Introduction
Dirina is a subtropical genus of lichenized fungi with a preference for areas with Mediterranean climates extending into adjacent temperate and tropical regions. It is predominantly distributed over the Northern Hemisphere (Tehler Reference Tehler1983; Tehler & Irestedt Reference Tehler and Irestedt2007). All species are mainly restricted to coastal habitats and may be very common locally. Dirina uses Trentepohlia as photobiont and the lichen life form is crustose with a white-greyish to greyish brown thallus; the cortex is composed of anticlinally or loosely anticlinally arranged hyphae and the medulla is normally white. The fruiting bodies are apothecioid, circular but often undulating in outline, usually sessile but sometimes also immersed, up to 2·5 mm in diameter with a pruinose disc. The thalline margin is well developed; all species have a characteristically carbonaceous hypothecium, distinct from the neighbouring white medulla. The asci are thick-walled, fissitunicate and contain eight fusiform, 3-septate, hyaline spores, within the size range of 15–35×4–8 µm. The conidia are filiform and curved; most species produce soredia for vegetative dispersal, and for which usually distinctly sorediate morphs have developed. Secondary metabolites are typically erythrin, lecanoric acid and the unidentified substance C (see below) present in all species.
The history of the genus Dirina and more details of the morphological and chemical aspects, as well as images of the species not shown here, can be found in earlier references (Tehler Reference Tehler1983, Reference Tehler1985, Reference Tehler1986, Reference Tehler1988, Reference Tehler, Nash, Ryan, Gries and Bungartz2002; Tehler et al. Reference Tehler, Feige and Lumbsch1995).
Much has happened in the past thirty years since the last revisionary study of the genus Dirina was published (Tehler Reference Tehler1983). At that time seven species, three subspecies, and two forms were recognized and by 2007 (Tehler & Irestedt Reference Tehler and Irestedt2007) only five species had been added to the list. In the present study, the number of Dirina species has risen to 24, of which nine are new to science. The primary source of the new knowledge comes from fresh material collected during numerous field trips made by two of the authors (AT and DE), to both old and new distribution areas around the world. Another major source of the new knowledge is data obtained from molecular studies.
Here, we will incorporate and analyze all new information not previously treated for the genus Dirina by combining data from molecular studies with new and old morphological and chemical evidence. We will also touch upon the biogeography of this exciting group in a phylogenetic context, and discuss it in relation to the recently revised sister genus Roccella and other genera in the family Roccellaceae (Tehler et al. Reference Tehler, Dahlkild, Eldenäs and Feige2004, Reference Tehler, Irestedt, Bungartz and Wedin2009a , Reference Tehler, Irestedt, Wedin and Ertz b , Reference Tehler, Irestedt, Wedin and Ertz2010; Tehler Reference Tehler2007; Tehler & Irestedt Reference Tehler and Irestedt2007; Ertz & Tehler Reference Ertz and Tehler2011).
Materials and Methods
Terminal taxa and taxon sampling
The study is based on collections made by Anders Tehler from Angola (2009), the Azores (2000, 2010), Canary Islands (2000, 2011), Cape Verde (2009/2010), Croatia (1980), France (2008), French Antilles (1981), Galapagos Islands (2005), Germany (1977), Gibraltar (2009), Great Britain (1979), Greece (1978), Hawaii (2010), Italy (1979, 2008), Mauritius (2003), Mexico (1976, 1993, 1995, 1996, 2007), Morocco (1978), Netherland Antilles (2006), Norway (1981), Peru (1981, 2006), Poland (1977), Portugal (1979, 2010), Senegal (2011), Socotra Island (2008), Spain (1976, 1979, 2007, 2009), Sweden (1976, 1977, 1982, 1983, 1998, 2007), United States (1976, 1981, 1992, 1993, 1994, 1995, 1997, 2008, 2010); on collections made by Damien Ertz from Belgium (s.ann.), Canary Islands (2009, 2011), Cape Verde (2011), France & Corsica (2011), Madagascar (2008), Madeira (2007), Portugal (2011), Spain (2011), United States (2008); and on specimens from the herbaria: ABL, B, BG, BM, BR, C, CANB, CBFS, COLO, DUKE, E, FH, FI, G, GBG, GZU, H, L, LD, LISU, LWG, M, MEL, MSC, NY, O, PAD, PC, RO, S, TUR, U, UC, UPS, US, W, ZT.
A total of 203 Dirina samples were used for the molecular investigations (Appendix 1), representing all Dirina species recognized worldwide. We tried to achieve a sample series with large morphological variation and with a large and representative geographical distribution. DNA voucher collections were also used for the TLC investigations. Designated voucher specimens are deposited in S and BR. All material in S is searchable and all types are imaged through the database Krypto-S at http://www.nrm.se/krypto-s.
Amplification, sequencing and alignment
The phylogeny of Dirina was examined by analyzing DNA sequences obtained from four loci: the nuclear ribosomal internal transcribed spacers ITS 1 and ITS 2 (and the intermediate 5.8s region), the nuclear large subunit ribosomal RNA gene (LSU), the second largest RNA polymerase subunit (RPB2) and the β-tubulin gene. Extractions, amplifications and sequencing procedures generally follow Tehler & Irestedt (Reference Tehler and Irestedt2007) and Tehler et al. (Reference Tehler, Irestedt, Bungartz and Wedin2009a , Reference Tehler, Irestedt, Wedin and Ertz b , Reference Tehler, Irestedt, Wedin and Ertz2010).
For the β-tubulin gene several new primers were designed, based on some initial Dirina sequences obtained using the primers Bt3-LM and Bt-10LM (Myllys et al. Reference Myllys, Tehler and Lohtander2001). The β-tubulin region was subsequently amplified in two partially overlapping fragments. For the first fragment the primer pair Bt3-MIa (ATC ACA ACG CGA ACA ACT GCT GA) and Bt-intR (CAC AAG TTG ATG CAC TGA AAG AGT) was primarily used. For samples that produced weak or unspecific PCR-products with the former primer combination, the primer Bt3-MIa was replaced with Bt3M-MIb (CAA AAG AAA TAT GTT CCG CGT GC). The second fragment was amplified with the primers BtintF (AAG AGT TCC CTG ACC GCA TGA T) and Bt-10LM (Myllys et al. Reference Myllys, Tehler and Lohtander2001). The thermocycling program for both β-tubulin fragments started with initial denaturation at 95°C for 5 min, followed by two cycles of 95°C for 30 s, 54°C for 30 s, 72°C for 60 s, followed by another two cycle phase and one 36-cycle phase with identical temperatures and intervals, except that the annealing temperatures were reduced to 52°C and 50°C, respectively. The thermocycling program ended with an extension at 72°C for 5 min.
The sequence fragments were assembled to complete sequences with SeqMan II(tm) (DNASTAR Inc.). Ambiguous nucleotide positions were coded with the appropriate IUPAC codes. Alignments were carried out using the computer program ClustalX 2.0.9 (Larkin et al. Reference Larkin, Blackshields, Brown, Chenna, McGettigan, McWilliam, Valentin, Wallace, Wilm and Lopez2007) under Multiple Alignment Mode, with all parameters set to default. Before the final phylogenetic analyses, some minor manual adjustments were made in the ITS alignments at positions where ClustalX had obviously failed to create the most parsimonious solution.
Choice of outgroup
The outgroup taxa and the rooting taxon were chosen from the fruticose genus Roccella which is the well-corroborated sister group to Dirina (Tehler Reference Tehler1983; Ertz & Tehler Reference Ertz and Tehler2011 and references therein).
Phylogenetic analysis
For the phylogenetic analyses, we used the programs T.N.T. Tree Analysis Using New Technology 1.1 (Goloboff et al. Reference Goloboff, Farris and Nixon2008) and MrBayes 3 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). In all analyses gaps were treated as missing data.
The parsimony analyses used the New Technology search with sectorial search, ratcheting, drifting, tree fusing and driven search options in effect, all using default settings. Resampling tree searches were done with parsimony jackknifing (Farris et al. Reference Farris, Albert, Källersjö, Lipscomb and Kluge1996) under the New Tech search as implemented in T.N.T. (Goloboff et al. Reference Goloboff, Farris and Nixon2008); 1000 replicates submitted to TBR branch swapping were conducted. In parsimony jackknifing the data are internally resampled with a jackknifing technique to find well-supported groups. Resampling works by calculating a tree for each of a large number of subsamples (pseudoreplicates) of characters from the data, then finding a summary tree, which comprises the groups occurring in the majority of trees for subsamples. The tree for each pseudoreplicate is found by parsimony analysis, and each pseudoreplicate is formed by randomly selecting characters from the data without replacement, each character having a fixed chance 1/e (about 36%) of being excluded. With this resampling technique, the actual number of characters used may vary from replicate to replicate. Groups found in less than 50% of the trees for pseudoreplicates were discarded, thus eliminating unjustified (poorly supported) resolution caused by ambiguous data sets.
Bayesian inference (Huelsenbeck et al. Reference Huelsenbeck, Ronquist, Nielsen and Bollback2001; Holder & Lewis Reference Holder and Lewis2003) was used to estimate the phylogenetic relationships. The models for nucleotide substitutions used in the analyses were selected for each gene individually by applying the Akaike Information Criterion (AIC) (Akaike Reference Akaike, Petrov and Csaki1973) and the program MrModeltest 2.2 (Nylander Reference Nylander2005) in conjunction with PAUP* (Swofford Reference Swofford1998). Posterior probabilities of trees and parameters in the substitution models were approximated with MCMC and Metropolis coupling using the program MrBayes 3.2 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). The analyses of the combined data set were performed with the models selected for the individual gene partitions, as well as with the parameter proportion of invariable sites (I) excluded for partitions with a gamma distribution included in the model. The exclusion of invariable sites could be justified since the gamma distribution already allows for sites with very low rates (Yang Reference Yang2006) and as applying many model parameters in a Bayesian analyses may lead to over-optimization where model parameters have difficulties converging on the same target distribution (Nylander et al. Reference Nylander, Ronquist, Huelsenbeck and Nieves-Aldrey2004). All parameters in the selected models were allowed to vary among the partitions, except the topology that was constrained to be the same. All chains were run for 50 million generations, with trees sampled every 1000th generation. The trees sampled during the burn-in phase (i.e. before the chain had reached its apparent target distribution) were discarded. The Bayesian Inference analyses were run on the University of Oslo Bioportal (www.bioportal.uio.no). The plots of the likelihood and model parameters against generation provided by the Bioportal were used to check for convergence and to estimate when the chains had reached their apparent target distribution.
Chemistry
For routine screening of secondary chemistry, ‘spot tests’ on thalli and ascomata were performed with a water solution of potassium hydroxide (K), and with a commercial solution of potassium hypochlorite (C). The reaction with p-phenylendiamine was not performed, for health reasons and because it is known to be invariable for Dirina (Tehler Reference Tehler1983). Thin-layer chromatography (TLC) of acetone extracts was performed on 20×20 cm silica gel 60 F254 layer aluminium or glass plates using solvent systems C, EA and G. For the visualization of the spots, 10% sulphuric acid was used as a reagent (Orange et al. Reference Orange, James and White2001). Punctelia subrudecta and Trapelia glebulosa were used as control species for the identification of lecanoric and gyrophoric acids, respectively.
Results
Loci and alignments
Altogether we analyzed ß-tubulin, ITS rDNA, nuLSU rDNA and RPB2 sequences from 203 Dirina samples and three Roccella outgroup species (Appendix 1). Most sequences were newly produced for this study and have been deposited in GenBank (KC107837–KC108627). In a few cases we were not able to read the full target region and a few sequences consequently miss short fragments, mainly in the beginning or at the end of the sequences. Taking this into account, the individual gene alignments vary as follows: for the protein coding RPB2 gene, no insertion or deletions were detected and the analyzed partition consisted of 923 sites; the LSU partition consisted of 906 sites and the individual sequences varied in length between 886 bp and 891 bp; the ITS partition consisted of 824 sites and the individual sequences varied in length between 575 bp and 681 bp; the β-tubulin partition consisted of 836 sites and the individual sequences varied in length between 836–834 bp. The concatenated data set contained 3489 aligned sites, of which 1278 were variable and 1100 parsimony informative.
We were unable to sequence eight samples which were included in the data set as missing data: Dirina madagascariensis 13215 for β-tubulin; D. immersa 9322 and D. madagascariensis 13086 for ITS; D. indica LWG-111, D. indica LWG-112, D. indica LWG-115 and D. indica LWG-117 for LSU; D. fallax 16421 for RPB2. The aligned data set contained 206 terminals and 3489 aligned sites.
Analyses of the ITS region before and after the manual adjustments also produced trees that were topologically fully congruent (only nodal support values were marginally changed), suggesting that modest adjustments in ambiguous regions in ITS only have minor effects on the topological results. Similarly, Tehler et al. (Reference Tehler, Irestedt, Wedin and Ertz2009b , Reference Tehler, Irestedt, Wedin and Ertz2010) found that analyses of alternative ITS alignments of New and Old World Roccella species had minor effects on the topological results. In addition, the independent gene trees were found to have no significant topological conflicts. Based on these observations, we found it was warranted to not exclude any positions for the final analyses of the concatenated data set, as the most variable regions may be potentially important for resolving particularly terminal branches in the tree.
Phylogenetic analyses and species concepts
There were no significant conflicts between the different gene tree topologies. The parsimony jackknife (PJ) tree (not shown) and the Bayesian tree (Figs 1–4) were mutually without topological conflict as obtained from the combined analyses. The most parsimonious trees had a best score of 4459 and the strict consensus tree (not shown) was of the same general topology as that of the PJ tree.

Fig. 1. Overview tree showing Dirina species, redrawn from the Bayesian tree (Figs 2–4). Posterior probability values shown above nodes; parsimony jackknife frequencies shown below nodes, frequencies below 50% indicated by X. New World species indicated by bold font.
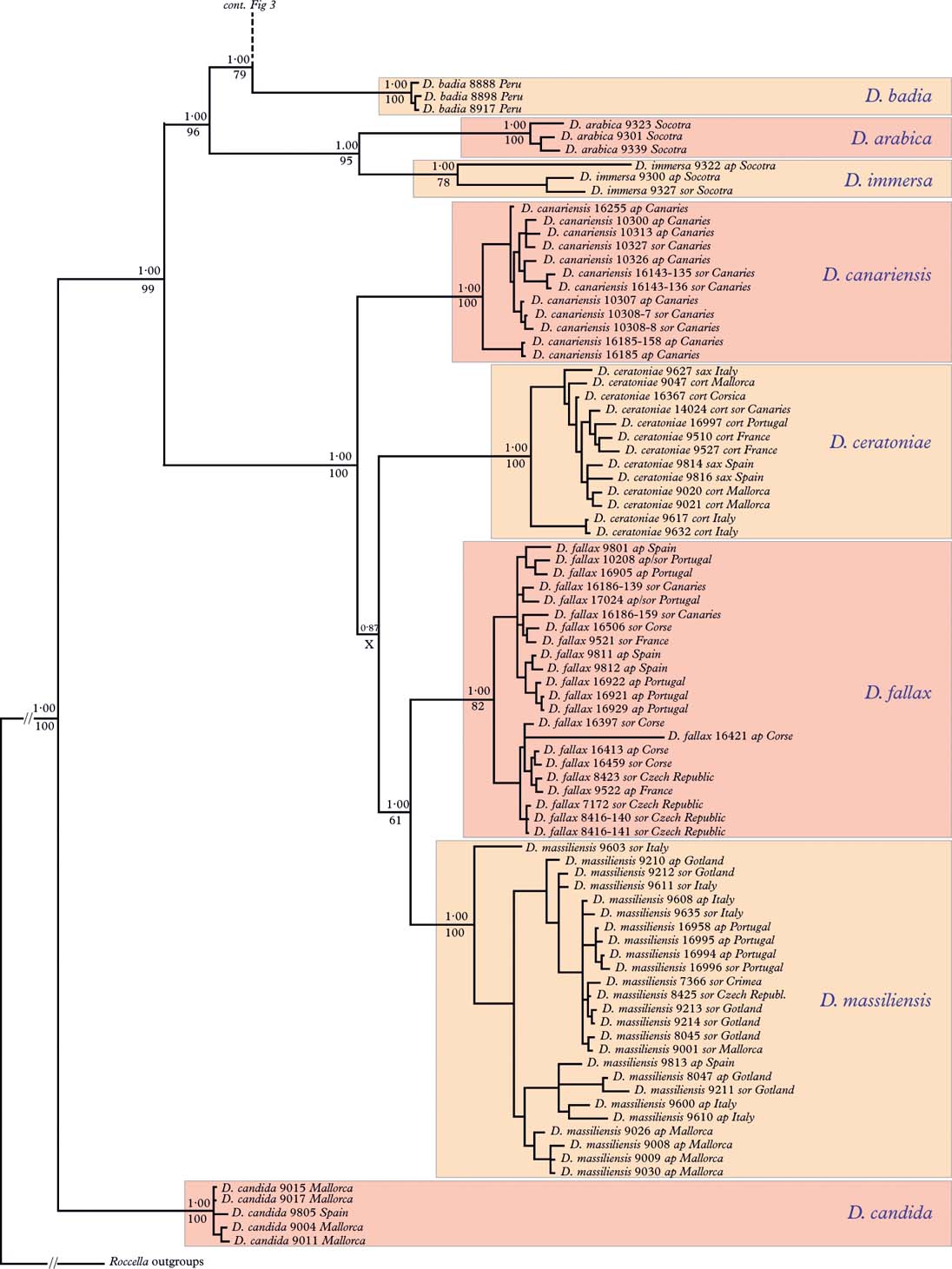
Fig. 2. Part one of tree received from Bayesian analysis with posterior probability values shown above nodes. Parsimony jackknife frequencies received from parsimony analyses using TNT, shown below nodes, frequencies below 50% indicated by X; ap=apothecial, sor=sorediate, sax=saxicolous, cort=corticolous. Support values not shown for intraspecific nodes. Specimen numbers correspond to collection number (cf. Appendix 1). Geographical origin indicated for each specimen. Boxes for readability only. Parts two and three are shown in Figs 3 and Reference Ayala4.

Fig. 3. Part two of tree received from Bayesian analysis with posterior probability values shown above nodes. Parsimony jackknife frequencies received from parsimony analyses using TNT, shown below nodes, frequencies below 50% indicated by X; ap=apothecial, sor=sorediate, sax=saxicolous, cort=corticolous. Support values not shown for intraspecific nodes. Specimen numbers correspond to collection number (cf. Appendix 1). Geographical origin indicated for each specimen. Boxes for readability only. Parts one and three are shown in Figs 2 and 4.

Fig. 4. Part three of tree received from Bayesian analysis with posterior probability values shown above nodes. Parsimony jackknife frequencies received from parsimony analyses using TNT, shown below nodes, frequencies below 50% indicated by X; ap=apothecial, sor=sorediate, sax=saxicolous, cort=corticolous. Support values not shown for intraspecific nodes. Specimen numbers correspond to collection number (cf. Table 1). Geographical origin indicated for each specimen. Boxes for readability only. Parts one and two are shown in Figs 2 and 3.
The Bayesian tree was generally better resolved and received stronger node support than the parsimony jackknife tree. Nodes without parsimony jackknife support also had poor posterior probability (PP) values, except for the major group Dirina angola through D. madagascariensis which received full PP support but a PJ frequency below 50% (Figs 1–4).
The a priori selection of nucleotide substitution models suggested that the GTR+I+G model had the best fit for ITS, LSU, and RPB2, while HKY+I+G had the best fit for β-tubulin. In the Bayesian inference analyses of the combined data set, where we strictly used these models, the likelihood value, as well as several model parameters, did not converge on the same target distribution. The posterior of the proportion of invariable site parameter in particular fluctuated considerably. In contrast, in the runs where the proportion of invariable sites parameter (I) was excluded from the models, all parameters as well as the likelihood value converge on the same target distribution. The results suggest that we have problems with over-optimization in the analyses where the posterior of the proportion of invariable sites parameter was included. However, when comparing the resulting tree topologies from the individual runs with the proportion of invariable site parameter included, as well as comparing them with the runs where the proportion of invariable site parameter was excluded, the tree topologies were found to be almost identical. The only topological differences observed were found in a few weakly supported nodes (posterior probabilities<0·80), while all strongly supported nodes (posterior probabilities>0·95) remained the same. Overall, the results suggest that strongly supported nodes are robust regardless of whether the proportion of invariable site (I) parameter is included or not, which makes sense since the gamma distribution also allows for sites with very low rates (Yang Reference Yang2006). However, due to the convergence problem in the analyses, where the proportion of invariable site parameter was included, we here present the tree based on the analyses where the proportion of invariable site parameter was excluded (Figs 2–4). This final inference was based on a total of 100 000 samples from the posterior, after discarding the burn-in phase.
Phylogenetic species recognition similar to that of Grube & Kroken (Reference Grube and Kroken2000) and Taylor et al. (Reference Taylor, Jacobson, Kroken, Kasuga, Geiser, Hibett and Fisher2000) has been applied for the recognition of species in Dirina, that is, concordance of multiple gene trees to identify the smallest congruent monophyletic groups between which recombination, as expressed by incongruence between single-gene trees, normally does not occur. This type of species concept has recently been successfully applied in lichen fungal phylogeny (Tehler et al. Reference Tehler, Irestedt, Bungartz and Wedin2009a , Reference Tehler, Irestedt, Wedin and Ertz b , Reference Tehler, Irestedt, Wedin and Ertz2010; Wedin et al. Reference Wedin, Döring and Gilenstam2004, Reference Wedin, Westberg, Crewe, Tehler and Purvis2009). The specimens examined in the combined data set of the present study (Figs 1–4) cluster into logical entities recognized as species. Each of the species entities receives significant PP and PJ support for all except one, Dirina jamesii, which lacks both PP and PJ support and which is recognized only in the strict consensus tree. The Dirina jamesii specimens will for the time being be retained as a species on the basis of the consensus tree and on the relative morphological and anatomical homogeneity, such as sessile to semi-sessile ascomata and the pseudohypothecial extension.
