Introduction
Lichenologists tend to consider that lichens disperse in two ways; either by vegetative diaspores or by sexual meiospores. Although both modes of dispersal may occur simultaneously, thalli producing diaspores by vegetative processes seem to be much less fertile compared to lichens normally producing ascomata. This common observation is often manifested in the recognition of two groups of lichens, sterile and fertile, where the former was presumed to be more genetically uniform than the latter (cf. Fahselt & Hageman Reference Fahselt and Hageman1990). However, molecular methods have revealed a large amount of genetic variation in mainly sterile or clonal species (Printzen & Ekman, Reference Printzen and Ekman2002; Printzen et al. Reference Printzen, Ekman and Tønsberg2003, Cassie & Piercey-Normore, Reference Cassie and Piercey-Normore2008).
Clonal thalli are constantly regarded as individuals instead of individual parts of one fragmented individual. We think this is a possible explanation for the enigmatic situation concerning the species pair concept. The overview of some of the theories explaining the evolution of asexual species is presented by Buschbom & Mueller (Reference Buschbom and Mueller2006) who show how deeply founded is the notion that lichens primarily disperse by one mode; i.e. either vegetatively or sexually.
A possible explanation for the occurrence of high genetic diversity within some clonal lichen species is that the occurrence of fruiting (indicating sexual reproduction), however rare, may make a significant contribution to the genetic variation within the species. Thus, the situation for vegetatively dispersed lichens is similar to the sexually dispersed lichens with long life spans and late development of fruiting bodies, although the latter lack the ability to move spatially during the time span from spore germination to meiosis. Vegetatively dispersed lichens produce soredia within two years after germination and establishment and hence readily propagate and establish in the vicinity of the original thallus. This is frequently observed, for example, on spruce twigs colonized by Hypogymnia physodes. If only some of the old individuals produce apothecia, the average time span between meiotic events will be no longer than 50 years for lichens in boreal forests, and probably much shorter. This time span is of similar magnitude to that for predominantly sexually propagated corticolous species (cf. Lättman et al. Reference Lättman, Brand, Hedlund, Krikorev, Olsson, Robeck, Rönnmark and Mattsson2009) and may explain the observation that genetic diversity can be as high in primarily vegetatively propagating lichens as it is in sexually propagated ones.
Hypothetically, the substratum preferences of lichens may be reflected in their genetic constitution. Using molecular markers it is possible to assess haplotypic identities of individuals in the material studied. If a large number of specimens are included it may be possible to find patterns in this variation and to potentially reveal the substratum preferences of different haplotypes. In the case of neutral or near neutral markers, observed preferences would hypothetically be due to linkage with genes coding for adaptive traits. In cases of genetic recombination this might be more obscure, but some alleles, or combinations of them, may be more common on some substrata indicating substratum preferences. Knowledge of this variation is important for understanding the ecology and dispersal strategy of lichens and hence for decision making in conservation.
The main objective of the present study was to assess the variability of the nrDNA ITS region and its relationship to substratum preferences in Hypogymnia physodes, a common species which is dispersed by soredia. The putatively close relative H. tubulosa, which is also sorediate, was included in order to minimize the risk of excluding material with intermediate morphology and also to estimate the genetic distance between the two species. In addition, we wished to examine whether there is evidence of genetic recombination within the chosen molecular marker and, if so, to assess the consequences for different interpretations for taxonomic and population studies. This study is part of the project Ecological and Societal Systems in Interaction, which integrates ecological studies on higher plants and lichens in order to examine biodiversity on different levels and to develop simplified methods for biodiversity assessments. One subproject is mainly focused at describing genetic variation of the angiosperm Daphne mezereum and its habitat characteristics, including occurrence of epiphytic macrolichens (cf. Vinter Reference Vinter2006).
Materials and Methods
Sampling
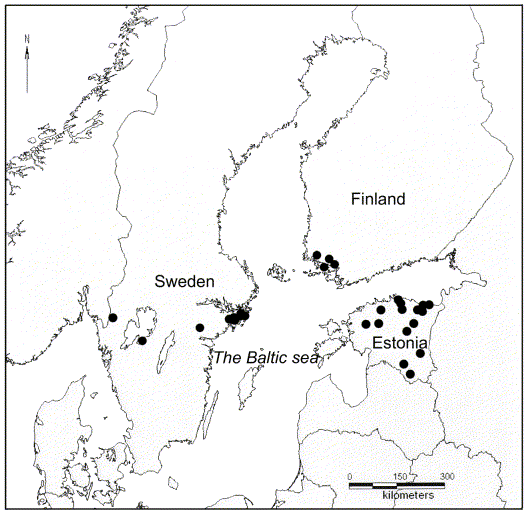
One specimen of all epiphytic macrolichens was collected from each phorophyte substratum occurring on 31 Daphne mezereum sites in Estonia, Finland, and Sweden in March–August, 2005 (Fig. 1). The sorediate Hypogymnia physodes was selected as the main object of this study because it was present at most of the sites included in the survey. Hypogymnia physodes often grows together with H. tubulosa (Moberg & Holmåsen Reference Moberg and Holmåsen1990). The two taxa are sometimes difficult to separate, because morphologically intermediate thalli occur regularly (J.-E. Mattsson, pers obs), hence, H. tubulosa was also included in the study.
