Introduction
Recent fieldwork on the Falkland Islands, along with the continued investigation of previously collected material from the archipelago, has made possible the compilation of an annotated checklist of lichens reported from the islands (Fryday et al. Reference Fryday, Orange, Ahti, Øvstedal and Crabtree2019). However, despite several recent publications describing new taxa from the islands (Fryday & Common Reference Fryday and Common2001; Coppins & Fryday Reference Coppins and Fryday2006; McCarthy & Fryday Reference McCarthy and Fryday2009; Lumbsch et al. Reference Lumbsch, Divakar, Messuti, Mangold and Lücking2010; Fryday & Øvstedal Reference Fryday and Øvstedal2012; Fryday & Hertel Reference Fryday and Hertel2014; Søchting et al. Reference Søchting, Søgaard, Sancho, Fröden and Arup2016; Fryday et al. Reference Fryday, Ertz and Jørgensen2017a, Reference Fryday, Schmitt and Pérez-Ortegab; Orange Reference Orange2018; Øvstedal et al. Reference Øvstedal, Lindblom, Knudsen and Fryday2018), many more remain to be fully investigated. In order to make the checklist from the islands as comprehensive as possible, this contribution describes 11 new crustose, mainly saxicolous, species in nine genera.
Materials and Methods
This study is based upon specimens collected by Henry Imshaug and Richard Harris (1968) and housed in the herbarium of Michigan State University (MSC), supplemented by those of the author and Alan Orange (Cardiff) collected in January–Febrary and November 2015 from various localities on the Falkland Islands (Fig. 1). The author's collections are also in MSC, whereas those of Orange are in the herbarium of the National Museum of Wales (NMW) with some duplicates in the Falkland Islands National Herbarium, Stanley (FINH).

Fig. 1. Map of the Falkland Islands showing main settlements and islands (black text and black filled circles) and localities mentioned in the text (grey text (red online) and black stars). Inset showing the position of the Falkland Islands in relation to South America. In colour online.
Apothecial characteristics were examined by light microscopy on hand-cut sections mounted in water, 10% KOH (K), 50% HNO3 (N), 10% HCl (H) or Lugol's reagent (0·15% aqueous IKI). Thallus sections were investigated in water, K and lactophenol cotton-blue. The ascus structure was studied in IKI, both without prior treatment and after pretreatment with K. Measurements of ascospores and paraphyses were made in K. Ascospore dimensions are given as (smallest measured–)arithmetic mean ± standard deviation(–largest measured). All hamathecial filaments are referred to as ‘paraphyses’, regardless of their origin. Thin-layer chromatography follows the methods of Orange et al. (Reference Orange, James and White2001). Nomenclature for apothecial pigments follows Meyer & Printzen (Reference Meyer and Printzen2000).
The Taxa
Bacidia marina Fryday sp. nov.
MycoBank No.: MB 829175
Characterized by its saxicolous habit, conglomerate apothecia, sordid blue-green K−, N+ violet epihymenium, K+ purple exciple and acicular multiseptate ascospores, 32–48 × 2·5–3·5 µm.
Type: Falkland Islands, East Falkland, Cape Pembroke, 51·692279°S, 57·763977°W, 3 m, sloping rocks in upper splash zone, 21 January 2015, Fryday 10818 (MSC—holotype).
(Fig. 2)
Thallus mostly endolithic or between grains of the substratum, except near apothecia where a few areoles may be present, better developed in shaded situations (underside of rocks) where white to hyaline areoles are present; areoles angular, 0·2–0·4 mm across; medulla I−. Photobiont trebouxioid; cells 6–15 µm diam.

Fig. 2. Bacidia marina. A, thallus with apothecia (Fryday 10818, holotype); B, ascospore; C, ascospores in ascus (in 10% KOH); D, ascus in IKI; E, hymenium in 10% KOH; F, exciple in 10% KOH with cyanobacterial cells at the outer edge (B–F Imshaug 40551). Scales: A = 1 mm; B–D & F = 10 µm; E = 50 µm. In colour online.
Apothecia lecideine, black, 0·4–0·6 mm diam., orbicular but larger apothecia becoming flexuose, usually in groups of 4–5, 0·8–1·0 mm across, rarely single; disc flat to slightly convex; margin thick, 0·05–0·07 mm wide, persistent, barely raised. In section: proper exciple red-brown, K+ purple, well developed, 70–85 µm wide, composed of radiating hyphae, 4–5 µm wide, cortical cells 8–10 µm diam. Hymenium 70–80 µm high; paraphyses simple, thin, 1·5 µm wide, widening slightly at apex to 4 µm, lax and readily separating in water; epihymenium 5–12 µm high, sordid blue-green K−, N+ violet. Hypothecium pale brown above and composed of vertical hyphae, darker brown below and composed of inflated, randomly orientated hyphae, K−. Asci cylindrical 45–55 × 10–12 µm, Bacidia-type; ascospores acicular, 7-septate, (32–)40·67 ± 4·31(–48) × (2·5–)3·00 ± 0·21(–3·5) μm, l/w ratio (11·14–)13·58 ± 1·36(–16·00), (n = 12), rounded at upper end tapering to narrow rounded lower end, loosely spirally arranged in the ascus.
Conidiomata not observed.
Chemistry
K−, C−, KC−, Pd+(f) brownish; thallus too thin for TLC.
Etymology
Named after its habitat of maritime rocks.
Ecology and distribution
Known only from the Falkland Islands, where it is reported only from maritime rocks at the eastern tip of East Falkland. Associated species: Tephromela lirellina (Darb.) Fryday, Buellia sp., Caloplaca s. lat. sp., Cliostomum sp. and Verrucaria sp.
Remarks
Anatomically the new species closely resembles Bacidia tuberculata Darb., but the epihymenium of that species is dilute reddish brown and the hypothecium is more consistently red-brown, K+ purple. However, B. tuberculata differs most noticeably in gross morphology, having a thick, granular thallus in which the apothecia, which are only rarely conglomerated, are immersed. Also similar is the recently described B. littoralis Kantvilas from Tasmania. However, that species has a distinctly epilithic, grey-green to olive brownish thallus, and apothecia that are rarely conglomerated and become convex and immarginate. It also has narrower excipular hyphae, a more uniformly red-brown hypothecium, paraphyses that do not separate in water and ascospores that are sometimes spirally arranged in the ascus. It appears more similar to B. tuberculata than to B. marina. Bacidia littoralis is also not known from New Zealand and appears to be confined to Tasmania and adjacent mainland Australia (Kantvilas Reference Kantvilas2018).
Additional specimens examined. Falkland Islands: East Falkland: Kidney Island, on SE shore between landing bay and SE Pt., sea level, 1968, Imshaug 40551, 40568 & Harris; Port William, N side of Hell's Kitchen, sea level, coastal rocks, 1968, Imshaug 41641 & Harris; ibid., N side of Gypsy Cove, sea level, 1968, Imshaug 41664A & Harris.
Bacidia pruinata Fryday sp. nov.
MycoBank No.: MB 829176
Characterized by the thick thallus, orange-brown, pruinose apothecia and multiseptate, acicular ascospores, 33–45 × 5–6 µm. Further distinguished from other Bacidia species by the minute granules in the apothecia that dissolve in K to give a yellow solution.
Type: Falkland Islands, Saunders Island, Rookery Hill, 51·311717°S, 60·108491°W, 241 m, N-facing rock, 4 November 2015, Fryday 11314 (MSC—holotype).
(Fig. 3)
Thallus effuse, cream to grey, thick and warted, 0·5–2·0 mm thick, areolate; areoles 0·3–0·4 mm across with a rough, granular surface; medulla I−. Photobiont trebouxioid; cells 6–15 µm diam. with a thick hyaline wall.

Fig. 3. Bacidia pruinata (A, G & H, Imshaug 42100; B–F, Fryday 11314, holotype). A, thallus with apothecia; B, asci in IKI; C, ascospores in IKI; D, hymenium in water; E, hymenium in 10% KOH; F, section in water; G, exciple in water, occluded by minute granules; H, exciple in 10% KOH showing fine, branched and anastomosing hyphae. Scales: A = 1 mm; B, C, G & H = 10 µm; D & E = 25 µm; F = 100 µm. In colour online.
Apothecia biatorine, pale orange-brown to grey, 1·0–1·2 mm diam., constricted below, orbicular becoming slightly flexuose, flat to slightly convex; disc heavily white, granular pruinose; margin thick (0·1–0·15 mm wide), not pruinose, slightly raised and persistent. In section: proper exciple well developed, c. 300 µm wide, cupular, pale yellow-brown laterally, hyaline below the hypothecium, inspersed with fine granules that dissolve in K to give a yellow solution but insoluble in N, composed of radiating branched and anastomosing hyphae, 2–3 µm wide, not enlarged at the outer edge. Hymenium 90–120 µm high; paraphyses ±simple, sparingly branched and anastomosing, very thin, 1·0 µm wide, widening slightly at apices to 2 µm, lax and readily separating in water except at the apex; epihymenium hyaline but upper 10–20 µm inspersed with fine brown granules that dissolve in K to give a yellow solution but are insoluble in N. Hypothecium pale yellow brown, c. 100–120 µm high, inspersed with fine granules that dissolve in K to give a yellow solution but are insoluble in N, composed of vertical hyphae above and inflated, randomly orientated hyphae below. Asci cylindrical, slightly swollen towards upper end, 65–70 × 18–20 µm, Biatora/Lecanora-type with a prominent ocular chamber when immature; ascospores acicular, 7–9(–12)-septate, (33–)42·08 ± 5·84(–45) × (5·0–)5·375 ± 0·380(–6·0) μm, l/w ratio (7·09–)7·86 ± 1·19(–10·00), (n = 12), not spirally arranged in the ascus.
Conidiomata not observed.
Chemistry
K+ yellow, C−, Pd−. TLC: atranorin, yellow spot at Rf 6·5 in solvent C, ±norstictic acid (holotype).
Etymology
Named after the densely pruinose apothecia.
Distribution and ecology
Known only from the Falkland Islands. Reported from siliceous rocks on both main islands as well as two of the smaller ones, usually at relatively high altitude (180–240 m), although one collection is from <100 m. Associated species: Ramboldia petraeoides (Nyl. ex C. Bab. & Mitt.) Kantvilas & Elix, Pertusaria cerebrinula Zahlbr. and Usnea sp.
