Introduction
Many extreme terrestrial habitats, especially those in the polar regions, are dominated by lichens. The poikilohydric nature of lichens and the production of UV-screening substances (e.g. quinones, xanthones and melanins) allow these organisms to thrive in areas that are hostile to other life forms. Green-algal lichens are the most successful species under these extreme conditions as they do not depend on the presence of liquid water for reactivation from their dry inactive state, in contrast to cyanobacterial lichens. This is why cyanobacterial lichens are completely absent in the Antarctic cold deserts (Lange et al. Reference Lange, Kilian and Ziegler1986; Kappen Reference Kappen2000).
‘Lecideoid’ lichens (Hertel Reference Hertel1984) are species described under the generic name Lecidea sensu Zahlbruckner (Reference Zahlbruckner1925) but not necessarily belonging to the genus in its strict sense. They are mainly characterized by a crustose thallus with green-algal photobionts, apothecia without algae in the exciple and colourless, aseptate ascospores. Most of the species are saxicolous and show a preference for polar and alpine habitats. Consequently, several lecideoid lichen species from the genera Carbonea, Lecanora, Lecidea and Lecidella appear in continental Antarctica (Hertel Reference Hertel2007; Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012), where they belong to the most abundant species (Hertel Reference Hertel2007; Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012).
The green-algal photobionts of lecideoid lichens have not been studied in detail so far, but the few available studies show that all belong to different species of the genus Trebouxia (Hildreth & Ahmadjian Reference Hildreth and Ahmadjian1981; Ettl & Gärtner Reference Ettl and Gärtner1995; Beck Reference Beck1999). Because of this, and their cosmopolitan distribution, they are ideal objects to study global distribution patterns of cold-adapted lichen photobionts.
Several studies on the mycobiont-photobiont interactions in lichens have shown that both green-algal and cyanobacterial lichens can switch photobionts (Nelsen & Gargas Reference Nelsen and Gargas2009; Otálora et al. Reference Otálora, Martínez, O'Brien, Molina, Aragón and Lutzoni2010; Wornik & Grube Reference Wornik and Grube2010; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011), even if the photobiont is transmitted vertically in the form of vegetative propagules (Nelsen & Gargas Reference Nelsen and Gargas2009; Wornik & Grube Reference Wornik and Grube2010). Lichens that are specific to certain photobiont species often associate with different genotypes, sometimes even within a single thallus (Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Helms Reference Helms2003; Blaha et al. Reference Blaha, Baloch and Grube2006; Guzow-Krzeminska Reference Guzow-Krzeminska2006; Piercey-Normore Reference Piercey-Normore2006; Casano et al. Reference Casano, del Campo, García-Breijo, Reig-Armiñana, Gasulla, del Hoyo, Guéra and Barreno2011). This ability to accept different algae as photobionts might be a survival strategy. Blaha et al. (Reference Blaha, Baloch and Grube2006) suggested that low photobiont specificity might extend the ecological range of lichens. The results of some recent studies suggest that ecological factors, especially climate, may have an impact on photobiont selection (Beck et al. Reference Beck, Kasalicky and Rambold2002; Yahr et al. Reference Yahr, Vilgalys and DePriest2006; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011; Peksa & Škaloud Reference Peksa and Škaloud2011). Helms (Reference Helms2003) demonstrated that the occurrence of certain Trebouxia clades correlates with the macroclimate, but the type of substratum (calcareous or siliceous rock, different tree species) seems also to be a major factor in photobiont choice (Helms Reference Helms2003; Werth & Sork Reference Werth and Sork2010). Since most lecideoid lichens in Antarctica grow on siliceous rock, substratum can probably not explain differences in photobiont occurrence and selection. The most important ecological factor to influence the photobiont selection of mycobionts can therefore be assumed to be the (micro-)climate. Furthermore, photobiont availability could be purely stochastic, in which case photobiont selection could be entirely defined by the specificity and preferences of the mycobiont. In general, Antarctic lichens are quite resistant to cold and arid conditions (Kappen & Valladares Reference Kappen, Valladares, Pugnaire and Valladares2007; Green Reference Green2009). Because the algal partner has to perform net photosynthesis under much lower temperatures than in most other biomes of the world, temperature has often been invoked as a key criterion for photobiont choice in Antarctic lichens (Kappen Reference Kappen1993; Green et al. Reference Green, Schroeter, Sancho, Pugnaire and Valladares1999; Pannewitz et al. Reference Pannewitz, Green, Schlensog, Seppelt, Sancho and Schroeter2006). An influence of temperature on Trebouxia photobionts was indeed revealed in several studies (e.g. Tschermak-Woess Reference Tschermak-Woess and Galun1988; Casano et al. Reference Casano, del Campo, García-Breijo, Reig-Armiñana, Gasulla, del Hoyo, Guéra and Barreno2011).
Systematic revisions of lichenized fungi mostly ignore the photobionts. As a result, the identity of the photobionts at species level is known only for a small fraction of all lichens described (Honegger Reference Honegger and Nash1996). Lecideoid lichens from Antarctica are no exception, the more so because these lichens are hardly accessible. This contribution is part of a comprehensive study on lecideoid lichens, mainly from continental Antarctica (Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012). Our major goals were: 1) to get a first overview on the genetic diversity of photobionts of Antarctic lecideoid lichens; 2) to identify algal clades with special ecological (mainly climatic) preferences; 3) to identify photobionts that occur exclusively in Antarctica and might be particularly well adapted to the cold and dry climate of the continental Antarctic.
Material and Methods
Collecting sites and material
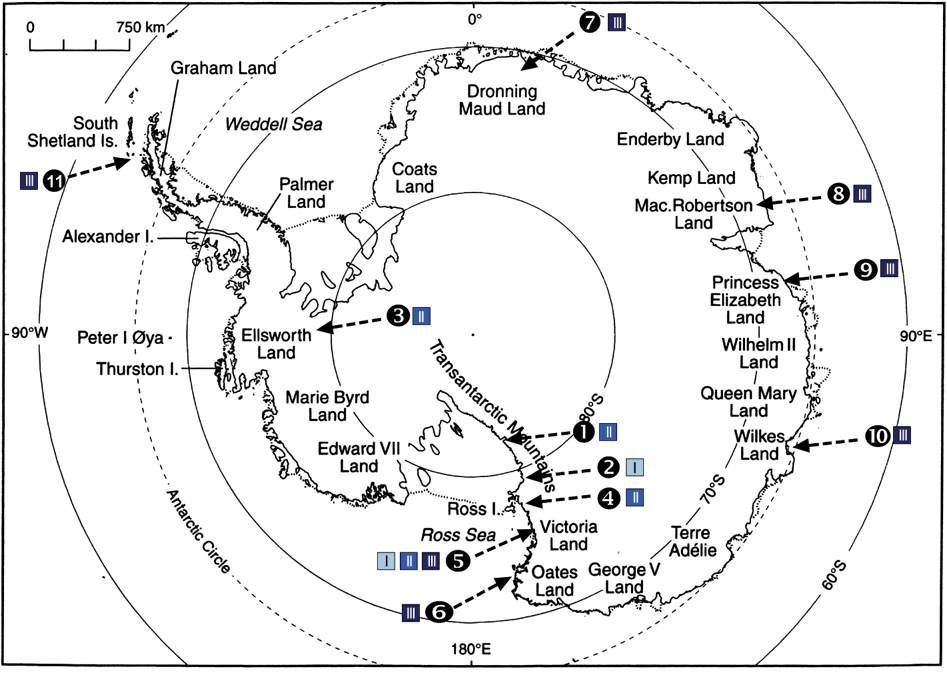
Saxicolous lecideoid lichens were collected in several regions of the Antarctic continent (Fig. 1, Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012). Terrestrial life is limited to ice-free areas that make up only 0·34% of Antarctica (Peat et al. Reference Peat, Clarke and Convey2007). Water availability is the main factor for lichen growth in the extreme cold deserts. The open sea and/or frequent fog are the main providers of humidity and have a dominant effect on the climatic conditions (Adams et al. Reference Adams, Bardgett, Ayres, Wall, Aislabie, Bamforth, Bargagli, Cary, Cavacini and Connell2006; Green et al. Reference Green, Schroeter, Sancho, Pugnaire and Valladares2007; Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012).
