Introduction
Bryoria Brodo & D. Hawksw. is the largest genus in the alectorioid clade of the family Parmeliaceae (Divakar et al. Reference Divakar, Crespo, Wedin, Leavitt, Hawksworth, Myllys, McCune, Randlane, Werner and Ohmura2015), which inhabits temperate to alpine regions worldwide. It has been comprehensively studied in North America and northern Europe (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Myllys et al. Reference Myllys, Velmala and Holien2011a ); however, morphological simplicity and chemical variability make its taxonomy difficult, and recent molecular data have resulted in several changes (Velmala et al. Reference Velmala, Myllys, Halonen, Goward and Ahti2009, Reference Velmala, Myllys, Goward, Holien and Halonen2014; Myllys et al. Reference Myllys, Velmala, Lindgren, Glavich, Carlberg, Wang and Goward2014). Recent studies have discovered additional new species in Bryoria from east-central Asia (Myllys et al. Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ; Jørgensen et al. Reference Jørgensen, Myllys, Velmala and Wang2012), southern South America and the Antarctic (Olech & Bystrek Reference Olech and Bystrek2004; Fryday & Øvstedal Reference Fryday and Øvstedal2012). Temperate South America is a region with an unexpectedly low reported Bryoria diversity, suggesting that additional species may be awaiting discovery in the region. In the course of lichen research by one of us (JV) in the Conguillío National Park in Chile, samples of Bryoria growing on Araucaria araucana trees were collected. Molecular, morphological and chemical analyses of the specimens revealed the presence of two species: Bryoria glabra (Motyka) Brodo & D. Hawksw. and another that did not group with any known species in our ongoing morphological, chemical, and phylogenetic analyses. This second species is therefore described here as new.
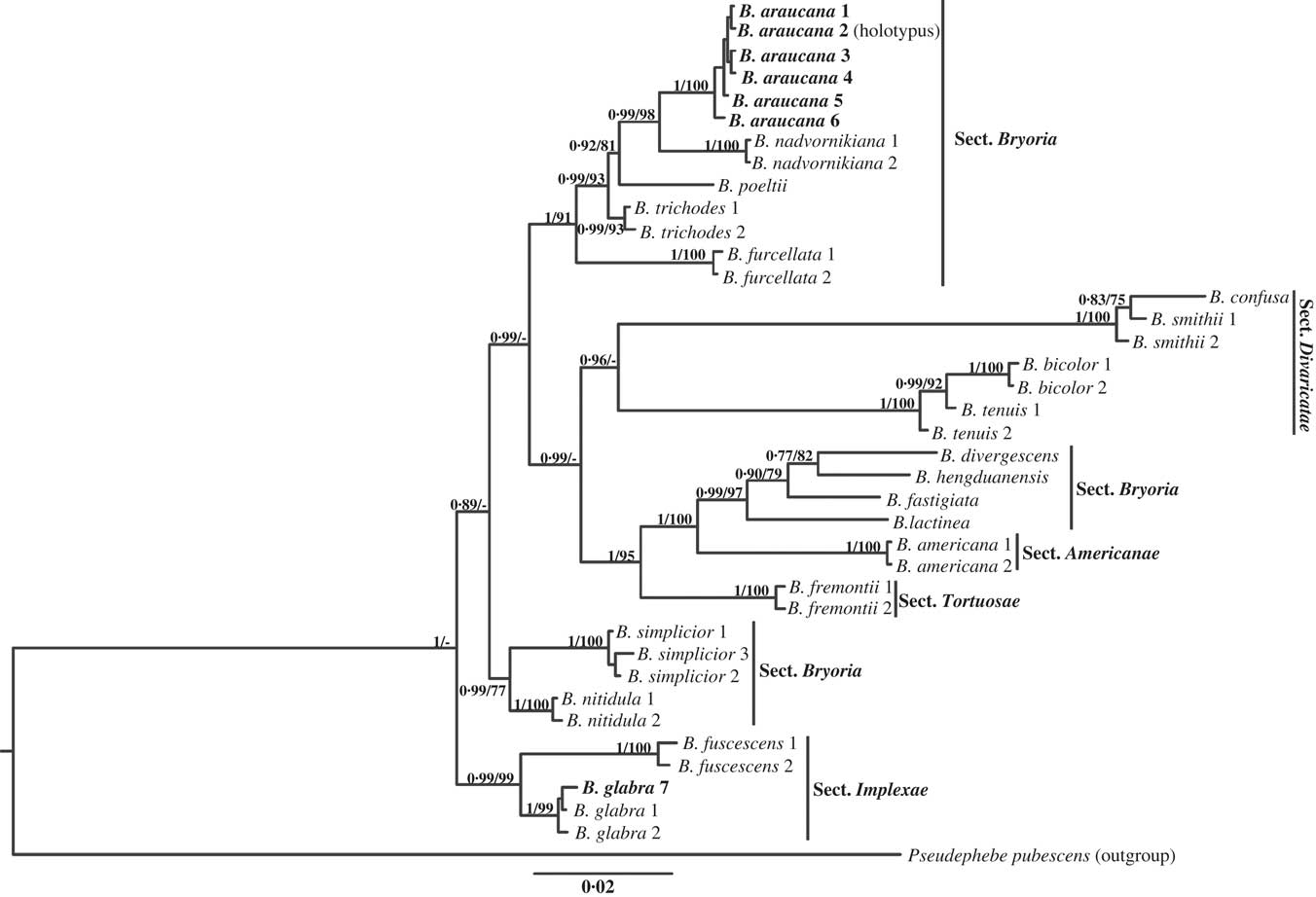
Fig. 1 Phylogenetic relationships of Bryoria species used in this study, 38 samples representing 19 species, based on ITS, mtSSU, and MCM7 markers analyzed in a concatenated data matrix. Tree topology depicts the results of the Bayesian Markov chain Monte Carlo (B/MCMC) analysis. Posterior probabilities and bootstrap values, when coincident with the Bayesian tree, are given on the node branches. Sections according to Myllys et al. (Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ). B. glabra (bold)=Chilean specimen. B. araucana (bold)=new species. Peudephebe pubescens used as outgroup.
Table 1 Specimen information and GenBank accession numbers for the taxa used in this study. Newly obtained sequences are in bold

