Introduction
Lecanora (Lecanoraceae, Ascomycota) in its broad sense is defined as a large, cosmopolitan genus comprising nearly 1000 currently recognized species, 250 of which have been described in the last 50 years (Lücking et al. Reference Lücking, Hodkinson and Leavitt2016). Species of Lecanora are characterized by a crustose (or placodioid) thallus with mostly lecanorine apothecia, Lecanora-type asci and simple hyaline ascospores (Edwards et al. Reference Edwards, Aptroot, Hawksworth, James, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) and can be found on rock, bark, wood, soil and detritus. The main phylogenetic lineages within Lecanora were recently demonstrated using molecular phylogenetic analyses (Zhao et al. Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016). However it is one of the last genera of lichens that remains largely undivided into more natural units.
Secondary metabolite content has traditionally played an important role in distinguishing taxonomic groups within Lecanora, with species-rich groups being characterized by the presence of atranorin or usnic acids (Printzen Reference Printzen2001). The pulvinic acid derivative calycin has so far been found in very few Lecanora species as either a major or accessory compound (Obermayer & Poelt Reference Obermayer and Poelt1992; Morse & Ladd Reference Morse and Ladd2016). One of these species is Lecanora somervellii Paulson from the Himalayas, which is distinctive within the genus because of its bright yellow thallus and apothecia, due to the presence of calycin and usnic acid.
In Russia, 87 species of Lecanora have been reported for South Siberia (Urbanavichus Reference Urbanavichus2010). During our field studies, we collected a bright yellow, calycin-containing species of Lecanora which differs from L. somervellii and is described below. The phylogenetic relationships of the two species and their position within Lecanora s. l. were analyzed based on ITS rDNA and mtSSU DNA sequence data.
Materials and Methods
Specimens and phenotype studies
The specimens that constituted the core material for this study were collected by the authors from the Altai Mountains (Siberia) during field trips in 2014 and from the Magadan Region (Russian Far East) in 2012. In addition, herbarium material deposited in GZU and FR was examined.
Morphological and anatomical characters were analyzed by employing standard light microscopy methods. Cross-sections of apothecia and thalli were hand cut and observed after mounting in water. Measurements are given as follows: (smallest value recorded) (![]() $\bar{x}$ − SD) –
$\bar{x}$ − SD) – ![]() $\bar{x}$ – (
$\bar{x}$ – (![]() $\bar{x}$ + SD) (largest value recorded), where
$\bar{x}$ + SD) (largest value recorded), where ![]() $\bar{x}$ is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values and the sample mean are given to the nearest 0·5 μm.
$\bar{x}$ is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values and the sample mean are given to the nearest 0·5 μm.
Secondary metabolites present in the thallus were analysed by means of thin-layer chromatography (TLC) (Culberson & Kristinsson Reference Culberson and Kristinsson1970) using solvent system B (hexane: methyl tert-butyl ether: formic acid, 140: 72: 18) (Culberson & Johnson Reference Culberson and Johnson1982).
Sequences and phylogenetic reconstructions
