Introduction
Inland dunes provide a unique but threatened environment for drought-tolerant organisms. In Europe, in addition to the European Aeolian Sand Belt, located in the northern European lowlands from Great Britain to Belarus and beyond Moscow (Koster Reference Koster2009), they can also be found in Italy and Hungary. Depending on their origin, they could be morainic or fluvioglacial and are extremely oligotrophic (Schmidt Reference Schmidt1986). Some of them are drift dunes; soil crusts and vascular plants bind others. Despite harsh environmental conditions (extreme irradiation, high temperature variability), the species richness is typically high in these habitats and rare specialists can also be present (Molnár Reference Molnár2003). Investigations on lichens are known from the Netherlands (Ketner-Oostra & Sýkora Reference Ketner-Oostra and Sýkora2009; Sparrius Reference Sparrius2011; Ketner-Oostra et al. Reference Ketner-Oostra, Aptroot, Jungerius and Sýkora2012), Northern Italy (Gheza et al. Reference Gheza, Assini and Valcuvia-Passadore2015, Reference Gheza, Assini and Valcuvia2016), Denmark (Schmidt Reference Schmidt1986), Poland (Zielińska Reference Zielinska1967; Adamska Reference Adamska2010; Adamska & Adamski Reference Adamska and Adamski2014) and Estonia (Jüriado et al. Reference Jüriado, Kämärä and Oja2016; Tilk et al. Reference Tilk, Ots and Tullus2018).
Although terricolous lichens, which are a relevant part of the biological soil crusts (BSC), are essential elements of the semi-arid sandy grassland community (Belnap & Lange Reference Belnap and Lange2003), we still have limited information on their assemblages and the main drivers that shape them in these ecosystems. In a European-wide study, it was demonstrated that latitude has a strong effect on inter- and intraspecific variability within BSC communities (Büdel et al. Reference Büdel, Colesie, Green, Grube, Lázaro Suau, Loewen-Schneider, Maier, Peer, Pintado and Raggio2014). The richness and diversity of BSC communities are significantly affected by both climate and microhabitat conditions (Escolar et al. Reference Escolar, Martínez, Bowker and Maestre2012; Concostrina-Zubiri et al. Reference Concostrina-Zubiri, Pescador, Martínez and Escudero2014). Moreover, soil properties and the characteristics of the neighbouring vegetation (Ochoa-Hueso et al. Reference Ochoa-Hueso, Hernandez, Pueyo and Manrique2011), as well as the herb and moss layer, have a detectable effect on species richness, for example, in alvar grasslands (Leppik et al. Reference Leppik, Jüriado, Suija and Liira2013). The increased density of the herb layer can cause a shift in the composition of lichen growth forms.
Disturbances such as those caused by fire and livestock in the Great Basin (North America) (Condon & Pyke Reference Condon and Pyke2018) have been shown to decrease the cover of BSC lichens and impact the lichen communities. Ullmann & Büdel (Reference Ullmann, Büdel, Belnap and Lange2001) gathered information about the relationships between environmental factors and the floristic composition of BSCs at local to regional scales in Africa and Australia. They emphasized soil characteristics (chemical, physical), vegetation cover, geological attributes and climate variables as the most critical drivers of BSC floristic variation. The studies mentioned above revealed that numerous variables could have an impact on lichen species richness, functional diversity and composition in different ecosystems. However, there is limited information available on the effect of soil surface variables on terricolous lichen cover and species composition in inland dune ecosystems in Europe.
Inland dune ecosystems are considered to be vulnerable and extremely sensitive to human impact (e.g. trampling, fire, grazing). They are also threatened by the effects of climate change, especially the increasing severity and frequency of drought events in this region (Bartholy et al. Reference Bartholy, Pongrácz, Torma, Pieczka, Kardos and Hunyady2009; Ferrenberg et al. Reference Ferrenberg, Reed and Belnap2015). According to the European Red List of Habitats, grasslands of the Pannonian and Pontic sandy steppe habitat types (E1.1a) are critically endangered (Janssen et al. Reference Janssen, Rodwell, García Criado, Gubbay, Haynes, Nieto, Sanders, Landucci, Loidi and Ssymank2016). As with the majority of European dry habitats, continental dunes in open sand steppes are protected by the European Habitat Directive (European Commission 2013) and belong to the Natura 2000 network to ensure their long-term persistence and the survival of their valuable and threatened species assemblages. Cryptogamic species are important in dry ecosystems; however, as Gheza et al. (Reference Gheza, Assini, Lelli, Marini, Mayrhofer and Nascimbene2020) stressed, these organism groups are often neglected during both habitat characterization and conservation management planning. Nevertheless, for conservation purposes, it is essential to discover the composition of the lichen communities, as well as the environmental factors affecting them, because they dominate large areas between vascular plant patches in these vulnerable habitats.
The open sand steppes, providing habitats for terricolous lichens in duneland ecosystems, cover c. 10 840 ha (0.12%) in Hungary (MÉTA database: Molnár et al. Reference Molnár, Bartha, Seregélyes, Illyés, Tímár, Horváth, Révész, Kun, Botta-Dukát and Bölöni2007). This vegetation type (EU habitat directive code 6260 – Pannonic sand steppes: European Commission 2013) is of high conservation value. This habitat also provides ideal environmental conditions for several lichen species that are rare and protected in Hungary. Some of them are regarded as endemic by Verseghy (Reference Verseghy1958, Reference Verseghy1994). Most (71%) of the lichen species protected in Hungary are terricolous (Farkas & Lőkös Reference Farkas and Lőkös2006; Farkas et al. Reference Farkas, Lőkös, Molnár and Lipnicki2012; Hungarian Ministry of Agriculture 2013; Sinigla et al. Reference Sinigla, Lőkös, Varga and Farkas2014, Reference Sinigla, Lőkös, Varga and Farkas2015). Five of these were found at the investigated sites: Cladonia magyarica, Xanthoparmelia pokornyi, X. pulvinaris, X. ryssolea, and X. subdiffluens (authorities can be found in Table 1). Xanthoparmelia pulvinaris and X. subdiffluens are regarded as endangered (Lőkös & Tóth Reference Lőkös, Tóth, Tóth and Horváth1997; Lőkös &Verseghy Reference Lőkös, Verseghy, Lőkös and Rajczy2001), and all except X. ryssolea were described from the Pannonian steppe areas in Hungary. Comprehensive floristic surveys of lichens in the region have been performed by Gallé (Reference Gallé1973) and Lőkös & Verseghy (Reference Lőkös, Verseghy, Lőkös and Rajczy2001). Nevertheless, there is only limited information about the effect of environmental conditions caused by the topography of inland dunes on the composition of lichen communities in Pannonian semi-arid sandy grasslands.
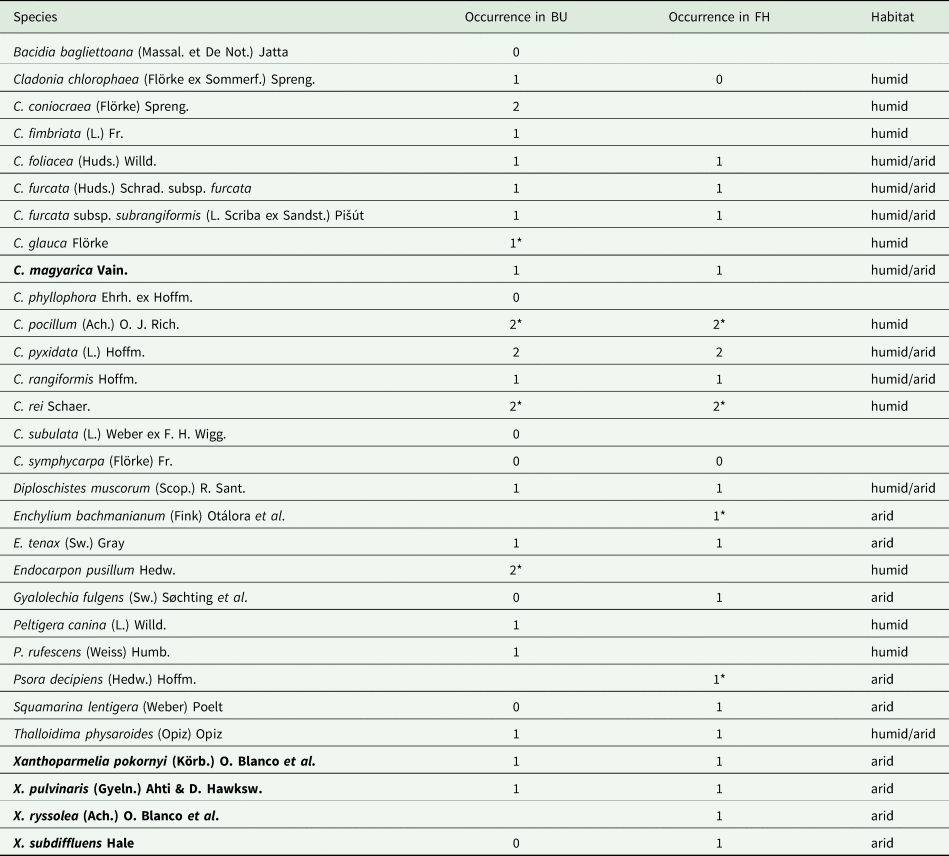
Table 1. The list of terricolous lichen species at the Bugacpusztaháza (BU) and Fülöpháza (FH) sites according to previous authors and collectors (Gallé Reference Gallé1973; Lőkös & Verseghy Reference Lőkös, Verseghy, Lőkös and Rajczy2001) and including data from the current investigation. Protected species are highlighted in bold.