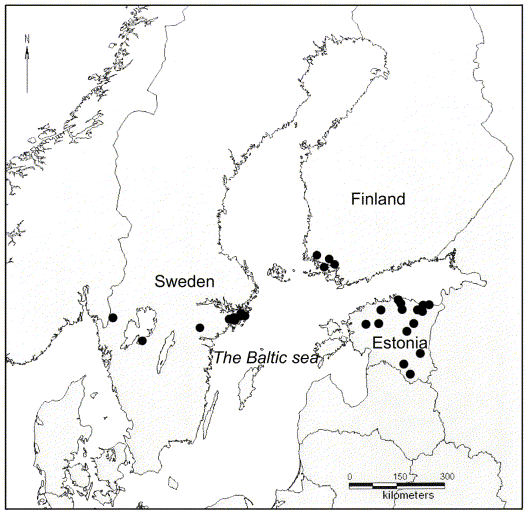
Fig. 1. The location of the 31 sites from which Hypogymnia spp. was collected for the present study.
Molecular methods
Total DNA was extracted using the Qiagen DNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions with the exception that the DNA was eluted in sterile water. Polymerase chain reaction (PCR) amplifications were performed using Amersham Pharmacia Biotech Ready-To-Go PCR Beads according to the manufacturer's instructions, with the following settings: initial denaturation 94°C for five minutes, followed by five cycles (94°C for 30 seconds, 55°C for 30 seconds and 72°C for 60 seconds) and finally 30 cycles (94°C for 30 seconds, 52°C for 30 seconds and 72°C for 60 seconds). Fungal nrDNA was amplified using the primers ITS1F-5′ (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4-3′ (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990). The PCR products were sequenced using the DYEnamicTM ET terminator cycle sequencing kit (Amersham Pharmacia Biotech) with the following settings: 96°C for 60 seconds followed by 25 cycles (96°C for 10 seconds, 50°C for 5 seconds and 60°C for 4 minutes) and finally 25 cycles (95°C for 20 seconds, 50°C for 15 seconds and 60°C for 60 seconds).
Sequences were aligned using the ClustalW algorithm in the MegAlign 5.06 program of the LaserGene 1.66 package (DNASTAR Inc.) followed by manual optimization.
Data analyses
The total sequence obtained was used in the initial analyses of recombination and genealogy. In order to estimate genealogical relationships between haplotypes, statistical parsimony (Templeton et al. Reference Templeton, Crandall and Sing1992) as implemented in the software TCS version 1.21 was used (Clement et al. Reference Clement, Posada and Crandall2000). An unrooted tree was constructed under the 95% parsimony probability criterion. Gaps were treated as a fifth character state.
We used the software DnaSP version 3.99.4 (Rozas & Rozas Reference Rozas and Rozas1999) which implements the four-gametic test (Hudson & Kaplan Reference Hudson and Kaplan1985) to estimate the minimum number of recombination events in the history of the sequence sample and to predict recombination breakpoints. 95% confidence intervals were obtained after coalescence simulations including 50 000 permutations. Because possible recombination was detected, each part of the sequences, the SSU intron, ITS1, 5.8S, and ITS2 section of sequences were studied in different combinations and separately in order to reveal any existing co-variation between genetic variability and substratum preferences.
A sample of the substratum from which each haplotype was collected and its nutrient richness (poor, intermediate, and rich) was estimated according to DuRietz (Reference DuRietz1945). An exact test of population differentiation based on haplotype frequencies (Raymond & Rousset Reference Raymond and Rousset1995) was performed using the software Arlequin 3.1.1 (Excoffier et al. Reference Excoffier, Laval and Schneider2005) and significance estimated using Markov chain Monte Carlo with 1 000 000 steps, 100 000 of which were dememorization steps.
