Introduction
Geographical ranges of lichen species are often underestimated, mainly because of the very unbalanced intensity of lichen diversity research in various regions of the world (Arcadia Reference Arcadia2013). Some species of microlichen (lichen crusts) have a distribution that is probably known reliably, often because of special circumstances, such as species of Dirina (Tehler et al. Reference Tehler, Ertz and Irestedt2013), most of which are restricted to coastal sites, a habitat that can be sampled fairly effectively because of its limited area. However, for most species distributional data are scarce, which might result in seemingly implausible disjunctions in known distributions, such as in Rinodina capensis (Mayrhofer et al. Reference Mayrhofer, Obermayer and Wetschnig2014), Sclerophora amabilis (Tibell Reference Tibell1999) and many others. Another reason for underestimated geographical ranges is the poor, but all too common, taxonomic practice of redescribing a lichen when it is found in different geographical regions, without adequately considering previous work. For instance, Sheard (Reference Sheard2010) provided some cases of crustose species that have been described and redescribed even in recent times.
The opposite problem, too extensive a reported geographical range, can be caused by insufficient taxonomic knowledge. According to the world biodiversity database GBIF (http://www.gbif.org/), some ‘prominent’ lichen names (e.g. Caloplaca citrina and C. holocarpa) are mapped throughout the world, but these species have not been confirmed outside temperate regions of the Northern Hemisphere (Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009, Reference Vondrák, Halıcı, Güllü and Demirel2016). The use of mainly European literature to determine lichens from other parts of the world has led to error in these cases and probably many others.
Russia includes most of northern Eurasia between 28°E and 169°W longitude and investigations of lichen diversity within its territory are essential to discover the real distributions of lichen taxa, especially those previously known only from Europe or North America (Davydov & Printzen Reference Davydov and Printzen2012). Although the lichen biota of Russia has been quite well studied, it is less known than that of western Eurasia, mainly because the territory is very large and some regions are difficult to access. Here we report on selected examples, supported with molecular data, where our Russian records have changed the previous understanding of a species’ range.
Materials and Methods
Specimens
Assessed specimens belong to nine species of Athallia, Calogaya, Caloplaca, Flavoplaca and Gyalolechia (Teloschistaceae). Specimens were collected by the authors from various regions of Russia. Acronyms of the author followed by the author’s herbarium numbers are used to identify specimens in the figures and in Table 1. Most specimens are precisely localized by WGS 84 coordinates. Vouchers collected by IU, IZ, JV, GU (Genadii Urbanavichus) and EM are deposited in PRA, those collected by LK and SC in LE, by ED and L. Yakovchenko in ALTB, by DH in LECB, by TS (Toby Spribille) in GZU and by IF in the private herbarium of the author. All specimens were examined and identified by the first author. For the molecular analyses we sequenced the ITS of selected samples from Russia, and also from other countries if GenBank data were scarce, to produce more comprehensive phylogenetic trees (Table 1).
Table 1 New ITS nrDNA sequences for Teloschistaceae used in this study together with locations, substrata and herbarium (hb) information
Sequences and phylogenetic reconstructions
DNA was extracted with a CTAB-based protocol (Aras & Cansaran Reference Aras and Cansaran2006). Primers for PCR amplification of ITS were ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee and Taylor1990). The PCR parameters included an initial hold at 94 °C for 5 min, and then 45 cycles with denaturating at 94 °C (30 s), annealing at 62 °C with the touchdown to 56 °C during the first 7 cycles (30 s), and an extension at 72 °C (60 s).
ITS nrDNA sequence data were used in our study for practical reasons: they are easily generated; the NCBI database (GenBank) includes a number of ITS sequences for reasonable fingerprinting; ITS single-locus genealogies are usually consistent with phenotypic data (seen in numerous ITS-based studies on Teloschistaceae) and are generally congruent with the loci nrLSU and mtSSU (e.g. Arup et al. Reference Arup, Søchting and Frödén2013). New sequences were submitted to NCBI’s BLAST website (Johnson et al. Reference Johnson, Zaretskaya, Raytselis, Merezhuk, McGinnis and Madden2008; http://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm taxonomic identity.
The 69 sequences from this study (Table 1) were arranged into five alignments for five genera together with close GenBank sequences (Table 2). Alignments were done in BioEdit 7.2.5 free software (Hall Reference Hall1999) with the use of ClustalW application (Thompson et al. Reference Thompson, Gibson, Plewniak, Jeanmougin and Higgins1997) and corrected by hand. Most of the GenBank data used are from Arup (Reference Arup2006), Arup & Grube (Reference Arup and Grube1999), Arup et al. (Reference Arup, Søchting and Frödén2013), Gaya et al. (Reference Gaya, Redelings, Navarro-Rosinés, Llimona, De Cáceres and Lutzoni2011), Himelbrant et al. (Reference Himelbrant, Stepanchikova, Motiejūnaitė, Vondrák, Tagirdzhanova, Gagarina and Kuznetsova2015), Joshi et al. (Reference Joshi, Vondrák, Vondráková, Nguyen and Hur2011), Kasalicky et al. (Reference Kasalicky, Döring, Rambold and Wedin2000), Malíček et al. (Reference Malíček, Palice and Vondrák2014), Powell & Vondrák (Reference Powell and Vondrák2011), Redchenko et al. (Reference Redchenko, Vondrák and Košnar2012), Šoun et al. (Reference Šoun, Vondrák, Søchting, Hrouzek, Khodosovtsev and Arup2011), Vondrák et al. (Reference Vondrák, Šoun, Hrouzek, Říha, Kubásek, Palice and Søchting2008, Reference Vondrák, Říha, Arup and Søchting2009, Reference Vondrák, Khodosovtsev, Šoun and Vondráková2012a , Reference Vondrák, Šoun, Vondráková, Fryday, Khodosovtsev and Davydov b ) and Wedin et al. (Reference Wedin, Baloch and Grube2002). Maximum likelihood (ML) phylogenetic analyses were run in the application Phylogeny.fr (Dereeper et al. Reference Dereeper, Guignon, Blanc, Audic, Buffet, Chevenet, Dufayard, Guindon, Lefort and Lescot2008) without Gblocks, with 250 bootstrap replicates and the GTR+I+G nucleotide substitution model. Outgroup sequences were selected from closely related genera on the basis of analyses by Arup et al. (Reference Arup, Søchting and Frödén2013) and our broader unpublished analyses.
Table 2 Alignment of the 69 sequences from this study for five genera of Teloschistaceae

* without 21 BP insertion in one sequence
Results
Athallia alnetorum (Giralt et al.) Arup et al.
See Arup et al. (Reference Arup, Søchting and Frödén2013) for nomenclatural details.
Caloplaca alnetorum Giralt et al. was combined into Athallia by Arup et al. (Reference Arup, Søchting and Frödén2013). It resembles some morphotypes of Gyalolechia flavorubescens s. lat., but according to Giralt et al. (Reference Giralt, Nimis and Poelt1992) differs in ascospore size and shape of conidia. We confirm that ascospore size is diagnostic, but we observed bacilliform conidia, characteristic of G. flavorubescens, in some specimens of A. alnetorum (specimens from Latvia; Frolov 663, 664). Athallia alnetorum is well known in Mediterranean mountains and the Alps (e.g. Giralt et al. Reference Giralt, Nimis and Poelt1992; Vondrák & Wirth Reference Vondrák and Wirth2013). It is new to Russia from the western foothills of the Caucasus Mountains but it is also common on the Baltic Sea coast in Latvia (I. Frolov, unpublished data), thus more northern Russian records are possible. The ITS sequence of the Russian specimen is within the A. alnetorum clade (see Supplementary Material Figure S1, available online).
Russian specimen. Russia: Krasnodar Krai: Caucasus Mts, Utrish Reserve, forested mountain c. 20 km SE from Anapa, alt. 430 m, 44·7212°N, 37·4684°E, broadleaved forest, on branch of Quercus petraea, 2014, I. Urbanavichene s. n. (PRA).
