Introduction
The lichen genus Siphula Fr. s.l. was long considered a challenge for taxonomists, principally because it was never observed with ascomata. Thus without the benefit of characters such as apothecial anatomy, morphology and ontogeny, ascus structure and ascospore morphology, inferences regarding its systematic position were at best speculative. Authors, such as Hafellner (Reference Hafellner1988), regarded the genus as incertae sedis although Poelt (Reference Poelt1973) recognized the family Siphulaceae Reichenb., containing the genera Endocaena Cromb., Siphula and Thamnolia Ach. ex Schaer., and placed it close to the Cladoniaceae on account of its chemistry. The advent of molecular methods offered great insights into the relationships of Siphula and its relatives. Thamnolia and some species of Siphula were shown to be members of, or at least close to, the Icmadophilaceae (Stenroos & DePriest Reference Stenroos and DePriest1998; Platt & Spatafora Reference Platt and Spatafora2000; Stenroos et al. Reference Stenroos, Myllys, Thell and Hyvönen2002). Subsequently Grube & Kantvilas (Reference Grube and Kantvilas2006), studying a broader sample of taxa, found that Siphula was heterogeneous and comprised two unrelated groups with remarkable morphological convergence. The core of Siphula was confirmed to lie within the Icmadophilaceae but a complex of strictly austral species was found to be unrelated to Siphula and transferred to a new genus, Parasiphula Kantvilas & Grube within the Coccotremataceae. More recently, molecular work by Ludwig et al. (Reference Ludwig, Knight and Kantvilas2016) has suggested that a further subdivision of Siphula might be warranted with the erection of a separate genus for the S. decumbens Nyl. group, although these results are yet to be formally published. These authors (loc. cit.) also reported the discovery of ascomata in S. decumbens and its relative, S. fastigiata (Nyl.) Nyl., which confirmed their close relationship to the Icmadophilaceae.
Species of Siphula s.l. are frequently hosts for lichenicolous fungi and, historically, some early reports or observations of supposed fruiting bodies were based on fungal parasymbionts (e.g. Nylander Reference Nylander1888) or on fungus-induced galls. For example, Hue (Reference Hue1914) erected the genus Nylanderiella Hue for Siphula decumbens, but what he thought to be ascomata were the fruiting bodies of a lichenicolous fungus from the genus Cercidospora Körb. (R. Santesson, in litt.). Lichenicolous fungi, especially those that are commensalistic (Fleischhacker et al. Reference Fleischhacker, Grube, Kopun, Hafellner and Muggia2015), are known to have potentially very selective host relationships and the possibility that they might help clarify Siphula taxonomy was already alluded to by Kantvilas (Reference Kantvilas2002).
Even though the lichens ascribed to Siphula s.l. are widespread and diverse, and lichenicolous species have been observed on them for a long time, knowledge of the fungi inhabiting these lichens was surprisingly scant until recently. The first siphulicolous species, Plectocarpon gayanum Etayo, growing on an unidentified species of Siphula, was described only a decade ago (Etayo & Sancho Reference Etayo and Sancho2008). Subsequently three further species were described: Aabaarnia siphulicola Diederich (Diederich Reference Diederich2014) and Pyrenidium coccineum Aptroot (Aptroot Reference Aptroot2014), both on Siphula decumbens Nyl., and Sphaerellothecium siphulae Zhurb., together with an unidentified species of Epithamnolia Zhurb. (reported as Hainesia sp.), on S. ceratites (Wahlenb.) Fr. (Zhurbenko Reference Zhurbenko2015).
The origins of the present study lie in a preliminary unpublished survey by JM and GK of lichenicolous fungi occurring in Tasmania, a centre of speciation for Siphula-like lichens. The study was subsequently expanded to include collections of Siphula s.l. (Siphula and Parasiphula) from other regions, enabling a broader overview of the fungi occurring on these hosts. In the present paper, two new genera and seven new species of lichenicolous fungi growing on Siphula s.l. are described, new data on the geographical distribution and the host range of siphulicolous fungi are presented, and a key to all taxa studied is provided.
Material and Methods
The study is based on 135 specimens of fungi found on Siphula s.l. deposited in the following herbaria: BILAS (1 specimen), H (25), HO (49), O (8), TNS (10), TU (4) and UPS (38). Microscopy and microimaging were undertaken using light and stereo microscopes; for some anatomical features, Nomarski interference contrast was employed. Microscopic characters were studied using hand-cut sections or squash preparations mounted in tap water, a 10% aqueous solution of potassium hydroxide (KOH; K), Lugol’s Iodine solution, directly (I) and after KOH pretreatment (K/I), Brilliant Cresyl Blue (BCr), a 50% aqueous solution of nitric acid (N), phloxine and Congo red. Measurements were made in water. When>10 measurements are summarized in the text, they are presented in the following format: (minimum–) (– SD)−(
![]() $$\bar{x}$$
+ SD)(–maximum) (n), where
$$\bar{x}$$
+ SD)(–maximum) (n), where
![]() $$\bar{x}$$
is the arithmetic mean, SD the standard deviation and n the number of measurements. Where ≤10 measurements are available, only extreme values are given. The length/width ratios of ascospores and conidia are indicated as ‘l/w’ and presented in the same way.
$$\bar{x}$$
is the arithmetic mean, SD the standard deviation and n the number of measurements. Where ≤10 measurements are available, only extreme values are given. The length/width ratios of ascospores and conidia are indicated as ‘l/w’ and presented in the same way.
The Species
Aabaarnia siphulicola Diederich
Bull. Soc. Nat. Luxemb. 115: 144 (2014); type: New Zealand, Auckland Islands, Rose Island, Observation Point, on Siphula decumbens, 1963, James 992/1 (UPS—holotype).
Notes. This fungus is characterized by pale, immersed, gall-inducing, cleistohymenial, hyaline ascomata, 100–200 μm diam., a K/I+ blue hymenium, subcylindrical, 4–6-spored, K/I− asci with a massive apical cap, and oval to shortly cylindrical, 3-septate ascospores, 23–29×7–8·5 μm (Diederich Reference Diederich2014). Our specimens correspond well with the protologue, although we observed a wider variation of gall forms. Galls on S. decumbens and S. dissoluta were identical to those described by Diederich (Reference Diederich2014); that is, laminal, strongly basally constricted and often slightly more pinkish than the host thallus. However, galls on S. fastigiata were marginal, causing only slight thickening and no discoloration of the host thallus.
Ecology and distribution. Aabaarnia siphulicola was first described from Siphula decumbens (Diederich Reference Diederich2014) and is here documented as occurring also on S. dissoluta and S. fastigiata. All host taxa have wide ecological amplitudes, from growing as epiphytes in wet forests to being terricolous in treeless vegetation. A wide range of specimens representing all habitats was examined but A. siphulicola was found only on terricolous host lichens, mainly from alpine areas. Similar trends have been observed by other workers. For example, Ihlen (Reference Ihlen1998) showed that different lichenicolous species may inhabit the same host (Baeomyces rufus (Huds.) Rebent.) at different altitudes. Furthermore, Muggia & Grube (Reference Muggia and Grube2018) showed that the lineages of lichenicolous fungi differ considerably between sheltered, temperate, boreal environments and exposed arctic and high-altitude environments. On Siphula fastigiata, A. siphulicola was found together with Sphaerellothecium sp., whereas on Siphula decumbens it was accompanied by Cercidospora santessonii and Plectocarpon gayanum.
Specimens examined. Australia: Tasmania: Mount Anne, 42°55'57·3''S, 146°26'25''E, 1090 m, on Siphula decumbens, 2016, Kantvilas 71/16 (HO 583062); Mount King William I, 42°14'S, 146°08'E, 1300 m, on S. fastigiata, 1984, Kantvilas 108/84 (HO 319763); Lawson Range, 42°58'S, 145°41'E, 480 m, on S. fastigiata, 1986, Moscal 11954 (HO 114215); Mount Norold, 41°15'S, 146°15'E, 950 m, on S. decumbens, 1994, Kantvilas 31/94A (HO 585488); tarn above Lake Oberon, 43°09'S, 146°16'E, 880 m, on S. decumbens, 2002, Felton (HO 520187); Wylds Craig, 42°28'S, 146°23'E, 1250 m, on S. fastigiata, 1998, Kantvilas 272/98 (HO 0232).—New Zealand: South Island: Arthur’s Pass, 1006 m, on S. fastigiata, 1962, James 1902 (UPS); ibid., 975–1036 m, on S. dissoluta, 1962, James 1907a (UPS); Rock and Pillars, Taieri County, Museum Rock, 1260 m, on S. dissoluta, 1972, Imshaug 56044 (HO 309723). Auckland Island: saddle between Meggs Hill and Mount Eden, on S. decumbens, 1963, James 865/1 (UPS); Port Ross, above Erebus Cove, on S. decumbens, 1927, G. E. Du Rietz 2283:1 (UPS L-860156a, UPS L-860157); ibid., on S. dissoluta, 1927, G. E. Du Rietz 2283:1 (UPS L-860150a).
Amylogalla fava Suija, Motiej. & Kantvilas gen. et sp. nov.
MycoBank No.: MB 827417 (genus) and MB 827434 (species)
Gall-inducing lichenicolous fungus. Ascomata cleistohymenial, immersed in the host thallus, yellowish to orange. Vegetative structures covered with a granulose, I+ blue, K/I+ violet pigment. Asci unitunicate, non-amyloid, with eight, aseptate, hyaline ascospores.
Type: Australia, Tasmania, South West National Park, Mount Scorpio, 43°10'S, 146°21'E, 1020 m, alpine heathland, on thallus of Parasiphula georginae, growing on soil in rock crevices, 16 December 1984, G. Kantvilas 720/84 (HO 132436—holotype).
(Fig. 1)
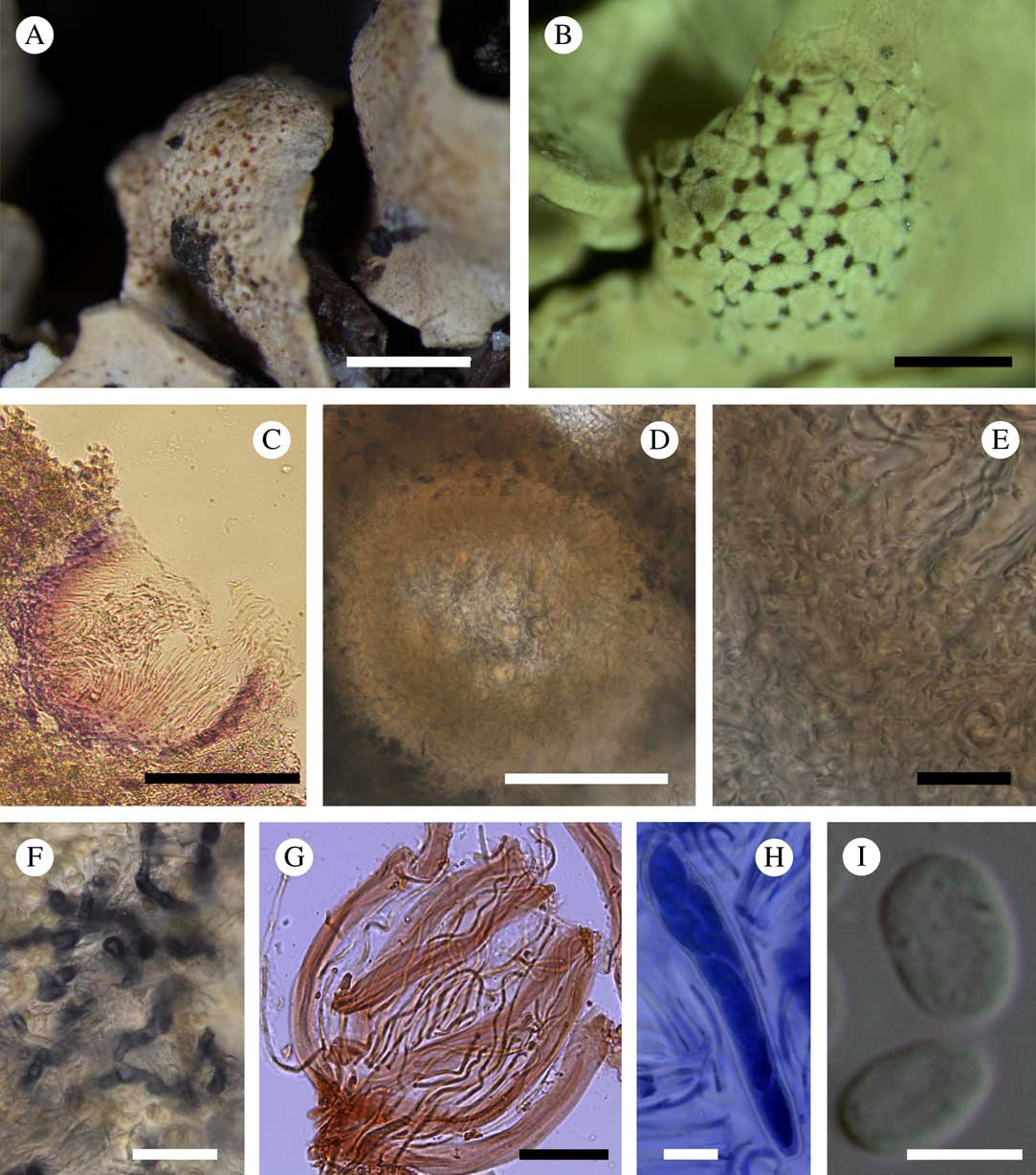
Fig. 1 Amylogalla fava. A, host thallus with honeycomb traces of former infection of the fungus (Jarman, HO 113707); B, galls with sunken ascomata (holotype); C, vertical section through an ascoma showing I+ violet reaction of the exciple (Kantvilas, HO 520227); D, vertical section through an immature ascoma (mounted in water); E, lower part of an ascoma showing the structure of the ascomatal wall (in water); F, vegetative hyphae after treatment with Lugol’s solution; G, asci and paraphyses (in Congo red); H, ascus with ascospores (in BCr); I, ascospores (D–I, Kantvilas, HO 585552). Scales: A & B=1 mm; C=100 μm; D=50 μm; E, F, H & I=10 μm; G=20 μm. In colour online.
