Introduction
Terrestrial, ice-free habitats in Antarctica are restricted to less than 0·5% of the entire continental area (British Antarctic Survey 2004). Environmental conditions of Antarctica present challenges to its biota that lie at the extremes of the spectra available globally (Peck et al. Reference Peck, Convey and Barnes2006). Of the macroscopic flora present on the continent, lichens colonizing exposed rock and soil habitats are subject to some of the most extreme conditions (Peck et al. Reference Peck, Convey and Barnes2006), including high levels of UV radiation, extremely low and very variable temperatures, lack of liquid water and desiccation stress, and high wind speeds and abrasion. With 427 recorded species, lichens form the dominant and most widespread element of the Antarctic flora (Ochyra Reference Ochyra1998; Bednarek-Ochyra et al. Reference Bednark-Ochyra, Vána, Ochyra and Smith2000; Øvstedal & Smith Reference Øvstedal and Smith2001). Communities of cryptogams (lichens, bryophytes) and soil inhabiting microbiota appear to tolerate such harsh conditions and to form the dominant vegetation at ice-free terrestrial habitats across the Antarctic Peninsula and the Antarctic continent (Olech Reference Olech, Beyer and Bölter2002; Seppelt Reference Seppelt, Beyer and Bölter2002; Kanda et al. Reference Kanda, Ohtani, Imura, Beyer and Bölter2002).
The present study was carried out on Coal Nunatak. Unlike most habitats described from the maritime Antarctic, this region is remote from the influence of maritime conditions, being isolated to the east and south by the permanent George VI Ice Shelf, and to the west and north by the bulk of Alexander Island and its western ice shelves. Ecosystems are characterized by critically low soil nutrient contents (Lawley et al. Reference Lawley, Ripley, Bridge and Convey2004; Engelen et al. Reference Engelen, Convey, Hodgson, Worland and Ott2008) and the harsh climatic conditions (A. Engelen et al. unpublished). Instability of the simple mineral soils, largely through freeze-thaw processes, is thought to be a limiting factor for the initial establishment and survival of biota (Smith Reference Smith and van der Maarle1993; Wynn-Williams Reference Wynn-Williams, Miles and Walton1993) and the subsequent development of ecosystems.
The macroflora of Coal Nunatak is not extensive. The margins of frost-sorted soil polygons support limited development of small bryophyte cushions and tiny lichen populations. Only six bryophyte species belonging to six genera (British Antarctic Survey, unpublished data) and 14 lichen species (11 genera) (A. Engelen, J. Buschbom, P. Convey & S. Ott unpublished) are currently known. Additionally, a variety of soil inhabiting microbiota has been identified using molecular biological techniques (Brinkmann et al. Reference Brinkmann, Pearce, Convey and Ott2007). Lichens possess a range of features that equip them to cope with harsh and unpredictable environmental conditions, being able to take advantage of short periods suitable for metabolic activity, interspersed with varying periods of anabiosis during which they may experience extreme temperatures, high radiation loads, desiccation and physical abrasion (Kappen & Lange Reference Kappen and Lange1969; Longton Reference Longton1988). However, to date, little research has been devoted to understand the life history features that underlie the evident ability of lichens to dominate terrestrial communities under more severe environmental conditions.
The majority of lichens recorded from Coal Nunatak are epilithic [e.g. Usnea lambii (syn. U. sphacelata R. Br. Wirtz), Pseudephebe minuscula (Nyl. ex Arnold) Brodo & D. Hawksworth, Buellia papillata (Sommerf.) Tuck., Lecidella pataviana (A. Massal.) Knoph & Leukert], with only a few species colonizing the soil surface (e.g. Candelariella flava (C. W. Dodge & Baker) Castello & Nimis, Psoroma tenue Henssen). The sterile, leprose, species Lepraria borealis Lohtander & Tønsberg is the most widespread crustose species found on Coal Nunatak. At this site, L. borealis can be described as lichenicolous, frequently found colonizing several different lichen species and can be found in different microsites defined by particular interactions.
