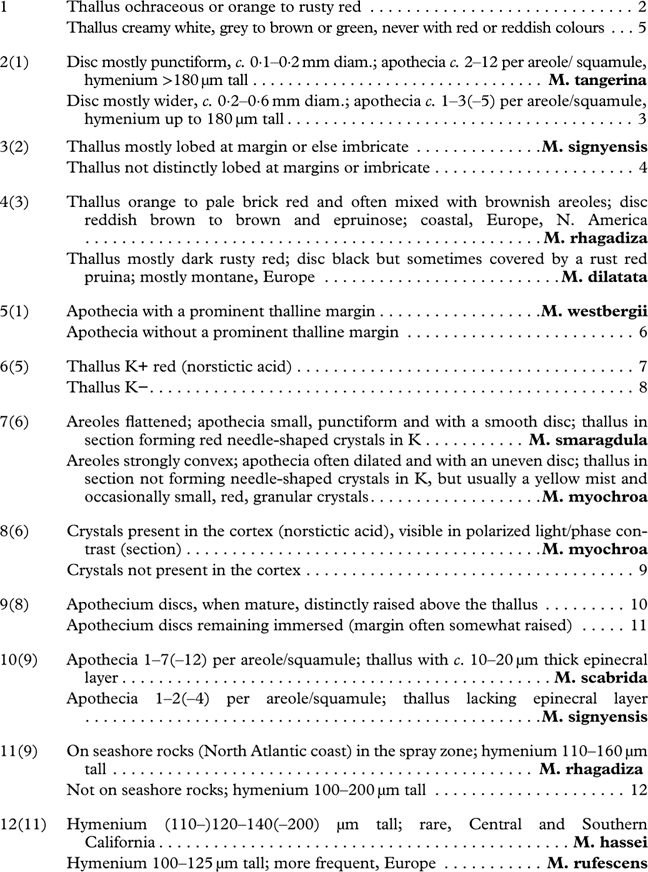
Introduction
Acarosporaceae is a distinct group of crustose lichenized fungi, occurring on exposed rocks and soil on all continents (Magnusson Reference Magnusson1929; Knudsen Reference Knudsen2007; Fletcher et al. Reference Fletcher, James and Purvis2009; Westberg et al. Reference Westberg, Crewe, Purvis and Wedin2011). It is probably the largest lichenized group having multi-spored asci with small, simple ascospores, and among the earliest lichenized ascomycete groups to diverge according to phylogenetic dating studies (Prieto & Wedin Reference Prieto and Wedin2013). The number of genera recognized within the monophyletic Acarosporaceae has steadily increased, with eight genera currently identified by molecular phylogenies (Westberg et al. Reference Westberg, Millanes, Knudsen and Wedin2015). Twelve species belonging to Acarosporaceae were recorded from Antarctica in the standard identification guide to the region (Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001). This includes 10 species of Acarospora, of which six are considered endemics: A. austroshetlandica (C. W. Dodge) Øvstedal, A. convoluta Darb., A. flavocordia Castello & Nimis, A. gwynnii C. W. Dodge & E. D. Rudolph, A. macrocyclos Vain. and A. williamsii Filson. Other genera reported from the area were Pleopsidium Körb., represented by a single species P. chlorophanum (Wahlenb.) Zopf, and Sarcogyne Flot., represented by S. privigna (Ach.) A. Massal. (Seppelt et. al. Reference Seppelt, Nimis and Castello1998) (=S. hypophaea (Nyl.) Arnold (Knudsen et al. Reference Knudsen, Kokourkova and Westberg2013)). Later, Kantvilas & Seppelt (Reference Kantvilas and Seppelt2006) added Polysporina frigida Kantvilas & Seppelt, bringing the total number of Antarctic Acarosporaceae to 13. Acarospora is considered a difficult genus and some Antarctic taxa have been collected only once or twice, with no field studies carried out on the variability of any taxa. Furthermore, their relationships to species in the Northern Hemisphere are not well understood (Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001).