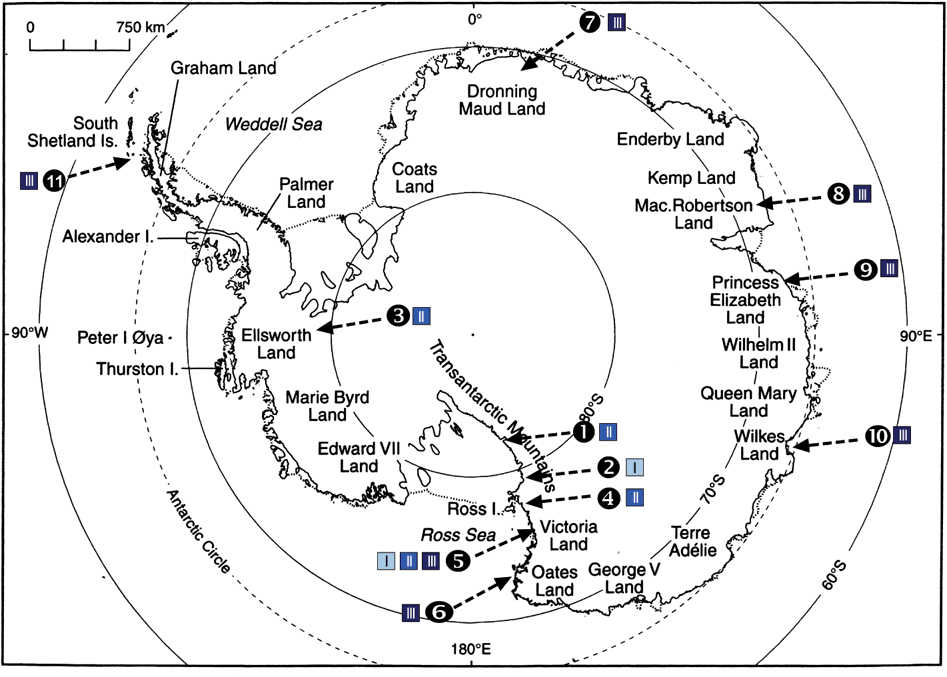
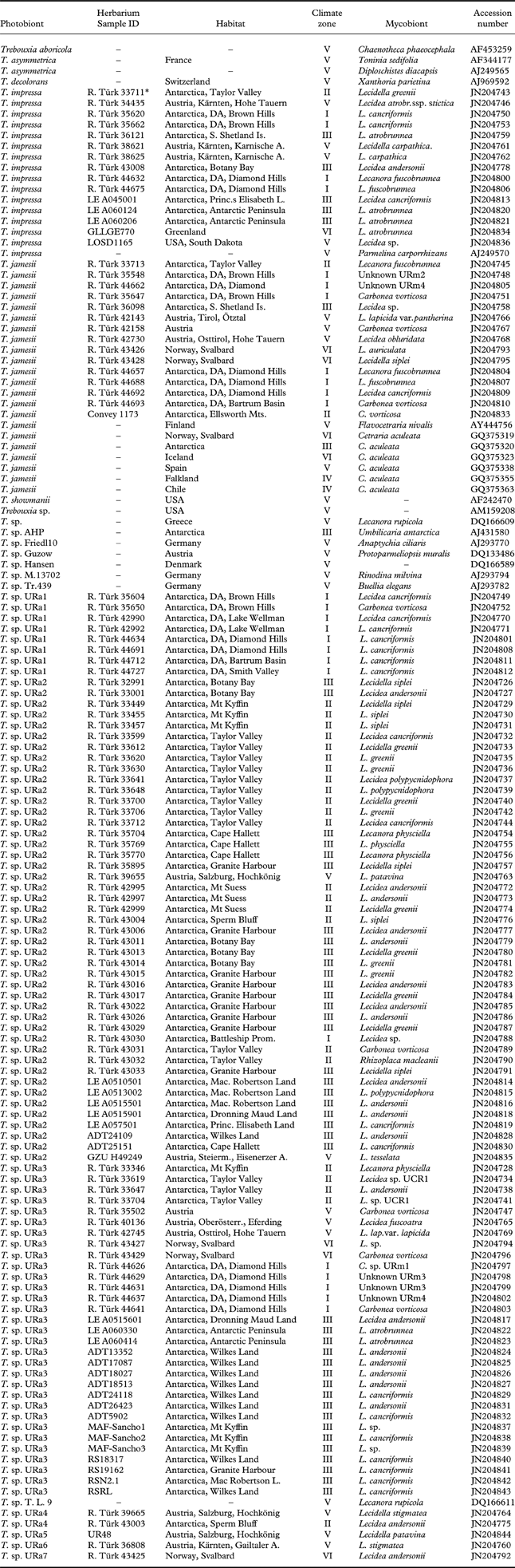
Fig. 1. Location of collecting sites in the maritime and continental Antarctica. Site numbers (ordered by latitude) are indicated together with the signs for the three habitat types (see text). 1) Mt Kyffin, 2) Darwin Area, 3) Ellsworth Land, 4) Taylor Valley, 5) Granite Harbour, Sperm Bluff, Battleship Promontory, 6) Cape Hallett, 7) Dronning Maud Land, 8) Mac. Robertson Land, 9) Princess Elisabeth Land, 10) Wilkes Land, 11) Antarctic Peninsula, South Shetland Islands. General map from Øvstedal & Lewis Smith (Reference Øvstedal and Lewis Smith2001). In colour online.
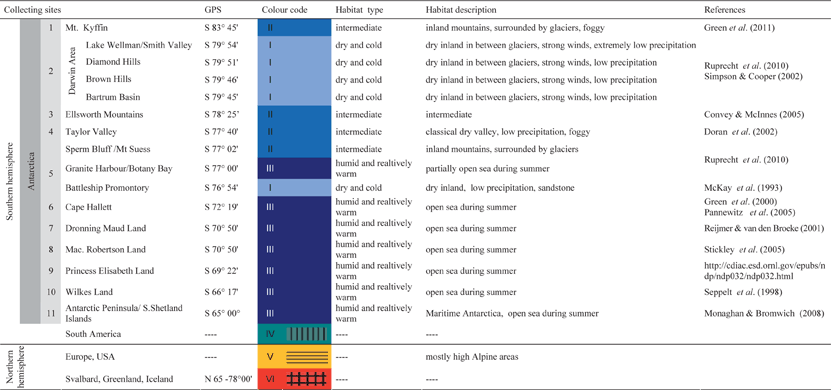
According to temperature and humidity, we roughly divided the snow-free areas into 3 different climatic regions (Table 1):