To test the phylogenetic relationships of calycin-producing species of Lecanora and their relationship to other species represented in GenBank (www.ncbi.nlm.nih.gov), the ITS region of the nrDNA (ITS1, 5.8S and ITS2) and the mitochondrial small subunit of the ribosomal RNA (mtSSU) were sequenced from eight specimens (Table 1). These markers were chosen because they were also used in the most comprehensive analyses of Lecanora by Zhao et al. (Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016) and many sequences are present in GenBank, whereas sequences from other loci are only available for very few species. DNA extraction, amplification and sequencing followed Davydov & Yakovchenko (Reference Davydov and Yakovchenko2017). Sequences were aligned with those of 75 other species preferably representing type material, from Zhao et al. (Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016) using MAFFT in Geneious 6.0 (Biomatters Ltd., New Zealand) with default settings (auto algorithm selection, gap open penalty 1·53, offset 0·123) and manually optimized. Before combining sequences into a joint ITS + mtSSU data matrix, the unambiguously alignable regions of 81 specimens for which both marker regions were obtained were used for calculations using RAxML 8.0.26 (Stamatakis Reference Stamatakis2014) to generate single-marker phylograms (not shown), which were tested for conflicts. The cladograms were similar regarding well-supported clades and lacked conflicts, therefore all sequences were combined into one matrix consisting of 1159 sites and used for RAxML and Bayesian analyses. The optimal substitution model was inferred initially assuming four independent subsets: ITS1, 5.8S, ITS2 and mtSSU using PartitionFinder version 1.1.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012). The Kimura 2-parameter model with a proportion of invariable sites (K80 + I) was inferred for the 5.8S partition. ITS1 and ITS2 were inferred to follow the same general time reversible model with site-specific rates modelled by a gamma-distribution and with a proportion of invariable sites (GTR+I+G). The Hasegawa-Kishino-Yano model with a proportion of invariable sites and gamma-distributed, site-specific rates (HKY+I+G) was inferred as optimal for mtSSU. Bayesian inference with the Markov chain Monte Carlo (BMCMC) method (Larget & Shimon Reference Larget and Simon1999) was performed using MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). We applied the above-mentioned substitution models, a variable rate prior and an unconstrained exponential branch-length prior with a mean of 0·12. The mean of the branch-length prior was calculated based on Maximum Likelihood (ML) tree reconstructions using the procedure described by Ekman & Blaalid (Reference Ekman and Blaalid2011). Three parallel analyses each with 6 incrementally heated chains using the default heating factor of 0·2 were run for 40 million generations; every 200th generation was sampled until the average standard deviation of split frequencies had dropped to 0·015. This was the case after 20 million generations. The first 50% of trees was discarded as burn-in and a 50% majority-rule consensus tree was calculated from the remaining trees of the three runs with the sumt command implemented in MrBayes 3.2.3. The most likely tree and 1000 bootstrap replicates were calculated using RAxML 8.0.26 (Stamatakis Reference Stamatakis2014) by raxmlGUI software version 1.3.1 (Silvestro & Michalak Reference Silvestro and Michalak2012), applying the GTRGAMMA model of substitution to the subsets. Species of Ramboldia were used as an outgroup because this genus has been shown to be closely related to Lecanora s. l. (Zhao et al. Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016). Bootstrap support values and posterior probabilities from the BMCMC analysis were mapped onto the ML tree from RAxML because the Bayesian 50% majority-rule consensus tree had the same topology.
Table 1. Lichen species used in the phylogenetic analyses of calycin-producing species of Lecanora together with specimen information and GenBank Accession numbers. Sequences produced in the present study are in bold.

Results
ITS and mtSSU sequences were successfully obtained from four specimens of the putative new species, described below as Lecanora solaris. The phylograms are combined in Fig. 1. Four sequences of L. solaris are combined in a well-supported clade (MrBayes: 1·0 PP; RAxML: 100% BS). Two sequences of L. somervellii cluster separately in a well-supported sister clade (1·0 PP, 100% BS). Both species appear as closely related to L. polytropa and L. intricata (1·0 PP, 89% BS), with which they form a well-supported clade (the Lecanora polytropa group) sister to Protoparmeliopsis, Rhizoplaca, Myriolecis and L. conizaeoides (1·0 PP, 84% BS).
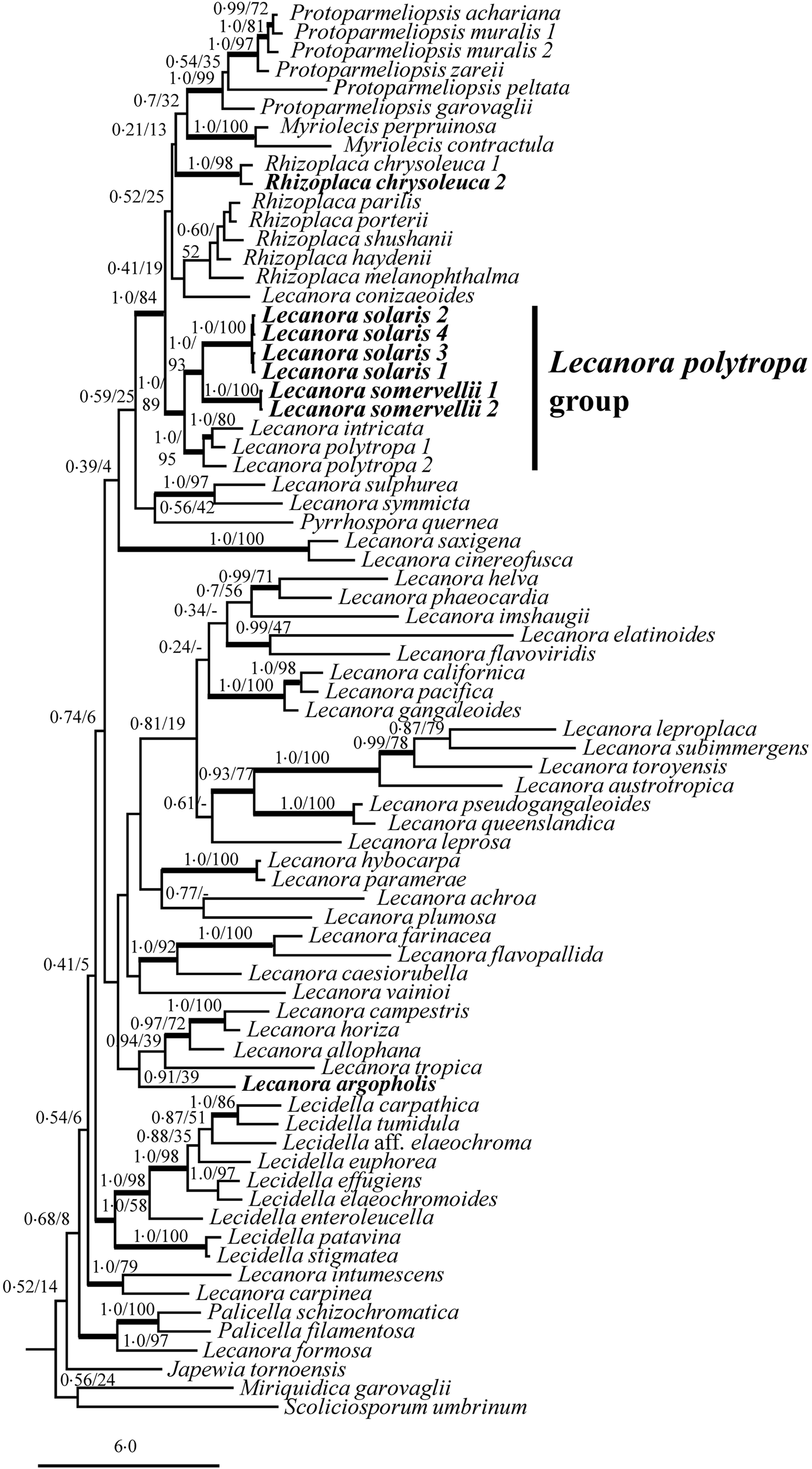
Fig. 1. The phylogenetic relationships of calycin-producing species of Lecanora and their relationship to other species represented in GenBank using ITS and mtSSU sequences. The reliability of each branch was tested by Maximum Likelihood (ML) and Bayesian methods. Numbers at tree nodes indicate bootstrap values of Bayesian inference with the Markov chain Monte Carlo (BMCMC) posterior probabilities (left of slash) and ML (right of slash). Thicker branches indicate BMCMC posterior probability values ≥ 0·95 or ML bootstrap values ≥ 70%. GenBank Accession numbers are given in Table 1. Sequences produced in the present study are marked in bold. Outgroup not shown.
Lecanora solaris Yakovchenko & Davydov sp. nov.
MycoBank No.: MB 824845
Similar to L. somervellii but differs by its squamulose growth form and plane to moderately convex apothecial discs with a distinct, persistent thalline margin.
Type: Russia, Republic of Altai, Kosh-Agachsky District, Sailjugem Range, right bank of the Bayan-Chagan River 2·5 km S of its junction with the Karasu River, on siliceous rocks, 49°31′55″N, 88°46′45″E, 2630 m a.s.l., 15 June 2014, E. A. Davydov 16532 & L. S. Yakovchenko (LE-L-13174—holotype; ALTB—isotype).
Thallus squamulose, ± circular, up to 10–15 mm diam.; squamules (0·2–)0·7–1·3–1·9(–3·5) mm diam., crowded, moderately to strongly convex, rounded initially but soon becoming incised and irregular in outline, sometimes peltate. S urface bright yellow, shiny, initially smooth, later strongly rugose, epruinose. Vegetative propagules absent. Cortex 42·5–57·5 μm, of compacted irregularly-oriented hyphae, up to 3·5 μm thick, with elongate lumina becoming rounded towards the uppermost part of the cortex and covered by cortical crystals. Cortical crystals ± rounded, irregular in shape, angular or bacilliform. Algal layer continuous, 135–215 μm thick, algae chlorococcoid, 17·5–22·5 μm diam.; medulla of loose hyphae of up to 5 μm diam.
