Within the family Graphidaceae, Graphis is by far the largest genus, containing over 300 currently recognized species (Lücking Reference Lücking2009; Lücking et al. Reference Lücking, Archer and Aptroot2009; Barcenas Peña et al. Reference Barcenas Peña, Lücking, Miranda-Gonzalez and Herrera-Campos2014). The great majority of species grow on trees and the ascomata often develop below the bark, eventually bursting through in order to release the ascospores. Graphis species are some of the most conspicuous crustose lichens in temperate and tropical forests. They possess elongate ascomata with their hymenium contained within raised excipular tissue that are commonly referred to as lirellae (Favre & Letrouit-Galinou Reference Favre and Letrouit-Galinou1964; Janex-Favre Reference Janex-Favre1964). They differ markedly from the majority of ascomata, most of which are radially symmetric. The latter expand radially to form mainly discoid apothecia in which the hymenium containing the asci and ascospores is permanently exposed to the atmosphere. In contrast, lirellae gradually elongate as approximately linear structures where the hymenium may be exposed only during periods of high humidity and wetting. At other times it is frequently enclosed by the raised excipular tissue. The Graphidaceae differ from most other lirellate lichens (e.g. Lithographa, Opegrapha) in their non-fissitunicate asci, the ascospores being released through an apical pore.
Our understanding of the growth and development of the reproductive structures of lichens has been seriously neglected, leaving a gap in our knowledge of their dispersal and ecology. There are also taxonomic implications since the size and shape of ascomata are often used to define species and genera. Size differences may simply reflect the growth stage of the lichen, rather than be genetically determined.
This investigation aimed to measure the growth, development and fertility of ascomata in two common members of the temperate Graphidaceae: Graphis elegans (Borrer ex Sm.) Ach. and G. scripta (L.) Ach.
Recent studies indicate that G. scripta, as currently understood, is a species complex (Pentecost Reference Pentecost2003; Neuwirth & Aptroot Reference Neuwirth and Aptroot2011) but the material used here all belonged to G. scripta s. str. as currently understood. Kraichak et al. (Reference Kraichak, Lücking, Aptroot, Beck, Dornes, Volker, Lendemer, Nelsen, Neuwirth and Nutakki2015) have recently found six to seven putative species within this complex using molecular phylogeny, but these did not correspond to morphologically recognizable taxa.
Thallus and ascoma growth were monitored using close-up photography. Photographs were taken with a micro-Nikkor lens fitted with a ring flash at focal lengths ranging from 5–25 cm using ASA 400 colour print film. Graphis elegans was monitored on the roots of beech (Fagus sylvatica) while G. scripta was monitored on stems of hazel (Corylus avellana). All ascomata were photographed when dry. Although the humidity of the site varied from visit to visit, there was no evidence that it affected the measurements, although this remains a possibility. All studied thalli were collected after the final measurements. Site details are provided in Table 1. Ascoma elongation rates were estimated by placing photographic prints under a Wild binocular microscope at ×25 magnification and comparing them in sequence with the lichen samples collected. Photographic distances were standardized using marked reference points adjacent to the lichen. Resolution on the photographs was c. ±20 μm and ±10 μm for the samples collected. Elongation rates were estimated for intervals between photographs and expressed as both µm d−1 and mm y−1. This method is similar to that used for measurements of Arthonia ascomata (Pentecost Reference Pentecost2014), but in the case of Graphis, the lengths of some of the ascomata could only be estimated by their appearance below the ruptured bark, and their true length was thus underestimated. Since differences in length rather than the absolute length was of interest in the growth studies, the method provided comparable results. To assess fertility, ten ascomata ranging from 1·8–2·2 mm in length were sampled at five points from the middle to the end (Fig. 1A). Thin sections were cut at each of these points with a razor, then mounted in water, gently squashed under a coverslip and examined at ×250 to determine the relative proportion of juvenile, mature and senescent/dehisced (brown) asci and spores. For this a scoring system was applied: 1, scarce in section; 2, frequent; 3, abundant.