A field visit to Manhaul Rock, a nunatak, entirely covered by the McLeod Glacier as recently as 50 years ago, was made by the first author and Bruce Maltman on 16 November 2009, when the former discovered a small, inconspicuous ‘Acarospora’ colonizing iron-stained quartz-biotite mica schist growing more or less perpendicular to the mineral cleavage plane (Fig. 1) just above the glacier. Thallus colours ranged from rust-coloured in exposed situations to grey-brown (green when moist) in shaded crevices (Fig. 2) (Purvis et al. Reference Purvis, Convey, Flowerdew, Peat and Najorka2012, Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013; Purvis Reference Purvis2014). Following the treatment by Øvstedal & Lewis Smith (Reference Øvstedal and Lewis Smith2001), it was provisionally named ‘Acarospora cf. badiofusca (Nyl.) Th. Fr.’ (Purvis et al. Reference Purvis, Convey, Flowerdew, Peat and Najorka2012, Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013; Purvis Reference Purvis2014) as previous investigations by Øvstedal & Lewis Smith indicated that Antarctic specimens named as A. badiofusca differed in apothecial anatomy compared with northern European specimens.

Fig. 1 A, Myriospora signyensis sampling site (yellow cross), lower slope of Manhaul Rock, 16 November 2009; B, close-up of sampling site showing Myriospora distribution (yellow box); C, field photograph showing colour variation from black, melanised to rust orange-brown and its tendency to colonize schist growing more or less perpendicular to the mineral cleavage plane; D photo-diagram of C showing the relationship between Myriospora thalli and mineral components. Scales: B, 15 cm engineering rule (mid image); C & D, mm scale using engineering rule.

Fig. 2 Myriospora signyensis showing range of pigmentation from partly melanised, black to strongly rust-coloured. A, holotype (Smith RILS 8673; AAS); B, Purvis & Maltman OWP S2_8 (BM); C, Purvis OWP S6_8 (BM); D, Smith RILS 8667 (AAS); E, Smith RILS 8868 (AAS); F, Smith RILS 7037 (AAS). Scales=1 mm (×5·6). See under ‘Other specimens examined’ for locality information.
Lichen pigmentation is often considered an adaptation for coping with high illumination (Gauslaa & McEvoy Reference Gauslaa and McEvoy2005; Elix & Stocker-Wörgötter Reference Elix and Stocker-Wörgötter2008). An initial study (Purvis et al. Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013) considered the hypothesis that the rust colour reflects element content and considered substance localization in response to stress. Analyses suggested mixed iron oxide/hydroxide mineral phases were present that would limit the light reaching potentially sensitive lichen photobionts (Wynn-Williams et al. Reference Wynn-Williams, Edwards, Newton and Holder2002; Purvis et al. Reference Purvis, Kearsley, Cressey, Crewe and Wedin2008a , Reference Purvis, Kearsley, Cressey, Batty, Jenkins, Crewe and Wedin b , Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013). In section, unidentified organic substance(s) (not oxalates) were localized between the discontinuous photobiont arrays arranged in more or less vertical clusters (Fig. 2e in Purvis et al. Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013). The distinctive photobiont arrangement observed also has taxonomic significance as it is one of the most distinctive characteristics of the genus Myriospora Nägeli ex. Uloth (formerly known as the ‘Acarospora’ smaragdula group, or Silobia M. Westb. & Wedin; Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009; Westberg et al. Reference Westberg, Crewe, Purvis and Wedin2011; Arcadia & Knudsen Reference Arcadia and Knudsen2012).
Myriospora is one of the earliest diverging taxa in the Acarosporaceae (Wedin et al. Reference Wedin, Wiklund, Crewe, Döring, Ekman, Nyberg, Schmitt and Lumbsch2005; Crewe et al. Reference Crewe, Purvis and Wedin2006; Westberg et al. Reference Westberg, Millanes, Knudsen and Wedin2015). Since this clade was acknowledged as distinct from Acarospora in a strict sense and accepted to accommodate seven species mainly reported from the Northern Hemisphere (Westberg et al. Reference Westberg, Crewe, Purvis and Wedin2011), only two further species have been added: M. hassei (Herre) K. Knudsen & Arcadia from North America (Knudsen Reference Knudsen2011; Arcadia & Knudsen Reference Arcadia and Knudsen2012) and M. westbergii K. Knudsen & Bungartz from the Galapagos (Knudsen & Bungartz Reference Knudsen and Bungartz2014). Here we describe Myriospora signyensis Purvis, Fdez-Brime, M. Westb. & Wedin as new to science based on the material previously identified as Acarospora cf. badiofusca from Signy Island (Øvstedal & Lewis-Smith Reference Øvstedal and Lewis Smith2001) and new collections from the same island. A revised key to all currently accepted species of Myriospora is presented.
