Introduction
Wirth & Vězda (Reference Wirth and Vězda1972) introduced the crustose genus Fuscidea V. Wirth & Vězda for species with a brown or grey areolate thallus, conspicuous brown prothallus and lecideine, brown to black-brown apothecia with asci of the Fuscidea-type containing eight, simple or 1-septate, mostly ellipsoid, sometimes medially constricted ascospores. The genus comprises c. 40 saxicolous and corticolous species, occurring on acidic substrata worldwide, mostly in areas with cool and maritime climates. Two corticolous, sorediate species, Fuscidea lightfootii (Sm.) Coppins & P. James and F. pusilla Tønsberg, are similar in thallus morphology and chemistry (see e.g. Gilbert et al. Reference Gilbert, Purvis, Skjolddal and Tønsberg2009). The commonly accepted distinguishing features are the presence (F. lightfootii) or absence (F. pusilla) of apothecia (Kantvilas Reference Kantvilas2001; Gilbert et al. Reference Gilbert, Purvis, Skjolddal and Tønsberg2009) and their geographical distribution (Tønsberg & Johnsen Reference Tønsberg and Johnsen2008).
Fuscidea lightfootii, described from the north of Ireland by Smith & Sowerby (Reference Smith and Sowerby1805), is usually fertile and has a thallus morphology that varies in colour, shape and size of the areoles, and the degree of soredia production. Its currently accepted distribution range includes Western Europe (Kalb & Hafellner Reference Kalb and Hafellner1992; Tønsberg & Johnsen Reference Tønsberg and Johnsen2008), Yunnan, China (www.tropicallichens.net), Brazil (Aptroot Reference Aptroot2002) and Tasmania (Kantvilas Reference Kantvilas2001, Reference Kantvilas2004). It is not known from North America (Tønsberg Reference Tønsberg2002; Fryday Reference Fryday2008).
Fuscidea pusilla, described from Norway by Tønsberg (Reference Tønsberg1992), is characterized as a small (less than 1 cm), sterile, sorediate crust occurring in colonies forming a mosaic of more or less confluent thalli. It occurs in Europe, in areas with continental as well as oceanic climates, and North America (Tønsberg Reference Tønsberg1993; Fryday Reference Fryday2008; Lendemer Reference Lendemer2011).
Fuscidea lightfootii and F. pusilla have been regarded as impossible to distinguish when sterile. They may qualify as cryptic species that are morphologically identical but genetically distinct from one another (see e.g. Crespo & Pérez-Ortega Reference Crespo and Pérez-Ortega2009). Tønsberg & Johnsen (Reference Tønsberg and Johnsen2008) suggested that F. lightfootii and F. pusilla may be conspecific and Gilbert et al. (Reference Gilbert, Purvis, Skjolddal and Tønsberg2009) proposed F. pusilla as a morph of F. lightfootii that forms small, sterile rosettes. Tønsberg & Johnsen (Reference Tønsberg and Johnsen2008), Gilbert et al. (Reference Gilbert, Purvis, Skjolddal and Tønsberg2009) and Lendemer (Reference Lendemer2011) recommended a taxonomic treatment of these species using molecular methods. Genes from two genomes of ribosomal DNA (i.e. mitochondrial and nuclear) may be sufficient for species delimitation (see e.g. Spribille et al. Reference Spribille, Klug and Mayrhofer2011; Bendiksby & Timdal Reference Bendiksby and Timdal2013; Resl et al. Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016).
Bylin et al. (Reference Bylin, Arnerup, Högberg and Thor2007) investigated the taxonomic position of the family Fuscideaceae by studying seven different species of Fuscidea, including one specimen of F. lightfootii and two of F. pusilla. Their results showed that both species were located in the Fuscidea-group with high support in a maximum parsimony analysis and, within this clade, they were located in two separate subgroups.
Several papers deal with the phylogenetic relationships between sterile, sorediate and fertile taxa, so-called species pairs, to test if they are conspecific. For example, Spribille et al. (Reference Spribille, Klug and Mayrhofer2011) investigated the taxonomy of the often sterile Mycoblastus alpinus (Fr.) Kernst. and the mostly fertile M. affinis (Schaer.) T. Schauer. Resl et al. (Reference Resl, Mayrhofer, Clayden, Spribille, Thor, Tønsberg and Sheard2016) studied the Rinodina degeliana Coppins (sorediate)/R. subpariata (Nyl.) Zahlbr. (esorediate, fertile) species complex. In these two studies, the species of interest were shown to be conspecific. In contrast, Bendiksby et al. (Reference Bendiksby, Haugan, Spribille and Timdal2015) showed that sterile, sorediate specimens of the Calvitimela aglea complex were two distinct lineages, impossible to distinguish morphologically but differentiated in chemistry and ecology.