The sister species to the rest of the genus Dirina is D. candida, which has immersed ascomata. Three other species also have immersed ascomata (D. insulana, D. mexicana and D. immersa) but they are all placed in unrelated groups in various places in the tree. The most parsimonious hypothesis is that they have reverted to the primitive character state with immersed ascomata (see Discussion below).
The greatest species diversity is found in the Mediterranean and Macaronesian region, with 10 species: Dirina candida, D. massiliensis, D. ceratoniae, D. fallax, D. canariensis, D. monothalamia, D. sorocarpa, D. teichiodes, D. cretacea and D. insulana (Figs 1–4).
On basis of the phylogenetic tree, the general distribution patterns in Dirina are best explained by vicariance, although several events involving long distance dispersal must be postulated to account for the distribution of some species.
Morphology
Thallus. All species are crustose and little variation is seen in thallus morphology. Species growing on calciferous rock are generally more whitish and have a thicker, more chalk-like consistency of the thallus and medullary texture. Species growing on acidic or volcanic rock are usually darker, thinner and have a less chalk-like thallus and medullary texture.
Medulla. Corticolous species have a medulla with a more or less observable hyphal texture, particularly near the substratum. Saxicolous species generally have a medulla of chalk-like texture, in which the hyphae are less prominent and hardly discernible but sometimes loose hyphae may develop in contact with the substratum. To observe these hyphae, use a stereomicroscope and gently lift off small pieces of the thallus from the substratum with a sharp knife or razor. Some species have a C+ red reaction, both in the medulla and the cortex layer, whereas most species have a C+ red reaction only in the cortex layer. To observe the medulla C reaction, make a perpendicular cut in the thallus, preferably at the edge of the piece of rock on which the specimen was collected. Apply the C solution exclusively to the medulla by using a sharp needle or knife.
Ascomata. Nearly all species of Dirina have pluricarpocentral discothecia-type ascomata. One species, Dirina cretacea, has stromatoid-like pluricarpocentral synascomata. A few species have immersed, monocarpocentral, solitary ascomata [see Tehler (Reference Tehler1990) for a discussion on ascoma development in Arthoniales]. The discothecia-type of ascomata are usually sessile with a clearly constricted base. Sessile or semi-sessile ascomata with an unconstricted base may be present in some species.
Disc. The pruinose surface of the disc is in many species smooth and even or only finely rimose with very intricate cracks, such as in Dirina canariensis (Fig. 5E). In other species the disc surface is more or less rimose, that is marked with numerous cracks running in all directions along the disc surface as in Dirina fallax (Fig. 6A). This feature may be difficult to evaluate. The distinction between an even surface and a surface with subtle rimulae may be hard to make. In some cases it may even be transitional, and variation between ascomata within single specimens may range from a completely even surface to one with a very intricately cracked surface. In these cases majority rule should be applied when the feature is studied, and at least ten ascomata and disc surfaces should be observed in high magnification under stereomicroscope before an opinion can be reached.

Fig. 5. Dirina species, habitus. A, D. angolana, holotype [Angola, 2009, Tehler 9730 (S-F210729)]; B, D. arabica, holotype [Socotra Island, 2008, Tehler 9301 (S-F210762)]; C, D. astridae, holotype [Mauritius, 2003, Tehler 8502 (S-L55012)]; D, D. indica, isotype [India, Gujarat, 2008, Rawal 09-011280 (S-F218410)]; E, D. canariensis, ascomata close up, holotype [Canary Islands, 2011, Tehler 10300 (S-F210768)]; F, D. canariensis, holotype [Canary Islands, Tehler 10300 (S-F210768)]. Scales: A–F=1 mm. Photographs Emma Hultén. In colour online.

Fig. 6. Dirina species, habitus. A & B, D. fallax, neotype [Sardinia, 1987, Ahti 47193 (S-F184389)], ascomata close up (A) and overview (B); C, D. fallax, [lectotype of Dirina repanda var. schistosa Bagl., Sardinia, s. ann., Gennari s. n. (S-F184363)]; D, D. madagascariensis, isotype [Madagascar, 2008, Ertz 13216 (S-F213217)]; E & F, D. pacifica, holotype [Hawaii, 2010, Tehler 10128 (S-F210836)], variation in thallus and thalline margin colour. Scales: A–F=1 mm. Photographs Ramona Ubral Hedenberg. In colour online.
Chemistry
We found erythrin, lecanoric acid and eleven unidentified substances in the genus Dirina in c. 140 specimens investigated by TLC (Table 1). Erythrin and the unidentified substance C (UV+ bluish before heating) were present in all species. Lecanoric acid was present in all species, with the exception of D. mexicana.
Table 1. Lichen substances detected by TLC in the species of Dirina.

* Rf values in solvents C and G are the ‘Relative Rf values’ as defined by Orange et al. (Reference Orange, James and White2001, p. 64) and calculated with reference to norstictic acid selected as standard.
+substance nearly always present; (+) substance sometimes or rarely present.
Traces of lecanoric acid have previously been detected by HPLC in Dirina mexicana (Tehler et al. Reference Tehler, Feige and Lumbsch1995). The substance is probably present in small amounts in D. mexicana and thus difficult to detect by TLC. This might also explain its absence in a few samples in some other species. Interestingly, several substances are more or less apomorphic to various groups and support the species circumscription for those clades. For instance, the very characteristic substance F (greyish with very low Rf value) is present only in Dirina mexicana, D. pacifica and sometimes in D. catalinariae, three neotropical species. It is absent in Dirina pallescens, the sister species of the clade D. catalinariae + D. mexicana, and in D. paradoxa, the sister species of D. pacifica. Substance J is autapomorphic for Dirina madagascariensis and substance A is autapomorphic for D. angolana. Substance B is always present in Dirina angolana, D. catalinariae, D. pallescens and D. sorocarpa.
Taxonomy
Dirina Fr.
Fries 1825: 244.—Dirina Fr. sect. Eudirina Redinger 1936:126. nom. illeg.; type: Dirina repanda Fr.=Dirina ceratoniae (Ach) Fr.
Dirinopsis De Not. 1846: 187; type: Dirinopsis massiliensis De Not.=Dirina massiliensis Durieu et Mont.
Thallus crustose, effuse, smooth, surface becoming cracked, often slightly verruculose or areolate, sometimes bullate, usually creamy white to white-grey or white-green, sometimes dark grey or grey-brown or white-yellow, usually pruinose sometimes subglabrous, prothallus brown or sometimes nearly white, loose, but when contiguous crust-like and black. Soralia when present punctiform, rimiform, globose or maculiform, usually of paler colour than the surrounding thallus. Cortex with the hyphae mainly anticlinally arranged; the separate hyphae are hyaline or nearly so in a hyaline or pale yellow-brown gelatinous substance; numerous crystals often incorporated in the cortex layer making the separate hyphae hard to distinguish. Medulla usually white and chalk-like, but near the substratum often composed of loose hyphae. Photobiont Trentepohlia.
Ascomata apothecioid or stromatoid, lecanorine, nearly always present and numerous (except in sorediate forms), solitary or aggregated, usually sessile with constricted base, sometimes immersed or sub-immersed, rarely substipitate, evenly dispersed over thallus surface but not near the margins; disc pruinose, white-grey to dark grey; thalline margin when young entire, when old undulating or strongly undulating; proper exciple (parathecium) thin or inconspicuous. Hypothecium carbonaceous or dark brown, usually sharply defined towards the white medulla. Hymenium hyaline, 50–140 µm thick; paraphysoids unbranched or sparsely branched, 1–2 µm diam.; hymenial strands thin or inconspicuous. Epithecium brownish, 35–80 µm thick; paraphysoids continued from the hymenium, branched and intertwined, with smooth or slightly ornamented tips, 1–3 µm diam.; incorporated crystals often make the separate hyphae hard to distinguish. Asci hyaline, clavate, bitunicate, constantly 8-spored, fissi- or semifissitunicate, 70–120×12–18 µm. Ascospores hyaline and smooth, when old sometimes brownish, fusiform or obtusely fusiform, usually curved but also straight, often with one end tapering more than the other, 3-septate; gelatinous sheath missing or inconspicuous.
Pycnidia numerous, few or absent, evenly dispersed over the thallus surface, often with a preference for the margins or the immediate margin, immersed or slightly elevated like black or dark brown dots. Conidia, only microconidia seen, thread-like and sickle-shaped, 12–16×1 µm.
Chemistry
Erythrin, lecanoric acid, and the unidentified substances A–K. Spot tests: thallus surface C+ red, K−, KC+ red, PD−; medulla C− or sometimes C+ red, K−, sometimes KC+ red, PD−; hymenium itself I+ red-brown but, depending on iodine concentration, a slightly I+ blue reaction may occur in the transition zones epithecium/hymenium and hypothecium/hymenium and in the hymenial strands, K/I+ blue; hypothecium K+ dark olive green.
Distribution and habitat
Except for a few species, the genus is confined to the Northern Hemisphere. All species grow on high vertical cliffs and rocks and/or on trees and shrubs along arid and mainly subtropical coasts around the world. Habitats are on the immediate coastline, usually not ranging more than a couple of km inland. The species grow with a northerly or southerly exposition depending on hemisphere, well above the littoral and away from direct sea-spray, usually at altitudes between 10–200 m depending on exposure to the sea.
Key to Dirina species
Many, or even most, Dirina species are very similar in habit. Discrete characters are few and determination may be difficult since indiscrete, more or less overlapping characters will also have to be used in the key. Most species are endemic or have restricted distributions and the likelihood of a correct species determination can be correlated to its geographical distribution with great accuracy. However, some very similar sympatric species, such as Dirina monothalamia and D. teichiodes, cannot be reliably distinguished from one another except by examining their DNA-data. This is also true for sterile, sorediate morphs of Dirina fallax and D. canariensis (see Discussion). Furthermore, the character with C− or C+ red medulla is very difficult to examine in specimens with thin thalli which is often the case with sterile morphs. In such cases, Dirina insulana, which is also present in its sterile form in the Canary Islands, may be indistinguishable from D. fallax and D. canariensis. Another difficult and variable character is the medulla with loose hyphae near the substratum. Saxicolous Dirina ceratoniae is very hard to distinguish from D. massiliensis, but in D. ceratoniae the medullary hyphae near the substratum are usually more or less loose or byssoid. However, in some specimens, particularly those with thin thalli, this character may fail or be very difficult to see.
-
1 Specimens mainly fertile, ascomata present ... 2
Specimens mainly sterile, soralia present ... 31
-
2(1) Saxicolous ... 3
Corticolous ... 23
-
3(2) Growing on calciferous rock ... 4
Growing on acidic rock ... 11
-
4(3) Ascomata sessile with constricted base ... 5
Ascomata immersed or semi-sessile with unconstricted base ... 9
-
5(4) Medulla chalk-like, but with loose hyphae near the substratum ... 6
Medulla chalk-like, also near the substratum ... 7
-
6(5) Disc pruina with rimose surface (Europe, usually corticolous sp.) ... D. ceratoniae
Disc pruina with even surface (Caribbean, usually corticolous sp.) ... D. paradoxa
-
7(5) Ascospores 25–35 µm long [mean value 30 µm] ... D. madagascariensis
Ascospores 17–26 µm long [mean value 22 µm or less] ... 8
-
8(7) Medulla C+ red (Socotra Island) ... D. arabica
Medulla C− ... D. massiliensis
-
9(4) Ascomata pluricarpocentral, stromatoid ... D. cretacea
Ascomata monocarpocentral, non-stromatoid ... 10
-
10(9) Disc faintly C+ red (Socotra Island) ... D. immersa
Disc C− (Mediterranean) ... D. candida
-
11(3) Ascomata immersed ... 12
Ascomata sessile or semi-sessile ... 13
-
12(11) Medulla C+ red ... D. insulana
Medulla C− ... D. mexicana
-
13(11) Disc pruina with rimose surface (cf. Fig. 6A) ... 14
Disc pruina with even or only finely rimose surface (cf. Fig. 5E) ... 17
-
14(13) Medulla C+ red ... D. catalinariae
Medulla C− ... 15
-
15(14) Ascospores 27–33 [mean length 30 µm] (Peru) ... D. badia
Ascospores [mean length 24 µm] ... 16
-
16(15) Thallus surface faintly red, ascospore width mean 5·5 µm (Europe and Macaronesia) ... D. fallax
Thallus surface red, ascospore width mean 4·5 µm (Hawaii and Galapagos) ... D. pacifica
-
17(13) Disc C− or faintly C+ red, only sometimes C+ red ... 18
Disc C+ red ... 20
-
18(17) Medulla chalk-like also near the substratum (Ascension, St Helena, Angola) ... D. jamesii
Medulla chalk-like but with loose hyphae near the substratum ... 19
-
19(18) Ascomata common, usually in the absence of soralia (Caribbean) ... D. paradoxa
Ascomata uncommon, always in the presence of soralia (Cape Verde) ... D. sorocarpa
-
20(17) Ascospore mean width 5·5 µm (Mascarenes) ... D. astridae
Ascospore mean width 4·5 µm ... 21
-
21(20) Disc pruina with even surface, ascomata slightly smaller >1·5 mm diam. ... (Canaries) ... D. canariensis
Disc pruina with finely rimose surface, ascomata slightly larger >2 mm diam. ... (Cape Verde) ... 22
-
22(21) Thalline margin usually slightly undulating, thallus surface plane (cf. Fig. 7D) ... D. teichiodes
Thalline margin usually undulating to strongly undulating, thallus surface plane to slightly rugose-verruculose (cf. Fig. 7A) ... D. monothalamia
Saxicolous species
-
23(2) Ascospores longer, mean length 29–31 µm ... 24
Ascospores shorter, mean length 23–26 µm ... 28
-
24(23) Disc C− or faintly C+ red ... 25
Disc C+ red ... 26
-
25(24) Ascomata slightly smaller, up to 2·0 mm diam. (Madagascar) ... D. madagascariensis
Ascomata slightly larger, up to 2·5 mm diam. (Baja California) ... D. pallescens
-
26(24) Disc pruina with even surface (cf. Fig. 5F) ... D. indica
Disc pruina with rimose or only finely rimose surface (cf. Fig. 6A) ... 27
-
27(26) Thallus creamy brown to dirty brownish ... D. badia
Thallus creamy white to white-brownish ... D. approximata
-
28(23) Disc pruina coarsely or finely rimose ... 29
Disc pruina even ... 30
-
29(28) Disc pruina coarsely rimose (Mediterranean) ... D. ceratoniae
Disc pruina finely rimose (Cape Verde, Senegal) ... D. monothalamia
-
30(28) Ascomata base constricted ... D. paradoxa
Ascomata base unconstricted or semi-constricted ... D. angolana
Corticolous species
-
31(1) Corticolous ... 32
Saxicolous ... 34
-
32(31) Soralia maculate to confluent, pinkish (Canaries) ... D. ceratoniae
Soralia punctiform to maculiform, of thallus colour ... 33
-
33(32) Medulla white (Galapagos) ... D. approximata
Medulla dirty white (Peru) ... D. badia
-
34(31) Growing on calciferous rock ... 35
Growing on acidic rock ... 36
-
35(34) Medulla C+ red (Socotra) ... D. immersa
Medulla C− (Europe) ... D. massiliensis
-
36(34) Medulla chalk-like but with loose hyphae near the substratum ... 37
Medulla chalk-like also near the substratum ... 39
-
37(36) Medulla C+ red (California, Baja California) ... D. catalinariae
Medulla C− ... 38
-
38(37) Thallus pale, usually creamy white to white-brownish (Cape Verde) ... D. sorocarpa
Thallus dark, usually greyish or greenish brown ... D. fallax (mainly Europe)
or ... D. canariensis (Canaries)
-
39(36) Medulla C+ red (Macaronesia) ... D. insulana
Medulla C− ... 40
-
40(39) Medulla dirty white (Atlantic islands, Angola) ... D. jamesii
Medulla white ... 41
-
41(40) Unidentified substance F present (Hawaii, Galapagos) ... D. pacifica
Unidentified substances H or K present (Mascarenes) ... D. astridae
Sterile specimens

Fig. 7. Dirina species, habitus. A, D. monothalamia [Cape Verde, 2010, Tehler 10074 (S-F210827)], saxicolous specimen with strongly undulating thalline margins; B, D. monothalamia, lectotype [Senegal, s. ann., Perrottet 4 (G-291546)], corticolous specimen; C, D. sorocarpa, holotype [Cape Verde, 2010, Tehler 10026 (S-F210855)]; D, D. teichiodes, epitype [Cape Verde, 2010, Tehler 10071 (S-F210861)]; E, D. pallescens, holotype [Baja California Sur, 2007, Tehler 9181 (S-F210843)]; F, D. immersa [Socotra, 2008, Tehler 9300 (S-F210796)], DNA voucher specimen. Scales: A–F=1 mm. Photographs Ramona Ubral Hedenberg. In colour online.
Dirina angolana Tehler & Ertz sp. nov.
MycoBank No.: MB 802894
Thallus obligately corticolous, epruinose. Ascomata sessile with base not constricted or semi-constricted. Disc pruina with even surface. Unidentified secondary metabolites A and B present.
Type: Angola, Luanda prov., 35 km S Luanda, 1 km N of Palmeirinhas, 2009, Anders Tehler 9730 (S-F210729—holotype; BR—isotype).
(Fig. 5A)
Thallus obligately corticolous, surface plane to slightly rugose-verruculose, epruinose, creamy white, 0·1–0·7 mm thick; cortex 25–45 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base not constricted or semi-constricted, >1·5 mm diam.; disc pruina with even surface, white-grey; thalline margin present, entire sometimes undulating; ascospores 23–30×4–5 µm, mean length 26·1 µm, mean width 4·7 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ faintly red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances A, B, C.
Etymology
The name refers to the geographical region of Angola.
Distribution and habitat
Dirina angolana has been collected only in Angola. It grows preferably on the trunks of Adansonia trees near the sea, but sometimes several km from the coast in the provinces of Luanda and Bengo, Angola.
Remarks
Dirina angolana is characterized by its epruinose thallus and sessile ascomata with unconstricted base, but most distinctly by its chemistry and the presence of the unidentified substance A which is autapomorphic for the species.
Additional material examined. Angola: Bengo: 2–3 km S of Barra do Dande, along the Hwy, 8°29·036′S 13°22·326′E, 2009, Tehler 9716 (S-F210728). Luanda: just N of Luanda, c. 5 km N of Cacuaco, 8°4·700′S 13°26·357′E, 2009, Tehler 9703 (S-F210727).
Dirina approximata Zahlbr.
Ann. Mycol. 29: 78 (1931).—Dirina paradoxa ssp. approximata (Zahlbr.) Tehler, Lichenologist 18: 296 (1986); type: Ecuador, Galapagos Islands: I. Seymour (South Seymour Island), 1929, Albert W.C.T. Herre s. n. [W—lectotype sel. by Tehler (Reference Tehler1983); B, BM, G, GBG, H, KASSEL, L, LD, M, NY, S-L6, UC, UPS—isolectotypes].
Dirina herrei Zahlbr., Ann. Mycol. 29: 78 (1931); type: Galapagos, I. Santa Maria (Charles Isl.) Post Office Bay, 1929, Albert W.C.T. Herre s. n. [LD—lectotype sel. by Tehler (1983); UPS—isolectotype and as Zahlbr. Lich Rar. Exs. n. 269 in B, W].
(Figs – see Tehler Reference Tehler1983)
Thallus obligately corticolous, surface plane to slightly rugose-verruculose, slightly pruinose, creamy white to white-brownish, 0·1–0·7 mm thick; cortex 10–50 µm thick; medulla chalk-like, but with loose hyphae near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·0 mm diam.; disc pruina with finely rimose surface, white-grey; thalline margin present, entire; ascospores 28–33×4–5 µm, mean length 30·8 µm, mean width 4·2 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Distribution and habitat
Dirina approximata is endemic to the Galapagos Islands. It is a strictly corticolous species growing on the bark of various trees and shrubs.
Remarks
Dirina approximata was earlier regarded as one of three subspecies under D. paradoxa Tehler (Tehler Reference Tehler1983, Reference Tehler1986). On the basis of the present molecular data, Dirina approximata was placed with high support in a sister species relationship to one of its former subspecies, D. sorocarpa (earlier D. paradoxa ssp. africana), endemic to the Cape Verde Islands. It was not closely related to the former main species D. paradoxa. On the basis of this evidence, Dirina approximata is now raised from subspecies to species level. The disjunction with the sister pair species distributed in the Galapagos and Cape Verde (Figs 1 & 2) is bewildering, and can only be explained by an ancient long distance dispersal event (see Biogeography below).