Materials and Methods
Sample location from field photographs
The original Myriospora signyensis sample location on Manhaul Rock was established by examining general habitat, close-up and macro photographs taken in the field using the layers and opacity function in Adobe Photoshop CC 2017. A photo-diagram showing the relationship between Myriospora thalli and mineral components was prepared by tracing a field macro photograph using Adobe Draw and an Apple Pencil on an iPad Pro, and thalli filled according to their colour (rust-coloured/dark melanised). The layered image was further modified using Adobe Illustrator CS5 and saved as a composite photo-diagram using the opacity function in Adobe Photoshop CC 2017.
Morphology and anatomy
The study is based mainly on material deposited in the herbaria AAS, BM and S (acronyms follow Index Herbariorum (Thiers Reference Thiers2017). Hand-cut sections of thalli and apothecia mounted in water were studied with light microscopy. In the apothecial studies, only the disc was measured and the margin (although distinct in many specimens) was excluded from the measurements. Hymenial reactions were studied with Lugol’s idodine solution, before and after the addition of 10% KOH solution. Ascospores and conidia were studied under oil immersion at ×100 magnification. Areole size is given for fertile areoles only and given as (min. value observed–) range including 85% of the variation (–max. value observed). The description of the subhymenium includes the hypothecium.
Chemistry
A 10–15% potassium hydroxide solution (K) was used on squash preparations of the thallus cortex and medulla to test for the presence of norstictic acid (K+ red), the only secondary substance reported from Myriospora (Westberg et al. Reference Westberg, Crewe, Purvis and Wedin2011). A commercial bleach solution (C) and para-phenylendiamine dissolved in alcohol (Pd) were further used on selected samples.
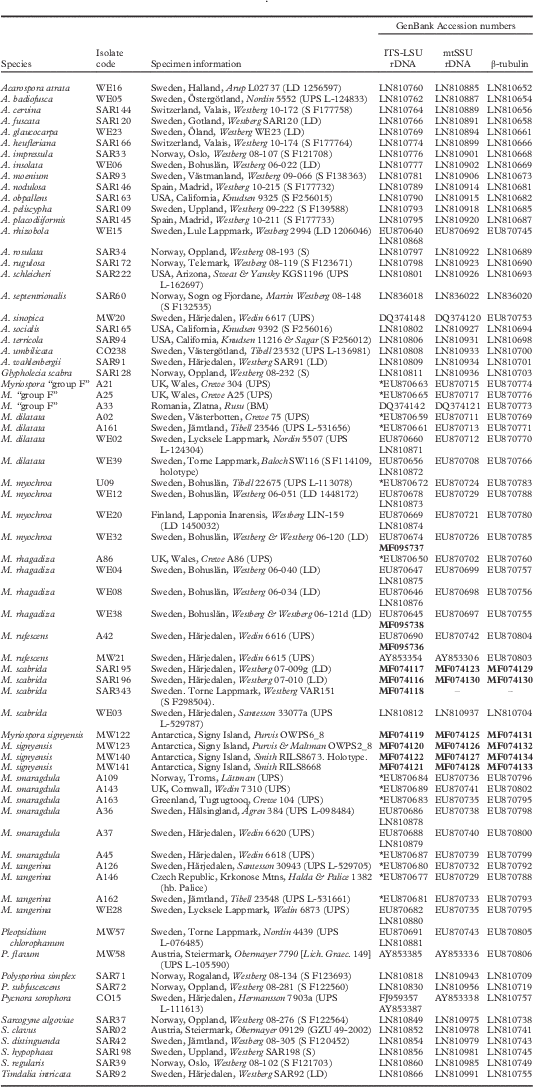
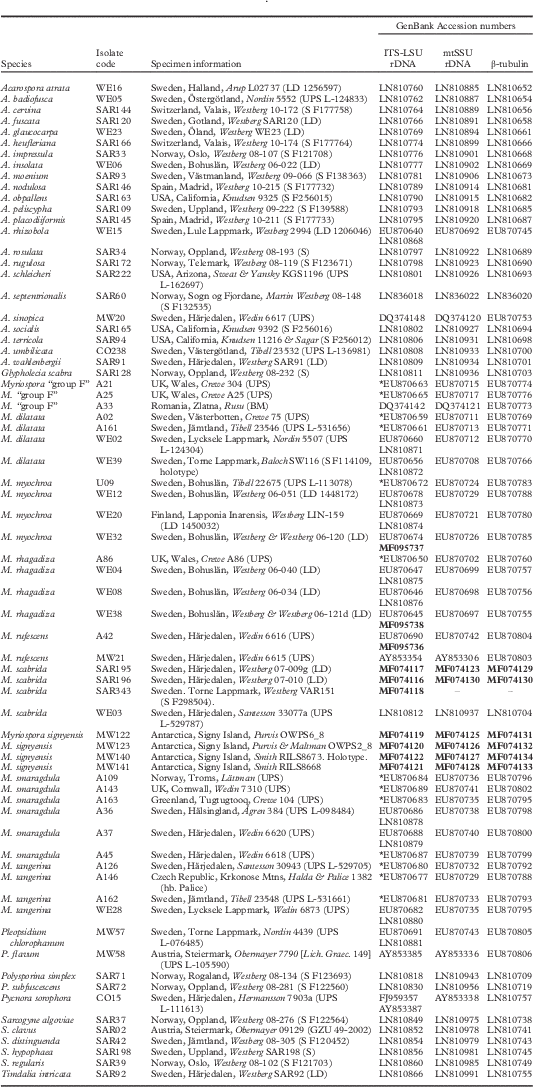
Molecular data
As morphology and preliminary BLAST analyses suggested that the new species belonged to Myriospora, we utilized a reduced version of the data matrices from Westberg et al. (Reference Westberg, Millanes, Knudsen and Wedin2015) that included all accepted Myriospora species and a selection of other Acarosporaceae-clades as background sampling to test this assumption, and to infer its putative monophyly and relationships further. New molecular data were gathered from seven samples (four of the new species and three additional samples of M. scabrida) listed in Table 1, and DNA extractions, PCR amplification of the markers (internal transcribed spacer complete repeat (ITS) and partial large subunit (nLSU) of the nuclear ribosomal DNA, the small subunit of the mitochondrial ribosomal DNA (mtSSU) and the protein coding β-tubulin (BT)) and Sanger sequencing were performed following methods summarized in Westberg et al. (Reference Westberg, Millanes, Knudsen and Wedin2015).