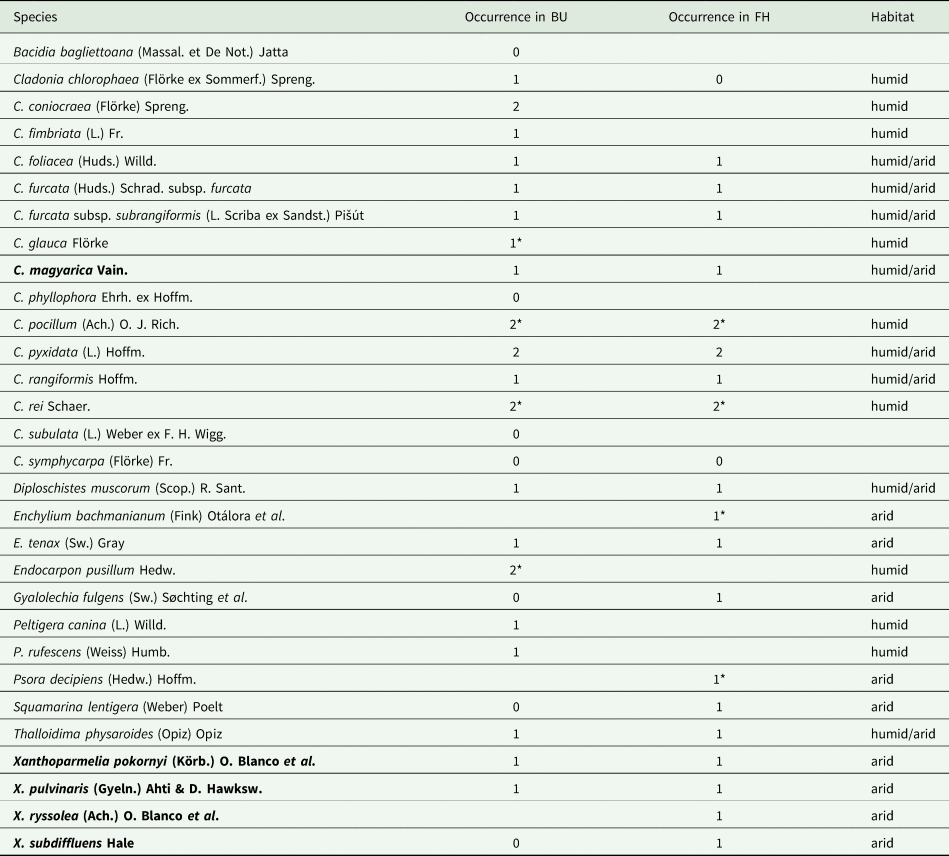
Blank cells – species not yet recorded at this site or the habitat preference is not known.
0 - species was not found in the present study but has been recorded previously.
1 - species was found in the present study and has been recorded previously.
2 - species was discovered in the present study for the first time at the site.
* - species was found in a studied stand but only outside the sampled quadrats.
The present study aims to fill the knowledge gaps mentioned above by asking the following questions. What is the difference in species richness and composition on a coarse scale, and does disturbance history have an impact at this spatial level? How different are the terricolous lichen communities at smaller scales? What kind of environmental variables could cause this difference? To answer these questions, total species number, species composition and coverage of the three main growth forms (crustose, fruticose and foliose) were analyzed by site, by dune side and by micro-quadrat environment. We hypothesized that disturbance history might cause differences in the species pool. In addition, we supposed that because of different light and humidity conditions, there could be a difference in environmental variables at the scale of dune side and even smaller scales. We hypothesized that this could lead to a difference in terricolous lichen species composition and that the coverage of the various growth forms would be different with different dune aspects.
Materials and Methods
Study sites
The two sites investigated are located in the Kiskunság National Park near Bugacpusztaháza (‘BU’) (46°39′11.81″N, 19°35′45.17″E; Natura 2000 site code HUKN20024) and near Fülöpháza (‘FH’) (46°52′21.45″N, 19°24′18.29″E; Natura 2000 site code HUKN20011), in Central Hungary (Fig. 1A). Sites are located 28 air km from each other. Both sites are situated in nature conservation areas and have been protected with no agricultural activity since 1975.

Fig. 1. A, location of the two study sites in Hungary. B, the landscape of Fülöpháza (FH). C, the landscape of Bugacpusztaháza (BU). D, a diagrammatic representation of the study design; ‘plots’ = quadrats. In colour online.
The natural vegetation has been exposed to intensive human impact for at least two millennia in the Kiskunság region (Bíró & Molnár Reference Biró and Molnár1998; Molnár et al. Reference Molnár, Biró, Bartha, Fekete, Werger and van Staalduinen2012). Trees were absent from both territories in the 19th century (earlier data are not available). The extensive grazing of Hungarian grey cattle followed by rural grazing of sheep resulted in open, strongly degraded grasslands in both sites and moving dunes in Fülöpháza. After the grazing was gradually abandoned in the 1960s, both sites were used for military purposes (1952–1974), where digging and trampling were characteristic. Nevertheless, these areas (especially the inner parts) were also protected from other activities (Molnár Reference Molnár2003). At the Bugacpusztaháza site, the spread of juniper (Juniperus communis L.) and poplar (Populus spp.) after extensive grazing was abandoned (until the mid-19th century and then after the 1950s) is noticeable (Bíró & Molnár Reference Biró and Molnár1998). However, the increased population of the European rabbit (Oryctolagus cuniculus L.) reshaped the landscape at Bugacpusztaháza (1985–1994; Ónodi et al. Reference Ónodi, Kertész and Botta-Dukát2006). Low intensity (only a goat herd) grazing was present until 1994 at the Fülöpháza site (Bíró et al. Reference Biró, Iványosi Szabó, Molnár and Szabó A2015). Another difference between the two sites is the two anthropogenic, accidental fire events at Bugacpusztaháza, when the area was partly burned. The first was in 1976 (c. when 80% of damage occurred), and the second happened in 2012. The investigation site was partly damaged during the second fire (Szatmári et al. Reference Szatmári, Tobak and Novák2016); however, one year later, there were no visible signs of burns in the open grasslands (Szatmári et al. Reference Szatmári, Tobak and Novák2016). Lichens seemed healthy without any signs of burning in our study site during the data recording. The Bugacpusztaháza site had more dense tree vegetation (consisting of poplar and juniper); meanwhile, Fülöpháza, surrounded by pine (Pinus nigra J. F. Arnold and P. sylvestris L.) plantations, exhibits a more open landscape where signs of grazing were still visible in the 2000s and trees still grow sparsely (Molnár Reference Molnár2003).
The area's climate is moderately continental with a sub-Mediterranean influence on both study sites (Péczely Reference Péczely and Pécsi1967). The mean regional annual air temperature is 10.4 °C and the yearly precipitation is 505 mm based on a 30-year average (for details see: Bíró & Molnár Reference Biró and Molnár1998; Lellei-Kovács et al. Reference Lellei-Kovács, Kovács-Láng, Botta-Dukát, Kalapos, Emmett and Beier2011; Bíró et al. Reference Biró, Szitár, Horváth, Bagi and Molnár2013). The region is mostly covered by calcareous sand from the deposits of the Danube River. The wind, as a secondary effect, reshaped the landscape, resulting in north-east, south-west facing dune sides with interdune depressions between them (Pécsi Reference Pécsi and Pécsi1967).
The Kiskunság National Park varies in topography and consists of nine separated areas surrounded by fields, plantations (Pinus spp., Robinia pseudoacacia L.) and fallows (Molnár Reference Molnár2003). The duneland in Bugacpusztaháza is double the size of Fülöpháza (2000 ha). Our knowledge of the similarities and differences between these dune fields before the 19th century is incomplete. However, it is generally accepted that Bugacpusztaháza dunes are closer to the natural state and became forested (as climax association) after grazing was abandoned, while the Fülöpháza site bears the signs of deforestation and grazing (Molnár Reference Molnár2003).