Additional material examined. Ecuador: Galapagos Islands: Daphne Major on the SW side, 00° 25·789′S 090° 22,498′W, 2005, Tehler 8716 (S-F96203); Floreana, lava flow behind beach at SE side of Punta Cormorán (“White Beach”), c. 100 m inland, permanent plot 40, alt. 4 m, 1°13′44·5″S 90°25′27·7″W, 2011, A. Yánez 2141 (S-F210742); c. 150 m inland, alt. 6 m, 1°13′45·6″S 90°25′28·7″W, 2011, Bungartz 9485 (S-F210737); Floreana, trail going to Post Office Bay off the dirt road between highlands and Puerto Velasco Ibarra, lower slope of Cerro Post Office, alt. 73 m, 1°14′33″S 90°26′43·5″W, 2010, A. Yánez 2022 (S-F210738); on smooth bark of Bursera between the Post-Office and cinder cone, 1971, Weber (S-L60672); Islote Gardner por Española, SW-part of the island, walking up to the top, alt. 21 m, 1°20′41·5″S 89°38′49·2″W, 2010, Bungartz 9205 (S-F210735); Rábida, NE point, 00°24·965′S 090°42·105′W, 2005, Tehler 8762 (S-F96211); small point on the SE side, (Jervis) 0°25·720′S 90°42·628′W, 2005, Tehler 8702 (S-F96192); Santa Cruz, NE coast between Punta Carrión and Cerro Colorado, 0°33·519′S 90°11·968′W, 2005, Tehler 8671 (S-L72898); Santa Maria (Charles Isl.), Post Office Bay, 1929, Herre s. n. (LD, UPS); South Seymour, 1929, Herre s. n. (GBG, S-L6); s. ann., Herre s. n. (W).
Dirina arabica Tehler & Ertz sp. nov.
MycoBank No.: MB 802895
Thallus obligately saxicolous on calciferous rock. Medulla C+ red. Soralia absent. Ascomata numerous, sessile, circular in outline, base constricted, >2·0 mm in diam. Disc pruina with even or finely rimose surface.
Type: Yemen, Socotra Island, Homill near the village, in the eastern part of the island, alt. 350 m, 2008, Anders Tehler 9301 (S-F210762—holotype; BR—isotype).
(Fig. 5B)
Thallus obligately saxicolous on calciferous rock, surface plane, slightly pruinose, creamy white, 0·1–0·5 mm thick; cortex 20–40 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·0 mm diam.; disc pruina with even surface or finely rimose surface, white-grey; thalline margin present, entire to undulating; ascospores 17–26×4–7 µm, mean length value 21·77 µm, mean width value 5·35 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C+ red; disc C+ faintly red or nearly negative. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Etymology
The name refers to the geographical region Arabia.
Distribution and habitat
Dirina arabica is endemic to Socotra Island. It grows on the Eocene limestone rocks.
Remarks
Dirina arabica is the sister species to D. immersa. They are sympatric and grow in the same localities, but are easily distinguished by the appearance of the ascomata, which are sessile in Dirina arabica but immersed in D. immersa. Dirina arabica is habitually and morphologically very similar to D. massiliensis but differs by its C+ red medulla, a feature shared only by four other species, D. immersa, D. candida, D. insulana and D. catalinariae.
Additional material examined. Yemen: Socotra Island: in the southern part of the island along the road where it leads up into the mountains from the south, alt. 300 m, 12°35·581′N 54°18·298′E, 2008, Tehler 9323 (S-F210763); Mumi plateau, Ant Kashara, eocene limestone blocks, 1994, Mies & Printzen 236a2 (S-F60885); Sefflah, the ridge just S of the village on S coast at the E most part of the island, alt. 400–600 m, 12°30·723′N 54°26·037′E, 2008, Tehler 9339 (S-F210764); s. loc., 1881, Balfour s. n. (G).
Dirina astridae Tehler sp. nov.
MycoBank No.: MB 802896
Thallus obligately saxicolous on acidic rock. Soralia present (usually in the absence of ascomata). Ascomata present (usually in the absence of soralia), sessile, with base constricted. Disc pruina with even surface or finely rimose surface. Unidentified secondary metabolites D, H, I and K present or sometimes present.
Type: Mauritius, Port Louis Distr., Port Louis, Mt. Signal, on the peak c. 200 m east of the tele station, 2003, Anders Tehler 8502 (S-L55012—holotype; BR—isotype).
(Fig. 5C)
Thallus obligately saxicolous on acidic rock, surface plane to slightly rugose-verruculose, slightly pruinose, creamy white to white-grey, 0·1–1·0 mm thick; cortex 35–45 µm thick; medulla chalk-like also near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >1·5 mm diam.; disc pruina with even surface or finely rimose surface, white; thalline margin present, entire to undulating and often strongly undulating; ascospores 22–30×5–6 µm, mean length 25·41 µm, mean width 5·31 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, D, H, K occasional and I rare.
Etymology
The name refers to the first author's daughter Astrid, who led the way to this species on a joint field trip to Mauritius.
Distribution and habitat
Dirina astridae is endemic to the Mascarene Islands and has only been collected on Mauritius. It is strictly saxicolous, growing on acidic or volcanic rock.
Remarks
Dirina astridae has its closest relative, Dirina jamesii, on the African West Coast in Angola and the Atlantic islands of St. Helena and Ascension Island. The topology suggests some level of incomplete sorting of two recently split lineages, thus the sample group including Dirina astridae and D. jamesii should not be considered conspecific (Fig. 4). No other Dirina species exhibits such a conspicuous species disjunction and, furthermore, Dirina astridae differs in some important aspects such as clearly sessile ascomata, thicker spores and a complex chemistry with substances D, H, I and K, none of which are present in D. jamesii.
Additional material examined. Mauritius: Black River: Mt St. Pierre, the eastern peak near Bambous, c. 7 km E Quatre Bornes, fertile specimen, 20°16·2′S 57°25·5′E, 2003, Tehler 8524 (S-L55029); sorediate specimen, Tehler 8525 (S-L55030). Port Louis: Mt. Signal, on the peak c. 200 m east of the tele station, 20°11′S 57°30′E, 2003, Tehler 8503 (S-L55013). Savanne: Maconde on the south coast, c. 500 m W of Baie du Cap, fertile specimen, 20°29·700′S 57°22·648′E, 2003, Tehler 8511 (S-L55022); sorediate specimen, Tehler 8510 (S-L55021).
Dirina badia (Tehler) Tehler & Ertz comb. nov.
MycoBank No.: MB 802897
Roccellina badia Tehler, Opera Bot. 70: 67 (1983); type: Peru, Dept. Lambayeque, Prov. Chiclayo, Cerro de Reque, c. 12 km SE of Chiclayo, alt. 200–500 m, 1981, Rolf Santesson & Anders Tehler P132:8 (S-L7934—isotype; B, BM, C, FH, G, H, LD, LIL, MSC, NY, O, R, TNS, UPS, US, W—isotypes).
Dirina approximata ssp. hioramii f. sorediata Tehler, Opera Bot. 70: 38 (1983).—Dirina paradoxa ssp. paradoxa f. sorediata (Tehler) Tehler, Lichenologist 18: 296 (1986); type: Peru, Dept. Tumbes, 14 km (road distance) NE of Puerto Pizarro, alt. <50 m, 1981, Rolf Santesson & Anders Tehler P123:3 (S-L1—holotype; BM, R, S-L12, UPS—isotypes).
(Figs – see Tehler Reference Tehler1983)
Thallus facultatively saxicolous/corticolous on acidic rock, surface plane, rugose-verruculose to nearly squamulose or suffruticose, slightly pruinose, creamy brown to brown, 0·3–1·5 µm thick; cortex 20–40 µm thick; medulla chalk-like, but with loose hyphae near substratum, white to white-brown, rarely white rusty red; soralia present (usually in the absence of ascomata), punctiform.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·5–2·0 mm diam.; disc pruina with rimose surface, white; thalline margin present, entire to undulating; ascospores 27–33×4–5 µm, mean length 29·9 µm, mean width 4·8 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, I.
Distribution and habitat
Dirina badia is restricted to northern Peru where it is common on the small desert mountains near the sea, several kilometres inland. It is usually saxicolous growing on acidic rock but it occurs also on twigs, branches and the bark of small trees and shrubs.
Remarks
Dirina badia was earlier referred to the genus Roccellina, primarily on the basis of its apparent hypothecial extension (Tehler Reference Tehler1983). However, as a result of the molecular analysis in combination with a reciprocal illumination (Hennig Reference Hennig1966) of specimens in Dirina badia, it was evident that the extensions developed were pseudo-hypothecial and analogous to the true hypothecial extensions found in Roccellina. The latter are prolongations that originate from the same carbonaceous tissue as that of the hypothecium. The pseudo-hypothecial extension in Dirina badia originates and develops from the dirty brownish medullary tissue. This is indicated by the KOH reaction in which the dark brown true hypothecial extension turns olive-green like the hypothecium, whereas the pseudo-hypothecial extension remains dirty brownish. The cortex tissue is made up of bifurcate, intertwined hyphae but still they are mainly anticlinally arranged as with the cortex type in Dirina.
At the same localities where Dirina badia was present, we also collected corticolous specimens that are very similar to D. badia in most respects except for their corticolous habitat. No corticolous specimens were available for molecular study and thus we cannot verify that these specimens belong to the same species as D. badia, but they have the same type of pseudo-hypothecial extension and contain the unidentified substance I as in Dirina badia. No saxicolous specimens of Dirina badia have been found with soredia. Thus, the sorediate Dirina specimens in Peru that were earlier placed under D. paradoxa f. sorediata are only tentatively placed under D. badia since these specimens might belong to D. approximata. Future molecular studies will reveal their accurate position.
The similar and sympatric species Roccellina nigrocincta is readily distinguished by its shorter ascospores, different secondary metabolites, the C− disc and the thallus surface which is C− or only faintly C+ red.
Additional material examined. Peru: Lambayeque: Chiclayo, el Cerro la Guitarra [saxicolous], 6°58′37·9″S 79°26′38″W, 2006, Tehler 8898 (S-F210766); Cerro de Reque, [saxicolous], 6°52′57·5″S 79°46′41·1″W, 2006, Tehler 8917 (S-F210767); c. 12 km SE of Chiclayo, [corticolous], 6°52′57·5″S 79°46′41·1″W, 1981, Santesson & Tehler P132:3 (S-F90334). Libertad: Pacasmayo, Cerro Chilco, c. 8 km SE of San Pedro de Lloc, [saxicolous], 7°28′16″S 79°26′38″W, 2006, Tehler 8888 (S-F210765); [corticolous], 1981, Santesson & Tehler P127:1 (S-F90354). Piura: Piura, Desierto Sechura, [corticolous], 6°5′S 81°55′W, 1981, Santesson & Tehler P114:22 (S-F90346); Paita, Cerro Chocán, [corticolous], 5°9′S 80°58′W, 1981, Santesson & Tehler P115:11 (S-F171196). Tumbes: NE of Puerto Pizarro, [corticolous], 3°28′S 80°23′W, 1981, Santesson & Tehler P123:1 (S-L7936).
Dirina canariensis Tehler & Ertz sp. nov.
MycoBank No.: MB 802909
Thallus obligately saxicolous on acidic rock. Soralia present (usually in the absence of ascomata). Ascomata present (usually in the absence of soralia). Disc pruina with even surface, C+ red. Ascospores mean width value 4·83 µm
Type: Spain, Canary Islands, Gran Canaria, Playa de Tasarte c. 6 km N Puerto de Mogán, in slope S of the playa, alt. 130–250 m, 2011, Anders Tehler 10300 (S-F210768, holotype; BR—isotype).
Thallus obligately saxicolous on acidic rock, surface plane, epruinose or slightly pruinose, creamy white to white-brownish, 0·1–0·7 mm thick; cortex 25–35 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >1·5 mm diam.; disc pruina with even surface, white-grey; thalline margin present, entire to undulating; ascospores 20–25×4–5 µm, mean length 25·02 µm, mean width 4·83 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, H occasional.
Etymology
The name refers to the geographical region of the Canary Islands.
Distribution and habitat
Dirina canariensis is endemic to the Canary Islands. It grows on vertical rocks and cliffs, often several km inland.
Remarks
Dirina canariensis belongs to the European monophyletic group with D. ceratoniae, D. massiliensis and D. fallax, and is placed as the sister species to these three species (Figs 1 & 2). Dirina canariensis also appears in a sterile, sorediate form. Sorediate specimens of Dirina canariensis are morphologically indistinguishable from sorediate specimens of the partly sympatric species D. fallax, and very hard to distinguish from sorediate specimens of D. insulana. The latter has a C+ red medulla but this character may be very difficult to examine as thalli are often very thin.
Additional material examined. Spain: Canary Islands: Gran Canaria, Jardin Canario, 27°3,912′N 15°27,777′W, 2011, Tehler 10313 (S-F210771); Gran Canaria, Mirador de Tasartico, 27°56·216′N 15°45·845′W, [fertile specimen], 2011, Tehler 10326 (S-F210772); [sorediate specimen], Tehler 10327 (S-F210773); Montañeta Redonda, just S of el Mirador de Fataga, 27°46′15″N 15°37′1″W, 2011, [fertile specimen], Tehler 10307 (S-F210769); [sorediate specimen], Tehler 10308 (S-F210770); La Gomera, Arure, trail N of Mirador Ermita del Santo, 28°07′55″N 17°19′23″W, 2011, Ertz 16255 (BR); San Sebastián de la Gomera, Roque de Berruga, 28°05′34″N 17°11′17″W, 2011, Ertz 16185 (BR); N of road to Hermigua, La Gerode, path to Casas de Jaragán and Montaña Ismael, 28°07′45″N 17°08′50″W, 2011, Ertz 16143 (BR); Tenerife, Anaga Peninsula, Pico del Ingles, 1978, Sipman 9540 (U); Ladera de Guyimar, Lomo Marrero near Mirador Don Martin, 1978, Walker 103 (BM); near La Laguna, 1977, Straka & Pichler 2236 (GZU); NW part S of Los Silos, 1976, Santesson 26652 (UPS).
Dirina candida (Müll. Arg.) Tehler & Ertz comb. nov.
MycoBank No.: MB 802910
Chiodecton candidum Müll. Arg., Rev. Mycol. (Toulouse) 6: 19 (1884); type: Egypt, Alexandria, 1879, Ascherson s. n. [G-66241—lectotype sel. by Tehler (1983)].
(Figs – see Tehler Reference Tehler1983)
Thallus obligately saxicolous on calciferous rock, surface plane, pruinose, white to white-grey, 0·2–2·0 mm thick; cortex 30–50 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, monocarpocentral, numerous, immersed, circular or linear in outline, 0·1–0·8 mm diam.; disc pruina with rimose surface, white to white-grey; thalline margin absent or sometimes with a thin margin; ascospores 18–20×5–7 µm, mean length 18·75 µm, mean width 5·75 µm.
Chemistry
Spot tests: thallus surface C+ red, but often faint or negative if the uppermost layer is not scraped or cut away; medulla C+ red; disc C−. Secondary metabolites: erythrin; lecanoric acid occasional, weak or absent; unidentified substances C sometimes absent.
Distribution and habitat
Dirina candida is distributed in the southern part of the Mediterranean region, from Andalusia in Spain, east to Libya and Egypt. It is restricted to calciferous rocks near the sea.
Remarks
Dirina candida was earlier referred to as D. immersa (Tehler Reference Tehler1983), a species described by Müller Argoviensis on material collected by Balfour from Socotra Island. It has the same morphology, anatomy and chemistry as Dirina candida and, in the lack of distinguishing characters, the two taxa were considered conspecific by Tehler (Reference Tehler1983). In this new study, the molecular data from four phylogenetic markers indisputably indicate that Dirina candida and D. immersa should be acknowledged as two distinct and unrelated species.
Dirina candida has not been found sorediate, in contrast to the otherwise virtually identical D. immersa. Fortunately, their distribution areas are not sympatric.
Additional material examined. Libya: Cyrenaica: Wadi Derna, 25 km SW of Derna, 1982, Anderberg 1051 (S-F63434).—Gibraltar: along the Mediterranean Steps, 2009, Tehler 9805 (S-F210774).—Spain: Alicante: Javea, Cap de Sant Antoni, 38°48′15″N, 0°11′47″E, 2011, Ertz 17120, 17129 (BR). Almeria: Carboneras, Punta de los Muertos, 1975, Tehler 1208 (S-F63433). Cadiz: Parque Natural de la Breña y Marismus de Barbate, Torre del Tajo at Mirador Acantilado, 36°10·734′N 5°58·372′W, 2009, Tehler 9818 (S-F210775). Mallorca: Cala Figuera, 5 km SE of Santanyí, 39°19′49·9″N 003°10′03·0″E, 2007, Tehler 9015 (S-F66049); Torre d'eu Beu, 39°19′41·1″N 003°10′40·0″E, 2007, Tehler 9017 (S-F66051); Cala Santanyi at Caló de N'Estrany, 1979, Tehler 4673 (S-F60758); Formentor 25 m before the tunnel, 1979, Tehler 4705 (S-L3479); N-part, Punta la Nao, 1978, Thor 628 (S-F60759); Playa de Cala Santanyí, 39°19′36·2″N 003°08′51·5″E, 2007, Tehler 9004 (S-F66088); 4 km SE of Santanyí, 300 m north of the inlet, 39°19′35·5″N 003°09′09·0″E, 2007, Tehler 9011 (S-F66077). Murcia: Mazarron, 30 km W of Cartagena, 1976, Tehler 1245 (S-F63432).
Dirina catalinariae Hasse
Bryologist 14: 102 (1911); type: USA, California, Los Angeles Co., St. Catalina Isl., on beach boulders near Avalon, 1911, H. E. Hasse 3020 [FH—lectotype sel. by Tehler (Reference Tehler1983)].
Dirina catalinariae f sorediata Tehler, Opera Bot. 70: 36 (1983); type: Mexico, Baja California, Punta Banda just by La Bufadora, 1976, Anders Tehler 1665 (S-L3—holotype; BM, COLO, E, H, L, LD, M, NY, RO, U, UC, UPS, US, W, ZT—isotypes).
(Figs – see Tehler Reference Tehler1983)
Thallus obligately saxicolous on acidic rock, surface plane, rugose-verruculose to nearly squamulose or subfruticose, slightly pruinose, creamy white to white-greyish, 0·2–1·1 mm thick; cortex 35–65 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia and ascomata often present side by side, punctiform, maculiform, flat or often capitate.
Ascomata present, often side by side with soralia, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·5–2·0 mm diam.; disc pruina with rimose surface, white-grey; thalline margin present, entire to undulating, strongly undulating or even so strongly undulating as to become stromatoid; ascospores 23–29×5–6 µm, mean length 26·1 µm, mean width 5·4 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C+ red; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances B, C, F occasional.
Distribution and habitat
Dirina catalinariae grows on rocks and cliffs along the coast from Monterey Co. in California (USA) to Laguna Manuela, Baja California (Mexico).
Remarks
Dirina catalinariae is the only Dirina species in which vegetative and sexual reproduction are not separated into two distinctively either fertile or sterile, sorediate morphs. Ascomata and soralia are frequently found side by side on the same thallus.
Dirina catalinariae f. sorediata was earlier treated as a separate taxon (Tehler Reference Tehler1983), but is now included under the nominal species Dirina catalinariae.