Table 1 Specimen voucher information and GenBank Accession numbers for collections included in the molecular analyses. Specimens for which only ITS was available are marked with an asterisk and for those where ITS and LSU were sequenced separately but from the same sample, both GenBank Accession numbers are included. Newly produced sequences are in bold
Molecular phylogeny
Sequence fragments were assembled and edited with Sequencer v4.9 (Gene Codes Corp., Ann Arbor, MI) and compared through BLASTn against the NCBI nucleotide database to rule out contaminations. Sequences were aligned with the online version of MAFFT v7 (http://mafft.cbrc.jp/alignment/server/) using the algorithms L-INS-I for ITS and nLSU, and E-INS-I for mtSSU and BT. Ambiguously aligned regions were delimited using Gblocks v0.91b (http://molevol.cmima.csic.es/castresana/Gblocks_server.html) and excluded from the analysis. Alignments were submitted to TreeBase (http://www.treebase.org; ID number 20967).
Maximum likelihood (ML) analyses were run for each locus separately with RAxML v8.2.0 (Stamatakis Reference Stamatakis2014), using the rapid bootstrap analysis and search for best-scoring ML algorithm (-f a), the GTRGAMMA model and 1000 bootstrap replicates (ML-BS). A conflict was considered significant when two alternative relationships corresponding to different loci were both supported with bootstrap values ≥70% (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996). As no conflicts were detected, the individual locus alignments were concatenated into a single alignment in which eight partitions were set (ITS1, 5.8S, ITS2, nLSU, mtSSU and each codon position of BT).
The concatenated dataset was subjected to ML and Bayesian inference (B) analyses. For the ML analyses, the same settings from the individual gene analyses were used in RAxML, with GTRGAMMA (the only true evolutionary model available in the software) applied to each partition. The Bayesian analysis was performed using MrBayes 3.2.1 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). The Bayesian Information Criterion (BIC) as currently implemented in jModelTest v0.1.1 (Posada Reference Posada2008) was used to select the models of evolution for each partition: GTR+I+G for mtSSU, BT 1st and BT 3rd; SYM+I+G for ITS1 and ITS2; SYM+I for BT 2nd; and K80+I+G for 5.8S and LSU. We performed two parallel runs with four independent chains, each for 20 million generations, with every 500th tree saved. The first 25% of the trees was discarded for each run as burn-in and the post-burn-in parameters inspected with Tracer v1.6 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2014). As the results showed proper mixing by the runs, the remaining trees were used to estimate branch lengths and posterior probabilities (B-PP).