The hypothesis for this study was that F. lightfootii and F. pusilla are conspecific (Tønsberg & Johnsen Reference Tønsberg and Johnsen2008). The objective was to clarify the interspecific relationship between F. lightfootii and F. pusilla using ITS, LSU and mtSSU rDNA.
Materials and Methods
Taxon sampling
The material for this study came from herbarium collections in BG, HO and MSC, and from recently collected material from Norway, the USA (Alaska), Czech Republic, Great Britain, Ireland and Poland. Specimens collected by the authors were deposited in BG. The specimens are listed in Table 1. All specimens were subjected to thin-layer chromatography (TLC) according to the method described by Culberson & Kristinsson (Reference Culberson and Kristinsson1970), Culberson (Reference Culberson1972) and Menlove (Reference Menlove1974). All three solvents (A, B′ and C) were used; glass plates and solvent C were used for the detection of fatty acids.
Table 1 List of voucher specimens used in the phylogenetic analysis of Fuscidea lightfootii and F. pusilla with their GenBank Accession numbers (see Fig. 1)

DNA extraction, PCR amplification and sequencing
DNA was extracted from apothecia (fertile specimens) or soredia (sterile specimens) of Fuscidea lightfootii and F. pusilla using the DNeasy Plant Mini Kit (Qiagen). Primers for amplification were as follows: 1) ITS, ITS1f (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee and Taylor1990), 2) LSU, nuLSU-155-5′ (Döring et al. Reference Döring, Clerc, Grube and Wedin2000) and nuLSU-1125-3′ (Vilgalys & Hester Reference Vilgalys and Hester1990), and 3) mtSSU, mtSSU1 and mtSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The PCR mixture consisted of 1× GeneAmp® PCR Buffer II (Applied Biosystems), 2·5 µM MgCl2 (Applied Biosystems), 20 µM dNTPs (Promega), 0·6 µM of each primer, 0·036U AmpliTaq® DNA Polymerase (Applied Biosystems), 5·0 µl of genomic DNA extract and distilled water to a total volume of 25 µl.
Thermal cycling parameters for the PCR reaction were as follows. For ITS, initial denaturation at 94 °C for 5 min, followed by 40 cycles starting with denaturation at 94 °C for 30 s, annealing with a 63–58 °C touchdown procedure decreasing 1 °C per cycle, ending at 57 °C for 30 s, 72 °C for 1 min 45 s, and a final elongation at 72 °C for 10 min. For LSU, initial denaturation at 94 °C for 5 min, followed by 40 cycles starting with denaturation at 94 °C for 30 s, annealing at 58–55 °C for 30 s, and polymerization at 72 °C for 1 min 45 s decreasing 1 °C per cycle for the first 6 cycles, and a final elongation at 72 °C for 10 min. For mtSSU, initial denaturation at 94 °C for 5 min, followed by 40 cycles starting with denaturation at 94 °C for 30 s, touchdown of the annealing temperature, decreasing from 62–56 °C for the first 6 cycles ending at 56 °C for 30 s, polymerization at 72 °C for 1 min 45 s, and a final elongation at 72 °C for 10 min.
PCR products were visualized on a 1% RedGel-stained agarose gel under UV light and purified using Exo-Sap-IT (GE Heathcare). Amplification primers were used for direct sequencing using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems) and run on an ABI Prism 3700 XL DNA Analyzer (Applied Biosystems) at the DNA Sequencing Laboratory, University of Bergen, Norway. Sequences were assembled using SeqMan II, version 4.05 (DNASTAR). GenBank Accession numbers are given in Table 1.
Phylogenetic analyses
MUSCLE (Edgar 2004 Reference Edgara , Reference Edgarb ) implemented in Geneious version 8.1.8 (Biomatters Ltd.) was used to align sequences, with the 65% similarity option (Gap penalty=14·5, Gaps extension penalty=5), followed by manual adjustment. Ambiguous positions were manually removed from the alignment prior to the analyses. Ropalospora lugubris (Sommerf.) Poelt was used as an outgroup.
A concatenated data set of ITS1, 5.8S, ITS2, LSU and mtSSU was used to study the interspecific relationships between F. lightfootii and F. pusilla. Because of differences in substitution rates among ITS1, 5.8S and ITS2, it was decided to treat these as separate partitions with individual substitution rates. The best-fit substitution models for individual fragments were identified by a likelihood ratio test (Huelsenbeck & Crandall Reference Huelsenbeck and Crandall1997) incorporated in the software jModelTest version 2.1.7 (Posada Reference Posada2008). The best-fit models with the lowest AIC scores were chosen for analyses (Table 2).
