Introduction
Lichens with the ascospore discharging into a mazaedium were traditionally united in the order Caliciales, together with similar taxa which are non-lichenized and/or do not form a mazaedium but have cup-shaped apothecia on slender stalks, just as most mazaediate lichens. It was always felt that neither the presence of a mazaedium nor the presence of stalked apothecia constituted a strong rationale for relatedness, and the neotropical calicioid fungi were already distributed over seven families by Tibell (Reference Tibell1996), with some remaining genera that belong to families in the Arthoniales that are still partly unnamed. Phylogenetically, the species with this morphology turned out to be unrelated, and they are now distributed over at least seven orders (Tibell Reference Tibell1996; Tibell & Wedin Reference Tibell and Wedin2000; Wedin et al. Reference Wedin, Döring, Nordin and Tibell2000; Prieto et al. Reference Prieto, Baloch, Tehler and Wedin2013). Species of this group are usually locally rare in the tropics, where the species are mostly confined to overhanging trees with rough or dry bark. In the Neotropics (Central and South America between the tropics of Cancer and Capricorn), around 50 species are known (Tibell Reference Tibell1996). Many of the c. 200 calicioid species that are known worldwide are thought to be widespread, and some of the more common species are thought to be cosmopolitan or at least palearctic or pantropical.
Calicioid lichens are relatively well known and since 1996 few if any additional species have been described from the Neotropics. In this paper, we describe three new species, all in small genera: the fourth species of the genus Mazaediothecium, the first lichenicolous Mycocalicium, and the first Stenocybe reported from the Neotropics.
The genus Mazaediothecium comprises three species (Aptroot Reference Aptroot1991; Harada & Yamamoto Reference Harada and Yamamoto2007), two of which are neotropical (Tibell Reference Tibell1996). This genus belongs to the Pyrenulaceae (Aptroot Reference Aptroot1991), but so far no species have been sequenced. The genus Mycocalicium comprises 12 accepted species (Tibell Reference Tibell1996, Reference Tibell1998, Reference Tibell2001; Titov Reference Titov2006), four of which are neotropical (Tibell Reference Tibell1996). It belongs to a small separate order, the Mycocaliciales (Tibell & Wedin Reference Tibell and Wedin2000), which comprises two families with five genera, of which the genus Chaenothecopsis (Tibell & Vinuesa Reference Tibell and Vinuesa2005; Titov Reference Titov2006) is by far the largest. The genus Stenocybe also belongs to this order and comprises about a dozen accepted species, many of which are bark parasites with a single tree host genus, but also some general bark-inhabiting species. No species has ever been reported from the Neotropics (Tibell Reference Tibell1996). Only one species is known from the Southern Hemisphere, viz. S. bartlettii Tibell from New Zealand (Tibell Reference Tibell1987).
Material and Methods
Identification and descriptive work was carried out in Soest using an Olympus SZX7 stereomicroscope and an Olympus BX50 compound microscope with interference contrast, connected to a Nikon Coolpix digital camera. Sections were mounted in tap water, in which all measurements were also taken. The specimens from this study are preserved in ISE. The chemistry was investigated by performing thin-layer chromatography (TLC) using solvent A (Orange et al. Reference Orange, James and White2001).
The Species
Mazaediothecium uniseptatum Aptroot sp. nov.
MycoBank No.: MB 812868
Mazaediothecium with ascospores 1-septate, distoseptate, a thin median euseptum also sometimes present, 7·0–12·0×5·0–7·5 μm, lumina angular, 1·5–2·0 μm diam.
Type: French Guiana, Saül, sentier limonade, on higher trunk of Protium sp. in mixed forest on lateritic soil, alt. c. 200 m, 3°32'N, 53°12'W, 24 September 1986, D. Montfoort & R. Ek 383 (L—holotype; ABL—isotype).
(Fig. 1)

Fig. 1 Mazaediothecium uniseptatum, holotype. A, habitus; B, ascospores. Scales: A=0·2 mm; B=20 μm. In colour online.
Thallus greyish white, not corticate, dull and almost hyphal, surrounded by a dark brown hyphal prothallus line. Associated algae trentepohlioid.
