Introduction
The photoautotrophic inhabitants of lichen thalli are distinctly less intensely studied than the lichen-forming fungi themselves. Before the advent of molecular techniques, photobiont morphospecies were distinguished by light microscopy. In the case of the unicellular representatives of Trebouxiophyceae, the most widespread photobionts of lichen-forming fungi, this was a difficult task. The prerequisites were sterile cultured isolates maintained under defined conditions and cultures of type species for comparison (Tschermak-Woess Reference Tschermak-Woess and Galun1988). Later, the photobiont diversity within thalli of conspecific lichen-forming fungi were biochemically analysed, the focus being on isoenzyme patterns (Kilias Reference Kilias1988; Kilias et al. Reference Kilias, Gelfi and Righetti1988; Fahselt Reference Fahselt1989). With molecular techniques and algal-specific primers applied to whole lichen DNA, the photobionts can be conveniently identified at the species level without any isolation and culturing (Beck et al. Reference Beck, Friedl and Rambold1998; Friedl et al. Reference Friedl, Besendahl, Pfeiffer and Bhattacharya2000; Dahlkild et al. Reference Dahlkild, Kallersjo, Lohtander and Tehler2001; Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001; Beck et al. Reference Beck, Kasalicky and Rambold2002). Thanks to this type of investigation our knowledge about the range of photobiont taxa associated with particular species of lichen-forming fungi has significantly increased in recent years. In most of these studies photobiont diversity was explored at the species level on the basis of ITS phylogenies of samples collected in geographically different locations. In only a few studies was photobiont diversity examined within populations. Photobiont diversity was studied in lichen communities growing on heavy-metal rich rock (Beck et al. Reference Beck, Friedl and Rambold1998; Beck Reference Beck1999; Beck et al. Reference Beck, Kasalicky and Rambold2002). Photobiont diversity was explored in the families Cladoniaceae (Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Piercey-Normore Reference Piercey-Normore2004, Yahr et al. Reference Yahr, Vilgalys and DePriest2004), Parmeliaceae (Piercey-Normore Reference Piercey-Normore2009) and Physciaceae (Dahlkild et al. Reference Dahlkild, Kallersjo, Lohtander and Tehler2001; Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001, Helms Reference Helms2003), in the genera Caloplaca Th. Fr. (Vargas Castillo & Beck Reference Vargas Castillo and Beck2012), Chaenotheca (Th. Fr.) Th. Fr. (Tibell Reference Tibell2001), Letharia (Th. Fr.) Zahlbr. (Kroken & Taylor Reference Kroken and Taylor2000), and Umbilicaria Hoffm. (Romeike et al. Reference Romeike, Friedl, Helms and Ott2002). At the intraspecific level, genetic diversity of green algal photobionts was studied in populations of a number of lichen-forming fungi (Opanowicz & Grube Reference Opanowicz and Grube2004; Blaha et al. Reference Blaha, Baloch and Grube2006; Guzow-Krzeminska Reference Guzow-Krzeminska2006; Ohmura et al. Reference Ohmura, Kawachi, Kasai, Watanabe and Takeshita2006; Piercey-Normore Reference Piercey-Normore2006; Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Muggia et al. Reference Muggia, Zellnig, Rabensteiner and Grube2010; Werth & Sork Reference Werth and Sork2010; Wornik & Grube Reference Wornik and Grube2010; Casano et al. Reference Casano, del Campo, Garcia-Breijo, Reig-Arminana, Gasulla, del Hoyo, Guera and Barreno2011; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Domaschke et al. Reference Domaschke, Fernández-Mendoza, García, Martin and Printzen2012; Francisco De Oliveira et al. Reference Francisco De Oliveira, Timsina and Piercey-Normore2012). Overall, these studies revealed that lichen fungi are able to associate with a range of photobionts although some species are more specialized on particular types of photobionts than others. Some species showed large-scale geographic trends in the distribution of photobionts (Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Wornik & Grube Reference Wornik and Grube2010; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011), others showed the ecological specialization of photobionts (Werth & Sork Reference Werth and Sork2010; Peksa & Skaloud Reference Peksa and Skaloud2011).
Many of the previous studies have relied on phylogenies of the Internal Transcribed Spacer Region (ITS) to identify photobiont species, based on the assumption that samples grouping with a known species in a well-supported clade are conspecific. ITS phylogenies are based on only one locus, but genetic diversity at the subspecific level is best explored with a multilocus approach. Fingerprinting techniques can provide valuable data on the genetic diversity of populations and these have been successfully used for characterizing lichen-forming fungi at the subspecific level: microsatellite-based fingerprinting (Walser et al. Reference Walser, Sperisen, Soliva and Scheidegger2003; Walser et al. Reference Walser, Holderegger, Gugerli, Hoebee and Scheidegger2005; Werth et al. Reference Werth, Wagner, Holderegger, Kalwij and Scheidegger2006; Werth et al. Reference Werth, Gugerli, Holderegger, Wagner, Csencsics and Scheidegger2007; Widmer et al. Reference Widmer, Dal Grande, Cornejo and Scheidegger2010; Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2011; Dal Grande et al. Reference Dal Grande, Widmer, Wagner and Scheidegger2012), tRNA fingerprinting (Schmitt et al. Reference Schmitt, Mangold and Lumbsch2002), or randomly amplified polymorphic DNA (RAPD) analysis applied to either pure fungal material such as central strands of Usnea sp. (Heibel et al. Reference Heibel, Lumbsch and Schmitt1999) or apothecial discs (Printzen et al. Reference Printzen, Lumbsch, Schmitt and Feige1999), or to sterile cultured fungal isolates (Murtagh et al. Reference Murtagh, Dyer, McClure and Crittenden1999; Dyer et al. Reference Dyer, Murtagh and Crittenden2001; Honegger et al. Reference Honegger, Zippler, Scherrer and Dyer2004b; Honegger & Zippler Reference Honegger and Zippler2007; Itten & Honegger Reference Itten and Honegger2010). Recently, microsatellite markers were also applied to investigate the reproductive mode and phylogeography in Dictyochloropsis reticulata (Tscherm.-Woess) Tscherm.-Woess photobionts (Dal Grande et al. Reference Dal Grande, Widmer, Wagner and Scheidegger2012; Widmer et al. Reference Widmer, Dal Grande, Excoffier, Holderegger, Keller, Mikryukov and Scheidegger2012). RAPD-PCR applied to either randomly selected single spore isolates from one apothecium (Murtagh et al. Reference Murtagh, Dyer and Crittenden2000; Seymour et al. Reference Seymour, Crittenden, Dickinson, Paoletti, Montiel, Cho and Dyer2005) or to the single spore isolates derived from a single ascus (i.e. the progeny of one meiosis, Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004a) was used for studying the mating systems of lichen-forming ascomycetes. Fingerprinting techniques such as short (STRR) or long (LTRR) tandemly repeated repetitive sequences were used for characterizing conspecific cyanobacterial symbionts of angiosperms (Rasmussen & Svenning Reference Rasmussen and Svenning1998), whilst M13 minisatellite fingerprinting facilitated the distinction of different genotypes among conspecific unicellular or filamentous green algae (Oppermann et al. Reference Oppermann, Karlovsky and Reisser1997).