Remarks
The apical ascus structure of the new species appears to be intermediate between the Biatora and Lecanora types (Hafellner Reference Hafellner1984). It has an amyloid tholus with a darker staining region immediately adjacent to the non-amyloid apical cushion, which penetrates to the upper wall of the ascus with sides that are either vertical or narrowing upwards (Fig. 3B)
The ascus type and other apothecial characters, especially the granular exciple composed of thin, branched and anastomosing hyphae, clearly exclude this species from Bacidia s. str. An ITS sequence was obtained from one collection (Orange 22970) but a BLAST search failed to reveal any close relatives, with no published sequence being more than 86% identical (Bacidina and Biatora spp.). Rather than erect a new monotypic genus, the species is retained in Bacidia pending further molecular investigation.
Additional specimens examined. Falkland Islands: East Falkland: Stanley, Goat Ridge, 600 ft, outcrops along ridge, 1968, Imshaug 41528 & Harris. West Falkland: Fox Bay, summit of East Head, 600 ft, 1968, Imshaug 42100 & Harris. Saunders Island: Rookery Mountain, 51·31201°S, 60·10918°W, 2015, A. Orange 22970 (NMW). Westpoint Island: settlement side of Woolly Gut Pt. peninsula, 300 ft, 1968, Imshaug 40830 & Harris.
Provisional key to species of Bacidia occurring in the Falkland Islands
1 Corticolous, on Empetrum nigrum stems; apothecia pale to mid brown, often piebald, not pruinose; ascospores 50–65 µm long, 9–15-septate ………Bacidia sp. ‘A’
Saxicolous………2
2(1) Apothecia black………3
Apothecia yellow or pallid………4
3(2) Thallus thick, granular; ascospores 35–40 µm long; hymenium 50–60 µm high; epihymenium brown………B. tuberculata
Thallus areolate or absent; ascospores 35–55 µm long; hymenium 80–90 µm high; epihymenium sordid blue-green………B. marina
4(2) Apothecia pallid, pruinose; exciple with minute crystals dissolving in K ……… B. pruinata
Apothecia yellow, not pruinose; exciple lacking minute crystals………Bacidia sp. ‘B’
Buellia gypsyensis Fryday sp. nov.
MycoBank No.: MB 829177
Distinguished from all other species of the genus by a combination of filiform conidia and the thallus containing 5-O-methylhiascic acid as the major substance.
Type: Falkland Islands, East Falkland, Stanley, Gypsy Cove, 51·673920°S, 57·809325°W, 10 m, shaded N-facing rock, 2 November 2015, Fryday 11286 (MSC—holotype).
(Figs 4 & 5)
Thallus areolate, cream or appearing pale yellow, occurring as a closed mosaic of thalli separated by black margins; individual thalli angular, mostly <5 mm across but up to 8 mm; areoles 0·2–0·4 mm across, angular, flat with vertical sides, 0·2–0·4 mm thick; cortex absent but a thin epinecral layer 5–10 µm high is patchily developed above an upper, 40–50 µm high, algal-free zone composed of vertically orientated hyphae (Fig. 4B) that are 3–4 µm wide and coated with extra-cellular crystals (Figs 4E & 5); medulla I+ pinkish. Photobiont trebouxioid; cells 6–12 µm diam. with a thick hyaline wall, distributed throughout the medulla, often in loose vertical columns.

Fig. 4. Buellia gypsyensis (Fryday 11286, holotype). A, thallus with apothecia; B, section through thallus; C, section through apothecium; D, conidia; E, fungal hyphae from thallus; F, ascus in IKI; G, ascus and ascospores; H, parasitic hyphomycete cells. Scales: A = 2 mm (insert = 0·2 mm); B, F–H = 10 µm; C = 50 µm; D = 20 µm; E = 5 µm. In colour online.

Fig. 5. Buellia gypsyensis (Fryday 11286, holotype). A & B, under DIC illumination; C & D, under polarized light with a red filter. A & C, hyphae in water, before treatment with 10% KOH; B & D, hyphae after treatment with 10% KOH. Scales = 10 µm.
Apothecia frequent, covering much of the thallus, black, lecideine, adnate to sessile with a broad base, 0·25–0·3(–0·4) mm diam.; disc flat, matt; margin thin, 0·02–0·03 mm wide, black, shiny, slightly raised. In section: proper exciple 15–25 µm wide, dark brown-black, annular but extending part way under the hymenium. Hymenium 70–75 µm high; paraphyses c. 2 µm wide, unbranched except near the apices that are up to 5 µm wide and have a brown cap; epihymenium brown, c. 10 µm high. Hypothecium composed of vertically aligned hyphae, upper 25 µm dilute red-brown, becoming darker below and extending 100 µm into the thallus (Fig. 4C). Asci clavate, c. 30 ×15 µm; apical apparatus indistinct but apparently Biatora-type with a wide apical cushion (Fig 4F); ascospores pale grey when immature, becoming brown, 1-septate, with a slight median wall thickening (Fig. 4G), (10–)11·33 ± 1·56(–15) × (4·5–)5·0 ± 0·37(–6·0) μm, l/w ratio (2·00–)2·27 ± 0·31(–3·0) (n = 12).
Conidiomata rare, red-brown, ±flat, 0·04 mm diam.; wall composed of vertically aligned hyphae c. 2–3 µm wide that gradually widen to 4–5 µm at the surface, upper 20–25 µm becoming increasingly brown-pigmented with dark brown caps (similar to the paraphyses); conidiophores not observed; conidia Amandinea-type, filiform, curved, 17–20 µm long (Fig. 5D).
Chemistry
K−, C+ pink, KC−, Pd−; 5-O-methylhiascic acid (major), gyrophoric acid (minor or trace) and lecanoric acid (minor or trace) by TLC.
Etymology
Named after Gypsy Cove, the type locality.
Ecology and distribution
Known only from the Falkland Islands, where it is reported from only a single collection from rocks near the coast at the eastern tip of East Falkland.
Remarks
The new species is known only from the type collection but is quite distinct as it is the only known buellioid lichen with Amandinea-type conidia and a thallus containing 5-O-methylhiascic acid as the only major substance. All other buellioid species containing 5-O-methylhiascic acid have short bacilliform conidia except Buellia carballaliana Paz-Berm. & Giralt, a lignicolous species known only from Portugal (Paz-Bermúdez et al. Reference Paz-Bermúdez, Giralt and Elix2009), for which conidia were not observed. The most similar species would appear to be B. eganii Bungartz described from New Mexico, but that species has short, bacilliform conidia and immersed apothecia with a reduced, hyaline proper exciple (aethalea-type; Bungartz & Nash Reference Bungartz and Nash2004; Bungartz et al. Reference Bungartz, Nordin, Grube, Nash, Gries and Bungartz2007).
The filiform conidia would suggest a placement of this species in Amandinea M. Choisy ex Scheid. & H. Mayrhofer, but phylogenies have consistently shown intermixed species with filiform and bacilliform conidia (e.g. Wedin et al. Reference Wedin, Baloch and Grube2002; Prieto & Wedin Reference Prieto and Wedin2016), indicating that the species with filiform conidia do not form a monophyletic group. Sheard & May (Reference Sheard and May1997) transferred four North American species from Buellia and Rinodina into Amandinea but acknowledged that other important morphological characters were variable and that even the length and type of conidia overlapped between species referred to Buellia and Amandinea. The indiscriminate transfer of buellioid species with filiform conidia to Amandinea should, therefore, be discouraged and a widely circumscribed Buellia is adopted here instead. Bungartz et al. (Reference Bungartz, Nordin, Grube, Nash, Gries and Bungartz2007) provide an excellent discussion of this and other problems in the acceptance of segregate genera in buellioid lichens.
Under normal light microscopy the thalline hyphae appear to be papillate (Fig. 4E) but further investigation using DIC and polarized light revealed that the hyphae are covered with extra-cellular crystals (Fig. 5). Under DIC microscopy the hyphae have an irregular surface that becomes smooth after treatment with 10% KOH (Fig. 5A & B), whereas with polarized light the birefringence caused by the crystals is completely eliminated by treatment with 10% KOH (Fig. 5C & D).
The thallus of the holotype is covered with the conidia of a hyphomycete similar to Intralichen or Trimmatostroma. The conidia are singular or in pairs, rarely in short chains, and measure 5–6 µm diam. (Fig. 4H).
Provisional key to species of Buellia and similar lichens occurring in the Falkland Islands
This key is very provisional. Buellioid lichens are common and diverse in the region and have yet to be comprehensively studied.
1 Ascospores 3-septate to submuriform; on bark………Diplotomma alboatrum
Ascospores 1-septate; on various substrata………2
2(1) Lichenicolous or on organic material………3
On rock………6
3(2) Lichenicolous on Poeltidea………Sclerococcum australe
On organic material………4
4(3) On moribund Bolax, peat and bryophyte detritus………Buellia sp. ‘A’
On twigs; conidia Amandinea-type………5
5(4) Thallus well developed; ascospores with thickened septum………B. skottsbergii
Thallus poorly developed; ascospores without thickened septum………B. punctata
6(2) Thallus yellow, C+ orange or C+ pink………7
Thallus white, grey or brown; KC− and C−………9
7(6) Xanthones present, C+ orange, UV+ orange………8
Xanthones absent, C+ pink (fleeting), UV+ yellow………B. gypsyensis
8(7) Thallus of convex areoles; medulla I+ violet; apothecia sessile………B. anisomera
Thallus of flat areoles; medulla I−; apothecia innate………B. ocellata
9(6) Ascospores with thickened walls, polarilocular when immature; conidia Amandinea-type; thallus lacking lichen products………10
Ascospores not polarilocular; conidia Amandinea- or Buellia-type; thallus with or without lichen products………13
10(9) Subhymenium inspersed………B. nitrophila
Subhymenium not inspersed………11
11(10) Ascospores 12–16 × 6–10 µm………B. discreta
Ascospores 18–24 × 6–12 µm………12
12(11) Thallus yellowish grey; apothecia 0·5–1·0 mm diam.………B. falklandica
Thallus pale grey; apothecia 0·3–0·4 mm diam.………B. decedens
13(9) Epihymenium brown (N−)………14
Epihymenium aeruginose or olivaceous (N+ red)……… 15
14(13) Apothecia 0·25–0·50 mm diam., persistently plane and marginate, hymenium c. 100 µm high; ascospores 15–18 × 9–11 µm………B. subcervina
Apothecia 0·4–1·0 mm, becoming convex and immarginate, hymenium 70–75(–95) μm high; ascospores 14–17 × 7–9 µm………B. coniops
15(13) Thallus lacking lichen substances or with unidentified substances (K−)………16
Thallus with atranorin or norstictic acid (K+ yellow or red)………17
16(15) Thallus well developed, lacking lichen substances; apothecia with thin, indistinct margin………B. cf. illaetabilis
Thallus poorly developed, containing two unidentified substances (TLC); apothecia with thick proper margin………Buellia sp. ‘B’
17(15) Thallus lacking norstictic acid, atranorin present (K+ yellow); apothecia adnate to sessile with thin proper margin………B. stellulata
Thallus containing norstictic acid (K+ red crystals in section), atranorin present or absent……… 18
18(17) Apothecia innate, concave, with proper margin not apparent; thallus white, containing atranorin; medulla I+ violet………B. spuria
Apothecia adnate to sessile, with well-developed proper margin; thallus creamy to pale brown, lacking atranorin; medulla I− ………B. russa
Cliostomum albidum Fryday sp. nov.