Results
The concatenated dataset included a total of 70 specimens, for which ITS, mtSSU and BT were available for all and nLSU available for 56. The data matrix contained 2341 aligned characters after the exclusion of the sites corresponding to ambiguous regions (ITS1 81 bp, 5.8S rDNA 183 bp, ITS2 59 bp, nLSU 590 bp, mtSSU 723 bp and β-tubulin 705 bp). Of the total number of characters analyzed, 1687 were constant and 659 were variable, 495 of which were parsimony-informative. In total, 25 new sequences were produced for this study (Table 1).
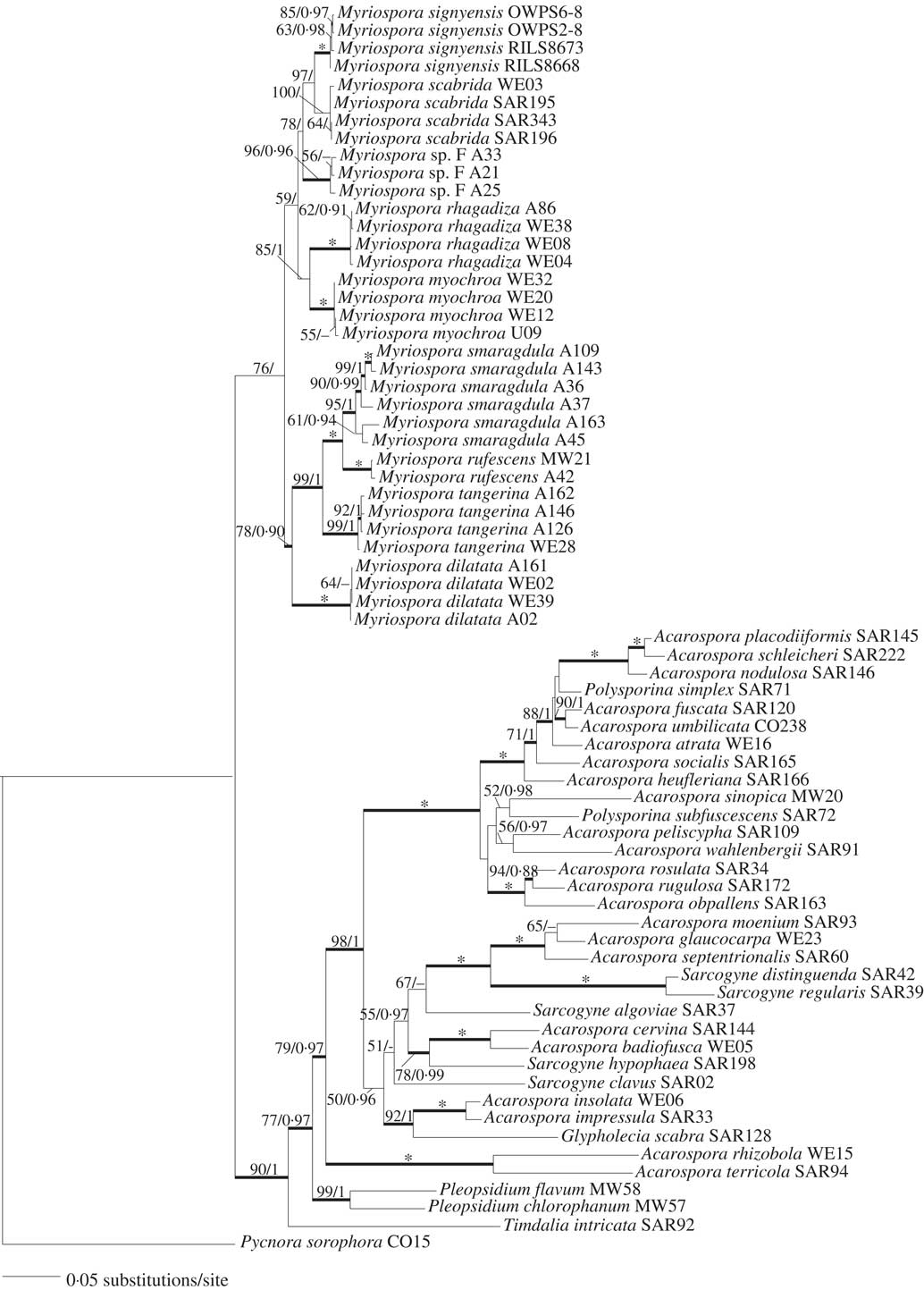
The two phylogenetic analyses resulted in fully congruent tree topologies, and we show here only the most likely tree from ML (ln likelihood=−15746·705) with the internode support for both analyses (ML-BS and B-PP; Fig. 3). The four M. signyensis specimens formed a strongly supported monophyletic group (ML-BS 100%, B-PP 1·0) in the ML and B analyses; however, only ML strongly supported (ML-BS 97%) a sister relationship with M. scabrida. Myriospora formed a monophyletic group and the patterns of relationships between the remaining Acarosporaceae members included in the phylogeny were congruent with previous studies (Wedin et al. Reference Wedin, Westberg, Crewe, Tehler and Purvis2009; Westberg et al. Reference Westberg, Millanes, Knudsen and Wedin2015).