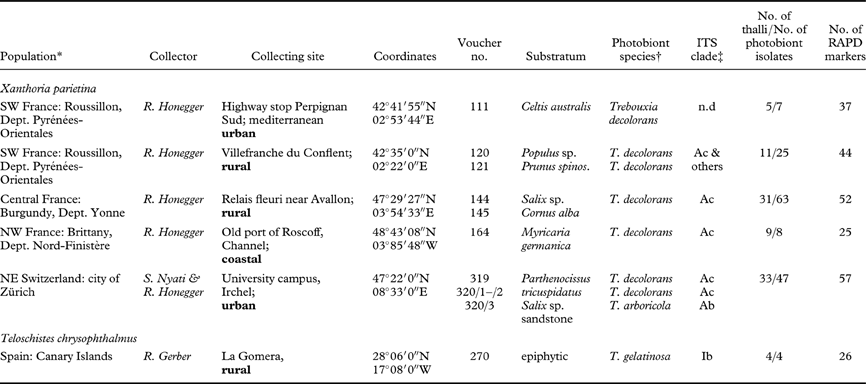
The present study aims at elucidating photobiont diversity in Trebouxia de Puymaly photobionts at the subspecific level in different populations of the yellow wall lichen, Xanthoria parietina (L.) Beltr., by means of RAPD-PCR fingerprinting applied to sterile cultured isolates. Thalli were sampled in populations at coastal, rural or urban sites in NW, SW or central France or in NE Switzerland. Some of these populations were old and undisturbed over prolonged periods of time (voucher numbers 120 and 121, 144 and 145), others grew at sites which had been newly built within the last 20 years (voucher numbers 111, 319 and 320). As Trebouxia species are assumed to reproduce exclusively asexually (Friedl & Büdel Reference Friedl, Büdel and Nash1996) and be rare outside lichen thalli (Ahmadjian Reference Ahmadjian1988), we were interested to see whether the photobionts of local populations of X. parietina exhibited low diversity, as expected if the algae are reproducing clonally.
Materials and methods
Specimen collection, photobiont isolation and culturing
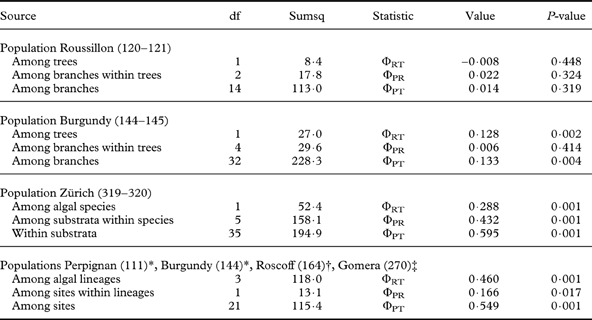
Lichen thalli from five populations of Xanthoria parietina and of one small population of Teloschistes chrysophthalmus (L.) Norman ex Tuck. (Table 1; Fig. 1A–B) were stored in a desiccated state at −20°C where they stay viable for prolonged periods (Honegger Reference Honegger2003). Each collecting site and sample was photographically documented. Sample codes included the site number (three-digit Arabic numbers), the substratum number (Arabic numbers), the thallus number (Roman numerals), and the isolate number (letters). Voucher specimens have been deposited in the herbarium of ETH Zürich (Z+ZT). In the Swiss population (319/320) the thalli were left in situ with only small fragments being collected.
Table 1. Collecting sites of lichen thalli and their Trebouxia photobionts isolated and analyzed in the present study
* Collecting sites from the same area are grouped as one population. †Photobiont species identified according to ITS and rbcL phylogenies; n.d. not determined.
‡ ITS phylogenies in Fig. 2; “others” indicates T. decolorans isolates falling in unresolved part of phylogram between clade A3 and A4.

Fig. 1. A, Population 320/2, with Xanthoria parietina growing on a vertical branch of Salix sp. at the pond in the University park Zürich Irchel; B, Population 270, Teloschistes chrysophthalmus growing intermixed with Physcia spp. on a vertical branch; C, RAPD-PCR of samples of populations 164, 111 and 144/3 (all X. parietina) and 270 (Telo. chrysophthalmus). C: control. M: molecular marker. See analysis of this sample set in Fig. 3D.
One, rarely two, mature apothecia per thallus (in the latter case termed a and b) were selected and ascospores were allowed to be ejected for parallel experiments on the genetic diversity within the fungal partner (Honegger & Zippler Reference Honegger and Zippler2007; Itten & Honegger Reference Itten and Honegger2010). In the herbarium sample the outline of each thallus was drawn on a transparent overlay and the site marked where apothecia had been removed. Photobiont cells were scraped out of the bottom (below subhymenial layer) and thalline margin of apothecia with a sterilized platinum needle and spread over the surface of agarized non-nutrient mineral medium (Bold's basal medium [BBM] according to Deason & Bold Reference Deason and Bold1960) with double the amount of nitrogen and 0.·005% w/v doxycycline (Sigma) as an antibiotic. Plates were maintained at 15±1°C at a 16h light/8 h dark cycle at approximately 5 µE m–2 s–1 for 2–3 weeks until cells started to divide. All cultures were screened regularly and fungal contaminants were removed. Dividing algal cells were transferred to Trebouxia medium II according to Ahmadjian (Reference Ahmadjian1967), with only ¼ amount of glucose and casmino acids (Honegger Reference Honegger2004). Most cultures were multi-cell isolates, with cells originating from a very small area within a single apothecium; however, a few cultures were single cell isolates. A total of 150 photobiont isolates from five X. parietina populations were investigated in this study. For comparison four isolates of Teloschistes chrysopthalmus growing side by side were analyzed. In parallel experiments the fungal partner was brought into sterile culture.
Genomic DNA isolation
Genomic DNA was isolated using the GFX PCR, DNA and Gel Band Purification Kit (Amersham Biosciences, Little Chalfont) with modifications to the manufacturer's protocol as follows. Algal isolates were frozen in liquid nitrogen and ground with a pre-cooled motor-driven micropestle. After addition of 100 µl of capture buffer to ground material, the samples were incubated at 60°C for 10 minutes and subsequently centrifuged. The supernatant was transferred to a GFX column preloaded with 100 µl of capture buffer, incubated for 3 minutes at room temperature, centrifuged and washed with 500 µl of washing buffer. DNA was eluted in 50 µl of elution buffer (10 mM Tris-HCL, pH 8.0) and stored at 4°C.
ITS amplification and sequencing
The nuclear ribosomal ITS region (ITS 1, 5.8S rDNA and ITS 2) was amplified with primer pair ITS 5 and ITS 4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990). Internal primers at 5.8S rDNA were used for sequencing; these were TreSeq1 (fwd): 5′-CAA CTC TCA ACA ACG GAT ATC-3′, TreSeq2 (rev): 5′-GAC GCT GAG GCA GAC ATG CTC-3′, and TreSeq3 (rev): 5′-CCG AAG CCT CGA GCG CAA TTT-3′. For comparison, three ITS sequences were obtained from whole lichen DNA extracts using forward primer AL1500bf situated in the 18S region (Helms et al. Reference Helms, Friedl, Rambold and Mayrhofer2001) and reverse primer LR3 located in the 26S region (Friedl & Rokitta Reference Friedl and Rokitta1997). Amplifications were performed in 50 µl reaction volume containing 2 µl genomic DNA (15–100 ng), 50 nM each dNTP, 5 µl 10× PCR buffer (100 mM Tris-HCl pH 8·3, 500 mM KCl, 15 mM MgCl2, 0·01% gelatin), 0·6 µM of each primer, 1·5 U Taq DNA polymerase (Sigma) and 35·75 µl autoclaved ddH2O under the following conditions: initial denaturation at 95°C for 3 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s and elongation/extension at 72°C for 60 s and final extension at 72°C for 5 min.
Sequencing was done in a 10 µl reaction mix containing 0·8 µl BigDye Terminator Mix V3.1, 120 nM primer, 1× reaction buffer, and 10–20 ng purified DNA. This reaction mixture was modified from the manufacturer's protocol as follows: initial denaturation at 94°C for 2 min, 60 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 3 min (0·9°C/s ramp). The products were analyzed on an Applied BioSystem/HITACHI ABI 3730 DNA Analyzer (Life Technologies, Rotkreuz, Switzerland).