Lichenicolous ascomycete inducing galls on the host thallus; galls mostly distinct, becoming rounded, not constricted at the base, with numerous immersed ascomata, sometimes indistinct and simply forming breaks through the cortex of the host thallus and leaving a characteristic honeycomb pattern when the fungus dies. Vegetative hyphae extending between the mycobiont hyphae and photobiont cells of the host, reaching to the medullary layer of the lichen, colourless, irregularly branched, c. 3 μm wide, I+ blue, K/I+ violet, walls covered with minute I+ dark blue granules.
Ascomata cleistohymenial, hemioangiocarpic (closed when young and developing within the medullary layer of the lichen, hymenial layer opening at maturity when emerging through breaks in the lichen cortex); mature ascomata aggregated, immersed, 90–185 μm diam. (n=7), concave, irregular in shape when viewed from above, globose to subglobose in section, orange to red to dark red. Exciple yellowish to orange in the upper part, 10–30 μm wide, K−, I+ blue, K/I+ violet, composed of rectangular cells (textura angularis) 5–8×3–6 μm, without excipular hairs; layer below the exciple composed of interwoven hyphae (textura intricata), c. 30 μm wide, K−, I+ blue, K/I+ violet. Hymenium 90–110 μm thick, orange in the upper part, colourless in the lower part; hymenial gel I−, K/I−; subhymenium colourless, composed of interwoven hyphae (textura intricata). Paraphyses mostly simple, dichotomously branched only at the base, separating easily in squash preparations, distantly septate, 1·5–2·0 μm wide, not or slightly swollen to 2·0–3·0 μm in upper part and covered with an orange gel (some gel-covered paraphyses up to 3 μm wide); gel patchily I+ blue, K/I−; contents of paraphyses Congo red+ reddish, BCr+ dark blue. Asci unitunicate, inoperculate, clavate to subcylindrical, 65–81×10–16 μm (n=7), 8-spored, thin-walled, non-stipitate, without apical apparatus, I−; croziers not observed. Ascospores (11·0–)11·7–14·3(–15·0)×( 5·0–)5·7–7·3(–7·5) μm, l/w=1·7–2·4 (n=16), aseptate, hyaline, ellipsoid, with rounded apices, irregularly biseriate in the asci; contents BCr+ blue; wall smooth, <1 μm wide, BCr−. Asexual morph not observed.
Etymology. The generic name refers to the unique amyloid reaction of the fungal structures and to the induction of galls, whereas the specific epithet ‘fava’ alludes to the characteristic ‘honeycomb’ pattern of the gall surface.
Notes. In the absence of molecular data, the relationships of this monotypic genus remain unclear. At first glance, the new fungus is reminiscent of Thamnogalla crombiei (Mudd) D. Hawksw. (Cordieritideaceae, Helotiales) in having ascomata immersed in galls, aseptate ascospores and non-amyloid asci (Hoffmann & Hafellner Reference Hoffmann and Hafellner2000). Aabaarnia siphulicola is likewise characterized by cleistohymenial ascomata, non-amyloid asci and the formation of galls but differs in having 3-septate ascospores (Diederich Reference Diederich2014). The most conspicuous difference between these two species and Amylogalla fava is the granular pigment on vegetative structures of Amylogalla which reacts persistently blue in I and violet in K/I. Such strong and persistent hemiamyloidy is quite uncommon in ascomycetes. A similar reaction was recorded in a few species of Patellariales (Kutorga & Hawksworth Reference Kutorga and Hawksworth1997), whereas in Trypetheliaceae and Graphidaceae the hymenial gel or ascospores may sometimes react I+ violet to purple (Hawksworth Reference Hawksworth1985; Staiger Reference Staiger2002). In some species of lichenized genera, for example Bellemerea Hafellner & Cl. Roux, Immersaria Rambold & Pietschm. and Lecidea Ach., the hyphae of the mycobiont are also I+ violet (Calatayud & Rambold Reference Calatayud and Rambold1998; Purvis & Gilbert Reference Purvis and Gilbert2009; Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010; Knudsen & Kocourková Reference Knudsen and Kocourková2014). On the other hand, the hyphae surrounding the exciple in Halospora deminuta (Arnold) Tomas. & Cif. are reported to occasionally react I+ violet but this character is not constant (Orange Reference Orange2009). Likewise Polycoccum stictaria (Linds.) D. J. Galloway is reported to have asci that turn I+ deep violet (Galloway Reference Galloway2004). In Helotiales, a faint hemiamyloid reaction (I+ rose, K/I+ pale violet) was reported in the ectal exciple of several species of Lachnellula P. Karst., Proliferodiscus J. H. Haines & Dumont and Perrotia Boud. by Baral (Reference Baral1987, Reference Baral2008), who noted that this feature is important taxonomically and illustrates the relationships between these genera. The structure and composition of fungal polysaccharides, responsible for different reactions with iodine solutions, are acknowledged as important characters in fungal taxonomy (Ruiz-Herrera & Ortiz-Castellanos Reference Ruiz-Herrera and Ortiz-Castellanos2010), albeit mostly above the rank of genus.
Ecology and distribution. This species is known only from Tasmania where it grows on the thalli of Parasiphula complanata, P. elixii, P. fragilis and P. georginae. Although extensive herbarium collections were available for study, it was not found on other species of Parasiphula (P. comata, P. foliacea, P. jamesii) even though these occur sympatrically with the infected taxa, often in close proximity. Species of Parasiphula are chiefly alpine and occur on peaty soil in a variety of microhabitats, ranging from exposed situations in rock crevices, on the ground in gaps in heathland, or seasonally submerged in puddles and around the fringes of tarns. Host thalli infected with A. fava occurred within the entire range of such habitats. Microscopic examination revealed no obvious damage to either the mycobiont or the photobiont; thallus colour remains unchanged at the locus of infection, although the fungus usually induces galls on the host thallus.
On Parasiphula complanata, A. fava was found associated with Endococcus hafellnerianus and an unindentified species of Sphaerellothecium, whereas on Parasiphula fragilis it was associated with Pyrenidium macrosporum and an undetermined agent inducing galls on the host thallus.
Additional specimens examined. Australia: Tasmania: Mount Algonkian, 42°24'S, 146°03'E, 1010 m, on Parasiphula fragilis, 1990, Kantvilas 79/90 (HO 122448); Mount Bobs, 43°18'S, 146°36'E, 1080 m, on P. fragilis, 1987, Jarman (HO 113707); Mount Campbell, NE of Lake Dove, 41°39'S, 145°59'E, on P. fragilis, 1972, Bratt 72/1224 (HO 45778); ibid., on P. complanata, 1972, Bratt 72/1223 (HO 45777); Clear Hill, 42°41'S, 146°16'E, 1190 m, on P. elixii, 1996, Kantvilas 30/96 (HO 316441); Crest Range, 43°17'31''S, 146°30'26''E, 960 m, on P. fragilis, 2016, Kantvilas 167/16A (HO 585552); Dove Lake, 41°39'S, 145°58'E, 950 m, on P. fragilis, 1984, Kantvilas & James 320/84 (HO 124471); Mount Ironstone, 41°43'S, 146°28'E, 1440 m, on P. complanata, 2005, Kantvilas 327/05 (HO 534844); Travellers Rest Lake, 42°03'S, 146°14'E, 950 m, on P. complanata, 2003, Kantvilas 2/03 (HO 520227); Mount Tyndall, 41°56'S, 145°34'E, 1060 m, on P. georginae, 1989, Kantvilas 253/89 (HO 129677), 254/89 (HO-544736).
Cercidospora santessonii Motiej., Zhurb., Suija & Kantvilas sp. nov.
MycoBank No.: MB 827416
Lichenicolous fungus similar to Cercidospora epipolytropa (Mudd) Arnold but distinguished mainly by host selection (Siphula s.s. instead of Lecanora), richly branched and anastomosed pseudoparaphyses, 1·5–2 µm thick, and consistently 8-spored asci, with the ascospores (0–)1-septate, 14·4–20·8×4·4–7·5 µm.
Type: Australia, Tasmania, Walls of Jerusalem National Park, c. 0·5 km NE of Twin Spires, 41°53'S, 146°07'E, 1250 m, alpine heathland, on thallus of Siphula fastigiata, 20 March 1999, G. Kantvilas 73/99 (HO 442924—holotype).
(Fig. 2)
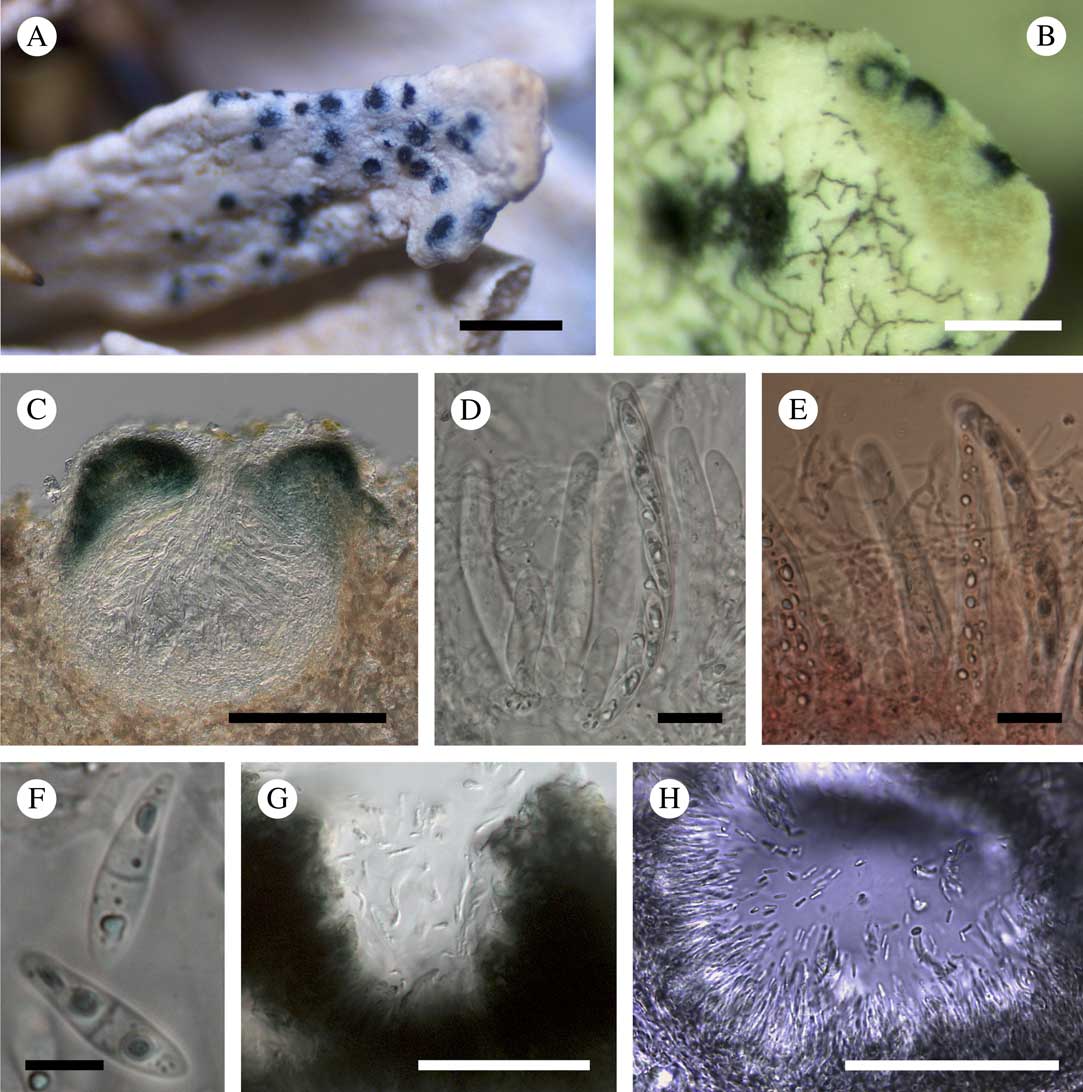
Fig. 2 Cercidospora santessonii. A, ascomata on the host thallus (holotype); B, ascomata of C. santessonii intermixed with hyphae of Sphaerellothecium sp. (Felton, HO 520187); C, vertical section through an ascoma (in water) (Malcolm & Vězda, H); D, asci with ascospores and pseudoparaphyses (in water); E, asci with ascospores and pseudoparaphyses (in Congo red); F, ascospores (in water; D–F, Felton, HO 520187); G, pycnidia with conidia (Du Rietz & Du Rietz, UPS L-860067c); H, pycnidia, conidiogenous cells and conidia (in water) (Felton, HO 520187). Scales: A=1 mm; B, G & H=500 μm; C=100 μm; D & E=20 μm; F=5 μm. In colour online.