Dominant plants in the semi-arid sandy grassland (Festucetum vaginatae ‘danubiale’ Rapaics ex Soó em. Borhidi association) at the investigation sites are Festuca vaginata W. et K., Stipa borysthenica Klokov, Fumana procumbens (Dun.) Gren. et Godr., Alcanna tinctoria (L.) Tausch, Dianthus serotinus W. et K., Onosma arenaria W. et K. and Sedum hillebrandtii Fenzl (Fekete Reference Fekete, Fekete, Molnár and Horváth1997; Borhidi et al. Reference Borhidi, Kevey and Lendvai2012). Most of the terricolous lichen species found here are members of the ‘Bunte Erdflechten-Gesellschaft’ (Gams Reference Gams1938), also known as the complex of Fulgensietum fulgentis Gams and Cladonietum symphycarpiae Doppelb. associations, which are typical for this region (Büdel Reference Büdel, Belnap and Lange2001).
Sampling method
Overall, ten stands of open sand grasslands were randomly selected, representing the duneland vegetation of the area. Sampling was carried out in late autumn and during wintertime because it was more advantageous to estimate the cover of the different lichen growth forms than in summer when they are usually in a dry condition. Our previous long-term study (Veres et al. Reference Veres, Farkas and Csintalan2020) revealed a significant difference in microclimate between the north-east (NE) and south-west (SW) facing dune sides or aspect of the sites. The more exposed, SW-facing slopes are considered arid; the NE-facing sides are considered less arid (hereafter ‘humid’) dune sides. At Bugacpusztaháza, three stands were located on randomly selected SW and three on NE aspects of different dunes (six dunes in total). Two stands for each dune side were similarly selected at Fülöpháza (four dunes in total). On each stand (i.e. dune side), one 1 m × 1 m sampling quadrat was placed in the central part of the stand (10 quadrats in total). The quadrat has 10 × 10 sections (100 contiguous 10 cm × 10 cm micro-quadrats per quadrat) (Fig. 1). The size of the quadrat was selected based on the characteristics of the aggregated vegetation (Scheidegger et al. Reference Scheidegger, Groner, Keller, Stofer, Nimis, Scheidegger and Wolseley2002). In the present study, only soil surface attributes were investigated. In each micro-quadrat, the coverages of lichen growth forms (% fruticose, foliose and crustose), moss (%), herb (%), litter (%) and bare soil (%) were evaluated with the Braun-Blanquet method (Braun-Blanquet Reference Braun-Blanquet1964). The average height of the herbs (mm) was also measured with a measuring tape. The presence or absence of each lichen species was recorded in each micro-quadrat. Thus in this study, lichen data and the potential explanatory variables were recorded at the micro-quadrat level (n = 100 micro-quadrats/quadrat for each of 10 quadrats) across two studied sites and two types of dune side at each site.
Many lichen species were identified in the field. Species that were difficult to identify in the field were transported to the laboratory and examined with HPTLC using the method of Arup et al. (Reference Arup, Ekman, Lindblom and Mattsson1993). They were then identified using Verseghy (Reference Verseghy1994), Smith et al. (Reference Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), and Wirth et al. (Reference Wirth, Hauck and Schultz2013). Nomenclature follows Index Fungorum (CABI 2020) and Farkas & Lőkös (Reference Farkas and Lőkös1994). Lichen species that occurred outwith the sampling units were also recorded and added to a complete species list, including authorities (Table 1).
Data analysis
First, we compiled detailed species lists for the two study sites, incorporating previous and present occurrences. Besides study site, dune side where a certain species occurred (i.e. ‘arid’ or ‘humid’ preference) was also recorded.
Only data for lichens in micro-quadrats were used for quantitative analyses. For these analyses, data for some species were merged, because of their very similar thallus structure, when they occurred in the same microhabitat type or their abundances were low (Cladonia chlorophaea and C. fimbriata only on humid dune sides; C. furcata and C. rangiformis, and C. magyarica and C. pyxidata in both types of microhabitats; and only occurring on arid dune sides were Xanthoparmelia pokornyi and X. ryssolea, and X. pulvinaris and X. subdiffluens). These five pairs are hereafter known as ‘groups’ and are presented, for example, as follows: Cladonia chlorophaea/fimbriata. Cladonia rangiformis occurred only in a small number of micro-quadrats (1%); therefore, we decided to merge the species together with the more frequent C. furcata (9%). They were the only two several-branched to many-branched Cladonia species in our study. Both species were represented by only 1–3 podetia in the micro-quadrats.
The effects of site (‘BU’ or ‘FH’) and dune side (‘arid’ or ‘humid’) on the environmental (cover of bryophytes, herbs, bare soil, litter and height of herbs) and lichen-related (species number, total lichen cover, cover of three growth forms) variables, as well as the relationship of environmental variables to lichen variables, were evaluated. Non-metric multidimensional scaling ordination and generalized mixed-effects models (random intercept models; Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009) were performed on data in micro-quadrats. PERMANOVA was performed on lichen frequency by quadrat. All analyses were carried out using R version 4.0.3 (R Core Team 2020).
Lichen assemblages were the first to be studied. A global non-metric multidimensional scaling (NMDS) was performed on the dataset of all single lichen species (13) and groups (five) present in the micro-quadrats (with a Jaccard dissimilarity matrix) to visualize micro-quadrat separation along with the dune sides and sites simultaneously using the ‘metaMDS’ function of vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson and Solymos2019); details can be found in Supplementary Material Section 1 (available online). A two-dimensional solution was selected based on stress as a function of dimensionality (Supplementary Material Fig. S1A, available online). The final stable solution was the lowest stress from 100 random starting configurations. The final configuration was centred and rotated to maximize orthogonality with the highest point dispersion on the first axis (see Borcard et al. Reference Borcard, Gillet and Legendre2018; Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson and Solymos2019), goodness-of-fit was assessed by Kruskal's stress formula one (Legendre & Legendre Reference Legendre and Legendre2012) and by a Shepard diagram, and proportion of original variation was estimated (Supplementary Material Fig. S1B, available online). Mantel tests were used to estimate the proportion of variation explained by individual NMDS dimensions, and environmental variables were fitted passively onto the ordination space (both using functions in vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson and Solymos2019)).
Besides ordination, separation of the lichen assemblages among sites and dune sides as main questions were tested at quadrat-level; therefore, samples were pooled and frequency data were calculated for the analysis. Permutational multivariate analysis of variance (PERMANOVA; Anderson Reference Anderson2001) based on Manhattan metrics with 9999 permutations was applied to test the aspect and site effects in one model. PERMANOVA is a flexible, dissimilarity-based, semi-parametric method to test differences in centroids and dispersions of groups with no assumption of multivariate normality. The method compares within-group to between-group variances using an F-test, distribution-free inferences achieved by random permutations of the objects between the compared groups. PERMANOVA was performed with the ‘adonis’ function in vegan (Oksanen et al. Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson and Solymos2019). Species richness and cover of lichen growth forms, as well as the different substratum forms as potential drivers of lichen communities, were further investigated. In the mixed-effects models, site (two sites) and dune side (two dune aspects at each site) were fixed factors, quadrat was a random factor (10 states), with response variable values for each micro-quadrat (100 per quadrat, 1000 in total). Since the distributions of most of the studied variables were highly skewed, generalized mixed-effects models (GLMMs) (random intercept models) with a restricted maximum likelihood (REML) method were used (Bolker et al. Reference Bolker, Brooks, Clark, Geange, Poulsen, Stevens and White2009; Dunn & Smyth Reference Dunn and Smyth2018). Prior to the analyses, all fixed factors were centred and scaled (z-score standardization). GLMMs were fit for most using lme4 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015) with different link functions depending on the distribution of a variable. For some, Tweedie models were built using glmmTMB (Brooks et al. Reference Brooks, Kristensen, van Benthem, Magnusson, Berg, Nielsen, Skaug, Maechler and Bolker2017) with the tweedie package (Dunn Reference Dunn2017). P-values were provided by lmerTest (Kuznetsova et al. Reference Kuznetsova, Brockhoff and Christensen2017). Details of the models are provided in Supplementary Material Section 2 (Tables S1.1–1.3 and S2.1–2.3, respectively, available online). Site and dune side (within separate sites) contrasts were then evaluated by estimated marginal means that were based on the constructed GLMMs using emmeans (Lenth Reference Lenth2021).
Finally, the presence of each of the six most abundant individual lichen species or groups (Cladonia foliacea, C. furcata/rangiformis, C. magyarica/pyxidata, Diploschistes muscorum, Enchylium tenax, Xanthoparmelia pokornyi/ryssolea) was tested by logistic mixed-effects models using lme4 (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). Site, dune side and substratum cover variables were used as fixed effects in micro-quadrats (details for logistic mixed-effects models provided in Supplementary Material Table S1.3, available online). Prior to these analyses, all tested explanatory variables as fixed factors were centred and scaled (z-score standardization). Details for logistic GLMMs can be found in Supplementary Material Table S3 (available online).