Phylogenetic analysis of DNA sequences
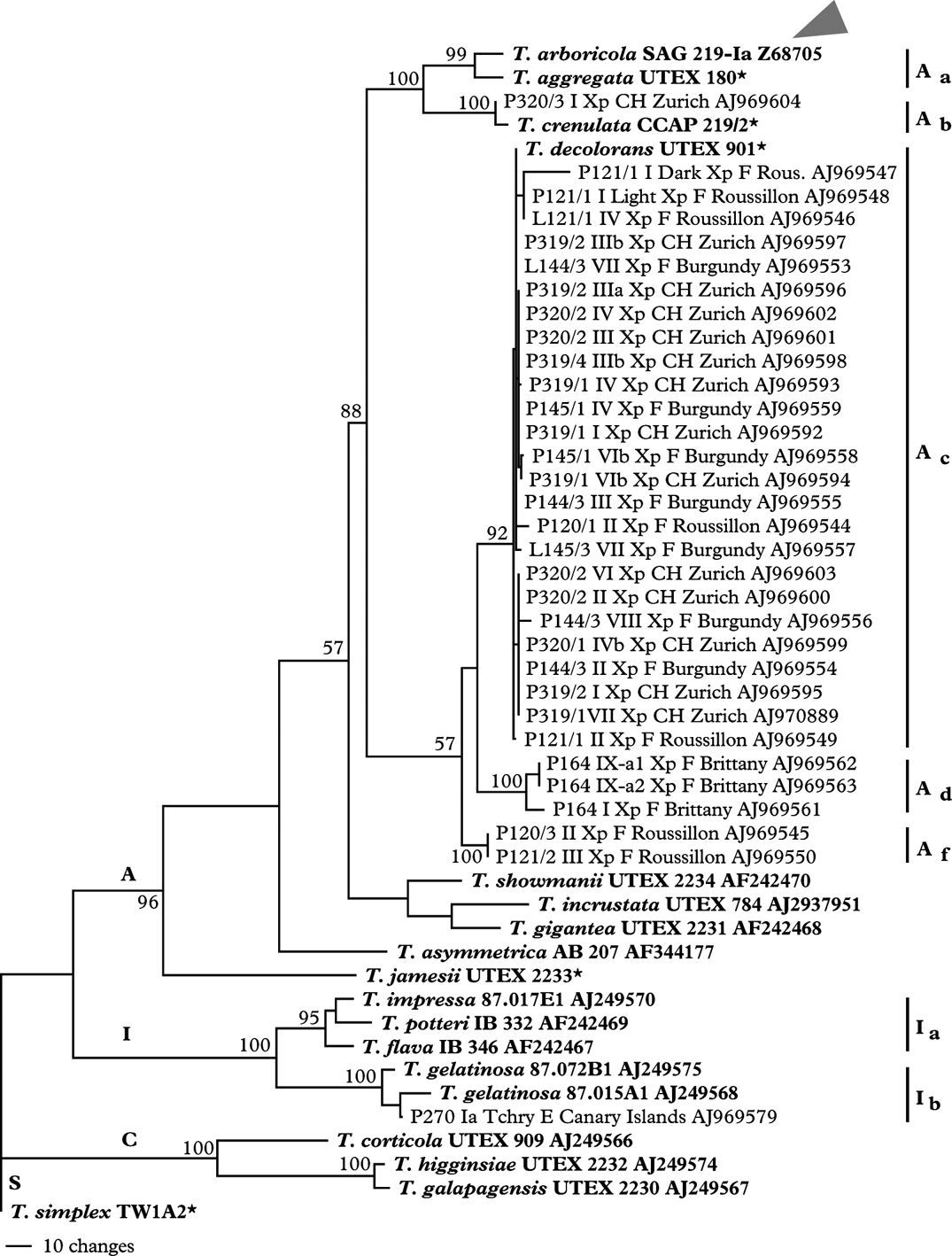
Sequences were analyzed with SequencherTM 4.2.2 (Gene Codes Corp. Ann Arbor, USA) and aligned automatically with Clustal X 1.81 (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997). The resulting alignment was manually aligned on MacClade 4.06 (Maddison & Maddison Reference Maddison and Maddison2002). Phylogenetic analysis was carried out with PAUP 4.0 b10 (Swofford Reference Swofford1998) by Maximum Parsimony (MP), Maximum Likelihood (data not shown), and Neighbor Joining (data not shown) methods. Bootstrap values for 1000 replicates were calculated. The sequence of Trebouxia simplex Tscherm.-Woess was used as an outgroup. This species is not a close relative of Trebouxia decolorans Ahmadjian, but we were able to align the ITS sequences. We used the grouping of samples within ITS clades that included known morphospecies of Trebouxia to identify the algal species, assuming that unknown samples would group with samples of the known morphospecies within the trees.
RAPD amplification and fingerprinting analysis
RAPD amplification was carried out with decamer primers (Operon Technologies Alameda, CA). Initial primer screening was carried out with 80 primers (Kit A–D) applied to DNA extracted from 2 Trebouxia isolates. 11 primers were found to be algal specific, but 5 of them gave only a very weak reaction. 38 primers amplified DNA derived from photobiont and mycobiont isolates. In the current investigation, 4 primers (OPA-05: AGGGGTCTTG; OPA-09: GGGTAACGCC; OPB-10: CTGCTGGGAC, and OPC-06: GAACGGACTC) were used for amplification. Reactions were performed with the Perkin Elmer Gene Amp PCR system 9600. 25 µl reaction mix was prepared, containing 1 µl genomic DNA, 1× reaction buffer (Sigma), 0.2 mM dNTPs, 1·25 U Taq polymerase (Sigma), and 0·2 mM single primer. PCR conditions were as follows: initial denaturation for 3 min at 94°C, followed by 40 cycles of 30 s at 93°C, 40 s at 37°C and 80 s at 72°C with final extension of 5 min at 72°C. The ramp was 1°C/s. Negative controls were included in all experiments to detect contamination. 10 µl of each PCR product was loaded onto a 1·2 % agarose gel and run for ∼6 hrs for good separation of bands. DNA molecular weight marker VI (Roche Diagnostics, Mannheim, Germany) was used as a fragment size marker (Fig. 1C).
DNA from all algal isolates obtained from the same population was processed in the same run. A few selected isolates were independently amplified to check the reproducibility of band patterns. In three experiments the suitability of RAPD fingerprinting was checked using phenotypically similar single cell and multi-cell isolates from the same apothecia. Five algal isolates were sub-cloned into 5 isolates each and amplified to test uniformity of isolates. Only clearly visible strong bands were considered, their presence or absence being marked in a binary data matrix per primer and finally in a combined data matrix for each population or dataset. Isolates for which satisfactory amplification was not obtained with all primers were removed from the data matrix. This binary data matrix was then used for phenetic analysis (NJ and UPGMA) on FreeTree (Pavlicek et al. Reference Pavlicek, Hrda and Flegr1999; Hampl et al. Reference Hampl, Pavlicek and Flegr2001) and for analyses of molecular variance (AMOVA). The robustness of the phenetic trees was tested with bootstrapping and jackknifing methods. Resulting phenograms were depicted with TreeView (Page Reference Page1996). Sequential agglomerative hierarchical nested cluster analysis (SAHN clustering) and ordination analysis were carried out on NTSYS-PC (Rohlf Reference Rohlf2000). In SAHN clustering, cophenetic-values were used to compute cophenetic correlation as a measure of goodness of fit, and results were plotted in the form of a dendrogram. Principal coordinates analysis (Cox & Cox Reference Cox and Cox2001) was performed in NTSYS-PC to show the relationships between isolates from different populations and substrata. Analysis of molecular variance was carried out with GenAlEx version 6.3 (Peakall & Smouse Reference Peakall and Smouse2006) grouping isolates by algal species, populations, trees and branches, depending on the hierarchy levels included within a given dataset. AMOVA was performed on four data sets. One dataset included multiple algal lineages and sites, another included two algal species and several substrata from which these species had been collected. Last but not least, two data sets contained isolates collected from multiple branches of several trees. Hence, we were able to study population structure in the photobionts at different scales.