A characteristic feature of lichen communities are interactions. Interactions between lichens growing in close association have been described from more temperate regions (Ott & Scheidegger Reference Ott and Scheidegger1992; Ott et al. Reference Ott, Meier and Jahns1995). The interactions are often defined by parasitic behaviour of one of the mycobionts involved. For instance, the mycobiont of Fulgensia bracteata parasitizes the lichen species Toninia sedifolia in order to take over the photobiont and to form a new thallus (Ott et al. Reference Ott, Meier and Jahns1995). The phenomenon of these interactions raises questions about the compatibility and selectivity of lichen symbiosis. Selectivity can be described as the degree to which symbionts interact preferentially with one another (Galun Reference Galun and Galun1988), while the degree of compatibility between two potential symbionts influences the process of recognition and is an essential prerequisite for successful lichenization (Schaper & Ott Reference Schaper and Ott2003). Throughout the life cycle of a lichen, the mycobiont while in the lichenized state, can be associated with different species of photobionts (Friedl Reference Friedl1987). The intensity of contact and interaction between the symbionts may vary in lichen species belonging to different genera and growth forms.
The present study focuses on the life history strategy of L. borealis and interactions between L. borealis and Usnea lambii, Ochrolechia frigida (Sw.) Lynge and Tephromela disciformis Øvstedal sp. nov. often found growing nearby and their photosynthesising bionts.
Materials and Methods
Study area
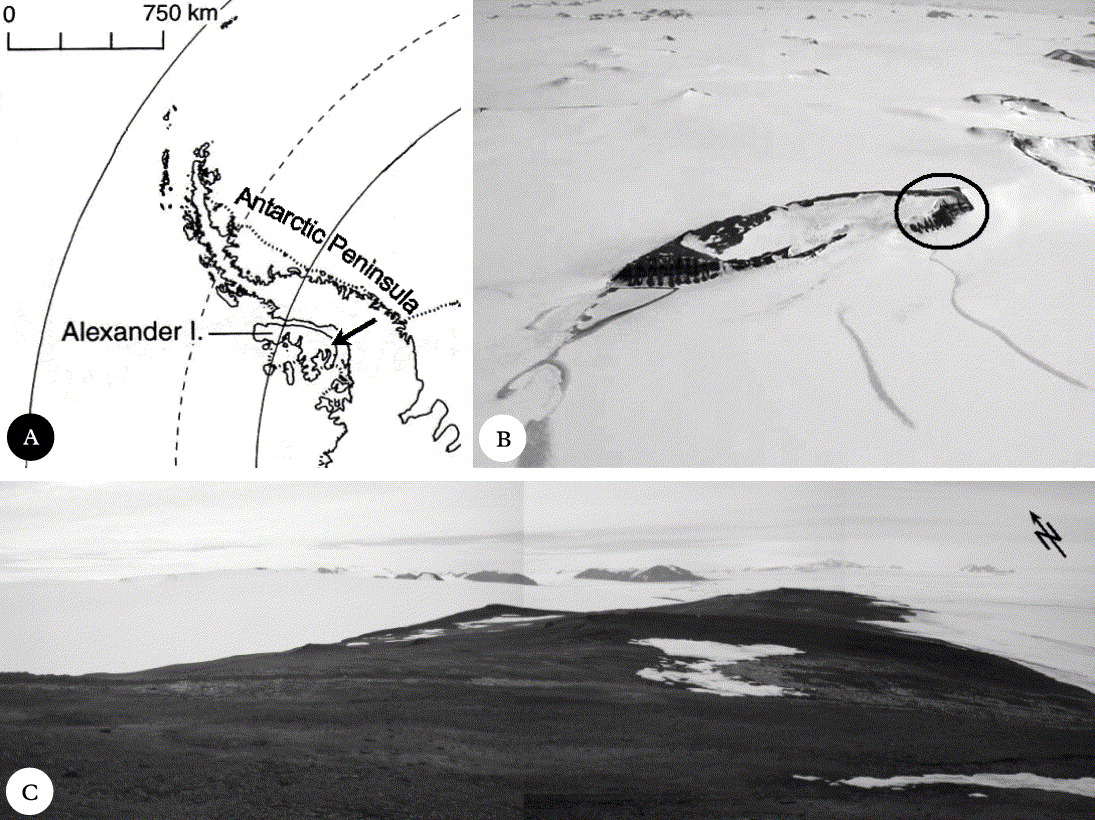
Coal Nunatak is a broad mountain summit ridge located on south-east Alexander Island (72°03′S 068°31′W) (Fig. 1). The nunatak has a north-east to south-west orientation and its summit is 467m above sea level. The development of cryptogamic communities is largely restricted to the shallow north-west slopes of the ridge at c. 400–430m a.s.l. Surface geomorphology is characterized by extensive development of patterned ground and other typical periglacial features (e.g. frost-sorted soil polygons, solifluction slopes), and exposed bedrock (Engelen et al. Reference Engelen, Convey, Hodgson, Worland and Ott2008). Climatic conditions are more typical of inland locations, and are considered intermediate between those of the maritime and continental Antarctic (Smith Reference Smith and Laws1984).