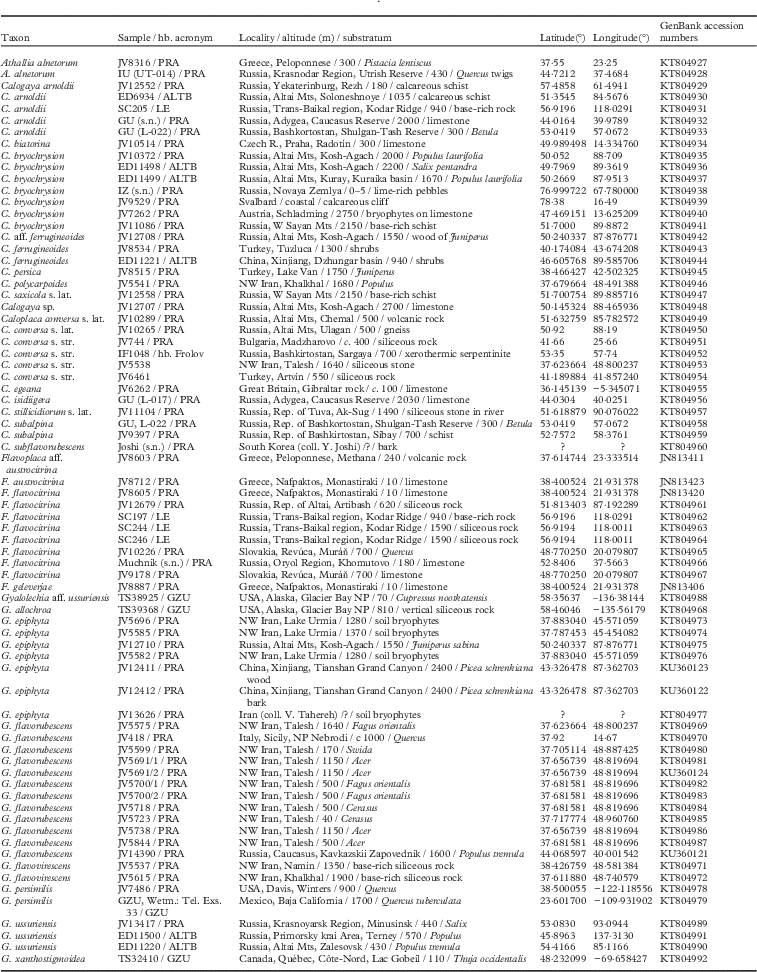
Calogaya arnoldii (Wedd.) Arup et al.
See Arup et al. (Reference Arup, Søchting and Frödén2013) for nomenclatural details.
A common lichen which has been called Caloplaca saxicola (Hoffm.) Nordin by numerous Russian lichenologists (cf. Urbanavichus Reference Urbanavichus2010) but proved to be Calogaya arnoldii (sensu Gaya Reference Gaya2009; Gaya et al. Reference Gaya, Redelings, Navarro-Rosinés, Llimona, De Cáceres and Lutzoni2011). Calogaya arnoldii and Calogaya saxicola (the combination proposed below) are closely related and the differences are subtle; they mostly concern shape and size of ascospores. However, both taxa are phenotypically variable, their characters overlap, and they cannot be identified with certainty from their phenotype. The Russian specimens were identified from their ITS sequences (Fig. 1). One Russian specimen from the Western Sayan Mountains (JV12558) has an ITS sequence (KT804947) similar to Calogaya saxicola sensu Gaya et al. (Reference Gaya, Redelings, Navarro-Rosinés, Llimona, De Cáceres and Lutzoni2011) and could be considered conspecific with C. saxicola.
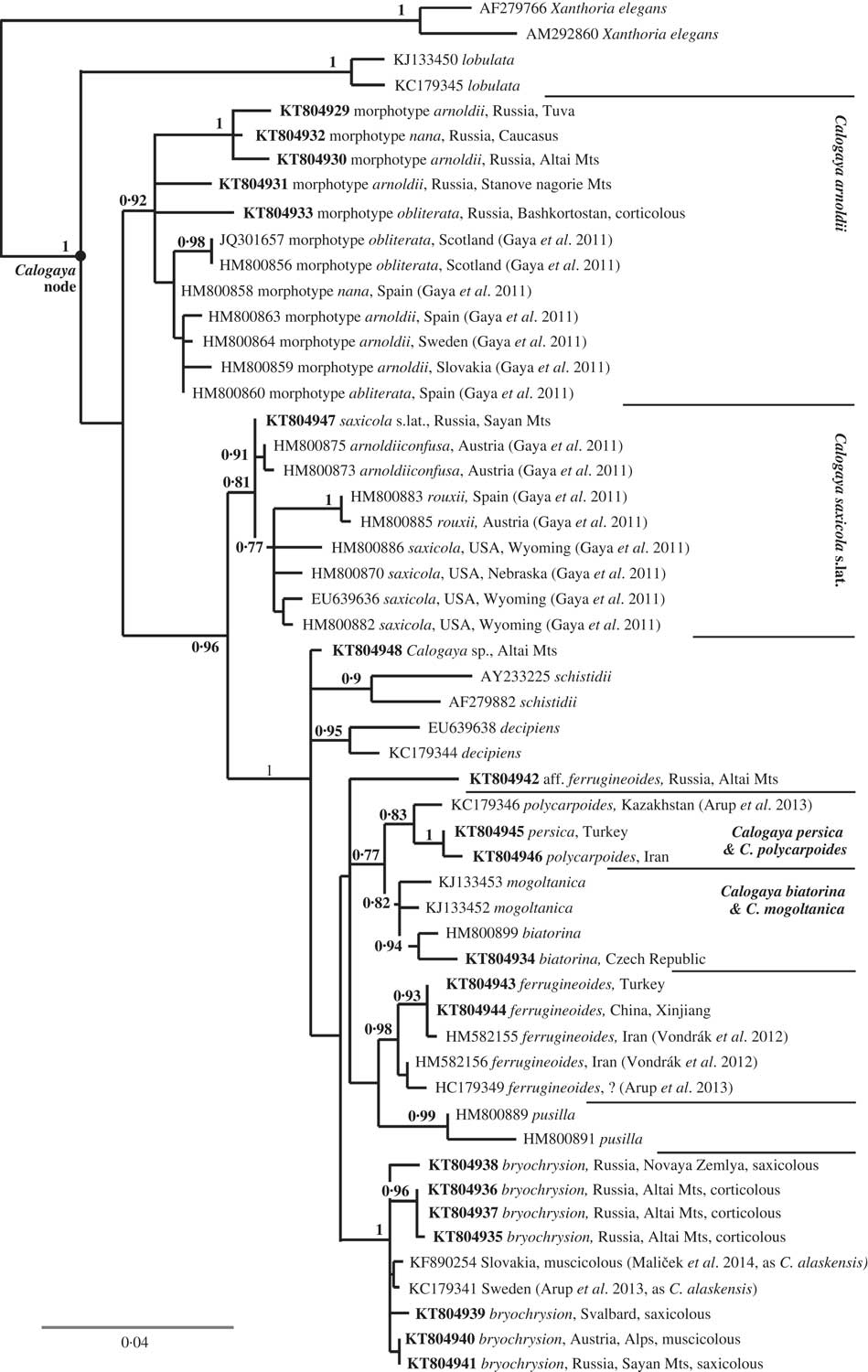
Fig. 1 Maximum likelihood ITS phylogeny of Calogaya showing positions of C. arnoldii, C. bryochrysion and C. ferrugineoides. New sequences are in bold; bootstrap supports (BS>0·7) are shown at nodes.
We consider the subspecies arnoldii, nana, and obliterata proposed by Gaya (Reference Gaya2009) to be merely expressions of phenotype plasticity within the species C. arnoldii, and our opinion is reflected in the ITS tree (Fig. 1).
Russian specimens. Russia: Republic of Adygea: Caucasus Mts, Caucasus Reserve, Kamennoe More Ridge, the edge of a cliff above Armyanka River, 44·0164°N, 39·9789°E, alt. 2000–2030 m, on limestone, 2011, G. Urbanavichus s. n. (PRA). Republic of Bashkortostan: Southern Ural Mts, Shulgan-Tash Reserve, cliff above Kapova Cave at the banks of Belaya River, 53·0419°N, 57·0672°E, alt. 300 m, on bark of Betula, 2007, G. Urbanavichus s. n. (PRA). Altai Krai: Soloneshensk district, Bashchelaksky Range, valley of Shinok River, alt. 1035 m, 51·3545°N, 84·5676°E, on stone, 2003, E. Davydov 6934 (ALTB). Sverdlovsk Region: Yekaterinburg, Rezh, Glinskoe, 0·5 km E of Chepchugovo Village, 57·4858°N, 61·4941°E, on lime-rich schist, 2013, J. Vondrák 12552 (PRA). Zabaikalsky Krai: Kodar Ridge, 56·9196°N, 118·0291°E, on lime-enriched siliceous rock, 2013, L. Konoreva s. n. (LE).