Results
Lichen communities of the two investigated sites and dune sides
In the present study, a total of 20 terricolous lichen taxa were recorded at the Bugacpusztaháza site and 19 at the Fülöpháza site, including species outside of the quantitatively measured quadrats (Table 1). Five of these are new to the sites from this investigation (Table 1). While Cladonia pocillum, C. pyxidata and C. rei were new occurrences to both sites, C. coniocraea and Endocarpon pusillum were found only at the Bugacpusztaháza site. A previous study (Lőkös & Verseghy Reference Lőkös, Verseghy, Lőkös and Rajczy2001) documented 22 terricolous lichen taxa from Bugacpusztaháza and 18 from the Fülöpháza site. Though C. convoluta (Lam.) Anders and C. foliacea were mentioned as separate species, they are currently considered conspecific (Pino-Bodas et al. Reference Pino-Bodas, Burgaz, Ahti and Stenroos2018). Eight formerly reported species were not confirmed during the present investigation (Table 1). Cladonia subulata was not detected at either site in the present study, although the morphologically very similar but chemically distinguishable C. rei was verified. Twelve species (13 taxa) were shared between the two sites, and six species were found outside of the quantitatively measured quadrats (Table 1).
Nine species were found only on humid dune sides (Table 1): Cladonia chlorophaea, C. coniocraea, C. fimbriata, C. glauca, Endocarpon pusillum, Peltigera canina and P. rufescens were found only at Bugacpusztaháza, while C. pocillum and C. rei occurred at both sites. Nine other species occurred only on arid sides of the dunes: Enchylium bachmanianum, Gyalolechia fulgens, Psora decipiens, Squamarina lentigera, Xanthoparmelia ryssolea and X. subdiffluens were found only at Fülöpháza, while Enchylium tenax, Xanthoparmelia pokornyi and X. pulvinaris occurred at both sites. The seven species (8 taxa) that were recorded on both dune sides were the same at the two sites (Table 1).
The lichen communities of the two sites differed, especially the arid lichen communities of Bugacpusztaháza from the arid lichen communities of Fülöpháza (Fig. 2). The core species composition of the Fülöpháza arid dune sides varied more among micro-quadrats along both NMDS1 and NMDS2 (Fig. 2) than the other three groups (FH humid, BU arid, BU humid), and is very different from the other three groups along NMDS1. In a weaker pattern expressed on both axes, Fülöpháza humid and Bugacpusztaháza humid core lichen species composition show a notable overlap, with core Bugacpusztaháza arid composition mainly being a subset of both.
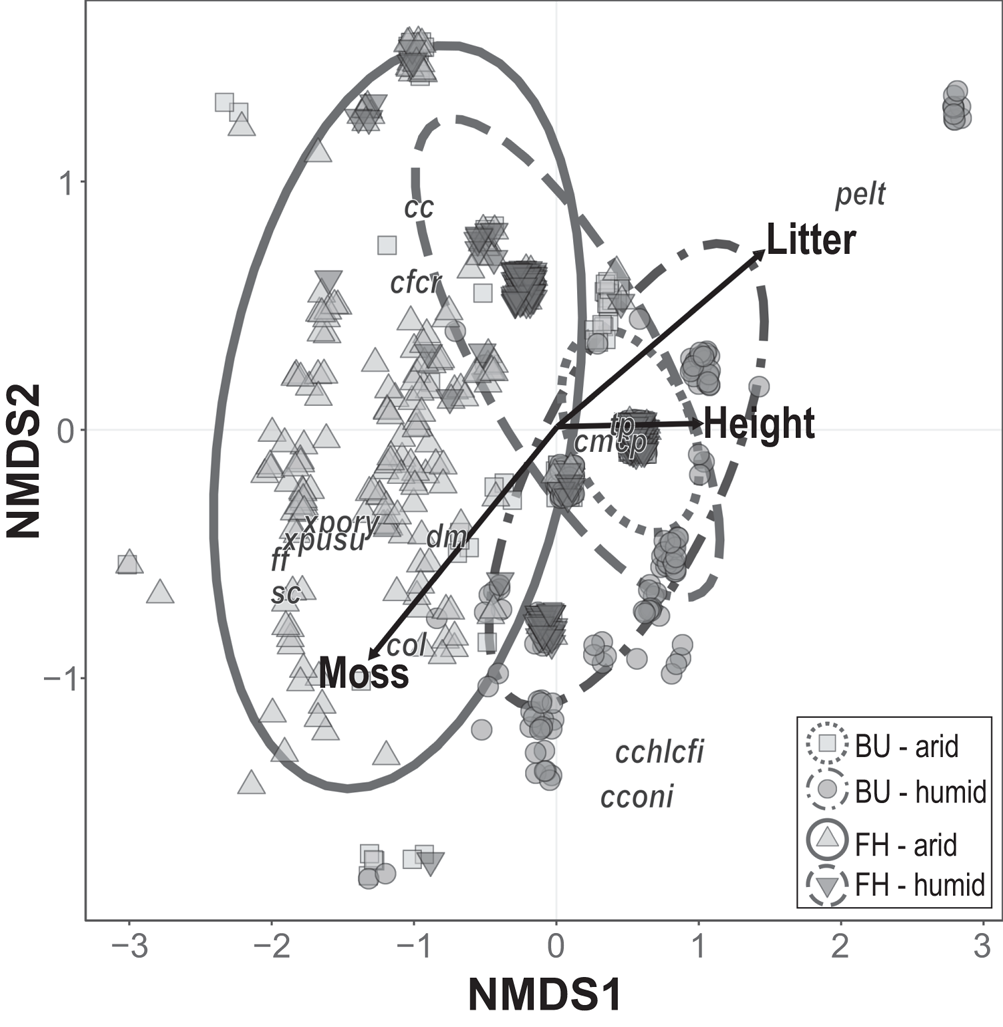
Fig. 2. Ordination diagram from the non-metric multidimensional scaling (NMDS, stress = 0.102) showing the pattern of terricolous lichen assemblages and micro-quadrats based on sites and the dune side with passively projected explanatory variables. Black arrows represent significant correlations (P < 0.05) with the sample scores. PERMANOVA statistics: Site, R 2 = 0.343, F(1,7) = 5.429, P = 0.0046; Dune side, R 2 = 0.215, F(1,7) = 3.399, P = 0.0231. BU = Bugacpusztaháza site; FH = Fülöpháza site. Abbreviations of species and group names: cc – Cladonia foliacea; cchlcfi – Cladonia chlorophaea/fimbriata; cconi – Cladonia coniocraea; cfcr – Cladonia furcata/rangiformis; cmcp – Cladonia magyarica/pyxidata; col – Enchylium tenax; dm – Diploschistes muscorum; ff – Gyalolechia fulgens; pelt – Peltigera canina and P. rufescens; sc – Squamarina lentigera; tp – Thalloidima physaroides; xpory – Xanthoparmelia pokornyi/ryssolea; xpusu – Xanthoparmelia pulvinaris/subdiffluens.
The site, dune side and environmental factors in micro-quadrats were found to be important factors determining terricolous lichen assemblages in these sandy areas. According to the pseudo F statistics, the site had higher explanatory power than the aspect of the dunes at a quadrat level (P < 0.0046 and P = 0.0231, respectively). The NMDS (stress = 0.102) showed a separation among dune sides and site (Fig. 2). The ordination using two orthogonal dimensions cumulatively represented 59.61% of the variation of species composition at the micro-quadrat scale (Mantel tests, NMDS 1: R 2 = 0.349, P < 0.001; NMDS 2: R 2 = 0.247, P < 0.001).
The position of the species in the ordination space indicated that a higher coverage of leaf litter had a strong association with P. canina. Enchylium tenax and Diploschistes muscorum, on the other hand, preferred growing in and on moss patches. The NMDS emphasized that C. foliacea, C. furcata/rangiformis, Xanthoparmelia species (Xanthoparmelia pokornyi/ryssolea and X. pulvinaris/subdiffluens), Squamarina lentigera and the crustose Diploschistes muscorum and Gyalolechia fulgens were more abundant in patches where herb height was lower.
The variation related to herb height along the first axis is distinct from variation attributable to a stronger factor correlated with litter and moss cover because their vectors are divergent (Fig. 2). The variation in herb height is correlated with both site and dune side, each varying greatly along NMDS1. In contrast, variation in a micro-quadrat environmental factor linked to litter and moss cover varies almost equally with NMDS1 and NMDS2.
