Introduction
Despite their rather low lichen diversity, the subantarctic islands of the Indian Ocean are known to harbour endemic lichen taxa with atypical thallus morphologies. The genus Orceolina Hertel is notably characterized by a distinct cortex anatomy with very thick cortical layers (Lumbsch Reference Lumbsch1997; Poulsen et al. Reference Poulsen, Schmitt, Søchting and Lumbsch2001); Protopannaria alcicornis P. M. Jørg. & R. S. Poulsen and Thamnolecania antarctica C. W. Dodge have unusual growth forms, the first having squamules like small moose antlers (Jørgensen Reference Jørgensen2001) and the second being a subfruticose bacidioid species (Dodge Reference Dodge1948). In addition, Usnea taylorii Hook. f. is a large, erect, fruticose species forming astonishing corneous thalli with a broad, subdivided axis (Walker Reference Walker1985), allowing the species to thrive in the harshest environmental conditions despite its large size (up to 10 cm tall). The discovery of the first umbilicate Trapeliaceae is another example of a remarkable endemic species occurring in these islands of the southern Indian Ocean.
Trapeliaceae is a medium-sized family that was described by Hertel (Reference Hertel1970) for the genera Orceolina Hertel, Placopsis (Nyl.) Linds., Trapelia M. Choisy and Trapeliopsis Hertel & Gotth. Schneid., and placed in the Baeomycetales based on phylogenetic studies (Lumbsch et al. Reference Lumbsch, Schmitt, Mangold and Wedin2007). The family currently incorporates about seven genera of lichenized species with crustose to placodioid thalli, but with Trapelia forming a paraphyletic assemblage (Kantvilas et al. Reference Kantvilas, Leavitt, Elix and Lumbsch2014; Resl et al. Reference Resl, Schneider, Westberg, Printzen, Palice, Thor, Fryday, Mayrhofer and Spribille2015). Members of the genus Placopsis and its close relatives (i.e. the genera Aspiciliopsis and Orceolina) are mainly distributed in the Southern Hemisphere with, for example, 39 species recorded in New Zealand (Galloway Reference Galloway2013), 22 species in southern South America (Galloway Reference Galloway2010) and 10 in Îles Kerguelen (Schmitt et al. Reference Schmitt, Lumbsch and Søchting2003; Galloway Reference Galloway2011).
Îles Crozet and Îles Kerguelen are among the most remote archipelagos in the world. They are of volcanic origin and located in the southern Indian Ocean near the Antarctic Convergence. The climate is subantarctic and strongly oceanic with low average temperatures but comparatively mild winters, frequent rain and snow falls, almost permanent winds throughout the year and frequent stormy conditions (Mawson Reference Mawson1934). Arborescent vegetation is absent and few native phanerogam species are present, 22 on Îles Kerguelen and 17 on Île de la Possession (Crozet) (Frenot et al. Reference Frenot, Gloaguen, Massé and Lebouvier2001), including the Kerguelen cabbage (Pringlea antiscorbutica R. Br. ex Hook.f.), famous for its antiscorbutic properties. The lichen biota is more diverse with mainly crustose saxicolous species. While the Îles Kerguelen have been the topic of many publications (see notably Galloway Reference Galloway2011 for references and a history of lichen recording), very few data are available for Crozet (Zahlbruckner Reference Zahlbruckner1906; Dodge Reference Dodge1948; Lamb Reference Lamb1974; Massé Reference Massé1982; Walker Reference Walker1985; Jørgensen Reference Jørgensen2000; Poulsen et al. Reference Poulsen, Schmitt, Søchting and Lumbsch2001; Schmitt et al. Reference Schmitt, Lumbsch and Søchting2003).
During fieldwork on Crozet in 2012 and 2015, material of an unknown conspicuous umbilicate species was collected by the first author (DE). Sequences obtained from these specimens revealed that they represent a distinct, new species of Trapeliaceae having close affinities with the genera Aspiciliopsis (Müll. Arg.) M. Choisy, Orceolina and Placopsis. Subsequent discussion with US and RSP revealed that they had also collected sterile but typical material of this taxon during their expedition to Îles Kerguelen in 1998. The present study aims to describe this peculiar new species of lichen, being considered a genus of its own, and to determine its phylogenetic position.