Calogaya bryochrysion (Poelt) Vondrák comb. nov.
MycoBank No.: MB 814538
Caloplaca bryochrysion Poelt, Feddes Repertorum 58: 175 (1955); type: Germany, Wettersteingebirge, Gipfel der Alpspitze, in feinen Felsspalten an Vogelblöcken, 1954, Poelt (M-0024347—holotype seen).
Syn. nov. Caloplaca alaskensis Wetmore, Bryologist 107: 507 (Reference Wetmore2004); type: USA, Alaska, valley of Mancha Creek with Firth River, 1958, Sharp 6531 (MIN—holotype).—Calogaya alaskensis (Wetmore) Arup et al. (Reference Arup, Søchting and Frödén2013: 38).
(Fig. 5A)
The name Caloplaca bryochrysion was synonymized with C. epiphyta by Hansen et al. (Reference Hansen, Poelt and Søchting1987). Søchting & Tønsberg (Reference Søchting and Tønsberg1997), however, considered C. epiphyta synonymous with C. xanthostigmoidea (=Gyalolechia xanthostigmoidea), but recognized C. bryochrysion as distinct. Caloplaca xanthostigmoidea and related taxa (now the genus Gyalolechia) contain fragilin and some chlorinated anthraquinones, but the type of C. bryochrysion has parietin as the main anthraquinone and lacks substances characteristic of Gyalolechia (Søchting & Tønsberg Reference Søchting and Tønsberg1997). Those authors therefore suggested that C. bryochrysion is related to C. citrina, a morphologically similar taxon with the same pigments.
We examined Caloplaca bryochrysion specimens from the Austrian Alps (in GZU, PRA) and also obtained an ITS sequence (JV7262 in Table 1) that groups with two C. alaskensis sequences (Fig. 1). We further compared the type of C. bryochrysion (Poelt Reference Poelt1955) with numerous samples of Calogaya alaskensis and consider both names synonymous. The epithet bryochrysion has priority over alaskensis, so a new combination is required.
Wetmore (Reference Wetmore2004) described Caloplaca alaskensis (now Calogaya) from only two localities in Alaska, but within a few years it had been reported from numerous arctic and boreal-alpine localities in North America, Europe, Svalbard and Greenland (Søchting et al. Reference Søchting, Lorentsen and Arup2008). The latter authors also provided ITS sequence data showing that geographically distant samples called C. alaskensis belong to the same species. Recently it was also found in central Europe, in the Carpathians (Malíček et al. Reference Malíček, Palice and Vondrák2014).
We obtained five ITS sequences from five Russian samples of Calogaya bryochrysion. Two are from arctic-alpine habitats and typical substrata (calcareous rock, calciphilous bryophytes), but the other three are from dry continental, semi-desert habitats in the Altai Mountains. They were collected on Populus laurifolia and Salix pentandra growing along rivers in high mountains mostly covered by dry steppe communities. This corticolous population may eventually prove to be an incipient species that is already distinct from the arctic-alpine population, but that is not evident from the ITS (Fig. 1) and morphological data, and so for the present we include it in C. bryochrysion.
Russian specimens. Russia: Republic of Altai: Kosh-Agach district, SE part of Kuray Ridge, NE of Chagan-Uzun Village, alt. 2000 m, 50·052°N, 88·709°E, on bark of Populus laurifolia, 2012, I. Frolov & J. Vondrák 10372 (PRA); Kosh-Agach district, left bank of Yustyd River, 2 km downstream of junction of Boguty and Naryngol Rivers, alt. 2200 m, 49·7969°N, 89·3619°E, on bark of Salix pentandra, 2013, E. Davydov 11498 (ALTB); Kosh-Agach district, Kurai, right bank of Kuraika River at 5 km N of Kurai, alt. 1670 m, 50·2669°N, 87·9513°E, on bark of Populus laurifolia, 2013, E. Davydov 11499 (ALTB). Republic of Tuva: West Sayan Mts, Ak-Dovurak, Ak-Sug, Enge-Beldir, glacier cirque in S-slope from pass ‘Sayanskiy’, 2200 m, at road A161, close to Republic of Khakasia border, alt. 2150–2200 m, 51·7000°N, 89·8872°E, on base-rich schist, 2013, I. Frolov & J. Vondrák 11086 (PRA). Arkhangelsk Region: Novaya Zemlya Archipelago, NE extremity of Severny Island, Karlsen Cape, alt. 0–5 m, 76·9997°N, 67·7800°E, on lime-rich pebbles at seashore, 2013, I. Zhdanov (LE). Zabaikalsky Krai: Kodar ridge, Hadytkanda valley, alt. 1230 m, 56·7480°N, 117·2650°E, 2015, S. Chesnokov 249 (LE); ibid., valley of Zolotoy brook, alt. 1410 m, 56·8389°N, 117·3064°E, 2015, S. Chesnokov 161 (LE).
Calogaya saxicola (Hoffm.) Vondrák comb. nov.
MycoBank No.: MB 815508
Psora saxicola Hoffm., Descr. Adumb. Pl. Lich. 1 (3): 82, Tab. 17, Fig. 3 (1790); type: Sweden (H-Ach 1019E “Lecanora murorum, Svecia”—neotype selected by Nordin Reference Nordin1972).
Caloplaca isidiigera Vězda
See Šoun et al. (Reference Šoun, Vondrák, Søchting, Hrouzek, Khodosovtsev and Arup2011) for nomenclatural details.
(Fig. 2A; distribution map)
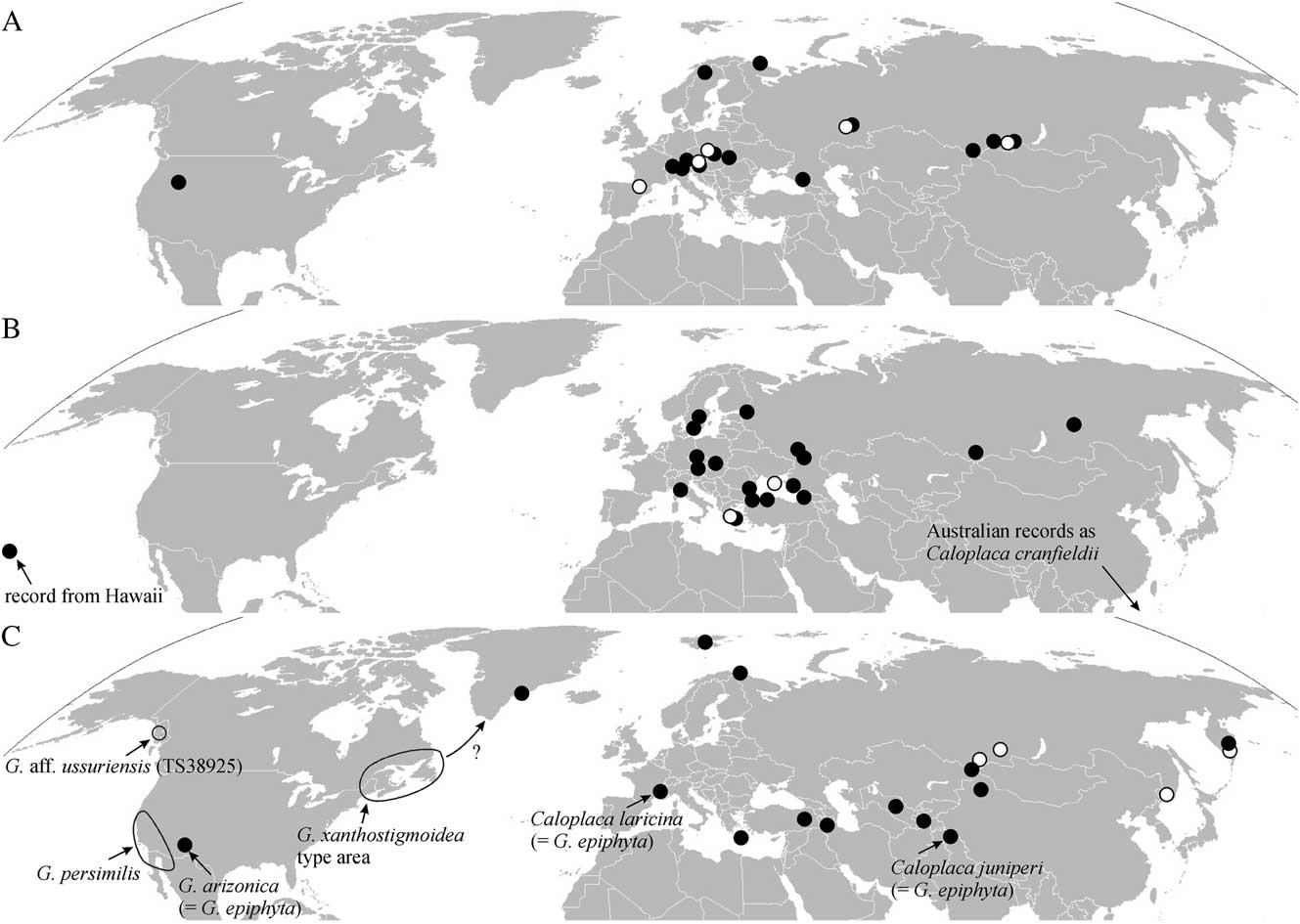
Fig. 2 Locations of specimens sequenced and confirmed in this study. A, Caloplaca isidiigera (black dots) and C. subalpina (white dots); B, Flavoplaca flavocitrina (black dots) and the closely related F. geleverjae (white dots); C, Gyalolechia epiphyta (black dots) and G. ussuriensis (white dots), with approximate ranges of other sorediate Gyalolechia (outlined).