MycoBank No.: MB 829178
Characterized by the saxicolous habit and apothecia lacking internal pigmentation.
Type: Falkland Islands, West Falkland, Port Howard, Mt. Maria, Castle Rock, 51·621332°S, 59·591020°W, 370 m, shaded, S-facing crags (underhang), 26 January 2015, Fryday 10902 (MSC—holotype).
(Fig. 6)
Thallus effuse, white, thin, <50 µm thick, with rock grains protruding through the surface, rimose to cracked-areolate; medulla I−. Photobiont trebouxioid; cells 9–15 µm diam.

Fig. 6. Cliostomum albidum (Fryday 10902, holotype). A, thallus with apothecia; B, Castle Rock on West Falkland. Cliostomum albidum was collected from a ledge below the overhang; C, ascus showing Biatora-type apical apparatus. Scales: A = 1 mm; C = 10 µm. In colour online.
Apothecia biatorine, white, pink to pale orange, 0·6–0·8–1·0 mm diam.; disc flat becoming convex, white pruinose; margin prominent when young, 0·15 mm wide, persistent but barely raised in older apothecia, 0·05 mm wide. In section: proper exciple well developed, c. 120 µm wide, composed of narrow, 1·5 µm wide, richly branched and anastomosing hyphae, inspersed with granular crystals that form bands towards the outer surface and dissolve in K to give a bright yellow solution; cortex absent; exciple extending in a narrow band under the hypothecium and forming a deep, conical ‘root’ 100–120 µm into the thallus. Hymenium 50–65 µm high, upper 10–20 µm with granular crystals that mostly dissolve in K; paraphyses thin, 1·5 µm wide, widening at the apex to 4 µm, sparingly branched and anastomosing. Hypothecium hyaline, 35–50 µm high, composed of randomly orientated hyphae, well differentiated from the hymenium. Asci Biatora-type, cylindrical, 35–40 × 15 µm, becoming clavate and 20 µm wide; ascospores hyaline, 1-septate, (11–)12·33± 0·89(–14) × (4·5–)4·96 ± 0·45(–5·5) μm, l/w ratio (2·18–)2·50 ± 0·23(–2·89), (n = 12).
Conidiomata not observed.
Chemistry
K+ yellow, C−, KC−, Pd+ orange-red; thallus too thin for TLC.
Etymology
Named after the white apothecia.
Distribution and ecology
Known only from the Falkland Islands. Reported from siliceous rocks on both main islands, usually at relatively high altitudes (180–370 m). Associated species: Cliostomum longisporum Fryday, Lecanora spegazzinii Müll. Arg., Lepra macloviana (Müll. Arg.) I. Schmitt et al., Ramboldia petraeoides, Rhizocarpon malvinae Fryday and R. geographicum (L.) DC. aggr.
Additional specimens examined. Falkland Islands: East Falkland: Stanley Common, Two Sisters, 51·685744°S, 58·016522°W, 289 m, slightly underhanging, E-facing rock face, 2015, Fryday 10732; ibid., 51·690161°S, 58·028866°W, 265 m, exposed rock near summit, 2015, Fryday 10740. West Falkland: Fox Bay, summit of East Head, 600 ft, 1968, Imshaug 42090 & Harris.
Cliostomum longisporum Fryday sp. nov.
MycoBank No.: MB 829179
Distinguished from all other species of the genus by its long ascospores (15–23 × 2·5–3·0 µm).
Type: Falkland Islands, East Falkland, Stanley Common, outcrops along Goat Ridge, 600 ft, 30 January 1968, Imshaug 41510 & Harris (MSC—holotype).
(Fig. 7)
Thallus effuse, white, thin, <50 µm thick, with rock grains protruding through the surface, rimose to cracked-areolate; medulla I−. Photobiont trebouxioid; cells 6–15 µm diam. with a thick hyaline wall.
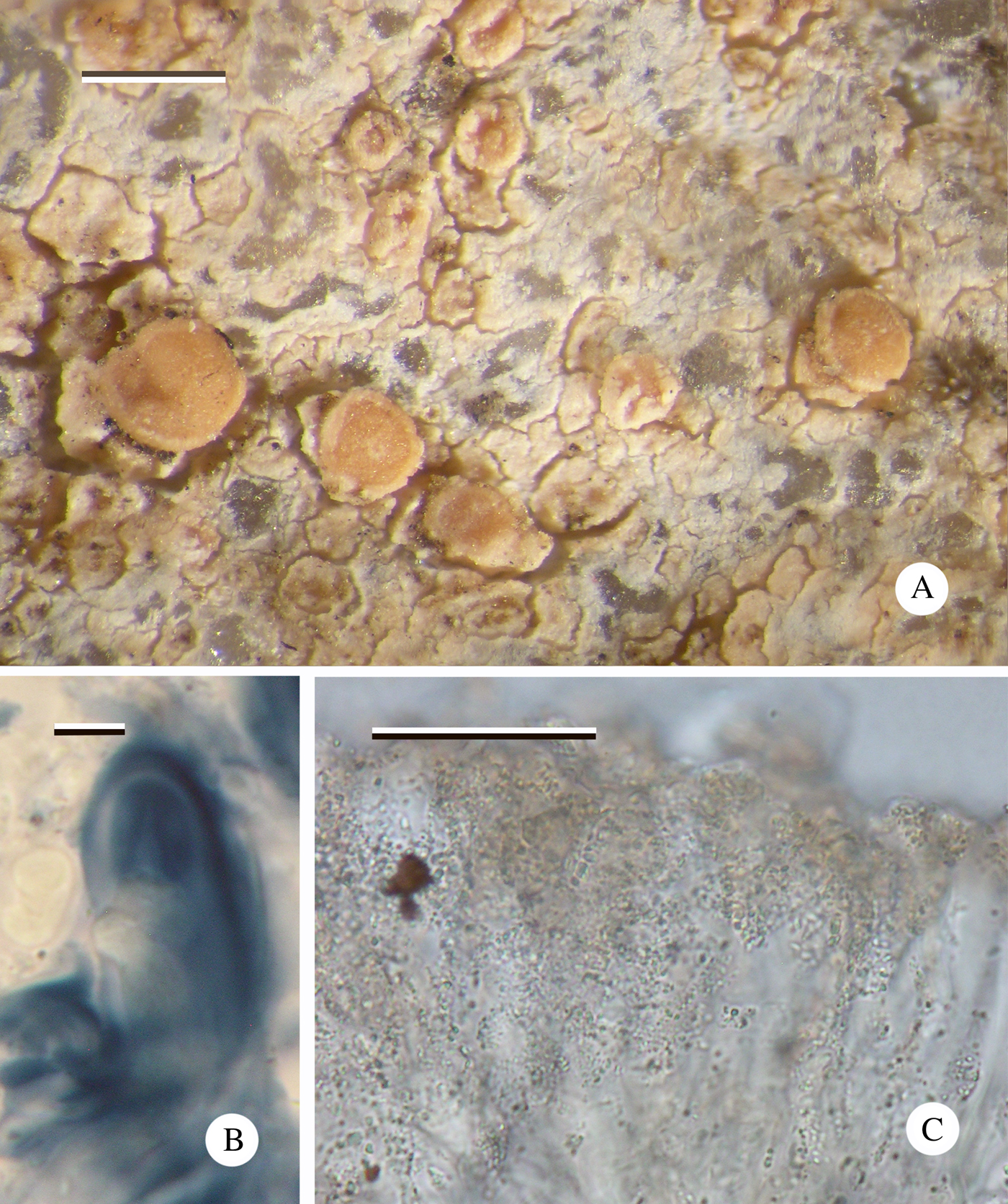
Fig. 7. Cliostomum longisporum (Imshaug 41510, holotype). A, thallus with apothecia; B, ascus showing Biatora-type apical apparatus; C, hymenium showing numerous minute granules. Scales: A = 0·5 mm; B = 5 µm; C = 25 µm. In colour online.
Apothecia biatorine, pale orange to pink, 0·3–0·5 mm diam.; disc flat, sometimes becoming convex, white pruinose; margin paler than disc, barely raised, 0·03 mm wide, barely visible in convex apothecia. In section: proper exciple 30 µm wide, composed of narrow (1·5 µm wide), branched and anastomosing hyphae, inspersed with granular crystals that dissolve in K to give a bright yellow solution; cortex absent. Hymenium 40–50 µm high, upper 10–20 µm with granular crystals that mostly dissolve in K; paraphyses narrow, 1·5 µm wide, widening at the apex to 4 µm, sparingly branched and anastomosing. Hypothecium hyaline 80–100 µm, well differentiated from the hymenium, composed of randomly orientated hyphae, inspersed with granular crystals that dissolve in K giving a bright yellow solution. Asci Biatora-type, initially cylindrical, becoming clavate, 25–30 × 10–12 µm; ascospores hyaline, acicular, 1(–3)-septate, tapering at lower end, usually straight, rarely curved, (15–)19·90 ± 2·02(–23) × (2·5–)2·80 ± 0·25(–3·0) μm, l/w ratio (5·00–)7·167 ± 0·99(–8·80), (n = 20).
Conidiomata not observed.
Chemistry
K−, C−, KC−, Pd−; thallus too thin for TLC.
Etymology
The name refers to the long ascospores, which are unique for the genus.