Materials and Methods
The external morphology was studied and measured using an Olympus SZX12 stereomicroscope. Macroscopic photographs were taken either with a Keyence VHX-5000 digital microscope and a VH-Z20R/W/T lens, or with a Canon EOS 5D Mark III camera mounted on the Olympus SZX12 stereomicroscope. Hand-cut sections of ascomata and thallus were mounted in water, a solution of 5% potassium hydroxide (K), or in Lugol’s iodine solution (1% I2) without (I) or with K pretreatment (K/I) and studied using an Olympus BX51 compound microscope. For the measurements, the minimum and maximum values are given, all values rounded to the nearest multiple of 0·5 mm. Measurements for ascospores are given as
![]() $$\bar{x}$$
±SD, with outlying values in parentheses. Measurements refer to dimensions in water. Photomicrographs were prepared using an Olympus BX51 compound microscope fitted with an Olympus ColorViewI digital camera. Colour reactions of the thallus were studied using 5% potassium hydroxide solution (K), common household bleach (C), 5% aqueous potassium hydroxide followed by common household bleach (KC), para-phenylenediamine (PD) and long-wave UV (366 nm).
$$\bar{x}$$
±SD, with outlying values in parentheses. Measurements refer to dimensions in water. Photomicrographs were prepared using an Olympus BX51 compound microscope fitted with an Olympus ColorViewI digital camera. Colour reactions of the thallus were studied using 5% potassium hydroxide solution (K), common household bleach (C), 5% aqueous potassium hydroxide followed by common household bleach (KC), para-phenylenediamine (PD) and long-wave UV (366 nm).
Extrolites were analyzed as follows. Dried lichen of the holotype (Ertz 20595) was extracted in acetone for 1 h, three times. The combined acetone extract was analyzed by TLC using solvents A, C and G (Huneck & Yoshimura Reference Huneck and Yoshimura1996). Plates were then observed under visible and UV lights (254 nm and 365 nm) and extrolites located using sulphuric anisaldehyde (ANS, 2%). Acetone extract was also injected onto a C18 Kinetex® column (100×4·6 mm, 2·6 µm- 100Å, Phenomenex®) connected to an HPLC-DAD (Shimadzu) in turn attached to an expression CMS single quadrupole (Advion) equipped with an ElectroSpray Ionization (ESI) detector in negative mode. Chromatograms were obtained using a gradient water/acetonitrile (both acidified by 0·1% formic acid) with a flow rate of 0·5 ml min−1, for 48 min. Direct Analysis in Real Time (DART) ionization is a mass spectrometry technique that permits the analysis of untreated samples and entire objects (Le Pogam et al. Reference Le Pogam, Legouin, Le Lamer, Boustie and Rondeau2015). We performed DART-MS in negative mode on the entire thallus of the holotype specimen.
Voucher specimens are deposited in the herbaria of the Botanic Garden Meise (BR), Botanic Garden Copenhagen (C), Université de Rennes 1 (REN) and Muséum National d’Histoire Naturelle de Paris (PC).
