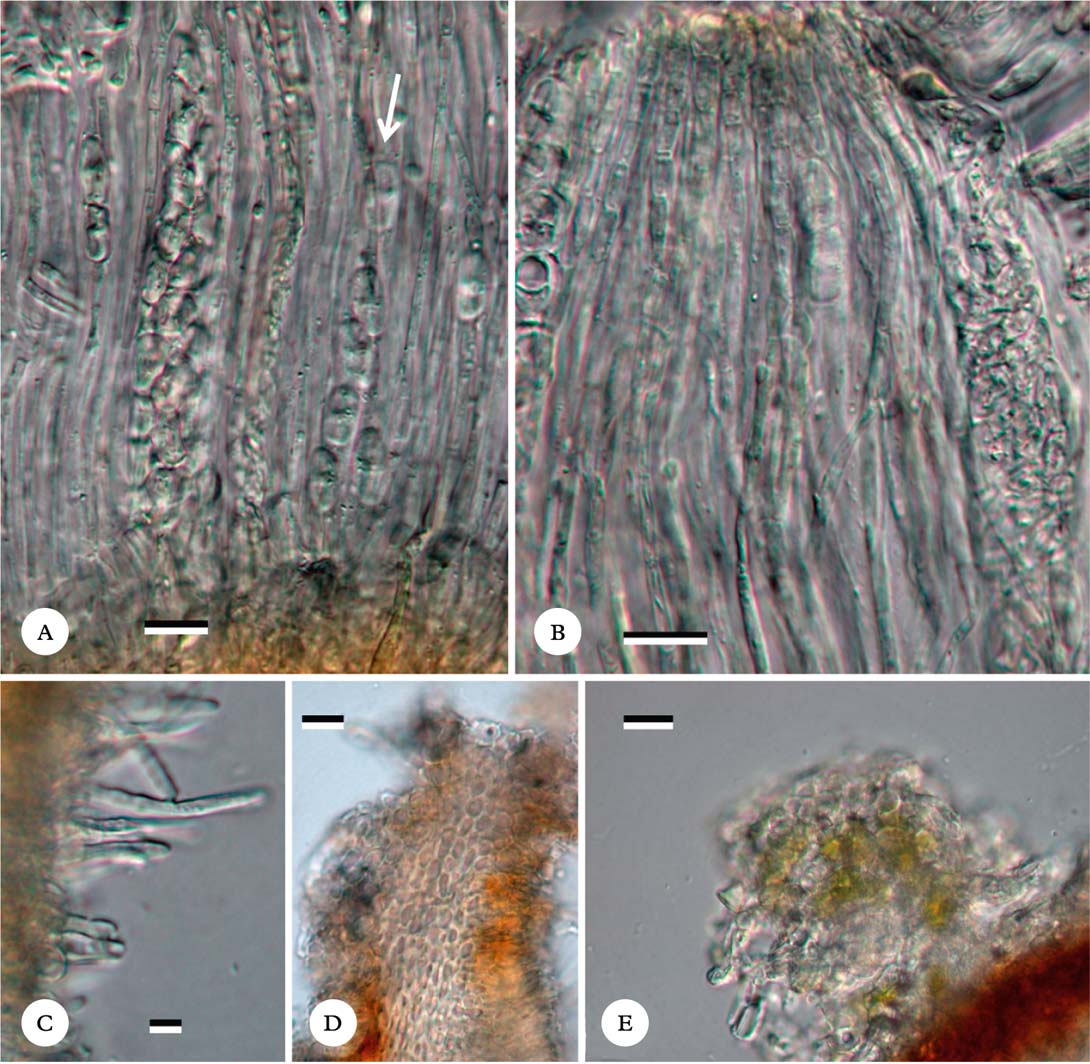
Fig. 2. Ramonia variospora, holotype. A, hamathecium showing paraphyses and polysporous ascus and octosporous ascus (at white arrow); B, hamathecium showing somewhat moniliform paraphyse tips; C, periphyses; D, section through ascoma rim showing cell lumina inside ascoma wall; E, thallus wart on ascoma, showing trentepohlioid algal and cortical cells. Scales: A–E=10 μm. In colour online.