Caloplaca isidiigera, described from the Carpathians (Vězda Reference Vězda1978), is known from numerous montane-alpine sites in Europe and North America (Šoun et al. Reference Šoun, Vondrák, Søchting, Hrouzek, Khodosovtsev and Arup2011). We newly report it from several localities in southern Siberia and suggest that it has a circumpolar distribution. Caloplaca isidiigera also occurs at low altitudes in continental Eurasia (e.g. JV9541 from the Chelyabinsk Region). An ITS sequence from the specimen from the Caucasus Mountains falls within the Caloplaca isidiigera clade (see Supplementary Material Figure S2, available online).
Russian specimens. Russia: Republic of Adygeya: Caucasus Mts, Caucasus Reserve, Kamennoe More Ridge, c. 0·85 km N of Mt. Nagoi Koshi, on limestone, 44·0304°N, 40·0251°E, alt. 2025 m, 3 vii 2011, G. Urbanavichus s. n. (PRA). Republic of Altai: Altai Mts, Choya district, Karakoksha, settlement Uymen’, Mt. Sagani (2036 m), c. 40 km S of Karakoksha, alt. 1700–2030 m, on vertical face of base-rich rock in subalpine zone, 2012, I. Frolov & J. Vondrák 10315 (PRA). Republic of Bashkortostan: Ural Mts, Irendik Range, Sibay, Gabelsha Village (c. 15 km W of Sibay), waterfall Gadelsha in upper stream of brook Khudolaz, alt. 500–800 m, 52·7572°N, 58·3761°E, on shaded base-rich siliceous stone in brook, 2011, I. Frolov & J. Vondrák 10512 (PRA). Republic of Tuva: West Sayan Mts, Ak-Dovurak, Ak-Sug, Enge-Beldir, glacier cirque in S-slope from pass ‘Sayanskiy pereval’, 2200 m, at road A161, close to border with Republic of Khakasia, alt. 2150–2200 m, 51·7000°N, 89·8872°E, on S-exposed schist outcrop, below overhang, in alpine zone, 2013, I. Frolov & J. Vondrák 11099 (PRA). Chelyabinsk Region: Magnitogorsk, in steppe c. 10 km S of town, alt. c. 300 m, 53·2613°N, 58·9263°E, on limestone boulders in steppe, 2011, O. Vondráková & J. Vondrák 9541 (PRA). Krasnoyarsk Krai: West Sayan Mts, Minusinsk, at road Minusinsk–Kyzyl, 2 km E of pass ‘Buybinskiy pereval’, E-exposed glacier cirque with mica-schist bedrock, alt. 1550–1600 m, 52·8491°N, 93·2808°E, on vertical mica-schist rock face in subalpine zone, 2013, I. Frolov & J. Vondrák 12653, 12654, 12697 (PRA). Murmansk Region: Pechenga, Kandalakskiy Reserve, Bolshoy Aynov Island, alt. 20 m, 69·8355°N, 31·5691°E, on siliceous stone, 2010, A. V. Melekhin s. n. (KPABG, det. I. Frolov).
Caloplaca subalpina Vondrák et al.
See Šoun et al. (Reference Šoun, Vondrák, Søchting, Hrouzek, Khodosovtsev and Arup2011) for nomenclatural details.
(Fig. 2A; distribution map)
Caloplaca subalpina was previously known from subalpine and alpine zones of the Alps, the Carpathians, the Pyrenees and the Sudetes (Vondrák et al. Reference Vondrák, Šoun, Hrouzek, Říha, Kubásek, Palice and Søchting2008), but according to our new data, its range extends much further eastwards, to the Western Sayan Mountains. No previous reports were corticolous, but one of our collections is from birch bark, where it is accompanied by two other generally saxicolous lichens, Caloplaca arnoldii and Xanthoria sorediata. ITS sequences of two Russian samples are placed in the Caloplaca subalpina clade (see Supplementary Material Figure S2, available online).
Russian specimens. Russia: Republic of Bashkortostan: Southern Ural Mts, Irendik Range, Sibay, Gadelsha Village (c. 15 km W of Sibay), waterfall Gadelsha in upper stream of brook Khudolaz, alt. 500–800 m, 52·7572°N, 58·3761°E, on vertical face of base-rich schist, with Leproplaca obliterans, 2011, I. Frolov & J. Vondrák 9397 (PRA); Southern Ural Mts, Shulgan-Tash Reserve, cliff above Kapova Cave at the banks of Belaya River, 53·0419°N, 57·0672°E, alt. 300 m, on bark of Betula, 2007, G. Urbanavichus s. n. (PRA). Krasnoyarsk Krai: West Sayan Mts, Minusinsk, at road Minusinsk–Kyzyl, 2 km E of pass ‘Buybinskiy pereval’, E-exposed glacier cirque with mica-schist bedrock, alt. 1550–1600 m, 52·8491°N, 93·2808°E, on vertical mica-schist rock face in subalpine zone, 2013, I. Frolov & J. Vondrák 12652, 12658, 12667, 12673 (PRA).
Flavoplaca flavocitrina (Nyl.) Arup et al.
See Arup et al. (Reference Arup, Søchting and Frödén2013) for nomenclatural details.
Syn. nov. Caloplaca cranfieldii S. Y. Kondr. & Kärnefelt in Kondratyuk et al., Bibl. Lichenol. 95: 352 (Reference Kondratyuk, Kärnefelt, Elix and Thell2007); type: Western Australia, Northampton, Lynton, on sandstone, 2004, Kärnefelt & Cranfield (Kondratyuk 20423, PERTH—holotype; GZU—isotype seen).—Flavoplaca cranfieldii (S. Y. Kondr. & Kärnefelt) Arup et al., Nord. J. Bot. 31: 45 (Reference Arup, Søchting and Frödén2013).
(Fig. 2B; distribution map)
Caloplaca flavocitrina (Nyl.) H. Olivier was synonymized with C. citrina by Laundon (Reference Laundon1965) and this view was accepted by many, including Russian authors (e.g. Stepanchikova et al. Reference Stepanchikova, Himelbrant and Konoreva2014). However, some recent authors have regarded C. flavocitrina as distinct from other yellow sorediate crusts of C. citrina s. lat. (cf. Vondrák et al. Reference Vondrák, Kocourková, Palice and Liška2007). ITS sequence data have confirmed that it is distinct (Arup Reference Arup2006; Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009). It is now placed in the genus Flavoplaca, which includes both sorediate and non-sorediate crusts (Arup et al. Reference Arup, Søchting and Frödén2013).
Flavoplaca flavocitrina s. lat. (including F. geleverjae) forms a well-supported clade (BS=1, Fig. 3), sister to a clade composed of F. austrocitrina and F. limonia that acts as outgroup. Flavoplaca flavocitrina differs from this outgroup in 13 nucleotide substitutions in our ITS alignment. Flavoplaca citrina, F. confusa and F. nigromarina, three morphologically similar taxa, are less closely related to F. flavocitrina in ITS. Flavoplaca geleverjae differs from F. flavocitrina in five nucleotide substitutions (two of them shared with the outgroup) and it may be a distinct species (Khodosovtsev et al. Reference Khodosovtsev, Kondratyuk and Kärnefelt2003; Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009). The sequence EU563389 (F. aff. flavocitrina, Bulgaria) is also included in the Flavoplaca flavocitrina s. lat. clade, but differs from F. flavocitrina in five substitutions (four of them shared with the outgroup). The corresponding specimen has F. flavocitrina morphology.