Additional material examined. Mexico: Baja California: Ensenada, Cabo Punta Banda by La Bufadora, 31°44′30″N 116°43′30″W, 1976, Tehler 1657 (S-F97389); Cabo Punta Banda, 31°44′38,2″N 116°44′15,8″W, 2007, Tehler 9086, 9087 (S-F210776, S-F148386); Cabo Punta Banda, 31°44′34,1″N 116°44′34,4″W, 2007, Tehler 9091 (S-F210777); Cabo Punta Banda, below Pico Banda, 31°44′50″N 116°44′30″W, 1996, Tehler 7689, 7690, 7691 (S-L1767, S-L1768, S-L1769); Cabo Punta Banda, W of La Bufadora blowhole, 31°44′30″N 116°43′30″W, 1995, Tehler 7580, 7581, 7582, 7584, 7585, 7586 (S-L4004, S-L4010, S-L4005, S-L4006, S-L4008, S-L4009); Cabo Punta Banda, Los Arbolitos, 1977, Tibell 7795 (S-L11737); Guadalupe Island, 28°57′30″N 118°15′W, 1996, Tehler 7669, 7670, 7671, 7676, 7677, 7678 (S-L1234, S-L1239, S-L1250, S-L1723, S-L1724, S-L1725); Guadalupe Island , at Fondeadero del Oeste, 28°58′50″N 118°18′50″W, 1996, Tehler 7656, 7657, 7658, 7659 (S-L1172, S-L1173, S-L1174, S-L1175); at Melpomene Cove, 1963, Weber & McCoy s. n (S-F97386); Laguna Manuela, 28°15′N 114°07′W, 1993, Tehler 7238 (S-L3394); 28°14′12,5″N 114°05′57,6″W, 2007, Tehler 9127 (S-F210778); La Mision, N Ensenada, 1976, Tehler 1587, 1595 (S-F97408, S-F97387); Punta Santa Rosallita, 28°39′20″N 114°14′56″W, 1997, Nash 40206 (S-L10071); Punta Santo Tomás, NE Puerto Santo Tomás, 1995, Tehler 7603, 7604, 7605 (S-L4024, S-L4025, S-L4026); Punta Santo Tomas, between Punta Santo Tomas and Punta Rif, 31°33′20″N 116°41′20″W, 1995, Tehler 7631 (S-L205); San Quintin, Cerro Kenton 1995, Tehler 7589, 7590 (S-L4012, S-L4013); Cerro Kenton by Oyster Plant, 1995, Tehler 7594, 7595, 7596 (S-L4017, S-L4018, S-L4019); Cerro Kenton at Chapalta, 1995, Tehler 7600, 7601 (S-L4023, S-L4027); 40 km north of El Rosario, 1976, Tehler 1607 (S-F97404). Baja California Sur: Vizcaíno peninsula, Punta Eugenia, 27°51′05·1″N 115°04′16·7″W, 2007, Tehler 9143, 9146 (S-F210779, S-F210780); Punta Eugenia, 27°50′46·1″N 115°03′09·3″W, 2007, Tehler 9151 (S-F210781); 3·5 km along road to Punta Abreojos from Highway 1·27°15′N 113°10′W, 1993, Tehler 7228 (S-L3385).—USA: California: Los Angeles Co., Santa Catalina Island, 1976, Tehler 1416 (S-F97417); Avalon, 1976, Tehler 1419 (S-F97420); between Cactus Bay and Starlight beach, 120–170 m, 33°28′45″N 118°30′30″W, 1993, Tehler 7320 (S-L3159); Catalina Harbor, between Ballast Point and Lobster Point, 33°25′45″N 118°30′45″W, 1993, Tehler 7335, 7336 (S-L3481, S-L3174); Big Fisherman's Cove, 33°26′30″N 118°29′W, 1993, Tehler 7301 (S-L3141); Catalina Harbor, Pin Rock, 1976, Tehler 1441, 1451 (S-F97394, S-F97402); Catalina Harbor, Wells beach, 1976, Tehler 1426, 1428 (S-F97395, S-F97410); Monterey Co., Seventeen Mile Drive, between Bird Rock and Point Joe, 1992, Tehler 7072 (S-L23169); Seventeen Mile Drive, Pescadoro Point, 1997, Tehler 7838 (S-L4045); between Monterey and Morro Bay, 2 km N of Willow Creek, 1997, Tehler 7859 (S-L4065); Point Lobos State Reserve, Punta de los Lobos Marinos, 1997, Tehler 7856 (S-L4062); Point Lobos State Reserve, Punta de los Lobos Marinos, 36°31·120′N 121°57·154″W, 2008, Tehler 9419 (S-F210782); S of Asilomar, China Rock, 36°36′N 121°57′W, 2008, Ertz 12476 (BR); Santa Barbara Co., Santa Rosa Island, Cañada Lobos, 34°01′15″N 120°05′30″W, 1994, Tehler 7426 (S-L3264); Santa Rosa Island, Black Mountain, 33°58′45″N 120°04′30″W, 1994, Tehler 7340 (S-L3177); Santa Rosa Island, NW Bee Canyon, 33°58′15″N 120°12′W, 1994, Tehler 7350 (S-L3189); Santa Rosa Island, East Point, 33°56′30″N 119°59′30″W, 1994, Tehler 7364 (S-L3202); Santa Rosa Island, Old Ranch Canyon, 33°58′15″N 119°59′30″W, 1994, Tehler 7366 (S-L3204); Santa Cruz Island, Prisoner's Harbor, 34°01′15″N 119°41′15″W, 1994, Tehler 7377 (S-L3217); Santa Cruz Island, NE of Frazer point, 34°03′45″N 119°54′45″W, 1994, Tehler 7388 (S-L3228); Santa Cruz Island, Willows Anchorage, 1976, Tehler 1485 (S-L23162); Santa Cruz Island, Coches Prietos Anchorage, 1976, Tehler 1548, 1554 (S-L23163, S-F97391); Santa Cruz Island, west of Coches Prietos beach, 1990, Bratt & Schmitt 6450 (S-L23168); Santa Cruz Island, N Frazer point, 1976, Tehler 1524, 1547 (S-F97390, S-F97399); San Luis Obispo Co., Diablo Canyon Nuclear Facility, Diablo Canyon, 1995, Tehler 7530 (S-L3133); Crowbar Canyon, 1995, Tehler 7531, 7534, 7535, 7536, (S-L3134, S-L3137, S-L3138, S-L3139); across from Lion Rock N Diablo Canyon, 35°14′N 120°52′W, alt. 10 m, 35°14′N 120°52″W, 1995, Nash & Bratt 36981 (GBG); Coon Creek Canyon, Montana de Oro State Park, 1989, Riefner 89-46 (S-L23170).
Dirina ceratoniae (Ach.) Fr.
Lichenogr. Eur. Reform. 194 (1831).—Lecanora ceratoniae Ach., Lichenogr. Universalis 361 (1810).—Parmelia ceratoniae (Ach.) Spreng., Syst. Veg. (ed. 16) [Sprengel] 4(1): 299 (1827).—Patellaria repanda var. ceratoniae (Ach.) Hepp, Flechten Eur. Fasc. 1–4: n. 408 (1853).—Lecania ceratoniae (Ach.) Stizenb., Ber. Tätigk. St. Gallischen Naturwiss. Ges. 1861–1862: 170 (1862).—Lecanora repanda f. corticola Harm., Lich. France 5: 1092; nom. illeg (1913); type: Spain, s. ann., Lagasca s. n, [H—lectotype sel. by Tehler (1983); UPS-L00636—isolectotype].
Dirina repanda Fr., Systema orbis vegetabilis: 285 (1825); type: “Eur. Austr.” corticola, s. ann., s. coll. s. n. (name rejected by the author).
Lecanora repanda f. lignicola Harm., Lichens de France. Catalogie systematique et descriptif 5: 1092; nom. nud. (1913).—Dirina repanda f. lignicola (Harm.) Zahlbr., Cat. Lich. Univ. 2: 509 (1924); type: France, s. ann., Saltel s. n. (not retrieved).
(Figs – see Tehler Reference Tehler1983)
Thallus facultatively corticolous/saxicolous, on calciferous rock when saxicolous, surface plane to slightly rugose-verruculose, epruinose or slightly pruinose, creamy white to white-greenish, 0·1–1·0 mm thick; cortex 40–60 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia present (usually in the absence of ascomata), maculate to confluent.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·5–3·0 mm diam.; disc pruina with rimose surface, white-grey to dark grey; thalline margin present, entire to undulating; ascospores 21–26×4–5 µm, mean length 23·6 µm, mean width 4·7 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ faintly red or negative. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Distribution and habitat
Dirina ceratoniae occurs mainly along the coasts and islands of the Mediterranean Sea, extending to the Atlantic coasts of S Portugal and N Morocco. It appears to be more common in the western part of its distribution area. Dirina ceratoniae prefers open, dry habitats, natural or cultural landscape with a long continuity, near the sea. It commonly grows on trunks, old branches and twigs of various trees and shrubs such as Ficus carica, Ceratonia siliqua, Rosmarinus officinalis, Juniperus phoenicea, Pinus halepensis, P. pinea, Pistacia lentiscus and others. It is usually corticolous but it may also occur on calciferous rocks.
Two specimens of Dirina ceratoniae have been collected on the Canary Islands (Tenerife), one of which is the single specimen of D. ceratoniae found with soredia. With the exception of these two specimens, Dirina ceratoniae is otherwise not known from Tenerife and records from other Canary Islands are very rare. Interestingly, the sorediate and fertile forms were found in the Botanical Garden of Puerto de la Cruz, and the sorediate form also in the Taoro Park within the same city. Our impression is that the specimens are unhealthy, as if the habitat conditions were not right and possibly, because of these improper habitat conditions, some developed soredia. We believe that Dirina ceratoniae was, on some occasion, introduced to the city in connection with importing plants to the Botanic Garden or other parks (possibly including those of hotels).
Remarks
Specimens growing on rock are confusingly similar to Dirina massiliensis, but in D. ceratoniae the medullary hyphae near the substratum are usually more or less loose or nearly byssoid. However, in some specimens, particularly those with thin thalli, this character may fail or be very difficult to see. Dirina ceratoniae can also be distinguished from D. massiliensis by its more greenish, less pruinose thallus surface and its longer ascospores.
Additional material examined. Algeria: Alger, s. ann., Paguy s. n. (S-F63566); Palestro bai Algier, 1879, Lahm s. n. (S-F63565).—Cyprus: Polis: Akamas peninsula, 40 km N of Paphos, 1980, Tehler 5048b (S-F63462).—France: Corse: An der Rinde von Cerat. siliqua u Junip. Lycea, s. ann., s. coll. s. n. (S-F63455); Bonifacio, 1989, Puntillo s. n. (S-F63560); Sud d'Ajaccio, presqu'île du Capu di Muru, 41°44′58″N 8°40′35″E, 2011, Ertz 16367 (BR); Bonifacio, Saint-Julien, 41°23′28″N 9°11′31″E, 2011, Ertz 16527 (BR). Var: Isles d'Hyeres, Isle de Port-Cros, Mont Vinaigre, 43°0·43′N 6°22·77′E, 2008, Tehler 9503 (S-F210783); Pointe du Cognet, 42°59·967′N 6°22·716′E, 2008, Tehler 9510 (S-F210784); Gien peninsula, La Madrague 43°2·410′N 6°5·732′E, 2008, Tehler 9527 (S-F210785).—Israel: Mt. Carmel: SE the Binyanaina Zikron-Yaakov R, 1964, Galem 4(2) (S-F63568).—Italy: Isole Ègadi: Favignana, 1979, Tehler 4500 (S-F97443). Isole Pelagie: Lampedusa, M. Imbricola, 1979, Tehler 4560, 4561, 4562, 4563, 4565, 4569, 4570 (S-F63444, S-F63445, S-F63446, S-F63447, S-F63464, S-F63451, S-F63456); Linosa, 1979, Tehler 4540, 4545, 4579 (S-F63448, S-F63450, S-F63457). Puglia: Lecce Distr., Diso (Comune), Marina di Marittimo 39°59·483′N 18°24·869′E, 2008, Tehler 9616, 9617 (S-F176052, S-F176053); Dolmen Li Scusi 40°5·695′N 18°25·945′E, 2008, Tehler 9618 (S-F176054); Galabone (Comune), La Reggia 1 km N of Lido Conchiglie 40°6·871′N 18°0·220′E, 2008, Tehler 9627 (S-F176058); Otranto (Comune), 40°8,731′N 18°29·316′E, 2008, Tehler 9631, 9632, 9633 (S-F176065, S-F176066, S-F176067). Sardinia: Alberi nei dintorni di Cagliari, 1865, Canepa s. n. (S-F63453); Tortoli, 1866, Marcucci s. n. (S-F63555); Cagliari, prope oppidum Villasimi, 1987, Vezda s. n. (S-F63562). Sicily: ai Gorghi Tonoli (Mazara okl Vallo, TP), 1994, Ottonello 18219 (S-L56062); Siracusa distr., N of Pachino, Pant. Roveta, 1979, Tehler 4582 (S-F63452); Prope Florentinam l. Biasols, s. ann., s. coll. 125 (S-F63454).—Morocco: Rabat: Ad cult Olea prope Rabat, 1933, Werner s. n. (S-F63563).—Portugal: Algarve: Lagos, 1·5 km W of Ponta de Piedade, 1979, Tehler 4606 (S-F63449); NW of Sagres, Cabo de Sao Vicente, 37°01′36″N 8°59′06″W, 2011, Ertz 16997 (BR).—Spain: Almeria: Carboneras, Punta de los Muertos, 1975, Tehler 1210b, 1237 (S-F63463, S-F135199); Punta de los Muertos, Torre Artillada de Mesa Roldán, 36°56·502′N 1°54·550′W, 2009, Tehler 9814 (S-F210786). Cadiz: Parque Natural de la Breña y Marismus de Barbate, Torre del Tajo at Mirador Acantilado, 36°10·734′N 5°58·372′W, 2009, Tehler 9816 (S-F210787). Canary Islands: Tenerife, Puerto de la Cruz, Botanical Garden, 2000, Tehler 8252 (S-L14018); Puerto de la Cruz, Taoro Park, 2009, Ertz 14024 (BR). Mallorca: Cabo Blanco, 1969, Wall s. n. (GBG); Costa de los Pinos, between Playa d'es Rivell and Port Vey, 1979, Tehler 4662, 4663 (S-F60739, S-F60740); Cabo Salinas, 1979, Tehler 4670, 4698 (S-F60741, S-F60743); Cala Santanyi, Calóde N'Estrany, 1979, Tehler 4693 (S-F60744); Cala Marsal, 1972, Santesson 24092a (S-F63458); Deia, 39°44′58·3″N 002°37′50·1″E, 2007, Tehler 9038 (S-F66045); Playa de la Rapita, 1979, Tehler 4653, 4667 (S-F60749, S-F60746); Playa de Cala Santanyí, 39°19′36·2″N 003°08′51·5″E, 2007, Tehler 9003 9012, 9013 (S-F66039, S-F66040, S-F66041); s'Amarodor, Parc Natural de Mondragó, 39°20′44·4″N 003°11′21·2″E, 2007, Tehler 9020, 9021 (S-F66043, S-F66044); Salines de Llevant 39°20′33·1″N 003°00′05·6″E, 2007, Tehler 9040 (S-F66046); Ses Covetes 39°21′16·3″NE 002°58′11·9″, 2007, Tehler 9046, 9047 (S-F66047, S-F66048). Murcia: 5 km N of La Azohia, 1976, Tehler 1260 (S-F63461); Los Belones, 1976, Tehler 1277, 1282 (S-F135175, S-F63460); Mazarron, 1976, Tehler 1252 (S-L3477). Tarragona: Tarragona, prope Cunit, 1964, Culberson & Culberson 12831 (S-F63459).—Tunisia: Sidi Bou Saïd: NE of Tunis, 1988, Svane 88 SS 7385-1 (S-F63569).
Dirina cretacea (Zahlbr.) Tehler
Opera Bot. 70: 44 (1983).—Chiodecton cretaceum Zahlbr., Österr. Bot. Z. 69: 245 (1899); type: Croatia (Jugoslavia), Pula, ad saxa calcaria, 1899, K. Stockert s. n. (W—holotype; NY, PAD, W—isotypes).
(Figs – see Tehler 1983)
Thallus obligately saxicolous on calciferous rock, surface plane to slightly rugose-verruculose, pruinose, white-grey, 0·3–1·0 mm thick; cortex 40–60 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, pseudomonocarpocentral (stromatoid), numerous, sessile, circular in outline, base not constricted, >3·8 mm diam.; disc pruina with even surface, white-grey; thalline margin present, entire; ascospores 19–23×5–6 µm, mean length 21·5 µm, mean width 5·8 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C−. Secondary metabolites: erythrin; lecanoric acid sometimes absent.
Distribution and habitat
Dirina cretacea is mainly distributed in the eastern Mediterranean region in Cyprus and along the coasts of the Adriatic, Ionian, and Aegean Seas. One outpost locality is known from Andalusia in Spain.
Remarks
Dirina cretacea is the only species with stromatoid, pseudomonocarpocentral ascomata and it is easily recognized by that feature. Dirina cretacea is the sister species to a group of four species (Figs 1 & 2), two from Cape Verde (D. teichiodes and D. sorocarpa), one from both Cape Verde and Senegal (D. monothalamia), and one from the Galapagos (D. approximata).
Additional material examined. Croatia: Dalmatia: Cres, s. ann., Baumgartner s. n. (S-F63527); Cres, Osor, 1980, Tehler 5107, 5108, 5109 (S-F63531, S-F63532, S-F63544); Korcula, Vela Luka, 1980, Tehler 5077 (S-F63538); Korcula, Brna, 1980, Tehler 5063 (S-F63542); Losinj, Veli Losinj, 1980, Tehler 5110 (S-F63541); Pag, Borovici, 1980, Tehler 5094 (S-F63534); Pag, 3 km NW of Pag, 1980, Tehler 5085 (S-F63537); Pag, Gradac, 1980, Tehler 5097 (S-F63540); Peljesac peninsula, Zuljana, 1979, Vezda s. n. (S-F63528); Peljesac, Rat by Sreser, 1980, Tehler 5062 (S-F63539); Siano, 30 km N of Dubrovnik, 1980, Tehler 5060 (S-F63543). Istria: Istria, 40 km S of Rijeka, 1980, Tehler 5103 (S-F63535); Pula, on walls of Arena amphitheatre, 1980, Tehler 5117 (S-F63533); Pula, Premantura, 1980, Tehler 5130 (S-F63536). Primorje-Gorski: Krk, 1980, Tehler 5101 (S-L3478).—Cyprus: Cape Greco, 20 km S of Famagusta, 1980, Tehler 4999 (S-F63548); Cape Pyla, 25 km E of Larnaca, 1980, Tehler 5049 (S-F63545); Paphos, Tombs of the Kings, 1980, Tehler 5028 (S-F63551); Polis, Akamas peninsula 1980, Tehler 5036 (S-F63549).—Greece: Crete: 1 km W of Chora Sphakion, 1976, Bråvander 4301 (S-F63530). Peloponnesos: Akhaia, Akr. Araxos, 1978, Tehler 4000 (S-F63529).—Italy: Puglia: Lecce Distr., Diso (Comune), Marina di Marittimo 39°59·483′N 18°24·869′E, 2008, Tehler 9614 (S-F176051); Galabone (Comune), Rivabella 40°5·904′N 18°1·347′E, 2008, Tehler 9625 (S-F176060); Galabone (Comune), La Reggia, 40°6·871′N 18°0·220′E, 2008, Tehler 9626 (S-F176057); Otranto (Comune), Torre San Emiliano, 40°5·444′N 18°29·781′E, 2008, Tehler 9606, 9607 (S-F176072, S-F176075); Santa Maria di Léuca (Comune), Capo Santa Maria di Léuca, 39°48·551′N 18°22·677′E, 2008, Tehler 9622 (S-F176096); Punta Ristola, 39°47·499′N 18°20·670′E, 2008, Tehler 9623 (S-F176097); S. Cesarea Terme (Comune), Torre Minervino, 40°3·978′N 18°28·754′E, 2008, Tehler 9605 (S-F176095); Porto Badisco, alt 50 m, 40°4·125′N 18°28·832′E, 2008, Tehler 9609 (S-F176092); S. Cesarea Terme, 1996, Nimis & Tretiach s. n. (S-F63525).—Libya: Cyrenaica: Wadi Derna, 25 km SW of Derna, 1982, Anderberg 1000 (S-F63553).—Montenegro: Budva: Trsteno, 1967, Vězda s. n. (S-F63526).—Spain: Cadiz: Parque Natural de la Breña y Marismus de Barbate, Torre del Tajo at Mirador Acantilado, 36°10·734′N 5°58·372′W, 2009, Tehler 9817 (S-F210788).
Dirina fallax De Not.
Giorn. Bot. Ital. 2(1): 189 (1846); type: Italy, Sardinia, s. ann., s. coll. s. n. (not retrieved); type: Italy, Sardinia, Prov. Sassari: Nurra, Capo (Punta) Falcone, Monte della Crocetta, near sea, alt. c. 50 m, Macchia on schistose (silicious) rocks, 1987, Teuvo Ahti 47193 (S-F184389—neotype sel. here; H—isoneotype).
Dirina repanda v. schistosa Bagl., Comment. Soc. Crittog. Ital. 1(5): 438 (1863).—Dirina repanda ssp. schistosa (Bagl.) Nyl., Bull. Soc. Linn. Normandie 6: 308 (1872).—Dirina schistosa (Bagl.) Nyl. in Hue, Rev. Bot. Bull. Mens. 5: 73 (1886).—Lecanora repanda var. schistosa (Bagl.) Harm., Lich. France 5: 1093 (1913); type: in rupibus schistosa ad promontorium di Carbonara in Sardinia meridionali, s. ann., Patrizio Gennari s. n. (S-F184363—lectotype sel. here).
Dirina repanda v. stipitata Nyl., Bull. Soc. Linn. Normandie 6: 309 (1872).—Lecanora repanda var. stipitata (Nyl.) Harm., Lich. France 5: 1092 (1913); type: Pyrénées Orientales, Port Vendres, Cap Béarn, 1872, William Nylander s. n. [H-NYL-23890—lectotype sel. by Tehler (1983)].
Lecidea praerimata Nyl., Flora 59(15): 235 (1876); type: Jersey, St John's, 1873, Larbalestier s. n. [H-NYL-10684—lectotype sel. by Tehler (1983)].
Dirina repanda v. basaltica Jatta, Nuovo Giorn. Bot. Ital. 18: 98 (1886); type: Italy, s. ann., s. coll. s. n. (not retrieved).
Dirina repanda f. lecideina Oliv., Bull. Acad. Int. Geogr. Bot. 11(3): 55 (1902); type: France, Port Vendres, s. ann., Goulard s. n. (not retrieved).
Dirina schistosa f. pedicillata Oliv., Bull. Acad. Int. Geogr. Bot. 11(3): 55 (1902).—Dirina repanda f. pedicellata (Oliv.) Zahlbr., Cat. Lich. Univ. 2: 509 (1924); type: France, Port Vendres, s. ann., Goulard s. n. (not retrieved).
Dirina repanda v. crassa Mahue & Gillet, Bull. Soc. Bot. France 68(21): 285 (1921); type: Morocco, Rabat, s. ann., Mahue & Gillet s. n. [UPS—lectotype sel. by Tehler (1983)].
Dirina repanda f. isidiosa Werner, Bull. Soc. Sci. Nat. Maroc 14(4–6): 148 (1934); type: Morocco, Rabat, plage de Temara, 1934, Werner s. n. [UPS—lectotype sel. by Tehler (1983)].
Dirina repanda f. sorediata Werner, (1975); type: Morocco, Rabat, 1931, R. G. Werner s. n. (not retrieved).
Thallus obligately saxicolous on acidic rock, surface plane to slightly rugose-verruculose, epruinose, grey-brown creamy, 0·1–1·5 mm thick; cortex 30–60 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·1–2·0 mm diam.; disc pruina with finely rimose surface, white-grey; thalline margin present, entire to undulating and often strongly undulating; ascospores 18–24×5–6 µm, mean length 21·24 µm, mean width 5·41 µm.
Chemistry
Spot tests: thallus surface C+ faintly red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, G occasional.