Fig. 3. Habitus of Ramonia species. A, R. variospora, holotype; B, R. valenzueliana, Ferraro et al. 10626; C, R. malmei, Ferraro et al. 10526; D, R. kandleri, Aptroot 40827; E, R. interjecta, leg. D. G. Zimmermann, 2011, Germany, Nordrhein-Westphalen, Solingen, picture by N. Stapper. Scales: A–E=0·3 mm. In colour online.
Thallus crustose, corticate, slightly shiny, grey, cracked into angular areoles of c. 0·1–0·3 mm diam. which soon become irregular hemispherical warts, at least towards the centre of the thallus where the thallus is c. 0·2 mm thick, not surrounded by prothallus; cortical cells almost globose, c. 3–6 µm diam. Photobiont trentepohlioid, cells c. 10–16×8–12 µm.
Apothecia numerous, dispersed, sessile, lentiform, on the outside covered with several thallus warts, round, 0·3–0·5 mm diam.; disc concave, pinkish, margin covered by thalline warts, c. 0·1 mm wide, almost closing inward over the disc leaving a hollow space over the hymenium. Wall almost surrounding the whole ascoma, reddish brown on the outside, hyaline inside; lumina elongated, in the central part c. 6–10×1·5–2·5 µm; near the ostiole almost globose. Ostiole almost closed when apothecia young, reddish brown when viewed from above and where not covered by thallus, granular. Excipulum a thin brown cupular layer, c. 50 µm thick. Hymenium not inspersed, after pretreatment with KOH with Lugol + blue, 120–170 µm high. Paraphyses unbranched, 2·0–2·5 µm wide, apices not swollen but filaments toward the tip slightly moniliform and up to 3·5 µm wide. Periphyses unbranched, numerous, hyaline, 13–29(–58)×4·5–5·5 µm, the longer ones visible from the outside as glassy hairs in the empty chamber below the ostiole. Asci soon disintegrating (no mature ascus tips observed), 90–129×10–35 µm, containing either 8 or c. 32–64 ascospores, variable within a hymenium; 8-spored asci cylindrical and slender; multispored asci fusiform and wide. Ascospores hyaline, young simple, 1-septate when mature; when 8 per ascus ellipsoid and (16–)16·4–18·3–20·2(–22)×(6–)6·8–7·4–8·0(–10) µm (n = 15); when c. 32–64 per ascus broadly ellipsoid and (10–)11·8–12·7–13·6–(14)×(6–)6·7–7·2–7·7(–8) µm (n=15), smooth, but surrounded by a 3–5 µm thick gelatinous sheath.
Pycnidia not observed.
Chemistry
TLC: no substances.
Ecology and distribution
On smooth bark of trees in Brejo de Altitude forest. Known only from Brazil.
Discussion
The new species is superficially similar to a tiny Ocellularia, but differs by the sessile apothecia. The similarity with Ramonia valenzueliana (Fig. 3B) is clear, although that species differs by the stellar slits around the ostiole, the constantly polysporous asci and the less verrucose thallus. The new species has the area around the ostiole obscured by thallus warts.
The new species is surprising because the asci within one apothecium vary as to the number of ascospores, either 8 or 32–64, and the ascospores are ellipsoid in 8-spored asci, while they are broadly ellipsoid in multispored asci; they are similar in width, but much longer in 8-spored asci. This is not an occasional aberration, as it was observed in all sectioned ascomata (3 out of c. 50 ascomata present on the type specimen were sectioned), and illustrations from three different ascomata are represented in the figure. The difference is especially striking in the youngest sectioned ascoma, where the ascospores are still aseptate: it can be seen that the 8-spored asci are much smaller than the multispored asci.