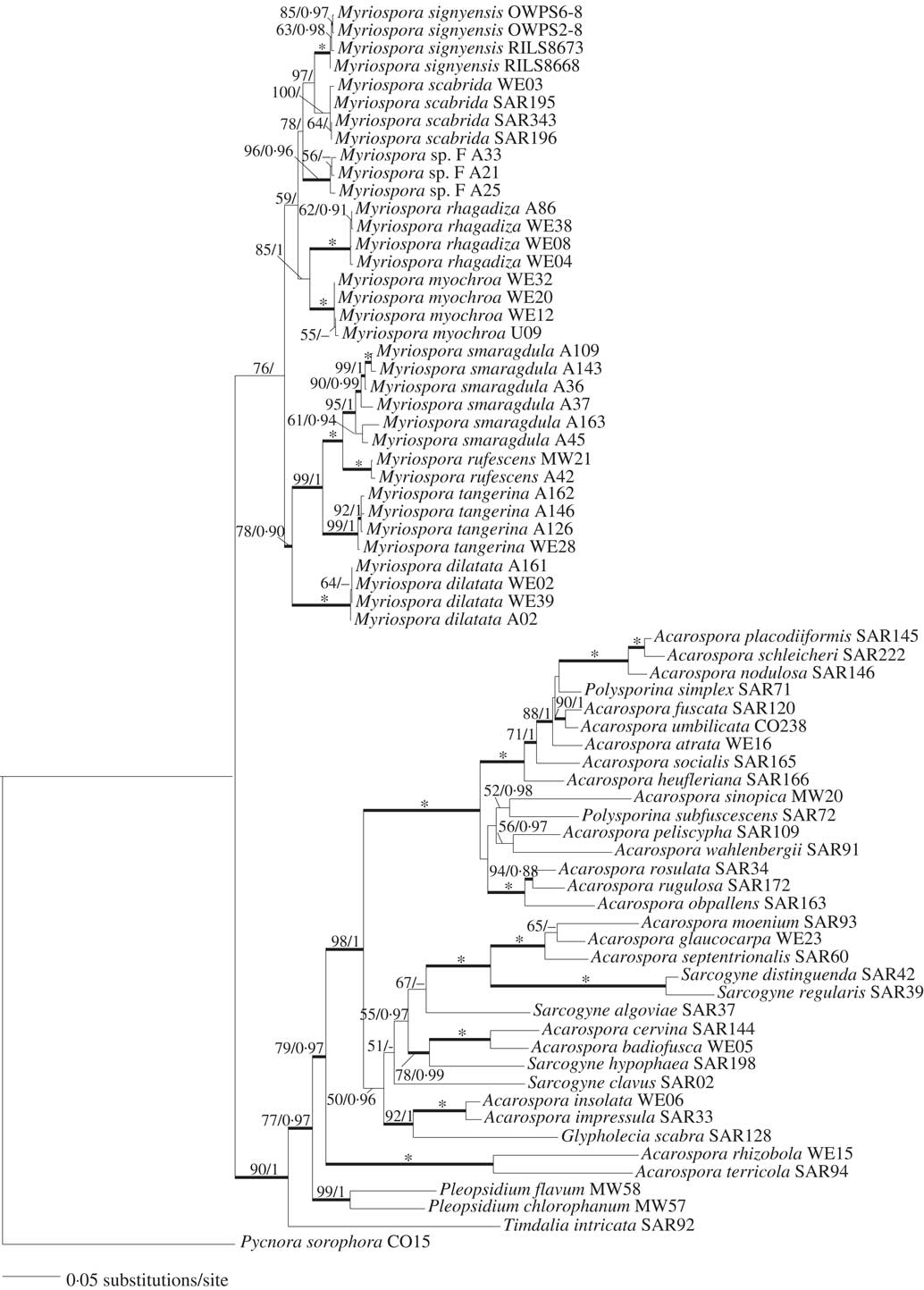
Fig. 3 Maximum Likelihood (ML) most likely tree based on a combined dataset of ITS, nLSU, mtSSU and β-tubulin for the taxa used in this study. Numbers above the internodes indicate ML-BS/B-PP. Thicker internodes indicate ML-BS≥70% and B-PP≥0·95; asterisks indicate ML-BS=100% and B-PP=1·0.