Molecular techniques
Well-preserved and freshly collected specimens lacking any visible symptoms of fungal infection were used for DNA isolation. Genomic DNA was isolated from fresh specimens using the CTAB extraction protocol (Doyle & Doyle Reference Doyle and Doyle1990). Amplification reactions were prepared for a 50 µl final volume containing 5 µl 10× DreamTaq Buffer (Fermentas), 1·25 µl of each of the 20 µM primers, 5 µl of 2·5 mg ml−1 bovine serum albumin (Fermentas #B14), 4 µl of 2·5 mM each dNTPs (Fermentas), 1·25U DreamTaq DNA polymerase (Fermentas), and 1 µl of template genomic DNA. A targeted fragment of c. 1·1 kb at the 5'end of the nuLSU rDNA was amplified using primers LIC15R (Miadlikowska et al. Reference Miadlikowska, McCune and Lutzoni2002) with LR6 (Vilgalys & Hester Reference Vilgalys and Hester1990), and a fragment of c. 0·8 kb of the mtSSU rDNA using the primers mrSSU1 and mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). The yield of the PCRs was verified by running the products on a 1% agarose gel using ethidium bromide. Both strands were sequenced by Macrogen® using amplification primers. The primer LR3 was used for the sequencing of nuLSU (Vilgalys & Hester Reference Vilgalys and Hester1990; Vilgalys’ website: http://www.botany.duke.edu/fungi/mycolab). Sequence fragments were assembled with Sequencher version 5.3 (Gene Codes Corporation, Ann Arbor, Michigan). Sequences were subjected to ‘megablast’ searches to verify their closest relatives and to detect potential contaminations.
Taxon selection and phylogenetic analyses
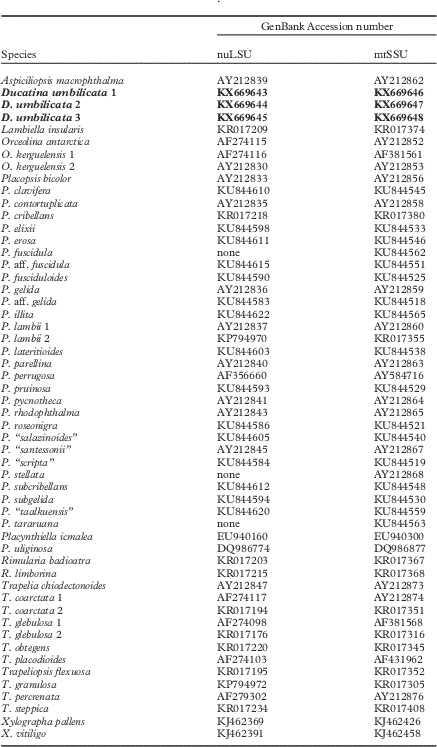
Six new sequences (3 nuLSU and 3 mtSSU) were obtained from this study and their closest relatives (i.e. Orceolina species) based on BLAST searches were retrieved from GenBank. Members representing all genera currently accepted in the Trapeliaceae as circumscribed by Resl et al. (Reference Resl, Schneider, Westberg, Printzen, Palice, Thor, Fryday, Mayrhofer and Spribille2015) were selected in addition to Placopsis species from Schmitt et al. (Reference Schmitt, Lumbsch and Søchting2003) and Schneider et al. (Reference Schneider, Resl and Spribille2016) (Table 1). We based our study on nuLSU and mtSSU sequences because these loci were available for the closest relatives of the new taxon: Aspiciliopsis and Orceolina. We only included samples for which at least mtSSU was available and, therefore, some clades were omitted. These included Trapelia antarctica Ertz et al., a species recently described from Antarctica (Ertz et al. Reference Ertz, Aptroot, Van de Vijver, Śliwa, Moermans and Øvstedal2014) that is the sister taxon to the clade from Aspiciliopsis macrophthalma (sub Placopsis macrophthalma) to Placopsis in Resl et al. (Reference Resl, Schneider, Westberg, Printzen, Palice, Thor, Fryday, Mayrhofer and Spribille2015). In total, 99 additional sequences were retrieved from GenBank for phylogenetic analyses. Lambiella insularis (Nyl.) T. Sprib. was chosen as the outgroup species. The sequences of taxa listed in Table 1 were aligned using MAFFT v.6.814b (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002) within Geneious and improved manually using Mesquite 3.04 (Maddison & Maddison Reference Maddison and Maddison2015). Terminal ends of sequences, ambiguously aligned regions, and introns were delimited manually and excluded from the datasets.