Chemistry as follows: Ale=alectorialic acid, Atr=atranorin, Bar=barbatolic acid, Cfum=confumarprotocetraric acid, Fum=fumarprotocetraric acid, Gyr=gyrophoric acid, Nor=norstictic acid, Pro=protocetraric acid, Pso=psoromic acid, Qua=quaesitic acid, Usn=usnic acid, Vul=vulpinic acid, No subs=no lichen substances detected.
Materials and Methods
The specimens collected were analyzed morphologically using standard methods (Smith et al. Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009) using a Nikon SMZ-1000 stereomicroscope and a Nikon Eclipse-80i microscope, and photographs were taken with a Nikon 105 mm f/2.8D AF Micro-Nikkor lens coupled to a Nikon D90 camera. Spot tests with C, K, KC, and Pd were carried out as explained in Brodo & Hawksworth (Reference Brodo and Hawksworth1977). For thin-layer chromatography (TLC), solvents A, B and C were used to run concentrated lichen extracts in 50 °C acetone spotted onto silica gel 60 F254 aluminium sheets (Merck, Darmstadt), according to standard methods (Orange et al. Reference Orange, James and White2010). For the best resolution in solvent C, the spotted plate was left to stand for 10 min before running in an acetic acid atmosphere.
DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Barcelona) with a slight modification to the manufacturer’s instructions (Crespo et al. Reference Crespo, Blanco and Hawksworth2001; Divakar et al. Reference Divakar, Del-Prado, Lumbsch, Wedin, Esslinger, Leavitt and Crespo2012). Three loci were amplified: 1) nrITS, with ITS1FKYO2 (5'-TAG AGG AAG TAA AAG TCG TAA-3') and ITS4KYO2 (5'-RBT TTC TTT TCC TCC GCT-3'; Toju et al. Reference Toju, Tanabe, Yamamoto and Sato2012) primers; 2) mSSU rDNA, with mtSSU1 (5'-AGC AGT GAG GAA TAT TGG TC-3') and mtSSU3R (5'-ATG TGG CAC GTC TAT AGC CC-3'; Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) primers; and 3) the low copy protein coding gene MCM7, with MCM71348rev (5'-GAY TTD GCI ACI CCI GGR TCW CCC AT-3') and MCM7-709f (5'-ACI MGI GTI TCV GAY GTH AAR CC-3'; Schmitt et al. Reference Schmitt, Crespo, Divakar, Fankhauser, Herman-Sackett, Kalb, Nelson, Nelson, Rivas-Plata and Schimp2009) primers. For amplification, a reaction mixture of 25 µl was used containing 18 µl sterile water, 2·5 µl ×10 buffer with 2 mM MgCl2, 0·5 µl dNTPs (10 mM of each base), 1·25 µl of each primer at 10 µM, 0·625 µl of DNA polymerase (1U µl−1), and 1–2 µl DNA template. In failed samples the PCR was repeated using PuReTaq Ready-To-Go PCR Beads (2·5 U of PuReTaq DNA Polymerase, 200 µM of each dNTP, BSA, buffer reaction and stabilizers: 10 mM Tris-HCl ph 9·0, 50 mM KCl, 1·5 mM MgCl2; GE Healthcare, Little Chalfont, UK) adding to the lyophilized bead 20 µl of sterile water, 1 µl of each primer at 10 µM, and 1·5 µl of DNA template.