-
I. Inland sites with extremely low precipitation and strong, katabatic winds (dry and cold).
-
II. Inland sites subject to coastal air-masses and/ or frequent fog (intermediate).
-
III. Coastal sites with open sea during the summer (humid and relatively warm).
Table 1. Investigation Areas sorted by latitude; the six climate regions are coded in different colours. The grey shaded numbers of the particular regions are consistent with the numbers in Fig. 1. A short habitat description summarizes the most important varieties. In colour online
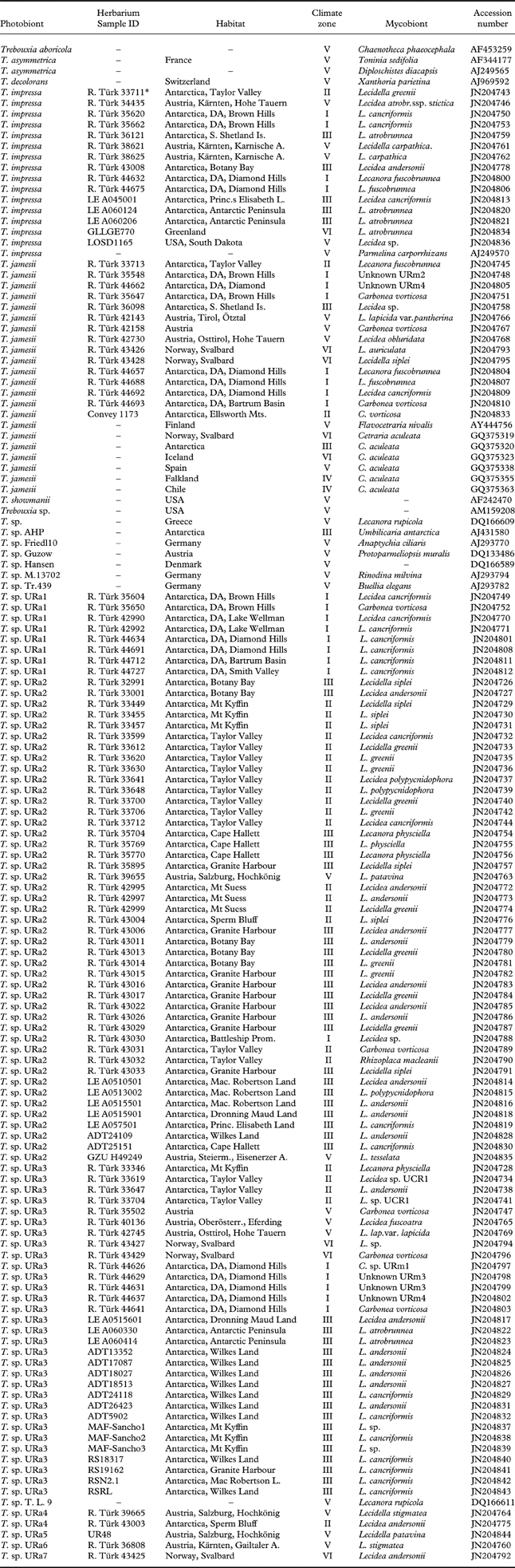
In total, we generated ITS sequences of 119 lecideoid lichens from eleven localities in the continental and maritime Antarctic (Fig. 1), and additional samples from the Alps and the Arctic (Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012). In addition, 22 Trebouxia sequences from GenBank were included in the dataset (Table 2) to infer the phylogenetic position of Antarctic photobionts within the genus Trebouxia. In order to identify already described species within our dataset we preferably included GenBank sequences from UTEX strains. Trebouxia samples from the Alps, Arctic regions and from South and North America were included in order to get information about intra-specific sequence variation and to see whether the species and haplotypes identified in Antarctic material also occur in other regions of the world. Information on the samples is summarized in Appendix 1.
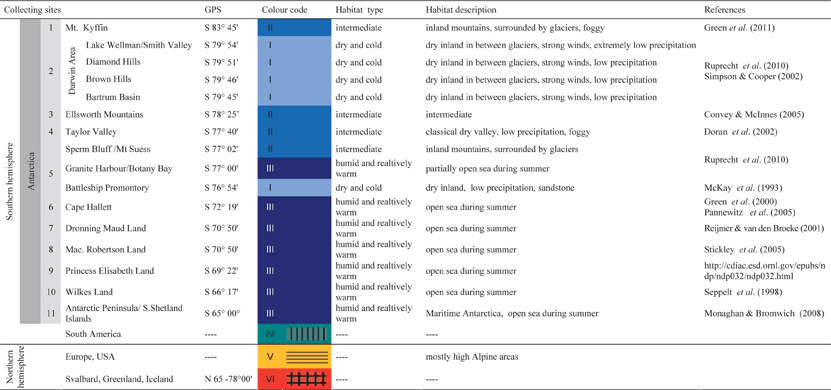
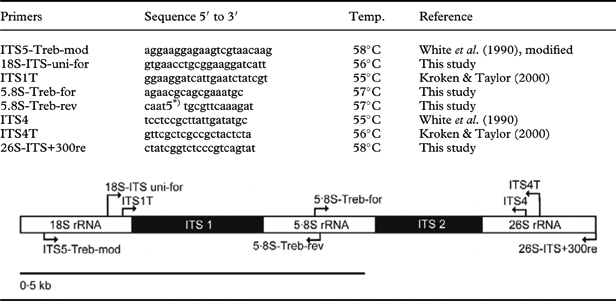
Table 2. List of primers used to amplify the internal transcribed spacer region rRNA (ITS) and approximate location of priming sites
* 5=inosine
DNA-amplification, purification, sequencing
Total DNA was extracted from the thallus or apothecia by using the DNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. The PCR mix contained 1 unit of Kapa HiFi polymerase (PeqLab), 0·2 nM of each of the four dNTPs, 0·3 µM of each primer and c. 1 ng genomic DNA. Nested PCRs were performed using GoTaq polymerase (0·5 units). The internal transcribed spacer region (ITS) of the photobionts' nuclear ribosomal DNA was amplified and sequenced with the primers described in Table 2. In cases where the PCR did not produce visible bands in the first reaction, we took 1 µl of this reaction as template for a nested PCR with internal primers. The most successful PCR protocol used the primers 18S-ITS-uni-for and ITS4T for the first PCR, and ITS1T and ITS4 for the nested PCR. PCRs using Kapa Hifi polymerase started with an initial denaturation of 98°C for 2 min, followed by 35 cycles at 98°C for 20 s, 56°C for 20 s and 72°C for 40 s. Cycling conditions for nested PCRs with GoTaq polymerase were as follows: initial denaturation at 95°C for 2 min, followed by 35 cycles at 95°C for 20 s, 55°C for 30 s and 72°C for 50 s. The PCR products were purified using the Qiaquick PCR purification kit (Qiagen) and re-dissolved in sterile distilled water. The purified PCR-products were labelled using the BigDye© Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Sequences were run on an ABI PRISM© 3700 DNA Analyzer (Applied Biosystems) using the PCR primers.
Phylogenetic analysis
Nuclear ITS sequences were assembled and edited using Geneious Pro 5.3.4 (www.geneious.com) and aligned with ClustalW (Thompson et al. 1994). The alignment was subsequently refined by using the MUSCLE algorithm implemented in the Geneious program. Poorly aligned positions and divergent regions were eliminated from the alignment using Gblocks 0.91b with default settings (Castresana Reference Castresana2000).
A nucleotide substitution model was chosen using Modeltest 3.7 (Posada & Crandall Reference Posada and Crandall1998). The Akaike information criterion selected the tranversional model (rAC=rCT≠rAG≠rAT≠rCG≠rGT, Prosada & Crandall Reference Posada and Crandall2001) including a discrete gamma distribution (TVM+G) as the optimal model. A maximum likelihood analysis (ML) was performed using the program Garli 0.96 (http://www.nescent.org/wg_garli/Main_Page) with the estimated TVM (0 1 2 3 1 4) + G model and default settings. A nonparametric bootstrap was used to assess robustness of clades, running 1000 pseudoreplicates.