Distribution and ecology
Known only from the Falkland Islands. Reported from siliceous rocks on both main islands, usually at relatively high altitude (180 m), although one collection is from <100 m. Associated species: Cliostomum albidum Fryday and Lithographa opegraphoides Coppins & Fryday.
Remarks
The collection Imshaug 41510 also supports an isotype of Lithographa opegraphoides Coppins & Fryday (Coppins & Fryday Reference Coppins and Fryday2006), which is known only from this collection. Cliostomum pallens (Kullh.) S. Ekman, a boreal corticolous species, also has 3-septate ascospores but these are only 9–18 µm long (Vainio Reference Vainio1922).
Additional specimens examined. Falkland Islands: East Falkland: Stanley Common, Tumbledown Mt., 51·690679°S, 57·937456°W, 100 m, underhang in low, N-facing crags, 2015, Fryday 11025. West Falkland: Fox Bay, summit of East Head, 600 ft, 1968, Imshaug 42090 & Harris.
Provisional key to species of Cliostomum occurring in the Falkland Islands
1 Thallus sorediate/leprose; corticolous………2
Thallus not sorediate or leprose; corticolous or saxicolous………3
2(1) Thallus and/or soralia Pd+ red (fumarprotocetraric acid); apothecia internally lacking pigmentation………C. flavidulum
Thallus and soralia Pd− (fumarprotocetraric acid absent); purple pigment (Melaena-red; K+ aeruginose, H+ purple, N+ red) in exciple and epihymenium ………C. violascens
3(1) Saxicolous………4
Corticolous………6
4(3) Apothecia dark, ±immersed in the thallus………C. falklandicum
Apothecia pale, sessile………5
5(4) Ascospores 15–23 × 2·5–3·0 µm………C. longisporum
Ascospores 11–14 × 4·5–5·5 µm………C. albidum
6(3) Apothecia brownish, with grey pruina ……… C. griffithii
Apothecia black, pruina absent………C. aeruginascens
Coccotrema rubromarginatum Fryday sp. nov.
MycoBank No.: MB 829203
Distinguished from all other members of the genus by the placodioid thallus with a red-brown margin and lower surface.
Type: Falkland Islands, West Falkland, Port Howard, Mt. Maria, between Freezer Rocks and Castle Rock, 51·613504°S, 59·576515°W, 325 m, stone run, 26 January 2015, Fryday 10894 (MSC—holotype).
(Fig. 8)
Thallus crustose-placodioid to subfruticose, white to pale grey, 4–6(–8) mm thick, composed of contiguous convex areoles, 0·4–1·0 mm across that, in well-developed specimens, are supported on short pseudopodetia 2–4 mm high and 0·1 mm thick; undersurface of placodioid margin red-brown, this pigment often extending to the edge of the upper surface; medulla I−. Soralia orbicular, discrete, arising from an areole that often forms a rounded margin 0·1 mm wide, rarely two or more becoming confluent; soredia granular, c. 0·05 mm diam., greenish when fresh, becoming pinkish cream with age. Primary photobiont Myrmecia?; cells (12–)15–20(–22) μm diam. with thick hyaline wall. Cephalodia frequent, grey, ±orbicular with shallow marginal lobes, often radially fissured, 1–2 mm across; secondary photobiont Chroococcus?; cells pale brown/orange 10–15 µm diam., 3–4(–8) enclosed in a yellow sheath 20–25 µm across, groups of smaller yellow-brown cells 3–4 µm diam. also present but not definitely part of the symbiosis.

Fig. 8. Coccotrema rubromarginatum. A, field photograph of a specimen in the stone run on Mt. Maria, West Falkland, from where the type was collected; B, thallus with soralia and cephalodia (Imshaug 40044); C, habitat in the stone run, C. rubromarginatum indicated by arrow. Scale = 1 mm. In colour online.
Apothecia poriform in thalline warts, 0·7–0·8 mm wide; ostiole pale, slightly depressed; internal cavity ±spherical, 140–160 µm diam. Hymenium I+ yellow, 120–130 µm high; paraphyses simple, thin, 1–1·5 µm wide, expanding at the apex to 5 µm. Hypothecium hyaline c. 30 µm high, composed of randomly orientated hyphae, well differentiated from the hymenium. Asci cylindrical, c. 150 × 25 µm; ascospores hyaline, simple, broadly ellipsoid, 8 per ascus (35–)40·75 ± 4·17(–48) × 18–21·4±1·88(–27) μm, l/w ratio (1·68–)1·91 ± 0·18(–2·25), (n = 20), cell wall c. 1–1·5 µm thick.
Conidiomata not observed.
Chemistry
Cortex K− or K+ dilute orange, medulla K+ bright yellow; stictic acid, constictic acid, ±norstictic acid and unknown pigment by TLC.
Etymology
The name refers to the red-brown margin of the thallus.
Distribution and ecology
Saxicolous on granitic rocks, usually in stone runs or fell-fields. Frequent on the Falkland Islands and also known from Tierra del Fuego (Isla de los Estados and Isle Grande). Associated species: Ochrolechia antarctica (Müll. Arg.) Darb., Ramboldia petraeoides and Lepra macloviana.
Remarks
The placodioid thalline morphology of Coccotrema rubromarginatum forms a link between the subfruticose thallus of C. coccophorum and the crustose thallus displayed by the rest of the genus.
Schmitt et al. (Reference Schmitt, Messuti, Feige and Lumbsch2001) showed that Lepolichen coccophorus (Mont.) Trevis. should be included within Coccotrema and made the new combination Coccotrema coccophorum (Mont.) I. Schmitt et al. However, they failed to realize that as Lepolichen Trevis. 1853 was an earlier name than Coccotrema Müll. Arg. 1889, this combination was invalid and that all species of Coccotrema should be transferred to Lepolichen. In the interests of nomenclatural stability, a formal proposal to conserve Coccotrema against Lepolichen is in preparation.
Additional specimens examined. Argentina: Tierra del Fuego: Isla de los Estados, Puerto Roco, summit of peak S of bay, 54°46′S, 64°15′W, 360 m, 1971, Imshaug 51117 & Ohlsson; Isla Grande (Tierra del Fuego), Sierra Alvear, W side of Paso Garibaldi, 54°42′S, 67°47′W, 460 m, 1971, Imshaug 54841 & Ohlsson.—Falkland Islands: East Falkland: Mt. Usborne, on ridge between Usbornes 1 & 2, 2250 ft, sheltered cliffs with seepage, 1968, Imshaug 39977 & Harris; ibid., below The Gap, 300 ft, stone run, 1968, Imshaug 40044, 40046A, 40063 & Harris; ibid., crags west of summit, 51·691550°S, 58·850976°W, 600 m, exposed NW facing crags, 2015, Fryday 11428; Mt. Kent, summit,1500 ft, cliffs on rock dome, 1968, Imshaug 40437 & Harris.
Provisional key to species of Coccotrema occurring in the Falkland Islands
1 Thallus subfruticose, with narrow, radiating marginal lobes; cephalodia absent………………C. coccophorum
Thallus crustose, placodioid in one species; cephalodia usually present ………2
2(1) Thallus placodioid with red-brown margin; discrete soralia present………C. rubromarginatum
Thallus not placodioid; soralia absent………3
3(2) Thallus without isidia; apothecia abundant………C. curcubitula
Thallus with isidia; apothecia scarce ………4
4(3) Isidia thick, 0·3–0·4 mm diam.………C. corallinum
Isidia fine, 0·10–0·15 mm diam.………C. magellanicum
Hymenelia microcarpa Fryday sp. nov.
MycoBank No.: MB 829204
Distinguished from all other species in the Hymenelia-Ionaspis complex by its minute apothecia (<0·1 mm diam.). Further characterized by the combination of a trebouxioid photobiont, apothecia with a granular epihymenium and lack of internal pigmentation or reaction with K or N.
Type: Falkland Islands, East Falkland, Estancia, SE side of inlet 6 km west of house, 51·657400°S, 58·262245°W, sea level, rocks at back of shore, 1 November 2015, Fryday 11254 (MSC—holotype).
(Fig. 9)
Thallus effuse, areolate, rarely rimose, pale cream to orange-pink; areoles contiguous or dispersed, orbicular when dispersed, 0·15–0·3 mm diam., irregular and larger when contiguous, 0·3–0·5 mm across; medulla I−. Photobiont trebouxioid; cells 6–12 µm diam.

Fig. 9. Hymenelia microcarpa (Fryday 11254, holotype). A, continuous, rimose thallus with minute apothecia; B, section through apothecium showing granular epihymenium; C, ascospores; D, form with dispersed, areolate thallus (Fryday 11081). Scales: A & D = 0·5 mm; B = 50 µm; C = 5 µm. In colour online.
Apothecia frequent, arising singly from the centre of each areole, pale pink (rarely hyaline), 0·06–0·1 mm diam., concave to flat; margin not apparent. In section: proper exciple poorly developed, hyaline. Hymenium 85–100 µm high; paraphyses simple, thin, c. 1 µm wide, not swollen at the apices; epihymenium not pigmented but with numerous small brownish granules not dissolving in K or N. Hypothecium hyaline, 12–15 µm high, composed of randomly orientated hyphae. Asci cylindrical, c. 50 × 15 µm, Hymenelia-type (outer coat I+ blue but inner walls and apical dome K/I−); ascospores simple, hyaline, simple, broadly ellipsoid, 8 per ascus (11–)12·50 ± 0·80(–14) × (6–)6·67 ± 0·78(–8) μm, l/w ratio (1·625–)1·89 ± 0·19(–2·17) (n = 12).
Conidiomata not observed.
Chemistry
No lichen substances detected.
Etymology
The name refers to the minute size of the apothecia.
Distribution and ecology
Known only from the Falkland Islands. Apparently quite frequent but easily overlooked because of the small size of the immersed apothecia. Known from four localities on East Falkland, all close to the sea. Associated species: Buellia sp., Lecidea sp., Porina austroatlantica P. M. McCarthy & Fryday, Porpidia cf. crustulata (Ach.) Hertel & Knoph, Porpidia sp., Rhizocarpon malvinae, R. infernulum (Nyl.) Lynge, Rinodina cf. peloleuca (Nyl.) Müll. Arg. and Verrucaria s. lat. sp.