Apothecia lecanorine, common, concentrated in centre of the thallus, at first arising singly on areoles that are then obscured, crowded to overlapping (1–4 apothecia per squamule), immersed initially soon becoming sessile and strongly constricted at the base, (0·4–)0·9–1·2–1·5(–2·0) mm diam., with raised margin; disc initially plane, later moderately convex, initially smooth, later smooth or scarcely rugose, rounded to irregular in outline, concolorous with the thallus to ochre-yellow, epruinose; thalline margin initially distinct, later disappearing, concolorous with disc, somewhat shiny, epruinose, (62·5–)79·0–97·0–115·0(–125·0) μm thick. Cortex of thalline margin same as the thalline cortex; medulla reaching into the thalline margin, consisting of loose hyphae of up to 5 μm diam., with green algal cells of up to 22·5 μm diam. Hymenium (42·5–)48·0–52·0–55·0(–57·5) μm tall, hyaline; subhymenium (12·5–)20·5–27·0–33·5(–42·5) μm tall, hyaline, with oil drops; epihymenium (12·5–)13·5–18·0–22·0(–25·0) μm tall, medium brown, densely incrusted by crystals, which penetrate down into the hymenium. Hypothecium (50·0–)70·0–91·5–113·5(–137·5) μm tall, hyaline with sulphur yellow pigment (calycin) in uppermost part, opaque, composed of compact, elongated, irregularly-oriented hyphae of 2·5–3·5 μm diam.; paraphyses simple to branched near the tips and in the mid-hymenium, septate, 2·0–2·5 μm wide, with clavate tips 3–5 μm wide. Asci clavate, 8-spored, (32·5–)36·5–40·5–44·0(–51·5) × (12·5–)14·0–15·0–16·5(–19·0) μm, Lecanora-type; ascospores simple, hyaline, ellipsoid to narrowly ellipsoid, (7·5–)10·0–11·5–13·0(–14·0) × (4·5–)5·0– 5·5–6·0(–7·5) μm.
Pycnidia rare, 90–110 μm diam., immersed, walls colourless, conidiogenous cells elongate-ampulliform; conidia filiform, colourless, simple, (9·5–)12·0–16·0–20·0(–22·0) × c. 0·8 μm.
Chemistry
Thallus K+ weakly reddish, KC−, C−, Pd−; calycin as well as usnic and rangiformic acids by TLC.
Etymology
The name refers to the bright yellow “sunny” colour of the lichen.
Substratum and ecology
The species grows on hard volcanic or weakly calcareous overhanging rocks in open pioneer communities within high mountain vegetation at elevations between 2630−3100 m (Fig. 2A). The species was scarce in this habitat. The following species co-occurred with Lecanora solaris: Acarospora cf. elevata H. Magn., Acarospora sp., Aspicilia sp., Lecanora sp., Protoparmeliopsis peltata (Ramond) Arup et al., Carbonea vorticosa (Flörke) Hertel.