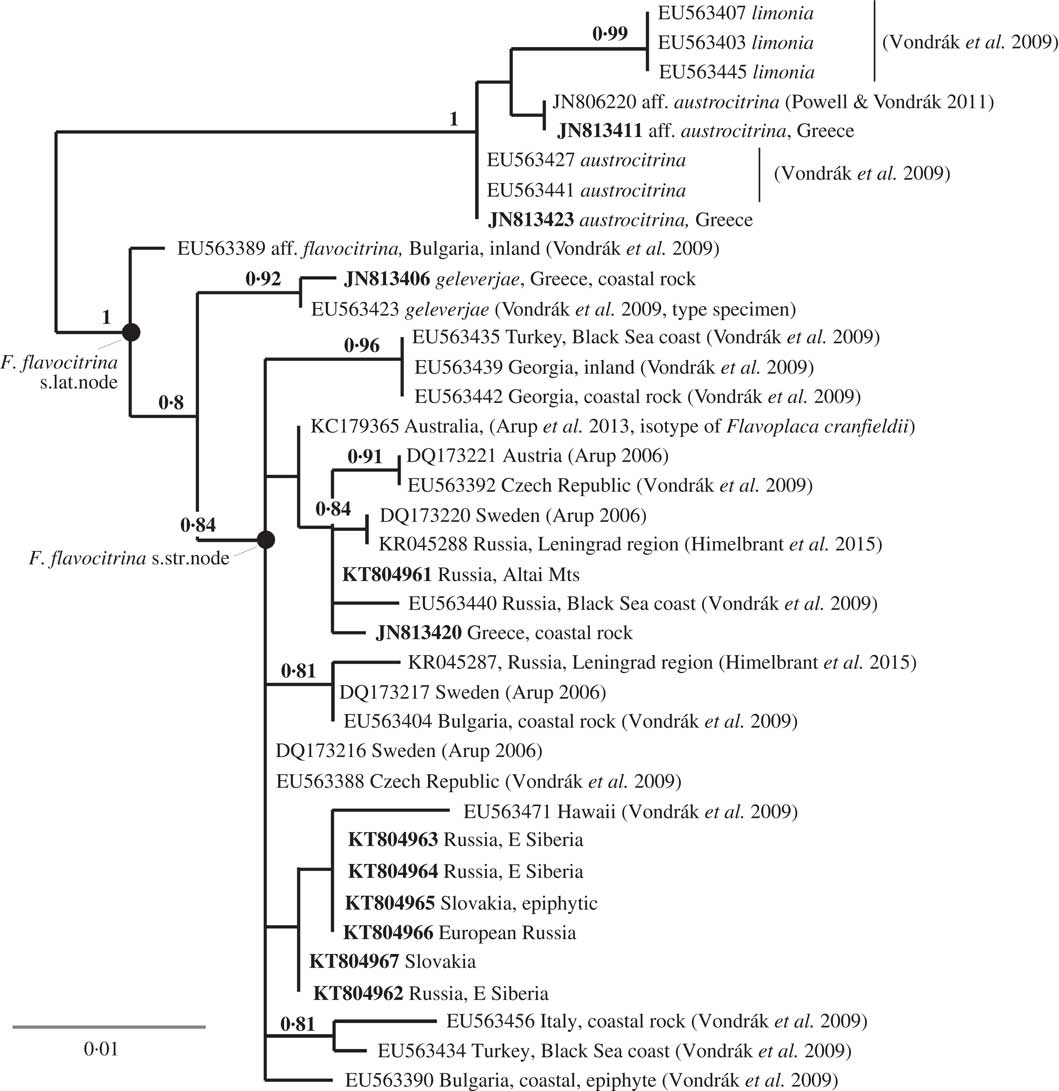
Fig. 3 Maximum likelihood ITS phylogeny of a section within Flavoplaca including F. flavocitrina. New sequences are in bold; bootstrap supports (BS>0·7) are shown at nodes.
Flavoplaca flavocitrina is exceptional among taxa of this genus owing to its very broad ecological range. It can grow on mineral-rich siliceous and calcareous rocks, numerous artificial substrata (e.g. tarmac, concrete), dust-impregnated wood and on base-rich bark (e.g. Acer platanoides, Ulmus glabra). No other species of Flavoplaca is so indifferent to substratum, and very few species anywhere in Teloschistaceae are so indifferent. It may be almost cosmopolitan in the Northern Hemisphere, which is also exceptional in Flavoplaca: as well as numerous European and Mediterranean records, it is known from North America (Brodo et al. Reference Brodo, Harris, Buck, Lendemer and Lewis2013), Hawaii (Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009) and Siberia (this paper).
Flavoplaca flavocitrina also occurs in the Southern Hemisphere (Australia), where it has been known as Caloplaca cranfieldii (Kondratyuk et al. Reference Kondratyuk, Kärnefelt, Elix and Thell2007; ≡ Flavoplaca cranfieldii). The isotype of C. cranfieldii in GZU matches F. flavocitrina morphologically, and the ITS sequence from the type (published by Arup et al. Reference Arup, Søchting and Frödén2013) falls into the F. flavocitrina clade in our phylogenetic reconstruction (Fig. 3). We consider C. cranfieldii to be a synonym of Flavoplaca flavocitrina.
There are several reports of Flavoplaca flavocitrina from European Russia (Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009; Muchnik et al. Reference Muchnik, Wilk, Vondrák and Frolov2014; Himelbrant et al. Reference Himelbrant, Stepanchikova, Motiejūnaitė, Vondrák, Tagirdzhanova, Gagarina and Kuznetsova2015). We can now add records from two Siberian localities, from siliceous rocks in natural habitats. It is definitely the most widely distributed species of Flavoplaca in Russia; most others are restricted to the Black Sea coast, such as F. arcisproxima, F. austrocitrina and F. communis (Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009), or to European Russia, such as F. dichroa (e.g. Vondrák et al. Reference Vondrák, Redchenko, Himelbrant, Stepanchikova and Kuznetsova2010). Identification of Flavoplaca flavocitrina should be confirmed by molecular barcoding (ITS sequences), because some taxa, including F. citrina (not confirmed from Russia), are very similar.
Russian specimens. Russia: Republic of Altai: Altai Mts, Turochak district, Artibash, c. 5 km NW of village, SW-exposed gneiss rocks above right bank of Biya River, alt. 450 m, on vertical face of siliceous rock, 2012, I. Frolov & J. Vondrák 12679 (PRA). Oryol Region: Krasnaya Zarya district, Khomutovo, alt. 180 m, 52·8406°N, 37·5663°E, on limestone, 2014, Muchnik s. n. (PRA). Zabaikalsky Krai: Kodar Ridge, alt. 940 m, 56·9196°N, 118·0291°E, 2013, L. Konoreva s. n. (LE, Chesnokov197); ibid., alt. 1590 m, 56·9194°N, 118·0011°E, on siliceous rock, 2013, L. Konoreva s. n. (LE, Chesnokov244, 246).
Gyalolechia epiphyta (Lynge) Vondrák comb. nov.
MycoBank No.: MB 815509
Caloplaca epiphyta Lynge, Skrifter om Svalbard og Ishavet 81: 119 (1940); type: [Greenland], Østgrønland, Jackson, Ø, 1929, Lynge (O-L-1279—holotype, seen in http://nhm2.uio.no/lav/web/index.html).
Syn. nov. Caloplaca arizonica H. Magn., Bot. Not. 1944: 69–70 (1944); type: USA, Arizona, Grand Canyon NP, Coconino Plateau, on Juniperus monosperma, 1926, E. & G. DuRietz 182/1 (UPS—holotype, not seen).—Gyalolechia arizonica (H. Magn.) Søchting et al. in Arup et al. (Reference Arup, Søchting and Frödén2013: 70).
Syn. nov. Caloplaca juniperi Poelt & Hinteregger, Bibl. Lichenol. 50: 150–152 (1993); type: Pakistan, Karakorum Mountains, Gilgit, Rakaposhi Range, Baghrot, N-facing flank opposite Sat, alt. 2600–2700 m (36°03'N, 74°35'E), on old Juniperus, 1991, J. Poelt (GZU—holotype, seen).