Remarks
The genera Hymenelia Kremp. and Ionaspis Th. Fr. were traditionally separated by their different photobionts, Trentepohlia in Ionaspis and a green chlorococcoid alga in Hymenelia (Magnusson Reference Magnusson1933; Jørgensen Reference Jørgensen1989), although it was generally accepted that this distinction was artificial (Clauzade & Roux Reference Clauzade and Roux1985; Coppins & Purvis Reference Coppins, Purvis, Purvis, Coppins, Hawksworth, James and Moore1992). Lutzoni & Brodo (Reference Lutzoni and Brodo1995) performed a cladistic analysis of morphological-anatomical and enzyme electrophoresis data and showed that two genera could be recognized, but that a rearrangement of the species was required. This new arrangement was generally accepted but the distinction between the two genera appeared to be just as artificial as the previous arrangement and was based on less distinctive or easily observable characters. Kantvilas (Reference Kantvilas2014) highlighted these issues and concluded that the best course of action, pending a full morphological-molecular revision, was to include all the species in a single genus. Under the system proposed by Lutzoni & Brodo (Reference Lutzoni and Brodo1995), the new species would be assigned to Ionaspis because of its granular epihymenium but I agree with Kantvilas (Reference Kantvilas2014) and, consequently, describe the new species in Hymenelia, which is the older of the two names.
The thallus of the new species is often areolate but the thallus of one collection (Fryday 11081) is continuous, cracked-rimose. However, intermediates occur and in one case the two different thalline morphologies occur on the same specimen. All four collections also have an identical apothecial anatomy and it is considered therefore that only one species is involved.
The only species of Hymenelia/Ionaspis previously reported from southern South America is Ionaspis fuegensis P. M. Jørg. & R. Sant. (Jørgensen & Santesson Reference Jørgensen and Santesson1989), but that species differs in having Trentepohlia as photobiont and apothecia with a dark disc and a pigmented epihymenium. Elsewhere in the region, Hymenelia glacialis Øvstedal was reported from Antarctica by Øvstedal & Lewis Smith (Reference Øvstedal and Lewis Smith2001) but this also has Trentepohlia as photobiont and apothecia with a dark disc and a pigmented epihymenium. Although Øvstedal & Lewis Smith (Reference Øvstedal and Lewis Smith2001) gave the author citation of this species as (C. W. Dodge) Øvstedal, the basionym, Aspicilia glacialis C. W. Dodge, is illegitimate because of A. glacialis (Arnold) Dalla Torre & Sarnth. and so Øvstedal's combination must be treated as a nomen novum (ICN Art. 58). The two species reported from Australasia, Hymenelia gyalectoidea Kantvilas and H. lacustris (With.) M. Choisy (Kantvilas Reference Kantvilas2014), both have larger apothecia, a rusty orange thallus and a different ecology: H. gyalectoidea is an alpine species whereas H. lacustris occurs on damp, usually semi-immersed rocks.
Among Northern Hemisphere species, the recently described Hymenelia parva Fryday & J. W. McCarthy (Fryday & McCarthy Reference Fryday and McCarthy2018) occurs in a similar habitat and has similarly minute apothecia, but lacks a granular epihymenium which, according to Lutzoni & Brodo (Reference Lutzoni and Brodo1995), is an important character in this group.
Additional specimens examined. Falkland Islands: East Falkland: Lafonia, Halfway House Arroyo, N side, E of road, 51·990196°S, 59·278492°W, 3–5 m, low S-facing rocks among Empetrum above river, 2015, Fryday 11081; ibid., inlet 3·5 km SW of Halfway House Arroyo, NW side of road, 52·012655°S, 59·319081°W, sea level, maritime rocks, 2015, Fryday 11448; Darwin, west side of cove at NE end of Darwin Harbour, 51·788657°S, 58·943417°W, sea level, pebbles in turf, 2015, Fryday 11416.
Lecania vermispora Fryday sp. nov.
MycoBank No.: MB 829205
Distinguished from all other members of the genus by the acicular, vermiform ascospores (23–36 × 2·0–2·5 µm).
Type: Falkland Islands, Westpoint Island, near the waterfall, 100 ft, Hebe-scrub, 20 January 1968, Imshaug 40692A & Harris (MSC—holotype).
(Fig. 10)
Thallus effuse, cream, leprose; individual granules <0·01 mm diam. but occasionally aggregating to up to 0·03 mm diam.; medulla I−. Photobiont trebouxioid; cells 6–12 µm diam.
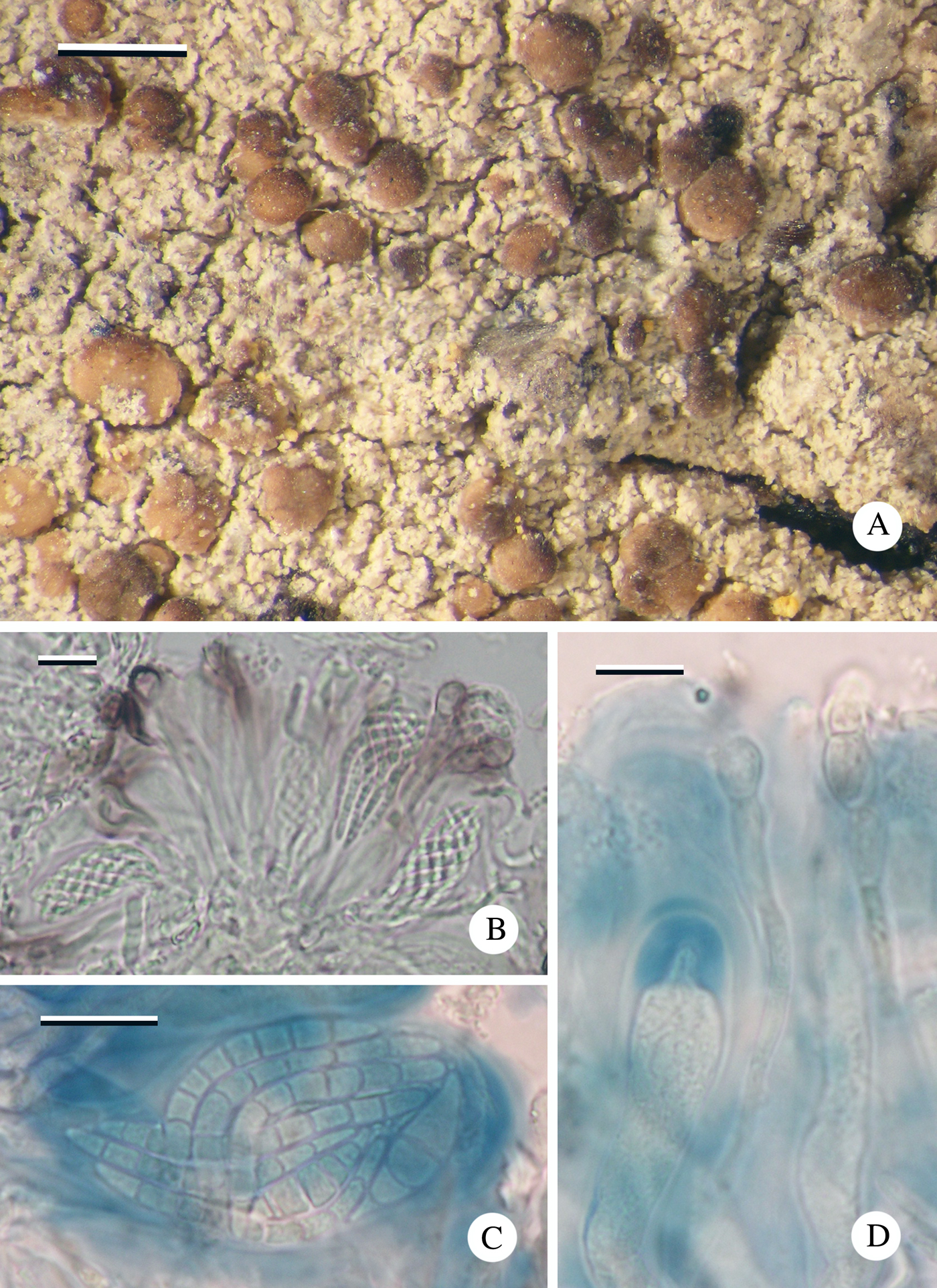
Fig. 10. Lecania vermispora (Imshaug 40692A, holotype). A, thallus with apothecia; B, section of hymenium showing ascospores in ascus and paraphyses with distinctive ‘Lecania-brown’ pigment in the epihymenium; C, mature ascospores in ascus; D, hymenium showing immature Biatora-type ascus and moniliform paraphyses. Scales: A = 0·5 mm; B–D = 10 µm. In colour online.
Apothecia frequent, biatorine, pale to dark brown, often piebald, initially flat, 0·15–0·2 mm diam. with a darker, slightly raised proper margin 0·02–0·04 mm wide, soon becoming convex, 0·2–0·3 mm diam. with an excluded margin. Thalline margin rarely apparent even in young apothecia. In section: proper exciple c. 50 µm thick, inner section pale brown becoming darker towards the cortex (K+ purple-brown; Lecania-brown); composed of narrow irregularly radiating hyphae c. 1 µm wide; cortex 10–20 µm wide, terminal cells 3–4 µm diam. Hymenium 35–40 µm high, upper 10–20 µm with patches of dilute brown (K+ purple-brown; Lecania-brown) pigment; paraphyses mostly simple, septate, c. 2 µm wide, gradually swelling towards the apices (to 5 µm) and becoming moniliform, sometimes with a brown (K+ purple-brown; Lecania-brown) cap. Hypothecium hyaline, c. 50 µm high, composed of randomly orientated hyphae. Asci Biatora-type, cylindrical, 25–30 × 10–12 µm, becoming clavate and up to 15 µm wide; ascospores hyaline, (3–)5–7-septate, spirally arranged in ascus, vermiform with rounded ends, (23–)30·08 ± 3·53(–36) × (2·0–)2·17 ± 0·25(–2·5) µm, l/w ratio (11·2–)13·96 ± 1·70(–16·5), (n = 12).
Conidiomata not observed.
Chemistry
K−, KC−, C−, Pd−; no substances detected by TLC.
Etymology
The name refers to the worm-like ascospores.