Amplifications were run in an automatic thermocycler (XP Cycler, Bioer, Hangzhou) using the following parameters for ITS rDNA and mtSSU rDNA: initial denaturation 5 min at 95 °C, then 35 cycles of 1 min at 95 °C, 1 min at 56 °C, 1·5 min at 72 °C, and a final extension of 10 min at 72 °C. For MCM7 we used a touchdown cycling process: initial denaturation 10 min at 94 ºC, then followed by 4 cycles of 45 s at 94 ºC, 50 s at 56 ºC, 1 min at 72 ºC; 4 cycles of 45 s at 94 ºC, 50 s at 54 ºC, 1 min at 72 ºC; 36 cycles of 45 s at 94 ºC, 50 s at 52 ºC, 1 min at 72 ºC and a final extension of 8 min at 72 ºC. PCR products were cleaned using illustraTM ExoProStar (GE Healthcare, Little Chalfont, UK), according to the manufacturer´s instructions. Sequencing was performed by the Unidad de Genómica (Parque Científico de Madrid).
DNA sequences obtained were manually adjusted using SeqMan version 7.0 (DNAstar, Madison) and MEGA5 (Tamura et al. Reference Tamura, Peterson, Peterson, Stecher, Nei and Kumar2011). Some GenBank sequences from Myllys et al. (Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ; Table 1) were added to the file and aligned using MAFFT version 7 (Katoh & Standley Reference Katoh and Standley2013), with the G-INS-I alignment algorithm, a scoring matrix of 1 PAM/k=2, and 0·1 as offset value. Gblocks version 0.91b (Castresana Reference Castresana2000) was used to delete non-conserved GAPs, allowing smaller final blocks, gap positions within the final blocks, and less strict flanking positions. The alignments of each region and the concatenated one were analyzed using maximum likelihood (ML) and Bayesian (B/MCMC) approaches, with Pseudephebe pubescens as outgroup to root the tree (Divakar et al. Reference Divakar, Crespo, Wedin, Leavitt, Hawksworth, Myllys, McCune, Randlane, Werner and Ohmura2015). For maximum likelihood (ML) tree reconstruction, the program RAxML v7.2.8 (Stamatakis Reference Stamatakis2006) implemented on the Cipres Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) was used. We selected the GTRGAMMA model, which includes a parameter (Γ) for rate heterogeneity among sites and chose not to include a parameter to estimate the proportion of invariable sites (Stamatakis Reference Stamatakis2006; Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008). Support values were assessed using the ‘rapid bootstrapping’ option with 1000 replicates. For Bayesian reconstruction, MrBayes v3.2.1 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) was used, assuming the general time reversible model (Rodriguez et al. Reference Rodríguez, Oliver, Marín and Medina1990) and a discrete gamma distribution with six rate categories (GTR+G). The nucleotide-substitution model and parameters were selected using the Akaike Information Criterion as implemented in jModelTest (Posada Reference Posada2008). A run with four million generations, starting with a random tree and employing eight simultaneous chains, was executed. Every 400th tree was saved to a file. We plotted the log-likelihood scores of sample points against generations using TRACER v1.5 (http://beast.bio.ed.ac.uk/Tracer) and determined that stationarity had been achieved when the log-likelihood values of the sample points reached an equilibrium value (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001), discarding the trees obtained before stationarity was reached. Posterior probabilities (PPs) were obtained from the 50% majority-rule consensus of sampled trees after excluding the initial 25% as burn-in. The phylogenetic trees were drawn using FigTree v1.4 (http://tree.bio.ed.ac.uk/software/figtree).