Syn. nov. Caloplaca juniperina Tomin, Bot. Materialy, Notulae System. e Sect. Cryptog. Inst. Bot. Nomine V. L. Komarovii Acad. Sci. URSS 9: 11–12 (1953); syntypes—Uzbekistan (Uzbekskaya SSR), northern slopes of Alay ridge, 1) Dzhaylayau Shayd, 26 vii 1948; 2) Dzhaylayau Mashelan’, 10 vii 1950; 3) ibid., 15 vi 1951; all syntypes collected by F. Shafeev (syntype 2 in LE seen and selected here as lectotype).—Gyalolechia juniperina (Tomin) Søchting et al. in Arup et al., Nord. J. Bot. 31: 71 (Reference Arup, Søchting and Frödén2013).
Syn. nov. Caloplaca laricina Rondon, Rev. Bryol. Lich. 32: 260 (Reference Rondon1963); type: France, Hautes-Alpes, Ville-Vieille en Queyras, alt. 1400 m, [44·4136°N, 6·2498°E], on wood of Larix decidua, 1957, Y. Rondon (G00288634—type not seen).
Syn. nov. Caloplaca tarani S. Y. Kondr. et al. in Kondratyuk et al., Acta Bot. Hungarica 55: 48–52 (Reference Kondratyuk, Lőkös, Zarei-Darki, Haji Moniri, Tchabanenko, Galanina, Yakovchenko, Hooshmand, Ezhkin and Hur2013); type: Russia, Sakhalin Island, Smirnykhovsky district, at the base of Mt Pogranichnaya, mixed deciduous and coniferous forest, on bark of Ulmus laciniata. 30.05.1997, A. A. Taran (SAKH—holotype, Fig. 4 in Kondratyuk et al. Reference Kondratyuk, Lőkös, Zarei-Darki, Haji Moniri, Tchabanenko, Galanina, Yakovchenko, Hooshmand, Ezhkin and Hur2013).
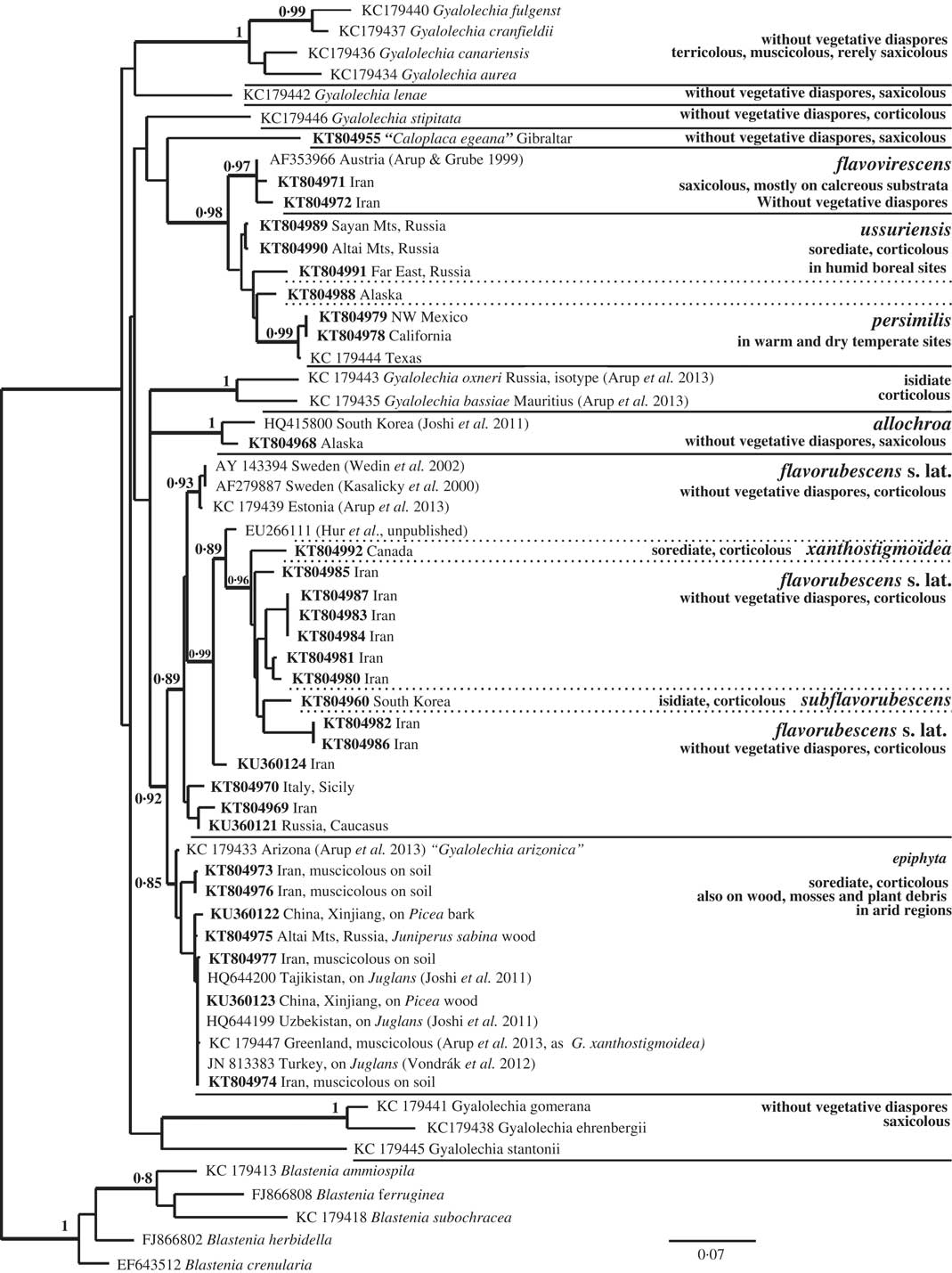
Fig. 4 Maximum likelihood ITS phylogeny of Gyalolechia showing positions of G. epiphyta and G. ussuriensis. New sequences are in bold; bootstrap supports (BS>0·7) are shown at nodes.
(Fig. 2C, distribution map; Fig. 5B)
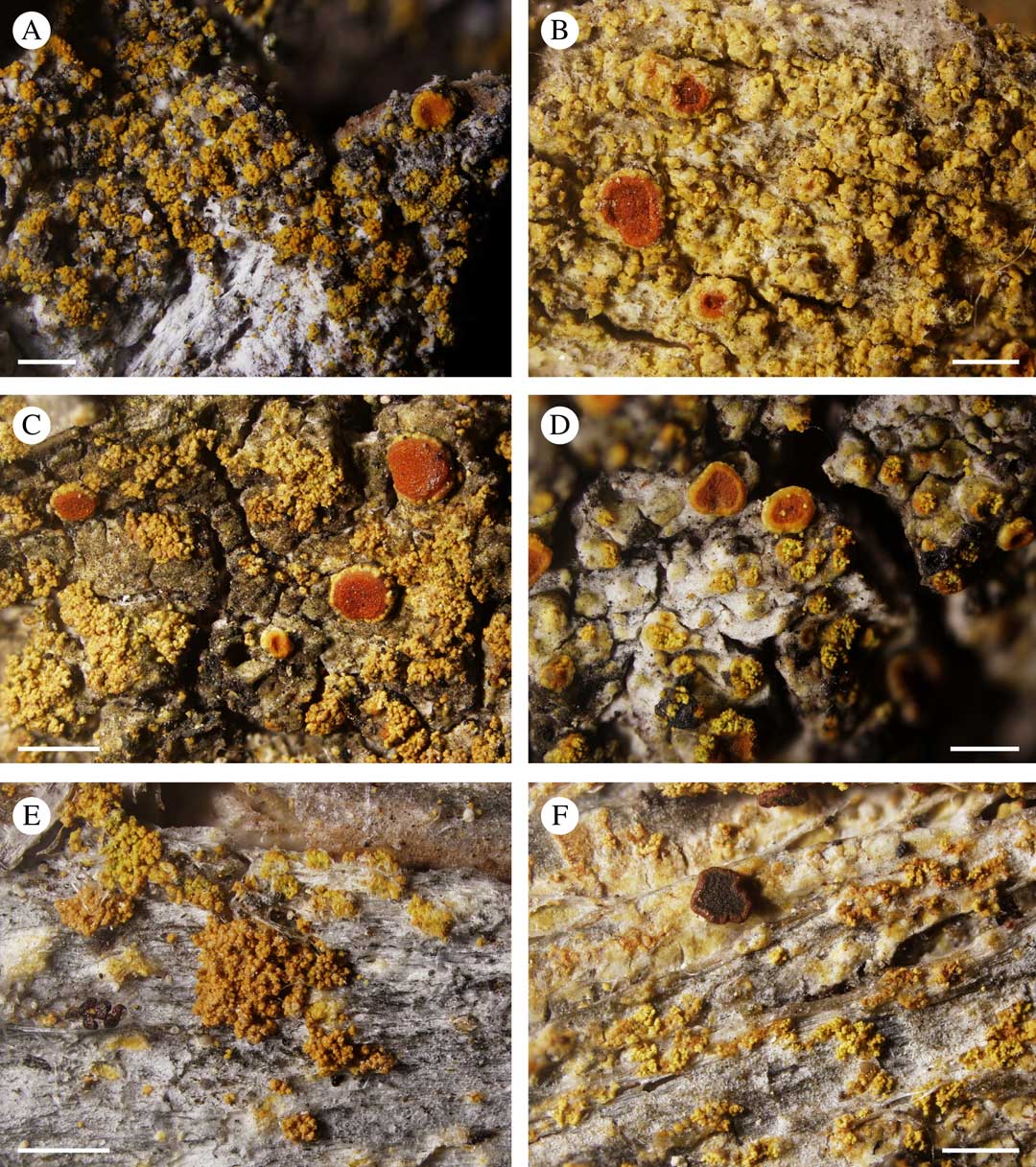
Fig. 5 A, Calogaya bryochrysion, corticolous specimen from Altai Mts (ED11499, KT804937); B, Gyalolechia epiphyta with blastidiate thallus and without soralia from Tajikistan (hb. Halda 174, HQ644199); C, Gyalolechia persimilis with pale yellow thallus and bright yellow soralia from California (JV7486, KT804978); D, Gyalolechia ussuriensis with pale yellow thallus and bright yellow soralia from the Russian Far East (ED11500, KT804991); E, Gyalolechia aff. ussuriensis with an inconspicuous endophloedal thallus and bright yellow soralia from Alaska (TS38925, KT804988); F, Gyalolechia xanthostigmoidea from eastern Canada (TS32410, KT804992), a taxon morphologically similar to G. persimilis/G. ussuriensis. Scales: A–F=0·5 mm.