Distribution and ecology
Known only from Hebe-scrub on stems of Hebe elliptica on Westpoint Island. Associated species: Buellia skottsbergii Zahlbr., Diplotomma alboatrum (Hoffm.) Flot., Enterographa sp., Gyalolechia xanthostigmoidea (Räsänen) Søchting et al., Myriolecis zosterae (Ach.) Śliwa et al., Opegrapha sp. and Ramalina laevigata Fr.
Remarks
The thallus of several collections appears minutely isidiose due to a hyaline filamentous alga, presumably an artefact of the less than ideal conditions under which the specimens were collected and preserved; all the specimens are from an offshore island and were collected over 60 years ago.
Additional specimens examined. Falkland Islands: Westpoint Island: near the waterfall, 100 ft, Hebe-scrub, 1968, Imshaug 40705B & Harris (topotype); in steep-sided coves at S side of the Woolly Gut, 200 ft, Hebe-scrub, 1968, Imshaug 40737B, 40743C, 40745H, 40747A & Harris; on steep slope and cliffs facing the Woolly Gut, Hebe-scrub, 1968, Imshaug 40891D & Harris.
Provisional key to species of Lecania occurring in the Falkland Islands
1 Corticolous on Hebe elliptica; ascospores 3–6-septate, spirally arranged in the ascus………L. vermispora
On bone; ascospores 1–3-septate, not spirally arranged in the ascus………L. subfuscula
Lepra argentea Fryday sp. nov.
MycoBank No.: MB 829206
Distinguished from all other species of the Pertusariales by its silver-grey thallus with isidia with dark pigmented caps and a distinctive thalline chemistry (?protolichesterinic acid).
Type: Falkland Islands, West Falkland, Mt. Adam, feldmark on summit ridge, 25 January 1968, Imshaug 41061 & Harris (MSC—holotype).
(Fig. 11)
Thallus effuse, silver-grey, thin, usually growing over terricolous bryophytes (one collection spreading onto rock) and following the contours of the substratum; isidia usually present, often in groups of 4–5 that probably arise through branching of the ‘stalk’; originating as dark green-grey pigmented warts emerging from the thallus, finally 0·4 mm high and with a short, unpigmented ‘stalk’ 0·2 mm wide, and a wider ‘cap’, 0·3 mm across that is usually dark pigmented and in section has a dark olivaceous brown cortex 25–30 µm thick (K+ brownish, H−, N+ blue-green); occasionally this pigment is absent and then the cap is creamy white throughout; medulla I−. Photobiont trebouxioid; cells 10–15(–17) μm diam.

Fig. 11. Lepra argentea. A, thallus with isidia (Fryday 10915); B, ascus with single large ascospore; C, ascus with ascoconidia; D, ascoconidia (B–D Imshaug 41056). Scales: A = 1 mm; B & C = 25 µm; D = 10 µm. In colour online.
Apothecia rare, lecanorine, 0·5–0·8 mm diam.; disc concave, black with grey pruina; margin raised, c. 0·05 mm wide and persistent. In section: proper exciple poorly developed, hyaline. Hymenium 140–170 µm high; paraphyses simple, c. 1 µm wide; epihymenium 25–30 µm, patchily olivaceous (K+ brownish, H−, N+ greenish). Hypothecium hyaline, 25–30 µm high, composed of randomly arranged hyphae. Asci clavate; ascospores poorly developed and dissolved into ascoconidia (see below) but notes and measurements on herbarium packets indicate they were hyaline, simple and one per ascus, (127–)151·00 ± 18·20(–172) × (58–)66·167 ± 5·63(–75) μm, l/w ratio (1·867–)2·29 ± 0·31(–2·57), (n = 6).
Conidiomata not observed; ascoconidia bacilliform 4–5 × 1·0–1·5 µm, formed from old ascospores within the ascus.
Chemistry
All spot tests negative; two or three pale, creamy yellow-pink spots at R f 6 in solvent C by TLC.
Etymology
Named for the silver-grey colour of the thallus
Distribution and ecology
Known only from the Falkland Islands. Reported from boulder fields on mountain summits on both main islands where it overgrows bryophytes, rarely spreading onto siliceous rocks. Associated species: Coccotrema corallinum Messuti.
Remarks
The new species is similar to Pertusaria pachythallina (Räsänen) Messuti but that species usually occurs on rocks and has a much thicker thallus that contains protocetraric and hypothamnolic acids (Messuti Reference Messuti2005). Pertusaria pachythallina also regularly supports cephalodia with Scytonema, these being present on all 11 collections of this species (from Isla de los Estados (Argentina) and SW Chile) held at MSC. Within the Pertusariales, cephalodia are characteristic of the genus Coccotrema Müll. Arg. (Coccotremataceae), but species of that genus have closed, perithecia-like apothecia. However, cephalodia have also been reported from a single species of Pertusaria, namely P. stellata Fryday (Fryday Reference Fryday2008), which has single-spored asci and should probably be transferred to the genus Lepra. However, this will not be undertaken here pending the outcome of the proposal to reject the name Lepra in favour of Variolaria Pers. (Jørgensen Reference Jørgensen2018). Apothecia have not been reported for P. pachythallina and because molecular data is not available, its systematic position is unclear. However, its similarity to L. argentea and the presence of cephalodia suggests it should also be transferred to Lepra.
Imshaug reported the chemistry of all his collections as containing protolichesterinic acid. However, thin-layer chromatography (TLC) using solvent C (Orange et al. Reference Orange, James and White2001) of one of his collections (Imshaug 41061) and one of the author's (Fryday 10915), along with a specimen know to contain this substance (Tuckermannopsis chlorophylla (Willd.) Hale; Imshaug 45060), revealed several pale, creamy yellow-pink spots at R f 6 in all three collections. These were in almost the same position and it is possible that protolichesterinic acid is present in L. argentea but a more detailed investigation is required to fully elucidate the chemistry of this species. What is certain is that L. argentea does not contain protocetraric and hypothamnolic acids and so is chemically distinct from P. pachythallina.
Imshaug wrote ascospore dimensions on the packets of two of the collections of the new species; one bryicolous (Imshaug 41061) and the other saxicolous (Imshaug 41056). Unfortunately, the bryicolous collection was poorly curated and no longer has any apothecia whereas the saxicolous collection consists of a large piece of rock with a thallus with scattered apothecia spreading onto bryophytes and several small, richly fertile pieces removed from their substratum that might have been bryicolous. The distinctive isidia of L. argentea are absent from this collection (Imshaug 41056) and, although the thalline chemistry reported by Imshaug (protolichesterinic acid) agrees with that of the other collections, it is possible that Imshaug 41056 represents a different species. However, even if this is the case, L. argentea is well characterized by its distinctive isidia and unusual chemistry and is certainly a new species.
The new species is unique in the Pertusariales in producing ascoconidia. Hawksworth et al. (Reference Hawksworth, Kirk, Sutton and Pegler1995) defined ascoconidia as “a conidium formed directly from the ascospore, esp. when still within the ascus”. Baral (Reference Baral1999) realized that two sharply distinct cases can occur and refined the term to refer only to the former case:
Case 1: conidia produced from ascospores within the living premature asci; each ascospore together with its ascoconidia is surrounded by a delicate membrane, forming more or less distinct “balls” when the ascus reaches maturity. These balls are violently ejected as single entities but they disintegrate if the asci die prior to discharge.
Case 2: conidia produced from ejected ascospores or rarely from ascospores within dead asci. They are never arranged as “balls”.
Baral (Reference Baral1999) further noted that these two types can only be distinguished within living asci. Because the asci of L. argentea are monosporus, it is not possible to distinguish between Baral's two cases.
Ascoconidia were first reported in lichenized fungi by Santesson (Reference Santesson1952), who recognized them in five foliicolous and three corticolous species, all with muriform ascospores. They have subsequently been described in at least four other species (Hafellner & Bellemère Reference Hafellner and Bellemère1983; Kantvilas & Vězda Reference Kantvilas and Vězda1992; Ertz & Diederich Reference Ertz and Diederich2004; Frisch & Kalb Reference Frisch and Kalb2006), three of which also produce muriform ascospores and are mostly referable to Baral's Case 2. The exception is Oevstedalia antarctica Ertz & Diederich in which Ertz & Diederich (Reference Ertz and Diederich2004) observed the development of eight “conidial balls” within the asci of fresh material at a very early stage of development and referred them to Baral's Case 1 (true ascoconidia).
Imshaug annotated his temporary herbarium packets containing the two fertile collections with details of the ascospores and made no mention of conidia, whereas currently no ascospores are apparent and only spore-shaped groups of conidia can be seen. However, Imshaug's annotation included the detail that the spores were “thin-walled”, which is unusual for species of Lepra that usually have thick-walled ascospores. It is possible that the thin-walled “spores” observed by Imshaug were actually “balls” of ascoconidia.
Additional specimens examined. Falkland Islands: East Falkland: Mt. Usborne, on leeward side of Mt. Usborne 1 summit, 1968, Imshaug 39928, 39931, 39949 & Harris; ibid., sheltered cliffs with seepage on ridge between Mt. Usbornes 1 & 2, 1968, Imshaug 39985 & Harris. West Falkland: Mt. Adam, feldmark on summit ridge, 1968, Imshaug 41056 & Harris; Port Howard, Mt. Maria, Lightning Rocks, 51·619028°S, 59·601849°W, 575 m, over bryophytes on S-facing rocks, 2015, Fryday 10915.