Species richness of the two investigated sites and dune sides
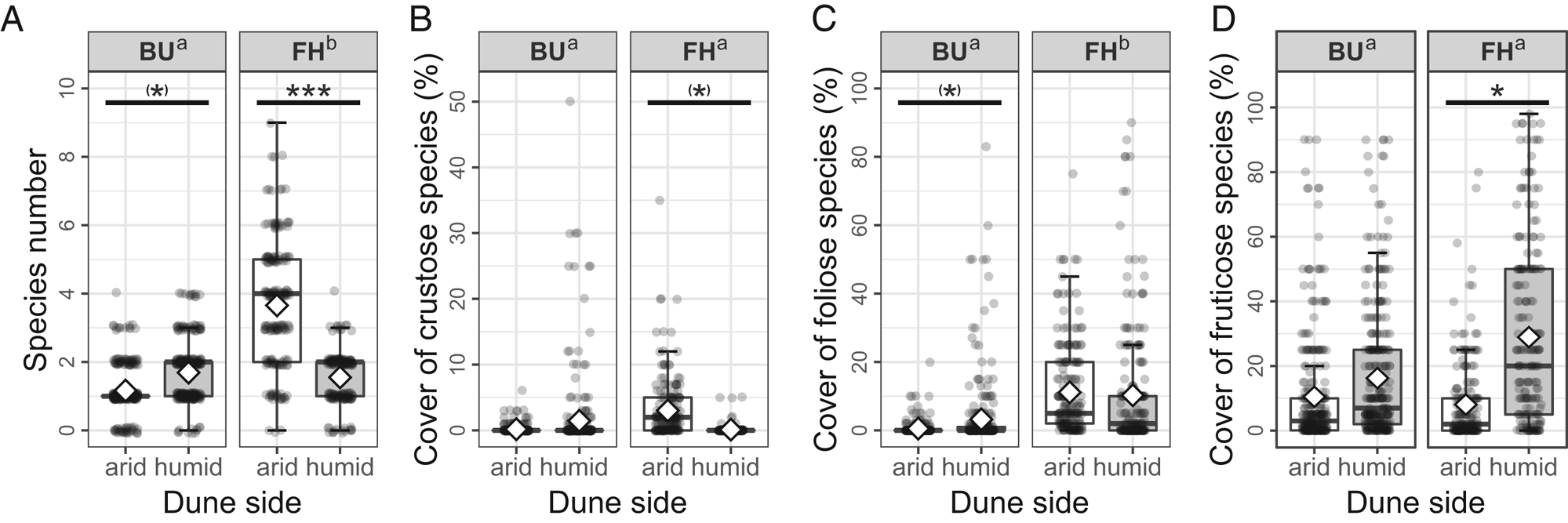
Species richness at the microplot scale was significantly higher at FH than at BU (t 994 = −3.502, P < 0.001) (Fig. 3A). At the FH site, the total species richness was significantly higher (t 994 = 3.671, P < 0.001) in arid micro-quadrats (mean 3.66, range 0–9) compared to humid micro-quadrats (1.55 [0–4]) (Fig. 3A). The opposite was detected at BU, where humid sides (1.69 [0–4]) hosted more species (t 994 = −1.909, P = 0.056) than arid ones (1.16 [0–4]) (Fig. 3A).
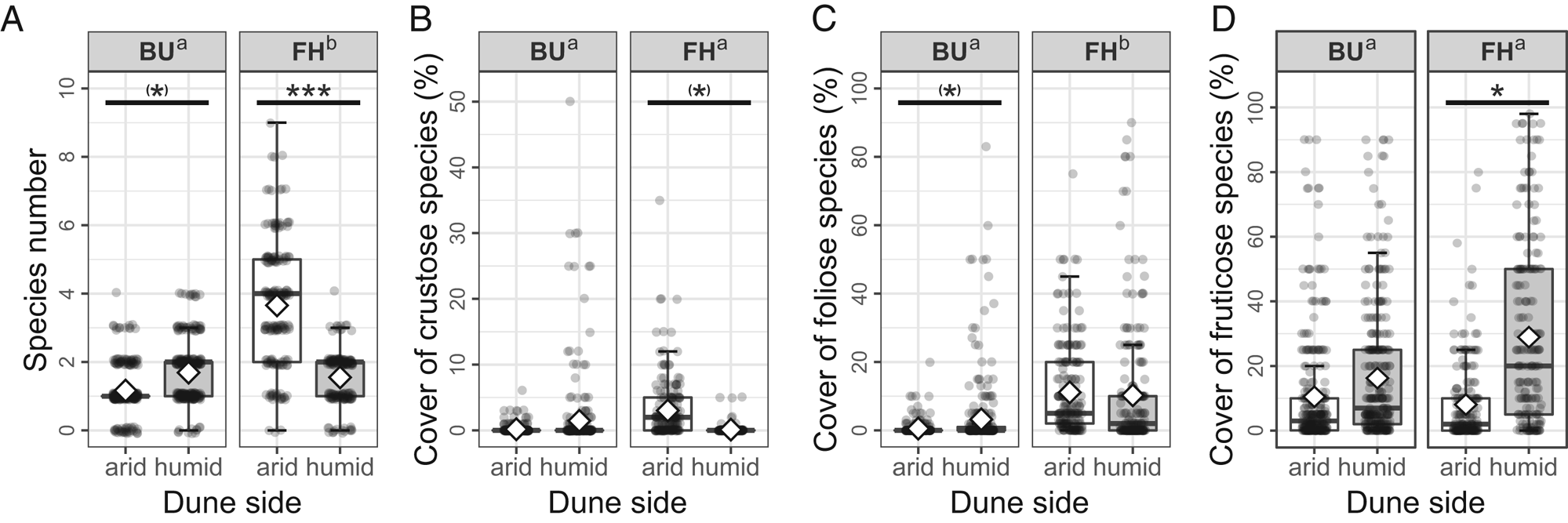
Fig. 3. Species richness of the two investigated sites and dune sides. The number of species per micro-quadrat (A), coverage (%) of crustose (B), foliose (C) and fruticose (D) species, by site (BU = Bugacpusztaháza, FH = Fülöpháza) and dune side (arid, humid). On the boxplots (median with the interquartile range = box, range = whiskers), both raw data (dots) and mean values (diamonds) are represented. Summary statistics of the Wald's Chi2 tests (Chi2 and P-values) are also shown on each graph. Different letters (‘a’ and ‘b’) indicate the statistical difference (P < 0.05) between the sites. The asterisks code the significant difference between the dune sides ((*) = P < 0.1, * = P < 0.05, *** = P < 0.001).
The site effect was not significant (t 994 = −0.389, P = 0.697) on the coverage of crustose species (Fig. 3B). Dune side had a marginally significant effect on the crustose abundance at FH (t 994 = 1.931, P = 0.054), where the cover in arid micro-quadrats was higher (3.01% [0–35%]) compared to humid ones (0.11% [range 0–5]), but not at BU (t 994 = 0.264, P = 792; arid 0.18% [0–6%], humid 1.45% [0–50%]).
At the FH site, foliose species had a significantly higher (t 994 = −3.280, P = 0.001) cover (arid 11.13% [0–75%]; humid 10.27% [0–90%]) than at BU (arid 0.52% [0–20%], humid 3.22% [0–83%]) (Fig. 3C). Contrary to the BU site, cover among dune sides at FH did not differ (t 994 = −1.777, P = 0.077 and t 994 = 0.753, P = 0.452, respectively) (Fig. 3C).
The coverage of fruticose species did not differ (t 994 = −1.026, P = 0.3049) between the two localities (Fig. 3D). In FH micro-quadrats, fruticose species reached significantly higher (t 994 = −2.087, P = 0.037) cover on humid (28.92% [0–98%]) than on arid (8.08% [0–80%]) sides, while at BU their coverage was similar (t 994 = −0.743, P = 0.458) on both dune sides (arid 10.48% [0–90%], humid 16.24% [0–90%]) (Fig. 3D).
Soil surface variables on the two investigated sites and dune sides
The cover of bare soil was significantly lower (t 994 = 4.075, P < 0.001) in FH than in the BU micro-quadrats (Fig. 4A). At the FH site, the average bare soil cover was significantly higher (t 994 = 3.599, P < 0.001) in arid (4.02% [0–40%]) compared to humid (0.2% [0–35%]) micro-quadrats (Fig. 4A). The two dune sides did not differ significantly (t 994 = −0.228, P = 0.8198) in their bare soil cover (arid 8.37% [0–90%], humid 13.33% [0–90%]) at BU.