Apothecia numerous, sessile, black, dull, with golden yellow pruina in upper half, c. 0·2 mm diam. and up to 0·6 mm high, cylindrical; margin not clearly visible. Excipulum black. Hamathecium not inspersed with oil droplets, covered by a thick mazaedium layer. Hamathecium filaments simple. Asci soon disintegrating (no mature intact asci observed); immature asci clavate-cylindrical, up to c. 60×8 μm. Ascospores pale grey, ellipsoid to broadly fusiform, 1-septate, distoseptate, a thin median euseptum also sometimes present, 7·0–12·0×5·0–7·5 μm, lumina angular, 1·5–2·0 μm diam.
Pycnidia unknown.
Chemistry. Pruina on apothecium a K+ violet anthraquinone.
Ecology. On higher tree trunks of Protium sp. in mixed forest on lateritic soil.
Discussion. All three Mazaediothecium species known so far have submuriform ascospores.
Mycocalicium enterographicola Aptroot & M. Cáceres sp. nov.
MycoBank No.: MB 812869
Mycocalicium with turbinate, green-pruinose apothecia, lichenicolous on Enterographa cf. quassiaecola Fée.
Type: Brazil, Sergipe, Povoado Pedrinhas, Mata da Fazenda Cafuz, alt. 75 m, 10°48'22''S, 37°16'46''W, 20 September 2013, M. Cáceres & A. Aptroot 18384 (ISE—holotype; ABL—isotype).
(Fig. 2)
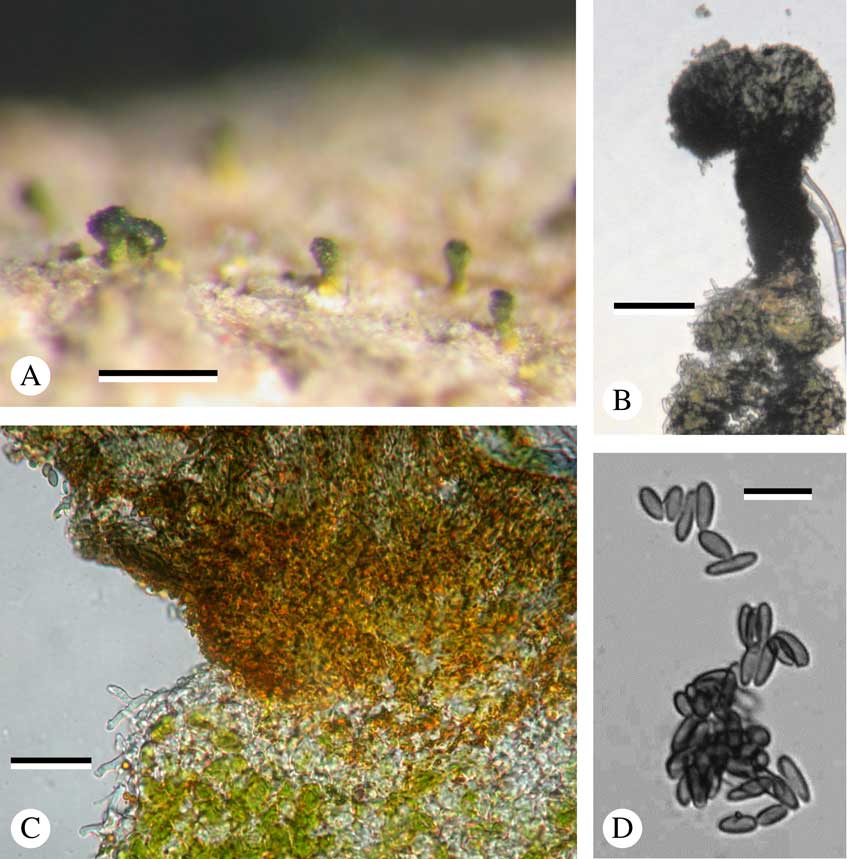
Fig. 2 Mycocalicium enterographicola, holotype. A, holotype; B, ascoma; C, stalk and host thallus; D, ascospores. Scales: A=0·25 mm; B=0·05 mm; C=25 μm; D=10 μm. In colour online.
Lichenicolous on Enterographa cf. quassiaecola Fée, whose thallus surface is broken up, and becoming fluffy and nearly leprose, not corticate, ochraceous grey. Associated algal cells (originating from the Enterographa) globose to ellipsoid, 7–11×6–10 μm, trentepohlioid but green.