Provisional key to species of Lepra and Pertusaria on the Falkland Islands
1 Corticolous or bryophilous ………2
Saxicolous………5
2(1) Corticolous on twigs ………P. microcarpa
Overgrowing terricolous bryophytes………3
3(2) Apothecia abundant, with pruinose disc (K−, Pd−)………L. panyrga
Apothecia usually absent; isidia with grey tips usually present………4
4(3) Thallus white, thick and areolate; protocetraric acid present (Pd+ orange-red) ………P. pachythallina
Thallus grey, thin and smooth; protocetraric acid absent (Pd−) ………L. argentea
5(1) Thallus with isidia or soralia; apothecia usually absent………6
Thallus with neither isidia nor soredia; apothecia present………10
6(5) Thallus with isidia………7
Thallus with soralia………9
7(6) Thallus dark grey with papillate isidia lacking a dark pigmented cap; containing protocetraric acid (Pd+ orange-red)………L. macloviana
Thallus white or silver grey, isidia with a short colourless ‘stalk’ and a wider, pigmented ‘cap'………8
8(7) Thallus white, thick and areolate; protocetraric acid present (Pd+ orange-red) ………P. pachythallina
Thallus grey, thin and smooth; protocetraric acid absent (Pd−) ………L. argentea
9(6) Thallus containing norstictic acid (K+ red crystals in section, Pd+ yellow); soralia convex, grey………L. excludens
Thallus containing fumarprotocetraric acid (K−, Pd+ red); soralia discrete, creamy coloured………L. aspergilla
10(5) Apothecia disciform………11
Apothecia verruciform………15
11(10) Ascospores 1 per ascus………12
Ascospores 2 or 8 per ascus………13
12(11) Thallus with papillae; protocetraric acid present (Pd+ orange-red,); on rocks………L. macloviana
Thallus without papillae or if present with grey tips; protocetraric acid absent (Pd−); usually overgrowing bryophytes and spreading onto rocks………L. argentea
13(11) Ascospores 2 per ascus; thallus containing picrolichenic and norstictic acids (K+ red, KC+ violet, Pd+ yellow)………P. alterimosa
Ascospores 8 per ascus; thallus lacking picrolichenic acid (KC−)………14
14(13) Thallus containing norstictic acid (K+ acicular crystals in section); salazinic acid absent ………P. erubescens
Thallus containing salazinic acid (K+ rhomboid crystals in exciple); norstictic acid absent ………P. salacinifera
15(10) Thallus containing norstictic acid (K+ red, Pd+ yellow) ………P. perrimosa
Thallus lacking norstictic acid (K−, Pd−)………16
16(15) Apothecia ±innate, mostly single; ascospores 4 per ascus, 90–150 µm long; confluentic acid present, 2′-O-methylperlatolic acid absent ………P. malvinae
Apothecia sessile or in raised warts, in groups of 3–5; 2′-O-methylperlatolic acid present, confluentic acid absent………17
17(16) Ascospores 8 per ascus, <100 µm long; thallus thin with raised fertile warts ………P. cerebrinula
Ascospores 4 per ascus, >150 µm long; apothecia innate to sessile, rarely in raised warts ………P. spegazzinii
Rhizocarpon malvinae Fryday sp. nov.
MycoBank No.: MB 829207
Similar to R. reductum but with a grey thallus and ±sessile apothecia, often with blue-green pigment in the epihymenium and a ± hyaline exciple with a large, heavily pigmented, blue-green ‘cap’.
Type: Falkland Islands, Weddell Island, Circum Peak, summit, 51·927272°S, 60·925263°W, 205 m, pebbles, 23 January 2015, Fryday 10845 (MSC—holotype).
(Fig. 12)
Thallus pale grey, effuse, thin, c. 120–150 µm thick, usually with a wide black, fimbriate hypothallus, rimose to cracked-areolate, usually continuous but sometimes reduced to scattered areoles on a black hypothallus; rarely with the thallus absent and apothecia occurring directly on the hypothallus; cortical cells pale brown, c. 5 µm diam. overlain by a thin epinecral layer c. 10 µm high; medulla poorly developed with the photobiont layer reaching almost to the substratum, I−. Photobiont trebouxioid; cells 8–12(–15) μm diam.

Fig. 12. Rhizocarpon malvinae. A, thallus and apothecia (Fryday 10845, holotype); B, exciple showing pale interior and pigmented ‘cap’ (Fryday 11255); C, ascospores (Fryday 11255, images marked with * from Imshaug 42369). Scales: A = 0·5 mm; B = 20 µm; C = 10 µm. In colour online.
Apothecia frequent, black, lecideine, sessile, 0·4–0·6 mm diam.; disc concave, becoming flat or slightly convex when overmature; margin thick, up to 0·1 mm wide, raised and persistent. In section: proper exciple brown, composed of ±vertically aligned hyphae c. 5 µm wide, with a dark blue-black (N+ red; Cinereorufa-green) pigmented “cap”; this cap is often massively produced, 80–100 µm wide (Fig. 12B) with a much reduced or±absent proper exciple below. Hymenium 85–100–150 µm high; paraphyses very thin, c. 1 µm wide, branched and anastomosing, swelling gradually to 3 µm at the apices, upper 10 µm olivaceous or blue pigmented (K+ intensifying blue, N+ red; Cinereorufa-green); epihymenium diffuse, olivaceous to blue (K+ intensifying blue, N+ red; Cinereorufa-green). Hypothecium dark brown. Asci narrowly clavate, Rhizocarpon-type, 75–85 × 30–35 µm; a scospores submuriform (rarely more than one primary longitudinal septum but with 3–5(–6) longitudinal septa), becoming pigmented when overmature, (20–)22·80± 2·44(–35) × (9–)10·20 ± 0·83(–11) μm; l/w ratio (1·91–)2·24 ± 0·21(–2·64), (n = 20).
Conidiomata not observed.
Chemistry
K+ yellow, C−, Pd+ orange; stictic acid and norstictic acid (trace) detected by TLC.
Etymology
Derived from Islas Malvinas, the Spanish name for the Falkland Islands.
Ecology and distribution
Apparently endemic to the Falkland Islands where it is common on siliceous rocks, especially at mid to high elevations. It is not present among the c. 10000 collections in MSC made by Imshaug and co-workers from southern South America (Fryday & Prather Reference Fryday and Prather2001). Associated species: Buellia anisomera Vain., B. russa (Hue) Darb., Fuscidea asbolodes (Nyl.) Hertel & V. Wirth, Lecanora capistrata (Darb.) Zahlbr., Lecidea sp., Myriospora smaragdula (Wahlenb.) Nägeli, Poeltidea perusta (Nyl.) Hertel & Hafellner, Porpidia crustulata (Ach.) Hertel & Knoph, P. tuberculosa (Sm.) Hertel & Knoph, Rhizocarpon distinctum Th. Fr., R. nidificum (Hue) Darb. and R. simillimum (Anzi) Lettau.
Remarks
Imshaug annotated his collections of this taxon “Rh. marginatum”, emphasizing the thick proper margin. Although noting that the Imshaug & Harris collections from the Falkland Islands had somewhat larger ascospores than European collections, Fryday (Reference Fryday2000) referred all their collections to R. reductum Th. Fr. However, having now seen specimens in the field it is apparent the Falkland Islands collections are morphologically and ecologically distinct. Rhizocarpon malvinae is frequent at higher altitudes, reaching 685 m on the summit of Mt. Adam, where it is part of mature communities on siliceous rocks, unlike R. reductum, which is a lowland species of pioneer communities. Morphologically it differs in the presence of a blue-green pigment in the upper exciple and epihymenium and the almost unpigmented lower exciple. Although lowland forms can be difficult to separate from R. reductum, the more typical upland form, with its grey thallus and sessile apothecia with a thick tumid margin, more closely resembles R. lavatum (Ach.) Hazsl. than R. reductum.
Additional specimens examined. Falkland Islands: East Falkland: Mt. Usborne, gap between Mt. Usborne 2 and Ceritos Rocks, 1550 ft, Cortaderia heath, 1968, Imshaug 40147 & Harris; Stanley Common, Goat Ridge, 600 ft, outcrops along ridge, 1968, Imshaug 41495 & Harris; ibid., Two Sisters, 51·690161°S, 58·028866°W, 265 m, pebbles at summit, 2015, Fryday 10733; ibid., exposed rock near summit, 2015, Fryday 10740; Mullet Creek, stream below fiord, 100 ft, 1968, Imshaug 41475, 41481, 41482, 41485 & Harris; Mt. Kent, summit, 1500 ft, cliffs on rock dome, 1968, Imshaug 40475 & Harris; ibid., E of military base, N of cliff, 51·673213°S, 58·105353°W, 440 m, pebbles in alpine heath, 2015, Fryday 10797, 10803; Lafonia, North Arm, between settlement and Garden Point, 52·140000°S, 59·371650°W, 0 m, pebbles in depression in track, 2015, Fryday 11079; ibid., 3·5 km west of Walker Creek, N of road, E of creek, 51·977064°S, 58·822842°W, 21 m, low outcrop in Empetrum heath above stream, 2015, Fryday 11436; Estancia, SE side of inlet 6 km west of house, 51·657400°S, 58·262245°W, sea level, rocks at back by shore, 2015, Fryday 11255; Darwin, west side of cove at NE end of Darwin Harbour, 51·788657°S, 58·943417°W, sea level, pebbles in turf, 2015, Fryday 11412. West Falkland: Hill Cove, NE base of French Peaks, 200 ft, stone run, 1968, Imshaug 41013 & Harris; Mt. Adam, E side of summit ridge, 2200–2297 ft, 1968, Imshaug 41089 & Harris; ibid., summit of southernmost peak, 2250 ft, 1968, Imshaug 41093 & Harris; ibid., ridge W of northern lake, 2000 ft, sheltered cliffs, 1968, Imshaug 41135 & Harris; Fox Bay, NE from Sullivan House, 500 ft, outcrops on ridge, 1968, Imshaug 42369 & Harris; Chartres, Patricia Luxton NNR, 51·725859°S, 59·984581°W, 15 m, low, exposed sandstone crags, 2015, Fryday 10987. Saunders Island: Rookery Cottage, 51·306687°S, 60·098780°W, 44 m, pebbles in Empetrum heath, 2015, Fryday 11354. Weddell Island: Circum Peak, NW slope, 51·925000°S, 60·928000°W, 140 m, pebbles, 2015, Fryday 10831; ibid., summit, 51·927272°S, 60·925263°W, 205 m, pebbles, 2015, Fryday 10840, 10847 (topotypes).
Specimens of Rhizocarpon reductum examined. Falkland Islands: East Falkland: Vantan Arroyo, 51·743908°S, 58·280770°W, 15 m, pebble in Leptinella scariosa (button weed) heath, 2015, Fryday 10747, 10758.