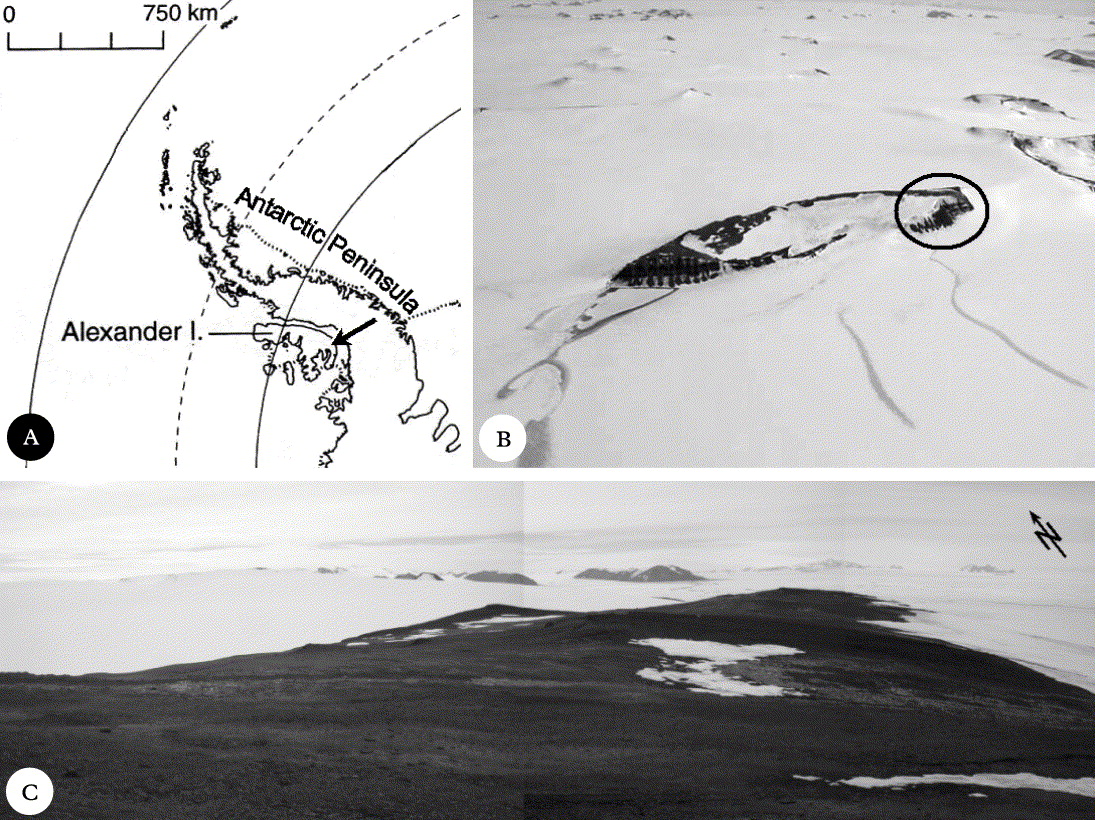
Fig. 1. The study area in Antarctica. A, Alexander Island, the arrow marks the location of Coal Nunatak; B, Coal Nunatak, the circle marks the location of the research area at the north-eastern end of the Nunatak; C, the study area on Coal Nunatak.