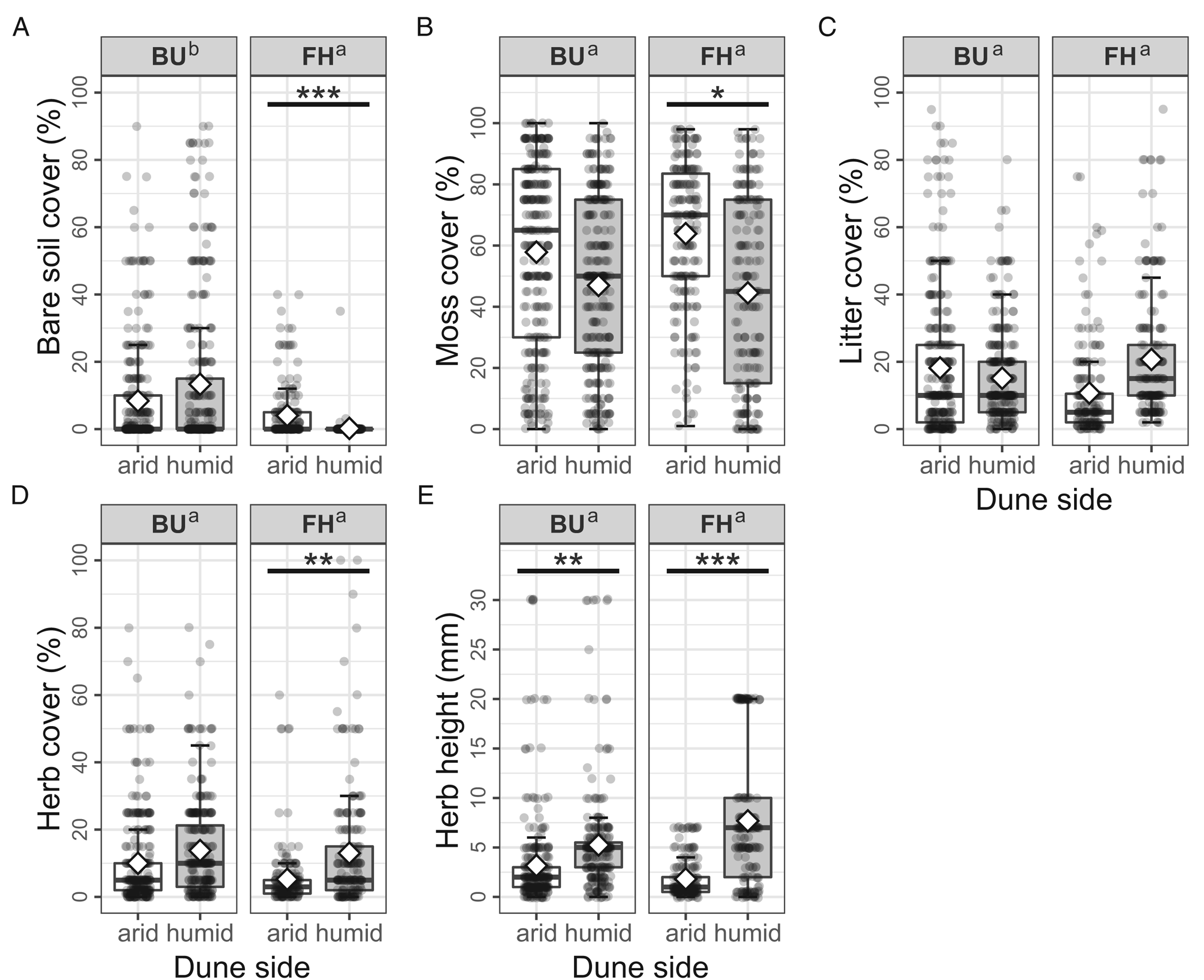
Fig. 4. Cover (%) of the studied soil surface types per micro-quadrat by site (BU = Bugacpusztaháza, FH = Fülöpháza). A, soil. B, moss. C, litter. D, herb. E, mean height (mm) of herbs. On the boxplots (median with the interquartile range = box, range = whiskers), both raw data (dots) and mean values (diamonds) are represented. Summary statistics of the Wald's Chi2 tests (Chi2 and P-values) are also indicated on each graph. Different letters (‘a’ and ‘b’) indicate the statistical difference (P < 0.05) between the sites. The asterisks code the significant difference between the dune sides (* = P < 0.05, ** = P < 0.01, *** = P < 0.001).
There was no remarkable difference detectable in the moss cover (t 994, P = 0.6877) between the two localities (Fig. 4B). However, at the FH site, the average moss cover was significantly higher (t 994, P = 0.0109) in arid (63.89% [1–98%]) compared to humid (44.52% [0–98%]) micro-quadrats, while at BU the coverage was similar (t 994 = 1.287, P = 0.1984) on both dune sides (arid 57.84% [0–100%], humid 46.95% [0–100%]) (Fig. 4B).
Site effect was not significant on the cover of litter (t 994, P = 0.8512) between the two sites, and it was similar on arid and humid dune sides at BU (t 994 = −0.076, P = 0.9396; arid 18.18% [0–95%], humid 15.12% [0–80%]) as well as at FH (t 994 = −1.546, P = 0.1225; arid 10.72% [0.1–75%], humid 20.70% [2–95%]) (Fig. 4C).
The herb cover did not differ significantly (t 994 = 1.469, P = 0.1422) between the two sites (Fig. 4D). The average herb cover was significantly lower (t 994 = −2.649, P = 0.0082) in arid (5.24% [0–60%]) compared to humid (13.00% [0–100%]) micro-quadrats at the FH site (Fig. 4D). The two dune sides did not differ significantly (t 994 = −0.802, P = 0.4227) in their herb cover (arid 9.99% [0–80%], humid 13.78% [0–80%]) at BU.
The average height of herbs was similar (t 994 = 0.499, P = 0.6180) at the two localities (Fig. 4E). At the same time, herbs reached a significantly lower height (t 994 = −6.696, P < 0.001) on arid (1.82 mm [0–7 mm]) than on humid (7.69 mm [0–20 mm]) dune sides at FH (Fig. 4E), as well as at BU (t 994 = −2.927, P = 0.0034; arid 3.169 mm [0–30 mm], humid 5.25 mm [0–30 mm]) micro-quadrats (Fig. 4E).
Factors determining abundant species
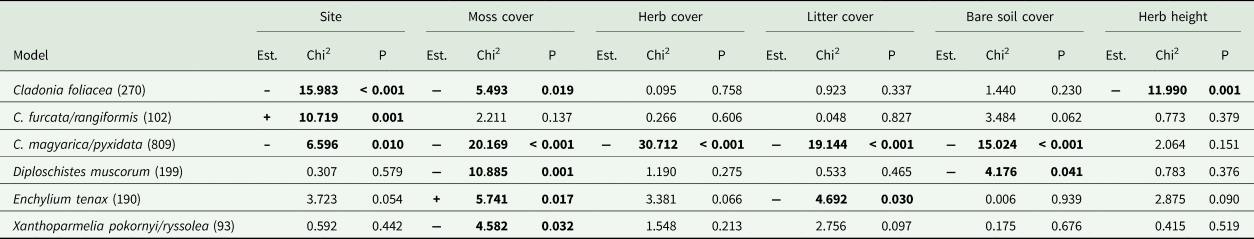
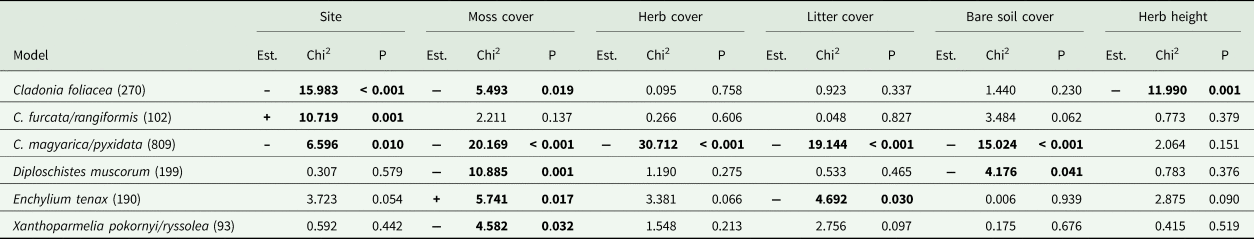
Abundance of the C. magyarica/pyxidata group was higher in the Bugacpusztaháza micro-quadrats (P = 0.01) (Table 2). In contrast, the C. furcata/rangiformis group (P < 0.001) and C. foliacea (P = 0.001) were more abundant at the Fülöpháza site (Table 2). Almost all abundant species (found in > 50 micro-quadrats and at both sites) were negatively influenced by a higher moss cover; only Enchylium tenax benefitted (Table 2). Herb cover negatively affected the C. magyarica/pyxidata group (P < 0.001), while litter cover negatively influenced the presence of both the former group and Enchylium tenax (Table 2). The cover of bare soil negatively affected the abundance of the C. magyarica/pyxidata group (P < 0.001) and Diploschistes muscorum (P = 0.041) (Table 2). Cladonia foliacea was negatively affected by higher herb height (P = 0.001) (Table 2).
Table 2. Summary table of the species-specific logistic generalized mixed-effects models studying the effects of potential explanatory variables on the most abundant (found in > 50 micro-quadrats and at each site) species and groups. For each tested variable, the results of the Wald's likelihood ratio-based Chi2-tests, estimate signs (Est.) and P-values are also presented (for further details see Supplementary Material Table S3, available online). Values in bold are statistically significant at P < 0.05. The number of records for each abundant species is also indicated in parentheses after the species name. Estimate sign = ‘+’ when the value is higher at Fülöpháza than at Bugacpusztaháza and ‘–’ when the value is higher at Bugacpusztaháza than at Fülöpháza.