Fig. 1 Relative frequency of three developmental stages of asci in Graphis scripta ascomata. A, sampling positions along an ascoma; B, juvenile asci; C, mature asci; D, senescent/dehisced asci.
Table 1 Details of sites used for growth monitoring of Graphis species

Radial growth rates of a small number of the thalli investigated are shown in Table 2. Data were limited owing to the frequent formation of mosaics and the often diffuse nature of the thalline edge. Thus these estimates are insufficiently precise for any detailed analysis. However, it appears that thallus diameter and thallus radial growth are not correlated.
Table 2 Radial growth rates of some of the Graphis thalli investigated in this study

Elongation rates for Graphis elegans ranged from 0·004–1·91 mm y−1 and averaged 0·39 mm y−1 for a sample of 69 ascomata collected from the roots of a beech tree (Table 3, Figs 2A, 3A & B). Ascomata averaged 1·06 mm in length and 0·27 mm in width. Most elongation rates ranged from 0·05–0·5 mm y−1 and only a few exceeded 1 mm y−1. Although ascoma length increased with age, the same was not true of the width, which soon reached its maximum value once the ascomata became exposed at the lichen surface. There was no significant correlation between ascoma length and elongation rate.

Fig. 2 Frequency histograms of ascoma elongation rate in Graphis. A, G. elegans; B, G. scripta.
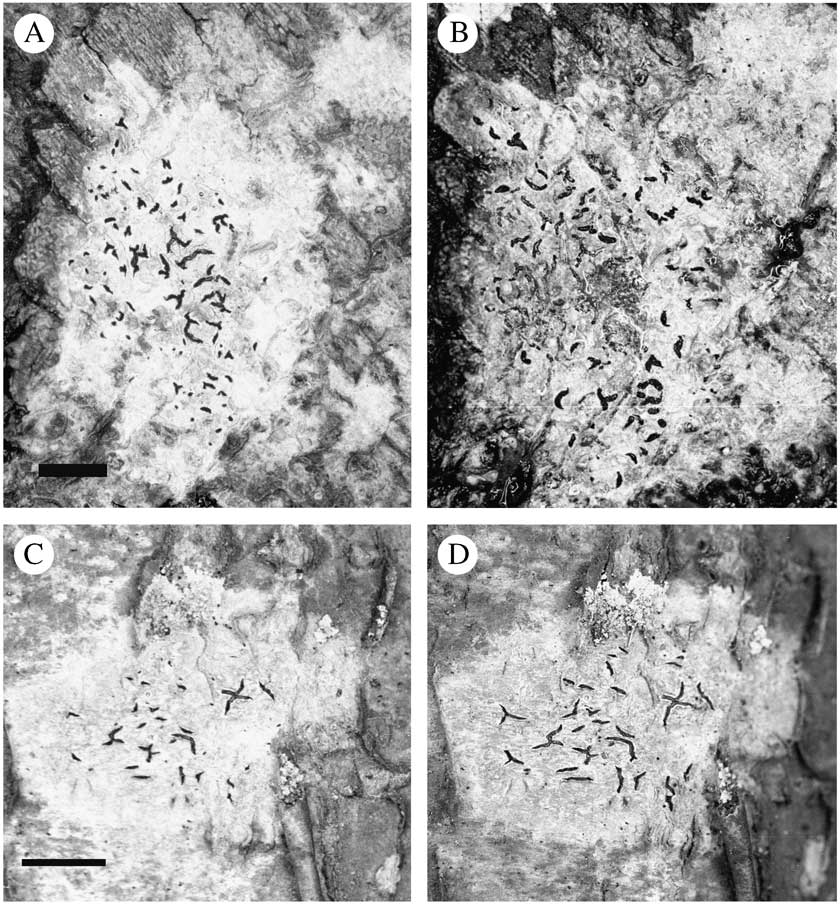
Fig. 3 Development and senescence of Graphis ascomata. A & B, G. elegans. A, 9th November 2000; B, 19th December 2001. C & D, G. scripta; C, 19th December 2001; D, 5th December 2002. Scale=5 mm.
Table 3 Data for ascoma dimensions and growth in Graphis species. All measurements refer to air-dried specimens and mean values are given ± 1SD