On Coal Nunatak Leparia borealis is the dominant crustose lichen species on both soil and rock. It is a bipolar species (Øvstedal & Smith Reference Øvstedal and Smith2001) predominantly colonizing other lichen species at Antarctic terrestrial sites and exceptionally bryophytes which serve as substrata. It does not develop a well-structured thallus, rather being characterized by a leprose thallus without a distinct medulla or marginal lobes and without the formation of apothecia (Crespo et al. Reference Crespo, Arguello, Lumbsch, Llimona and Tønsberg2006). Lepraria borealis forms soredia-like structures of 90–110μm, which agglomerate into larger consortia whose surface structure is characterized by a dense mass of loose hyphal ends. Colonies are generally 2–3mm in diameter, often coalescing into larger patches coloured white to pale grey (Øvstedal & Smith Reference Øvstedal and Smith2001). Reproduction occurs vegetatively by the soredia-like structures which form the thallus. Both bionts are dispersed together within a single propagule, and relichenization is presumably not required.
Sampling
Samples of lichen communities from three distinct microniches were collected. At each microniche L. borealis was associated with one different soil or rock inhabiting lichen: Usnea lambii, Tephromela disciformis and Ochrolechia frigida. Lepraria borealis and O. frigida colonize the soil surface and, more infrequently, bryophytes. Usnea lambii and T. disciformis colonize rock surfaces only. The photobionts both from the three L. borealis collections and from each of their associated lichens were examined for molecular differences (a total of six photobionts) (Table 1). For replicates a total of nine small samples of L. borealis were studied consisting of three samples removed from the thalli of each of the three associated lichen species. In addition nine samples consisting of three small thallus fragments of each of the three lichen species growing near L. borealis were selected for extraction. Photobiont identity was established through a molecular analysis of the internal transcribed spacer (ITS) region of the rDNA (Friedl & Rokitta Reference Friedl and Rokitta1997; Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001; Romeike et al. Reference Romeike, Friedl, Helms and Ott2002; Schaper & Ott Reference Schaper and Ott2003; A. Engelen, J. Buschbom, P. Convey & S. Ott, unpublished data). Identity was inferred from sequence similarity through BLAST searches. The samples of the lichen communities were temporarily stored dry under field conditions before being transported to the British Antarctic Survey Rothera Research Station (Adelaide Island), where they were frozen (−20°C) and subsequently sent to the laboratory in Düsseldorf. Taxonomic determination relied on standard morphological and anatomical features. For morphological and anatomical investigations on the hyphal connections between L. borealis and the three lichen species growing nearby, both light and scanning electron microscopy (LEO 1400, Cambridge, UK) have been used.
Table 1. NCBI accession numbers of the ITS sequences of the Trebouxia and Asterochloris photobionts of Lepraria borealis, Usnea lambii, Tephromela disciformis and Ochrolechia frigida.

DNA analysis
For photobiont identification a molecular approach was used. The nuclear internal transcribed spacer (ITS) region of the rDNA was analysed, including ITS1, ITS2 and the gene coding for the 5.8S ribosomal subunit. The method has been used routinely in molecular studies of green algal photobionts (Friedl & Rokitta Reference Friedl and Rokitta1997; Rambold et al. Reference Rambold, Friedl and Beck1998; Beck Reference Beck1999; Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001; Kroken & Taylor Reference Kroken and Taylor2000; Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Romeike et al. Reference Romeike, Friedl, Helms and Ott2002; Schaper & Ott Reference Schaper and Ott2003; Yahr et al. Reference Yahr, Vilgalys and DePriest2004, Reference Yahr, Vilgalys and DePriest2006).
In order to obtain photobiont DNA, clusters of photobiont cells were first removed from the lichen thalli to minimize contamination and the disruption of molecular procedures by secondary lichen metabolites such as phenolic substances. A total of nine extractions of L. borealis (three replicates from each sample) were made. In addition, three small samples of the algal layer of each associated lichen species were removed under a dissecting microscope and extracted.
DNA Extraction
The clusters of photobiont cells were fragmented using liquid nitrogen and quartz sand. For DNA extraction the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) was used. After extraction the isolated DNA was stored at −20°C.