Results and Discussion
The tree obtained from the concatenated ITS, mtSSU and MCM7 dataset (Fig. 1) is mainly based on sequences published by Myllys et al. (Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ), who performed a parsimony analysis obtaining five infrageneric sections. Here we subjected those sequences to maximum likelihood and Bayesian analyses, resulting in a different and better supported tree topology. This discrepancy may not be due to the phylogenetic reconstruction method, but to different sampling and the loci used. Sections Americanae, Divaricatae, Implexae and Tortuosae are resolved as monophyletic, but in different tree locations than those of Myllys et al. (Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ). Section Implexae appears as basal rather than derived, and sections Americanae and Tortuosae are no longer basal. Section Divaricatae seems justified, but section Bryoria was recovered as polyphyletic and split into three monophyletic groups. In view of this, sections Americanae, Tortuosae (with one sequenced species each) and Bryoria (polyphyletic) will evidently need revision after more detailed analysis has been undertaken. At the species level, Bryoria tenuis appears paraphyletic with B. bicolor, and B. smithii paraphyletic with B. confusa, but due to the small number of specimens included in this study it would be premature to propose any change here.
Analyses of the Chilean specimens (Table 1; Fig. 1) revealed the presence of Bryoria glabra, the first record from the Southern Hemisphere, and a set of different specimens that did not group with any known species. These were phylogenetically close to the Northern Hemisphere Bryoria nadvornikiana (Gyeln.) Brodo & D. Hawksw. but they contained fumarprotocetraric rather than barbatolic acid as the main substance. The Chilean specimens were quite different from B. nadvornikiana morphologically in that they lacked extensive blackened bases and short, perpendicular, lateral, spinule-like branches. Additionally, they were morphologically and chemically similar to the Northern Hemisphere Bryoria trichodes (Michx.) Brodo & D. Hawksw. but lacked soralia, although soralia are not found in every specimen of B. trichodes. The material is therefore described here as a new species.
The Species
Bryoria araucana Boluda, D. Hawksw. & V. J. Rico sp. nov.
MycoBank No.: MB811960
Resembles the Northern Hemisphere circumboreal Bryoria trichodes, but is distinct molecularly, without soralia, and with less conspicuous pseudocyphellae.
Type: Chile, IX Región de La Araucanía, Provincia de Cautín, Comuna de Melipeuco, Conguillío National Park, Tramo Contrabandistas, Sendero Las Araucarias, close to Conguillío Lake, 38°39'13·57''S, 71°37'05·27''W, 1215 m, Araucaria araucana forest, on the north side of an araucaria trunk, 31 August 2014, J. Villagra 2 (MAF-Lich. 19718—holotype). GenBank accession numbers: KP975405 (ITS), KP939082 (mtSSU), and KP975413 (MCM7).
(Fig. 2)

Fig. 2 Bryoria araucana, holotype. A, habitat; B, habit; C, detail of branching pattern; D & E, detail of pseudocyphellae. Scales: B=1 cm; C=1 mm; D=0·15 mm; E=0·25 mm.
Thallus pendent to subpendent, 6–12 cm long; isotomic to anisotomic dichotomously branched, angles between dichotomies mainly obtuse, rarely acute; branches terete, even, main branches at base 0·2–0·4 mm diam., tips to 0·1 mm diam.; terminal portions with few lateral branchlets acutely inserted. Surface dark grey to dark greyish brown, shiny, base ordinarily black; cortex prosoplectenchymatous. Soralia and isidia lacking. Pseudocyphellae inconspicuous, depressed, fusiform, concolorous to slightly darker than the thallus, sometimes faintly pruinose, straight or twisted, up to 1·5 mm long. Photobiont trebouxioid.
Apothecia and conidiomata unknown.
Chemistry
Inner cortex and medulla C−, K−, KC−, PD+ yellow turning red, sometimes faint. TLC: fumarprotocetraric acid as the main substance, with protocetraric and confumarprotocetraric acids in trace amounts.
Etymology
Named after the IX Región de la Araucanía in Chile, which is the only known area for the species, as was the case in the name Araucaria araucana.