Vegetative hyphae not observed. Ascomata perithecioid, subglobose in section, 120–270 μm diam. (n=9), immersed in the host thallus, with only the ostiolar region breaking through the surface of the host; ostiole indistinct. Exciple 30–40 μm thick, in the upper half bluish green around the ostiole to ± greenish brown at 1/3 of the peridium, or blue-green inside and olive-grey outside, in the lower half hyaline, composed of cells that are rectangular to somewhat elongate at the base, green coloration enhanced in K or K−. Pseudoparaphyses abundant, septate, richly branched and anastomosing, 1·5–2·0 μm wide, not swollen at the apices. Asci 8-spored, clavate to subcylindrical, (54–)56–77(–85)×(10–)10·5–14·5(–15)μm (n=13), I−, K/I−, with a short, indistinct ocular chamber and a short stalk. Ascospores (0–)1-septate, (14·4–)15·8–19·3(–20·8)×(4·4–)4·7–6·4(–7·5) μm, l/w=(2·3–)2·7–3·8(–4·3) (n=43), hyaline, ovate-ellipsoid to slightly fusiform, with a median septum and cells somewhat heteropolar, the lower cell slightly longer and narrower than the upper one, occasionally with a halo 0·5–1 μm thick, biseriate to diagonally uniseriate in the ascus.
Asexual morph pycnidia, 80–100 μm diam., intermixed with ascomata, immersed in the host thallus, globose, with the upper part of the pycnidial wall bluish green, and the lower part colourless or slightly greyish, consisting of 3–4 rows of rectangular cells c. 10 μm wide. Conidiophores absent. Conidiogenous cells lining the pycnidial wall, colourless, elongate, 15×1 μm. Conidia borne apically, 3·0–6·0×1·0 μm (n=10), bacilliform, aseptate, hyaline.
Etymology. This species is named after the eminent Swedish lichenologist, the late Rolf Santesson, in acknowledgement of his enormous contribution to the study of lichenicolous fungi, including those on Siphula.
Nomenclatural note. The specimen UPS L-860064 was previously examined by Rolf Santesson, whose handwritten note on the label indicates that he intended to propose a new combination “Cercidospora medioxima” based on Nylanderiella medioxima (Nyl.) Hue (Hue Reference Hue1914), which itself is based on Siphula medioxima Nyl. This new combination was never published and the name Nylanderiella medioxima (Nyl.) Hue has since been synonymized with Siphula decumbens (Kantvilas Reference Kantvilas1998).
Notes. According to Navarro-Rosinés et al. (Reference Navarro-Rosinés, Calatayud and Hafellner2009), the colour of the peridial pigment, the number of ascospores per ascus, ascospore shape and septation, the dimensions of all parts of the ascomata, and host selection are the main characters for distinguishing species of Cercidospora Körb. Of c. 30 Cercidospora species (Lawrey & Diederich Reference Lawrey and Diederich2018), seven are superficially similar to C. santessonii, with an exciple that is bluish green above and hyaline below, exclusively or mainly 1-septate ascospores, and persistently or predominantly 8-spored asci. The salient differences between these taxa are summarized in Table 1. The most similar species is C. epipolytropa s.l., which differs mainly by host selection (epilithic Lecanora vs. Siphula) and, according to our observations and published data, by its simple to sparsely branched, relatively narrower pseudoparaphyses, (1–1·5(–2) μm versus 1·5–2 μm in C. santessonii), and asci that can be less than 8-spored. Furthermore, the ascospores in C. epipolytropa are 1(–2)-septate, whereas in C. santessonii they are (0–)1-septate. There are also slight size differences between the two species: in C. santessonii, the ascomata, asci and conidia are larger, although these distinctions are subtle and require further examination. Branching of the pseudoparaphyses is not generally well described in Cercidospora species (see Table 1 and the references cited in the legend). Navarro-Rosinés et al. (Reference Navarro-Rosinés, Calatayud and Hafellner2004, Reference Navarro-Rosinés, Calatayud and Hafellner2009) state that a characteristic of Cercidospora is that they are simple or with some anastomoses. However, in at least one other species, C. alpina Ihlen & Wedin, the pseudoparaphyses are also branched and anastomosed (Ihlen & Wedin Reference Ihlen and Wedin2007).
Table 1 Comparison of Cercidospora species with 1-septate ascospores, 8-spored asci and an exciple bluish green above and hyaline below (following Navarro-Rosinés et al. Reference Navarro-Rosinés, Calatayud and Hafellner2004, Reference Navarro-Rosinés, Calatayud and Hafellner2009; Zhurbenko Reference Zhurbenko2012; Calatayud et al. Reference Calatayud, Navarro-Rosinés and Hafellner2013 and present paper)

Ecology and distribution. This species is known from the thalli of Siphula decumbens and S. fastigiata in alpine habitats, where these host lichens are common on peaty soil or on soil in crevices in large rock outcrops. No visible damage to the host thallus was observed, except for in Malcolm & Vězda (H) where it had turned grey under infection. The species is recorded from Tasmania and New Zealand. A further record from Venezuela on S. pteruloides is uncertain as no asci or ascospores were observed in that material.
In Tasmanian specimens, Cercidospora santessonii was associated with Sphaerellothecium sp. (on S. decumbens and S. fastigiata) and with Aabaarnia siphulicola and Plectocarpon gayanum (on S. decumbens).
Additional specimens examined. Australia: Tasmania: Mount Bobs, 43°18'S, 146°36'E, 1080 m, on Siphula fastigiata, 1987, Jarman (HO 113705); tarn above Lake Oberon, 43°09'S, 146°16'E, 880 m, on S. decumbens, 2002, Felton (HO 520187).—New Zealand: South Island: Arthur’s Pass, on S. fastigiata, 1927, G. E. & G. Du Rietz 1508:2 (UPS L-860067c, UPS L-860064); Mount Rochfort, 750 m, on S. decumbens, 1997, Malcom & Vězda (H). —Venezuela: Territorio Federal Amazonas: Atabapo Departamento, Cerro Marahuaca, 3°35’N, 65°20’W, 2480–2580 m, on S. pteruloides, 1982, Guariglia et al. (identification uncertain; H).
Comparative material of Cercidospora epipolytopa s.l. examined (all on Lecanora polytropa (Ehrh. ex Hoffm.) Rabenh.). Estonia: Hiiu County: Hõralaid islet, 58°54'N, 23°04'E, 2002, Jüriado & Suija (TU 23731b); Salinõmme, 58°50'N, 22°57'E, Jüriado & Suija (TU 25641a); Kadakalaid islet, 58°59'N, 23°00'E, 2004, Jüriado & Suija (TU 27792b); Hiiumaa Islets Nature Reserve, Vareslaid islet 58°59'N, 23°00'E, 2001, Jüriado (TU 27980a). Harju County: Lahemaa National Park, Mohni Island, 59°40'N, 25°47'E, 2008, Suija (TU 45439d); ibid., 59°40'N, 25°48'E, 2008, Jüriado (TU 45619i).—Greenland: Qassiarsiuk (Brattahlil), 61°06–10'N, 45°30–35'W, 100 m, 2005, Motiejūnaitė (BILAS 7567).—Lithuania: Panevėžys County: Biržai District, Juodžioniai, 56°14'N, 24°53'E, 60 m, 2006, Stončius (BILAS 9568). Vilnius County: Trakai District, Aukštadvaris, 54°36'N, 24°30'E, 150 m, 1997, Motiejūnaitė (BILAS 10708).—Russia: Karelia Keretina: Keret’ Archipelago, Keret’ Village, 66°16'N, 33°34'E, 5 m, 2000, Himelbrant (LE 210292). Putorana Plateau: Talnakh Town, 69°28'N, 88°30'E, 1985, Zhurbenko (LE 206994). Murmansk Region: Tumannyi, 69°01'N, 35°48'E, 100 m, 1997, Zhurbenko (LE 261644). Polar Ural: Mt. Rai-Iz, 66°57'N, 65°37'E, 150 m, 1986, Zhurbenko (LE 261554). Yakutiya: Laptevykh Sea coast, E part of Kunga Range, 71°16'N, 129°22'E, 240 m, 2000, Kunitskii (LE 260154). Chukotka Autonomous Okrug: Wrangel Island, Somnitel’naya River, 70°58'N, 179°35'W, 1985, Yurtsev (LE 261764:a); Bezymyannoe Lake, 66°39'N, 176°40'E, 1979, Makarova (LE 261525); Lorino, 65°29'N, 171°43'W, 1972, Makarova (LE 261534); Sireniki, 64°24'N, 173°54'W, 1983, Makarova (LE 261784).
Echinothecium sp.
Vegetative hyphae forming a conspicuous, dark, superficial reticulum. Ascomata perithecioid, superficial, 20–60 µm diam. (n=10), covered by protruding septate hyphae 2–5 μm thick and up to 50 μm long. Asci 35×13–15 μm (n=4), 8-spored. Ascospores hyaline to occasionally pale brown, soleiform, with a wider upper cell, (9·5–)9·8–11·0(–11·7)×(3·7–)3·9–4·7(–5·2)μm, l/w=(2·0–)2·2–2·6(–2·7) (n=18), 1-septate, smooth-walled, non-halonate. All specimens were collected at high elevations in Venezuela.
Notes. This fungus closely resembles Echinothecium reticulatum Zopf, a species reported from various genera of the Parmeliaceae as well as from Physcia (Halici & Aksoy Reference Halici and Aksoy2009; Zhurbenko et al. Reference Zhurbenko, Hermansson and Pystina2012; Brackel Reference Brackel2014). However, in a strict sense, E. reticulatum is confined to species of Parmelia s.s. and is characterized by somewhat shorter ascospores, 8–9·5×3·5–4·5 μm (Zopf Reference Zopf1898) in comparison to the material examined on Siphula. The second accepted species of this genus, Echinothecium aerophilum Alstrup & M. S. Cole, grows on species of Alectoria and is very distinct in its loosely interwoven vegetative hyphae with few connections to the host thallus, 4-spored asci and much larger ascospores, 27–37×7–9 μm (Alstrup & Cole Reference Alstrup and Cole1998). As the material available is rather limited and the taxonomic status of Echinothecium reticulatum s.l. requires further revision, this fungus is identified only to genus.
Specimens examined. Venezuela: Bolivar State: Roscio Municipality, top of Tramen-tepui, NW of Macizo del Ilu-(Uru-) tepui, 5°27'N, 61°01'W, 2650 m, on Siphula pteruloides, 1985, Huber (H). Tachira State: around Quebrada de Pata de Judio, near to La Linea, upper part of Páramo de Tamá, the Oirá basin, 3000 m, on Siphula sp., 1983, Lopez-Figueiras 30286 (H). Territorio Federal Amazonas: Atabapo Departamento, Marahuaca hill, 3°35'N, 65°20'W, 2480–2580 m, on S. pteruloides, 1982, Guariglia et al. (H).
Endococcus hafellnerianus Motiej., Suija & Kantvilas sp. nov.
MycoBank No.: MB 827418
Lichenicolous fungus characterized by ascomata 100–130 μm diam., predominantly 4-spored asci and soleiform ascospores, 15–24×5–10 μm.
Type: Australia, Tasmania, Mount Norold, 43°15'S, 146°15'E, 950 m, alpine heathland-sedgeland, on thallus of Parasiphula georginae growing on soil, 24 February 1994, G. Kantvilas 31/94 (HO 319643—holotype).