Discussion
Species pools of the two study sites
Fewer species were previously known from Fülöpháza than from the Bugacpusztaháza site, and the species composition of the two localities is slightly different. There may be several reasons for the differences in the species pools of the studied sites, which could affect the species composition separately and/or in interaction. Terricolous lichen communities are mainly soil type-specific but historical disturbances and land-use change also affect their composition (Leppik et al. Reference Leppik, Jüriado, Suija and Liira2013). The natural vegetation has been exposed to intensive human impact for at least two millennia in the Kiskunság region (Molnár et al. Reference Molnár, Biró, Bartha, Fekete, Werger and van Staalduinen2012). At the Bugacpusztaháza site, the noticeable spread of juniper and poplar could provide favourable microhabitat conditions for terricolous lichen species requiring shade, more humidity or increased humus content of the substratum (through the decomposition of wood and litter). This phenomenon could probably explain the significantly higher number of ‘tree/shrub-correlated’ species (e.g. Cladonia chlorophaea, C. coniocraea or Peltigera species) than at the Fülöpháza site. Two fire events influenced the vegetation and BSC communities of the Bugacpusztaháza site, in contrast to Fülöpháza where grazing was abandoned later.
Our results showed that the site had higher explanatory power than dune side. Since we could not find any significant difference in the climate between the two study site (Veres et al. Reference Veres, Farkas and Csintalan2020), and the investigated environmental variables differed significantly only in open soil cover, we hypothesize that the disturbance history could also play an important role in developing different lichen communities.
The dunes near Fülöpháza have been affected by several kinds of disturbances in the past (mainly grazing) (Molnár Reference Molnár2003, Reference Molnár2008). The species richness at Fülöpháza could be explained by the lack of fire events, as well as by the later abandonment of grazing. Such activities can dramatically reduce the total BSC cover, lichen cover and species richness (e.g. Johansen Reference Johansen, Belnap and Lange2001; Warren & Eldridge Reference Warren, Eldridge, Belnap and Lange2001). Grazing can also cause changes in the amount of lichen biomass and species richness in grasslands (Boch et al. Reference Boch, Prati, Schöning and Fischer2016; Balogh et al. Reference Balogh, Farkas, Lőkös, Papp, Budai, Antal, Novák and Matus2017). However, low-intensity grazing, especially by sheep, can cause higher species richness (Boch et al. Reference Boch, Prati, Schöning and Fischer2016) and may explain the higher number of terricolous species at Fülöpháza. The more open vegetation could also provide habitats for more species, as Ketner-Oostra & Sýkora (Reference Ketner-Oostra and Sýkora2000) pointed out in their study where both the terricolous lichen diversity and coverage was the highest in the early, more open vegetation stages on coastal dunes.
The cover of foliose lichens was significantly lower at the Bugacpusztaháza than at the Fülöpháza site. However, P. canina occurred only in the Bugacpusztaháza micro-quadrats; the higher abundance of C. foliacea and Xanthoparmelia species caused the significantly higher cover of foliose species at the Fülöpháza site. The cover of fruticose and crustose species was similar at the two study sites. Thalli of C. magyarica, C. pyxidata and the wood-correlated, scyphose Cladonia species were more abundant at Bugacpusztaháza, whereas C. furcata and C. rangiformis were more abundant at Fülöpháza. The most abundant crustose species was Diploschistes muscorum at both sites.
The Bugacpusztaháza site appears to have had a greater lichen species turnover and less species retention than Fülöpháza. Seven formerly mentioned species (Lőkös & Verseghy Reference Lőkös, Verseghy, Lőkös and Rajczy2001) were not confirmed during the present investigation at the Bugacpusztaháza site. The disappearance of the earlier rare Cladonia phyllophora and the relatively rare Squamarina lentigera is not entirely surprising. However, that of the earlier common Bacidia bagliettoana, C. symphycarpa and C. subulata and the frequent Gyalolechia fulgens is a serious loss. Similarly, C. chlorophaea and C. symphycarpa, previously common at the Fülöpháza site, were not found during the present study, indicating environmental changes in the last decades.
Differences in species composition between arid and humid dune sides
There are many microclimatic differences between the SW- and NE-facing dune sides. In the long-term, incoming light (photosynthetically active radiation), and air and soil temperatures are usually higher on SW- than NE-facing dune sides, whereas the relative air humidity and soil water content are lower (Veres et al. Reference Veres, Farkas and Csintalan2020). These diverse microenvironmental conditions provide different microhabitat types; therefore, species with various ecological requirements can live together in the same habitat.
We observed that fruticose species could reach a greater cover in the humid micro-quadrats where the cover and height of herbs were usually higher at both localities. Our results are consistent with the findings of Leppik et al. (Reference Leppik, Jüriado, Suija and Liira2013), who demonstrated that the increased density of the herb layer was one of the main drivers for the development of a terricolous lichen community. A shift was detected in the composition of lichen growth form from the dominance of crustose (and squamulose) to fruticose lichens. In our study, it appears that the branching C. furcata and C. rangiformis are more tolerant of the environmental stress of the arid sides than the cup Cladonia species. Cladonia magyarica and C. pyxidata are probably more sensitive to moisture fluctuation and prefer more stable microclimatic conditions on humid dune sides (e.g. Blum Reference Blum, Ahmajian and Hale1973; Büdel & Scheiddeger Reference Büdel, Scheidegger and Nash2008; Veres et al. Reference Veres, Farkas and Csintalan2020).
Another factor is that the competition with mosses seems to be more pronounced, especially on arid sides, where vegetation was lower and more sparse. Therefore, the lower coverage of plant-derived variables, and significantly higher moss cover in arid compared to humid micro-quadrats, can explain the lower total lichen cover in arid microhabitats. Our results are in agreement with the observation that vegetation generally becomes more open with increasing aridity (Walter & Breckle Reference Walter and Breckle1984) and lichens compete with mosses for free patches (Ketner-Oostra & Sýkora Reference Ketner-Oostra and Sýkora2000).
The higher cover of fruticose species in humid micro-quadrats is mainly due to C. pyxidata and C. magyarica. They often grow on moss cushions, which prolongs their assimilation time during dry days (Colesie et al. Reference Colesie, Scheu, Green, Weber and Büdel2012) and compensates for the lower incoming irradiation caused by a higher herb cover and amount of litter (e.g. Pintado et al. Reference Pintado, Sancho, Green, Blanquer and Lazaro2005). This might explain why these species can tolerate the disadvantageous light conditions caused by vascular plants.
Our results suggest that the arid and humid lichen communities are rather different at the two sites. An interaction effect between the sites and dune sides was also detectable. In Fülöpháza, arid micro-quadrats vary much more in lichen species composition than their humid counterparts. The opposite is true for Bugacpusztaháza arid and humid sides. The difference in soil surface variables between the arid and humid dune sides was also different at the two localities. While at Bugacpusztaháza only the average height of herbs was significantly higher in the humid compared to arid micro-quadrats, at Fülöpháza every soil surface variable showed significant differences (except litter cover) between the microhabitats. Because the vegetation is more open at the Fülöpháza site (Molnár Reference Molnár2003), the effect of topography could prevail to a greater degree than at Bugacpusztaháza, where the vegetation is further progressing to climax state (Molnár Reference Molnár2003). The more balanced microclimate (Veres et al. Reference Veres, Farkas and Csintalan2020) can mitigate the contrast between environmental conditions prevailing on arid and humid dune sides.
Differences in species composition at the microhabitat scale
Almost all species were negatively influenced by a higher moss cover because terricolous lichens usually compete with mosses for open patches between vascular plants (e.g. Ketner-Oostra & Sýkora Reference Ketner-Oostra and Sýkora2000). Only Enchylium tenax, which grows in moss cushions, might benefit from the greater moss cover, but this species was negatively influenced by higher litter cover. Our results suggest that among the most abundant species, C. magyarica and C. pyxidata are the most sensitive species, negatively affected by the measured microenvironmental variables. These species grow on moss cushions, preferring open spaces between vascular plants. The litter cover negatively influenced the presence of both the former group and Enchylium tenax, probably because of its shading effect. The higher cover of bare soil negatively affected the abundance of Diploschistes muscorum, whose host species are C. magyarica and C. pyxidata. Cladonia foliacea was negatively affected by higher herb height, probably because the spread of thalli is hindered due to scrolling by the wind.
Our results showed that the composition of dune lichen communities could be impacted by both site and dune side. The difference shown in herb height and herb cover between sites and dune sides might cause a difference in the light level reaching lichen thalli, resulting in different microclimatic conditions where lichens with different environmental needs could find their preferred niche. It was also shown that at lower herb cover and height, the chance of competition might increase between mosses and lichens.