Results
DNA sequences from 120 individuals were obtained: 104 from Hypogymnia physodes and 16 from H. tubulosa. A group 1 intron situated between positions 1516 and 1517 at the end of the small subunit (SSU) of the nuclear ribosomal DNA (Gargas et al. Reference Gargas, DePriest, Grube and Tehler1995) was detected in both species. When both species were included, the alignment of the intron, including the last part of the SSU, was 263 nucleotides long and the total ITS alignment contained 514 nucleotides. For H. physodes the alignment of the intron, including the last part of the SSU was 262 and for H. tubulosa 254 nucleotides long. For H. physodes the alignment of the total ITS region was 511 and for H. tubulosa 502 nucleotides long.
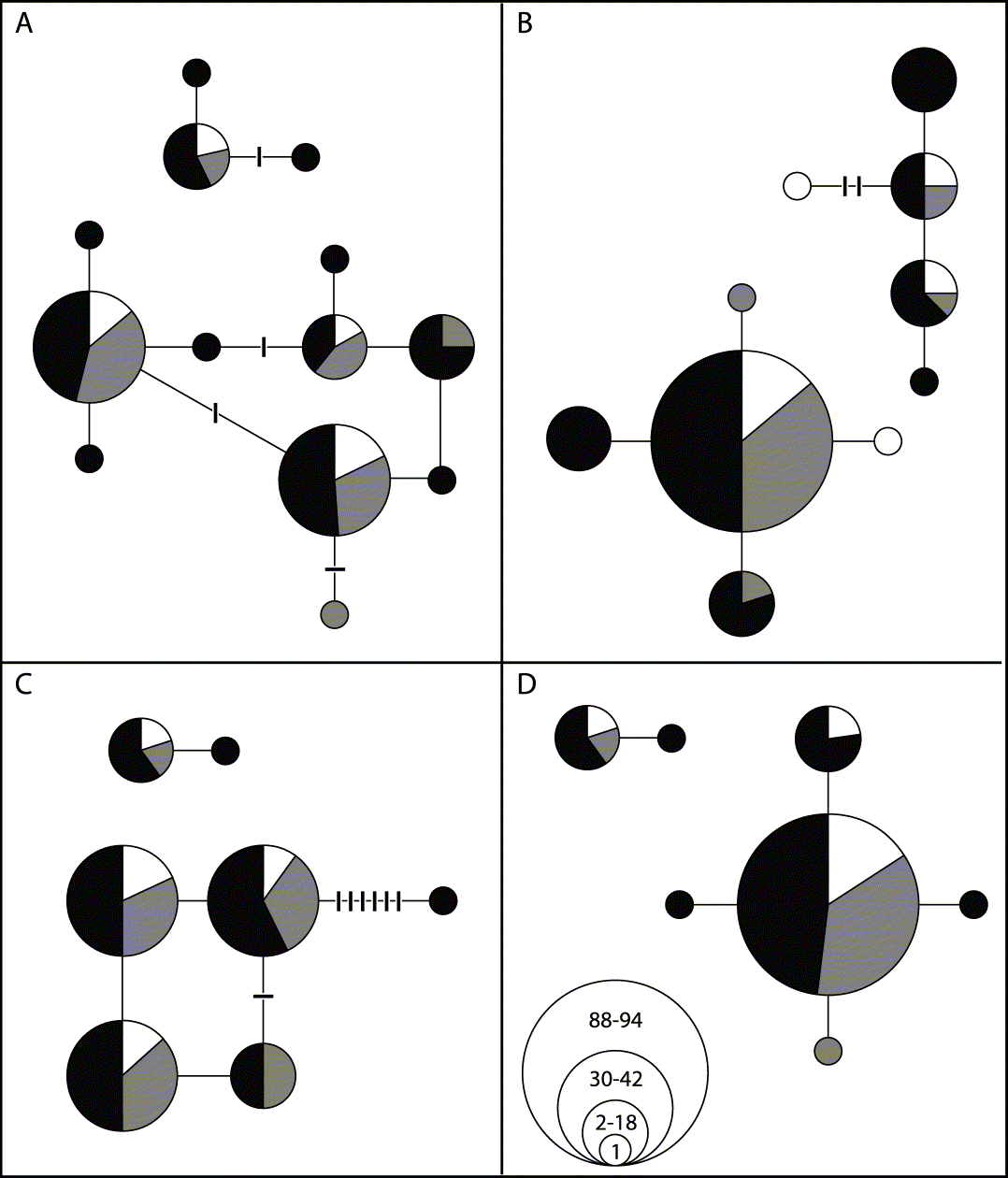
Fifteen haplotypes were found for H. physodes and seven for H. tubulosa for the combined alignment of the intron and the ITS.The parsimony network estimation resulted in two unconnected networks; one containing all the samples of H. physodes and the other containing the H. tubulosa samples (Fig. 2).