Maximum parsimony (MP) analyses were performed using PAUP* (Swofford Reference Swofford2003). Heuristic searches with 1000 random taxon addition replicates were conducted with TBR branch swapping and MulTrees option in operation, and equally weighted characters and gaps treated as missing data. Bootstrapping was performed based on 1000 replicates with random sequence additions. Homoplasy levels were assessed by calculating consistency index (CI), retention index (RI), and rescaled consistency (RC) index from each parsimony search. For Bayesian tree inference a Markov Chain Monte Carlo (MCMC) procedure as implemented in the program MrBayes 3.1.2 was used (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001). The analyses were performed assuming the general time reversible model of nucleotide substitution including estimation of invariant sites and a discrete gamma distribution with six rate categories (GTR + I + Γ, Rodriguez et al. Reference Rodriguez, Oliver, Marin and Medina1990). A run with 3·5 million generations starting with a random tree and employing 4 simultaneous chains was executed. Every 100th tree was saved into a file. We plotted the log-likelihood scores of sample points against generation time using TRACER 1.0 (http://evolve.zoo.ox.ac.uk/software.html?id=tracer) to test whether stationarity was achieved by checking if the log-likelihood values of the sample points reached a stable equilibrium value (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001). Subsequently, the first 3500 trees were deleted as the ‘burn-in’ of the chain. A consensus topology with posterior probabilities for each clade was calculated from the remaining 31 500 trees.
Only clades that received bootstrap support equal or above 70% under parsimony and likelihood and posterior probabilities ≥0·95 were considered as strongly supported.
Haplotype networks
In order to visualize the haplotype diversity and the occurrence of haplotypes in different regions, we calculated a 95% parsimony probability haplotype network for the cosmopolitan species inferred from the phylogenetic tree, using TCS v1.21 (Clement et al. Reference Clement, Posada and Crandall2000) with a fixed connection limit at 15 steps.
Analysis of nucleotide polymorphism
DnaSP v5 (Librado & Rozas Reference Librado and Rozas2009) was used for calculating haplotype and nucleotide diversities for the Antarctic photobiont clades, photobionts in different regions of the Antarctic and photobionts found in the eight lichen species that were represented by more than four individuals. Nucleotide diversities were calculated using P-distances, excluding gaps and missing data. We used two-sided t-tests to assess whether differences in genetic diversities between different regions or clades were significant.
Results
The final data matrix for the molecular phylogeny comprised 141 OTUs with a length of 640 positions. Of the alignment positions, 226 were parsimony-informative. The MP analyses yielded 6195 equally parsimonious trees 490 steps long (CI=0·647, RI=0·953, RC=0·617).
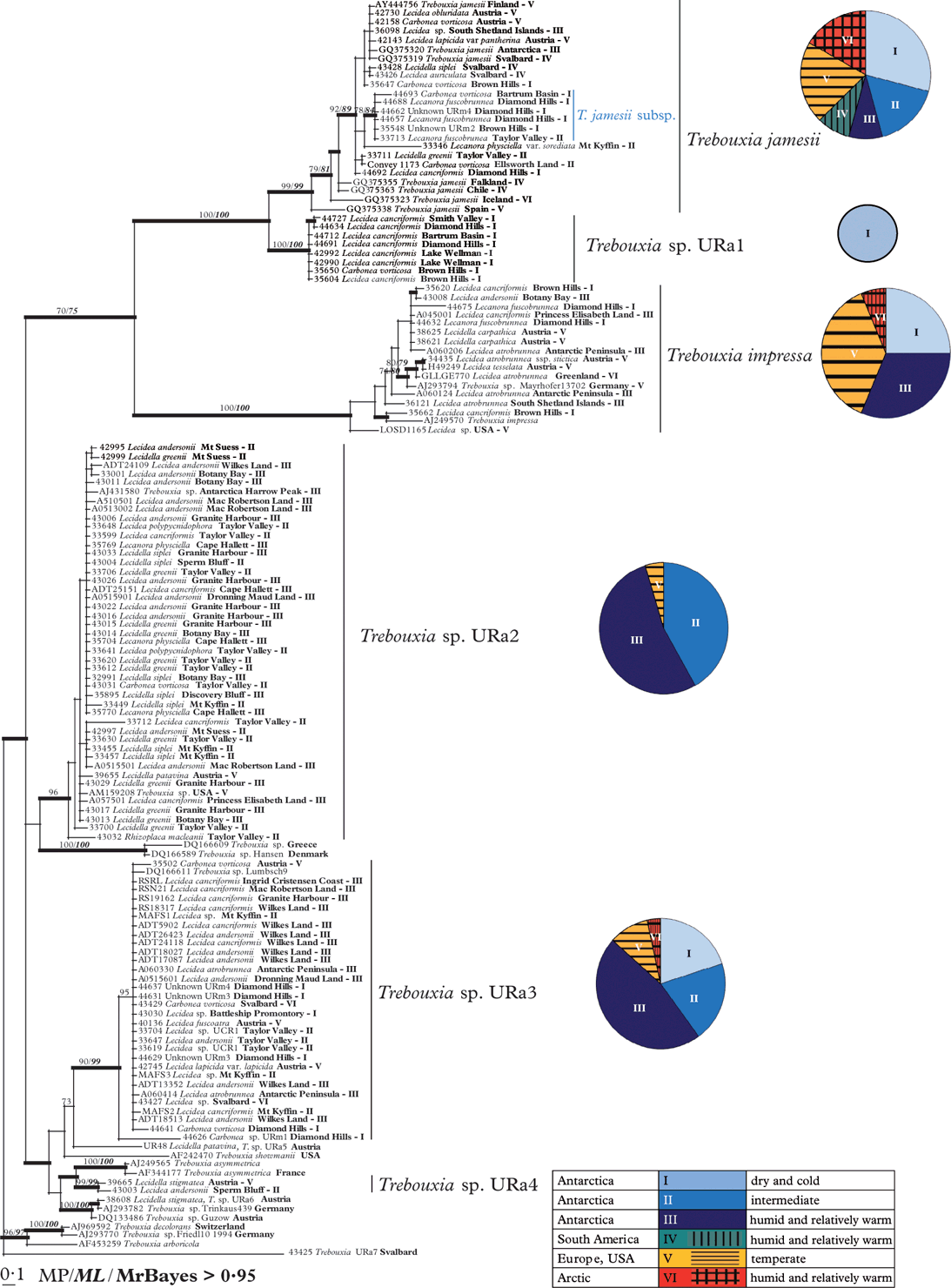
The ML and Bayesian analyses recovered the same well-supported clades as the MP analysis. The Bayesian consensus tree, with the support values of all three analyses, is shown in Fig. 2. Pie charts show the distribution of the members of each Trebouxia clade in different climate zones and habitat types (see also Table 1).