(Fig. 3)
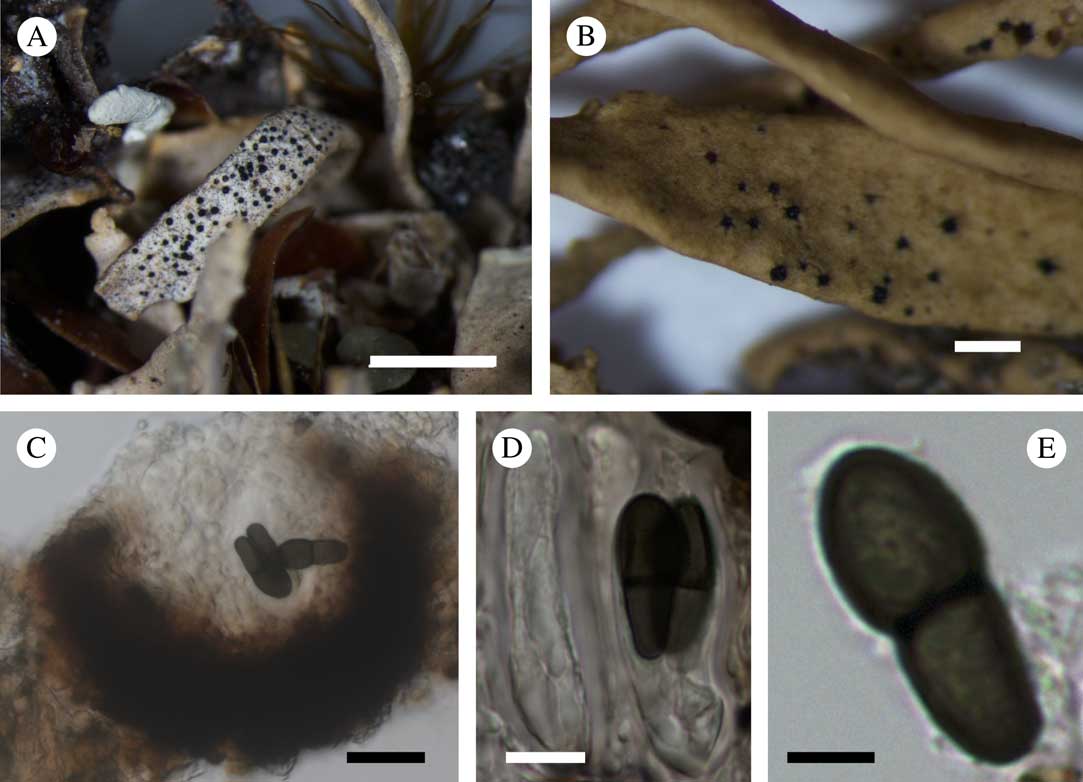
Fig. 3 Endococcus hafellnerianus. A & B, ascomata on the host thallus (A, holotype; B, Bratt, HO 45777); C, section through an ascoma; D, asci with ascospores; E, ascospore (in water, Kantvilas HO 122448). Scales: A=5 mm; B=1 mm; C=20 μm; D=10 μm; E=5 μm. In colour online.
Vegetative hyphae not observed. Ascomata pseudothecia, abundant, subglobose in section, black, 100–130 μm diam. (n=5), immersed in the host thallus, breaking through the thallus cracks with only the ostiolar region clearly visible. Ascomatal wall pseudoparenchymatous, composed of 3–4 layers of cells (textura intricata) 5–10 μm wide, cell walls thickened, dark brown, K−. Hamathecium of short, simple periphyses lining the ostiole; paraphysoids absent; interascal gel I+ orange. Asci (42·0–)42·0–49·6(–52·0)×(14·5–)14·7–20·9(–22·0) μm (n=11), I−, predominantly 4-spored, occasionally 3- or 6-spored, 8-spored when immature, saccate to clavate, shortly stalked or non-stipitate, with a small, rather wide, flat, apical extension. Ascospores 1-septate, (15·0–)17·2–21·8(–24·0)×(5·0–)6·6–8·9(–10·0) μm, l/w=1·9–3·6 (n=28), soleiform, the upper cell usually larger than the lower one, not or only slightly constricted at the septum, dark brown and smooth-walled when mature, hyaline to greyish and minutely verruculose when young, with a halo<1 μm thick. Asexual morph not observed.
Etymology. This species is named after the prominent Austrian lichenologist, Josef Hafellner, in acknowledgement of his enormous contribution to the study of lichenicolous fungi.
Notes. Of c. 40 species of Endococcus Nyl. (Lawrey & Diederich Reference Lawrey and Diederich2018), only five, including “Microthelia” calcaricola Mudd (Mudd Reference Mudd1861), which Hawksworth (Reference Hawksworth1979) with some doubts synonymized with Endococcus rugulosus Nyl., have ascospores exceeding 20 μm in length (Table 2). The most similar documented species to Endococcus hafellnerianus is an undescribed Endococcus sp. (Kocourková Reference Kocourková2000), which has larger ascomata, persistently 8-spored asci and a different, taxonomically unrelated host (Lecidella carpathica Körb.).
Table 2 Comparison of Endococcus species with ascospores exceeding 20 μm in length (following Mudd Reference Mudd1861; Keissler Reference Keissler1930; David & Etayo Reference David and Etayo1995; Kocourková Reference Kocourková2000; Kainz & Triebel Reference Kainz and Triebel2004; Zhurbenko & Brackel Reference Zhurbenko and Brackel2013; Zhurbenko et al. Reference Zhurbenko, Chesnokov and Konoreva2016 and present paper)

Ecology and distribution. This species is known only from Tasmania where it occurs on the thalli of Parasiphula complanata, P. fragilis and P. georginae, causing local discoloration of the host. These taxa all occur on boggy peaty soil in alpine, treeless vegetation. On Parasiphula complanata, the new species was associated with Amylogalla fava.
Additional specimens examined. Australia: Tasmania: Mount Campbell, NE of Lake Dove, 41°39'S, 145°59'E, on Parasiphula complanata, 1972, Bratt 72/1223 (HO 45777); Mount La Perouse, 43°30'S, 146°44'E, 1150 m, on P. fragilis, 1986, Kantvilas 184/86 (HO 585551).
Epigloea soleiformis Döbbeler
Beihefte zur Nova Hedwigia 79: 229 (1984); type: Österreich, Salzburg, Schladminger Tauern, zwischen Hundsfeldsee und Hundskopf, 1800–2000 m, auf veralgten Moosen, 1981, Döbbeler (M Dö 4720—holotype).
Notes. This non-lichenized fungus is typically associated with algal films over humus, rotten wood, rock and occasionally lichens, mostly species of Cladonia, Peltigera, Placynthiella, Stereocaulon or Trapeliopsis (Döbbeler Reference Döbbeler1984; Zhurbenko Reference Zhurbenko2010; Kukwa et al. Reference Kukwa, Kowalewska, Śliwa, Czarnota, Czyżewska, Flakus, Kubiak, Wilk, Dimos-Zych and Kolanko2012; Czarnota & Hernik Reference Czarnota and Hernik2013). The species is characterized by dull blackish green or grey perithecioid ascomata, 0·07–0·15 mm diam., 8-spored asci and 1-septate ascospores, 8·5–14×3·5–5 μm, without apical appendages (Döbbeler Reference Döbbeler1984). Our specimen concurs with the protologue. This is its first record from Siphula, as well as the first from South America.
Specimen examined. Chile: Magallanes Province: Puerto Bueno, 51°00'S, 74°12'W, over Siphula dissoluta, 1969, Imshaug 44580 & Ohlsson (H).
Neolamya sp.
(Fig. 4)
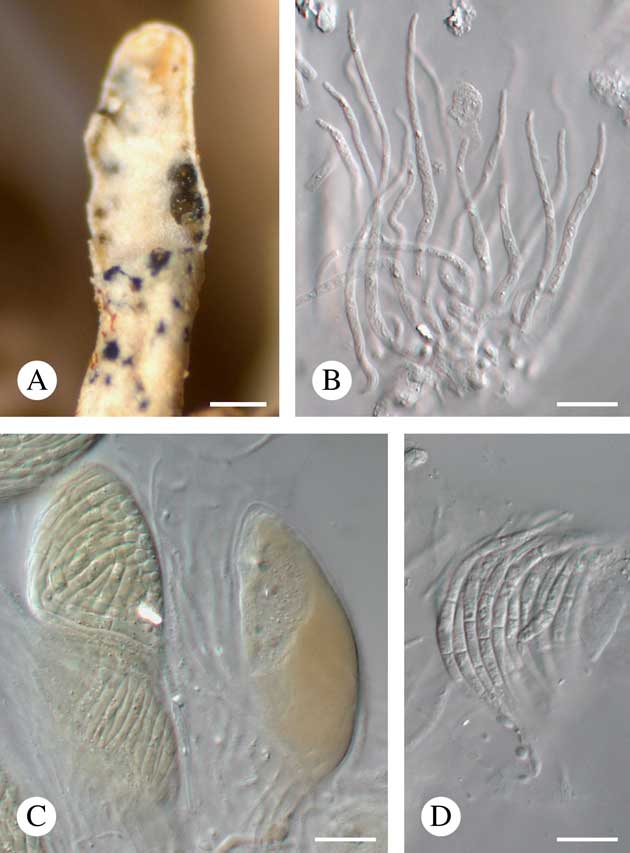
Fig. 4 Neolamya sp. (Ohmura 5255). A, ascomata on partly eroded wet host lobe tip; B, paraphyses (in K); C, asci (in K/I); D, ascospores (in water). Scales: A=200 μm; B–D=10 μm. In colour online.
Vegetative hyphae not observed. Ascomata perithecioid, aggregated, immersed to slightly protruding, exposed parts opaque, black and matt when dry, translucent, olive and somewhat glossy when wet, subglobose to broadly ellipsoid in section, 100–140 μm diam. (n=6), lacking a clypeus, with an irregular pore 20–30 μm long. Exciple patchily pale to dark olive-brown, darker above. Hymenial gel K/I−. Periphyses not observed. Paraphyses well developed, unbranched, distantly septate, c. 2·5 μm thick at the base, gradually tapering to 1–1·5 µm towards the apex, apically not enlarged. Asci unitunicate, K/I−, 47–67×17–21 μm (n=7),±ellipsoid, without a distinct ocular chamber, containing at least 20 spores. Ascospores hyaline, falcate to sigmoid (possibly due to packing inside the ascus), gradually attenuating towards the apices, 40–50×2–3 μm (n=10), smooth-walled, non-halonate, 3-septate, not constricted at the septa, arranged within the ascus in a bundle. Asexual morph not found. The fungus does not visibly damage the host thallus or induce gall formation in the host.
Notes. The material examined represents an undescribed species but is too scant to be formally described. It is referred to Neolamya Theiss. & Syd. but with some uncertainty as this genus is characterized by a dark brown exciple and distinct periphyses (Ertz Reference Ertz2004). It differs clearly from the other three known species of Neolamya by the septation and size of the ascospores and by its host selection. Neolamya ahtii Zhurb., growing on species of Cladonia P. Browne, is characterized by mainly 1-septate and much shorter ascospores, 6·5–34×2·5–6 μm; N. peltigerae (Mont.) Theiss. & H. Syd., growing on species of Peltigera Willd., has 3–6-septate ascospores that are much longer, 56–90×2–3·5 μm; N. xanthoparmeliae Kocourk., growing on species of Xanthoparmelia (Vain.) Hale, has 3–10-septate and likewise much longer ascospores, 55–111·5×3–5 μm (Ertz Reference Ertz2004; Kocourková Reference Kocourková2009; Zhurbenko & Pino-Bodas Reference Zhurbenko and Pino-Bodas2017). This fungus also strongly resembles Spirographa fusisporella (Nyl.) Zahlbr., the type of the genus Spirographa Zahlbr. However, the species of this genus are characterized by cleistohymenial ascomata (Diederich Reference Diederich2004).
Specimen examined. USA: Hawaii: Maui Island, trail between Puu Kukui and Kaulalewelewe, 20°53'N, 156°35'W, 1500–1600 m, on Siphula pickeringii, 1998, Ohmura 5255 (TNS).
Plectocarpon gayanum Etayo
Biblioth. Lichenol. 98: 171 (2008); type: Chile, Navarino, campamento del lago Róbalo, 54°58'23·3''S, 67°40'59·9''W, 290 m, on cf. Siphula indet., 2005, Etayo 2462, Sancho, Gómez-Bolea & Søchting (MAF—holotype; hb. Etayo, UMAG—isotype).
Notes. This fungus is characterized by black, stromatic ascomata, 350–700 μm diam., a hymenium that is I+ red, K/I+ blue, later turning red, clavate, 8-spored asci and ellipsoid, 1–2-septate ascospores (Etayo & Sancho Reference Etayo and Sancho2008). The specimens studied essentially concur with the protologue, although we recorded slightly larger ascospores ((14·8–)16·9–19·1(–20·5)×(4·8–)5·6–6·4(–7·0) μm, l/w=(2·2–)2·7–3·3(–3·7), n=80) with larger locules (100–240 µm diam.) compared to Etayo & Sancho (Reference Etayo and Sancho2008) (ascospores: 12·5–18×4·5–6 μm; locules: 110–150 µm diam.). Whereas the sterile, stromatic tissue is typically black-brown and K−, in one specimen (Imshaug 40154 & Harris) it was olivaceous brown to black-brown, K± greenish, or reddish brown and becoming less reddish in K. The brown pigment dissolves in K and is analogous to pigment 2 of Ertz et al. (Reference Ertz, Christnach, Wedin and Diederich2005). We also noted that the fungus occasionally causes local discoloration of the host thallus.