Distribution and ecology
Known only from the type locality and immediate surroundings in the Parque Nacional Conguillío, IX Región de La Araucanía (Chile), occurring on trunks of Araucaria araucana in mature open forests (Fig. 2A). Those forests are characteristic of the upper supratemperate bioclimatic belt with the ultraperhumid rainfall regime of the South American Temperate Region (Amigo & Ramírez Reference Amigo and Ramírez1998). Furthermore, the mean annual precipitation in the area, which falls mainly as snow, is c. 2000 mmy-1, and the mean annual temperature is 8·6 °C, with dry and hot short summers (Di Castri & Hajek Reference Di Castri and Hajek1976). Bryoria araucana is more frequent on the north-facing trunks exposed to humid winds, growing with Coelopogon epiphorellus, Protousnea dusenii, P. magellanica, P. poeppigii, and Platismatia glauca. On the south-facing sides of the trunks it is less frequent, growing with Nephroma antarcticum, Pseudocyphellaria coriifolia, P. flavicans, and P. granulata. It may be anticipated that B. araucana will be found to have a wider distribution in the temperate forests of the Southern Hemisphere that are almost unexplored for alectorioid lichens.
Conservation status
Although the new species seems not to be frequent, it occurs in a protected area (Parque Nacional Conguillío, Chile). No special actions to conserve the species are currently required.
Additional specimens examined. Bryoria araucana Chile: IX Región de La Araucanía, Provincia de Cautín: Comuna de Melipeuco, Parque Nacional Conguillío, Tramo Contrabandistas, Sendero Las Araucarias, close to Conguillío Lake, 38°39'14·83''S, 71°37'01·06''W, 1211 m, Araucaria araucana forest, on the north side of an araucaria trunk, 2013, J. Villagra 5 & 6 (MAF-Lich. 19723, 19724); ibid., 38°39'13·57''S, 71°37'05·27''W, 1215 m, Araucaria araucana forest, on the north side of an araucaria trunk, 2014, J. Villagra 1, 3 & 4 (MAF-Lich. 19719, 19720, 19721).
Bryoria glabra Chile: IX Región de La Araucanía, Provincia de Cautín: Comuna de Melipeuco, Parque Nacional Conguillío, Tramo Contrabandistas, Sendero Las Araucarias, close to Conguillío Lake, 38°39'13·57''S, 71°37'05·27''W, 1215 m, Araucaria araucana forest, on the north side of an araucaria trunk, 2014, J. Villagra 7 (MAF-Lich. 19722).
Bryoria araucana and the Northern Hemisphere species B. trichodes form divergent independent clades which are well supported (Fig. 1). The two species are very similar in morphology and chemistry (cf. Brodo & Hawksworth Reference Brodo and Hawksworth1977), but B. araucana develops less conspicuous pseudocyphellae, lacks atranorin, and apothecia and soralia are unknown. Bryoria nadvornikiana, B. poeltii (Bystrek) Brodo & D. Hawksw. and B. furcellata (Fr.) Brodo & D. Hawksw. are phylogenetically related species, but they can be distinguished by the characters shown in Table 2. Based on the molecular results, restricted distribution, development of inconspicuous pseudocyphellae, and absence of soralia, the new species is well supported.
Table 2 Comparison of the chemistry, main morphological characters and distribution of five phylogenetically related species of Bryoria including B. araucana based on our observations and bibliographic references (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Bystrek Reference Bystrek1969; Myllys et al. Reference Myllys, Velmala and Holien2011 a; Wang & Chen Reference Wang and Chen1994)

Ale=Alectorialic acid, Atr=Atranorin, Bar=Barbatolic acid, Chlor=Chloratranorin, Fum=Fumarprotocetraric acid.
The Bryoria glabra specimen appears to be the first record from the Southern Hemisphere (Fig. 1; Table 1). It is characterized by repeatedly oval, whitish soralia and regular branching, with rounded and obtuse angles between the branches, and contains fumarprotocetraric acid (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Myllys et al. Reference Myllys, Velmala, Holien, Halonen, Wang and Goward2011b ).
Five additional Bryoria species are reported in the literature from southern South America (Argentina and Chile): Bryoria bicolor (Ehrh.) Brodo & D. Hawksw. (Calvelo & Liberatore Reference Calvelo and Liberatore2002), B. chalybeiformis (L.) Brodo & D. Hawksw., B. mariensis Øvstedal et al. (Fryday & Øvstedal Reference Fryday and Øvstedal2012), B. implexa (Hoffm.) Brodo & D. Hawksw., and B. austromontana P. M. Jørg. & D. J. Galloway (Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2004). Bryoria araucana is distinguished from all these species by the corticolous pendent habit, lack of soralia, branches 0·2–0·4 mm diam., and the sometimes dark basal parts. However, we consider some of these literature records dubious, and in need of verification through molecular analyses.
This contribution was prepared with support from the Spanish Ministerio de Economía y Competitividad projects CGL2011-25003 and CGL2013-42498-P.