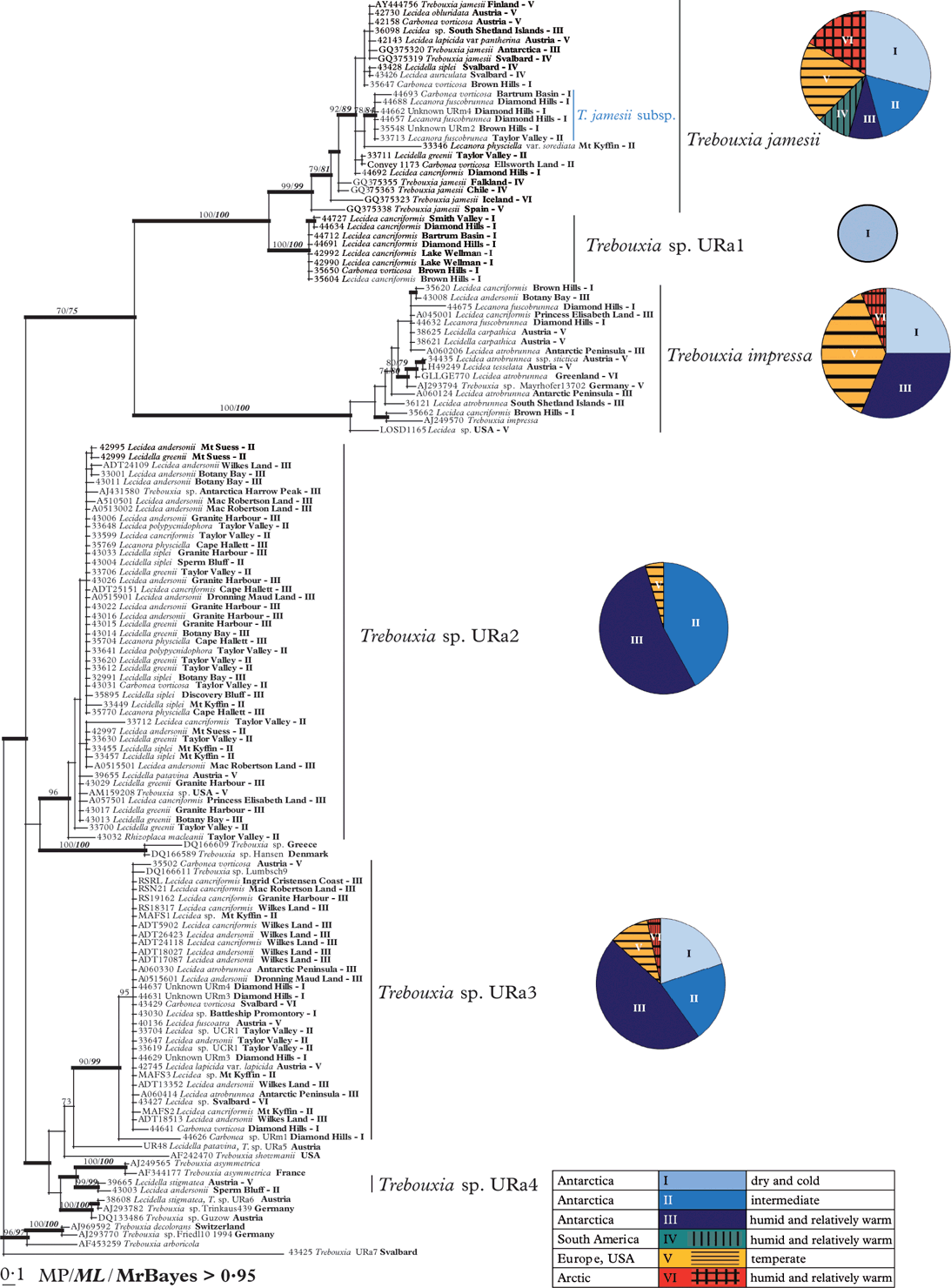
Fig. 2. Phylogeny of Antarctic Trebouxia species combined with samples from Austria, USA, the Arctic and downloaded sequences from Genbank. Herbarium numbers are combined with the species names of the mycobiont, the collecting place and the numbers of the habitat types. Antarctic Trebouxia species are labelled beside the respective clades. The diagrams beside the particular Trebouxia species show the distribution patterns of their occurrence in different climate zones. This Bayesian tree is based on a dataset of ITS sequences with >0·95 support and directly mapped bootstrap values with >70 support MP and ML analyses.
Phylogenetic analysis
The phylogenetic reconstruction revealed five strongly supported, monophyletic groups and one additional weakly supported clade of Trebouxia photobionts. The tree is rooted with T. decolorans, T. arboricola and two unidentified Trebouxia species from Germany and Svalbard. The crown group of the tree is formed by a well-supported clade including sequences of Trebouxia jamesii from Genbank and its equally well-supported sister group, here preliminarily called T. sp. URa1, which was only found in extremely cold and dry regions in Antarctica. The cosmopolitan and highly variable group of T. jamesii includes a well-supported sub-clade that seems to be restricted to dry regions of continental Antarctica, where it occurred in lichen species typical for these extreme habitats (Carbonea vorticosa, Lecanora fuscobrunnea, and two undetermined species).
The photobionts of clade URa1 are mainly restricted to Lecidea cancriformis, a dominant lichen species of the extremely dry and cold deserts.
The sister group relationship between T. jamesii and T. sp. URa1 was strongly supported in all analyses. Both clades are sister to a heterogeneous cosmopolitan group which includes Genbank accessions of T. impressa. Again, the relationship between these clades is well supported although not as strong as that between T. jamesii and T. sp. URa1.
The relationships among clades basal to this crown group are poorly resolved and supported, although two large and several smaller clades received high support values. The photobionts of the fourth clade Trebouxia sp.URa2 are associated with a wide range of lecideoid lichens (Fig. 2, Table 3). Representatives of this clade are not only found in the Antarctic but also in Europe and the USA. The species was not found in the extremely dry and cold continental Antarctic regions. This cluster is sister to two unnamed Genbank accessions from Greece and Denmark but the relationship lacks support.
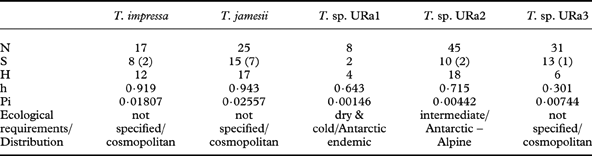
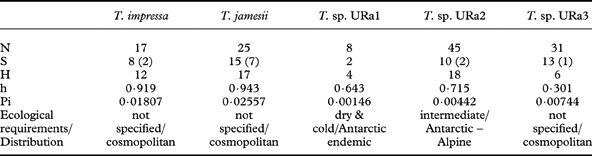
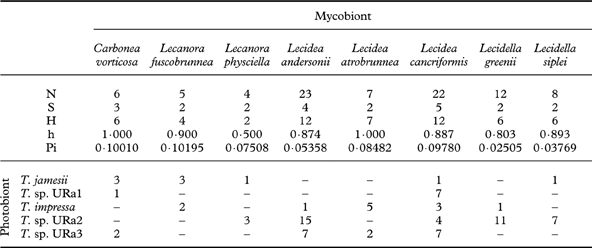
Table 3. Diversity statistics for the major clades of photobionts: number of sequences (N), associated mycobiont species (S) (numbers of GenBank accessions in brackets) and haplotypes (H), and diversities of haplotypes (h) and nucleotides (Pi).
The fifth group is also widely distributed and was preliminarily named Trebouxia sp. URa3. The voucher specimens were collected in temperate regions, as well as in coastal to dry and even extremely dry regions (Fig. 2).
A small group of well supported clades including T. asymmetrica and Trebouxia sp. URa4 is sister to T. sp. URa3, but the relationships between these clades are poorly resolved.