Gyalolechia epiphyta is diagnosed by its blastidiate/granulose thallus and absence of true soralia (Fig. 5B), but it is quite similar to the sorediate taxa Gyalolechia persimilis, G. ussuriensis and G. xanthostigmoidea. Gyalolechia epiphyta forms a supported clade in the ITS tree (Fig. 4). Variability among 12 sequences included in the ITS tree was detected in 19 positions, but this variability is rather randomly distributed; each sequence pair within the clade is more than 98·5% identical. The exception is KC179447 (from Greenland) which contains an indel of 21 bp length that is absent in other Gyalolechia species. The closest relative is G. flavorubescens s. lat. (including G. xanthostigmoidea and “Caloplaca” subflavorubescens) which forms a supported ITS clade with considerable internal variability (Fig. 4).
Gyalolechia epiphyta is widely distributed in the Arctic and temperate zones of the Northern Hemisphere. In continental regions it prefers steppes and dry forests. It is usually epiphytic or epixylic (often on Juniperus), but also epigeic or epibryic in rock crevices in arctic-alpine habitats or in steppes. Its epilithic occurrences are common in the Arctic. It is variable in thallus morphology; in particular, the size of vegetative diaspores (blastidia, granules) varies considerably, often within a single thallus. When it grows on bark, it is commonly fertile, but specimens from soil or bryophytes are usually sterile.
The wide geographical range of G. epiphyta and its occurrence in different climatic zones and on different substrata has resulted in it being described as new several times under different names. We consider Gyalolechia arizonica synonymous with G. epiphyta. We have not seen its type specimen, but the ITS sequence of the specimen “T.H. Nash 38931 (C)” is placed within the G. epiphyta clade. We have also appraised several specimens of G. arizonica from Arizona (T. H. Nash 16456 in PRA-V, T. H. Nash 21219 in PRA-V, O. Breuss 27.7.1991 in W) and morphologically they fit collections of G. epiphyta with coarse granules. They were collected from Juniperus, a typical substratum for Asian populations of G. epiphyta.
We have seen type specimens of Caloplaca juniperi from northern Himalaya and Gyalolechia juniperina from Central Asia and we consider them conspecific with G. epiphyta. Rondon (Reference Rondon1963) described Caloplaca laricina from the Alps; although we did not locate its type, we appraised the specimen collected by Rondon in 1963 from Larix wood in Basses-Alpes, Méolans (A. Vězda: Lich. Sel. Exs. 250 in PRA-V) and it has G. epiphyta morphology. The photograph showing thallus morphology in the description by Rondon (Reference Rondon1963) also represents G. epiphyta. The protologue of Calplaca tarani with a photograph of the type (Kondratyuk et al. Reference Kondratyuk, Lőkös, Zarei-Darki, Haji Moniri, Tchabanenko, Galanina, Yakovchenko, Hooshmand, Ezhkin and Hur2013) indicates that this taxon described from the Far East is also G. epiphyta. We have assessed specimens collected from Kamchatka in the Far East (in the list below) that have G. epiphyta morphology but, unfortunately, repeated attempts to sequence these specimens were unsuccessful.
Despite Gyalolechia epiphyta having been described from many parts of the world under different names, we disagree with the synonymization of G. xanthostigmoidea (Räsänen) Søchting et al. in Arup et al. (Reference Arup, Søchting and Frödén2013: 72) with G. epiphyta proposed by Søchting & Tønsberg (Reference Søchting and Tønsberg1997). Gyalolechia xanthostigmoidea, described from New Brunswick in Canada (Räsänen Reference Räsänen1933), is probably a distinct taxon more similar to G. persimilis/G. ussuriensis, because it forms soralia (Fig. 5F) and its ITS sequence (see Table 1 for specimen details) does not place it in the G. epiphyta clade (Fig. 4). Arctic-alpine, blastidiate specimens belong to G. epiphyta, as supported by the ITS sequence KC179447 from the Greenland specimen (Fig. 4), called “G. xanthostigmoidea” by Arup et al. (Reference Arup, Søchting and Frödén2013).
Russian specimens. Russia: Republic of Altai: Altai Mts, Kosh-Agach district, Kuray Steppe, limestone hills c. 4 km W of Kuray, alt. 1470–1680 m, on wood of Juniperus sabina, 2012, I. Frolov & J. Vondrák 12710 (PRA); ibid., on mosses in limestone crevices, J. Vondrák 10319 (PRA); Kosh-Agach district, SE part of Kuray Ridge, NE of Chagan-Uzun Village, alt. 3000–3100 m, over mosses on limestone outcrop in alpine zone, 2012, I. Frolov & J. Vondrák 10353 (PRA). Kamchatka Krai: Ust’-Bol’sheretsk district, Praviy Kihchik River basin, alt. 250m, 53·558224°N, 156·738025°E, on Lonicera caerulea, 2004, D. Himelbrant s. n. (PRA, ex LECB); ibid., alt. 220 m, 53·581380°N, 156·683090°E, on Populus suaveolens, 2004, D. Himelbrant s. n. (PRA, ex LECB); ibid., alt. 250 m, 53·548477°N, 156·697123°E, on Populus suaveolens, 2004, D. Himelbrant s. n. (PRA, ex LECB).
Gyalolechia ussuriensis (Oxner, S. Y. Kondr. & Elix) Vondrák comb. nov.
MycoBank No.: MB 814537
Caloplaca ussuriensis Oxner et al. in Kondratyuk et al., Folia Cryptogamica Estonica 48: 21–23 (2011); type: Russia, Primorsky Krai, in the vicinity of Okeanicheskaya [=Okeanskaya] railway station, on Acer pseudosieboldianum, 1927, A. Oxner (LE—isotype seen).