PCR
For a 25 μl PCR reaction, 2·5 μl template, 9 μl sterilized water, 12·5 μl HotStartTaq™ Master Mix (Qiagen) and 0·5 μl of each primer were used. The green algae specific primer with 5′-3′orientation is Al 1700f (Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001). The reverse primer used, LR3, (http://www.biology.duke.edu/fungi/mycolab/primers.htm) is not specific for green algae (Friedl & Rokitta Reference Friedl and Rokitta1997). For the amplification of the photobiont ITS-region a thermocycler (Biometra, Goettingen, Germany) was used with the following PCR program: at 95°C the taq-polymerase was activated for one minute. The DNA was denatured for one minute at 94°C. The annealing temperature of the primers was set to 53°C for one minute. The elongation of the annealed primers by taq-polymerase was in effect for 1·5 min at 72°C. The denaturation, annealing and elongation steps were repeated 35 times, after which the final extension of partially elongated products took 10 minutes at a temperature of 72°C. After final extension the PCR product was cooled at 4°C. The amplified PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany).
DNA sequencing
DNA sequencing was carried out by GATC-Biotech (Konstanz, Germany) using an ABI 3730 XL Sequencer and BigDye 3.1. Non algal specific primers used for sequencing were 1800f (5′-3′orientation) (Friedl Reference Friedl1996) and ITS4 (3′-5′orientation) (White et al. Reference White, Burns, Lee, Taylor, Innis, Gelfand, Sninsky, White and Orlando1990).
The resulting ITS rDNA sequences were edited using the application ‘Bioedit for Windows’ (www.mbio.ncsu.edu/BioEdit/bioedit.html). NCBI-BLAST (www.ncbi.nlm.nih.gov) searches of GenBank records were performed to confirm that the amplified and sequenced DNA fragments originated from the photobiont and to infer the taxonomic classification of the closest hit.
Results and Discussion
The ITS sequences of three distinct photobionts associated with Lepraria borealis were identified at Coal Nunatak, representing two different genotypes of the genus Trebouxia (similarity 86·1%) and one of the genus Asterochloris. The ITS rDNA sequences of the photobionts found in L. borealis were 100% similar to those of photobionts found in the respective lichens that were growing near each of the samples obtained. When growing close to T. disciformis (Fig. 2A) or U. lambii (Fig. 2B & C), L. borealis contained the same Trebouxia jamesii genotypes as the neighbouring lichens in the same habitat (Table 1: FJ406572, FJ406573, FJ406574, FJ406575). Similarly, in association with Ochrolechia frigida (Fig. 2D) the same Asterochloris genotype (100% similar) was present in both lichens (Table 1: FJ406576, FJ406577). No evidence of multiple genotypes of algae was found. Contrary to the results presented here, Nelsen & Gargas (Reference Nelsen and Gargas2008) found all Lepraria individuals from a range of tropical and temperate localities associated with Asterochloris species as their photobiont.

Fig. 2. Lepraria borealis in close association with Tephromela disciformis, Usnea lambii and Ochrolechia frigida. A, L. borealis (lb) associated with T. disciformis (td) growing on a small rock (r); B, L. borealis (lb) associated with U. lambii (ul), thalli of L. borealis (arrow) are attached to the thallus of U. lambii; C, thalli of L. borealis (lb) are attached by outgrowing hyphae (arrow) to the thallus of U. lambii (ul); D, L. borealis (lb) associated with O. frigida (of), arrows mark the border between O. frigida and L. borealis. Scales: A = 2mm; B = 1mm; C = 100μm; D = 5mm.
Molecular biological studies have shown that the selectivity of different lichen mycobionts towards their respective photobiont exhibits a continuum of intensity, both at species and generic levels (Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001; Beck et al. Reference Beck, Kasalicky and Rambold2002; Romeike et al. Reference Romeike, Friedl, Helms and Ott2002). For example, mycobionts of the genus Physcia demonstrate a high selectivity towards their photobionts (Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001), while lower selectivity was observed for the mycobiont of two species of Umbilicaria (Romeike et al. Reference Romeike, Friedl, Helms and Ott2002). Beck et al. (Reference Beck, Kasalicky and Rambold2002) suggest that lichens that depend on relichenization for successful colonization may express a low selectivity toward their photobionts. Some lichens can even use different species of algae as photobionts during their life-cycle (Friedl Reference Friedl1987). The ability to form its characteristic thallus with three different photobiont species from two different genera indicates a lower level of selectivity of the symbionts of L. borealis. ‘Selectivity’ describes the degree to which symbionts interact preferentially with one another (Galun Reference Galun and Galun1988). High levels of mycobiont selectivity lead to a low diversity of suitable photobionts being present in a lichen genus, as is found in the family Cladoniaceae (Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001), the genera Physcia (Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001) and Letharia (Kroken & Taylor Reference Kroken and Taylor2000). In contrast, a lower level of selectivity and a wider diversity of photobionts reported in the endemic Antarctic species Umbilicaria antarctica (Romeike et al. Reference Romeike, Friedl, Helms and Ott2002) has been interpreted as a form of plasticity that can be advantageous under extreme environmental conditions. Low selectivity describes the association of a lichen-forming fungus with more common suitable algal species in the habitat. A change of environmental conditions may cause a replacement of the algal symbiont towards a different species or genotype appearing as a preference by the fungal partner (Piercey-Normore Reference Piercey-Normore2006).