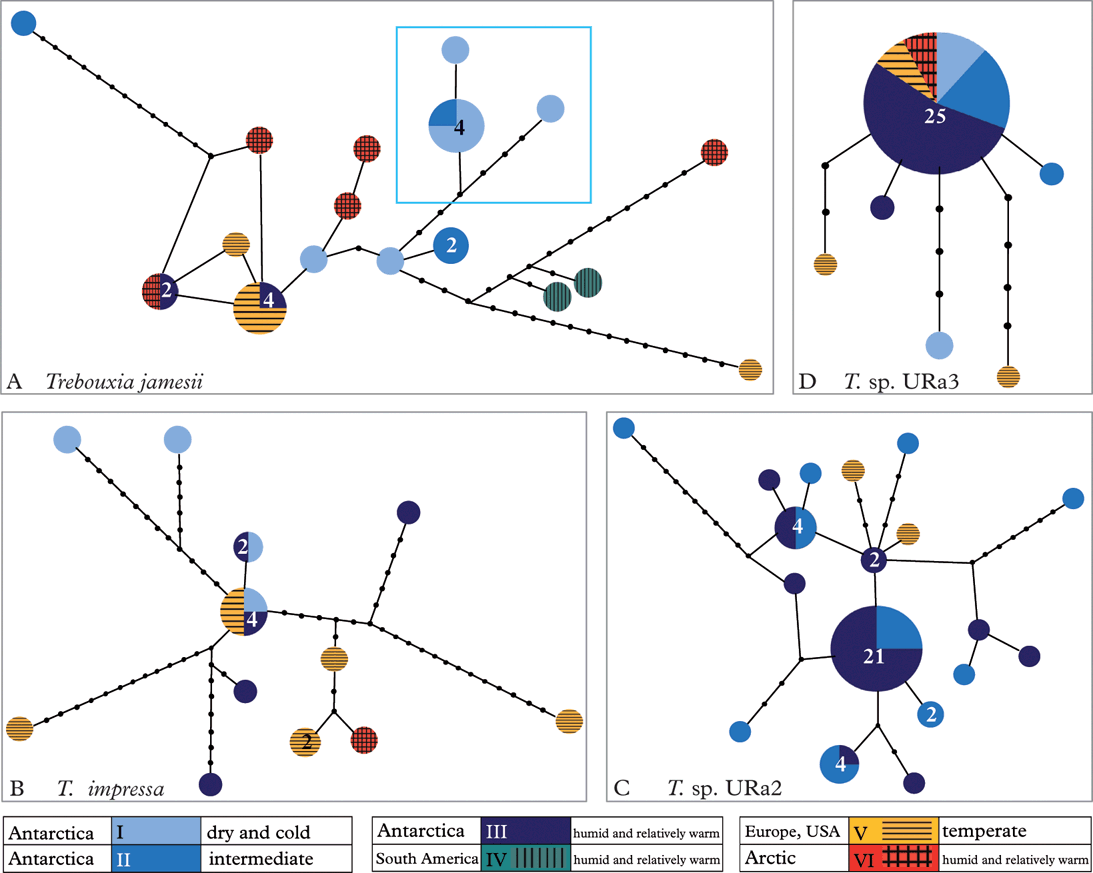
Haplotype networks
Figure 3 shows the haplotype networks (Clement et al. Reference Clement, Posada and Crandall2000) for Trebouxia jamesii, T. impressa, T. sp. URa2 and T. sp. URa3. We did not calculate a haplotype network for clade T. sp. URa1, because it consisted of only four haplotypes. The high diversity of T. jamesii (Fig. 3A) is indicated by a high number of haplotypes that are separated by many mutational steps. One of the haplotypes shows a bipolar distribution, while another was found in the maritime Antarctic and in alpine regions of the Northern Hemisphere. Six individuals belonging to three haplotypes form a clade that was only found in the cold and dry Antarctic deserts. The other haplotypes are scattered throughout the habitat types and geographic regions.
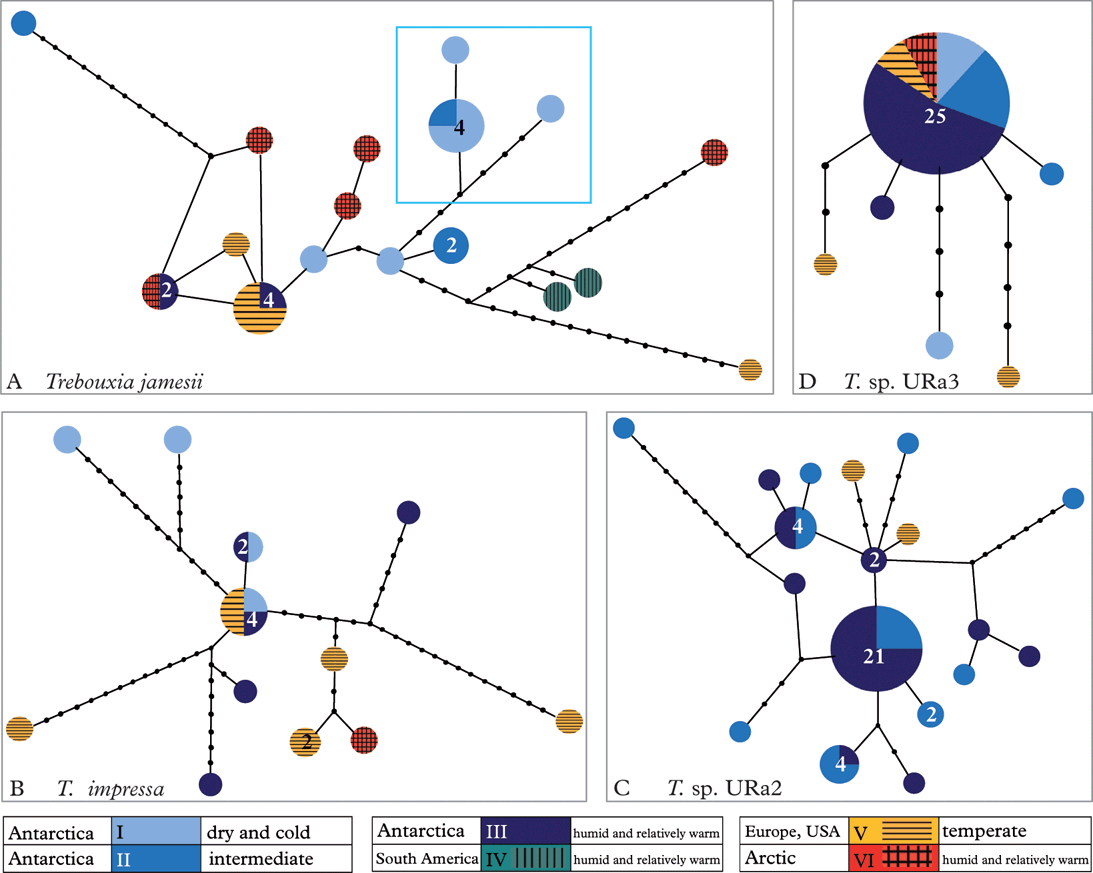
Fig. 3. Haplotype networks. A, Trebouxia jamesii with highlighted endemic sub-clade; B, T. impressa; C, Trebouxia sp. URa2; D, Trebouxia sp. URa3. Numbers of haplotypes ≥2 are indicated within the dots.
Trebouxia impressa (Fig. 3B) shows twelve haplotypes, again separated by several mutational steps. The central haplotype with four accessions occurs in humid and extremely dry Antarctic habitats, as well as in Alpine areas.
In contrast to these networks, T. sp. URa2 (Fig. 3C) consists of 13 relatively closely related haplotypes that were restricted to more humid Antarctic regions and also include two haplotypes from Austria and the USA. Twenty-five out of 31 individuals of T. sp. URa3 (Fig. 3D) belong to a single haplotype that was found in both polar regions and Alpine regions in Austria. Five haplotypes were each only represented by a single individual. One photobiont sequence of Carbonea sp. URm1 (44626, T. sp. URa3, see Fig. 2) was not included in the haplotype network, because it was separated from the other haplotypes by too many mutational steps.