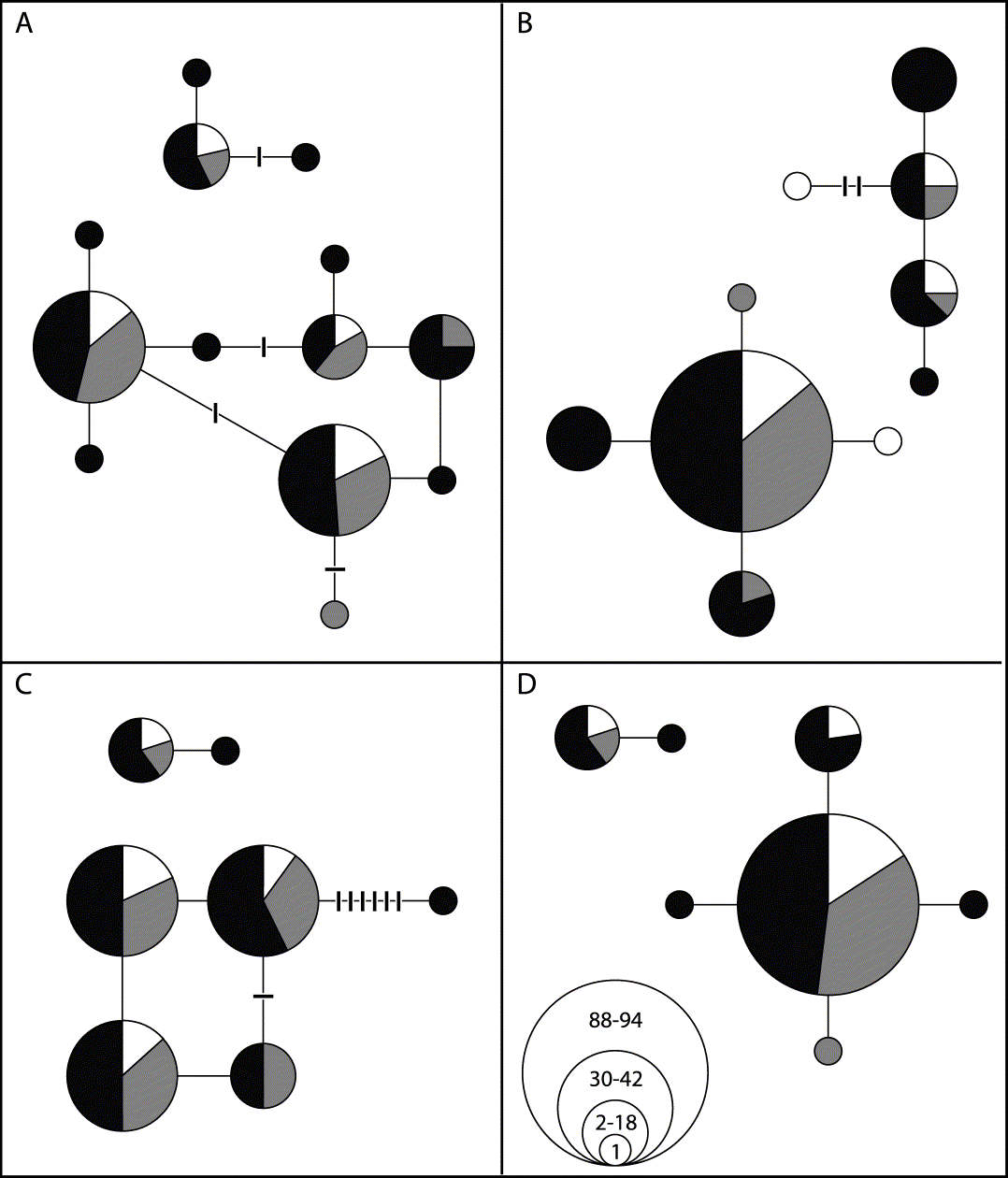
Fig. 2. Haplotype network and geographic distribution of H. physodes (lower, large networks) and H. tubulosa (upper, small networks). Lines between circles indicate one single mutational step, while hatch marks indicate unobserved haplotypes. Black colour marks individuals from Estonia, white from Finland, and grey from Sweden. The number of individuals of each haplotype is reflected in the size of the circles, divided into four classes as depicted in D. A, total ITS haplotypes; B, intron; C, ITS1; D, ITS2.