Distribution and habitat
Dirina fallax has its main distribution in the western part of the Mediterranean Region and the Atlantic coasts of Europe and Africa, north from Scotland and south to Morocco, with an outpost locality in the Canary Islands. In its sorediate form, it is also present in the Czech Republic in Central Europe. It is strictly confined to siliceous and acidic rocks.
Remarks
The type of Dirina fallax has been searched for unsuccessfully in all relevant Italian herbaria in Florence, Genoa, Modena, Padua, Pisa, Rome, Turin and Verona. Possibly the type is lost and therefore we chose to appoint a neotype for the name D. fallax. We chose a specimen from Sardinia, which was mentioned with no further specifications as the type locality by De Notaris (Reference de Notaris1846).
Dirina fallax is similar to D. massiliensis and is also closely related to that species. Dirina fallax has a thinner and usually a more grey-brownish thallus compared to the thicker, whitish and chalk-like thallus of D. massiliensis. However, Dirina fallax varies considerably in colour of the thallus and thalline margin, from dark brown to greyish to creamy white. Dirina fallax is strictly confined to acidic rock, in contrast to its sister species D. massiliensis, which is strictly confined to calciferous rocks. Sorediate specimens of Dirina fallax are morphologically indistinguishable from sorediate specimens of the partly sympatric species D. canariensis. They are also very difficult to distinguish from sorediate specimens of D. insulana. The latter has a C+ red medulla but this character may be very difficult to examine in the often very thin thalli.
Additional material examined. Bulgaria: Pontus: Burgas, Varvara et Micurin, 1977, Pisut & Vězda s. n. (S-F204743).—Czech Republic: Ceske Budijovice: Stredni Povltavi, Hlbuka nad Vltavou, 49°4′31″N 14°27′8″E, 2011, Vondrák 8423, 8424(CBFS). Prachatice: Sumava Mts foothills, Husinec, 49°2′23″N 13°59′41″E, 2011, Vondrák 8416 (S-F184109); Sumava Mts foothills, Husinec, 49°1′59·663″N 13°59′2·735″E, 2009, Vondrák & Merkulova 7172 (CBFS). Znojmo: Podyjí National Park, Lukov, 48°50′12″N 15°53′59″E, 2011, Vondrák 8426 (S-F184112).—France: Corse: Ajaccio, scopulus La Parata dictus, 1969, Lambinon, Rondon & Vězda s. n. (S-F184388); NE de Bonifacio, 41°25′10″N 9°14′39″E, 2011, Ertz 16506 (BR); Cap Corse, Pino, Minerviu, 2011, Ertz 16459 (BR); Nord d'Ajaccio, côte sud du Golfe de Sagone, 42°01′11″N 8°43′13″E, 2011, Ertz 16396, 16397 (BR); Marchese (Cargèse), Punta d'Omigna, 42°09′N 8°34′E, 2011, Ertz 16413, 16421 (BR). Var: Isles d'Hyeres, Isle du Levant, 43°1·485′N 6°26,540′E, 2008, Tehler 9521, 9522 (S-F210789, S-F210790).—Germany: Heidelberg: Handschuhsheim, 1880, Zwackh s. n. (S-F204744, S-F204745); Neuenheim, 1876, Zwackh s. n. (S-F204747); 1880, Zwackh s.n. (S-F204748); s.loc., s.ann., Bayrhoffer s. n. (S-F204746).—Great Britain: England: V.C. 2, East Cornwall: Boscastle between Tintagel Head and Trevalga, 1979, Tehler 4626, 4629 (S-F97437, S-F204756); near Boscastle, 1939, Lamb 817 (S-F184235); Logan Rock, S of Penzance, 1979, Tehler 4638 (S-F204758); Zennor, 1979, Tehler 4640, 4647 (S-F97438, S-F204757).—Greece: Kassandra: 2 km SSW of Paliuro, 39°54′N 23°40′E, 39°54′N 23°40′E, 1982, Karis 175, 176, 178 (S-F204749, S-F204753, S-F204754).—Italy: Isole Pelagie: Linosa 1979, Tehler 4581 (S-F97451); Linosa, between P. Paranzello and Scoaglio dei Bovi Marini, 1979, Tehler 4533, 4535 (S-F97449, S-F204755); [transitions with both ascocarps and soralia] 1979, Tehler 4537 (S-F97450).—Morocco: Grand Casablanca: Menseouri, [some specimens with both ascocarps and soralia], 1978, Tehler 2158 (S-F170248).—Portugal: Algarve: SW of Aljezur, 37°17′58″N 8°52′22″W, 2011, Ertz 16905 (BR); SW of Aljezur, 37°18′01″N 8°52′17″W, 2011, Ertz 16921, 16922, 16929 (BR); Vila do Bispo, Praia do Castelejo, 1979, Tehler 4601 (S-F184430). Leira: E of Nazaré, Monte de Sao Bartolomeu, 39°35′35″N 9°03′07″W, 2011, Ertz 17024 (BR). Lisbon: Azaio just S of Cabo da Roca 38°46,138′N 9°29,871′W, 2010, Tehler 10208 (S-F210794).—Spain: Almeria: Cabo de Gata, Punta Negra, Torre Vela Blanca, 36°43·557′N 2°10·194′W, 2009, Tehler 9811, 9812 (S-F210792, S-F210793). Cadiz: Cádiz, Punta de la Chullara, 36°18·690′N 5°16·084′W, 2009, Tehler 9801 (S-F210791); Lake Embalse de Celemin, 1978, Tehler 4300 (S-F135189); 20 km N of Tarifa, 1978, Tehler 2141 2145 2148 (S-F135190, S-F135193, S-F135194). Canary Islands: La Gomera, San Sebastián de la Gomera, Roque de Berruga, 28°05′34″N 17°11′17″W, 2011, Ertz 16186 (BR).
Dirina immersa Müll. Arg.
Proc. Roy. Soc. Edinburgh 11: 465 (1882); type: Socotra Island, 1881, B. Balfour 1322 [G-66178—lectotype sel. by Tehler (1983); G-66179—isolectotype].
Chiodecton socotranum Müll. Arg., Proc. Roy. Soc. Edinburgh 11: 470 (1882); type: Socotra Island, 1881, B. Balfour 1346 [G-66214—lectotype sel. by Tehler (1983); G-66215—isolectotype].
Dirina immersa f. sorediata Müll. Arg., Proc. Roy. Soc. Edinburgh 11: 466 (1882).—Dirina massiliensis f. sorediata (Müll. Arg.) Tehler, Opera Bot. 70: 33 (1983); type: Socotra Island, 1881, B. Balfour s. n. [G-66180—lectotype sel. by Tehler (1983)].
(Fig. 7F)
Thallus obligately saxicolous on calciferous rock, surface plane, pruinose, white to white-grey, 0·2–2·0 mm thick; cortex 30–50 µm thick; medulla chalk-like also near substratum, white; soralia present (usually in the absence of ascomata), punctiform.
Ascomata present (usually in the absence of soralia), monocarpocentral, numerous, immersed, circular or linear in outline, 0·1–0·5 mm diam.; disc pruina with rimose surface, white to white-grey; thalline margin absent; ascospores 16–20×5–7 µm, mean length 18·48 µm, mean width 5·97 µm.
Chemistry
Spot tests: thallus surface C+ red, but often faint or negative if the uppermost layer is not scraped or cut away; medulla C+ red; disc C+ faintly red. Secondary metabolites: erythrin; lecanoric acid rare; unidentified substance C.
Distribution and habitat
Dirina immersa is endemic to Socotra Island. It grows on calciferous rocks near the coast.
Remarks
Dirina immersa is the sister species to D. arabica. They are sympatric and grow in the same localities, but are easily distinguished from one another by the appearance of the ascomata which are immersed in Dirina immersa but sessile in D. arabica.
Dirina immersa earlier also included D. candida. They have similar morphology, anatomy and chemistry and, in the absence of any distinguishing characters, they were considered conspecific by Tehler (Reference Tehler1983). In this new study, the molecular data from four phylogenetic markers indisputably indicate that Dirina immersa and D. candida should be acknowledged as two distinct and unrelated species.
Dirina immersa is also found with soredia, in contrast to the otherwise virtually identical D. candida. Fortunately, their distributions are shown not to be sympatric.
Additional material examined. Yemen: Socotra Island: Homill, 12°35′16·9″N 54°18′31·2″E, 2008, Tehler 9300 (S-F210796); Mumi plateau, Ant Kashara, 1994, Mies & Printzen 244b2 (S-F159765); Sefflah, 12°30′43·4″N 54°26′02·2″E, 2008, Tehler 9340 (S-F210795); S part where the main road leads up into the mountains, 12°22′34·9″N 53°55′17·8″E, alt. 300 m, 2008, Tehler 9322, 9327 (S-F210797, S-F210798).
Dirina indica Upreti & Nayaka sp. nov.
MycoBank No.: MB 802911
Thallus obligately corticolous. Ascomata sessile, base not constricted or semi-constricted. Disc pruina with even surface, C+ red. Ascospore mean length value 29·11 µm.
Type: India, Gujarat, Jamnagar, Marine National Park, Pirton Island, 2008, J. Rawal 08-011280 (LWG—holotype; S—isotype).
(Fig. 5D)
Thallus obligately corticolous, surface plane to slightly rugose-verruculose, epruinose, creamy white to white greenish, 0·1 mm thick; cortex 20–30 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base not constricted or semi-constricted, >0·5 mm diam.; disc pruina with even surface, white; thalline margin present, entire; ascospores 23–33×4 µm, mean length 29·11 µm, mean width 4·46 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Etymology
The name refers to the geographical region of India.
Distribution and habitat
Dirina indica is distributed on the west coast of India (Gujarat), the southern coast of the Arabian Peninsula in Yemen, and on Socotra Island. It is strictly corticolous, growing on various trees and shrubs such as Avicennia, Adenium, Acacia, Salvadora and Ceriops.
Remarks
The sister species to Dirina indica is D. angolana (Figs 1 & 2). The latter is known only in Angola and the disjunction is interesting since a similar distribution pattern was observed in the fruticose genus Roccella, within groups of the large but heterogenous species R. montagnei (Tehler et al. Reference Tehler, Irestedt, Wedin and Ertz2010).
Additional material examined. India: Gujarat: Jamnagar, Marine National Park, Pathapir, 2009, Rawal 09-011540 (LWG); Rozy Bandar, 2009, Rawal 09-011520 (LWG); Chaneda Island, 2008, Rawal 08-011556/B (LWG).—Yemen: Abian Governorate: Djebel Urays, Wadi Asurai, above Bir Asurai, 2002, Schultz 14239 (hb. Matthias Schultz). Socotra Island: Homill, in the eastern part of the island, on the ridge facing the sea, 12°35′35·2″N 54°18′25·6″E, 2008, Tehler 9312 (S-F210799).
Dirina insulana (Tav.) Tehler
Opera Bot. 70: 43 (1983).—Enterographa insulana Tav., Portugaliae Acta Biol., Sér. B, Sist. 3(3): 325 (1952); type: Portugal, Madeira, near Montado dos Peceguieros, on basalitic cliffs, alt. 900 m, 1951, Tavares 4563 (not available—not retrieved); type: Portugal, Madeira, Camara de Lobos, August 1956, Baretto, n. 2. hb. Tavares n. 6155 (LISU—neotype sel. by Tehler 1983).
Dirina insulana f. sorediata Tehler, Opera Bot. 70: 44 (1983); type: Portugal, Madeira, Funchal, Botanical Garden NE of the town, 1983, Kaj Sundgren 1 (S-L4—holotype).
(Figs – see Tehler Reference Tehler1983)
Thallus obligately saxicolous on acidic rock, surface plane, epruinose or slightly pruinose, grey-green or grey-brown, sometimes white-grey, 0·1–0·5 mm thick; cortex 20–40 µm thick; medulla chalk-like also near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), monocarpocentral, numerous, immersed, circular or linear in outline, 0·1–0·9 mm diam.; disc pruina with even surface, white-grey; thalline margin absent; ascospores 22–27×5–6 µm, mean length 24·7 µm, mean width 5·6 µm.
Chemistry
Spot tests: thallus surface C+ red, sometimes faintly red; medulla C+ red; disc C−. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Distribution and habitat
Dirina insulana is a widespread species found throughout Macaronesia in the Azores, Madeira, the Canary Islands, Cape Verde and the coast of southern Portugal. It grows on the vertical, volcanic outcrops near the sea.
Remarks
Dirina insulana produces both ascomata and soredia, usually in distinctly either fertile or sterile morphs. Transitional specimens with both ascomata and soralia on the same thallus are uncommon. Sorediate specimens of Dirina insulana are morphologically indistinguishable from sorediate specimens of the partly sympatric species D. fallax, D. canariensis and D. sorocarpa, but D. insulana is the only one of these species with a C+ red medulla. However, that character may be very difficult to observe in the often very thin thalli.
Additional material examined. Cape Verde: Boa Vista: Mt. Rochinha, N von Sal-Rei, 1987, Mies 559h3 (S-F151310); Mt. Vigia-massiv, Leuchtturm oberhalb Ponta do Sol, 1987, Mies 561f3 (S-F151312). Brava: Cova Rodela, 600 m, 1987, Mies 734,2 (S-F151295). Fogo: Cova Matinho, Ponta Segue-Segue, 14°56′N 24°18′W, 1988, Mies 1216h 2 (S-L11769). Maio: Mt. Batalha, 1987, Mies 790h2 (S-F151308). Sal: Monte Leâo (Rabo da Junco), 16°42·030′N 22°58·929′W, 2010, Tehler 10080 (S-F210802). Santiago: N des Mt. Graciosa, N von Tarrafal, 1988, Mies 833g 2 (S-L11758). Santo Antao: Fontainhas, 17°12′N 25°06′W, 1988, Mies 1191i 2 (S-L11773). Sao Nicolau: Punta Espechim, W Ribeira Funda, 16°40′N 24°20′W, 1988, Mies 938c2, 941a2 (S-L11776, S-L11741); NW des Mt. Bissau, Ribeira zum Rib, Madeira Vermelha, 16°37′N 24°15′W, 1988, Mies 1080f 2 (S-L11770). Sao Tiago: between Sao Domingos and Rui Vaz, 1986, Mies 214m2 (S-F151307); Mt. Grande bei Milho Branco, 1986, Mies 331a2 (S-F151306). Sao Vicente: Madeiral, 16°49′N 24°56′W, 1988, Mies 1017c2, 1017d2 (S-L11757, S-L11740); Madeiral, 16°49·174′N 24°56·140′W, 2010, Tehler 10053 (S-F210801); NW of Calhau, Goa Baixo, 16°51′27″N, 24°52′42″W, 2011, Ertz 17279 (BR); Praia da Ceilada do Calhau, 16°51·552′N 24°52·741′W, 2009, Tehler 10011 (S-F210800).—Portugal: Azores: Faial, Castelo Branco, 38°31·299′N 28°42·961′W, 2000, Tehler 8215 (S-L12333); Horta, Conceicao, 1986, Arvidsson A289, A290, A291 (GBG); São Miguel, Ponta Delgada, opposite Clinica S. João de Deus in Fajã de Baixo 37°45·258′N 25°38·649′W, 2000, Tehler 8175b (S-L12326); 2010, Tehler 10224 (S-F210807); Ponta Delgada S of the airport, 37°44·454′N 25°42·381′W, 2010, Tehler 10212 [sorediate](S-F210803), 10213 [fertile] (S-F210804); Ponta Delgada, Parque da Alminhas 37°45·830′N 25°44·099′W, 2010, Tehler 10219 [fertile] (S-F210805), 10220 [sorediate] (S-F210806); Terceira, Angra do Heroismo, Monte Brasil 38°38·873′N 27°13·708′W, 2010, Tehler 10237 (S-F210809); Lajos, Ponta da Serra das Lajes, by Aeroporto da Lajos, 38°47·108′N 27°6·484′W, 2010, Tehler 10248 [sorediate] (S-F210810), 10249 [fertile] (S-F210811); Praia do Vitoria, Ponta da Ma Merenda, 38°44·049′N 27°2·881′W, 2010, Tehler 10230 (S-F210808). Lisbon: Azaio just S of Cabo da Roca 38°46·138′N 9°29·871′W, 2010, Tehler 10207 (S-F210812); SW of Sintra, SE of Cabo da Roca, 38°45′21″N 9°28′40″W, 2011, Ertz 17037 (BR). Madeira: Funchal, Botanical Garden, 1982, Dahlén & Baltscheffsky 999 (S-F63436); 1983, Sundgren 2 (S-L3480); 1981, Anderberg 632 (S-F63435); Ponta de São Lourenco, 2007, Ertz 10542 (BR); Porto Santo Island, Pico do Castelo, Ertz 10568 (BR).—South Atlantic Islands: Ascension Island: Norfolk Pine track, 1976, James s. n (S-L5079).—Spain: Canary Islands: El Hierro, Sabinosa, Punta de la Dehesa, 2009, Ertz 13532 (BR); Gran Canaria, Jardin Canario, 28°3·912′N 15°27·777′W, 2011, Tehler 10311 [fertile] (S-F210813),10312 [sorediate] (S-F210814); La Gomera, San Sebastián, La Gerode, path to Casas de Jaragán and Montaña Ismael, 28°07′45″N 17°08′50″W, 2011, Ertz 16142, 16328 (BR).
Dirina jamesii (Tehler) Tehler & Ertz comb. nov.
MycoBank No.: MB 802912
Roccellina jamesii Tehler, Bryologist 88: 131 (1985); type: Ascension Island, Cricket Valley, S.W.-facing aspect, alt. 1400 ft, 1976, P. W. James s. n. (BM—holotype; S-L502—isotype).
(Figs – see Tehler Reference Tehler1983)
Thallus obligately saxicolous on acidic rock, surface plane, slightly pruinose, creamy white to white-brownish, 0·1–1·0 mm thick; cortex 40–65 µm thick; medulla chalk-like also near substratum, dirty white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted or unconstricted, >1·5 mm diam.; disc pruina with even surface or finely rimose surface, white-grey; thalline margin present, entire to undulating; ascospores 23–27×4–5 µm, mean length value 23·31 µm, mean width value 4·74 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red, faintly red or negative. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Distribution and habitat
Dirina jamesii is distributed over the two Atlantic islands, St. Helena and Ascension Island, and mainland Africa in Angola, often forming extensive mosaics on more or less dry vertical faces or underhangs of boulders and cliffs, near the sea. It is strictly saxicolous.
Remarks
During the years since Roccellina jamesii was described (Tehler Reference Tehler1985), we have had the opportunity to study and collect new material of this species. The new morphological and molecular data obtained from that material clearly indicate that R. jamesii should be transferred to the genus Dirina.
Similar to the situation with Dirina badia, and as a result of the molecular analysis in combination with a reciprocal illumination of the hypothecial extensions developed in Dirina jamesii, it was evident that the extensions developed were pseudo-hypothecial and analogous to the true hypothecial extensions found in Roccellina. The latter are prolongations that originate from the same carbonaceous tissue as that of the hypothecium. The pseudo-hypothecial extension in Dirina jamesii is more inconspicuously pale brownish (sometimes missing) and develops from the bottom layer of the dirty brownish medullary tissue. This is indicated by the KOH reaction, in which the dark brown true hypothecial extension, like the hypothecium, all turns olive-green, whereas the pseudo-hypothecial extension remains K dirty brownish. The cortex tissue is made up of bifurcate, intertwined hyphae but still they are mainly anticlinally arranged, as with the cortex type in Dirina.
On the basis of the newly collected material from Angola, it also became clear that the fruiting bodies range from more or less immersed over semi-sessile with unconstricted base, sometimes developing into fully sessile ascomata with a constricted base.
Dirina jamesii is treated here as a species, although it is unsupported as paraphyletic (Fig. 4). There may be more than one species involved but the sampling group and the evidence in support of describing species are simply too poor. There were only three possible collections available for molecular investigation: one from St Helena, one from Ascension and one from Angola (two DNA samples were taken from the latter). These three collections were more or less unresolved or very poorly resolved. However, morphologically they have similarities, such as the sessile to semi-sessile ascomata, and they are grouped together in the consensus tree.
Additional material examined. Angola: Bengo: Caba Ledo, S of the village, 9°40·675′S 13°12·058′E, 2009, Tehler 9742 (S-F210815).—South Atlantic Islands: St Helena: Rupert's Valley, 15°55·547′S 5°42·436′W, 2006, Aptroot 66292 (ABL); above Jamestown, along track to Saddle Battery, 15°55·744′S 5°42·817′W, 2006, Aptroot 66423 (S-F86655). Ascension Island: Sisters Peak, 7°55·977′S 14°21·971′W, 2006, Aptroot 66748 (S-F86671); Cricket Valley, 1976, James s. n (S-L5078, S-L5077); Devil's riding school, 1976, James s. n (S-F196062); above Two Boats Village, 1976, James s. n (S-F196068).
Dirina madagascariensis Tehler, Ertz, Killmann, Razafin., Sérus. & Eb. Fisch. sp. nov.
MycoBank No.: MB 802913
Thallus facultatively saxicolous/corticolous, on calciferous rock. Medulla chalk-like also near substratum. Soralia absent. Ascomata sessile, base constricted, up to 2 mm diam. Disc C− rarely C+ red. Ascospores 25–35×4–5 µm, mean length value 30·6 µm. Unidentified secondary metabolite J present.
Type: Madagascar, Toalanaro (Fort Dauphin), alt. 4 m, 2008, Damien Ertz 13216 (BR-LICH 7484-15—holotype; S-F213217—isotype).