The interaction between L. borealis and its various host lichen species differs from the interactions described for Fulgensia bracteata and Toninia sedifolia (cf. Ott et al. Reference Ott, Meier and Jahns1995). As F. bracteata overgrows the thallus of T. sedifolia, the germinating hyphae invade the thallus of the latter, ‘capturing’ the photobiont for incorporation into its own thallus. Molecular genetic analyses based on the ITS region confirm that both lichen species always share for 100% the identical photobiont (Schaper & Ott Reference Schaper and Ott2003). The result of the interaction is that the thallus of T. sedifolia degenerates. In contrast, the interaction between L. borealis and its ‘host’ lichen species can be described as very loose. In this case, a limited number of short hyphae are involved in contact between the soredia-like structures of L. borealis and the thallus of U. lambii (Fig. 2C) and the other two species. Although it is clear that contact is made with the thallus surface, it has not been confirmed whether the thallus itself is penetrated because of substantial difficulties in preparing material for light microscopy and SEM. Repeated attempts failed because of the loose contact between L. borealis and the respective lichen species.
Expression of the lower photobiont selectivity of L. borealis might be modulated through the mycobiont always being associated with the specific photobiont of the interacting or ‘host’ lichen species. However, it remains unclear when or how the exchange or ‘capture’ of the photobiont takes place between lichen species. As L. borealis does not rely on sexual reproduction mechanisms (in which the two bionts are dispersed separately), it has no requirement for relichenization. Rather, it reproduces using soredia-like thallus pieces, in which both bionts are dispersed together. The thallus structure of L. borealis is relatively poorly defined, being a loose conglomerate of fungal hyphae and green algae, which suggests that the symbiotic contact between bionts may be unspecialized.
The ability to switch the photosynthesising partners might allow the mycobiont of L. borealis to improve the success of colonization of new and changing environments with different microclimatic conditions. Symbiont-switching could permit a fine-tuning of the symbiosis to survive new selective pressures (Bronstein 1997; Nelsen & Gargas Reference Nelsen and Gargas2008). At an Antarctic terrestrial site such as Coal Nunatak the life strategy of algal switching seems to be essentially advantageous for successful distribution and establishment of L. borealis in microniches. The ability to switch symbiont partners might be especially beneficial for clonal organisms as algal-switching may compensate the lack of genetic recombination in asexual reproducing lichen species such as L. borealis co-dispersing by both symbionts (Nelsen & Gargas Reference Nelsen and Gargas2008).
In the context of the harsh environmental conditions of Coal Nunatak, the particular low degree of selectivity found in the mycobiont of L. borealis can be interpreted as highly advantageous for colonization and adaptation and therefore for competition with other lichen species in extreme habitats.
We thank the British Antarctic Survey for logistic support and for allowing access to the study site on Alexander Island, and also its staff at Rothera Research Station for their support. We especially thank the BAS field assistants, Neil Stevenson and Robin Jarvis, for their kind and invaluable technical support in the field. Thanks are due to Dag Øvstedal and Hannes Hertel for determination of the lichen species. This project was funded by a grant of the Deutsche Forschungsgemeinschaft (DFG) to SO (Ot96/10-1/2/3) and the Düsseldorf Entrepreneurs Foundation to AE, and also forms an output of the BAS BIOFLAME and SCAR EBA scientific programmes. The authors also thank two anonymous reviewers for their comments on an earlier version of the manuscript.