Analysis of nucleotide polymorphism
Table 3 shows diversity statistics for the major clades of photobionts. Trebouxia jamesii is the most diverse of the five photobiont species recognized here. In our dataset, this photobiont also associates with the highest number of lecideoid lichen species (15). Trebouxia sp. URa2 and URa3 associate with 10 and 13 different lecideoid lichen species respectively, although they display a considerably lower haplotype and nucleotide diversity. Trebouxia impressa, although almost as diverse as T. jamesii, was found in only eight lichen species. Most pairwise comparisons between the clades are non-significant, but Trebouxia URa1 shows significantly lower diversity than T. impressa, T. jamesii and T. sp. URa2, and T. sp. URa2 is significantly less diverse than T. impressa (two-sided t-tests, data not shown).
Neither species composition nor genetic diversity and haplotype richness differ significantly between the three Antarctic climate zones (data not shown). Every habitat contains four photobiont species in different frequencies. The highest number of haplotypes was found in the coastal habitats (19), followed by the extremely dry habitats (15) and the foggy inland sites (14). The extremely dry habitats show the highest haplotype and nucleotide diversities (Table 4). Table 4 also shows that mycobiont species richness is considerably lower in dry interior sites than in intermediate and coastal sites.
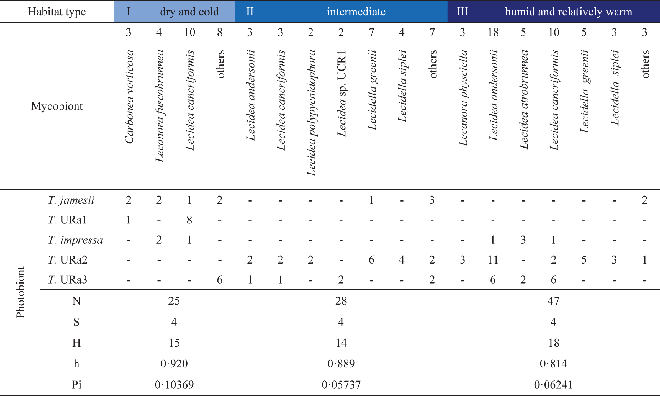
Table 4. Ecological characterization and diversity statistics of the three climate zones in Antarctica, calculated with dnaSP. Occurrence of lichen species (mycobiont) combined with the photobionts, Numbers of sequences (N), photobiont species (S) and haplotypes (H), and diversities of haplotypes (h) and nucleotides (Pi). In colour online
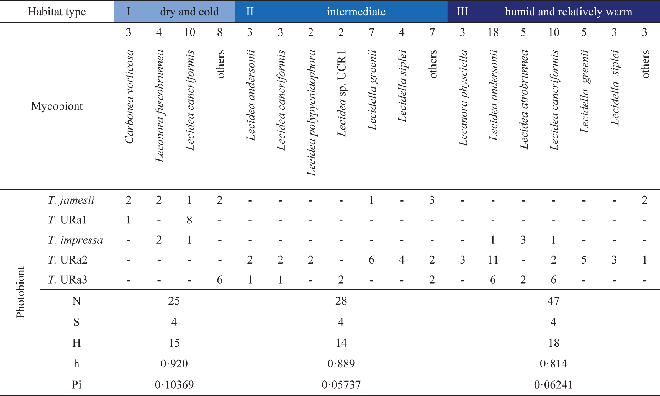
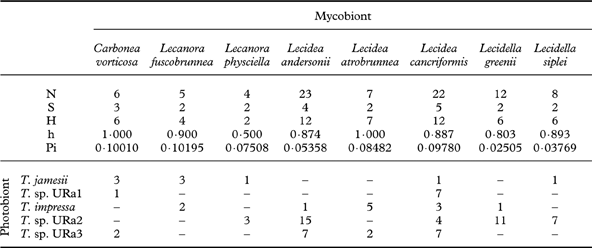
Eight lichen species were represented by more than four individuals in this phylogenetic study. Diversity statistics focusing on these eight investigated species of lecideoid lichens differ with regard to the number of associated photobiont species and also their haplotype diversity (Table 5). However, the number of photobiont species that were found to be associated with a lichen species only once is apparently correlated with the number of individuals investigated. With one notable exception, the haplotype diversities of photobionts do not differ significantly between mycobiont species. Regarding the photobiont diversity, Lecidea cancriformis, however, is significantly more diverse than L. andersonii (0·02>P>0·01), Lecidella siplei (0·02>P>0·01) and Lecidella greenii (0·005>P>0·001).
Table 5. Associations of mycobionts and photobionts: numbers of sequences (N), photobiont species (S) and haplotypes (H), and diversities of haplotypes (h) and nucleotides (Pi)
Discussion
In spite of decades of research on Antarctic lichens (see Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001), the lichen flora of this continent is still insufficiently known. While the physiological properties of Antarctic lichens and their photobionts have been studied intensely (Kappen Reference Kappen1993, Reference Kappen2000; Green et al. Reference Green, Schroeter, Sancho, Pugnaire and Valladares1999; Pannewitz et al. Reference Pannewitz, Green, Schlensog, Seppelt, Sancho and Schroeter2006; Barták et al. Reference Barták, Váczi, Hájek and Smykla2007 ), very little is known about the diversity and distribution of Antarctic lichen photobionts. In this study we therefore attempted to characterize the green-algal photobionts of a group of widely distributed crustose lichens, relating their occurrence with geographic origin and ecological differences of Antarctic ecosystems. Green algae of the genus Trebouxia are among the most common photobionts of lichens (Ettl & Gärtner Reference Ettl and Gärtner1995; Rambold et al. Reference Rambold, Friedl and Beck1998). Trebouxia impressa, T. jamesii and a few unidentified genetic lineages have previously been identified as photobionts of various Antarctic lichen species (Aoki et al. Reference Aoki, Nakano, Kanda and Deguchi1998; Romeike et al. Reference Romeike, Friedl, Helms and Ott2002; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011). In our analysis of lecideoid lichens, we could also identify these two photobionts in different lichen species (Fig. 2). In addition, we found three major clades (URa1, URa2, and URa3) that, based on ITS sequences, cannot be assigned at present to any described species of Trebouxia. Another clade (URa4) of Trebouxia present in Antarctica was detected in only two specimens, one Lecidella stigmatea from Austria and one Lecidea andersonii from the coastal continental Antarctic, and will not be discussed here.