The analysis of H. physodes revealed two recombination sites within the ITS region. One is located between nucleotides 170 and 182 and the other between 182 and 449. ITS1 is about 200, 5.8S 190, and ITS2 110 nucleotides long, respectively, although this may indicate a single homoplasious site around position 182. The results suggest recombination in the ITS regions and the possibility of independent heritage of the genes coding for SSU, 5.8S, and LSU. There are no indications in this study of recombination between the intron and the very short included part of SSU or between this part and ITS1.
Studied separately, the different regions (intron, ITS1, 5.8S, ITS2) show different degrees of variability both within as well as between the two species. For H. physodes the number of haplotypes of the four regions are 5, 5, 1, and 5 and for H. tubulosa 5, 2, 1, and 2, respectively. The theoretical number of possible combinations of these haplotypes is 125 and 40 respectively.
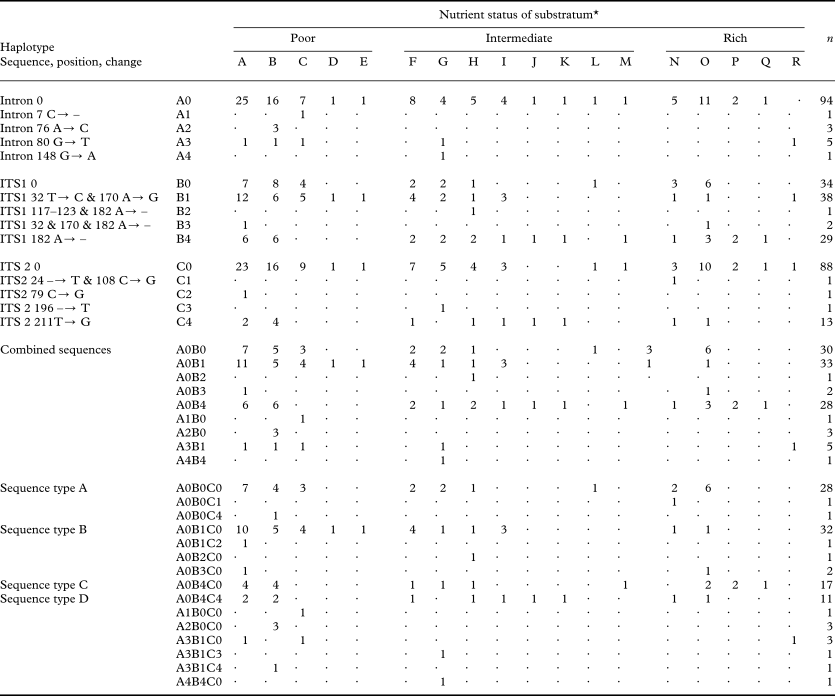
The different haplotypes of H. physodes show no differences in their substratum preferences (Table 1); either on the basis of the the combined DNA sequence or the different regions (Figs. 2 & 3). However, the exact test of differentiation between substratum richness (poor, intermediate or rich) did not lead to rejection of the the null hypothesis, i.e. no differentiation in haplotype composition between the three substratum richness classes (poor, intermediate or rich) for any haplotype; intron P = 0·79, ITS 1 P = 0·09271, ITS 2 P = 0·37. The H. physodes samples show a wide range of substratum preferences within the study area and also some variation in the preferences of the four most common haplotypes (Table 1). This species is most frequently found on ‘nutrient poor’ substrata although it occurs on almost all substrata regardless of their nutrient status (Fig. 3). No discernable geographic structure of the distribution of the haplotypes was found (Fig. 2).