(Fig. 6D)
Thallus facultatively saxicolous/corticolous, on calciferous rock when saxicolous, surface plane to slightly rugose-verruculose, slightly pruinose, creamy white to white-greyish, 0·1–1·5 mm thick; cortex 30–50 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·1–2·0 mm diam.; disc pruina with finely rimose surface, white; thalline margin present, entire to undulating; ascospores 25–35×4–5 µm, mean length value 30·6 µm, mean width value 4·74 µm.
Chemistry
Spot tests: thallus surface C+ red, sometimes upper pruina has to be removed to see reaction; medulla C−; disc C− rarely C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C & J.
Etymology
The name refers to the geographical region of Madagascar.
Distribution and habitat
Dirina madagascariensis is endemic to, and has only been collected in, the southern part of Madagascar. It is facultatively saxicolous and corticolous, growing on calciferous rocks or on the trunks of various trees and shrubs in forests with Didierea madagascariensis, Euphorbia stenoclada, Alluaudia comosa, Euphorbia laza, Delonix adansonioides and Kalanchoe grandidieri, near the coast in the districts of Toliara and Toalanaro.
Remarks
The unidentified secondary metabolite J is characteristic for this species.
The three saxicolous and three corticolous specimens investigated are divided into two clades and may represent two species (Fig. 4). However, only the saxicolous clade receives full PP and PJ support, whereas the corticolous clade receives somewhat less PJ support (90%). We will therefore refrain from describing a corticolous and saxicolous species until a hypothesis can be tested by the analyses of more specimens. Unexpectedly, the closest relatives and the sister group to Dirina madagascariensis is the strictly American species group containing D. catalinariae, D. mexicana, D. pallescens and D. paradoxa (see Biogeography below). It is only distantly related to the geographically much closer species Dirina astridae (Mauritius), D. arabica/D. immersa (Socotra) or D. indica (W. India).
Additional material examined. Madagascar: Toliara: La Table, 23°25′09·3″S 43°47′03·1″E, 2008, Ertz 13095 (BR-LICH 8734-04); La Table, 23°24′40·8″S 43°47′40·0″E, 2008, Ertz 13127 (BR-LICH 8765-35); Ifaty, 23°07′23·1″S 43°36′34·4″E, 2008, Ertz 13086 (BR-LICH 8724-91); S of Ifaty, 23°10′18·6″S 43°37′09·7″E, 2008, Ertz 13042 (BR-LICH 7391-19). Toalanaro: Fort Dauphin, 25°01′37·5″S 47°00′04·9″E, 2008, Ertz 13215 (BR-LICH 7487-18).
Dirina massiliensis Durieu et Mont.
Expl. Sci. Algerie. Cryptog. 1: 247 (1847).—Parmelia repanda Fr., Lichenogr. Eur. Reform. 177 (1831) [Dirina repanda auct. non Fr. (1825)].—Parmelia massiliensis Dufour ex Mont., Arch. Bot. (Paris) 295 (1833).—Dirinopsis massiliensis De Not., Giorn. Bot. Ital. 2(1): 187 (1846).—Urceolaria repanda (Fr.) Schaer., Enum. Crit. Lich. Eur. 92 (1850).—Secolegia repanda (Fr.) Norman, Nyt Mag. Naturvidensk. 230 (1853); type: France, Marseille, Montredon, 1806, Dufour s. n. (UPS—lectotype sel. by Tehler 1983).
Lecanora repanda Fr. ex Duby, Bot. Gall. (1830); type: France, Marseille, s.ann, Le Prevost s. n. [UPS—lectotype sel. by Tehler (1983); M—isolectotype].
Lecidea stenhammarii Fr. ex Stenham., Öfvers. Kongl. Vetensk.-Akad. Förh. 4: 197 (1848); Fries, Summa Veg. Scand. (Fries) 155, nom. nud.1845.—Lecanactis stenhammarii (Fr. ex Stenham.) Arnold, Flora 54: 196 (1871).—Bilimbia stenhammarii (Fr. ex Stenham.) Boistel, Nouv. Fl. Lichens 2: 33 (1903).—Dirina stenhammarii (Fr. ex Stenham.) Poelt & Follmann, Herzogia 1: 61 (1968).—Dirina repanda f. stenhammarii (Fr. ex Stenham.) Clauzade & Roux, Bull. Mus. Hist. Nat. (Marseilles) 35: 192 (1975); type: Sweden, Gotland, Kyllaj, Kylleiberget, “Gottlandia in grascruptio montium calcareorum, Kylleiberget, Klejven in picroccia, Sundre etc” Chr. Stenhammar scripsit, s.ann, Christian Stenhammar s. n. (S-L5—lectotype sel. by Tehler 1983).
Pyrenothea aponina A. Massal., Ric. Auton. Lich. Crost. 151 (1852).—Lecanactis aponina (A. Massal.) Zahlbr., Cat. Lich. Univ. 2: 533 (1924).—Dirina massiliensis f. aponina (A. Massal.) Tehler, Lichenologist 20: 398 (1988); type: ex hb. Massalongo, [Anzi, Lichenes rarioris Veneti no. 85]. Ad tophum, et rupes calcareas fontium thermalium aponinarum (di Abano) in collibus Euganeis, Mass., s. ann, A. Massalongo s. n. (S-F177479, S-F177477, UPS).
Dirina patronii Bagl., Mem. Reale Accad. Sci. Torino 2(17): 397 (1857).—Dirina repanda f. patronii (Bagl.) Zahlbr., Cat. Lich. Univ. 2: 509 (1924); type: Italy, “Cresce su d'un vecchio muro nel villaggio Creveri presso Voltri”, s. ann, Stefano Patrone s. n. (not retrieved).
Dirina repanda v. pelagosae J. Steiner et Zahlbr., Österr. Bot. Z. 53(5): 177 (1903); type: Croatia (Jugoslavia), Pelagosa Isl., s. ann, A. Ginzberger s. n. (W—holotype).
Dirina cyclosora Poelt & Nimis, Bull. Soc. Linn. Provence 45: 254 (1994); type: s. ann., s. coll. s. n. [TSB—holotype (not seen); GZU—isotype].
(Figs – see Tehler Reference Tehler1983)
Thallus obligately saxicolous on calciferous rock, surface plane, rugose-verruculose to nearly squamulose or suffruticose, pruinose, white-grey, 0·2–3·5 mm thick; cortex 40–70 µm thick; medulla chalk-like also near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, 0·5–3·0 mm diam.; disc pruina with rimose surface, white-grey to dark grey; thalline margin present, entire to undulating, strongly undulating or even so strongly undulating as to become stromatoid; ascospores 20–24×4–6 µm, mean length value 21·6 µm, mean width value 5·0 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ faintly red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, D, E, H rare.
Distribution and habitat
Dirina massiliensis is one of the most widespread species of Dirina. It grows on calcareous coastal outcrops along the coasts of the Mediterranean, Atlantic Ocean (from Bergen, Norway south to Morocco) and the Baltic Sea (Öland to Gotland). It also occurs on inland calcareous rocks in Central Europe, in localities with dry, open vertical cliffs and rocks resembling coastal cliffs but then only as sterile, sorediate thalli.
Remarks
Dirina massiliensis occurs more or less distinctly as either a fertile, ascocarpous morph or as a sterile, sorediate morph although transitional specimens with both ascomata and soralia on the same thallus occur, even if they are relatively uncommon. Dirina massiliensis also develops a pycnidiate morph (Tehler Reference Tehler1988). Both the sorediate morph and the pycnidiate morph have been treated as a separate taxa, named Dirina stenhammarii or Dirina massiliensis f. sorediata when sorediate and Lecanactis aponina or D. massiliensis f. aponina when pycnidiate. We no longer distinguish these or any other sterile morphs taxonomically, since supporting molecular data is absent. Ascomata formation is frequent in the Mediterranean region climate and rare in central and northern Europe. The northernmost localities with specimens abundantly forming ascomata are the southern point of Gotland and the small island Lilla Karlsö, close to Gotland in the Baltic Sea. The only ascoma-forming specimen on an inland locality originates from the Ardennes in southern Belgium (but no other fertile specimen has been seen since then in this country).
In Tehler (Reference Tehler1983), Dirina massiliensis on calciferous rock and D. fallax on acidic rock were treated as conspecific, but new data from the present investigation clearly indicate that they should each be treated at species level.
Dirina massiliensis is similar to D. fallax and is also closely related to that species. Dirina massiliensis has a thicker, whitish and chalk-like thallus compared to the thinner and more grey-brownish thallus of D. fallax. Furthermore, D. massiliensis is strictly confined to calciferous rocks whereas Dirina fallax is strictly confined to acidic rock.
Dirina massiliensis is confusingly similar to saxicolous D. ceratoniae, but the medullary hyphae in D. massiliensis are chalk-like throughout and never loose or byssoid near the substratum which is normally the case in D. ceratoniae. However, in some specimens of the latter species, particularly those with thin thalli, this character may fail or be very difficult to see. Dirina ceratoniae can also be distinguished from D. massiliensis by its more greenish, less pruinose thallus surface and its longer ascospores.
Additional material examined. Belgium: Ardennes, s. ann., s. coll., s. n. (FH); Wulveringem (Veurne), Beauvoorde, 2008, Ertz 12403 (BR).—Croatia: Dalmatia: Korcula, Brna, 1980, Tehler 5070, 5075 (S-F97436, S-F204729); Vela Luca, 1980, Tehler 5078, 5080 (S-F170255, S-F204730); Dubrovnik (Ragusa), s. ann., Micheletti s. n. (S-F184127); Primorje, Rab, 1926, Baumgartner 65 (S-F204702); Losinj, Veli Losinj, 1980, Tehler 5116 (S-F204704); Rab, Kampor, 1980, Tehler 5099 (S-F204705); Drvenik, 80 km SE of Split, 1980, Tehler 5061 (S-F204726); Risan, 50 km S of Dubrovnik, 1980, Tehler 5059 (S-F204727); Karlobag, 80 km N of Zadar, 1980, Tehler 5093 (S-F204728); Krk, Pinenzioi SW Fuska, 1980, Tehler 5102 (S-F204731); Pag, Gradac, 1980, Tehler 5098 (S-F204732); Pag, 3 km NW of Pag, 1980, Tehler 5091 (S-F204733); Sibenik, 1980, Tehler 5082 (S-F204734). Istria: Pula, on walls of the Arena amphitheatre, 1980, Tehler 5122, 5123, 5129 (S-F97432, S-F97444, S-F204707); Premantura, [transitional, apothecia-soredia], 1980, Tehler 5132, 5135, [transitional apothecia-soredia], 5140, (S-F97435, S-F97433, S-F204710); Rijeka, 1980, Tehler 5105 (S-F204706); Rovinj, 35 km NE of Pula, 1980, Tehler 5144 (S-F204712).—Cyprus: Cape Greco: 20 km S of Famagusta, 1980, Tehler 5010, 5015 (S-F204697, S-F97445). Cape Pyla: 25 km E of Larnaca, 1980, Tehler 5056, 5057 (S-F97448, S-F204719). Paphos: Tombs of the Kings, just outside Paphos, 1980, Tehler 5027, 5034 (S-F204720, S-F97446). Polis: Akamas peninsula 40 km N of Paphos, 1980, Tehler 5042, 5044 (S-F97447, S-F204696).—Czech Republic: Horazd'ovice: Sumava Mts, Zichovice, 49°16′28·93″N 13°35′30·59″E, 2007, Vondrák 5125 (CBFS). Znojmo: Podyjí National Park, Lukov, 48°50′17″N 15°54′25″E, 2011, Vondrák 8425 (CBFS).—France: Antibes: s. loc., 1866, Metzler s. n. (S-F184362). Bouches-du Rhône: Châteauneuf-les Martiques, 1965, Marcucci s. n. (S-F184218). Sarthe: Brùlon calcaire de Pissegrèle, 1906, Monguillon s. n. (S-F204701).—Germany: Bavaria: Eichstätt, 1876, Arnold s. n. (S-F204698); 1873, Arnold 560 (S-F204699); 1876, Arnold s. n. (S-F204700); 1977, Tehler 4212 (S-F204695). Würtemberg: s. loc., 1895, Zhieber s. n. (GBG).—Gibraltar: along the Mediterranean Steps, 2009, Tehler 9803 [fertile], 9804 [sorediate] (S-F210816, S-F210817).—Greece: Peloponnesos: Akhaia, Akr. Araxos, 35 km W of Patras, 1978, Tehler 4014 (S-F204722).—Hungary: Bükk Mts, Bükk National Park, Borsod-Abauj-Zemplen, Malyinka, 48°08′00·1″N 20°30′03·1″E, 2008, Vondrák & Khodosovtsev 6379 (CBFS); s. loc., s. ann., Lojka s. n. (S-F184415).—Italy: Isole Ègadi: Favignana, 1979, Tehler 4510, 4517 (S-F97442, S-F204725). Isole Pelagie: Lampedusa, 1979, Tehler 4547, 4553, 4580 (S-F97441, S-F204724, S-F97440). Puglia: Lecce, Otranto, 1996, Nimis & Tretiach s. n. (S-F97431); Otranto, 2008, Tehler 9600, 9603, 9604, 9608, 9635, 9636 (S-F176070, S-F176063, S-F176064, S-F176073, S-F176062, S-F176068); S. Cesarea Terme, 40°4·125′N 18°28·832′E, 2008, Tehler 9610, 9611 (S-F176093, S-F176091); Diso, 39°59·483′N 18°24·869′E, 2008, Tehler 9612, 9613 (S-F176049, S-F176050); Santa Maria di Léuca, 39°47·499′N 18°20·670′E, 2008, Tehler 9624 (S-F176098); Foggia, Isole Tremiti, 1997, Nimis s. n. (S-L3972). Sardinia: Carolim, s. ann., Canepa s. n. (GBG, S-F184125, S-F184230); Cagliari, s. ann., Canepa s. n. (S-F184183, S-F184361); Capo St. Elia,1866, Marcucci s. n. (S-F184205, S-F184234).—Libya: Cyrenaica: 32 km WSW of Derna, 13 km ESE of Lamludah, 1982, Thor 2926 (S-F127872).—Morocco: Agadir: Cap Rhir unweit Tamri, 1967, Follmann & Follmann-Schrag s. n. (GBG). Casablanca: Mensouri, c. 50 km N of Casablanca, 1978, Tehler 2159 (S-F204737). El Jadida: Mazagan, 1932, s. coll. s. n. (S-F184126).—Norway: Hordaland: Austevoll, Litlakalsøy, 1981, Skjolddal 5802 (S-F204666). Rogaland: Tysvaer, Kårstø, 1981, Skjolddal 5801 (S-F204667). Sund: Store Sotra by Golta, 1981, Tehler 5803 (S-F204668).—Poland: Karpaty: Beskidy Zachodnie Mts., 1977, Tehler 4202 (S-F204721). Nowy Targ: Pieniny, Wawoz Sobczanski, 1957, Tobolewski s. n. (S-F204723).—Portugal: Algarve: Bordeira, 37°11′47″N 8°55′00″W, 2011, Ertz 16952, 16958 (BR); Cabo de Sao Vicente, 2011, Ertz 16994, 16995, 16996 (BR); 1979, Tehler 4584, 4600 (S-F184426, S-F184427); Beliche, 1944, Palhinha 3675 (S-F204703); 1951, Tavares s. n. (S-F184237). Lisbon: Praia da Adraga, circa Colares, 1951, Tavares s. n. (S-F184226).—Spain: Almeria: Carboneras, Punta de los Muertos, 1975, Tehler 1206, 1209 (S-F135200, S-F135201); 2009, Tehler 9813 (S-F210818). Mallorca: Cala Marsal and Cala Brafi, 1972, Santesson 24086a (S-F184124); Cala Santanyi, 1979, Tehler 4680, 4684, 4686 (S-F60779, S-F60767, S-F60768); Formentor, 1979, Tehler 4673, 4681, 4683 (S-F60770, S-F60772, S-F60777); Formentor, 2007, Tehler 9026, 9030 (S-F66008, S-F66010); Playa de Cala Santanyí, 2007, Tehler 9001, 9002, 9008, 9009 (S-F66003, S-F66005, S-F66006, S-F66007); Punta la Nao, 1978, Thor 629 (S-F60764). Murcia: La Azohia, 1976, Tehler 1255, 1256 (S-F135188, S-F135186); Los Belones, 1976, Tehler 1272, 1273 (S-F135176, S-F135177); Mazarron, 1976, Tehler 1244, 1247 (S-F135170, S-F135168).—Sweden: Gotland: Hellvi, Kyllaj, 1982, Tehler 5950 (S-F204645); 2007, Tehler 9213 (S-F96537); Lilla Karlsö, 1977, Thor 253, 253a(1), 253a(2), 253b (S-F204661, S-F97426, S-F97429, S-F204662); Rute, Furilden, 57°45′17·3″N 18°56′52·5″E, 2007, Tehler 9214 (S-F96544); Stenkyrka, Stenky rke huk, 1980, Anderberg 603 (S-F204664); Sundre, Gullstajnen, 1977, Tehler 3015 (S-F204665); Hoburgen, 1977, Thor 55 (S-F204659); 1980, Anderberg 602 (S-F204663); 1983, Tehler 6000 (S-L193); 1998, Tehler 8044, 8045, 8046, 8047 (S-L5649, S-L5650, S-L5651, S-L5652); 2007, Tehler 9210, 9211, 9212 (S-F96531, S-F96534, S-F96536); Klejven, s. ann., Stenhammar s. n. (S-L197, S-F204649, S-F204658); Visby, Galgberget, 1982, Thor 2706 (S-F204646); Snäckgärdsbaden, 1977, Thor 252 (S-F204660); s. loc., s. ann., Stenhammar s. n. (S-F204647). Öland: Borgholm, 1860, Hellbom s. n (GBG); 1871, Molér s. n. (GBG, S-F204642); Köping, Köpings branter, 1867, Zetterstedt s. n. (S-F204643); 1976, Tehler 2113 (S-F204641).—Switzerland: Baselland: Liestal, Kant, s. ann., Hepp 757 (S-F204685).—Ukraine: Crimean Peninsula: Sevastopol, Balakalava, 44°29′42″N 33°36′4″E, 2009, Vondrák, Merkulova & Khodosovtsev 7366 (CBFS).
Dirina mexicana Tehler
Lichenologist 27: 256 (1995); type: Mexico, Estado de Sinaloa, 3 km NNE of Higuera de Zaragoza, hill surrounded by agricultural fields, on vertical rocks, alt. 50 m, 1993, Anders Tehler 7175 (S-L7—holotype; AZU, BM, ESS, H, M, NY, US, UPS—isotypes).
(Figs – see Tehler et al. Reference Tehler, Feige and Lumbsch1995)
Thallus obligately saxicolous on acidic rock, surface plane to slightly rugose-verruculose, slightly pruinose, white-grey to white-yellowish, 0·2–0·5 mm thick; cortex 20–40 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, monocarpocentral, numerous, immersed, circular or linear in outline, 0·4–0·8 mm diam.; disc pruina with rimose surface, white; thalline margin absent; ascospores 21–27×5–6 µm, mean length 24·3 µm, mean width 5·7 µm.
Chemistry
Spot tests: thallus surface C+ red in rimulae only, otherwise faintly red or C−; medulla C−; disc C−. Secondary metabolites: erythrin; unidentified substances C, F, occasional G (lecanoric acid absent but perhaps present in very low amount, see Chemistry).
Distribution and habitat
Dirina mexicana has been found in coastal areas of Mexico, from Laguna Manuela in Baja California south to Cabo San Lucas, and including near Higuera de Zaragoza in Sinaloa. It grows on vertical or overhanging rocks and cliffs. Occasionally it may be abundant with individuals forming large mosaic patterns.
Remarks
Dirina mexicana has immersed ascomata similar to the non-related species D. insulana, D. immersa and D. candida. Dirina mexicana is partly sympatric with its sister species D. catalinariae and to the closely related D. pallescens. However, both Dirina catalinariae and D. pallescens are easily distinguished from D. mexicana by their conspicuously sessile ascomata with constricted base. Dirina pallescens is furthermore strictly corticolous whereas D. mexicana and D. catalinariae are strictly saxicolous. Dirina mexicana is also characterized by the combined presence of the two unidentified substances F and G, a combination that it shares only with Dirina pacifica.
Additional material examined. Mexico: Baja California Sur: Isla Santa Margarita, 24°24′N 111°43′W, 1993, Tehler 7215 (S-L10); La Palma, NW of Cabo San Lucas, 23°00′38·3″N 110°05′49·3″W, 2007, Tehler 9193 (S-F210821); Laguna Manuela, 28°15′N 114°07′W, 1993, Tehler 7236 (S-L8); Migriño, NW of Cabo San Lucas, 23°04′55·0″N 110°06′41·8″W, 2007, Tehler 9196 (S-F210822); Playa Punta Lobos S of Todos Santos, 23°28′N 106°50′W, 1993, Tehler 7156 (S-L11); S of the beach, 23°24′31·5″N 110°14′05·0″W, 2007, Tehler 9173 (S-F210819); 1991, Nash 29741 (S-F69910); Playa San Pedro, S of Todos Santos, 23°23′02·1″N 110°12′45·0″W, 2007, Tehler 9187 (S-F210820); Punta Gaspareno, N of Cabo San Lucas, 23°15′27·3″N 110°09′43·1″W, 2007, Tehler 9198 (S-F210823); 3·5 km along road to Punta Abreojos from Highway 1·27°15′N 113°10′W, 1993, Tehler 7229 (S-L9); W of San Ignacio at junction of road to Punta Abreojos, 1989, Wetmore 63893 (S-F69911). Sinaloa: 3 km NNE of Higuera de Zaragoza, 26°01′N 109°16′W, 1993, Tehler 7175 (S-L7).