The number of ascospores is the result of multiple divisions of the ascospore-mother cell. During mitosis, ascospores divide simultaneously and (usually) repeatedly (Webster & Weber Reference Webster and Weber2007). Therefore, the number of ascospores is often a factor of 2; 1, 2, 4, 8, 16, 32, 64 and 128 are the usual numbers. Multispored asci occur in various monophyletic lichen groups, the majority in three families (Acarosporaceae, Biatorellaceae and Thelocarpaceae) with species predominantly with multispored asci, as well as in 16 complete genera (Aptroot & Schumm Reference Aptroot and Schumm2012), but also in isolated species or species groups within 40 other genera (such as Ramonia). See Aptroot & Schumm (Reference Aptroot and Schumm2012) for a listing of these groups, to which Amandinea and Relicina should still be added. Seemingly multispored asci also occasionally originate in a different way by means of the formation of ascoconidia (Hafellner & Bellemère Reference Hafellner and Bellemère1983; Ertz & Diederich Reference Ertz and Diederich2004; Lücking Reference Lücking2008). This is the formation by larger ascospores of tiny conidia, while the ascospores are still within the ascus. In lichenized fungi, this is occasionally seen, but only in species with large muriform ascospores, viz. so far in Brigantiaea (Hafellner & Bellemère Reference Hafellner and Bellemère1983), Calopadia (Santesson Reference Santesson1952), Gyalidea (personal observation), Oevstedalia (Ertz & Diederich Reference Ertz and Diederich2004), Topeliopsis (Frisch & Kalb Reference Frisch and Kalb2006), and Tricharia (Ferraro Reference Ferraro2000). Although the final developmental stage of ascoconidia can seem similar to those of multispored asci with mitotic ascospores, it is usually possible to distinguish the two phenomena by the observation of intermediate stages. So the ascoconidia of Oevstedalia are clustered in 8 balls representing the parent ascospores (Ertz & Diederich Reference Ertz and Diederich2004). There is no such indication that the spores in the multispored asci in the new Ramonia are formed by ascoconidiogenesis.
Reports of species that have asci either 8-spored or multisporous are scarce. The only cases seem to be two species of Cryptolechia (which is also in the Gyalectaceae), viz. C. nana (Tuck.) D. Hawksw. & Dibben with ascospores (8–)12–16 per ascus and C. myriadella (Nyl.) D. Hawksw. & Dibben also with ascospores 8–12(–16) per ascus (Kalb Reference Kalb2007). This is, however, a much less spectacular case than the dimorphy of the new species described here, which combines within each studied hymenium asci with 8 and with 32–64 ascospores, without intermediates.
World key to the species of Ramonia
This key also mentions the world distribution and the substratum if not tree bark.
-
1 Asci within one apothecium with variable number of ascospores, either 8 or 32–64; ascospores 16–22×6–10 µm when 8 per ascus, 10–14×6–8 µm when asci polysporous; Brazil ... R. variospora Sobreira et al.
Asci either polysporous or not, not variable within one apothecium ... 2
-
2(1) Asci polysporous (>8 spores per ascus) ... 3
Asci with 8 (or fewer) spores per ascus ... 10
-
3(2) Ascospores simple ... 4
Ascospores septate ... 7
-
4(3) Ascospores globose, 2·2–3·5 µm diam.; Kenya ... R. micrococca Vězda
Ascospores ellipsoid ... 5
-
5(4) Ascospores <3 µm wide, narrowly ellipsoid; tropical America, Papua New Guinea & Indonesia. ... R. microspora Vězda
Ascospores >3 µm wide; ellipsoid ... 6
-
6(5) Ascospores 4–6×3 µm; apothecia 0·2–0·4 mm diam.; Brazil ... R. intermedia Kalb
Ascospores 8–9×4·0–4·5 µm; apothecia 0·2–0·6 mm diam.; Brazil ... R. kandleri Kalb
-
7(3) Ascospores 16–24 per ascus, 1–3-septate to submuriform, 9–14×4–5 µm; Nepal ... R. nepalensis Thor & Vězda
Ascospores >24 per ascus, 1–3-septate ... 8
-
8(7) Ascospores 1-septate, 10–14×5–6 µm; tropical America ... R. valenzueliana (Mont.) Stiz.