Provisional key to species of Rhizocarpon occurring in the Falkland Islands
1 Thallus yellow-green (rhizocarpic acid present); ascospores pigmented………2
Thallus white-grey or brown, not yellow-green (rhizocarpic acid absent); ascospores pigmented or hyaline………4
2(1) Ascospores 1-septate; apothecia sessile; thallus with norstictic acid (K+ red, needle-shaped crystals in section)………R. superficiale
Ascospores 1–3-septate or submuriform, usually with less distinct longitudinal and diagonal septa; apothecia innate; thallus with psoromic acid (K+ yellow, no crystals in section)……… 3
3(2) Ascospores 1–3-septate, usually with less distinct longitudinal and diagonal septa; thallus consisting of dispersed areoles on a black hypothallus ………R. nidificum
Ascospores submuriform; thallus ±continuous………R. geographicum
4(1) Ascospores pigmented dark blue-green (N+ red; Cinereorufa-green) 12–16 × 6–8 µm; medulla I+ violet ………R. simillimum
Ascospores hyaline, although sometimes becoming brown when overmature………5
5(4) Ascospores with only transverse septa, 1–3-septate………6
Ascospores with transverse and longitudinal septa………9
6(5) Ascospores 3-septate; medulla I− ………R. submodestum
Ascospores 1-septate; medulla I− or I+ violet………7
7(6) Epihymenium red-brown, K+ purple-red (Atra-red); ascospores occasionally becoming pigmented or 3-septate; medulla I+ violet ………R. polycarpum
Epihymenium brown, olivaceous or blue, K−; ascospores occasionally becoming pigmented but not 3-septate; medulla I−………8
8(7) Ascospores >24 µm long; paraphyses not distinctly capitate ………R. hochstetteri
Ascospores <22 µm long; paraphyses distinctly capitate………R. infernulum
9(5) Medulla I+ violet; epihymenium K+ purple-red (Atra-red) ………R. distinctum
Medulla I−; epihymenium K+ purple-red (Atra-red) or K−………10
10(9) Epihymenium K+ purple-red (Atra-red)………R. subpostumum
Epihymenium without K+ purple-red pigment………11
11(10) Epihymenium olivaceous; excipulum brown, N− ………R. reductum
Epihymenium and excipulum blue-black, N+ crimson ………R. malvinae
Tephromela lignicola Orange & Fryday sp. nov.
MycoBank No.: MB 829208
Separated from other sterile sorediate species by a combination of its yellow to blue-grey soralia, its unique chemistry (atranorin and alectoronic acid) and lignicolous habit.
Type: Falkland Islands, Weddell Island, Weddell Settlement, behind Mountain View Cottage, 51·891525°S, 60·912416°W, 15 m, fence post, 24 January 2015, Fryday 10863 (MSC—holotype).
(Fig. 13)
Thallus not apparent, endoxylic, completely within the substratum. Soralia erumpent, initially orbicular to 0·3 mm diam., becoming elongate to 0·6 × 0·3 mm, plane to concave, following the grain of the wood, but soon confluent and convex and forming a ±continuous crust; soredia farinose, pale green below becoming blue-grey at the surface (pigment K-, N+ purple-red), 20–25 µm diam., aggregating to 75–100 µm across. Photobiont trebouxioid; cells (6–)10–15 µm diam.

Fig. 13. Tephromela lignicola (Fryday 10863, holotype) thallus with soredia. Scale = 1 mm. In colour online.
Apothecia unknown.
Conidiomata not observed.
Chemistry
K+ yellow, KC−, C−, Pd−; atranorin and alectoronic acid by TLC.
Etymology
The name reflects the substratum on which the species occurs.
Ecology and distribution
Known only from the Falkland Islands, where it occurs on fence posts and worked timber on both main islands and Weddell Island. It is possible that this species was imported with the fence posts, which probably originated in Chile. Associated species: Blastenia circumpolaris Søchting et al., Buellia punctata (Hoffm.) A. Massal., Cliostomum griffithii (Sm.) Coppins, Lecanora expallens Ach. and Xylographa vitiligo (Ach.) J. R. Laundon.
Remarks
Tephromela lignicola superficially resembles Buellia griseovirens (Turner & Borrer ex Sm.) Almb. but that species contains atranorin and norstictic acid, and reacts K+ red. The collections do not correspond with any of the known corticolous, sorediate species of Tephromela (e.g. T. sorediata Kalb & Elix from Australia has unpigmented soralia) but an ITS sequence from one collection (Orange 22552) is almost identical to that obtained from Tephromela alectoronica Kalb from Australia, which is a non-sorediate species also reported from South America (Elix Reference Elix2009). It is possible that T. lignicola is the sorediate morphotype of T. alectoronica.
The lichenicolous fungus Skyttea violacea Etayo, previously reported only on a sorediate, corticolous morph of Tephromela atra (Huds.) Hafellner from Tierra del Fuego (Etayo & Sancho Reference Etayo and Sancho2008), was present on the thallus of the holotype.
Additional specimens examined. Falkland Islands: East Falkland: Darwin, Darwin House, 51°48·36′S, 58°57·49′W, 10 m, on wooden fence of garden by pasture, 2011, A. Orange 19679 (NMW C.2015.004.95); San Carlos Water, Bonners Bay, head of the Bay Brook, 51°36·366′S, 59°00·845′W, on fence post, 2011, A. Orange 19831 (NMW); Goose Green Farm, Darwin, 51·80798°S, 58·95824°W, 2015, A. Orange 23157 (NMW). West Falkland: Port Howard, 51°37·74′S, 59°32·39′W, on old wood lying on ground, 2011, A. Orange 20142 (NMW C.2015.004.96); north-west of Fox Bay, Lake Sullivan, 51·91055°S, 60·16769°W, 10 m, on wood of fence post, 2015, A. Orange 22552 (NMW C.2015.004.75).
Provisional key to species of Tephromela occurring in the Falkland Islands
1 Thallus sorediate, soralia green-grey; apothecia unknown; on fence posts……… T. lignicola
Thallus not sorediate; apothecia present; on rocks……… 2
2(1) Thallus in small patches (<1 cm diam.); containing stictic or norstictic acid only ………T. minor
Thallus more widespreading (>5 cm diam.); containing atranorin, α-collatolic acid and ± alectoronic acid………3
3(2) Apothecia ±lirellate; paraphyses apices blue-black………T. lirellina
Apothecia consistently orbicular; paraphyses apices purple-brown………4
4(3) Hymenium inspersed ………T. skottsbergii
Hymenium not inspersed………5
5(4) Apothecia innate………T. atroviolacea
Apothecia sessile………6
6(5) Thalline margin poorly developed, usually ±excluded; apothecia large, up to 5 mm diam.; hypothecium with a wide hyaline band adjacent to the hymenium ………T. superba
Thalline margin well developed; apothecia <1·5 mm diam.; hypothecium with a narrow hyaline band adjacent to the hymenium ………7
7(6) Thalline margin not corticate, not raised above the level of the disc ………T. atrocaesia
Thalline margin corticate, raised above the level of the disc………T. atra
Conclusion
Including this contribution, there have been 31 taxa described from the Falkland Islands since the year 2000 (Fryday & Common Reference Fryday and Common2001; Coppins & Fryday Reference Coppins and Fryday2006; McCarthy & Fryday Reference McCarthy and Fryday2009; Lumbsch et al. Reference Lumbsch, Divakar, Messuti, Mangold and Lücking2010; Fryday & Øvstedal Reference Fryday and Øvstedal2012; Fryday & Hertel Reference Fryday and Hertel2014; Fryday et al. Reference Fryday, Ertz and Jørgensen2017a, Reference Fryday, Schmitt and Pérez-Ortegab; Orange Reference Orange2018; Øvstedal et al. Reference Øvstedal, Lindblom, Knudsen and Fryday2018) but, as can be seen from the number of ‘Associated Species’ identified only to genus or included in the keys only as “sp.”, many more remain to be fully investigated. Notes on many of these taxa can be found on the websites Lichens of the Southern Subpolar Region (Fryday Reference Fryday2018) or Lichens of the Falkland Islands (Fryday Reference Fryday2019). These additional taxa are often known from only a single collection, or material is otherwise insufficient to formulate an adequate description or to select a good type specimen. In particular, collections of crustose Teloschistaceae, Lecideaceae and Verrucariaceae have been deliberately excluded from the present contribution because they are being investigated by specialists in these groups and more new species are confidently expected from their research. As an example of this underexplored biodiversity, the genus Trapelia consists of c. 20 species worldwide and none of these had previously been reported from the Falkland Islands. However, Orange (2018) reported seven species from the archipelago: two were previously described, two were newly described endemics, and three were species for which insufficient material was available for formal recognition.
The lichen biota of southern South America and many other austral regions is poorly understood and little researched genetically, so any conclusions concerning lichen distribution should be treated with extreme caution. Although all but one of the species described here are known only from the Falkland Islands, that number is certain to decrease as the lichen biota of other areas in the region (and beyond) becomes better known. However, as stated above under Rhizocarpon malvinae, Imshaug and co-workers made c. 10000 collections from southern South America, including c. 3550 from Isla de los Estados and c. 2200 from Isla Grande de Tierra del Fuego, the areas geographically closest to the Falkland Islands (Fryday & Prather Reference Fryday and Prather2001), and only one of the 11 species described here has been found among those collections. Conversely, several collections from the Falkland Islands have been identified as species with a predominately Northern Hemisphere distribution (e.g. Lepra aspergilla and L. excludens) and it is possible that, as has recently been demonstrated for Ochrolechia antarctica (Müll. Arg.) Darb. (Ertz et al. Reference Ertz, Fryday, Schmitt, Charrier, Dudek and Kukwa2016), when these species are subjected to molecular investigation, they will be shown to be genetically distinct and represent different taxa.
Fieldwork on the Falkland Islands by the author was funded by the United Kingdom Government through DEFRA and the Darwin Initiative as part of the project Lower Plants Inventory and Conservation in the Falkland Islands (Reference number DPLUS017). Support for fieldwork and advice regarding landowners was provided by Falklands Conservation. The US National Science Foundation (NSF) is thanked for funding Dr Imshaug's expedition to the islands in 1968. I thank Ralph Common (East Lansing) for permitting me to use his photographic equipment and image processing software, and for providing images for Fig. 4A, D & H and Fig. 5; Alan Orange (Cardiff) for providing additional records of several species and DNA sequence data for Bacidia pruinata and Tephromela lignicola; John (Jack) Elix (Canberra) for identifying the unknown substance in the thallus of Buellia gypsyensis; Paul Deiderich (Luxembourg) and Javier Etayo (Pamplona) for identifying the lichenicolous fungi on the thalli of Buellia gypsyensis and Tephromela lignicola respectively. I further thank Alan Orange (Cardiff) and Gintaras Kantvilas (Hobart) for comments on a draft version of this manuscript, and the curators of the herbarium of the British Antarctic Survey (AAS) and the Swedish Museum of Natural History (S) for the loan of collections in their care.