Ecology and distribution. This species was initially described from Chile, growing on an unindentified species of Siphula (Etayo & Sancho Reference Etayo and Sancho2008). Here it is also recorded from Tasmania, New Zealand and the Falkland Islands, where it grows on Siphula decumbens, S. dissoluta and S. fastigiata. These three host taxa have wide ecological amplitudes, ranging from relatively lowland heathland or forest habitats to treeless alpine elevations. The distribution of infected thalli reflects this broad range. On S. fastigiata, Plectocarpon gayanum was found together with unidentified species of Sphaerellothecium, whereas on Siphula decumbens, it was accompanied by Cercidospora santessonii and Aabaarnia siphulicola.
Specimens examined. Australia: Tasmania: Mount Cameron, 40°58'33''S, 147°55'59''E, 300 m, on Siphula fastigiata, 1997, Kantvilas (HO 320821); track to Mount Cameron, 40°59'S, 147°56'E, 300 m, on S. fastigiata, 1995, Kantvilas 39/95 (HO 312570); Mount Field, 760 m, on S. decumbens, 1963, James AU 2081 (H, UPS); c. 1·5 km S of Frodshams Pass, 42°49'S, 146°23'E, 370 m, on S. decumbens, 2004, Kantvilas 193/04 (HO 526342); Mount Mawson, 1000 m, on S. decumbens, 2007, Motiejūnaitė 10915 (BILAS); peak 1 km E of Mount Mueller, 42°47'S, 146°28'E, on S. dissoluta, 1998, Kantvilas 234/98 (HO 329289); lower Olga River, 42°44'S, 145°47'E, on S. decumbens, 1975, Gilbert (75/419) (HO 45829); Lake Osborne, 850 m, on S. decumbens, 1981, James & Kantvilas 507/81 (UPS); tarn above Lake Oberon, 43°09'S, 146°16'E, 880 m, on S. decumbens, 2002, Felton (HO 520187); Lake Osborne Track, 43°13'S, 146°45'E, 850 m, on S. decumbens, 1981, Kantvilas & James 507/81 (HO 118134); Mount Read, 41°51'S, 145°33'E, 1110 m, on S. decumbens, 1964, Bratt & Lakin 1156 (HO 46048); Ragged Range, 42°46'S, 146°19'E, 720 m, on S. decumbens, 1995, Kantvilas 8/95 (HO-310587); Lake Seal, 42°40'S, 146°34'E, 920 m, on S. decumbens, 1981, Tibell 11247 (UPS); Shadow Lake, 42°06'S, 146°08'E, on S. decumbens, 1987, Moscal 14344 (HO 62586); South Sister, 41°32'S, 148°10'E, 800 m, on S. fastigiata, 2004, Kantvilas 407/04 (HO 529054); track from Waterfall Valley to Windermere Hut, 41°46'S, 145°58'E, on S. decumbens, 1977, Matthews 77/151 (HO 33791); Gordon Road, c. 2 km S of Needles picnic area, 42°46'S, 146°24'E, 450 m, on S. gracilis, 2007, Kantvilas & de Villiers 287/07 (HO 544941); Savage River National Park, E side of Baretop Ridge, 41°18'37''S, 145°26'51''E, 580 m, on S. decumbens, 2015, Kantvilas 65/15 (HO 576874); Mount Read, 41°51'S, 145°33'E, 1110 m, on S. decumbens, 1964, Bratt & Lakin 1156 (HO 46048).—New Zealand: South Island: Arthur’s Pass National Park, Avalanche Peak Track, on S. dissoluta, 1971, Harris 647 (HO 309743); Arthur’s Pass, 975–1036 m, on S. dissoluta, 1962, James 1907b (UPS); ibid., on S. decumbens, 1927, G. E. & G. Du Rietz 1508:2 (UPS L-860148); ibid., on S. fastigiata, 1927, G. E. & G. Du Rietz 1508:2 (UPS L-860067a); track from Flora Saddle to Mount Arthur Hut, 41°11'S, 172°44'E, 1100 m, on S. decumbens, 1995, Wedin 4968 (UPS); 1·5 km ENE of Mount Arthur Hut, 41°12'S, 172°44'E, 1050 m, on S. decumbens, 1993, Tibell 19616a (UPS); Mount Brewster, Haast Pass, 1433–1524 m, on S. dissoluta, 1962, James 480/1 (UPS); Hokitika, on S. decumbens, 1927, G. E. & G. Du Rietz 1570b:1 (UPS). North Island: Pouakai Range, Dover Track, 39°17'S, 174°05'E, 950 m, on S. decumbens, 1990, Wedin 2146 (UPS). Auckland Island: Port Ross, above Erebus Cove, on S. decumbens, 1927, G. E. Du Rietz 2283:1 (UPS L-860156b); ibid., on S. dissoluta, 1927, G. E. Du Rietz 2283:1 (UPS L-860150b); Ranui Cove, 60 m, on S. decumbens, 1962, James 709 (H, UPS).—Falkland Islands: East Falkland: between Mount Usborne 2 and Ceritos Rocks, 470 m, on S. dissoluta (H, TNS), 1968, Imshaug 40154 & Harris (H, TNS).
Polycoccum sp.
Vegetative hyphae not observed. Ascomata perithecioid, black, 70–100 μm diam. (n=10), slightly protruding or occasionally semi-immersed; wall brown, K−, in surface view composed of strongly elongate cells 1·5–3·5 μm wide. Hymenial gel K/I−, I−, sometimes I+ blue. Interascal filaments abundant, septate, branched, 1–3 μm diam., sometimes indistinct. Asci 43–70×19–28 μm (n=6), (2–)4(–8)-spored, I−, K/I−, clavate, ellipsoid, oblong or narrowly ovoid, with a short or indistinct stalk. Ascospores (16·5–)18·7–23·9(–27·6)×(6·6–)7·6–9·2(–10·4) μm, l/w=(1·9–)2·2–2·8(–3·1) (n=85), medium to dark greyish olive to olive at maturity, irregularly uni- to biseriate in the ascus, 1-septate, usually slightly constricted at the septum, narrowly obovoid, with a wider and slightly longer upper cell (ratio 6:5); wall distinctly granular, sometimes with a halo 0·5–1·5(–3) μm thick. The fungus does not visibly damage the host thallus or induce gall formation, although the ascomata may sometimes lift surrounding host tissues and induce gall-like swellings.
Notes. The material examined is very similar to Endococcus hafellnerianus as described above, which differs by lacking interascal filaments. It is possible that both taxa belong to the same species in which some specimens have poorly developed interascal filaments but additional collections are required to verify this hypothesis.
Specimens examined (all on Parasiphula complanata). Chile: Magallanes Province: Puerto Bueno, 51°00'S, 74°13'W, 1868, Cunningham (UPS).—Argentina: Isla de los Estados: Capitan Canepa Bay, 54°49'S, 64°27'W, 120 m, 1971, Imshaug 53147b & Ohlsson (H).—New Zealand: Stewart Island: track from Rakeahua Hut to Mount Rakeahua Peak, 46°56'S, 167°53'E, 640 m, 1995, Wedin 4885b (UPS).
Pyrenidium actinellum Nyl. s.l.
Flora (Regensburg) 48: 210 (1865); type: England, Co. of Kent (Maidstone), Boxley Hill, on Leptogium teretiusculum, Jones (H-NYL 41028—lectotype).
Notes. This fungus is characterized by the following features: pseudothecia subglobose, ovoid or obpyriform, 150–200 μm diam., with a brown wall; hamathecium of abundantly branched and anastomosing paraphysoids, 1·5–2·0 μm diam., and periphyses which are green-pigmented at the top of the ostiolar region; asci clavate to subcylindrical, 4–8-spored, 60–90×12–18 μm; and ascospores (2–)3(–4)-septate, brown, with paler apices, 19–32×7–12 μm (Hafellner & Mayrhofer Reference Hafellner and Mayrhofer2007; Navarro-Rosinés & Roux Reference Navarro-Rosinés and Roux2007). Our material concurs well with these descriptions, including the characteristic blue-green pigmentation of the hamathecium near the ostiole, and the ascospore size: (18·9–)22·6–27·0(–31·9)×(7·6–)9·2–11·6(–13·0) µm, l/w=(1·8–)2·1–2·7(–3·5) (n=100). In its broad sense, this is a common cosmopolitan species (or group of species) which has been reported on all continents from many, distantly related lichen genera (Brackel Reference Brackel2014). It is newly documented here as occurring on Siphula.
Specimens examined. South Africa: Western Cape Province: Caledon District, 610 m, on Siphula aff. decumbens, 1944, Leighton 709 (UPS); Outeniqua Mountains, Duiwelskop Pass, c. 1000 m, on S. decumbens, 1970, Degelius SA 207 (UPS); Stellenbosch Flats, on S. torulosa, 1930, Duthie 5038 (UPS).
Pyrenidium cf. coccineum Aptroot
Bryologist 117: 286 (2014); type: Solomon Islands, Guadalcanal Island, central part, Mount Popomansiu, summit area, c. 2200 m, on Siphula decumbens, 1965, Hill 10854 (BM—holotype; ABL—isotype).
Notes. This taxon is characterized by 3-septate ascospores, black ascomata covered by a red, K+ purple pruina and by the way it changes the colour of the infected host to pink (Aptroot Reference Aptroot2014). Our specimen agrees with the protologue with respect to the host species and ascospore length (Aptroot Reference Aptroot2014) but it has slightly narrower ascospores (19–22 × 6–7 μm vs. 17–21×7·5–8·5 μm), does not have red pruina on the ascomata, and does not induce a change of colour in the infected host thallus. More material is needed to confirm our hypothesis regarding their conspecifity.
Ecology and distribution. Pyrenidium coccineum was hitherto known only from the type locality. The Tasmanian specimen is from a thallus of Siphula decumbens growing on peaty soil in crevices in outcrops of Ordovician conglomerate in scrubby heathland.
Specimen examined. Australia: Tasmania: Ragged Range, 42°46'S, 146°19'E, 720 m, on Siphula decumbens, 1995, Kantvilas 8/95 (HO-310587).
Pyrenidium macrosporum Motiej., Zhurb., Suija & Kantvilas sp. nov.
MycoBank No.: MB 827420
Lichenicolous fungus similar to Pyrenidium actinellum s.l. but distinguished by the larger ascospores, 32–55·5×12·5–18 μm compared to 19–32×7·5–13 μm.
Type: Australia, Tasmania, The Hermit, 42°49'S, 146°08'E, 450 m, buttongrass moorland, on thallus of Parasiphula jamesii, 19 January 1984, G. Kantvilas 56/84 (HO 113212—holotype).
(Fig. 5)

Fig. 5 Pyrenidium macrosporum. A & B, galls with ascomata (A, holotype; B, Roivainen, H); C, vertical section through an ascoma (in water); D, ostiolar region showing green pigmentation (in water); E, ascospore (in K); F, lower part of an ascoma, showing structure of the ascomatal wall and ascospores; G, paraphyses (in Congo red); H, fissitunicate ascus (in water); C–H: Kantvilas, HO 113190. Scales: A & B=1 mm; C=100 μm; D, F & H=50 μm; E & G=5 μm. In colour online.
Vegetative hyphae brown, 4–5 μm wide, torulose. Ascomata single or aggregated in groups of 50–100 or more, 90–415 μm diam. (n=10), perithecioid, ostiolate, black, subglobose in section, immersed to 1/3 protruding. Exciple brown, K+ green-black throughout, 40–65 μm thick in the ostiolar region, 25–35 μm thick below, pseudoparenchymatous, composed of several layers of elongate cells 6–10×5–6 μm. Periphyses 20–30×3·0–3·5 μm, unbranched or sometimes bifurcate. Paraphysoids hyaline, abundant, branched and anastomosing, 1·0–4·0 μm thick; both periphyses and paraphysoids associated with dispersed pale to medium green to mostly blue-green granules, most abundant in the ostiolar area. Hymenial gel I−, K/I−. Asci clavate, (50–)80–120 (–160)×(11–)20–35(–40) μm (n=13), 4–8-spored, I−, with an apical dome and short, blunt ocular chamber. Ascospores (32·0–)42·5–51·1(–55·3)×(12·5–)14·5–17·1(–18·0) μm, l/w=(1·8–)2·7–3·3(–3·6) (n=96), hyaline or very pale brown when immature within the asci, later dark brown, sometimes with paler apical cells, fusiform to ellipsoid, straight or slightly curved, smooth-walled, with a halo c. 1 μm thick, 3-septate, with apical cells smaller than central ones, slightly constricted at the septa (more markedly so at the central septum), with a pore in the central part of each septum and a single guttule in every cell. Asexual morph not observed.