Ascospores more than 1-septate ... 9
-
9(8) Apothecia 0·4 mm diam.; ascospores 3-septate, 13–20×4–6 µm; subtropical North America. ... R. absconsa (Tuck.) Vězda
Apothecia 0·3–0·6 mm diam.; ascospores 1–3-septate, 8–14(–16)×4–6 µm; Tanzania ... R. elixii Kalb
-
10(2) Ascospores vermiform to acicular (length: breadth ratio >10:1) ... 11
Ascospores ellipsoid to obovoid or fusiform (length: breadth ratio <10:1) ... 13
-
11(10) Thallus corticolous; ascospores >8-septate ... 12
Thallus saxicolous; ascospores 3-septate, 45–60×3–4 µm; Southern California ... R. vermispora Lendemer & K. Knudsen
-
12(11) Apothecia 0·2–0·4 mm diam.; ascospores 8–10-septate, 50–60×3–5 µm; Western Europe ... R. subsphaeroides (Tav.) Vězda
Apothecia 0·4–0·7 mm diam.; ascospores 8–14-septate; 45–75×3·5–4·0 µm; Western Europe ... R. chrysophaea (Pers.) Vězda
-
13(10) Ascospores muriform ... 14
Ascospores transversely septate ... 16
-
14(13) Apothecia pale; ascospores 21–38(–45)×9–14 µm; British Isles ... R. dictyospora Coppins
Apothecia black ... 15
-
15(14) Ascospores 28–45(–50)×8–13 µm; British Isles ... R. nigra Coppins
Ascospores 18–22×12–15 µm; on Andreaea moss on rock, Marion Island ... R. subantarctica Øvstedal
-
16(13) Thallus saxicolous or terricolous; ascospores 3-septate ... 17
Thallus corticolous; septation various ... 20
-
17(16) Apothecia pale; on calcareous rocks; ascospores fusiform, 18–23×4–6 µm; on limestone, Western Europe. ... R. calcicola Canals & Gómez-Bolea
Apothecia black; on weakly or non-calcareous rocks or soil ... 18
-
18(17) Ascospores obovate, 23–27×7–8 µm; on weakly calcareous soils, California ... R. ablephora (Nyl. ex Hasse) R. C. Harris
Ascospores fusiform; on non-calcareous rocks ... 19
-
19(18) Ascospores 17–25 µm long; on granite and decomposed granite, California ... R. gyalectiformis (Zahlbr.) Vězda
Ascospores 25–31 µm long; on serpentinite, California ... R. extensa Lendemer et al.
Ascospores 3–4 septate, 12·5–20·0×3·5–5·0 μm ... R. himelbrantii Gagarina
-
20(16) Ascospores >3-septate, >20 µm long ... 21
Ascospores 1–3-septate, <20 µm long ... 22
-
21(20) Ascospores with perispore, 20–30×4–6 µm; apothecia 0·25–0·40 mm diam.; Europe ... R. luteola Vězda
Ascospores without perispore, 24–43×4·5–7·0 µm; apothecia 0·3–0·4 mm diam.; not lichenized; immersed in bark or in soft wood, Europe ... R. interjecta Coppins
-
22(20) Ascospores 1-septate, 9–12×4–5 µm; tropical America ... R. malmei Vězda
Ascospores 3-septate ... 23
-
23(22) Apothecia 0·1–0·2 mm diam.; ascospores narrowly ellipsoid; Australia ... R. leptospora (Müll. Arg.) Vězda
Apothecia >0·2 mm diam.; ascospores ovoid-ellipsoid ... 24
-
24(23) Ascospores >15 µm long; Australia ... R. eungellae Kalb
Ascospores <15 µm long ... 25
-
25(24) Apothecia 0·5–0·7 diam.; Indonesia, Brazil, Australia ... R. cupellina Vězda
Apothecia 0·3–0·4 diam.; Florida ... R. rappii Vězda
PNBS thanks the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for a Master's scholarship. AA thanks the Stichting Hugo de Vries-Fonds for travel support and Damien Ertz for facilitating his visit to BR. MESC thanks CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a research grant (Processo 311706/2012-6). Norbert Stapper is warmly thanked for providing Figure 3E.