Apothecia numerous, dark greenish black, dull, with green pruina below, c. 0·1 mm diam., turbinate (broadly pin-shaped); margin not clearly visible. Stalk bright yellow below, dark green at the tip, to 0·15 mm tall, c. 0·05 mm wide, internally yellowish, of densely branched c. 2·0–2·5 μm wide hyphae, unchanged in K. Excipulum aeruginose. Hamathecium often covered by a thick mazaedium layer. Hamathecium filaments simple, c. 1·0 μm wide. Ascogenous hyphae c. 2·0–3·0 μm wide, with croziers. Asci cylindrical, rather persistent, 30–35×3·0–3·5 μm, with 8 uniseriate ascospores, tip tapering, with apical thickening of c. 2·0×1·0 μm without central perforation. Ascospores dark grey, ellipsoid, not septate, 7·0–8·0×2·0–2·5 μm, wall not ornamented.
Pycnidia unknown.
Chemistry. Pulvinic acid on the stalk and apothecium head.
Ecology. On thallus of Enterographa cf. quassiaecola on tree trunks in Atlantic rainforest.
Discussion. All Mycocalicium species known so far are saprobes, mostly growing on decaying wood. This is the first lichen parasite in the genus. It is strongly reminiscent of M. calicioides (Nádvorník) Tibell (Tibell Reference Tibell1996), which also has a green pruina, but with a thinner layer on the capitulum of the apothecium only, not on the stalk. The incurved apothecium margin and the abundant presence of pulvinic acid are characters that it has in common with several species of Mycocalicium, but not with other genera in the Mycocaliciales. The presence of a distinct mazaedium layer is unusual in the whole order. The lifestyle of the new species is interpreted here as lichenicolous because it was found to occur only on thalli of an Enterographa, the morphology of which species was altered, making its identification to species level dubious.
Stenocybe tropica Aptroot sp. nov.
MycoBank No.: MB 812870
Stenocybe on mangrove tree bark, ascospores 3-septate, distoseptate, 46–71×14–21 μm, remaining clustered in the mouth of the apothecium, asci persistent.
Type: Brazil, São Paulo, Praia de Peruíbe near Itanhaém, alt. 1 m, 9 July 1979, K. Kalb & J. Poelt (GZU—holotype).
(Fig. 3)

Fig. 3 Stenocybe tropica, holotype. A, habitus; B, ascus; C, ascospores. Scales: A=0·5 mm; B & C=15 μm. In colour online.
Saprotrophic on bark.
Apothecia sparse, glossy black, without pruina below, capitulum c. 0·3 mm diam. and up to 0·5 mm high, overall shape cylindrical to clavate; margin clearly visible, glossy, incurved when young. Stalk glossy black, to 0·8 mm tall, c. 0·15 mm wide, internally dark brown, dense, brittle; individual hyphae not very discernible. Excipulum black. Ascospores remaining clustered on the mouth of the apothecium in a mazaedioid way. Asci cylindrical, persistent, up to c. 300×25 μm, with 8 uniseriate ascospores, tip thick-walled, with ocular chamber. Ascospores dark brown, fusiform to broadly fusiform, 3-septate, distoseptate, 46–71×14–21 μm, tips mostly pointed, lumina regularly to irregularly angular to diamond-shaped or partly rounded, middle lumina mostly pentangular with an outward pointed tip, outer lumina much smaller than central lumina and not separated from the wall by endospore; often seemingly protruding as an almost hyaline tip.
Pycnidia unknown.
Chemistry. No secondary substances detected.
Ecology. On tree trunks in mangrove forest.
Discussion. This species belongs without doubt to the genus Stenocybe, which is characterized by the combination of calicioid apothecia and brown distoseptate ascospores with angular to diamond-shaped lumina. This species has much larger ascospores than any of the consistently 3-septate Stenocybe species known. It is close to the European-Macaronesian S. septata (Leight.) A. Massal, which differs by the variously 1–6-septate ascospores and the evanescent asci (Giavarini & Purvis Reference Giavarini and Purvis2009), and generally occurs on Ilex bark. The shape of the lumina of this species, as illustrated by Tibell (Reference Tibell1984: 629), also seems to be different; the outward tips of the larger lumina are flattened, so that the middle lumina are clearly sexangular. Still, it is possible that the new species rather represents an aberrant disjunct population of S. septata.
MESC thanks the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for a research grant (Processo 311706/2012-6). AA thanks the Stichting Hugo de Vries-fonds for a travel grant and the staff of Graz Herbarium for hospitality during his stay there.