We can also assume that individual lichen species are influenced by the progression to climax vegetation as a principal effect of site. The Bugacpusztaháza site is more closed (Molnár Reference Molnár2003), where woody species are more abundant, providing substratum, shade, humidity, humus or other advantages over these harsh conditions for wood-correlated species. Some species were detected only here, such as the wood-correlated species, or the Peltigera species and C. chlorophaea appearing only at later successional stages (Gallé Reference Gallé1973; Jun & Rozé Reference Jun, Rozé, Herrier, Mees, Salman, Seys, Van Nieuwenhuyse and Dobbelaere2005). In contrast, other species seem to prefer the more open habitats with scarce vegetation, since Psora decipiens, Gyalolechia fulgens and Squamarina lentigera were found only at the Fülöpháza site.
Comparison with communities from other European countries
The number of terricolous lichen taxa found on the dunes of the investigated sites (Bugacpusztaháza 20; Fülöpháza 18; overall 22 species) is slightly lower than those reported from other European countries (Denmark 23 (Schmidt Reference Schmidt1986); Poland 29 (Adamska Reference Adamska2010); Netherlands 34 (Ketner-Oostra et al. Reference Ketner-Oostra, Aptroot, Jungerius and Sýkora2012); Estonia 28 (Jüriado et al. Reference Jüriado, Kämärä and Oja2016); Italy 33 (Gheza et al. Reference Gheza, Assini, Lelli, Marini, Mayrhofer and Nascimbene2020)). In line with those countries, half of the species recorded in these habitats in Hungary belong to the genus Cladonia.
The communities found in the present study differed from communities presented in dunelands of other European countries mainly because of the characteristics of the soil (sand) and vascular plant vegetation. The sandy soil of the Kiskunság region (and most of the Hungarian sandy soils) is calcium-rich, contains more nutrients because of the extended grazing (Molnár Reference Molnár2003) and hosts different lichen associations compared to most of the cited study sites that are acidic and poor in nutrients (e.g. Ketner-Oostra et al. Reference Ketner-Oostra, Aptroot, Jungerius and Sýkora2012). Limited research has focused on the exploration of lichen associations in calcareous sandy grasslands between inland dunes (Jüriado et al. Reference Jüriado, Kämärä and Oja2016; Gheza et al. Reference Gheza, Assini, Lelli, Marini, Mayrhofer and Nascimbene2020), therefore our data contribute significantly to the knowledge of the lichen communities of these habitats. Enchylium tenax, Gyalolechia fulgens, Psora decipiens and Thalloidima sedifolium (Scop.) Kistenich et al., very similar to Thalloidima physaroides, were also detected by Gheza et al. (Reference Gheza, Assini, Lelli, Marini, Mayrhofer and Nascimbene2020) in Italian calcareous grasslands. Even though most of the cited studies were performed on lichen associations of acidophilous sand, several species overlap with the current research. Cladonia chlorophaea, C. fimbriata, C. foliacea, C. furcata, C. glauca, C. pyxidata, C. rangiformis, C. subulata, C. rei, Diploschistes muscorum, Peltigera canina and P. rufescens are also present in Denmark, Estonia, Italy, Netherlands or Poland. Cladonia foliacea, C. furcata, C. rangiformis, C. pyxidata and Diploschistes muscorum were the most frequent species in the present study. These species have a broad ecological tolerance and can be found on both acidic and calcareous soils, and in arid as well as humid microhabitats. Besides the Hungarian record, C. coniocraea was detected only on Estonian dunes.
Five of the lichen species recorded in the study area are listed on the Red List of lichens as occurring in Hungary and lacking in other mentioned European areas. Cladonia magyarica, Xanthoparmelia pulvinaris and X. subdiffluens have been protected since 2005, while X. pokornyi and X. ryssolea since 2013 (Farkas & Lőkös Reference Farkas and Lőkös2006; [Hungarian] Ministry of Rural Development 2013). The four Xanthoparmelia species were found only on arid dune sides, and their thalli were more abundant at Fülöpháza in the present study.
Conservation implications
From a conservation perspective, our results indicate that lichens could significantly contribute to the biodiversity of dry calcareous grasslands due to their heterogeneous community structure. In relevés where the cover of bare soil was higher (due to previous, low-intensity wildlife disturbances, e.g. trampling or rooting) the number of lichen species was lower, therefore activities resulting in more open surfaces (trampling, removal of vegetation etc.; Belnap & Eldridge Reference Belnap, Eldridge, Belnap and Lange2001) should be reduced to avoid species loss. However, low-intensity grazing can lead to a higher terricolous lichen species richness (Boch et al. Reference Boch, Prati, Schöning and Fischer2016), as was exhibited at the Fülöpháza site. Since the closing vegetation at Bugacpusztaháza is at an advanced stage towards the climax association, the territory can provide a less favourable environment for the steppe species moved by wind or animals.
It was also pointed out that arid and humid dune sides host special lichen communities (nine species occurred only on arid and another nine only on humid sides), unambiguously influenced by site. Disturbances in the past, which have been greater at Fülöpháza, might be required to maintain an open dune habitat facilitating establishment and growth of the endangered and protected species (Xanthoparmelia ryssolea, X. subdiffluens) and of the characteristic species of the Fulgensietum fulgentis association (Gyalolechia fulgens, Psora decipiens, Squamarina lentigera). Our results indicate that the further progression to climax vegetation shown at Bugacpusztaháza might eliminate those endangered species that should be protected. Nevertheless, the wood-correlated species were more abundant at the Bugacpusztaháza site, and they preferred humid dune sides, increasing species diversity. Determining the various needs of the different species provides further proof for the importance of habitat diversity.
Another threat for the cryptogamic communities is the introduction of non-native annual plant species (e.g. Asclepias syriaca L. in the studied regions). The probability of the adverse effects of biological invasions in these habitats is higher after soil disturbances when bare soil areas are created and, in general, these effects are more pronounced in the case of crustose species (Belnap & Eldridge Reference Belnap, Eldridge, Belnap and Lange2001).
We found that past disturbances can play a significant role in the species composition of terricolous lichen communities in inland duneland ecosystems and may result in an alteration in lichen species richness. The ability to compete with herbs and mosses may also be a crucial factor that determines the occurrence of different terricolous lichen species.
From a conservation and restoration point of view, these results could contribute to the development of conservation recommendations for achieving a more diverse terricolous lichen community (and therefore BSC community). In setting the ‘target state’ of an ecosystem, it is essential to determine the past disturbance history of the region and also be aware of the present state.
Conclusions
It was revealed, as a proxy for local species pool and land-use history, that the effect of site was stronger on terricolous lichen community composition than was the impact of dune aspect, and the effect of the side was distinct at the two localities, probably due to the different disturbance history and successional stage of the vegetation under the same climate. The dune aspect leads to distinct microclimatic patterns that induce differences in soil surface variables (especially those which are connected to the herbaceous layer) and lichen species composition. At sites with more open vegetation, cover of the fruticose growth form and herbs, as well as their height, decreased with aridity, unlike the cover of moss. Therefore, we can postulate that as a result of climate change (drier and warmer conditions are projected in this region), lichens can gain more space due to the shrinkage of vascular plant vegetation (Kröel-Dulay et al. Reference Gy, Ónodi, Szitár, Lhotsky, Mojzes and Kertész2018). However, lichens have to compete with mosses for these free surfaces. These changes will supposedly lead to higher cryptogamic biodiversity. On the other hand, species preferring arid microhabitats could appear, leading to a higher terricolous lichen diversity. Conversely, in microhabitats which might become more humid or where grazing is abandoned, lichen communities can be suppressed due to the closing vegetation. Some lichen species were found only on arid dune sides or more open sites (e.g. the protected steppe species or the key species of the Fulgensietum fulgentis association), while others occurred only on humid dune sides or on the site hosting more closed vegetation and trees (e.g. the wood-correlated species), therefore it is important to protect and maintain a diverse topography and landscape structure hosting various lichen communities when considering habitat-specific conservation and monitoring (Gheza et al. Reference Gheza, Assini, Lelli, Marini, Mayrhofer and Nascimbene2020) in inland dunelands. It is worth considering that the different species forming duneland communities have various microenvironmental needs and are very sensitive to different environmental factors.
Acknowledgements
The authors would like to express their thanks to Anita Juhász and Zoltán Zsíros for help with the fieldwork, and to the Kiskunság National Park for permission to conduct research. The authors are grateful to László Lőkös for the useful comments and to Gábor Ónodi for sharing information and experience on the investigation sites. The authors would also like to thank Scott LaGreca for revision of the English text. The present work was supported by the Hungarian Scientific Research Fund OTKA-T101713 and the National Research Development and Innovation Fund NKFI K 124341.
Author ORCIDs
Katalin Veres, 0000-0001-5818-4891; Zsolt Csintalan, 0000-0001-7744-3393; Bence Kovács, 0000-0002-8045-8489; Edit Farkas, 0000-0002-5245-1079.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282921000360