Table 2 Best-fit models calculated for individual and concatenated data sets. The number of parsimony-informative and conservative sites are given

Individual trees were inspected for conflicts on nodes with values >70%, using the results from the maximum likelihood analysis. The analyses were performed under the same settings as described below. One significant conflict between Maronea A. Massal. and the clade containing F. pusilla and F. verruciformis Mas. Inoue was detected in the LSU tree. We did not exclude any taxa and thus combined all data matrices in one final concatenated alignment.
The phylogenetic analysis of the concatenated data set was performed with Bayesian Inference using Markov chain Monte Carlo (MCMC) as implemented in MrBayes version 3.2.1 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003). Two parallel runs of MCMC, each with four chains, starting from a random tree and using the default temperature of 0·2, were performed for six million generations. Gaps were treated as a fifth character state. Trees were sampled every 10th generation, including branch lengths. To test whether the MCMC chains had converged, the average standard deviation of split frequencies (ASDSF) of two parallel runs was monitored. The generations before the ASDSF had reached 0·01 were deleted as burn-in. A 50% majority-rule consensus tree was constructed from 540 000 trees and visualized in Geneious. Branches were considered significantly supported when posterior probabilities were≥0·95.
The concatenated data set was used for the ML tree reconstruction and the branch support calculation in the program RAxML version 7.2.8 alpha (Stamatakis Reference Stamatakis2014) implemented in Geneious. Bootstrapping was carried out on 1000 replicates under the GTR+I+G model. Only clades with bootstrap values >70% were considered to be significant. The PTP (Poisson Tree Processes) model (Zhang et al. Reference Zhang, Kapli, Pavlidis and Stamatakis2013) was run for species delimitation in the ML tree based on the concatenated data set. The default options were applied and 200 000 MCMC generations were used, with the outgroup removed. The species tree was plotted using the PhyloMap visualisation (Zhang et al. Reference Zhang, Mamlouk, Martinetz, Chang, Wang and Hilgenfeld2011).
Results
The final aligned concatenated data set comprised 11 taxa with 2283 characters, of which 1694 were constant and 359 parsimony-informative. There were 89 sequences newly generated. The Bayesian 50% majority-rule consensus (BI) tree, average branch lengths and posterior probabilities of branches for all specimens are given in Fig. 1. The average −ln likelihood of the tree was 8585·33 and the final ASDFS was 0·0031 at termination.
The bootstrap supports of the ML analysis were added to the BI consensus tree (Fig. 1). The incongruences between the BI and ML trees are indicated by an open circle in Fig. 1. The individual and final alignments, together with the resulting BI and ML trees of the concatenated data set, were deposited in treebase.org (ID: 21993). The resulting BI and ML trees showed that the species of interest were grouped in two distinctly supported clades.
Within the Fuscidea lightfootii-clade (PP=1·0/ML=100%), some genetic differentiation was shown but not corresponding to any geographical or ecological traits. Sequences of sterile and fertile F. lightfootii were almost identical since their pairwise identity was 99·9%. The sequences of sterile and fertile F. pusilla were clustered in one robust clade supported by PP=0·99 and ML=97% and their pairwise identity was also 99·9%.
The BI and ML supports from the PTP model of the concatenated data set showed that the two species are distinct (data not shown). In the plot reconstructed by PhyloMap, the first axis explained 83·35% of variance and the second axis explained 8·43% (see Fig. 2). Fuscidea lightfootii and F. pusilla, the species in question, were well separated from each other and occurred on different branches.
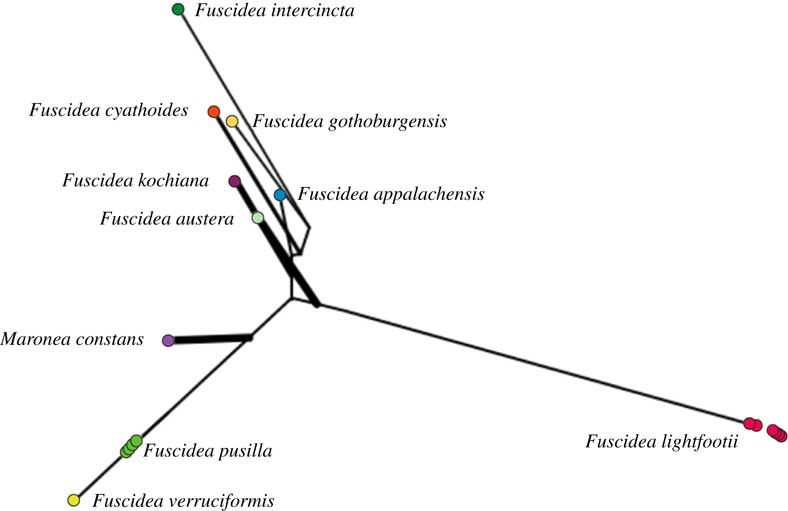
Fig. 2 PhyloMap visualization of the PTP (Poisson Tree Processes) model run for species delimitation of the ML tree based on the concatenated data set for Fuscidea. 200 000 MCMC generations were used, with the outgroup removed. In colour online.