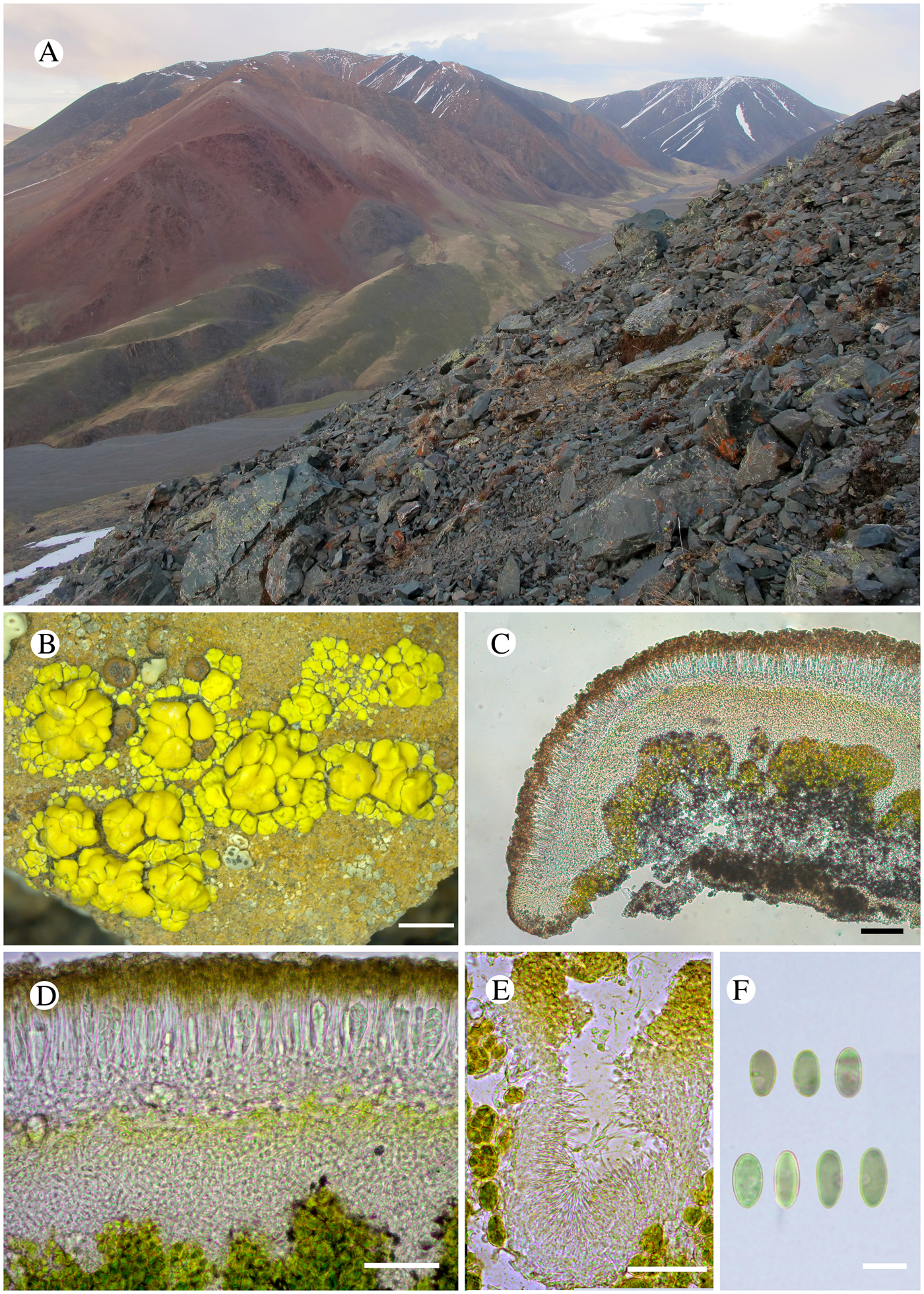
Fig. 2. A–F, Lecanora solaris; A, type locality, Sailjugem Range, right bank of the Bayan-Chagan River; B, holotype (LE-L-13174); C, section of apothecium; D, section of apothecium showing clearly the layer of calycin; E, pycnidia with broadly falcate conidia; F, ascospores. Scales: B = 2 mm; C = 100 μm; D = 50 μm; E = 25 μm; F = 10 μm.
Distribution
Lecanora solaris is so far known only from the Altai Mountains where it was collected in the Sailjugem Range and the Mongun-Taiga massif.
Notes
The examination of the neotype of L. somervellii revealed that L. solaris differs in the thallus morphology: L. somervellii has a large, well-developed, placodioid thallus and lecanorine apothecia which soon become strongly convex with an excluded thalline margin; whereas L. solaris has a squamulose thallus with flattened to moderately convex, lecanorine apothecia with a persistent thalline margin. According to our field observations in the Magadan Region the two species differ also in their habitat; L. somervellii occurs on sunny exposed siliceous rocks while L. solaris favours shaded, weakly calcareous rocks. Morphologically, L. solaris also resembles species of Pleopsidium but can easily be distinguished anatomically by having 8-spored asci. Sterile samples differ from Pleopsidium by the small size and shape of the thallus, which is never placodioid and large, as well as by its habitat: Pleopsidium species occur on exposed, well-insolated surfaces of siliceous rocks.