Table 1. Haplotypes of Hypogymnia physodes, their characteristics, and occurrence on different substrata
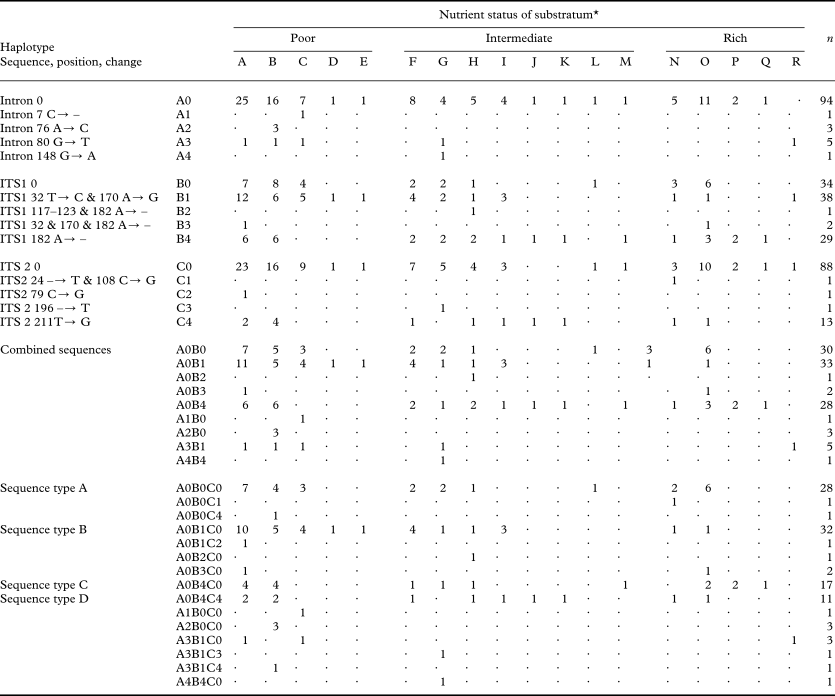
* A=Betula sp.; B=Picea abies; C=Pinus silvestris; D=Juniperus communis; E=Larix decidua; F=Alnus glutinosa; G=Quercus robur; H=Sorbus aucuparia; I=Prunus padus; J=Syringa vulgaris; K=Prunus avium; L=Amelanchier spicata; M=Corylus avellana; N=Populus tremula; O=Salix caprea; P=Tilia cordata; Q=Acer platanoides; R=Populus canescens.
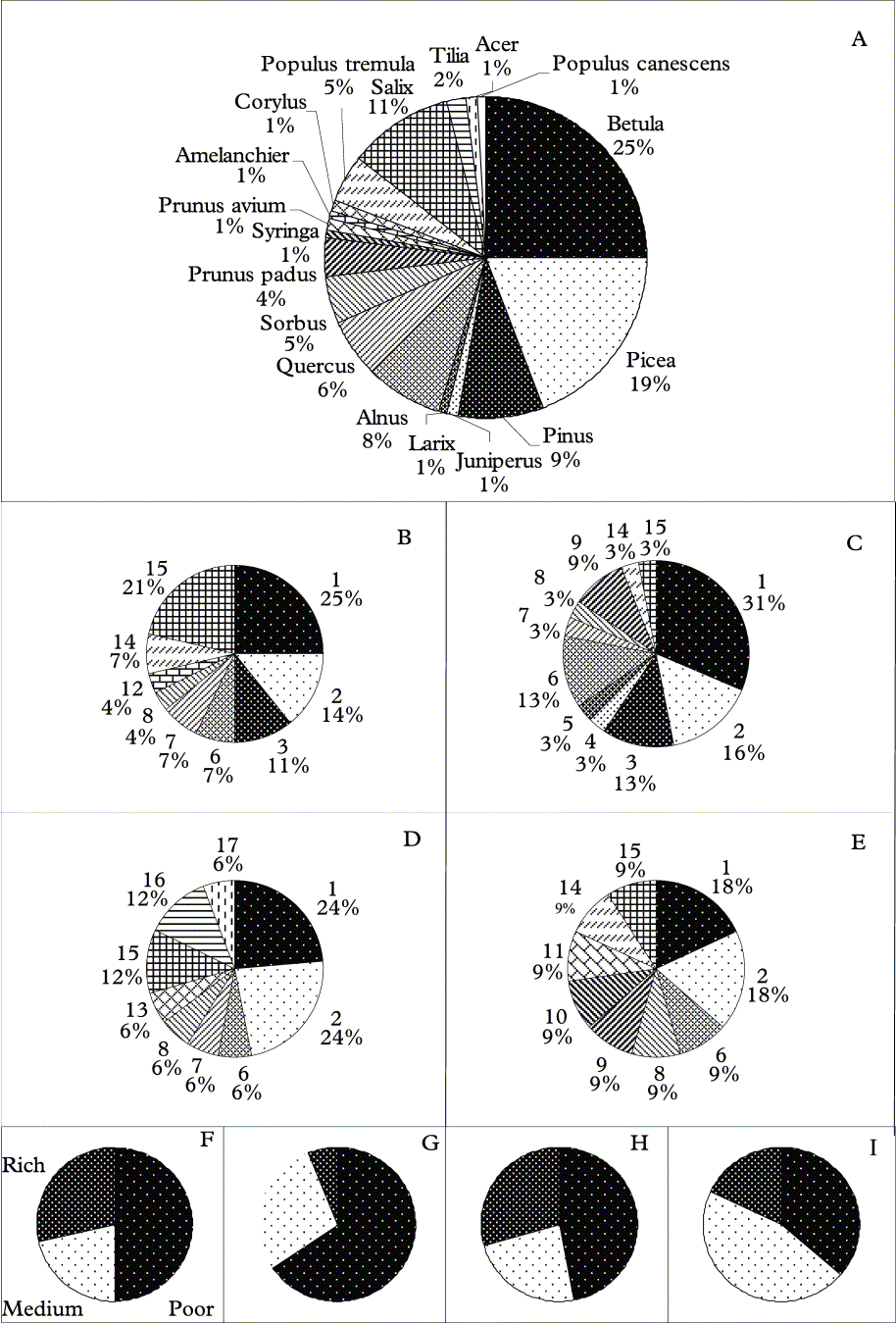
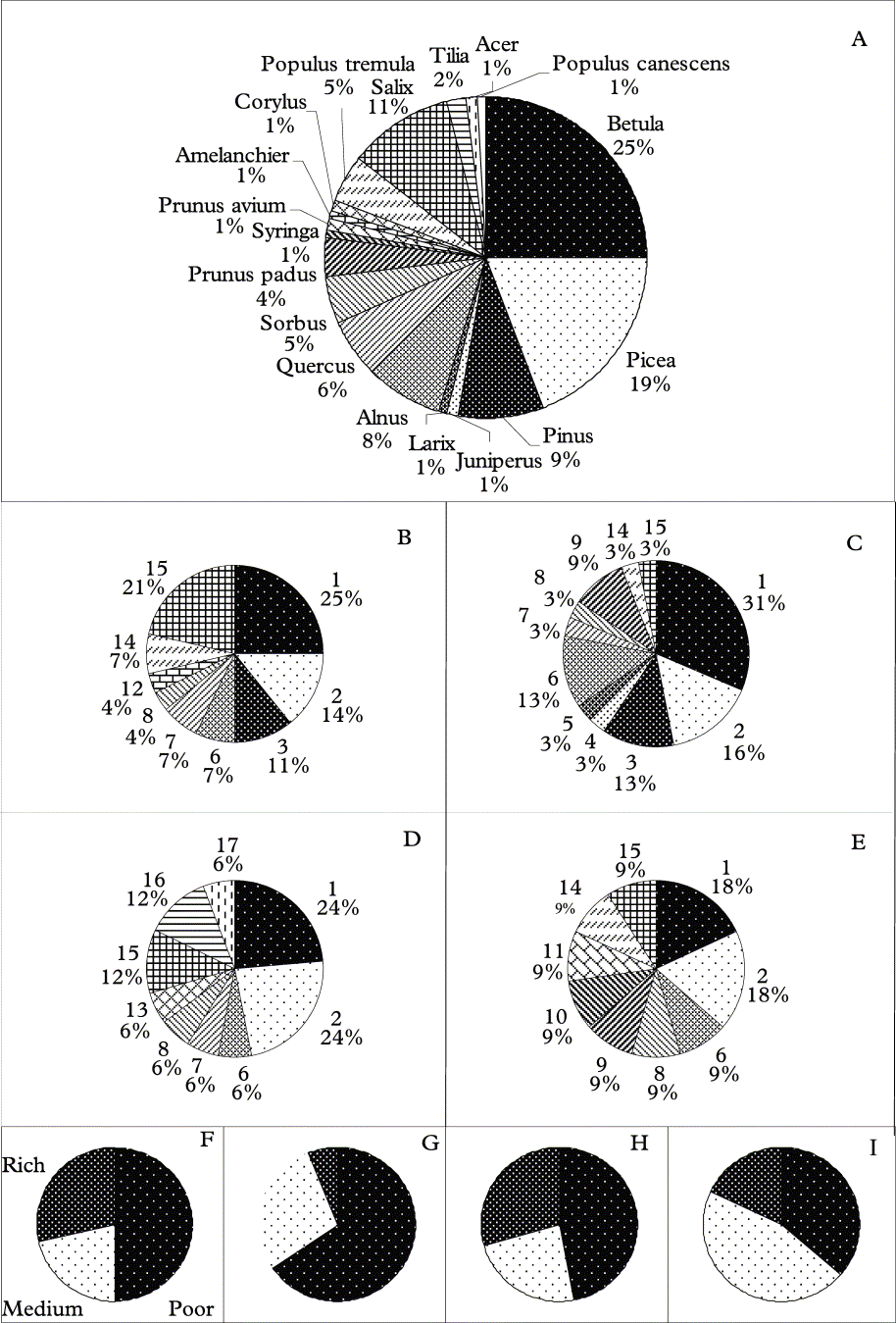
Fig. 3. Frequency of substrata of the total sample (A) and of substrata (B–E) and nutrient conditions of substrata (F–I) of the most common haplotypes in Hypogymnia spp. A, 18 substrata of the material collected (n=104); B, haplotype A (n=28); C, haplotype B (n=32); D, haplotype C (n=17); E, haplotype D (n=11); F, haplotype A (n=28); G, haplotype B (n=32); H, haplotype C (n=17); I, haplotype D (n=11). Species: 1, Betula sp.; 2, Picea abies; 3, Pinus sylvestris; 4, Juniperus communis; 5, Larix decidua; 6, Alnus glutinosa; 7, Quercus robur; 8, Sorbus aucuparia; 9, Prunus padus; 10, Syringa vulgaris; 11, Prunus avium; 12, Amelanchier spicata; 13, Corylus avellana; 14, Populus tremula; 15, Salix caprea; 16, Tilia cordata; 17, Acer platanoides; 18, Populus canescens.