Etymology. The specific epithet refers to the large size of the ascospores.
Notes. Ten species are currently accepted in the genus Pyrenidium Nyl. (Lawrey & Diederich Reference Lawrey and Diederich2018), among which P. macrosporum stands out clearly by its much larger asci and ascospores. Furthermore, Pyrenidium macrosporum is known only from Parasiphula, whereas two other species of the genus occur on Siphula s.s.: P. actinellum s.l., found on S. decumbens and S. torulosa, and P. coccineum, recorded on S. decumbens. Hafellner & Mayrhofer (Reference Hafellner and Mayrhofer2007) and Navarro-Rosinés & Roux (Reference Navarro-Rosinés and Roux2007) considered the green to blue-green pigmentation of the ostiolar area to be a character peculiar to Pyrenidium actinellum. However, P. aggregatum Knudsen & Kocourk., described subsequently by Knudsen & Kocourková (Reference Knudsen and Kocourková2010), as well as P. macrosporum, both share this feature. The new species does not cause distinct discoloration of the host thallus but it does induce gall-like swellings around the ascomata.
Ecology and distribution. The new species has been found in Tasmania, Argentina and Chile, growing on thalli of Parasiphula complanata, P. fragilis, P. georginae and P. jamesii. The Tasmanian specimens are from host thalli growing on boggy, peaty soil in treeless alpine heathland and buttongrass (Gymnoschoenus)-dominated moorland.
Additional specimens examined. Australia: Tasmania: Green Head, c. 3 km SSE of Greystone Bluff, 43°06'S, 146°04'E, 880 m, on Parasipula georginae, 1991, Kantvilas 67/91 (HO 129674); head of Spring River, Port Davey Track, 180 m, on P. jamesii, 1984, Kantvilas 514/84 (HO 113190); Turrana Bluff, 41°46'S, 146°21'E, 1330 m, on P. fragilis, 2012, Kantvilas (HO 564518).—Chile: Fuegia occidentalis: Bahia Cordoba, 350 m, on P. complanata, 1929, Roivainen (H). Magallanes Province: E side of Juan Island, 50°39'S, 74°36'W, on P. complanata, 1969, Imshaug 44233a & Ohlsson (H).—Argentina: Isla de los Estados: Bahia Capitan Canepa, 54°49'S, 64°27'W, 120 m, on P. complanata, 1971, Imshaug 53147a & Ohlsson (H); ibid., 54°50'S, 64°30'W, 150 m, on P. complanata, 1971, Imshaug 52995a & Ohlsson (H).
Saania mobergii Zhurb. gen. et sp. nov.
MycoBank No.: MB 827282 (genus) and MB 827283 (species)
Lichenicolous fungus. Ascomata stromatic, multilocular, superficial, blackish, convex, up to 1200 μm wide and 350 μm thick, in section brown, K+ olive, N+ red, composed of ± isodiametric or tangentially elongate cells. Hymenial gel I−, K/I−. Exciple indistinct. Hamathecium of persistent periphysoids. Asci bitunicate, elongate-clavate, 51–105×17–31 μm, 4(–8)-spored; wall I−, K/I−. Ascospores narrowly obovate to ellipsoid, (17·5–)21·5–26·0(–29·0)×(7·0–)9·5–12·0(–15·0) μm, 1(–3)-septate, irregularly biseriate to diagonally uniseriate in the ascus, initially hyaline and smooth-walled, later sometimes brown and verruculose.
Type: South Africa, Cape Province, Cape District, Table Mountain, near Upper Cableway station, on thallus of Siphula torulosa growing on ground, 9 August 1953, O. Almborn 1935 (UPS—holotype).
(Fig. 6)
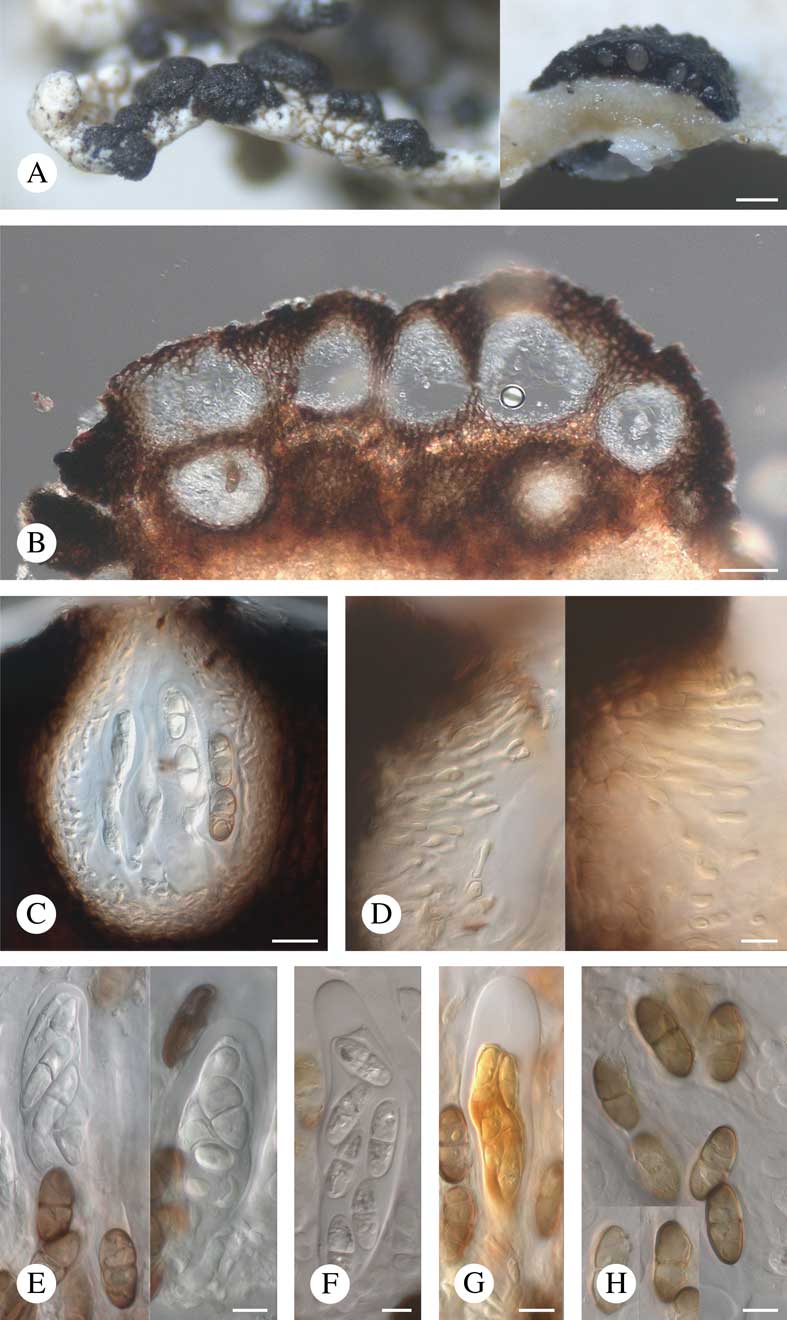
Fig. 6 Saania mobergii. A, stromatic ascomata, note section through ascoma on the right (holotype on the left, Esterhuysen 12279 on the right); B, stromatic ascomata in section in water (1942, Stoke); C, locule in section in water (Esterhuysen 12279); D, hamathecial filaments in K/I (Almborn 5083 on the left, Moberg 11757a on the right); E, asci and ascospores in water (Almborn 5083 on the left, holotype on the right); F, ascus and ascospores in K (Almborn 5083); G, ascus and ascospores in K/I (Almborn 5083); H, ascospores in K (Almborn 5083). Scales: A=200 μm; B=50 μm; C–H=10 μm. In colour online.
Vegetative hyphae not observed. Ascomata stromatic, multilocular, superficial, single or sometimes contiguous to confluent, dark brown to almost black, glossy, convex, often irregular in shape, (120–)230–710(–1200) μm wide (n=68), (80–)150–290(–350) μm thick (n=19), with a clearly delimited margin; surface tuberculate due to elevations above the locules, sometimes inconspicuously radially striate. Ascostromata in section brown, darker brown towards the outer edge, K+ olive, N+ red, composed of ± isodiametric (5–10×5–8 μm) or tangentially elongate (around locules) cells, with intercellular spaces 2–4 μm wide; pores in cell walls not observed. Locules up to 40 within the stroma, arranged in one or occasionally several layers, sometimes merging with each other, roundish, broadly ellipsoid, ovoid or ampulliform in section, (40–)60–110(–180) μm wide (n=60), without a distinct ostiolar canal, but extending to the surface of the stroma and exhibiting gaping holes at later stages; hymenial gel I−, K/I−. Exciple similar in structure and colour to the stromatal tissue. Hamathecium of persistent periphysoids, consisting of (1–)2–3(–4)-celled filaments 20–40 μm long and 1·5–2·5(–5·5) μm thick, constricted at the septa, sometimes dichotomously branched but not anastomosing, attached at the apex and walls of the locules and not reaching their base when mature; since the ostiolar canal is indistinct, periphyses are not clearly differentiated. Subhymenium hyaline, pseudoparenchymatous, c. 20 μm thick. Asci bitunicate, elongate-clavate, shortly stalked, (51–)70–97(–105)×(17–)21–29(–31) μm (n=30), 4(–8)-spored, I−, K/I−, except for the ascoplasm which stains I+ orange, K/I+ orange, with apex thickened to 4–8(–13) μm and lacking a distinct internal apical beak. Ascospores (17·6–)21·5–26·1(–28·8)×(7·1–)9·3–12·1(–15·1) μm, l/w=(1·7–)2·0–2·6 (–3·4) (n=169), 1(–3)-septate, occasionally slightly constricted at the septa, central pore in the septa not observed, narrowly obovate (with slightly wide upper half) to ellipsoid, irregularly biseriate to diagonally uniseriate in the ascus, initially hyaline, smooth-walled and occasionally with a halo<1 μm thick, later sometimes evenly pale brown to brown, verruculose and non-halonate.
Asexual morph rare, similar in structure and colour to the locules containing asci; conidiophores absent; conidiogenous cells hyaline, short-ampulliform; conidia hyaline, bacilliform, 6·5–7·2×1·6–1·9 μm (n=8).
Etymology. The generic name refers to the “San” or “Saan”, the first nation people of southern Africa, whereas the specific epithet honours the eminent Swedish lichenologist, Roland Moberg, who collected one of the paratypes of the new species.
Notes. This new species does not correspond with other genera of lichenicolous fungi with stromatic multilocular ascomata and consequently a new genus is proposed. Clypeococcum D. Hawksw., Homostegia Fuckel, Macrographa Etayo, Perigrapha Hafellner and Plectocarpon Fée all differ from the new genus by having interascal filaments (Hafellner Reference Hafellner1996; Ertz Reference Ertz2004; Hawksworth et al. Reference Hawksworth, Atienza and Cole2004; Ertz et al. Reference Ertz, Christnach, Wedin and Diederich2005; Etayo & Sancho Reference Etayo and Sancho2008). Furthermore, the stromata of Clypeococcum form a clypeus; Homostegia differs in having 8-spored asci and usually pigmented, 3-septate ascospores; Macrographa differs in having N− stroma and hyaline, 1-septate ascospores; Perigrapha and Plectocarpon differ in their K/I+ blue hymenial gel and their asci with a K/I+ blue apical ring. A further genus, Lasiosphaeriopsis D. Hawksw. & Sivan, differs from Saania in having unitunicate asci without any distinct apical apparatus and in the occurrence of pores in the stromatic cell walls (Hawksworth Reference Hawksworth1980).
Stigmidium eucline (Nyl.) Vězda, growing on species of Varicellaria Nyl., forms a stroma-like plexus of vegetative hyphae around tightly aggregated ascomata (Zhurbenko Reference Zhurbenko2017) and thus may resemble the new species. However, it differs from the latter by having interascal filaments (Sérusiaux et al. Reference Sérusiaux, Diederich, Ertz and van den Boom2003; Kocourková & Knudsen Reference Kocourková and Knudsen2010), and the stroma-like structures of this species are unusual for the genus Stigmidium Trevis. in general, as typified by S. schaereri (A. Massal.) Trevis. (Roux & Triebel Reference Roux and Triebel1994; Triebel & Cáceres Reference Triebel and Cáceres2004). Lichenostigma Hafellner s.l. also has stromatic ascomata but does not form locules and lacks hamathecial filaments (Ertz et al. Reference Ertz, Lawrey, Common and Diederich2014).
When compared to other siphulicolous fungi, in habit Saania mobergii most closely resembles Plectocarpon gayanum. However, the latter clearly differs in having anastomosing interascal filaments attached at the base of the locules, I+ red, K/I+ blue hymenial gel and consistently hyaline, smaller ascospores.