Dirina monothalamia Tehler & Ertz nom. nov.
MycoBank No.: MB 802914
Chiodecton africanum Fée, Essai Crypt. Écorc. Suppl. 53 (1837) [non Dirina africana Kremp.].—Schismatomma africanum (Fée) C. W. Dodge, Beih. Nova Hedwigia 12: 91 (1964).—Dirina approximata ssp. africana (Fée) Tehler, Opera Bot. 70: 39 (1983).—Dirina paradoxa ssp. africana (Fée) Tehler Lichenologist 18: 296 (1986); type: In Senegambia, presqu'île du cap vert (afrique) sur un vieux figuier près de Kounoun, Caramano sur les pierres, s. ann., Perrottet 4 [G-291546—lectotype sel. by Tehler (1983); G-291545, G-291548, H-NYL-23882, M—isolectotypes].
Thallus facultatively saxicolous/corticolous, on acidic rock when saxicolous, surface plane to slightly rugose-verruculose, slightly pruinose, creamy white to white-brownish sometimes brown to nearly dark brown, 0·1–0·7 mm thick; cortex 10–50 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·0 mm diam.; disc pruina with even surface or finely rimose surface, white; thalline margin present, entire to undulating to often strongly undulating; ascospores 23–28×4–5 µm, mean length 26·13 µm, mean width 4·66 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid rarely absent; unidentified substance C.
Etymology
The epithet ‘monothalamium’ was first written by Fée on the sheet containing the type material Chiodecton africanum. He later crossed out ‘monothalamium’ and replaced it with ‘africanum’. Therefore we will select and use monothalamium, the first, original epithet made by Fée.
Distribution and habitat
Dirina monothalamia is known from Cape Verde and the nearby African mainland in Senegal. It grows on coastal outcrops or the bark of various trees, and often on Adansonia digitata in Senegal.
Remarks
Dirina monothalamia is morphologically nearly identical to D. teichiodes but differs from the latter by having a slightly more rugose thallus with convex areoles, a thalline margin which in saxicolous specimens is more undulating to strongly undulating (Fig. 7A), and a slightly higher mean value of ascospore length. However, none of these characters are discrete and they all overlap with the corresponding characters in Dirina teichiodes. As a result, it seems these two species can only be accurately determined with reference to each other by the analyses of molecular characters.
Corticolous specimens do not develop such undulating thalline margins as saxicolous specimens, in which they are usually not only undulating but also often strongly so.
Dirina monothalamia is also very similar to D. sorocarpa, but the latter is readily distinguished by the presence of soralia. Dirina monothalamia has never been found sorediate.
The species Dirina africana, currently referred to as Diploschistes africanus (Kremp.) Zahlbr., was described by Krempelhuber (Reference Krempelhuber1877) from Somaliland. Thus the epithet africana cannot be used for this taxon.
Additional material examined. Cape Verde: Boa Vista: Mt. Vigia, 1987, Mies 560a3 (S-F151297); Mt. Vigia-massiv, Ponta do Sol, 1987, Mies 561g3 (S-F151299). Brava: Sorno, 14°53′N 24°44′W, 1988, Kalnins 1275a2 (S-L11751); Vinagre, 14°52′N, 24°41′W, 1988, Kalnins 1273a2, 1274a2 (S-L11755, S-L11756, S-L11753); Furna, 1987, Mies 743a3 (S-F151290); S von Furna, 150 m, 1987, Mies 752a3, 747d3 (S-F151291, S-F151293). Maio: Mt. Batalha, 1987, Mies 790g2 (S-F151301). Sal: Mt. Grande, NW-Grat, 16°49′N, 22°54′W, 1988, Mies 1067i2, 1067j2 (S-L11747, S-L11754); Algudeiro, N Santa Maria, 16°37′N 22°56′W, 1988, Mies 1040a2 (S-L11752); Monte Grande, 16°49·700′N 22°56·249′W, 2010, Tehler 10067 (S-F210826); Buracona, 16°48·802′N 22°59·294′W, 2010, Tehler 10074 (S-F210827); Monte Leâo (Rabo da Junco), 16°42·030′N 22°58·929′W, 2010, Tehler 10079-69 (S-F210828). Santo Antao: Fontainhas, 17°12′N 25°06′W, 1988, Mies 1191h2 (S-L11750). Sao Nicolau: W Estancia Bras nach Ribeira Funda, 16°40′N 24°20′W, 1988, Mies 932d 2 (S-L11748). Sao Tiago: Serra da Antonia, 1987, Mies 225f2 (S-F151304); Mt. Graciosa, 1987, Mies 636h3 (S-F151303). Sao Vicente: Punta Santo Antonio, 16°53′N 24°56′W, 1988, Mies 1212a2 (S-L11749); Praia da Ceilada do Calhau, 16°51·552′N 24°52·741′W, 2009, Tehler 10009 (S-F210824); Praia Grande 16°51·549′N 24°53·195′W, 2010, Tehler 10028 (S-F210825); between Baia das Gates and Calhau, NE of Monte Verde, 16°52′29″N, 24°54′46″W, 2011, Ertz 17246 (BR).—Senegal: Dakar: Senegal, Dakar, Bot. Garten, 1972, Pittoni s. n. (GZU); between Flygplatz Dakaar-Yoff und N'Gor, 1972, Pittoni s. n. (GZU); N'Gor, 1972, Pittoni s. n. (GZU); Goree, 1972, Pittoni s. n. (GZU); Noflaye between Sangalkam and Bambilor, 14°47·194′N 17°12·579′W, 2011, Tehler 10400 (S-F210829); W of Sangalkam, 10 km NE of Rufisque, 14°46·516′N 17°14·765′W, 2011, Tehler 10401 (S-F210830); Deni Biram Ndao, 14°50·634′N 17°11·918′W, 2011, Tehler 10402 (S-F210831); 5 km N Sebikhoutane, 14°43·149′N 17°09·827′W, 2011, Tehler 10404 (S-F210832); Ile de Gorée, 14°39·958′N 17°23·922′2011, Tehler 10407 (S-F210834); Yoff, E of the airport S of Mbenguène, 14°44·850′17°17·587′2011, Tehler 10411 (S-F210835). Thiès: Popenguine N of Mbour, 14°32·484′N 17°6·282′W, 2011, Tehler 10405 (S-F210833).
Dirina pacifica Tehler & Ertz sp. nov.
MycoBank No.: MB 802915
Thallus obligately saxicolous on acidic rock. Soralia present (usually in the absence of ascomata). Medulla C-. Ascomata present (usually in the absence of soralia), sessile. Disc pruina with rimose surface. Ascospore mean length 23·82 µm and width mean value 4·47 µm. Unidentified secondary metabolite F present and G occasional.
Type: USA, Hawaii, Oahu, Koolaupoko Distr., Makapuu Point W of the lighthouse, 2010, Anders Tehler 10128 (S-F210836—holotype; BR—isotype).
Thallus obligately saxicolous on acidic rock, surface plane, epruinose, creamy white to greyish or brownish, 0·1–0·3 mm thick; cortex 25–35 µm thick; medulla chalk-like also near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata present (usually in the absence of soralia), pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >1·5 mm diam.; disc pruina with rimose surface, white-grey; thalline margin present raising above disc, entire to undulating; ascospores 19–27×4–5 µm, mean length value 23·82 µm, mean width value 4·47 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances C, F, G occasional.
Etymology
The name refers to the geographical region of the Pacific Ocean.
Distribution and habitat
Dirina pacifica is known from the Galapagos Islands and Hawaii. It grows on coastal outcrops.
Remarks
The Dirina pacifica specimens from Hawaii are closely related to the specimens from the Galapagos (Figs 1–4). The sister species to Dirina pacifica is D. paradoxa, originating from the Caribbean. Dirina pacifica also commonly appears in a sterile, sorediate form.
The thallus and the thalline margin vary considerably in colour from dark brown to greyish to creamy white and nearly white-yellowish (Fig. 6E & F).
The great distance between Hawaii and the Galapagos, and the fact that the Galapagos and Hawaiian specimens in all nodes form a 100% sister pair relationship, indicate that two distinct species may actually have evolved. However, there are no obvious morphological or chemical features to distinguish them. Furthermore, we have only two specimens sampled from the Galapagos. We will therefore refrain from describing two species until such a hypothesis can be tested by the analyses of more specimens.
Additional material examined. USA: Hawaii: Hawaii, South Kohala Distr., Waimea 20°1·400′N 155°39·950′W, 2010, Tehler 10138 (S-F210839); W.M. Keck Observatory Headquarters, 20°1·401′N 155°39·945′W, 2010, Tehler 10136 (S-F210838); Oahu, Koolaupoko Distr., Makapuu Point 21°18·539′N 157°39·432′W, 2010, Tehler 10129 (S-F210837); Makapuú cliffs, 1994, Smith 7537 (S-L5071); Makapuú cliffs, Tomtom Trail, 1981, Smith & James 7520 (S-L68959); 1996, Smith & Wetmore 7697, 7698a, 7698b, 7698c (S-L8703, S-L8700, S-L8701, S-L8702).—Ecuador: Galapagos Islands: Floreana, N of village 1°16′9·9″S 90°29′20·2″W, 2011, Bungartz 9487 (S-F210840); Floreana, Punta Luz del Dia, 01°14·181′S 090°28·501′W, 2005, Tehler 8693 (S-L68244); trail from Black Beach, 1976, Weber & Lanier L-82896 (COLO-294625); Pinzón, N coast, 00°35·851′S 090°41·221′W, 2005, Tehler 8748 (S-F96209); small point on the W side, 00°36·480′S 090°41·558′W, 2005, Tehler 8694 (S-F96088); Rábida, NE point, 00°24·852′S 090°42·205′W, 2005, Tehler 8778 (S-F96215); Santa Cruz, trail from Puerta Ayora to Bella Vista, 1976, Weber & Lanier L-82854 (COLO-294667); E of Puerto Ayora near CDRS, 00°44′45″S 90°17′39″W, 2005, Aptroot 63294 (hb. Aptroot); Puerto Ayora, CDRS, 0°44·869′S 90°18·408′W, 2005, Tehler 8600 (S-L61608); vicinity of Academy Bay, s. ann., Weber L-40824a L-40824b L-40824c (COLO-190270, COLO-190269, COLO-190330); Santa Fé 0°49′11″S 90°5′24″W, 2005, Tehler 8726 (S-L72897).
Dirina pallescens Tehler & Ertz sp. nov.
MycoBank No.: MB 802916
Thallus obligately corticolous. Soralia absent. Ascomata up to 2·5 mm diam. Disc faintly C+ red. Ascospores mean length 29·4 µm. Unidentified secondary metabolite B present.
Type: Mexico, Baja California Sur, Todos Santos, c. 5 km S of town along Highway 19, 2007, Anders Tehler 9181 (S-F210843—holotype; BR—isotype).
(Fig. 7E)
Thallus obligately corticolous, surface plane to rugose-verruculose, epruinose, creamy white to white-brownish, 0·1–0·5 mm thick; cortex 30–40 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·5 mm diam.; disc pruina with rimose or slightly rimose surface, white-grey; thalline margin present, entire to undulating; ascospores 27–30×4–5 µm, mean length value 29·4 µm, mean width value 4·4 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red, sometimes faintly red. Secondary metabolites: erythrin; lecanoric acid; unidentified substances B & C.
Etymology
The name refers to the often pale colour of the thallus.
Distribution and habitat
Dirina pallescens is distributed in Mexico and restricted to Baja California Sur, Sonora (Los Medanos) and Oaxaca (Puerto Angel). It grows near the sea on various trees and shrubs, such as Bursera and mangrove.
Remarks
Dirina pallescens is the corticolous sister species to D. catalinariae and D. mexicana; both are saxicolous. In addition to its corticolous habit, it can be distinguished from D. catalinariae by its longer and more slender ascospores. It shares the unidentified substance B with Dirina catalinariae but not with D. mexicana. Unidentified substance B is also present in the more distantly related species D. angolana, endemic to Angola, and D. sorocarpa, endemic to Cape Verde.
Additional material examined. Mexico: Baja California Sur: Cabo San Lucas, 22°58′ 16·3″N 110°02′42·1″W, 2007, Tehler 9189 (S-F210844); Todos Santos, 23°28′N 110°09′W, 1993, Tehler 7165 (S-L3327); Punta Lobos beach S of Todos Santos, 23°28′N 106°50′W, 1993, Tehler 7154 (S-L8216); just S of beach, 23°24′31·5″N 110°14′05″W, 2007, Tehler 9164 (S-F210841); on the ridge just south of beach, 23°24′31·5″N 110°14′05″W, 2007, Tehler 9176 (S-F210842); 23°28′N 106°50′W, 1993, Tehler 7154 (S-L8216); 1993, Nash 33740 (GBG, S-F69917). Oaxaca: Puerto Angel, 1910, Oreutt, 4973 (FH, L). Sonora: Los Médanos, 27°09′N 110°08′W, 1993, Tehler 7130 (S-L3296); W of Ciudad Obregon, 1993, Wetmore, 71824 (S-F69919).
Dirina paradoxa (Fée) Tehler
Lichenologist 18: 296 (1986).—Chiodecton paradoxum Fée, Essai Crypt. Écorc. 64 (1824).—Platygrapha dirinea Nyl. (excl. Dirina multiformis Mont. & Bosch), Mém. Soc. Imp. Sci. Nat. Cherbourg 5: 131 (1857).—Schismatomma paradoxum (Fée) Zahlbr., Cat. Lich. Univ. 2: 563 (1924).—Schismatomma dirineum (Nyl.) Räsänen, Ann. Bot. Soc. Zool.-Bot. Fenn. “Vanamo” 21, Not. Bot. 16: 6, nom. illeg. (1946); type: ad corticem crotoni Cascarillae et Cinch. laccifera, Cassia lignea, s. ann., Ruiz & Pavon 126 (G-291552—holotype; G-291551—isotype).
Platygrapha psaroleucoides Stirt., Rep. Trans. Glasgow Soc. Field Naturalists 4: 166 (1876).—Schismatomma psaroleucoides (Stirton) Zahlbr., Cat. Lich. Univ. 2: 564 (1924); type: Trinidad, Port of Spain, 1875, Brodie s. n. [BM—lectotype sel. by Tehler (1986)].
Dirina hioramii B. de Lesd., Rev. Bryol. Lichenol. 7: 60 (1934).—Dirina approximata ssp. hioramii (B. de Lesd.) Tehler, Opera Bot. 70: 37 (1983); type: Cuba, Cuba oriente, Guantanamo, Boca del Jaibo, arboricola, 1921, B. Hioram 5552a [originally in KASSEL later probably moved to KÖLN and now donated to B—lectotype sel. by Tehler (1983)].
(Figs – see Tehler Reference Tehler1983)
Thallus facultatively saxicolous/corticolous, on calciferous or acidic rock when saxicolous, surface plane to slightly rugose-verruculose, when saxicolous more or less bullate slightly pruinose, creamy white to white-greenish or white-brownish, 0·05–0·70 mm thick; cortex 10–50 µm thick; medulla chalk-like but with loose hyphae near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·0 mm diam.; disc pruina with even surface, white; thalline margin present, entire to undulating; ascospores 21–30×4–5 µm, mean length value 25·49 µm, mean width value 4·72 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C− or sometimes faintly red. Secondary metabolites: erythrin; lecanoric acid; unidentified substance C.
Distribution and habitat
Dirina paradoxa is distributed in the Caribbean region, in the Bahamas, Florida Keys, Lesser Antilles, Netherland Antilles, Venezuela and Trinidad. It grows near the coast on the bark of various trees and shrubs or on rock, both calciferous and acidic.
Remarks
Dirina paradoxa was earlier (Tehler Reference Tehler1983, Reference Tehler1986) regarded as a species with three subspecies. The present analysis of molecular data clearly shows that Dirina paradoxa is the sister species to the geographically distant D. madagascariensis, and not to any of its former subspecies, here referred to as D. approximata, D. teichiodes and D. monothalamia, which all belong to another group of species. The specimens earlier determined as Dirina paradoxa from Peru, both fertile and sorediate, have been transferred to D. badia.
This is the only Dirina species growing on both calciferous and acidic rocks. We have had sequences only from calciferous specimens, and so further studies including more specimens growing on both types of substrata may reveal that two species are involved, similar to the situation that was found in Dirina massiliensis–D. fallax. But, for the time being, the calciferous and acidic growing specimens will be treated as conspecific under Dirina paradoxa.
Additional material examined. Bahamas: Eleuthera: 1907, Britton & Millspaugh 5640 (FH, NY). Great Exuma: 1905, Britton & Millspaugh 3033, 3052 (FH, NY). Long Key: 1905, Brace 4149 (FH, NY). Little San Salvador: 1907, Britton & Millspaugh 5688 (FH, NY). Nassau: 1905, Wight s. n. (FH). New Providence: 1904, Britton 855 (FH, NY); 1918, Brace 9398, 9504 (FH, NY); 1918, Brace 9446 (NY). Watling Island: 1907, Britton & Millspaugh 6162 (FH, NY).—Cuba: Guantánamo: Puerto Escondido, Finca Ocujal, 1933, Hioram s. n. (KASSEL).—France: French Antilles: St. Barthelemy, N of the airport, 1981, Tehler 5545 (S-L68953).—Netherland Antilles: Bonaire: 1977, Slageren 8374, 8388 (U); Seru Largu, 12°11′21·7″N 68°16′35·7″W, 2006, Tehler 8930, 8931, 8932 (S-F210845, S-F210846, S-F210847); Montana Mtn. in the Karpata region, 12°13′44·7″N 68°22′01·7″W, 2006, Tehler 8938, 8939 (S-F210848, S-F210849); Washington-Slagbaai National Park, Juwa Pass, 12°16′00″N 68°24′00″W, 2006, Tehler 8947 (S-F210850). St. Eustatius: SE-coast, White Wall, 1980, Sipman 14996 (S-L68954, U). Curaçao: 1977, Slageren 8221, 8239 (U). Saba: 1980, Sipman 15233 (U).—USA: Florida: The Lower Keys, Sugarloaf Key, S of the highway, 1981, Tehler 5595 (S-L3550); Pumpkin Key, 1921, Kelly s. n. (S-F69923 US); Suger Loaf Blvd c. 50 m S Cayman Dr., 24°37·535′N 81°32·588′W, 2010, Tehler 10113 (S-F210851); Middle Cape, 1916, Small s. n. (US).—Venezuela: Estado Falcon: Peninsula de Paraguaná, El Balsamal, 1979, Figueiras 21246, 21247 (S-L7673, S-L7674); Cerro de Santa Ana, 1979, Lopez-Figueiras & Wingfield 21750-A, 21750-B (S-L7675, S-L7676); Cuara, vía Encrucijada-Santa Ana, 1979, Lopez-Figueiras 19229 (S-L7677); Santa Ana, al sur del cerro frente a Santa Ana, 1979, Lopez-Figueiras & Wingfield 21830 (S-L7678); Monte Cano, 1979, Lopez-Figueiras 21255 (S-L7679); Cerro Colorado, cercanías de Santa Ana, 1979, Lopez-Figueiras 21318 (S-L7680); Punta Prudention, Tacuato, 1979, Lopez-Figueiras & Wingfield 32492 (S-F90366).
Dirina sorocarpa Tehler & Ertz sp. nov.
MycoBank No.: MB 802917
Thallus obligately saxicolous on acidic rock. Medulla with loose hyphae near the substratum, C−. Soralia present (usually in the absence of ascomata), punctiform to maculiform. Ascomata rarely present and then always together with soralia. Disc pruina with even or only finely rimose surface, C− or faintly red. Unidentified secondary metabolite B present.
Type: Cape Verde, Sao Vicente, Praia Grande on NE facing ridge above the beach, alt. 100–200 m, 2010, Anders Tehler 10026 (S-F210855—holotype; BR—isotype).
(Fig. 7C)
Thallus obligately saxicolous on acidic rock, surface plane to slightly rugose-verruculose, slightly pruinose, creamy white to white-brownish, 0·1–0·5 mm thick; cortex 25–40 µm thick; medulla chalk-like but sometimes with slightly loose hyphae near substratum, white; soralia present (usually in the absence of ascomata), punctiform to maculate.
Ascomata rarely present and then always together with soralia, pluricarpocentral, discothecia, few, sessile, circular in outline, base unconstricted or constricted, >1·8 mm diam.; disc pruina with even surface or finely rimose surface, white-grey to dark grey; thalline margin present, entire to undulating; ascospores 21–30×4–5 µm, mean length value 24·77 µm, mean width value 4·67 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C− or faintly red. Secondary metabolites: erythrin; lecanoric acid sometimes absent; unidentified substances B & C.
Etymology
The epithet sorocarpa refers to the feature of the ascomata (ascocarps), if present, being often partly sorediate, or under all circumstances produced together with soralia elsewhere on the thallus.
Distribution and habitat
Dirina sorocarpa is endemic to Cape Verde and hitherto known from the islands of Sao Vicente and Sal.
Remarks
Dirina sorocarpa is the sister species to D. approximata from the Galapagos Islands. It is easily distinguished from that species by its shorter spores and its saxicolous habitat. Dirina sorocarpa is morphologically very similar to the closely related species D. monothalamia and D. teichiodes, but differs from those two species by the presence of soralia and by the presence of the unidentified substance B. Fully sorediate specimens of Dirina sorocarpa are very difficult to distinguish from sterile, sorediate specimens of the distantly related D. insulana. Thin thalli of the latter are very hard to examine for the C+ red medulla reaction which is otherwise a characteristic feature of that species.