Discussion
A haplotype network based on putatively neutral or near neutral molecular markers (intron and ITS sequences) confirmed that H. physodes and H. tubulosa are two distinct species that share no haplotypes, i.e. complete lineage sorting is reached (Fig. 2). The genetic distance between these two species is large, c. 75 mutational steps in the genealogy constructed using statistical parsimony, thus indicating a more distant relationship compared to previous assumptions. In addition, there is considerable genetic variability within each species, shown in the pie charts (Fig. 3). As substratum nutrient richness and pH affect the lichen flora this genetic pattern may indicate evolutionary or adaptive processes underlying this variation. Thus, the differences in substratum preferences of thalli of different haplotypes may indicate genetic divergence at least partly as a result of adaptations for different substrata. The partial haplotypes show an even more pronounced pattern both for specific substrata (Fig. 3 B–E) as well as for the nutrient richness of the substrata (Fig. 3 F–I).
It was not possible to show any potential for the different haplotypes to establish on different substrata although all the most common haplotypes (A–D) are most frequent on Betula. The most common haplotype on Salix spp. is A whereas haplotype B seems to have a preference for Alnus and Betula sp. Haplotype B also seems to be more common on “nutrient poor” substrata while haplotype D is comparatively more common on other substrata. Also the partial haplotypes show similar patterns as, for example, one ITS1 haplotype (B4) is less common on “nutrient poor” substrata and on Pinus (n = 9) only one ITS2 haplotype (C0) is present although on other substrata (n > 2) at least two haplotypes were recorded (Table 1). However, this may be a sampling artifact as haplotype C0 accounts for 85 % of the haplotype population and only nine individuals were collected on Pinus.
The borders between the three different parts of the ITS region are at nucleotide 201 and 391. The first site for crossing over, according to four-gamete tests, is in the interval 178–190 which falls within ITS1. The second site is more uncertain but is probably in ITS2, because this is a presumed non-coding (neutral or nearly neutral) region although it theoretically could be anywhere between 182 and 457. If we assume a crossing over site in ITS2 it ought to be somewhere between nucleotide 391 and 457. A larger sample with more haplotypes included has to be analyzed to solve this question. Out of the ten sequences of Hypogymnia physodes currently deposited in GenBank, there are three ITS sequences not found in our data set
Each of the parts of the markers used (SSU, intron, ITS1, 5.8S, ITS2) should be regarded as an important piece of information on their own and should be evaluated in relation to morphology, substratum preferences, as well as migration history.
Although H. physodes is primarily a clonally reproducing species, it may be concluded that recombination is probably frequent enough to break up linkage between supposedly adaptive genes responsible for substratum choice and the gene(s) under study (ribosomal DNA incl. intron). The results are consistent with H. physodes behaving like a sexual species even though sex is probably rare.
This study is part of the Ecological and Societal Systems in Interaction project funded by Östersjöstiftelsen. The project is hosted at and part of the strategic development of environmental sciences at Södertörns högskola (Södertörn University), Sweden. We are grateful to all other participants in the project, especially Mikael Lönn, for scientific support, methodological development and fruitful discussions. Mikael Lönn also participated in the original design of the study together with Linda Svensson. The latter was responsible for supervising students during the field work. The work of Tiina Vinter in refining the field methods and for collecting most of the material is also acknowledged. Further, we thank all students collecting material during participation in courses on field methods (Estonia and Sweden) and ecology (Finland). Finally, we acknowledge help with the statistical analysis from Stefan Ekman and constructive suggestions from three reviewers. The map was drawn by Ulf Arup.