The accessions of T. jamesii form a genetically diverse clade with several sub-clades (Figs 2 & 3A, Table 3). Lichens associated with T. jamesii are found in all Antarctic habitats investigated by us. The comparison with sequences from other parts of the world revealed that one ITS haplotype from the maritime Antarctic (including the South Shetland Islands) also occurs in Austria and Finland. This result is in line with those of Fernández-Mendoza et al. (Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011), who found one ITS and one GPD-haplotype of T. jamesii with a bipolar distribution. The wide geographic distribution of T. jamesii is related to its large genetic diversity, its occurrence as a photobiont of many lichens (see e.g. Kroken & Taylor Reference Kroken and Taylor2000; Blaha et al. Reference Blaha, Baloch and Grube2006; Piercey-Normore Reference Piercey-Normore2006; Hauck et al. Reference Hauck, Helms and Friedl2007; Fernández Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011) and its wide ecological amplitude. In the Antarctic, T. jamesii is accepted by a whole range of different species of Carbonea, Lecidea, Lecidella and Lecanora (Fig. 2, Table 5) and occurs in cold deserts as well as in humid maritime regions. The delimitation of T. jamesii and T. simplex (synonymized by Friedl Reference Friedl1989) is still far from clear and it is likely that more than one phylogenetic species is hidden behind these names. Hauck et al. (Reference Hauck, Helms and Friedl2007), for example, found an apparently undescribed sister clade to T. simplex and T. jamesii f. angustilobata, which they preliminarily named T. hypogymniae. A closer look at the phylogenetic tree in Fig. 2 shows one sub-clade that was exclusively found in the extreme cold Antarctic deserts, and that is firmly embedded in the T. jamesii clade. All members of this sub-clade were collected in habitat types I and II (Table 1, Figs 2 & 3) and therefore seem to be well adapted to the extreme Antarctic climate. Extended sampling and additional molecular markers may eventually reveal that this is a cryptic species endemic to Antarctica.
The presence of undescribed lineages of Trebouxia in Antarctica is further supported by clade T. sp. URa1, a lineage that is preferentially associated with Lecidea cancriformis. None of the identified Trebouxia accessions in GenBank matched closely with our sequences in this clade. As in the case of the T. jamesii sub-clade, the fact that this clade appears restricted to the extremely dry and cold deserts suggests that it represents a new species that may be adapted to this hostile environment. Its preferred mycobiont, L. cancriformis, is in turn quite unselective and accepts almost all Trebouxia species detected in our study. Low photobiont selectivity has previously been reported from Antarctic lichens (Wirtz et al. Reference Wirtz, Lumbsch, Green, Türk, Pintado, Sancho and Schroeter2003) and explained by selection against photobiont-specific mycobionts and harsh environmental conditions. The fact that the unspecific L. cancriformis is one of the most dominant species in the extreme cold deserts and occurs in the entire continental Antarctic (Ruprecht et al. Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2010, Reference Ruprecht, Lumbsch, Brunauer, Green and Türk2012) further supports this interpretation.
A wide distribution of T. impressa was previously reported in the literature (Ettl & Gärtner Reference Ettl and Gärtner1995; Aoki et al. Reference Aoki, Nakano, Kanda and Deguchi1998; Romeike et al. Reference Romeike, Friedl, Helms and Ott2002). However, the other two unnamed lineages of Trebouxia in our dataset are more widely distributed. Trebouxia URa3 is almost as widely distributed as T. jamesii. T. sp. URa2 was also found in temperate areas (Austria and USA, Fig. 3C) but does not occur in cold desert regions. Specimens with this algal clade were all collected in coastal or coastal-influenced Antarctic habitats (Table 1, Figs 2 & 3). Trebouxia impressa and members of both undescribed clades form associations with almost all of the lichens sampled, although T. sp. URa2 was not found with Carbonea vorticosa and Lecanora fuscobrunnea, which prefer extremely dry climates (Table 5).
Perhaps the most surprising result of our study concerns the diversity of photobionts in different climatic regions of the Antarctic. Haplotype and nucleotide diversity of the photobionts show no significant difference among the three Antarctic climate zones (Table 4). Species composition differs slightly but there is no ‘typical’ species pattern in any of the three climatic zones. This result is counterintuitive because the harsh environmental conditions have often been postulated to exert strong selective pressure on Antarctic organisms (Kappen Reference Kappen1993; Broady & Weinstein Reference Broady and Weinstein1998; Domaschke et al. Reference Domaschke, Fernández Mendoza, García, Martín and Printzen2012), a force expected to limit genetic diversity. In the case of the lichen photobionts studied here, there is no evidence of reduced levels of genetic diversity due to selective pressure in more extreme cold desert regions. Figure 3C even indicates that haplotypes of T. sp. URa2 found in the humid, coastal regions are genetically more similar than haplotypes from the dryer regions, although the same overall number of haplotypes was found in both regions. T. sp. URa3 is genetically much more uniform and preferably found in the more humid Antarctic areas, but at least haplotype numbers do not differ among the three climatic regions. Domaschke et al. (Reference Domaschke, Fernández Mendoza, García, Martín and Printzen2012) reported significantly lower levels of genetic diversity and haplotype richness in Antarctic populations of Cetraria aculeata and offered two different explanations: demographic bottlenecks through colonization of the Antarctic or selection for a few well-adapted haplotypes. The data presented here indicates that demographic factors may play the more important role in shaping the genetic diversity of Antarctic lichens and their photobionts.
None of the mycobionts of 12 different lecideoid and 2 sterile Antarctic lichens studied here are specific to a single photobiont species or lineage, although Lecidella greenii and L. siplei are preferably associated with Trebouxia sp. URa2. The sampling areas of both species contain a pool of several other photobionts (Fig. 2; Appendix I). This supports the findings of other researchers that true selectivity for a particular photobiont species is found in only a few lichens (Otálora et al. Reference Otálora, Martínez, O'Brien, Molina, Aragón and Lutzoni2010). The major factor shaping photobiont-mycobiont associations seems to be the local availability of acceptable photobionts (Beck et al. Reference Beck, Friedl and Rambold1998; Kroken & Taylor Reference Kroken and Taylor2000; Piercey-Normore & DePriest Reference Piercey-Normore and DePriest2001; Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martín and Printzen2011). Our finding that Lecidea cancriformis associates with the highest number of photobionts (Table 5) can thus be explained in two different ways. Firstly, L. cancriformis is the most widespread lichen in our sample and may therefore simply have a better chance of encountering different photobionts. This may, for example, explain the contrast to L. andersonii which is mainly restricted to coastal influenced – dry habitats and shows slightly lower photobiont diversity than L. cancriformis. Carbonea vorticosa, a lichen with a preference for cold and dry habitats, also associates with fewer Trebouxia clades than L. cancriformis, but this may be a sampling artefact. Secondly, the harsh environmental conditions in dry parts of the continental Antarctic may enforce lower photobiont specificity, by allowing more unspecific lichens such as L. cancriformis to colonize more different habitats (Wirtz et al. Reference Wirtz, Lumbsch, Green, Türk, Pintado, Sancho and Schroeter2003). Our dataset does not allow testing of these hypotheses. It is interesting to note, however, that Lecidella greenii and L. siplei, which are largely restricted to the coastal humid to dry climate zones (II & III), were with a single exception only associated with Trebouxia URa2, although there is a pool of several Trebouxia species available in the area. If a larger sample supports this observation, it would indicate that photobiont specificity causes restricted occurrence and not vice versa.
We are grateful to H. T. Lumbsch (Chicago) and R. Türk (Salzburg) for advice and support. Selina Becker (Frankfurt) is thanked for technical assistance and Mikhail Andreev (St. Petersburg) and Rod Seppelt (Tasmania) for providing additional samples from other regions of continental Antarctica.
This study was financially supported by the research funding programme ‘LOEWE – Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz’ of Hesse's Ministry of Higher Education, Research, and the Arts. Antarctica New Zealand is thanked for logistic support and Shulamit Gordon for coordinating LGP, to which this research contributed. Waikato University is thanked for financial support. This manuscript is an output of the FRST-funded IPY Research Programme Understanding, valuing and protecting Antarctica's unique terrestrial ecosystems: Predicting biocomplexity in Dry Valley ecosystems located at Biological Sciences and ICTAR (International Centre for Terrestrial Antarctic research) at Waikato University.
Appendix 1. Trebouxia samples used in this study, with information on collecting localities, climate zones, voucher specimens, mycobionts, and Genbank accession numbers.
* hb.Türk (SZU)