Taxonomy
Myriospora signyensis Purvis, Fdez-Brime, M. Westb. & Wedin sp. nov.
MycoBank No.: MB 821620
Similar to Myriospora dilatata but differs in having distinctly sessile mature apothecia with a prominent margin and a lobed thallus.
Type: Antarctica, Signy Island, Gneiss Hills (south), alt. 230 m, on mica schist stones, 22 February 1992, R. I. Lewis Smith 8673 (AAS—holotype (Fig. 2A) [sequenced]).
Thallus epilithic, areolate; areoles rounded to angular, contiguous to irregularly dispersed, usually lobate towards the edges, sometimes imbricate, fertile areoles (0·3–)0·5–1·2(–1·4) mm wide; upper surface frequently pale orange to rusty orange-red or pale to dark brown, smooth to rough, concave to convex; epinecral layer absent; upper cortex 25–80 µm thick, often with orange-brown granules or pigmented region above, colourless below, cell lumina 2–5 µm diam.; photobiont chlorococcal; algal layer discontinuous and disrupted at irregular intervals by bundles of medullary hyphae reaching the cortex above the algal cells.
Apothecia 1–2(–4) per areole/squamule, sessile, round; disc 0·2–0·9 mm diam., mostly dark brown to black, sometimes with a rusty or orange-red pruina, flat, rough; margin raised and prominent, dark brown to black, sometimes rusty or orange-red pruinose. Thalline exciple lacking. Proper exciple colourless in section, often orange-pigmented externally, expanding to 180 µm across in the upper part, uppermost cells with pale to dark pigmented caps. Hymenium 150–170 µm tall, colourless, no oil droplets visible, I+ bluish in low concentration, red in higher concentration, K/I+ blue; paraphyses hyaline, simple or sometimes branched near the tips, 1–2 µm wide in mid-hymenium, tips clavate, to 4 µm wide; epihymenium orange-brown; subhymenium colourless to very pale brown with scattered oil droplets, I+ pale bluish to red, K/I+ blue. Asci up to 160×25 µm; no apical structures visible in K/I; ascospores c. 2–4×1·0–2·5 µm, hyaline, oblong.
Pycnidia rare; conidia c. 2×1 µm, oblong.
Chemistry. Thallus UV−, K− (norstictic acid absent), C−, Pd−.
Etymology. Signyensis refers to the type locality on Signy Island, Antarctica, which was named by the Norwegian whaler Captain Sørlle (1892–1988) after his wife Signy. Sørlle charted the island between 1912 and 1913 (Hattersley-Smith Reference Hattersley-Smith1991).
Substratum and ecology. This species grows predominantly on mica schist in montane situations. Signy Island forms part of the Scotia metamorphic complex (Mathews & Maling Reference Mathews and Maling1967; Tanner et al. Reference Tanner, Pankhurst and Hyden1982) comprising Permian-Triassic continental elements of predominantly semi-pelite juxtaposed with oceanic elements. These include pelite, marble, metachert and metabasites with an enriched-type MORB and alkaline ocean-island basalt chemistry (Storey & Meneilly Reference Storey and Meneilly1985), which were accreted onto the continent margin during an episode of subduction in the Early Jurassic (Flowerdew et al. Reference Flowerdew, Daly and Riley2007). An oceanic element of the Scotia metamorphic complex is exposed at Manhaul Rock. Structurally, pelite and semi-pelite with garnet porphyroblasts form most of the flat, elevated surfaces of the rock exposure from where Myriospora signyensis was exclusively collected (Purvis et al. Reference Purvis, Convey, Flowerdew, Peat, Najorka and Kearsley2013).