Results
Identity and diversity of photobionts
As shown in the ITS phylogram (Fig. 2) most of the isolates from samples collected in SW France (Roussillon), Burgundy and on our University campus in Zürich, Switzerland belonged to Trebouxia decolorans and revealed identical or near identical ITS genotypes. Numerous isolates contained a SSU 1512 group I intron of approx. 460 bases length, whose sequence was removed prior to alignment. For a detailed analysis of the group I introns in Trebouxia photobionts of Teloschistaceae see Nyati et al. (Reference Nyati, Bhattacharya, Werth and Honeggerin press). The isolates collected on sandstone from Xanthoria parietina belonged to T. arboricola and those isolated from Teloschistes chrysophthalmus belonged to Trebouxia gelatinosa (Fig. 2).
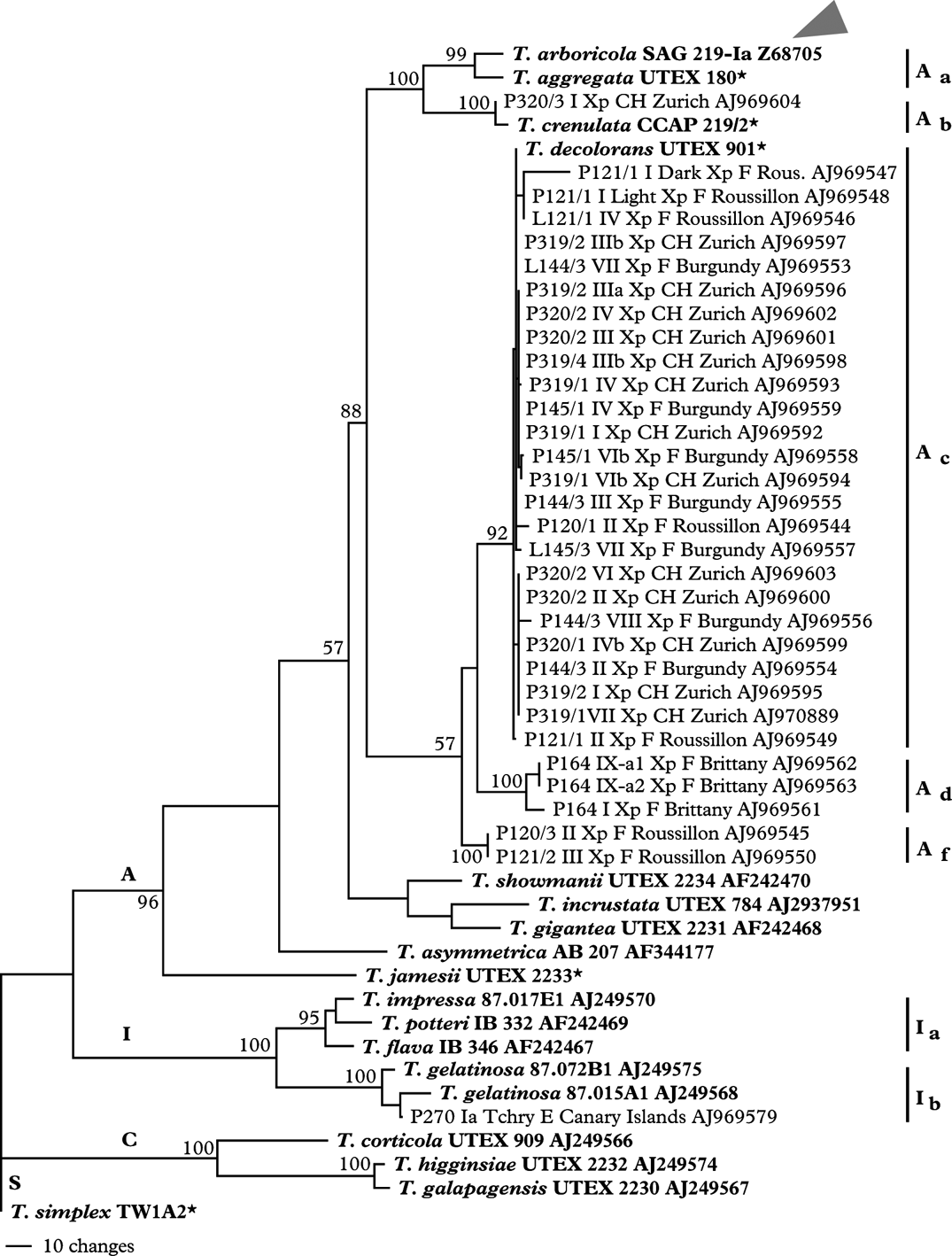
Fig. 2. Maximum parsimony phylogram of internal transcribed spacer regions (ITS1 and ITS2) along with 5.8S rDNA. Bootstrap values for 1000 replicates are given at the nodes. The letters A, I, C, and S indicate ‘arboricola’, ‘impressa’, ‘corticola’ and ‘simplex’ clades respectively. The tree is outgroup-rooted with a Trebouxia simplex sequence. The type species of the genus (T. arboricola, SAG 219-Ia) is indicated with arrowhead. Unpublished sequences provided by T. Friedl and G. Helms are marked with an asterisk. Each sample is labelled with voucher number followed by apothecia number, species abbreviation (Xp: Xanthoria parietina), country code, city/province and accession number. P denotes sequences obtained from a photobiont isolate while L indicates whole lichen DNA used for PCR amplification.
A total of 25 to 57 markers were obtained with four RAPD primers in our fingerprinting experiments (Table 1). The genetic variability of the isolated photobionts was very high, as assessed by RAPD fingerprinting. Reproducibility of the RAPD markers was high when freshly isolated DNA was used and procedures were standardized. RAPD-PCR with genomic DNA isolated from 8 different isolates (voucher 144) was repeated using 4 different RAPD primers which showed very similar band patterns. Nevertheless, as we could not guarantee identical banding patterns among different PCR reactions, we decided against combining the RAPD banding patterns of different PCRs. Hence, several multi-locus RAPD data sets were analyzed separately, each representing the samples that had been included into one PCR reaction.