Taxonomy
Anatomical and morphological measurements are given as (smallest value–)mean(–largest value) (n=the number of measurements). We refrained from cutting sections from more than two apothecia of Fuscidea pusilla since they are very rare.
Fuscidea lightfootii (Sm.) Coppins & P. James
Lichenologist 10: 201 (1978).—Lichen lightfootii Sm., in Sowerby, English Botany 21: tab. 1451 (1805); type: N. Ireland [in the protologue: “north of Ireland”], R. Scott (BM—lectotype selected by Coppins & James (Reference Coppins and James1978)); UK, Scotland: East Lothian, V.C. 82: Lammermuir Hills, Gifford, Hopes Reservoir, willow carr beside stream, 55°51′N, 2°43′W, alt. 260 m, on mature Salix, 30.10.2010, A. M. Fryday 9387 and B. J. Coppins (MSC0050473—epitype, designated here).

Fig. 3 A–D, Fuscidea lightfootii; A, fertile (A. M. Fryday 9387 and B. J. Coppins in MSC— epitype; B, fertile (T. Tønsberg 47026, BG-L-100387); C, asci and ascospores (same specimen as in B); D, sterile (J. I. Johnsen, BG-L-99466). E–H, Fuscidea pusilla; E, sterile (T. Tønsberg 40953, BG-L-96938—epitype); F, fertile (MRDS 118546 in hb. M. R. D. Seaward); G, asci and ascospores (same specimen as in F); H, sterile (T. Tønsberg 46018, BG-L-98963). Scales: A, D & F=0·5 cm; B=1 mm; C & G=20 µm; E & H=1 cm. Photographs: A, B, D–F, H & K by K. Abel.
Thallus crustose, green and brown, sometimes only green (herbarium material whitish, greyish green, occasionally tinged with brown), up to 0·9 mm thick, forming rosettes to a few cm diam. on Alnus sp. and Salix sp., but on Betula sp. a mosaic of small, thin thalli, areolate, becoming contiguous and confluent with other thalli forming larger patches, sorediate. Areoles convex, sometimes strongly convex, at first esorediate, green to pale brown and up to 0·13 mm diam., later usually sorediate and up to 0·15 mm diam., often becoming confluent. Soralia bursting from the apices of the areoles, green, often becoming confluent. Soredia green with a brown tinge, farinose, (12–)26(–31) μm diam.; consoredia (43–)44(–55) μm diam. Medulla up to 0·25 mm, I−, with crystals (in polarized light). Prothallus brownish or whitish, visible between the areoles and along the thallus margin. Photobiont Apatococcus F. Brand (Zahradníková et al. Reference Zahradníková, Andersen, Tønsberg and Beck2017), individual cells ≤24 μm diam.; walls ≤1·2 μm thick.
Apothecia ≤0·9 mm, rounded, often crenate; margin brown, thin, 0·05 mm, hyphae in section with narrow cells; disc black, mostly flat, occasionally convex or concave. Epithecium brown; hymenium brownish, 48–96 μm deep; hypothecium hyaline, ≤30 μm deep. Paraphyses (2·0–)2·6(–5·0) μm wide; tips brown, enlarged, to (3–)4(–5) μm. Asci clavate, of the Fuscidea-type, (24·0–)44·5(–60·0)×(6–)9(–13) μm. Ascospores simple, or occasionally 1-septate, colourless, elliptical and with median constrictions, (6–)9(–12)×(2·5–)4·0(–5·0) μm (n=50).
Pycnidia not observed.
Chemistry. Divaricatic acid. Spot tests: K−, C−, KC−, Pd−, UV+ bluish white (thallus).
Distribution and ecology. Fuscidea lightfootii is corticolous on branches and twigs, rarely trunks, of Salix caprea (38% of the total specimens sequenced), S. aurita (25%), Alnus glutinosa (19%), Betula spp. (6%) and Prunus (6%). It has also been found on worked timber. Revised (sequenced) material is from Great Britain and Rogaland in SW Norway; it would seem to be a species of oceanic climate. The list of species examined can be found in Appendix A (see Supplementary Material, available online).
Notes. As the likelihood of successful DNA amplification of the type specimen from 1805 is very low, a successfully sequenced specimen was designated as the epitype, following Article 9 of the International Code of Botanical Nomenclature (McNeill et al. Reference McNeill, Barrie, Buck, Demoulin, Greuter, Hawksworth, Herendeen, Knapp, Marhold and Prado2012).