Ecology and distribution. The new species is known only from South Africa where it grows on thalli of Siphula decumbens, S. torulosa, S. verrucigera and an undescribed species of Siphula related to S. fastigiata. It does not visibly damage or induce gall formation in the host thallus.
Additional specimens examined. South Africa: Western Cape Province: Bainskloof Pass, on Siphula decumbens, 1953, Almborn 5083 (UPS); 4 miles N of Bainskloof Pass, on S. verrucigera, 1953, Almborn (H); N Cedarberg, 1600 m, on Siphula sp., 1945, Esterhuysen 12279 (UPS L-860155, UPS L-860078); Deception Peak, 1524 m, on Siphula sp., 1942, Stoke (UPS); Groot Drakenstein Mountains, on S. decumbens, 1943, Esterhuysen 9559 (UPS); top of Mount Paarl, on S. torulosa, 1963, Kofler (UPS); Piketberg, N of Noupoort Farm, 32°47'S, 18°39'E, 800 m, on S. torulosa, 1996, Moberg 11757 (H); Table Mountain, 914 m, on S. decumbens, 1953, Almborn 1747 (UPS).
Skyttea sp.
Vegetative hyphae not observed. Ascomata apothecioid, urceolate, erumpent, superficial when mature, greyish black, 100–150 μm diam. (n=4); pore c. 20% of the ascomatal diameter; margin whitish, with several fissures. Exciple greenish, comprised of rectangular cells (textura angularis), K+ olivaceous then brown, N+ olivaceous green, with marginal, hyaline to slightly greenish excipular hairs with roundish tips, 6–9×2·5–3 μm. Epihymenium hyaline to slightly greenish, K−, N−. Hymenium hyaline, I−, 30–40 μm thick. Paraphyses filiform, simple, distantly septate, c. 1 μm thick, not easily separating in K; apices not thickened. Asci 37–39×5–12 μm (n=3), 8-spored, I−, unitunicate, subcylindrical, with apex thickened and without a distinct apical apparatus. Ascospores hyaline, fusiform or slightly falcate, (9·0–)9·5–11·5(–12)×(2·5–)2·6–3·0(–3·0) μm, l/w=3–4·4 (n=15), aseptate, smooth, halonate. Asexual morph not observed.
Notes. The inclusion of this presumably undescribed species in the genus Skyttea Sherwood et al. is provisional due to the limited material available and additional specimens are needed to ascertain its taxonomic relationships. Almost all known Skyttea species grow on crustose lichens, with the few exceptions of S. aff. fusispora growing on Physciaceae (Diederich & Etayo Reference Diederich and Etayo2000) and S. anziae Etayo & Diederich on Anzia sp. (Etayo Reference Etayo2002). In its general characteristics, our specimen is most similar to S. lecanorae Diederich & Etayo, a species growing on Lecanora with ascospores of similar length but wider (7–13×2–4·5 μm; Diederich & Etayo Reference Diederich and Etayo2000). Furthermore, the excipular hairs are longer in S. lecanorae (8–13 μm) and both fungi inhabit different, taxonomically unrelated hosts.
Specimen examined. Australia: Tasmania: Mount Eliza, on Parasiphula georginae, 1985, Kantvilas (HO 316101).
Sphaerellothecium siphulae Zhurb.
Nova Hedwigia 101: 420 (2015); type: Russia, Murmansk Region, Barents Sea coast, mouth of Olenka River, 69°02'N, 36°24'E, 50 m, tundra with puddles, on Siphula ceratites, 1997, Zhurbenko 97398 (LE 264400—holotype!; GZU—isotype!).
Notes. The species is characterized by densely aggregated ascomata, only sometimes associated with a macroscopically visible net of dark hyphae, the absence of interascal filaments, 1-septate ascospores, (8·5–)10·0–11·5(–14·0)×(3·5–)4·0–5·0(–6·0) μm, with the wall BCr−, the host genus and a strong pathogenicity (Zhurbenko Reference Zhurbenko2015). Ascomata are rarely associated with conidiomata, which contain hyaline, fusiform, 1(–3)-septate, smooth-walled conidia, (8·3–)10·3–13·3(–13·6)×(1·1–)1·3–1·7(–1·8) μm, l/w=(5·5–)6·6–8·8(–10·2) (n=26). Hitherto, no asexual morph had been reported for this species.
Thirty additional specimens of Sphaerellothecium species from South America, South Africa and Australasia, growing on various species of Parasiphula and Siphula, were also examined. The morphological variations and host selection of these specimens suggest that more than one species is involved. For the moment, however, these specimens remain unnamed pending a comprehensive revision of Sphaerellothecium using molecular methods. Although some of these specimens are similar to S. siphulae, this name is retained only for specimens growing on Siphula ceratites from the Northern Hemisphere, from where this species was described.
Ecology and distribution. Sphaerellothecium siphulae was known previously from the Russian Arctic (Zhurbenko Reference Zhurbenko2015) and the British Isles (Paul Reference Paul2016) but it has a wide, circumpolar distribution similar to its host Siphula ceratites, a characteristic species of moist habitats in northern coastal regions. It is recorded here for the first time from North America (Canada and the USA), Finland and Norway.
Specimens examined (all on Siphula ceratites). Norway: Finnmark: East Finnmark, Båtsfjord, between Gammelelve and Hursi, 1974, Kvist (H); Elvenes, 1864, Fries (H); Porsanger, Stabbursdalens nasjonalpark, Coarvosavzze, 1970, Ryvarden 6432 (O-L28875). Nordland: Tysfjord, Bognes (E6) south of Lødingen, 40 m, 1985, Ross (O-L28793); Brønnøy, near Horn at 10·5 km marker N of Brønnøysund, 50 m, 1989, Ross (O-L28787); Salten, Salfjell ved Balvann, 1966, Ryvarden (O-L28850). Nord-Trøndelag: Flatanger Municipality, Nordstraumen, 64·50522°N, 10·79483°E, 2015, Suija (TU-75652). Sør-Trøndelag: Oppdal, Falkfangarhøa h. 1399 SW, 1350 m, 1983, Sæbo & Sivertsen (O-L28827); Oppdal, Skrinkø sør., 1060 m, 2003, Bjørn & Ognedal Bpl-L10355 (O-L123492); Rissa, Traugheia, 350 m, 1960, Rui (O-L28837). Troms: Kvænangen/Nordreisa, Kvænangsfjellet, 135 km S of Alta, 300 m, 1987, Ross (O-L28785).—Russia: Chukotka: Cape Schmidt, 68°55'N, 179°27'E, 1981, Laanetu (TU-56799); Murmansk Region: Borisoglebski (Kolttaköngäs), 69°39'N, 30°08' E 1927, Lippmaa (TU-63682, TU-63684).—USA: Alaska: Seward Peninsula, Cape Prince of Wales, 65°48'N, 168°W, 1978, Flock FL-737 (H).—Canada: British Columbia: Kaien Island, Prince Rupert, Mount Hays, 1963, Schofield & Boas 21542 (H); without exact locality, 1931, Kujala (H); Kunghit Island, 52°00'N, 131°03'W, 1971, Schofield & Brodo 17674 (H, TNS).
Stigmidium kashiwadanii Zhurb. sp. nov.
MycoBank No.: MB 827284
Lichenicolous fungus similar to Sphaerellothecium siphulae but distinguished by the (1–)3-septate or, rarely, submuriform vs. 1-septate, larger ascospores, (12–)14·5–17·5(–20)×(5–)6–8(–9) μm vs. (8·5–)10–11·5(–14)×(3·5–)4–5(–6) µm.
Type: Australia, Western Australia, Snake Rocks, 28 miles SE of Perth, 32°22'S, 116°03'E, 270 m, on thallus of Siphula coriacea growing on exposed rocks, 13 December 1965, S. Kurokawa 6631 (TNS—holotype).
(Fig. 7)
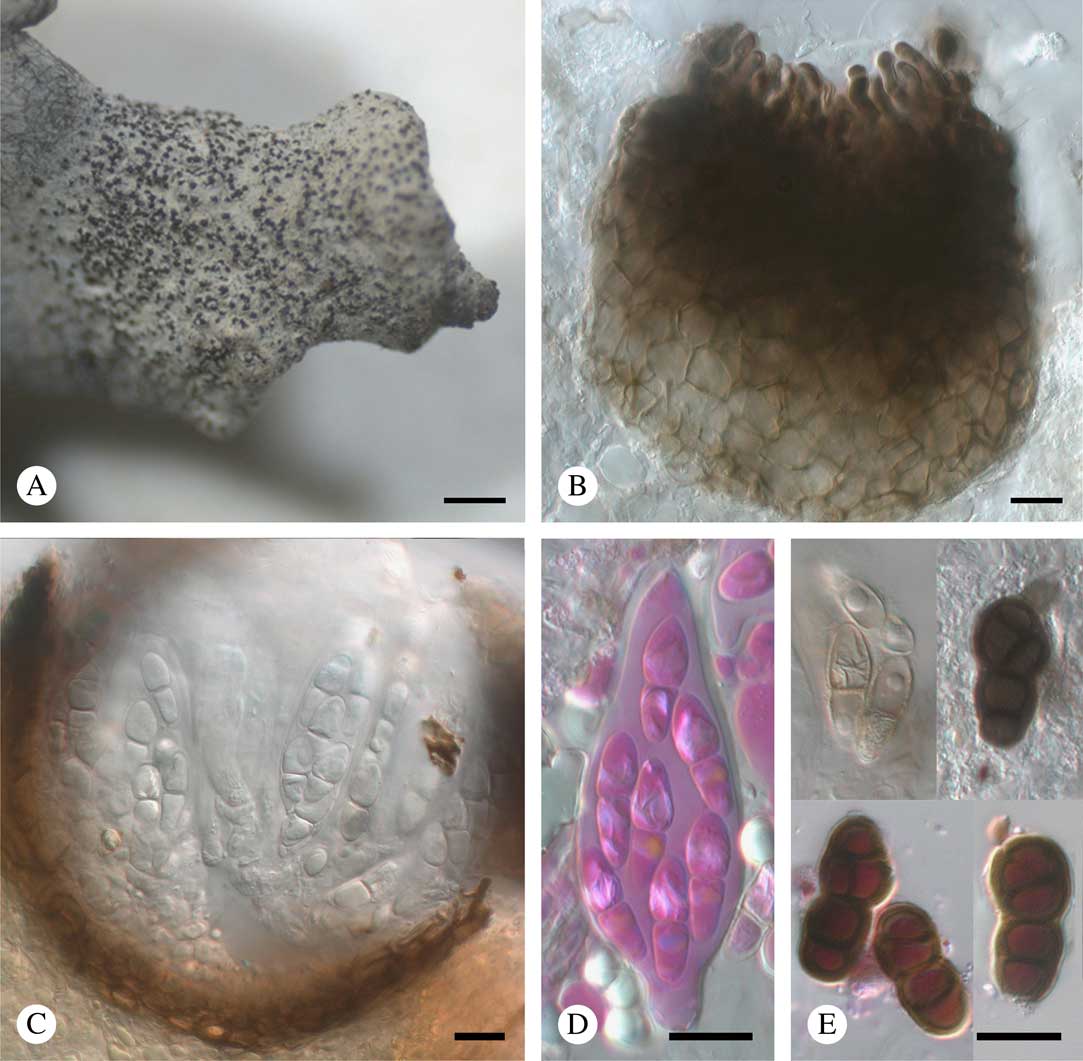
Fig. 7 Stigmidium kashiwadanii (holotype). A, ascomata aggregated on host lobe tip; B, squashed ascoma in K, showing abundant external ostiolar filaments; C, ascoma in section with hyaline ascospores; D, ascus in phloxine after K; E, aged ascospores in K (above) and phloxine after K (below). Scales: A=500 μm; B–E=10 μm. In colour online.
Vegetative hyphae mostly immersed, pale to medium brown, 2·5–4·5 μm diam., not forming a dark, superficial reticulum. Ascomata perithecioid, black, dull or slightly glossy, subglobose in section, sometimes slightly papillate, ostiolate, without appendages, 60–110 μm diam. (n=10), protruding in the upper part to rarely half-exposed, separate to occasionally contiguous, aggregated in clusters of up to 200. Exciple 6–10 μm thick, composed of 2–4 layers of tangentially compressed cells, in surface view composed of polygonal cells 3–15 μm long, dark brown above, medium to pale brown below, K+ olive-grey, pigmentation evenly amorphous. Ostiolar filaments well developed, brown, more or less isodiametric, rounded at the apex, not branched, 0(–2)-septate, 9–19×3–4·5 μm. Short pseudoparaphyses of type ‘a’ sensu Roux & Triebel (Reference Roux and Triebel1994), rather inconspicuous, 4–9×1·5–3 μm. Long interascal filaments absent. Interascal gel I−, K/I−. Asci (41–)44·5–51·5(–56)×(14·5–)16·5–21·5(–23) μm (n=16), 8-spored, bitunicate, narrowly pyriform-ovoid (wider below the middle), shortly stipitate; endoascus markedly thickened above; wall BCr−, I−, K/I−. Ascospores subclavate to narrowly ovoid, (12·2–)14·7–17·7(–20·1)×(5·0–)6·0–7·8(–8·8) μm, l/w=(1·8–)2·1–2·7(–3·7) (n=129), initially hyaline, smooth-walled and 1-septate (septum median or rarely disposed towards the upper part), later sometimes brown, verruculose and (2–)3-septate or, rarely, submuriform, with an additional longitudinal or oblique septum, constricted at the median septum (at later stages often markedly so) but not disintegrating into semi-spores, in K rarely with a thin gelatinous sheath around immature ascospores, never pseudotetrablastic, irregularly arranged in the ascus; wall BCr−; plasma BCr+ blue. Asexual morph not seen.