Population 120/121 from “Roussillon” (Departement Pyrenées Orientales), SW France
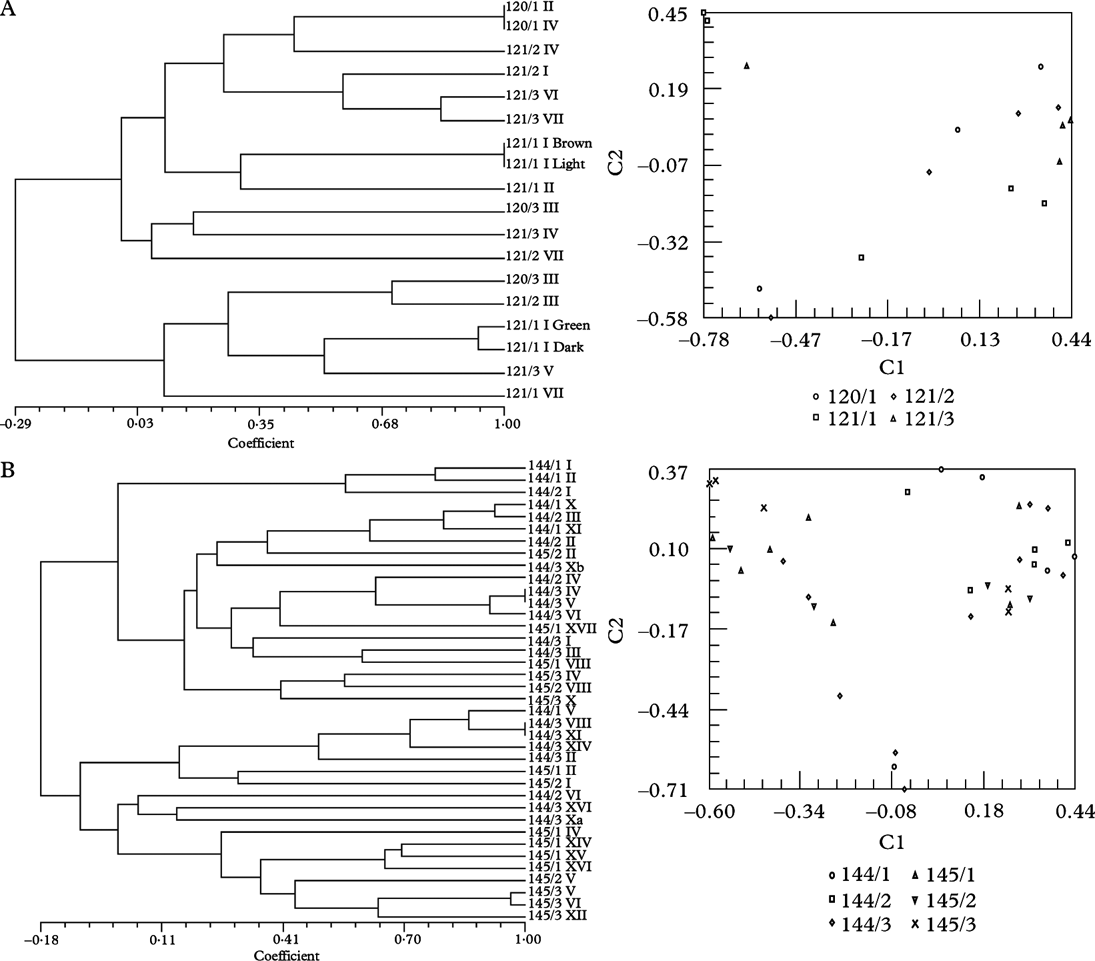
Four thalli of Xanthoria parietina growing on two fragments (120/1, 120/3) of the same branch of Salix sp. and 11 thalli growing on three small branches of Prunus spinosa (121/1-121/3) were collected just outside Villefranche-du-Conflent situated in a steep valley running parallel to the Pyrenees in SW France near the Spanish border. These samples are termed “Roussillon” in the ITS phylogram (Fig. 2), the local name of the larger area. All trees and shrubs at this site were completely covered by this golden-yellow lichen, indicating massive eutrophication. Both collecting sites were c. 10 m apart from each other. All thalli revealed the same phenotype and contained T. decolorans as photobiont. From sample 121/1 I (one apothecium) four different algal phenotypes were isolated, which could be distinguished by their colour: brown, green, light green and dark green. Two different ITS genotypes were distinguished (Fig. 2). With fingerprinting techniques three different genotypes were identified among these four different algal phenotypes (Fig. 3A). This was the first and only time that different genotypes from the same algal species were isolated from a very small area, i.e. the thalline margin of an apothecium. In the remaining 14 samples 13 algal genotypes were found (Fig. 3A). SAHN clustering and principal coordinates analysis showed no tendency of samples to group by phorophyte species.
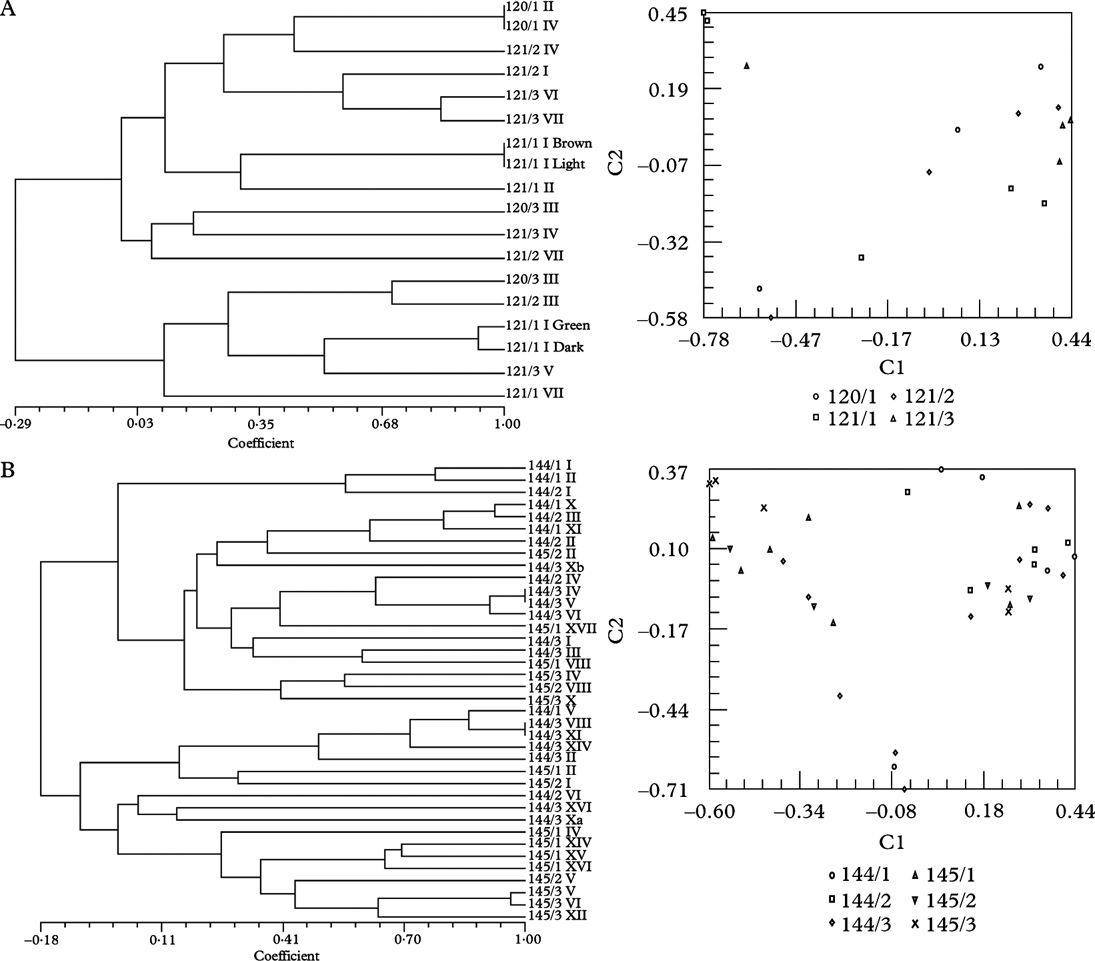
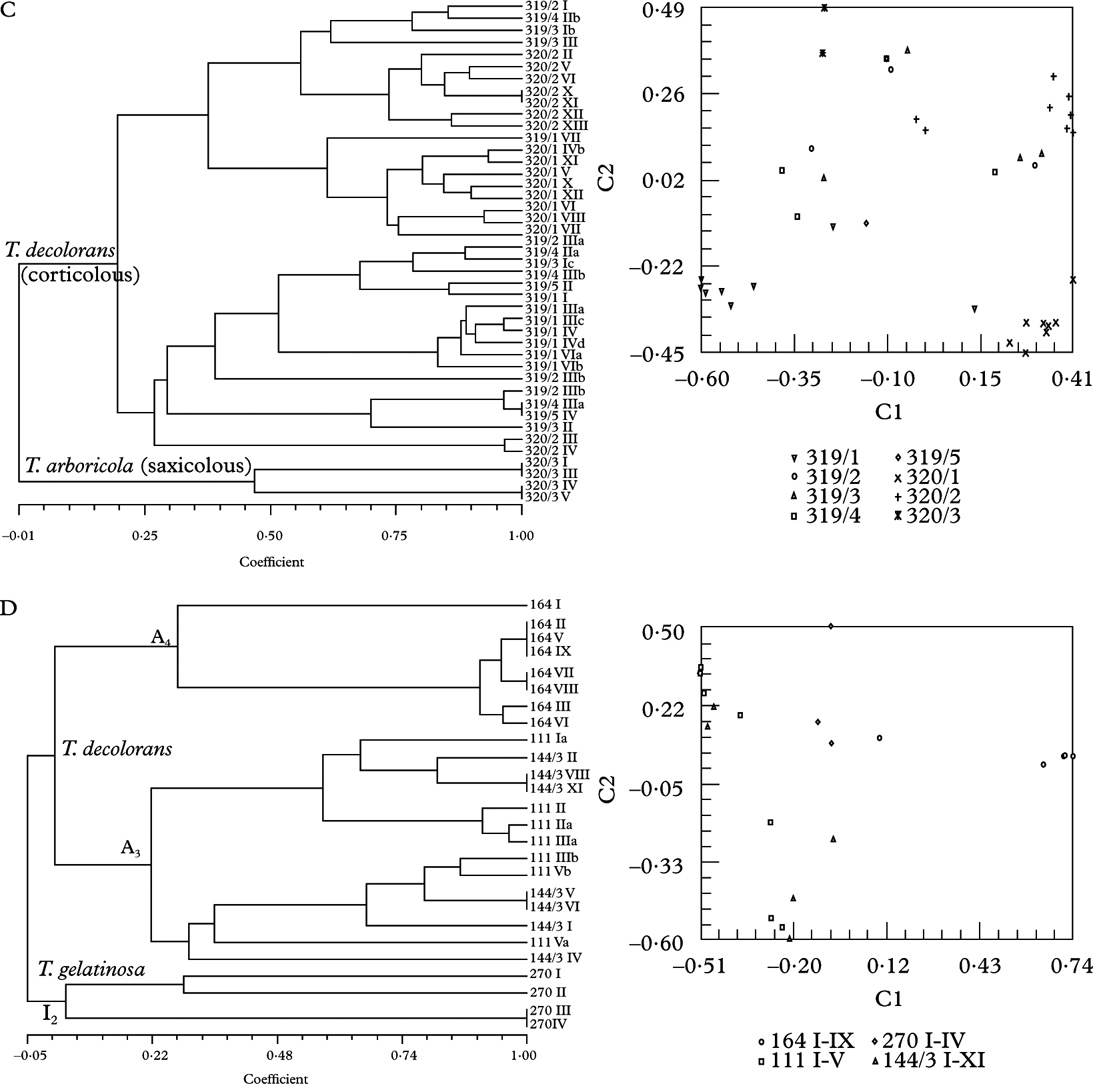
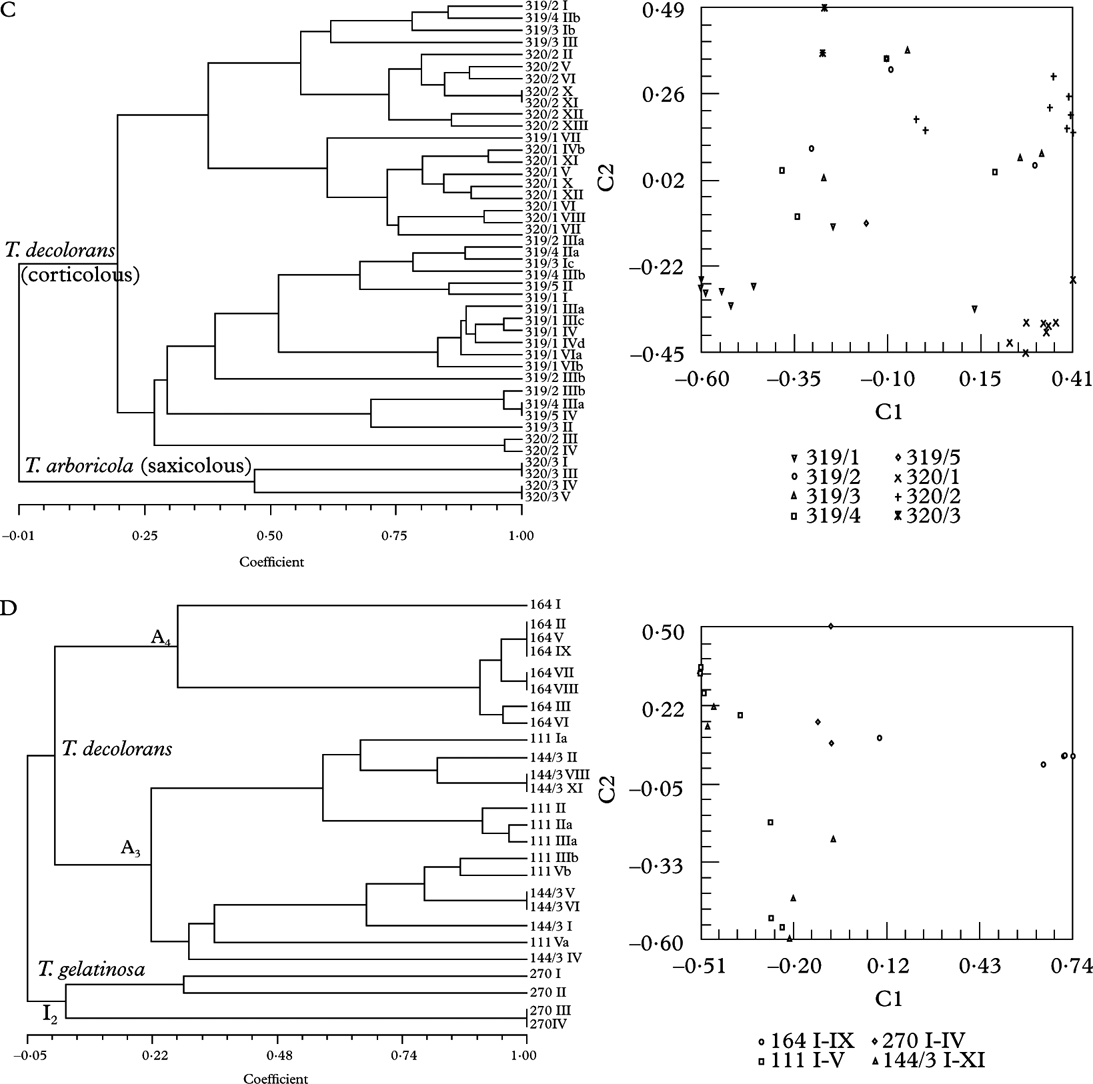