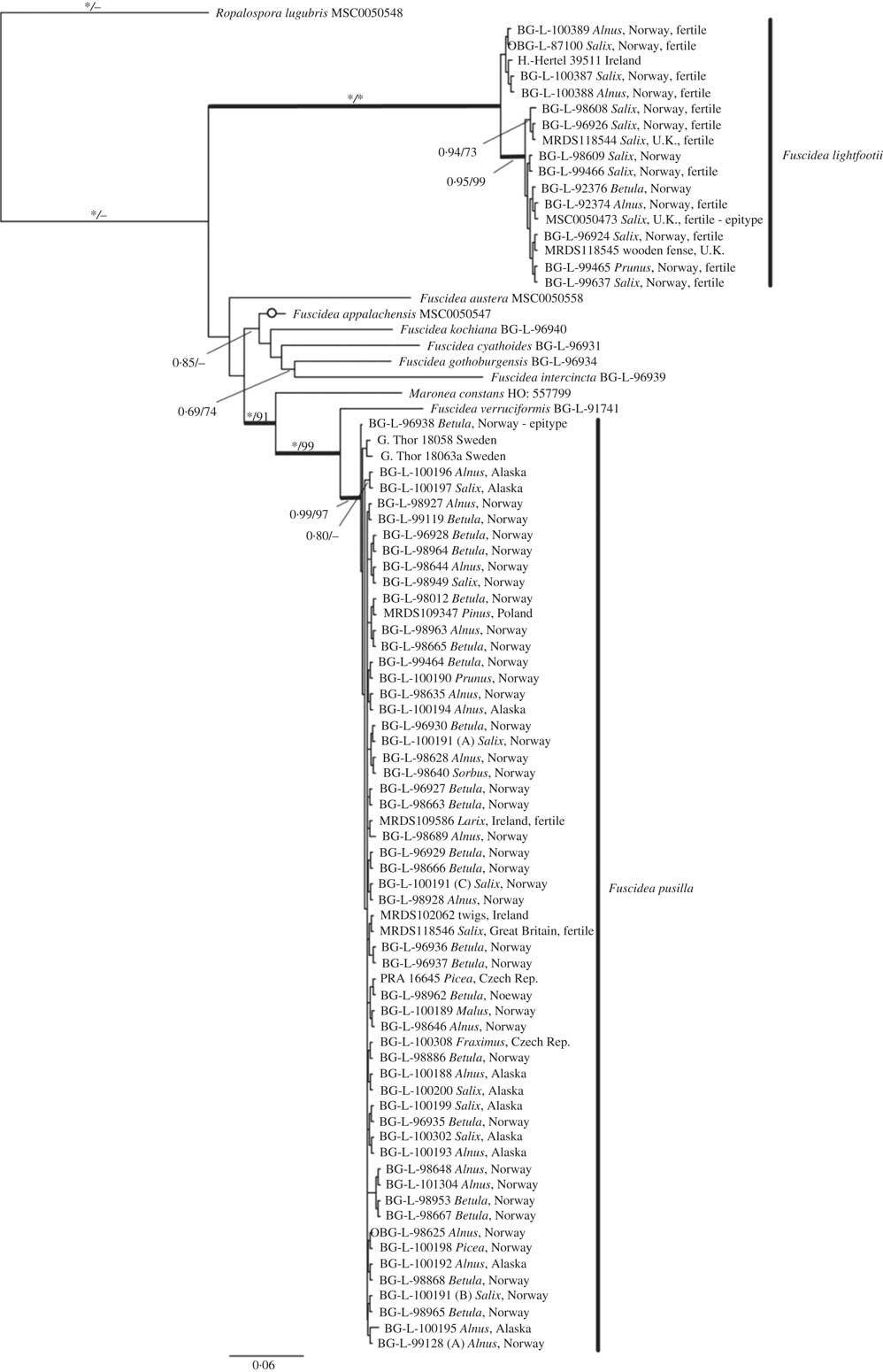
Fig. 1 Phylogenetic relationships of Fuscidea lightfootii and F. pusilla displayed as a 50% majority-rule consensus tree of a B/MCMC analysis based on ITS, LSU and mtSSU sequences (–ln=8585·33). Posterior probabilities (PP)/bootstrap support (BS) values are displayed above the branches. PP=1·0 and BS=100% indicated by an asterisk. Fertile specimens are indicated. Thick branches indicate well-supported clades. Open circles on a branch denote an incongruent topology with the ML tree. Sequences from Fuscidea specimens downloaded from GenBank lack information regarding type of phorotype and reproductive stage.
Fuscidea pusilla Tønsberg
Sommerfeltia 14: 138 (1992); type: Norway, Hedmark: Åmot, between Åset and Bechsminne [“Åset-Bechsminne”], along State Road 3, UTM grid ref.: 32W PN 2674 (1917 II) [c. 61·0805°N, 11·3359°E], alt. 240 m, on Betula pubescens/pendula (roadside tree), 6 August 1983, T. Tønsberg 8041 (BG-L-22659—holotype [vidi]; E, UPS—isotypes); Norway, Hedmark: Åmot, along and just W of State Road 3, between Åset and Bechsminne, 61°05·08′N, 11°21·07′E, alt. 240–250 m, on trunk of young Betula on east-facing, steep, unstable slope near gravel pit and 20 m from busy road, 4 June 2011, T. Tønsberg 40953 (BG-L-96938—epitype, designated here).