Table 1 GenBank Accession numbers for the specimens included in the phylogenetic inference depicted in Figure 1. Newly described species and sequences in bold
Because it was not possible to complete the nuLSU and the mtSSU sequences for the same set of 54 samples, analyses for topological incongruence among loci were carried out on datasets with 51 samples for which both genes were available (Table 1). To detect significant conflicts among datasets, Bayesian analyses were carried out on the single-locus dataset using the Metropolis-coupled Markov chain Monte Carlo (MCMCMC) method in MrBayes v.3.2.6 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) on the CIPRES web portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Best-fit evolutionary models were estimated using the Akaike information criterion (AIC; Akaike Reference Akaike1973) as implemented in Modeltest v.3.7 (Posada & Crandall Reference Posada and Crandall1998). The TIM+I+G and GTR+I+G models were selected respectively for the nuLSU and mtSSU datasets. Two parallel MCMCMC runs were performed, each using four independent chains and 40 million generations, sampling trees every 1000th generation. Tracer v.1.6 (Rambaut & Drummond Reference Rambaut and Drummond2007) was used to ensure that stationarity was reached by plotting the log-likelihood values of the sample points against generation time. Convergence between runs was also verified using the PSRF (potential scale reduction factor), where values were equal or close to 1·000. Posterior probabilities (PP) were determined by calculating a majority-rule consensus tree generated from the 60 002 post burn-in trees of the 80 002 trees sampled by the two MCMCMC runs using the sumt option of MrBayes. All topological bipartitions with PP values≥95 were compared for the two loci. A conflict was assumed to be significant if two different relationships (one being monophyletic and the other being non-monophyletic) for the same set of taxa were both supported with PP values≥95. Based on this criterion, no conflict was detected and therefore the nuLSU and mtSSU datasets were concatenated. It must be noted that relationships within the Placopsis clade in the nuLSU tree were poorly resolved, probably due to several short sequences (often shorter than 400 bp) published by Schneider et al. (Reference Schneider, Resl and Spribille2016).
Bayesian analyses were carried out on the two-locus dataset under the selected models for two partitions (nuLSU and mtSSU) and using the same settings as for the analyses for topological incongruence among loci. In addition, a Maximum Likelihood (ML) analysis was performed on the two-locus dataset using GARLI (Zwickl Reference Zwickl2006, v. 0.951 for Mac OSX) with default settings. We used 1000 bootstrap pseudoreplicates to calculate a majority-rule consensus tree in PAUP* 4.0b10 (Swofford Reference Swofford2002) to assess the Maximum Likelihood bootstrap values (ML-bs).
The Bayesian tree did not contradict the ML trees topology for the strongly supported branches. Therefore, only the majority-rule consensus tree of the Bayesian analysis is shown, with the posterior probabilities of the Bayesian analysis added above the internal branches and the bootstrap support values of the ML analysis added below the internal branches (Fig. 1). ML-bs≥70% and PP≥95 were considered to be significant. Phylogenetic trees were visualized using FigTree v.1.4.2 (Rambaut Reference Rambaut2012).