We observed two different morphs of L. solaris: squamulose (typical) and umbilicate (Davydov 14336 & Yakovchenko). The umbilicate morph differs in the thallus margin having a lobate, umbilicate (not uniformly squamulose) growth form; a smooth to strongly echinate (not rugose) thallus surface and apothecial discs that become more strongly rugose with age. Both morphs were collected at the same locality and under similar ecological conditions. The ITS sequences of both morphs were identical but the mtSSU sequence of the umbilicate morph (MH520111) differs by five substitutions from that of the squamulose morphs, including a specimen from the same locality. The short and unsupported branches within the L. solaris clade, however, indicate that these differences reflect intraspecific variability. The mtSSU sequence of the umbilicate morph also includes an insertion at position 638 (ACCC-GCG-GCAAAGCATCAGTGAGCC) which is lacking in all sequences of squamulose specimens, and very similar to the homologous part of the sequence of Rhizoplaca chrysoleuca (Sm.) Zopf (ACCTTGCGTTGCAAAGCATCAGTGAGTGCC: differences marked in bold). However, this insertion is extremely variable among Lecanora and Rhizoplaca species and was excluded from the alignment.
Additional specimens examined
Russia: Republic of Altai: Kosh-Agachsky District, Sailjugem Range, watershed of Bayan-Chagan and Sarzhemoty Rivers, 4 km S of its junction, 49°32′00″N, 88°45′01″E, 2750 m a.s.l., in crevices of rocks, 2014, E. A. Davydov 14333 & L. S. Yakovchenko (ALTB); ibid., right bank of the Bayan-Chagan River, 2·5 km S of its junction with the Karasu River, 49°32′01″N, 88°46′36″E, 2680 m a.s.l., in crevices of rocks, 2014, E. A. Davydov 14334 & L. S. Yakovchenko (ALTB). Republic of Tuva: Mongun-Taiginsky District, Mongun-Taiga massif, headwaters of the Mugur River, 27·5 km W of Mugur-Aksy, 50°18′22″N, 90°04′26″E, 3000–3100 m a.s.l., alpine meadows and mountain tundras with stones, on rocks, 2014, E. A. Davydov 14335, 14336 & L. S. Yakovchenko (ALTB).
Lecanora somervellii Paulson
J. Bot., Lond. 63: 192 (1925); type: Nepal, Langtang area, huge rocks near Kyangjin, 3750 m, 8–10 September 1986, J. Poelt N86-L257 [GZU—neotype!, selected by Obermayer & Poelt, Lichenologist 24: 112 (1992)].
This species is characterized by its effigurate, citrine-yellow thallus (due to the production of calycin). The specimens collected from the Magadan Region (Yakovchenko et al. Reference Yakovchenko, Zheludeva, Ohmura and Davydov2018) are a good match to the protologue and description by Obermayer & Poelt (Reference Obermayer and Poelt1992). The chemical substances detected by TLC were calycin, usnic acid, rangiformic acid, norrangiformic acid, and an unidentified fatty acid (Rf class 3 in solvent B). Lecanora somervellii is known from the Himalayas, both in Nepal and Tibet, where it grows on steep to overhanging sides of very hard siliceous rocks at an altitudinal range of c. 3750 m to c. 5540 m (Paulson Reference Paulson1925; Obermayer & Poelt Reference Obermayer and Poelt1992).
Specimens examined
Nepal: Central Himalaya: Langtang area, huge rocks near Kyangjin, elev. c. 3750 m, 8–10 September 1986, J. Poelt N86-L257 (GZU).—Russia: Magadan Region: small mountain c. 120 km NE of Atoka, 61°11′47·7″N, 153°58′10·5″E, elev. 1130 m, on rock, 11 August 2012, Y. Ohmura 10109, 10111, L. S. Yakovchenko & E. Zheludeva (TNS-L-125468, 125469).
Discussion
A similarity between Lecanora somervellii and L. polytropa f. illusoria (Ach.) Leight. was already noted in the protologue of L. somervellii by Paulson (Reference Paulson1925), who nevertheless assumed a closer relationship for his new species with Aspicilia. Our phylogeny based on ITS and mtSSU and the six-gene phylogeny of Zhao et al. (Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016) both support the hypotheses that the calycin-producing L. solaris and L. somervellii represent sister taxa within the L. polytropa-group and that Myriolecis, Protoparmeliopsis, Rhizoplaca and the L. polytropa-group are closely related to each other. Apart from showing a close relationship between L. formosa and Palicella (also inferred by Zhao et al. (Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016)), our phylogeny does not allow further conclusions on the relationships within Lecanora s. l. because its backbone is not supported.