Thallus crustose, ≤0·32 mm thick, greyish green to green (in herbarium greyish green to green), usually forming small rosettes up to 10 mm diam. on Betula spp., sparingly sorediate in patches; up to 2 cm on Alnus incana. Areoles discrete, convex, up to 0·3 mm diam., easy to squash, developing beneath and penetrating through the uppermost layer of bark, becoming dissolved into soredia, especially at the thallus centre. Soralia green to pale yellowish with brown tinge, bursting from the apices of the areoles, irregular, becoming confluent. Soredia mostly farinose (10–)12(–14) μm diam.; consoredia (36·0–)40·5(–45·0) μm diam. Medulla ≤0·2 mm) or indistinct or absent, I−; crystals present. Prothallus distinct, pale to dark brown, visible between the areoles, sometimes ramifying the thallus. Photobiont Apatococcus fuscideae A. Beck & Zahradn., having globose to broadly ellipsoid cells dividing by binary fission (Zahradníková et al. Reference Zahradníková, Andersen, Tønsberg and Beck2017); individual cells (12–)19(–36) μm diam.; walls≤2 μm thick.
Apothecia sessile, constricted at base, roundish, up to 0·9 mm diam., dark grey-brown to black; margin paler or concolorous with disc, flexuose; rim of hyphae with elongated cells, thin, 0·04 mm. Disc black, mostly flat, occasionally convex or concave. Epithecium brown; hymenium brownish, ≤100 μm; hypothecium hyaline, ≤15 μm. Paraphyses (1·5–)2·0(–3·5) μm wide; tips enlarged, brown, (3–)4(–6) μm. Asci clavate, of the Fuscidea-type, (30–)35(–40)×(8·0–)8·5(–11·0) μm. Ascospores simple, colourless, elliptical, medially constricted (6–)8(–10)×(2·5–)3·0(–4·5) μm (n=18).
Pycnidia not observed.
Chemistry. Divaricatic acid. Spot tests: K−, C−, KC−, Pd−, UV+ blue-white (soralia).
Distribution and ecology. Based on the sequenced material only, F. pusilla is a corticolous species occurring in continental as well as oceanic climates at altitudes ranging from about sea-level to 800 m. Its presently known distribution includes Central Europe, Great Britain, Ireland, Norway and the USA (Alaska). It has been collected mainly on Betula spp. (38% of the specimens sequenced), Alnus incana (31%), Salix caprea (13%) and Picea abies (3·6%), and occasionally (less than 2%) on other phorophytes such as Alnus viridis, Fraxinus excelsior, Larix sp., Malus domestica, Prunus sp. and Sorbus aucuparia (see Table 1). Most of the specimens from Betula were collected on young trees with flaking bark. The list of species examined is provided in Appendix A (see Supplementary Material, available online).
Discussion
The resulting BI and ML trees demonstrate that Fuscidea lightfootii and F. pusilla are grouped in two clearly supported clades and that they are phylogenetically distinct. The hypothesis that they are conspecific, mentioned by Tønsberg & Johnsen (Reference Tønsberg and Johnsen2008) and suggested by Gilbert et al. (Reference Gilbert, Purvis, Skjolddal and Tønsberg2009), is therefore rejected. Our result agrees with Bylin et al. (Reference Bylin, Arnerup, Högberg and Thor2007), where F. lightfootii and F. pusilla appeared in different groups.
Based on the material studied here, F. lightfootii and F. pusilla differ in the size of their thalli, the species reaching a few cm in diameter and up to 10 mm in diameter, respectively. This difference is probably due to differences in phorophyte bark structure and uneven specimen sampling. For F. lightfootii, most collections are from Salix (63% of the total specimens sequenced) and Alnus (19%), while F. pusilla has most frequently been collected on Betula (38%) and Alnus (33%). The small size of the F. pusilla thalli is apparently due to the bark of young Betula trees being an unstable substratum where the uppermost, colourless layer tends to peel away. In one collection of F. lightfootii (J. I. Johnsen, BG-L-92376) from the trunk of Betula (see Fig. 4), the thalli form a mosaic of small, thin rosettes similar to those typical for F. pusilla when growing on this phorophyte. When growing on Alnus and Salix, F. pusilla thalli are thicker and may exceed 2 cm in diameter, for example T. Tønsberg 44828 (BG-L-98635) and T. Tønsberg 44774 (BG-L-100191). In the material from those phorophytes, there is no difference in size between thalli of F. lightfootii and F. pusilla.