Mean elongation rates for G. scripta were significantly lower than those of G. elegans (Table 3, Figs 2B, 3C & D), as demonstrated by an analysis of variance (F=6·7; P=0·01). Rates ranged from 0·004–1·77 mm y−1 and averaged 0·28 mm y−1 while ascoma length averaged 1·28 mm. Most elongation rates were≤0·5 mm y−1 with a small number having rates exceeding this. This species grew on the smooth, young bark of hazel and it was suspected that the expansion of bark tissues as the tree branches increased in size could influence the elongation rate of the ascomata, since these developed under the bark. To test this, 70 elongation rates were measured on ascomata that grew approximately parallel to the direction of stem elongation, and another 70 at right angles (i.e. in the direction of bark expansion). An analysis of variance demonstrated no significant difference in elongation rates between the directions (P>0·05) so the data sets were combined. Again, ascoma length was not significantly correlated to ascoma elongation rate (r=0·099, P>0·05). For G. scripta, the largest ascomata rarely exceeded 3·5 mm in length. Using the mean elongation rate, these long ascomata would be 12–15 years old. Given a mean thalline radial growth rate of c. 0·47 mm y−1, such ascomata could be produced only in thalli exceeding c. 15 mm in diameter. Thalli from which the measurements were taken ranged from 20–35 mm in diameter. The age of ‘average’ ascomata in the G. elegans and G. scripta samples was 2·7 and 4·6 y respectively.
In G. scripta juvenile and mature asci appear to be more common near the ends of ascomata while at their centres, there are more senescent/dehisced and fewer mature asci (Fig. 1B–D). Ascomata longer than 2·2 mm were uncommon, but a small sample measuring up to 3·7 mm in length showed the same trend. Ascomata of G. elegans rarely attained a length of 2 mm in the thalli examined, but a small sample measuring 1·0–1·4 mm long showed a similar pattern of development. Examination of young ascomata in the length range 0·2–0·5 mm showed that asci in G. scripta only became visible in ascomata >0·35 mm in length. In these small fruit bodies, asci at all levels of development were found. In one case, a young ascoma 0·53 mm in length contained 17 juvenile, 6 mature and 3 senescent/dehisced asci. Using the mean elongation rate of 0·76 μm d−1 (Table 3) and a constant elongation rate over time, it appears that ascospore production begins approximately 1·5 years after ascoma initiation in G. scripta.
In a small sample (n=6) of lirellae of ‘average’ length (i.e. 1·0 mm and 1·3 mm for G. elegans and G scripta, respectively) the absolute number of juvenile, mature and senescent/dehisced asci were determined at the centre of the lirellae and the results shown in Table 4. The measurements confirm the high abundance of senescent/discharged asci and also demonstrate that young asci continue to be formed in the oldest region of these ascomata.
Table 4 Absolute numbers of asci found in thin sections of Graphis taken at the centre of ‘average’ ascomata. Values are per mm ascoma length

Lirellate ascomata are also found in the Arthoniaceae and Lecanographaceae, and in one species of the latter, Alyxoria (Opegrapha) varia, their elongation rate was found to decline with age (Pentecost Reference Pentecost2014). No evidence was found for this in Graphis however. Also, the mean elongation rate of both Alyxoria varia and Arthonia (Opegrapha) calcarea was in the range 0·15–0·16 mm y−1, considerably lower than the measurements obtained here. In other respects, these ascomata show similarities, since they all demonstrated early initiation of asci and ascospores, longevity of ascomata, and some evidence of decline in fertility with age of the hymenial tissues. Thin sections of Graphis ascomata revealed the regular appearance of asci containing brown and withered spores or appeared empty with brown collapsed walls which appeared with increasing frequency as the ascomal tissues aged. Three possibilities present themselves: the ascospores had failed to develop fully; asci had failed to discharge spores, or the brown-walled empty asci represented the remnants of asci that had discharged their spores. Ascomata should evolve to produce a high proportion of successfully discharged spores unless the habitat is in some way unsuitable for the full development of these lichens, so a high proportion of moribund ascospores would not be predicted although it has been noted in other lichens (Carmen et al. Reference Carmen, Divakar, Zhang, Gonzalez and Struwe2013) and could frequently occur in the lichenized ascomycetes. The increase in abundance of moribund asci with tissue age demonstrates a cumulative effect of abortive/discharged asci over time. The Graphis study sites were not heavily impacted by air pollution although total atmospheric nitrogen depositions (http://www.pollutantdeposition.ceh.ac.uk/data) were moderately high at 16–19 kg ha−1 y−1.
The greater rate of elongation in G. elegans could be due to a number of factors such as the growth rate and vigour of the thalli, the different substratum, differences in their morphology or the different environmental setting, so it may not be species-specific. In G. elegans, the lirellae are of the compound type, where the mature hymenium is divided into two or more separate channels or ‘slits’ separated by excipular tissue. As a result the lirellae are broader than those in G. scripta. The ranges of elongation rates of the two taxa were similar despite the significant difference in their means, and more extensive study is required to clarify these differences.
The timing of the growth measurements did not indicate any seasonal variation in ascomal growth. Analysis of variance demonstrated no significant difference between G. scripta elongation rate during the summer compared with the rest of the year (F=0·81, P=0·37). However, the photographic sequence was normally conducted over long periods (>1 year) and was not designed to detect seasonal differences so further work will be required to confirm these findings. Future studies should also attempt to correlate microclimatic data with more regular imaging so that the seasonal growth of ascomata can be more fully understood.