Fig. 3. Dendrograms obtained by SAHN clustering (left) and principal coordinate analysis with the first two axes shown (right). Each symbol represents an isolate. All except voucher numbers 270 are Trebouxia isolates from thalli of Xanthoria parietina. Details are given in the legend to the right. A, Trebouxia decolorans isolates from ‘Roussillon’ population (voucher numbers 120 on Populus sp., 121 on Prunus spinosa). B, T. decolorans isolates from Burgundy population (144 on Salix babylonica, 145 on Cornus alba). C, T. decolorans isolates from epiphytic populations (319 on Parthenocissus tricuspidatus, 320/1-320/2 on Salix sp.) and T. arboricola from saxicolous population (320/3) in Zürich. D, T. decolorans isolates from populations in Brittany (164 on Myricaria germanica), Perpignan (111 on Celtis australis), Burgundy (144/3 on Populus sp.) and T. gelatinosa from Teloschistes chrysophthalmus (270) from Canary Islands.
Population 144/145 from Burgundy, (Departement Yonne), Central France
Twenty-two thalli of Xanthoria parietina were collected on three fragments of a branch of an old weeping willow (Salix babylonica) and 16 thalli on three twigs of a Cornus albus shrub in the garden of the mansion Le Relais fleuri near Avallon, situated within large fields adjacent to woods in Burgundy. All thalli of X. parietina revealed the same phenotype. ITS genotypes were very similar (Fig. 2). With fingerprinting techniques 36 different genotypes were identified among the 38 isolates of Trebouxia decolorans from this site (Fig. 3B). Cluster analysis and principal coordinates of the RAPD data showed a tendency for algal isolates to group by phorophyte species.