Additional material examined. Cape Verde: Sao Vicente: Baia das Gatas, 16°53·420′N 24°55·605′W, 2009, Tehler 10017 (S-F210853); between Baia das Gates and Calhau, 16°52′29″N, 24°54′46″W, 2011, Ertz 17248 (BR); Madeiral, 16°49·174′N 24°56·140′W, 2010, Tehler 10052 (S-F210858); Mato Inglés, 16°51·640′N 24°55·847′W, 2010, Tehler 10041 (S-F210856); Mato Inglés, 16°51·57′N 24°55·52′W, 2010, Tehler 10051 (S-F210857); Praia da Ceilada do Calhau, 16°51·552′N 24°52·741′W, 2009, Tehler 10010 (S-F210852); Praia Grande 16°51·549′N 24°53·195′W, 2010, Tehler 10027 [fully sorediate] (S-F210854). Sal: Ponta trás do Cagarral 16°46·590′N 22°53·335′W, 2010, Tehler 10060 (S-F210859).
Dirina teichiodes (Stirt.) Tehler & Ertz comb. nov.
MycoBank No.: MB 802918
Lecidea teichiodes Stirt., J. Linn. Soc., Bot. 14: 368 (1874); type: Cape Verde, Challenger Expedition 1873, 1873, Moseley s. n. [H-NYL-23903—lectotype sel. by Tehler (1983); H—isolectotype]. Epitype: Cape Verde, Sal, Monte Grande at the N foot of the mountain c. 1 km from the summit, alt. 50 m, 2010, Anders Tehler 10071 (S-F210861—epitype sel. here; BR—isoepitype).
(Fig. 7D)
Thallus obligately saxicolous on acidic rock, surface plane, slightly pruinose, creamy white, 0·1–0·5 mm thick; cortex 25–40 µm thick; medulla chalk-like also near substratum, white; soralia absent.
Ascomata present, pluricarpocentral, discothecia, numerous, sessile, circular in outline, base constricted, >2·0 mm diam.; disc pruina with even surface or finely rimose surface, white; thalline margin present, entire to slightly undulating; ascospores 20–29×4–5 µm, mean length 23·13 µm, mean width 4·62 µm.
Chemistry
Spot tests: thallus surface C+ red; medulla C−; disc C+ red. Secondary metabolites: erythrin; lecanoric acid sometimes absent; unidentified substance C.
Distribution and habitat
Dirina teichiodes is endemic to Cape Verde. It is strictly saxicolous and known only from three localities on Sal Island (the type specimen lacks locality details).
Remarks
Dirina teichiodes is morphologically very similar to D. monothalamia. Dirina teichiodes has a more even and finely rimose thallus with flat areoles, a thalline margin which is usually entire or only slightly undulating, and the mean value of ascospore length is slightly shorter. However, none of these characters are decisive and they all overlap with the corresponding characters in D. monothalamia. As a result, it seems these two species can only be accurately determined with reference to each other by the analysis of molecular characters.
Dirina teichiodes is also very similar to D. sorocarpa but the latter is readily distinguished by the presence of soralia.
Additional material examined. Cape Verde: Sal: Monte Leâo (Rabo da Junco), 16°42·030′N 22°58·929′W, 2010, Tehler 10079-70 (S-F210862); Ponta trás do Cagarral c. 2 km N of Pedra Lume, 16°46·590′N 22°53·335′W, 2010, Tehler 10059 (S-F210860).
Excluded taxa
Fulvophyton calcicola (Sparrius) Tehler & Ertz comb. nov.
MycoBank No.: MB 802919
Dirina calcicola Sparrius, Bryologist 107: 522 (2004); type: USA, Florida, Key West, 1898, Thaxter 415 (NY—holotype).
Remarks
On the basis of the shared features of pale brownish thallus, immersed ascomata and hyaline ascospores with a distinct gelatinous sheath (perispore), we believe this species is better placed in Fulvophyton (Ertz & Tehler Reference Ertz and Tehler2011).
Schismatomma insulae-howense (Sparrius) Tehler & Ertz comb. nov.
MycoBank No.: MB 802920
Dirina insulae-howensis Sparrius Biblioth. Lichenol. 89: 87 (2004); type: Australia, New South Wales, Lord Howe Island, Intermediate Hill via track to North Hummock, 1995, Elix 42042 (CANB CBG-9703492—holotype).
Remarks
We have no sequences from this species but the morphological and chemical evidence, long slender ascospores and absence of secondary substances indicate that it is better placed in Schismatomma.
Schismatomma neozelandicum (Redinger) Tehler & Ertz comb. nov.
MycoBank No.: MB 802921
Enterographa neozelandica var. ochracea Redinger, Feddes Repert. Spec. Nov. Regni Veg. 43: 72 (1938).—Dirina neozelandica (Redinger) Sparrius, Biblioth. Lichenol. 89: 89 (2004); type: New Zealand, Pahia Point, Foveaux Strait, s. l., coastal rock, 1935, Thomson A116 [W—lectotype sel. by Sparrius (2004)].
Enterographa neozelandica var. murina Redinger, Feddes Repert. Spec. Nov. Regni Veg. 43: 72 (1938); type: New Zealand, South Island, Bluff, 1933, Thomson s. n. [not in W—lectotype sel. by Galloway (2004)].
Placidiopsis novozelandica C. W. Dodge, Nova Hedwigia 19: 453 (1971); type: New Zealand, Stewart Island, Mutton Bird Islets, Big Stage Island, 1965, Fineran 912 (CANU—holotype).
Remarks
We have no sequences from this species but the morphological and chemical evidence, long slender ascospores and absence of secondary substances indicate that it is better placed in Schismatomma.
Incertae sedis
Dirina follmannii (C. W. Dodge) Sparrius
Biblioth. Lichenol. 89: 88 (2004).—Enterographa follmannii C. W. Dodge [as E. follmanni], Nova Hedwigia 12: 324 (1966); type: Los Molles, costa (prov. Aconcagua), 1960, Follmann 14660-D (FH-302903—holotype).
Remarks
The holotype specimen of Dirina follmannii is poorly developed. We have sequences from similar specimens to the holotype, collected by Tehler in Chile, but molecular results are inconclusive since they place it with either Pentagenella or Minksia. More material is needed before the position of this species can be finally settled.
Discussion
Phylogeny
Dirina candida is the sister species and sister group to all other Dirina species (Figs 1–4). Following the next node in the phylogenetic tree, Dirina is divided into two basal groups. One has four species distributed in Macaronesia, the Mediterranean and Europe; Dirina canariensis constitutes the sister group to the other species in that group. The second basal group contains the rest of the species with the species pair Dirina arabica and D. immersa as the sister group to the rest of the species in that group.
Surprisingly, the characteristic feature of monocarpocentral, solitary ascomata (Tehler Reference Tehler1990), [referred to below as immersed ascomata since they are always immersed (Fig. 7F)] seems to have evolved independently several times in Dirina as it is found in the unrelated species D. candida, D. immersa, D. insulana and D. mexicana (Fig. 1). The evolution involving immersed ascomata in Dirina has obviously, on several occasions, been subjected to either gains or losses. All species in Roccella, the sister genus to Dirina, have pluricarpocentral discothecia; thus, by definition, immersed ascomata are derived, and the most parsimonious hypothesis is that they have evolved on four separate occasions. However, taking the broader view including the whole order Arthoniales (Ertz & Tehler Reference Ertz and Tehler2011), it is more reasonable to suggest that the discothecium is a derived feature that evolved once at the node following Enterographa (Ertz & Tehler Reference Ertz and Tehler2011), which is the sister group to the rest of Roccellaceae and a genus with immersed ascomata. The overwhelming majority of the species in Roccellaceae have ascomata of the discothecium type, including Sigridea, Roccella, Dirina, Lecanactis, Roccellina and Dendrographa. One genus, Syncesia, has pluricarpocentral synascomata. Immersed ascomata are more commonly seen outside Roccellaceae, in other families of Arthoniales such as Opegraphaceae, Roccellographaceae and Lecanographaceae. Thus, a more parsimonious and plausible hypothesis is that the immersed ascomata seen in Dirina are all reversals to a plesiomorphic state.
Asexual morphs earlier described at the rank forma are no longer recognized as taxonomic units: Dirina catalinariae f. sorediata, D. insulana f. sorediata, D. massiliensis f. sorediata, D. paradoxa ssp. paradoxa f. sorediata and D. massiliensis f. aponina. None of these taxa constitute monophyletic, evolutionary units but are rather considered conspecific expressions of dispersal strategies since they are inclusive and nested in sexual lineages. Examples of such polymorphic lineages have been repeatedly investigated, described and exhaustively discussed in earlier papers and will not be further discussed here. We now take the final consequence of these results and cease to recognize any polymorphic sorediate taxa (Tehler Reference Tehler1982) included in the so-called species pair concept (Poelt Reference Poelt1970, Reference Poelt1972), unless they can be shown to have evolved into distinct monophyletic lineages.
The Dirina pacifica specimens from Hawaii are closely related to the specimens from the Galapagos, and the two sample groups form a sister pair. The great distance between Hawaii and the Galapagos, and the fact that the two sequenced specimens from the Galapagos form a significantly supported sister pair relationship to a likewise significantly supported sister group of specimens from Hawaii, would make a reasonable argument to recognize the two as distinct species. The specimens from Hawaii contain the unknown secondary product H, but we have not been able to confirm whether this product is also present in the Galapagos specimens since we have too little material available for TLC analyses. However, there are no morphological characters to distinguish the sample specimens and furthermore the DNA sampling from the Galapagos is too scanty, with only two specimens to support a two-species hypothesis. For the time being we will consider them conspecific.
The sister species to Dirina pacifica is D. paradoxa, originating from the Caribbean. Interestingly, the related genus Syncesia (Roccellaceae) contains two species, S. hawaiiensis and S. farinacea, which show a similar pattern to that of D. pacifica and D. paradoxa. We have sequences from RPB2 and LSU of Syncesia hawaiiensis, as well as a Syncesia specimen collected in the Galapagos, which show that the Hawaiian specimens and the Galapagos specimen form a supported sister pair (A. Tehler, D. Ertz & M. Irestedt, unpublished data). Syncesia farinacea is mainly distributed in the Caribbean and the South American mainland. Geographically, we seem to have two similar but independent distribution patterns to indicate a Caribbean-Galapagos-Hawaii connection: firstly, the two Dirina populations in Hawaii-Galapagos and their sister species D. paradoxa in the Caribbean; and secondly the Syncesia hawaiiensis and the specimen from the Galapagos, and their sister species S. farinacea in the Caribbean. Unfortunately, we have no sequenced specimens from the Caribbean Syncesia farinacea population to corroborate that hypothesis
The three saxicolous and three corticolous specimens of Dirina madagascariensis were divided into two clades and may thus actually represent two species. However, as with D. pacifica, we believe that three investigated specimens each are too few upon which to set up a two-species hypothesis. Furthermore, only the saxicolous clade receives full statistical PP and PJ support, whereas the corticolous clade receives somewhat less PJ support of 90%. Thus, for the time being, we refrain from describing separate corticolous and saxicolous species until such a hypothesis can be tested by the analysis of more specimens.
Most Dirina species are endemic or have restricted distributions but some very similar species are sympatric, most notably Dirina monothalamia and D. teichiodes. When saxicolous, these two species contain no discrete morphological characters on which they can be reliably distinguished from one another, and we have found no way to safely differentiate between them other than by examining DNA-data. Another example is the sterile, sorediate morph of Dirina fallax which has been collected a few times in the Canary Islands, where it is morphologically and chemically indistinguishable from sterile, sorediate specimens of D. canariensis. Other recent phylogenetic studies on lichen species complexes also showed that morphological and chemical data might be insufficient to delimit species boundaries in several genera, suggesting that species diversity has in many cases been underestimated in the past (Divakar et al. Reference Divakar, Figueras, Hladun and Crespo2010; del Prado et al. Reference del Prado, Divakar and Crespo2011; Leavitt et al. Reference Leavitt, Frankhauser, Leavitt, Porter, Johnson and St Clair2011; Lumbsch & Leavitt Reference Lumbsch and Leavitt2011; Molina et al. Reference Molina, Divakar, Millanes, Sánchez, del Prado, Hawksworth and Crespo2011; Núñez-Zapata et al. Reference Núñez-Zapata, Divakar, del Prado, Cubas, Hawksworth and Crespo2011; Orange Reference Orange2012; Pino-Bodas et al. Reference Pino-Bodas, Burgas, Martin and Lumbsch2012).
Biogeography
The main geographical pattern suggests that vicariance has played an important role in the distribution and radiation of the species in Dirina. For this to be the case, we have to assume that Dirina is very old and that speciation is generally slow. Twelve species from the Old World are distributed in Southern and Central Europe, Macaronesia including the Atlantic islands Ascension and St. Helena, and mainland Africa in Angola; five species are distributed in the region of the Indian Ocean, Socotra, northern India, Mauritius and Madagascar; and finally there is a monophyletic group of five species present in the Americas and islands of the Pacific Ocean (Figs 1–4).
The most basal species in Dirina is D. candida, which is present in the south-west Mediterranean region, and the most basal species in Roccella is R. allorgei from the northern Macaronesian region including southern Portugal, indicating that this region is the centre of origin and the distribution area of the ancestor to the clade including both Dirina and Roccella. The general distribution pattern for Dirina is similar to that in Roccella, with a paraphyletic group of Old World species and a monophyletic group of American species derived from one of the Old World groups, notably a group that includes many Macaronesian species. In Roccella, vicariance was proposed to explain its American–Macaronesian distribution (Tehler et al. Reference Tehler, Irestedt, Wedin and Ertz2009b ). The ancestor to the monophyletic genus Roccella was suggested to have been distributed over an ancient geographical region that successively broke up into the New and Old World areas. The American and Macaronesian populations became more and more isolated from one another during the late Jurassic period, 160–145 mya, and finally, in the late Cretaceous period, 100–65 mya, they became fully separated through the formation of the South Atlantic Ocean. Successively Roccella established itself and radiated into the present species in the two current distribution areas.
The pattern in Dirina is very interesting since it is not only the sister group to Roccella, but it reveals a similar American–Macaronesian distribution pattern to Roccella, clearly indicating that both genera have independent but similar evolutionary histories. Similar to Roccella, Dirina also contains a significantly supported monophyletic group with five American species, D. catalinariae through D. paradoxa, in a sister group relationship with a likewise significantly supported monophyletic group including Old World and mainly Macaronesian species. Hence, two independently evolved genera, Dirina and Roccella, support the same vicariance pattern.
The vicariance hypothesis determined by plate tectonics anticipates the group including Dirina and Roccella, altogether 48 species, to be extraordinarily old. The precursors to the genera must have evolved or had already evolved along the coasts of the western Tethys Sea, and they must have had an established distribution at the opening of the Atlantic Ocean when Pangaea broke up into Laurasia and Gondwana in the Mesozoic period over 200 million years ago, making them older than the first angiosperms. On the one hand, this implies a very slow evolutionary rate compared with many other organisms, and furthermore that the easy cosmopolitan dispersal in lichens is not always the rule. On the other hand, as a reflection of the fact that the hotspot islands of the Galapagos never connected to the mainland and despite the fact that conservative evolution would have prevailed for these organisms over time, it is still likely that dispersal and adaptive radiation also played a role in the evolution of some species groups, as for example the rapid evolution of the Roccella galapagoensis-complex (Tehler et al. Reference Tehler, Irestedt, Bungartz and Wedin2009a ) once the ancestor succeeded in spreading to the islands.
Interestingly, and also in support of the hypothesis of vicariance, neither Dirina nor Roccella have in any successful way succeeded in dispersing and establishing distribution areas much outside what can be considered the fragments of the ancient region of the Tethys Sea, now mainly present in the Northern Hemisphere. Remarkably, very few species are found south of the equator, and for no obvious reason Dirina, as well as Roccella, are both absent from areas outside the ancient region of the former Tethys Sea that otherwise would obviously provide perfect growing conditions with respect to both climate and habitat, such as the areas on the South American West Coast south of the equator, South Africa and the west and south coasts of Australia. The only real exception to this pattern is the widespread species R. montagnei, which has evidently succeeded in dispersing into South Africa and a few localities on the north-west coast of Australia (Tehler et al. Reference Tehler, Irestedt, Wedin and Ertz2010). In Dirina, only four species occur in the Southern Hemisphere at any notable distance south of the equator, each endemic to its particular locality: D. jamesii (the South Atlantic Islands of St. Helena and Ascension), D. angolana (Angola), D. astridae (Mauritius) and D. madagascariensis (Madagascar).
However, there are some important exceptions and differences between Dirina and Roccella. Firstly, Dirina includes several instances with long distance dispersal not seen in Roccella. Most notably, two dispersal events towards America must have occurred, of which one must have been prior to the evolution of the monophyletic American group, namely that of Dirina badia to Peru, possibly from an ancient region now split up into the Mediterranean and Socotra Island. The other, and probably much more recent American dispersal event, is that of D. approximata in the Galapagos originating from a group of species now present in southern Macaronesia. In addition, a long distance dispersal event must be proposed for Dirina madagascariensis going in the other direction back to the Old World, probably some time during the successive break into the New and Old World distribution areas. That scenario is indicated by the fact that D. madagascariensis is included with significant support in the monophyletic American group, but unresolved or only poorly supported as sister species to the rest of the American species within that group. Dispersal has obviously also occurred among the Old World Dirina species, most notably species in a poorly supported group containing D. angolana through D. astridae within the sister group to the American species otherwise containing Macaronesian species. However, two of the species in that group, D. angolana and D. jamesii, which are distributed in Angola and the Atlantic islands respectively, may simply be the result of a natural extension of the Macaronesian region into these areas during the opening of the South Atlantic Ocean. A parallel is seen in Roccella and the otherwise mainly Macaronesian species R. tinctoria, which has an outpost locality in Angola.
Recent dating studies (Lücking et al. Reference Lücking, Huhndorf, Pfister, Plata and Lumbsch2009; Berbee & Taylor Reference Berbee and Taylor2010; Amo de Paz et al. Reference Amo de Paz, Cubas, Divakar, Lumbsch and Crespo2011), which would probably estimate the Roccellaceae at around 100 mya, do not agree with the assumption that vicariance through plate tectonics is responsible for the similar distribution pattern in the two independently evolved lineages, Dirina and Roccella. These dating studies extrapolated to the genera Roccella and Dirina would estimate that clade to be at the most 50 mya and, as a consequence, the Dirina and Roccella nodes would be considerably younger, perhaps in the range of 25 mya. Thus, even if molecular dating studies have been questioned and should be treated cautiously (Ayala Reference Ayala1999; Schwartz & Maresca Reference Schwartz and Maresca2006), such dating analyses contradict, and differ from, the proposition of plate tectonics explaining the American vs Old World distribution pattern that we see in Dirina and Roccella by a range of nearly two hundred million years. Assuming that the correct dating of Dirina and Roccella is in the range 20–30 mya, then molecular dating analyses clearly suggest long distance dispersal to explain the cross Atlantic distribution of these two genera.
Even though most of the species in both Dirina and Roccella have developed means of both sexual dispersal (ascospores) and vegetative dispersal (soredia), they usually have very narrow distribution areas, clearly indicating a restricted mobility which in turn may indicate antiquity. The high number of endemic species in Dirina and Roccella may come as a result of this immobility. The majority of the species in both Dirina and Roccella are endemics, or even point endemics, for example D. astridae and R. boryi (Mauritius), D. arabica and R. phycopsioides (Socotra), D. mexicana and R. bajasurensis (Baja California), the latter distributed in only a 100 km western coast stretch in the most southern tip of Baja California.
Nonetheless, a few species in Dirina have obviously had the capability to move over oceanic distances in more recent times, thus long distance dispersal with subsequent regional species radiation is certainly plausible. In a dispersal scenario, to explain the transatlantic distribution, two ancestral species in Dirina and Roccella would independently from one another make the jump across the Atlantic Ocean, which by that time had opened to a considerable extent. Subsequently both of these species radiated into the five American Dirina species and nine American Roccella species that we see today. A similar scenario has been proposed for the Xanthoparmelia pulla group on the basis of a molecular dating study (Amo de Paz et al. Reference Amo de Paz, Cubas, Crespo, Elix and Lumbsch2012).
The distribution patterns in Dirina and Roccella need to be studied in more detail. All species in both Dirina and Roccella are mainly restricted to maritime coastal habitats and are often found endemic to islands such as the Galapagos Islands, the Canary Islands, the Azores, Socotra and Mauritius. For these islands, the geological age can be reasonably well determined and thus could serve as exact calibration points for the nodes in a phylogenetic tree. A molecular dating analysis is currently prepared for the Arthoniales-Roccellaceae and will be presented in a forthcoming publication (A. Tehler & M. Irestedt, unpublished data).
We wish to thank Stefan Bengtson (at the Palaeozoology Department of the Swedish Museum of Natural History) and Mats Wedin for valuable comments on the manuscript. One of us (DE) is indebted to the staff of the Parc Botanique et Zoologique de Tsimbazaza in Antananarivo for logistical support in Madagascar, and would like to thank Eberhard Fischer, Dorothee Killmann, Tahina Razafindrahaja and Emmanuël Sérusiaux who collected Dirina madagascariensis with him. We would also like to thank Dalip K. Upreti and Sanjeeva Nayaka for kindly putting the description and material for sequencing of their new species Dirina indica at our disposal. André Aptroot, Frank Bungartz, Jan Vondrák and A. Yánez have kindly contributed to collecting fresh material for our studies.
Appendix 1.
Species and voucher information of Dirina specimens used in phylogenetic analyses. All sequences are new to this study except those with GenBank accession numbers in italics which were published previously.