Etymology. This species is named after the eminent Japanese lichenologist, Hiroyuki Kashiwadani, who collected several of the specimens on which the description is based.
Notes. On the basis of its morphological and anatomical characters alone, the new species could be placed in either Sphaerellothecium Zopf or Stigmidium. The current delimitation of these genera is based mainly on the presence or absence of a distinct net of dark, thick-walled vegetative hyphae. This feature is characteristic of Sphaerellothecium araneosum (Arnold) Zopf, the type of Sphaerellothecium (Cáceres & Triebel Reference Cáceres and Triebel2004; Triebel & Cáceres Reference Triebel and Cáceres2004), but its interpretation can be quite subjective and there are a number of species that could be placed in either genus. One such species is Sphaerellothecium siphulae, which also grows on Siphula. It resembles Stigmidium kashiwadanii in its aggregated ascomata, distinct pathogenicity and most ascomatal characters but differs from that species in having 1-septate, smooth-walled, only exceptionally brown and much smaller ascospores, (8·5–)10–11·5(–14)×(3·5–)4–5(–6) µm (Zhurbenko Reference Zhurbenko2015). The new species is distinguished from most species of Sphaerellothecium and Stigmidium by the occurrence of 4-septate, submuriform ascospores. Ascospores with more than three septa were formerly reported in these genera only for Sphaerellothecium soechtingii Zhurb. & Alstrup growing on Arthrorhaphis alpina (Schaer.) R. Sant. (Zhurbenko Reference Zhurbenko2007), and Stigmidium psorae (Anzi) Hafellner growing on Psora spp. (Calatayud & Triebel Reference Calatayud and Triebel1999). Those of S. soechtingii are (1–)3(–5)-septate to occasionally submuriform but much smaller, (10–)11·5–14·5(–15)×(3·5–)4–5 μm; those of S. psorae are 1–3(–5)-septate but longer, (16–)17·5–22(–23·5)×(5–)5·5–7·5(–8) μm.
Ecology and distribution. The species is known from four collections from Western Australia, all on thalli of Siphula coriacea, a common terricolous species in heathland and dry sclerophyll forest. Host lobes turn grey when heavily infected.
Additional specimens examined (all on thalli of Siphula coriacea). Australia: Western Australia: Boyup Brook, 1967, Kashiwadani 4639 (TNS); Mount Chudalup at 31 miles S of Pemberton, 230 m, 1965, Kurokawa 6689 (TNS); Karagullen, 1967, Kashiwadani 4573 (TNS); Schannon, Mill Road, 1967, Kashiwadani 4681 (TNS).
Additional Fungi Recorded
In addition to the taxa treated above, our survey also encountered other taxa, the generic positions of which remain unclear. We list these here as a record of what was found and as a basis for future investigations:
(i) a discomycete with sessile black, shiny, apothecia lacking hairs, an olivaceous exciple and epihymenium, clavate to subcylindrical, 8-spored asci, and ellipsoid, hyaline, 1-septate ascospores, 9·0–13·0×4·0–5·0 μm (n=10). (Australia: Tasmania: Quamby Bluff, 41°39'S, 146°42'E, on Parasiphula complanata, 1985, Kantvilas, HO 315823);
(ii) a Niesslia-like pyrenomycete with superficial ascomata, 70–85 μm diam., with aseptate setae to 30 μm long, indistinct branched interascal filaments, 8-spored asci and hyaline, soleiform, 1-septate, smooth-walled ascospores, 10·0–14·0×4·0–5·0 μm (n=10) (New Zealand: South Island: 1·5 km ENE of Mount Arthur Hut, 41°12'S, 172°44'E, 1050 m, on Siphula decumbens, 1993, Tibell 19616b, UPS);
(iii) a Polycoccum-like pyrenomycete with ascomata 50–95 μm diam., surrounded by a brown mycelial net immersed in the host thallus, 4–6-spored asci and brown, 1-septate ascospores, 16·0–20·0×5·0–7·5 μm (n=9) (Australia: Tasmania: Twelvetrees Range, 690 m, on Parasiphula jamesii, 1984, Kantvilas 539/84, HO 113217);
(iv) a Pseudostigmidium-like pyrenomycete with black, glossy, protruding ascomata 40–80 μm diam., triangular in section, 8-spored asci and hyaline, soleiform, 1-septate, smooth-walled ascospores, 7·8–10·0×3·2–4·0 μm (n=4) (Chile: Tierra del Fuego: Isla Clarence, southern peninsula, 40 m, on Siphula ramalinoides, 1987, Stenroos 2584, H);
(v) a pyrenomycete with black, glossy, sessile, aggregated ascomata 80–120 μm diam., and brown, soleiform, (0–)1-septate, smooth-walled or occasionally verruculose ascospores, (8·8–)9·8–11·4(–12·2)×(3·9–)4·2–5·0(–5·5) μm, l/w=(1·9–)2·0–2·6(–2·9) (n=28) (USA: Hawaii: Maui Island, along the trail between Puu Kukui and Kaulalewelewe, 20°53'N, 156°35'W, 1500–1600 m, on Siphula pickeringii, 1998, Kashiwadani 41074, TNS);
(vi) a Lichenostigma-like coelomycete with dispersed conidiomata, 40–80 μm diam., and brown, verruculose conidia, 8·3–14·6 μm diam., composed of 3–10 cells, each 3·0–6·7 μm diam. (Colombia: Departamento de Santander: Páramo de Almorzadero, 3885 m, on Siphula pteruloides, 1978, Aguirre 1063 & Cleef, H);
(vii) two hyphomycetes of uncertain affinity (New Zealand: South Island: Arthur’s Pass, subalpine bog, on Siphula fastigiata, 1927, G. E. & G. Du Rietz 1508:2, UPS L-860067b; Otago, Old Man Range, S von Alexandra, beirn Obelisk, 1800 m, on S. foliacea, 1985, Henssen 30381a & Lumbsch, H).
Discussion
Our survey revealed 16 species in 14 genera of lichenicolous fungi, including the primarily algicolous Epigloea soleiformis which occurs occasionally on lichens. With the exception of that species and the ubiquitous Pyrenidium actinellum s.l., all are restricted to Siphula-like lichens; that is, the genera Siphula s.s. (Icmadophilaceae) and Parasiphula (Coccotremataceae). Seven species and two monotypic genera are described as new to science.
With respect to the key hypothesis underpinning this study, namely “do lichenicolous fungi support the generic delimitation of Siphula-like lichens (Grube & Kantvilas Reference Grube and Kantvilas2006)”, the answer is unequivocally in the affirmative. Five species (Amylogalla fava, Endococcus hafellnerianus, Polycoccum sp., Pyrenidium macrosporum and Skyttea sp.) are found exclusively on species of Parasiphula whereas the rest are restricted to species of Siphula s.s. This pattern also holds for the most part at generic level, with only the broadly ranging genus Pyrenidium spanning both host genera. This in itself is a significant finding given that Siphula-like lichens frequently occur as intimately intermixed swards comprising several species of both genera, and yet the lichenicolous fungi in such situations distribute themselves in a highly host-specific way.
At the species level, Siphula s.l. can present a bewildering range of morphological variation that complicates the delimitation of taxa. At the same time, it displays a range of discrete chemical spectra. The current taxonomy of the species (Kantvilas Reference Kantvilas1996, Reference Kantvilas1998, Reference Kantvilas2002, Reference Kantvilas2004; Kantvilas & Elix Reference Kantvilas and Elix2002) is essentially based on chemical characters and their correlation with morphological and biogeographical data. Using secondary chemistry as the critical species-level character, however, has not been without complications, and there is considerable scope in further exploring the taxonomy of Siphula. In this respect, the lichenicolous fungi studied offer little insight into species-level classification. Most of the more frequent taxa have been recorded from several, albeit related, host species and it is not unusual to find the same fungus on each and any of the related species, S. decumbens (thamnolic acid), S. fastigiata (baeomycesic and squamatic acids) and S. dissoluta (hypothamnolic acid). A possible exception is Stigmidium kashiwadanii, which is known only from Siphula coriacea (barbatic acid), but this is also the only lichenicolous fungi-supporting host recorded for continental Australia. Likewise, the restriction of Spharelleothecium siphulae to Siphula ceratites only (Zhurbenko Reference Zhurbenko2015) is also underlain by a geographical pattern, as this host is the sole Northern Hemisphere example.
In the Coccotremataceae, apart from Parasiphula, lichenicolous fungi are known only from Coccotrema itself (Etayo & Sancho Reference Etayo and Sancho2008). In the Icmadophilaceae, Thamnolia (with 23 species (Zhurbenko Reference Zhurbenko2012)) shows a richness in lichenicolous fungi comparable to Siphula. Previous molecular investigations of Siphula-like lichens (Stenroos & DePriest Reference Stenroos and DePriest1998; Platt & Spatafora Reference Platt and Spatafora2000; Stenroos et al. Reference Stenroos, Myllys, Thell and Hyvönen2002; Grube & Kantvilas Reference Grube and Kantvilas2006; Ludwig et al. Reference Ludwig, Knight and Kantvilas2016) have consistently suggested that within Siphula itself, the so-called S. decumbens group is distinct from S. ceratites, the generic type, and is possibly deserving of separate generic status. Siphula ceratites is distinguished, inter alia, by the presence of chromones whereas the S. decumbens group contains depsides (Kantvilas Reference Kantvilas2002). The problem with these analyses has been that none has included a good representation of neotropical collections where, within S. pteruloides and S. carassana, there are chromone-only, chromone plus depsides and depside-only races (Kantvilas & Elix Reference Kantvilas and Elix2002). Lichenicolous fungi might well offer some insights into this taxonomic question. Unfortunately, however, although numerous collections of these taxa were examined in the present study, they supported very few lichenicolous fungi and so this question remains unresolved.
With respect to geographical patterns, the austral region is clearly a centre of diversity for fungi inhabitating Siphula-like lichens, in the same way that this region is the centre of speciation for the hosts. Thus 12 of the species documented are known only from the Southern Hemisphere, including eight species known only from Tasmania. The apparent restriction of certain species to certain regions is probably an artefact of our sampling, and it is to be hoped that infected Siphula-like thalli attract more attention from collectors in the future. Of more interest is the fact that particular species of lichenicolous fungi are shared between Tasmania and New Zealand, Tasmania and South America or Tasmania, New Zealand and southern South America, patterns displayed by numerous lichen, bryophyte and vascular plant taxa. In contrast, the lichenicolous fungi recorded from hosts from southern Africa, Hawaii, the Northern Hemisphere, mainland Australia and the Neotropics were, in each case, restricted to their immediate geographic region. The two strictly siphulicolous species known from the Northern Hemisphere are Neolamya sp. (Hawaii) and Sphaerellothecium siphulae s.s., a fungus widely distributed across the entire arctic and boreal range of its host.

MZ thanks Paul Diederich, Javier Etayo and Andre Aptroot for most valuable discussions on the taxonomic status of Saania mobergii. The curators of H, O, TNS and UPS herbaria, and Leena Myllys, Yoshihito Ohmura and Stefan Ekman, assisted in locating and processing relevant Siphula specimens for study and loan. Financial support for AS was provided by IUT 20-30 and by the European Regional Development Fund (Centre of Excellence EcolChange). Financial support for JM was granted by the Lithuanian Research Council (grant no. MIP-17-5) and the Bilateral Exchange Programme between the Academies of Sciences of Lithuania and Estonia. The contribution by MZ was carried out within the framework of the research project of the Komarov Botanical Institute of the Russian Academy of Sciences “Biodiversity and spatial structure of fungi and myxomycetes communities in natural and anthropogenic ecosystems” (АААА-А18-118031290108-6) using equipment from its Core Facility Center “Cell and Molecular Technologies in Plant Science”; his visits to the TNS and UPS herbaria were supported by JSPS Invitation Fellowship for Research in Japan (no. S16173) and by the Rolf Santesson foundation, respectively. Some of the material studied was collected by GK during field surveys co-funded by the Australian Biological Resources Study (ABRS) and BHP Billiton under the Bush Blitz Programme, with laboratory work supported by an ABRS Tactical Taxonomy Grant.