Population 164 from Brittany (Departement Nord-Finistère), NW France
Eight thalli of X. parietina were collected on a tamarisk shrub at the easternmost mole next to the Chapelle Sainte Barbe in the old port of Roscoff on the Channel in NW France. This small population was unique because it comprised different thalline phenotypes with varying amounts of anthraquinone irrespective of illumination. Thalli growing side by side revealed colours from intense orange-yellow (164 I) to bright intense yellow (164 II) to greenish (164 V), some being greenish with intensely yellow lobe margins (164 IV). Juvenile thalli of X. parietina growing side by side on a fully illuminated wooden garden bench next to the Myricaria shrubs showed the same varieties of phenotypes (Itten & Honegger Reference Itten and Honegger2010). In TLC analyses all thalli of this population comprised parietin as the main anthraquinone (R. Honegger, unpublished data); thus belonging to chemosyndrome A, according to Søchting (Reference Søchting1997), a characteristic feature of X. parietina. All thalli of Xanthoria ectaneoides (Nyl.) Zahlbr. growing on granitic rock at this collecting site revealed chemosyndrome A3 (Søchting Reference Søchting1997), with parietin, teloschistin and fallacinal as the main anthraquinones (R. Honegger, unpublished data), and with Trebouxia arboricola de Puymaly as photobiont (Nyati et al. Reference Nyati, Schaerer, Werth and Honegger2014).
Together with other Trebouxia isolates from Xanthoria spp. from maritime collecting sites on the Canary Islands, in the Eastern Mediterranean area and in Southern Australia the algal isolates of this population belong to a unique subclade Ad (Fig. 2) within clade A sensu Helms (Reference Helms2003). With fingerprinting techniques, five different genotypes were found among the eight Trebouxia isolates from this site (Fig. 3), isolate Nr. 164/1 from the intensely orange coloured thallus, with a different ITS genotype (Fig. 2), being distinctly different from all others (Figs. 1 & 3).
Population 111 from Perpignan (Departement Pyrenées Orientales), SW France
A small population comprising seven thalli of X. parietina was collected on the stem of a Celtis australis tree at the stop of highway A9 (Narbonne to Barcelona) at Perpignan Sud in SW France. This site was newly constructed and trees planted less than 20 years ago. All thalli revealed an unusual phenotype with greenish grey colour and a slightly knobbly surface, but in a phylogenetic analysis with multi-locus approach they all turned out to be X. parietina (C. Eichenberger, unpublished data). In mediterranean and also in continental climates, the very smooth and hard bark of Celtis australis does not normally suppport lichen growth, probably due to its very poor water holding capacity.
Transplantation experiments would be needed to show whether these X. parietina thalli might differentiate a more typical phenotype on a different substratum. With fingerprinting techniques seven genotypes were found among the seven Trebouxia isolates from this site. None of them was sequenced, but as all isolates clustered with T. decolorans isolates from the Burgundy population 144, partly showing identical fingerprints (Fig. 3D), we conclude that they belong to the same species.
Population 319/320 from Zürich University campus, NW Switzerland
Twenty-one thalli of X. parietina were collected on the bottom part of five stems of a wild vine (Parthenocissus tricuspidatus; 319/1-319/5), nine on the stem (320/1), eight on a branch (320/2) of a willow tree (Salix sp.) by a pond and four on the sandstone underneath this willow tree (320/3) on our University campus at Zürich Irchel. All epiphytic samples comprised Trebouxia decolorans, all saxicolous samples T. arboricola as photobiont, as determined from sequences of the ITS region (Figs. 2, 3C). With fingerprinting techniques 36 genotypes were identified among the 38 epiphytic and two genotypes among the four saxicolous samples. Principal coordinates and cluster analysis showed a clear trend for isolates to group according to substratum.
Population 270: Teloschistes chrysophthalmus from La Gomera (Canary Islands)
The small population of Teloschistes chrysophthalmus from La Gomera, Canary Islands, was comprised of only four thalli growing on the same branch (Fig. 1B). With fingerprinting techniques, three distinctly different genotypes were found among the four isolates of Trebouxia gelatinosa Ahmadjian ex Archibald (Fig. 1C). Teloschistes chrysophthalmus is usually richly fertile but can also disperse vegetatively by means of the stiff marginal hairs at the tip of the fruticose thalli and at the apothecial margin, which break off very easily. They are predominantly fungal but contain algal cells in their basal part.
Analysis of molecular variance and cluster analysis
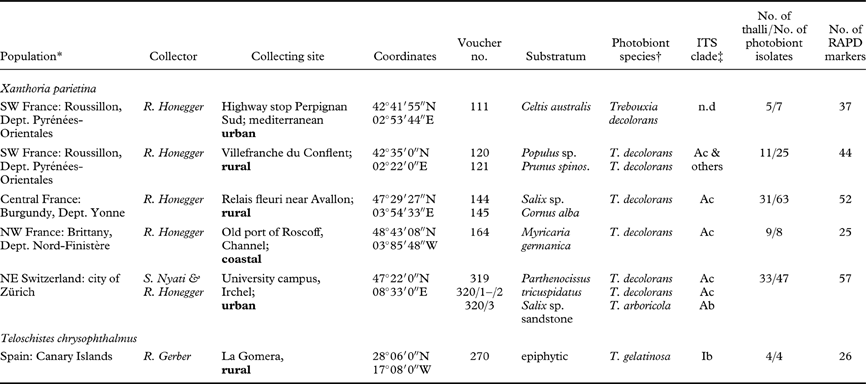
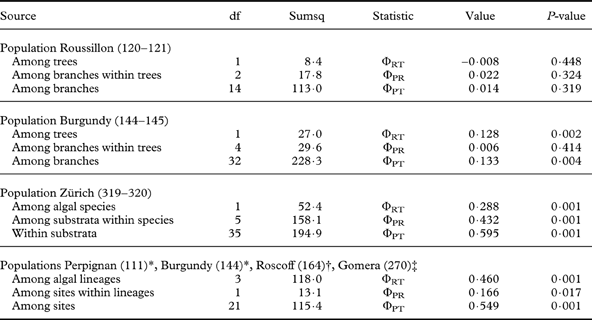
As expected, results from analysis of molecular variance indicated subdivision by algal lineages (Table 2). However, within algal lineages there was significant subdivision between populations or growth substrata, indicating that populations of the studied lichen fungi are structured. All studied sites except one (Roussillon) exhibited significant differentiation between lichen photobionts isolated from different phorophyte trees. Genetic differentiation among lineages of Trebouxia was high (ΦRT=0·28–0·46). Lower but significant differentiation was found between different sites (ΦPR=0·16). Isolates grouped by algal lineage and within algal lineages by sampling sites (Fig. 3D) or substrata (Fig. 3C) were in agreement with the cluster analysis as well as the analysis of molecular variance. The site which exhibited no differentiation between trees or branches in AMOVA (Roussillon) did not show any such structure in the cluster analysis either (Fig. 3A) but all other data sets showed some degree of population structure.
Table 2. Analysis of molecular variance based on RAPD data sets. The table gives the source of variance, the degrees of freedom (df), the sums of squares (Sumsq), the type and value of the Φ-statistic, and its P-value based on 1000 permutations.
* Trebouxia clade Ac.
† Trebouxia clade Ad.
‡ Teloschistes chrysophthalmus associated with Trebouxia gelatinosa and Trebouxia clade I2.
Discussion
The sexual lichen-forming ascomycete Xanthoria parietina associated with a diverse pool of T. decolorans strains in the sampling sites studied; saxicolous specimens associated with T. arboricola. Teloschistes chrysophthalmus had T. gelatinosa as a photobiont. As shown in the present study, RAPD-PCR is suitable for gaining valuable insights into the genetic diversity at the subspecific level in the green algal photobionts of lichen-forming fungi, provided that sterile cultured isolates are available. As it was technically not possible to combine all data from the present study into one large data set, the different populations were analysed separately.