Fig. 4 Fuscidea lightfootii, sterile (J. I. Johnsen, BG-L-92376) resembling F. pusilla (Rogaland, Norway). Scale=1 cm. Photograph by K. Abel.
The genetic variation within F. lightfootii and F. pusilla is very low. Only a small number of haplotypes are recognized within each of the two species and no geographical trends are found (data not shown).
Two specimens, originally identified as F. lightfootii based on the presence of apothecia, have been proved to represent fertile specimens of F. pusilla. These are from Larix in Ireland (MRDS 109586) and from Salix in Great Britain (MRDS 118546). Fertile specimens of F. pusilla have not previously been reported (e.g. Tønsberg Reference Tønsberg1992; Gilbert et al. Reference Gilbert, Purvis, Skjolddal and Tønsberg2009). Its apothecia appear to be morphologically and anatomically rather similar to those of F. lightfootii. In the present study, the asci of F. pusilla appear to be smaller (mean length=35 μm) than those of F. lightfootii (mean length=44·5 μm). As we refrained from making sections from more than two apothecia of F. pusilla, further data are needed to test this difference statistically.
The photobionts in Fuscidea have been identified as two distinct species of Apatococcus F. Brand (Zahradníková et al. Reference Zahradníková, Andersen, Tønsberg and Beck2017). Apatococcus fuscideae, characterized by a reticulate chloroplast, is the photobiont in most species of Fuscidea, including F. pusilla. Fuscidea lightfootii, on the other hand, is associated with a different species of Apatococcus, still undescribed. We do not know if it is possible to distinguish between these two photobionts using non-molecular methods such as cultivation or by examination in squash preparations of lichen thalli. According to Friedl & Büdel (Reference Friedl and Büdel2008), the chloroplast morphology and the life cycle of green algae in lichen thalli may differ from conspecific, free-living specimens.
We consider F. lightfootii and F. pusilla to represent cryptic species as it is apparently not possible to identify a specimen to one or the other species based on morphological methods alone. Of the two species, F. pusilla appears to have the broadest ecological range, occurring in both continental and oceanic areas. In the British Isles and SW Norway, F. lightfootii and F. pusilla are sympatric (see Figs 5 & 6).
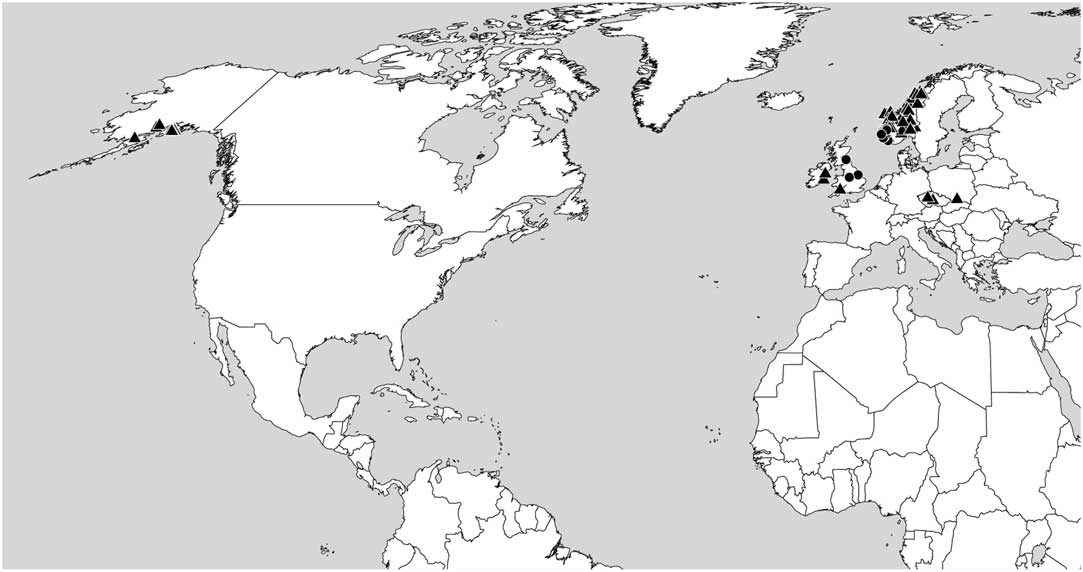
Fig. 5 Distribution of Fuscidea lightfootii (circles) and F. pusilla (triangles) based on the specimens cited and sequenced.