(Fig. 2C, distribution map; Fig. 5D)
Gyalolechia ussuriensis is a humid-temperate to boreal taxon described from the Far East (Kondratyuk et al. Reference Kondratyuk, Elix, Galanina, Yakovchenko, Kärnefelt and Thell2011). Although it is paraphyletic in our ITS tree with G. persimilis (Fig. 4), we consider these taxa to be distinct because G. persimilis is known from quite different conditions in dry, temperate regions of western North America (Wetmore Reference Wetmore2004) (see Fig. 2C). ITS sequences of G. ussuriensis also differ from those of G. persimilis in 15 nucleotide positions. The sequence of the Alaskan G. aff. ussuriensis (KT804988 in Fig. 4) is short, without the ITS2 region. It has affinities with both G. persimilis and G. ussuriensis, but it also has unique nucleotides in seven positions. This specimen (KT80498) may represent another taxon because it has a more reduced thallus than either G. persimilis or G. ussuriensis (compare Fig. 5C, G . persimilis and D, G. ussuriensis with E, G. aff. ussuriensis ), and it has a rather specific ecology, growing on the bark of Cupressus nootkatensis in places not favourable for other lichens. (Note that all published specimens of G. persimilis/G. ussuriensis have been collected from broad-leaved trees.) Gyalolechia xanthostigmoidea (Fig. 5F) is morphologically very similar to both G. persimilis and G. ussuriensis, but it is geographically distinct (Fig. 2C) and its ITS sequence KT804992 is not related to either (Fig. 4).
Gyalolechia ussuriensis was known only from a small territory in the Russian Far East (Kondratyuk et al. Reference Kondratyuk, Elix, Galanina, Yakovchenko, Kärnefelt and Thell2011), but our records from the Salair Range, Sayan Mountains and Kamchatka suggest a much broader range in humid taiga forests in Siberia.
Russian specimens. Russia: Altai Krai: Zalesovsky district, Salair Range, headwaters of Berd’ River at 20 km NE from the Kordon settlement, in Abies sibirica - Populus tremula forest, alt. 430 m, 54·4166°N, 85·1166°E, on Populus tremula, 2012, E. Davydov 11220 (ALTB). Kamchatka Krai: Mil’kovo district, Nature Reserve, S of Nikolka volcano, alt. 270 m, 55·0958°N, 159·9950°E, 2009, D. Himelbrant & I. Stepanchikova s. n. (LECB); ibid., 55·1013°N, 159·9894°E, on Populus suaveolens, 2009, D. Himelbrant & I. Stepanchikova s. n. (LECB); SW slope of Tolbachik Volcano, c. 40–43 km SE of Kozyrevsk, alt. 683 m, 55·7317°N, 160·1974°E, on Populus suaveolens, 2006, D. Himelbrant s. n. (PRA, ex LECB). Krasnoyarsk Krai: West Sayan Mts, Minusinsk, Shushenskoe, 10 km SE of Tanzibey Village, forest in valley of Bolshoy Kebezh River, alt. 440 m, 53·0830°N, 93·0944°E, 2013, I. Frolov & J. Vondrák 13417 (PRA). Primorsky Krai: Terney district, Northern Sikhote-Alin’, 30 km WNW of Amgu settlement, alt. 570 m, 45·8963°N, 137·3130°E, on bark, 2014, L. Yakovchenko & E. Davydov 11500 (ALTB).
Discussion
Work by the first author in and around the Mediterranean (Vondrák et al. Reference Vondrák, Říha, Arup and Søchting2009, Reference Vondrák, Šoun, Vondráková, Fryday, Khodosovtsev and Davydov2012b ) has previously suggested that many species of Teloschistaceae, for example those in Flavoplaca or the Caloplaca xerica group, have a narrow range. However, our recent data from Russia shows quite the opposite. Some species previously known only from Europe (e.g. Caloplaca subalpina) occur as far east as the Sayan Mountains in South Siberia. Caloplaca ussuriensis, formerly thought to be restricted to the Far East, occurs from Kamchatka to the Altai Mountains in South Siberia. Other species (Calogaya bryochrysion and Caloplaca isidiigera) are almost circumpolar, and Flavoplaca flavocitrina may be almost cosmopolitan.
Our earlier conclusion about narrow ranges is therefore not applicable to Teloschistaceae as a whole. It was biased by the particular characteristics of the Mediterranean region, where a combination of history, climate and geography has indeed resulted in a high degree of endemism (Blondel & Aronson Reference Blondel and Aronson1999). In contrast, our more recent data support the fact that numerous species known from Europe or North America have been merely unrecognized in North Asia (Davydov & Printzen Reference Davydov and Printzen2012).
Within species pairs (sensu Poelt Reference Poelt1970), lineages which reproduce vegetatively often have larger geographical ranges than their strictly sexual counterparts. Such contrasts in distribution can be found in, for example, Hypogymnia (Miądlikowska et al. Reference Miądlikowska, Schoch, Kageyama, Molnar, Lutzoni and McCune2011), Letharia (Kroken & Taylor Reference Kroken and Taylor2001), and Ramalina (Rundel & Bowler Reference Rundel and Bowler1976). In phylogenies of many genera within Teloschistaceae, lineages producing vegetative diaspores randomly alternate with strictly sexual lineages, that is, those with apothecia (and with or without pycnidia). This pattern was also observed, for example, by Buschbom & Mueller (Reference Buschbom and Mueller2006) in a section of Porpidia. Species that display only vegetative distribution are very few (e.g. Leproplaca spp.), but most Teloschistaceae that reproduce vegetatively produce both apothecia and vegetative diaspores (Table 3), although apothecia are not common in some cases. The ability to produce both sexual and vegetative diaspores combines all the advantages of evolutionary plasticity with the ability to retain favourable allele combinations (e.g. Williams Reference Williams1975; Maynard Smith Reference Maynard Smith1978). Vondrák et al. (Reference Vondrák, Frolov, Říha, Hrouzek, Palice, Nadyeina, Halıcı, Khodosovtsev and Roux2013, pages 710–711) reported some examples where species with vegetative diaspores have wider geographical ranges than their strictly sexual relatives and here we provide additional evidence. Six of the eight species discussed reproduce both sexually (via ascospores) and asexually (by soredia/blastidia/isidia and also by conidia) and have wider ranges than their strictly sexual relatives. These are as follows:
-
1) The continental and arctic-alpine Calogaya bryochrysion is related to a clade containing strictly sexual C. biatorina, C. ferrugineoides and C. polycarpoides (Fig. 1) that are widely distributed in Central Asia, but they are absent from arctic and alpine habitats. Another related sexual species, C. pusilla, is probably restricted to western Eurasia: our easternmost records are from Turkey (unpublished data).
-
2) Within the genus Caloplaca, three of ten species with vegetative diaspores are distributed in Eurasia and also in North America. Strictly sexual species, 15 lineages of C. cerina s. lat. and C. stillicidiorum s. lat. in Šoun et al. (Reference Šoun, Vondrák, Søchting, Hrouzek, Khodosovtsev and Arup2011), are usually known from rather small territories, with the exception of the lineage “stillicidiorum (5)”.
-
3) Sexual species closely related to Flavoplaca flavocitrina are F. havaasii, F. marina, F. maritima and F. ora (Arup et al. Reference Arup, Søchting and Frödén2013). All these have rather restricted geographical ranges.
-
4) Gyalolechia epiphyta is related to sexual G. flavorubescens s. lat. (Fig. 4), an entity that has a wide range, but which probably consists of several geographically more restricted taxa. Gyalolechia ussuriensis is related to the sexual G. flavovirescens known from western Eurasia, Greenland and North America, but its wide range has not been tested by molecular data, and so more species may exist within G. flavovirescens.
Table 3 Modes of reproduction of species in large Teloschistaceae genera with distribution centres in northern Eurasia

Note: species dispersed solely by vegetative diaspores are not known in these genera. Those producing both apothecia and vegetative diaspores (third column) can be without apothecia locally, but samples with apothecia are not exceptional. Large genera without modern taxonomic revision are not treated (e.g. Pyrenodesmia and Variospora)
Evidence is accumulating that various Teloschistaceae species have wide geographical ranges. Many of them are characterized by dual reproductive modes (producing sexual and asexual diaspores), but a few species without vegetative diaspores may also have broad ranges. The influence of reproductive mode on the fitness, competitive success and geographical range of lichens seems a promising area for research. The evolutionary grounds for switches between reproductive modes are also a related and promising topic for future study.
Linda in Arcadia and Toby Spribille kindly revised the manuscript. Toby Spribille also generously provided his lichen samples. We are supported by the Grant Agency of the Faculty of Environmental Sciences (CULS, Prague, 42900/1312/3114), a long-term research development project (RVO 67985939), the Russian Foundation for Basic Research (grants 14–04–10091, 14–04–01411, 14–04–31024, 15–04–05291, 15–04–05971 and 15–29–02396), Saint-Petersburg State University (research grant 1.37.151.2014), Komarov Botanical Institute RAN grant (01201255601), the RAS Fundamental Research Program “Biodiversity of natural systems”, and by the grant NSh-1858.2014.4.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0024282916000116