The discovery of L. solaris in the Altai Mountains returns us to the problem of the neotypification of L. somervellii by Obermayer & Poelt (Reference Obermayer and Poelt1992). As the holotype of L. somervellii was lost (or at least not found) and Paulson's original material was missing, a neotype was selected for L. somervellii from the material collected by Poelt in Nepal in 1986. The neotype is consistent with the protologue of the species published by Paulson (Reference Paulson1925), in which the unique citrine-yellow colour of L. somervellii and its 8-spored asci are noted. However, other characters of the neotype material were not consistent with the protologue (Obermayer & Poelt (Reference Obermayer and Poelt1992)). For example, Paulson described the thallus of L. somervellii as squamulose with a white lower surface and the apothecia as lecanorine, plane to immersed or slightly convex and with a persistent thalline margin. The neotype, on the other hand, has a distinctly placodioid thallus with closely attached marginal lobes and apothecia with a thalline margin only in the juvenile stage. Hence, morphologically L. solaris better fits the original description of Paulson (Reference Paulson1925). However, since Paulson's original material is not available, we must follow the neotypification by Obermayer & Poelt (Reference Obermayer and Poelt1992). Furthermore, no material similar to L. solaris has as yet been found in the Himalayas.
Among the yellow-coloured Lecanora species, only L. somervellii and L. solaris are so far known to contain calycin and usnic acid. Other species, such as Lecanora sulphurella Hepp, L. fulvastra Kremp. and L. inaurata C. A. Morse & Ladd, either contain only usnic acid as a yellow pigment or produce atranorin and/or chloroatranorin as major secondary compound(s) in addition to calycin (Follmann & Huneck Reference Follmann and Huneck1976; Leuckert & Mayrhofer Reference Leuckert and Mayrhofer1985; Lumbsch Reference Lumbsch1994; Morse & Ladd Reference Morse and Ladd2016).
This and previous phylogenetic studies (Peréz-Ortega et al. Reference Pérez-Ortega, Spribille, Palice, Elix and Printzen2010; Zhao et al. Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016), as well as phenotypic characters (Obermayer & Poelt Reference Obermayer and Poelt1992; Arup & Grube Reference Arup and Grube1998), indicate that Lecanora species with calycin and usnic acid, and the L. polytropa group, do not belong in Lecanora s. s. Our data suggest that L. somervellii and L. solaris should be included in the L. polytropa group sensu Zhao et al. (Reference Zhao, Leavitt, Zhao, Zhang, Arup, Grube, Pérez-Ortega, Printzen, Śliwa and Kraichak2016). Apart from the calycin production, both species appear phenotypically similar to L. polytropa in their concave or plane, marginate, broadly attached apothecia arising singly on areoles and soon becoming convex, immarginate, constricted below and clustered in the centre of the thallus obscuring the areoles. Well-developed thalli of L. polytropa may sometimes form squamulose thalli similar to those of L. solaris. The L. polytropa group in this new sense is strongly supported as monophyletic and probably requires taxonomic recognition at the generic level. However, additional data are necessary to fully understand the species composition of this clade before it can be accepted as a separate genus.
Author ORCIDs
Evgeny A. Davydov 0000-0002-2316-8506, Christian Printzen 0000-0002-0871-0803.
We thank Dr W. Obermayer (GZU) for his help with herbarium specimens of Lecanora somervellii. The authors are grateful to the following colleagues for organizing expeditions: Dr G. E. Insarov and Dr A. N. Kuksin to the Ubsunur Hollow Biosphere Reserve (Tuva), and D. G. Malikov to Sailugemsky National Park. We also thank the directors and staff of the above-mentioned institutions for their great support during these expeditions. We are grateful to two anonymous reviewers and Dr Stefan Ekman for their valuable comments and suggestions, and to Dr Alan Fryday for improving the text. This work benefitted from the sharing of expertise within the DFG priority program SPP 1991 Taxon-Omics and support from DFG PR 567/19 -1 to the project “Lecanomics”.