Distribution. Myriospora signyensis is known only from five localities on Signy Island. Further study of Acarosporaceae on the adjacent Coronation Island, South Orkney Islands, with oceanic elements of the Scotia metamorphic complex, is likely to yield further localities for this currently rare, though easily overlooked species. No Acarosporaceae are treated in the recent field guide ‘The Lichens of the Falkland Islands’, although the emphasis is on species that can be identified without the need of a microscope (Orange Reference Orange2016). Highly disjunctive patterns of distribution are well known in lichens. It would be interesting to establish if this species has a typical oceanic Gondwanan distribution (e.g. Fryday et al. Reference Fryday, Ertz and Jørgensen2017).
Notes. This distinctive species is easily recognized by having large sessile apothecia with prominent margins, its thallus is typically lobed at the margins and is orange-red or rust-coloured, or else brown-pigmented. It is closely related to M. scabrida (Fig. 3), which also has a somewhat lobed, imbricate thallus with large and frequently somewhat raised apothecia. Myriospora scabrida is, however, never rusty red in colour, frequently has a larger number of apothecia per areole/squamule, and has a thick and distinctive epinecral layer on the thallus. Young thalli of M. signyensis might be mistaken for M. dilatata when the apothecia are immersed in the thallus, but the thallus of M. dilatata is never imbricate-lobate and the ascomata of M. signyensis eventually develop into considerably larger and much more distinctly raised and sessile apothecia than this species. Myriospora signyensis was previously misidentified as Acarospora badiofusca (Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001) but A. badiofusca s. str. is not closely related to Myriospora (Fig. 3) and has an amyloid hymenium rather than the hemiamyloid reaction that all Myriospora species show. Myriospora signyensis is also morphologically so distinctive that we believe not all samples previously identified as A. badiofusca from the area are M. signyensis. These include one (AAS: DCL 3613) from South Georgia with immersed apothecia previously considered to represent an environmental modification (Øvstedal & Lewis Smith 2001) sampled from a nitrogenous habitat (penguin rookery). Material from a geothermal area on Deception Island (RILS 6906) is also excluded. On Signy Island, a rust-coloured taxon sampled as ‘Sarcogyne’ from a coastal locality on Bernstone Point (DCL 1388: AAS) is too fragmentary for identification.
Other specimens examined. Antarctica: South Orkney Islands: Signy Island, Gneiss Hills (south), alt. 230 m, on mica schist stones, 1992, R. I. Lewis Smith 8668a (AAS) [sequenced]; ibid., Gneiss Hills (north), alt. 240 m, on mica schist stones, 1992, R. I. Lewis Smith 8667b (AAS); ibid., Jane Col, alt. 150 m, fellfield, 1985, R. I. Lewis Smith 7037 (AAS); ibid., southern central region of McLeod Glacier, Manhaul Rock, on weathered iron-stained quartz mica schist just above the glacier, alt. c. 99 m, S60.72372 W45.61554, 2009, O. W. Purvis & B. Maltman OWPS2_8 (BM) [sequenced], OWPS2_7 (S); ibid., Khyber Pass, alt. c. 79 m, S60.71753 W45.61013, 2009, O. W. Purvis OWPS6_8 (BM) [sequenced] and others sampled from the same localities together with other lichens: OWPS2_3, OWPS2_4, OWPS2_5, OWPS2_6, OWPS6_7 (all BM).
OWP gratefully acknowledges the Swedish Museum of Natural History and financial support through the Synthesys project (grant agreement no. 226506) within the European Union’s Seventh Framework Funding and also from the NERC Antarctic Funding Initiative Collaborative Gearing Scheme (ref. CGS11/57). Nicholas J. P. Owens is thanked for his helpful assistance. Vladimir Blagoderov is thanked for assistance with photomicroscopy, NHM Image Resources for macro photography (Fig. 3F) and useful advice, and Mike Flowerdew for kindly suggesting the visit to Manhaul Rock. We are grateful to Per Magnus Jørgensen for his helpful assistance with literature. MW acknowledges support from the Swedish Research Council (grants VR 621-2012-3990 and VR 2016-03589). The analyses were performed using resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science under project UPPNEX b2014033. Special thanks to two anonymous reviewers and Alan Fryday for helpful advice and editorial assistance.