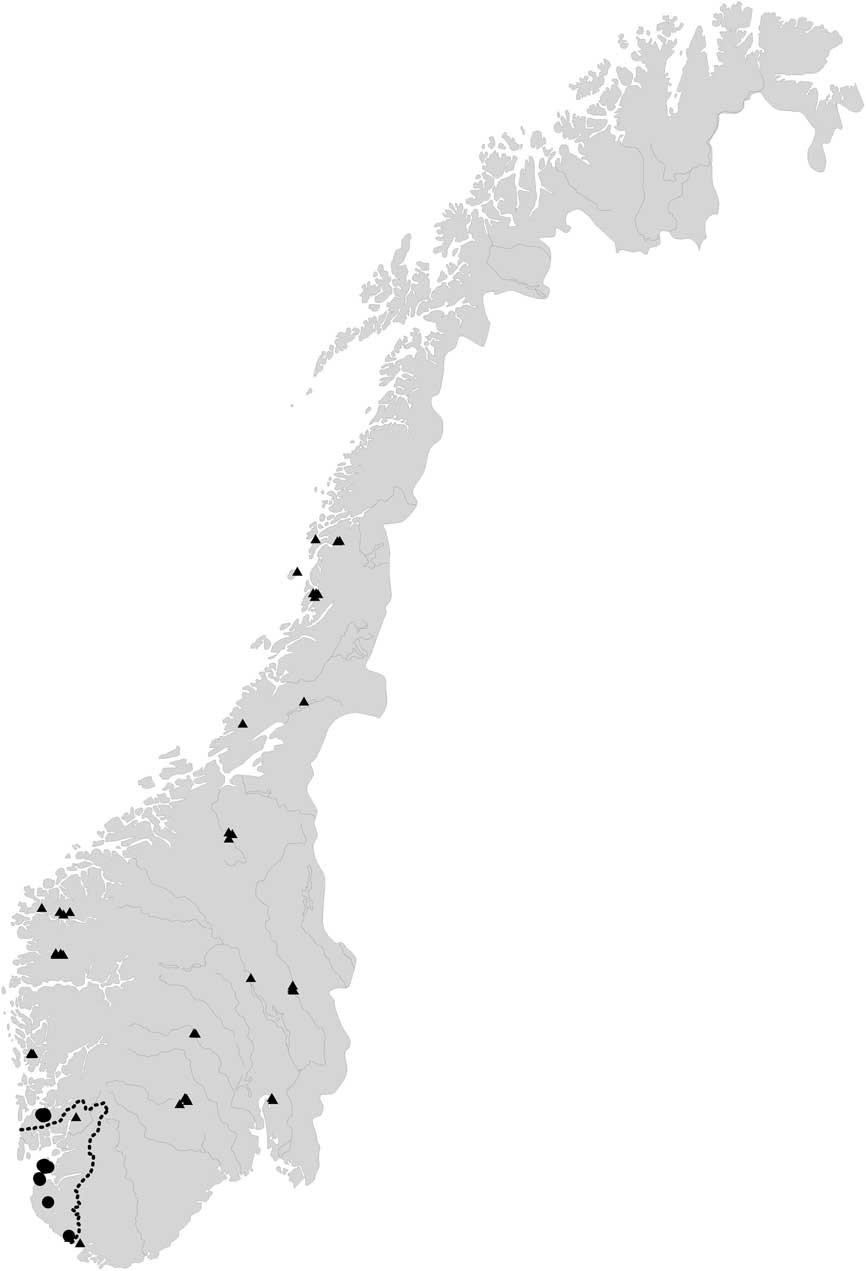
Fig. 6 Distribution of Fuscidea lightfootii (circles) and F. pusilla (triangles) in Norway based on the specimens cited and sequenced, showing their overlapping zone in or near Rogaland County (dotted line).
We conclude that F. lightfootii and F. pusilla are two distinct species, and that DNA sequencing is necessary for their identification.
We are grateful to Alan Fryday (Michigan State University), Mark R. D. Seaward (University of Bradford), Gintaras Kantvilas (University of Tasmania), Zdeněk Palice (Academy of Science of the Czech Republic) and Ondřej Peksa (West Bohemian Museum in Pilsen) for the loan of material, to Mark R. D. Seaward for linguistic improvements, Louise Lindblom (University of Bergen) for technical help with the molecular work, Per M. Jørgensen (University of Bergen) for help with botanical nomenclature, Kim Abel (Røyken) for taking photographs, and Mats Wedin (Swedish Museum of Natural History), Mika Bendiksby (Norwegian University of Science and Technology) and two anonymous reviewers for their helpful comments. The molecular work was carried out at the Biodiversity Laboratories at the University of Bergen. The authors acknowledge the Department of Natural History and the Grolle Olsen Fund (both the University of Bergen) for financial support for the DNA laboratory work and fieldwork.
TT gratefully acknowledges Bruce McCune (Oregon State University) for the invitation to carry out lichenological fieldwork in National Parks in Alaska during 2013–2015; the National Park Service (NPS), Southwest Alaska Network, Anchorage, for funding; Amy Miller and James Walton (both NPS) for project coordination and for organizing and executing field logistics.
Supplementary Material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0024282918000270