A combined analysis was carried out on 28 photobiont isolates from 3 different X. parietina populations (photobiont T. decolorans) and a single T. chrysophthalmus population (photobiont T. gelatinosa). The combined analysis was conducted to test the suitability of the RAPD technique in differentiating algal genotypes originating from different X. parietina populations and differentiating Trebouxia species (Fig. 1). SAHN clustering and principal coordinates analysis (Fig. 3) clearly grouped isolates into separate clades (clades Ac, Ad and Ib), as was the case in ITS phylogenetic analysis. A combined analysis was also carried out to infer population subdivision in Trebouxia photobionts. AMOVA results indicated that there was little population subdivision at the local scale (among trees and among branches within a site, Table 2), a pattern similar to that found for other photobionts (Werth & Scheidegger Reference Werth and Scheidegger2012). For T. decolorans photobionts associated with the epiphytic lichen Ramalina menziesii, differentiation was found between photobionts sampled from different phorophyte species, implying a possible ecological specialization of photobiont strains (Werth & Sork Reference Werth and Sork2010). In the present RAPD data, significant genetic differentiation between photobionts collected from different phorophytes was found in one site (Avallon) but a second site showed no such trend even though the lichens had been collected from multiple phorophyte species (Roussillon).
As expected, there was substantial genetic differentiation between algal lineages (i.e. species of Trebouxia and clades of T. decolorans) and within a given algal lineage, and there was substantial differentiation between geographically distant sampling sites. Similar large-scale geographic trends have been found in various photobionts of lichen-forming ascomycetes (Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Wornik & Grube Reference Wornik and Grube2010; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Widmer et al. Reference Widmer, Dal Grande, Excoffier, Holderegger, Keller, Mikryukov and Scheidegger2012).
Each population of Xanthoria parietina and even the small population of Teloschistes chrysophthalmus included in this study, turned out to consist of many different algal genotypes. In only a few of the 114 samples were identical fingerprints found. Some of them grew side by side (two isolates from each of six thalli), the others slightly apart from each other (3×2 and 1×3 isolates). The lowest diversity was found in the maritime epiphytic population 164 with five Trebouxia genotypes among eight samples of Xanthoria parietina.
How can the surprisingly high genetic diversity among Trebouxia isolates within the different populations be explained? One possible explanation for this pattern is recombination, leading to an increased number of algal multilocus genotypes. Even if they occurred in low frequency, recombination events would have a pronounced effect on the number of multilocus genotypes in a population. Based on DNA sequencing data, recombination has been inferred for Trebouxia jamesii photobionts of Letharia spp. in western North America (Kroken & Taylor Reference Kroken and Taylor2000). In contrast, in an analysis based on microsatellite genotypes, no recombination was found for the green alga Dictyochloropsis reticulata, the photobiont of Lobaria pulmonaria (Dal Grande et al. Reference Dal Grande, Widmer, Wagner and Scheidegger2012). Sexual reproduction has neither been demonstrated in the genus Trebouxia nor in other unicellular lichen photobionts of the genera of Asterochloris Tscherm.-Woess, Coccomyxa Schmidle, Dictyochloropsis, Myrmecia Printz etc. among the Trebouxiophyceae, which are all assumed to be asexual haplonts (Friedl & Büdel Reference Friedl, Büdel and Nash1996). In the absence of sexual reproduction and recombination, genetic diversity results either from parasexual events, of which we have no knowledge in the algae concerned, or from mutations. Asexually reproducing organisms may accumulate a large number of mutations over time, as long as their genome is stable and no ‘house-keeping’ genes are negatively affected by mutations. Examples of ancient asexual haplonts are the arbuscular mycorrhizal fungi, which have accumulated numerous mutations over time so that their multinucleate mycelia are heterokaryotic, i.e. harbour nuclei belonging to a wide range of different genotypes (Hijri & Sanders Reference Hijri and Sanders2004). Moreover, an example for the accumulation of mutations in an asexual haplont exists in lichen photobionts: the analysis of microsatellite data from many populations of L. pulmonaria demonstrated that the clonal green algal photobiont Dictyochloropsis reticulata has accumulated a substantial amount of somatic mutations (Dal Grande et al. Reference Dal Grande, Widmer, Wagner and Scheidegger2012).
As X. parietina does not form symbiotic vegetative propagules but ascospores, it is assumed to re-lichenize at each reproductive cycle. The present findings suggest that there is not a pool of one to few compatible algal genotypes present at each site, which might be accepted by ascospore-derived germ tubes, but many different genotypes. Alternatively, the populations of X. parietina and their Trebouxia photobiont, as investigated in the present study, might be not true populations as seen, for example in flowering plants, but an assembly of many different combinations of the fungal and algal partners, which were brought to the site from different external sources. Xanthoria parietina forms neither soredia nor isidia but it does have options for vegetative dispersal in the symbiotic state. Thallus fragments are one possibility (Honegger Reference Honegger1996), lichenivorous invertebrates and their faecal pellets another one. Faecal pellets of the ever present oribatid mites, which feed on apothecia and thalli of X. parietina, grazing them down to the medullary layer, were shown to contain viable ascospores and Trebouxia cells (Meier et al. Reference Meier, Scherrer and Honegger2002). Migratory and other birds, passively carrying faecal pellets with them, were assumed to play a largely underestimated role in short and long distance dispersal of lichens (Meier et al. Reference Meier, Scherrer and Honegger2002).
A study using a single cell manipulator to isolate algae from lichen thalli pointed towards the homogeneity of algal strains within the thalli of two lichen species (Beck & Koop Reference Beck and Koop2001). Here, we found evidence for multiple algal strains in a thallus of X. parietina. The same result has also been reported for some other species, including Evernia mesomorpha, which frequently associates with different photobiont strains in the same thallus (Piercey-Normore Reference Piercey-Normore2006) and Parmotrema tinctorum (Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2011). Helms et al. (Reference Helms, Friedl, Rambold and Mayrhofer2001) found two different photobionts within single thalli of crustose Physciaceae (Rinodina astrocinerea, R. tunicata and Rinodinella controversa). According to the findings of Casano and co-workers (2011), Ramalina farinacea always associates with two species of Trebouxia but such a pattern may be rare. The co-occurrence of multiple photobiont strains in the same thallus could be due to photobiont switching or to the fusion of thalli during development (Mansournia et al. Reference Mansournia, Wu, Matsushita and Hogetsu2011). However, it cannot be excluded with certainty that in some cases, the multiple strains may represent epibionts. In particular PCR-based studies based on whole thallus DNA extracts are prone to amplify algal epibionts. We isolated the algae from within the lichen thallus picking algal cells from the apothecial margin. Hence it is not likely that the two photobiont strains found in one apothecium represent the thallus photobiont and an epibiont.
An analysis of the genetic diversity of the fungal partner might show whether the X. parietina populations investigated in this study are mixtures of genotypes from different sources. Xanthoria parietina was shown to be a self-fertile species, in which the progeny of meiosis is genetically identical with the mycelium of the mother thallus (Honegger et al. Reference Honegger, Zippler, Gansner and Scherrer2004a; Scherrer et al. Reference Scherrer, Zippler and Honegger2005). RAPD-fingerprinting of ascospore-derived sterile cultured isolates of some of the respective fungal partners has been conducted previously (Itten & Honegger Reference Itten and Honegger2010).
To conclude, this is the first study of its type where axenically isolated Trebouxia photobionts from several populations of Teloschistaceae lichens have been analyzed by the RAPD fingerprinting technique. We detected extensive genetic variation within and between algal lineages (e.g. T. decolorans clades), between substratum, and between geographically distant sampling sites. It will be stimulating to combine data sets on the fungal exhabitant and the algal inhabitant of lichen thalli to gain invaluable insights into population structure.
Our sincere thanks are due to Undine Zippler for excellent technical assistance; to Prof. Jakob Schneller for providing accessibility to NTSYS-PC; to Urs Landergott for stimulating discussions; to the Swiss National Science Foundation for generous financial support (grant Nr. 31-103860 to R. H.).