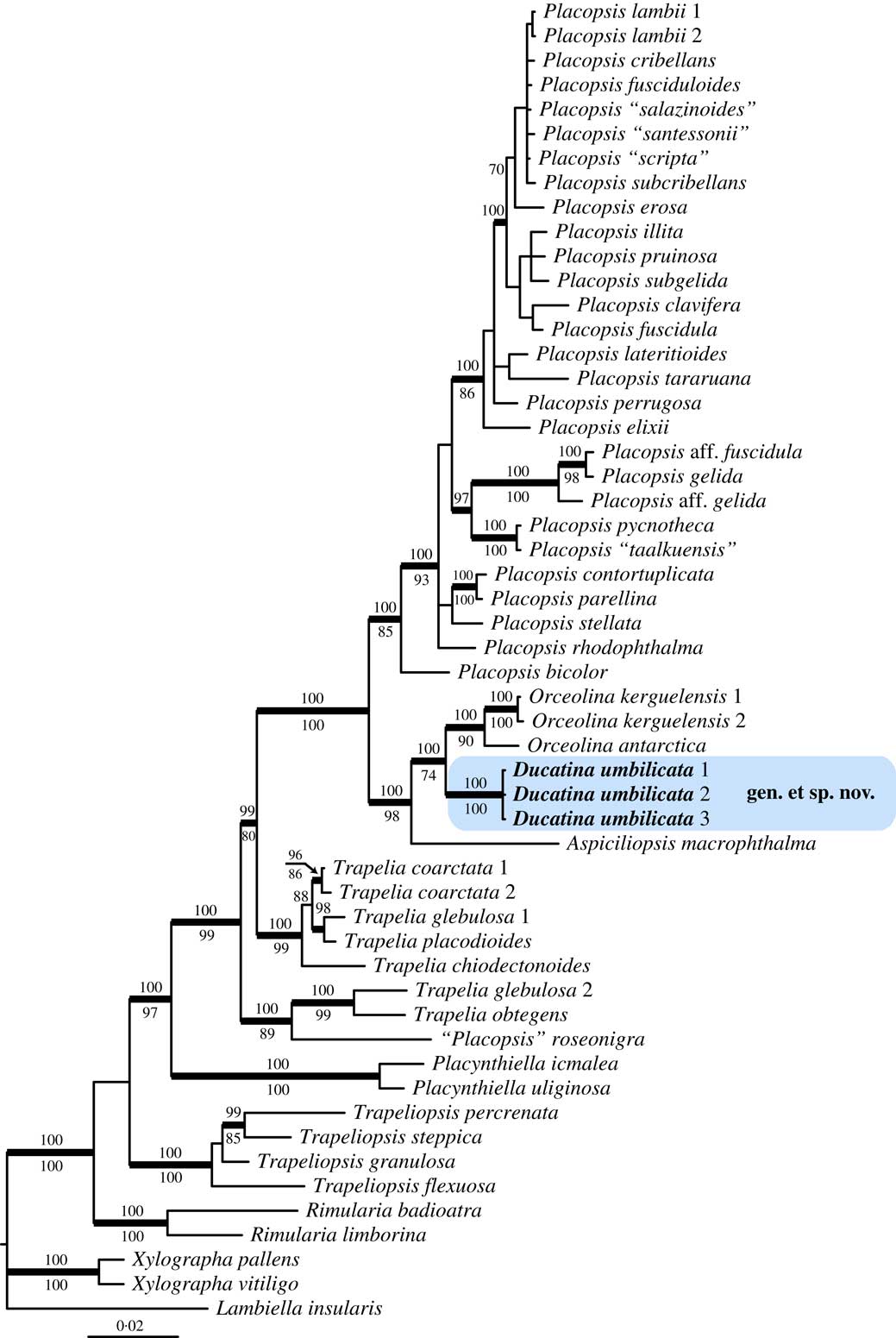
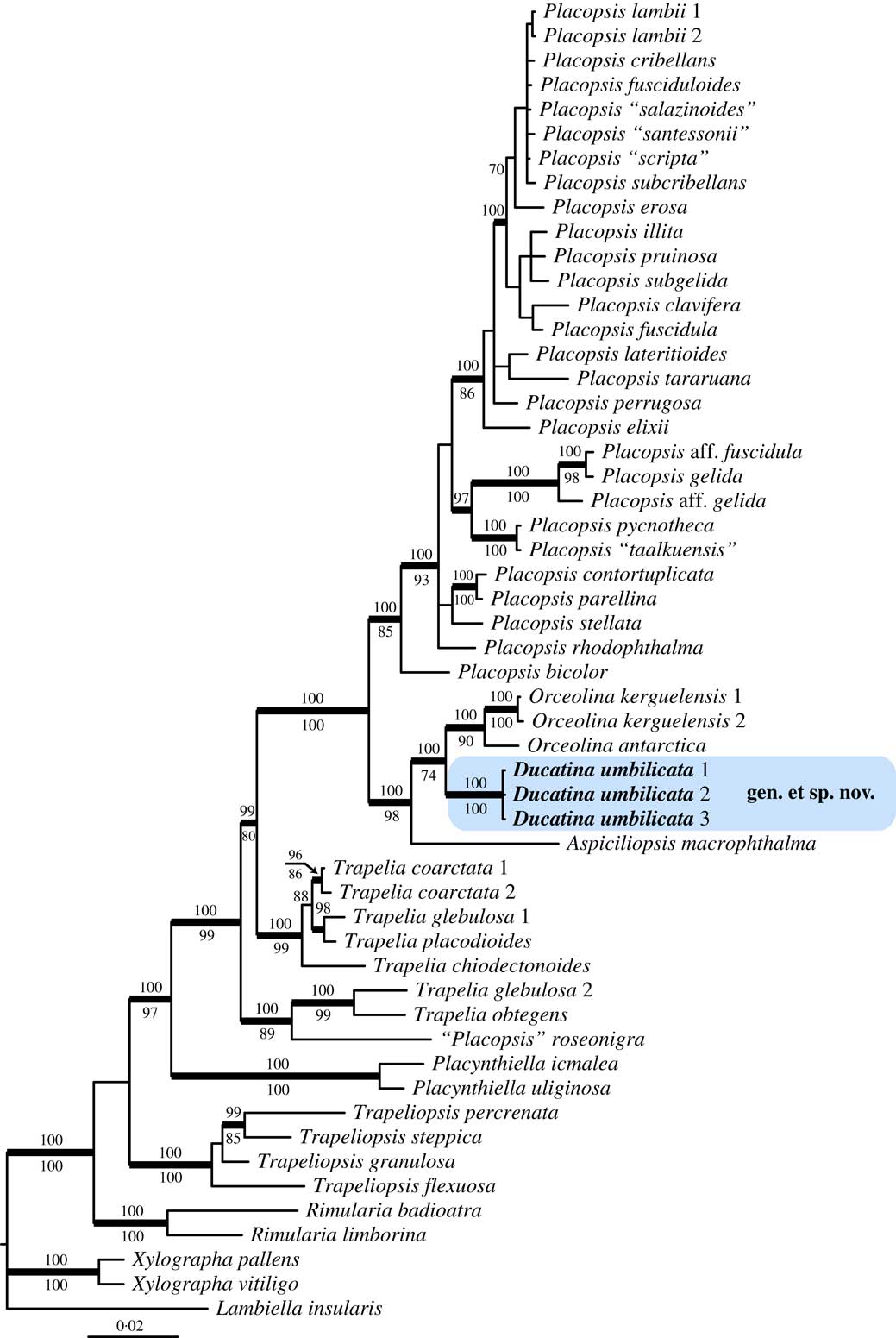
Fig. 1 Phylogenetic relationships within Trapeliaceae based on nuLSU and mtSSU sequences using Bayesian analysis. Posterior probabilities≥95 are shown above internal branches, and Maximum Likelihood bootstrap values≥70 shown below internal branches. Internal branches considered strongly supported by both analyses are represented by thicker lines. The three newly sequenced samples corresponding to the new taxon, Ducatina umbilicata, are highlighted.
Results
The combined two-locus dataset consisted of 54 specimens and 1571 unambiguously aligned sites, 911 sites for nuLSU and 660 for mtSSU. The majority-rule consensus tree of the Bayesian analysis is shown with the posterior probabilities of the Bayesian analysis added above the internal branches (Fig. 1). The genus Trapelia is paraphyletic with Placopsis nested within Trapelia, as already shown in previous studies (Kantvilas et al. Reference Kantvilas, Leavitt, Elix and Lumbsch2014; Resl et al. Reference Resl, Schneider, Westberg, Printzen, Palice, Thor, Fryday, Mayrhofer and Spribille2015; Schneider et al. Reference Schneider, Resl and Spribille2016). The clade Aspiciliopsis to Placopsis is strongly supported (PP=1) and divided into two main clades corresponding to Placopsis s. str. and a lineage including the new taxon and three species referred to here as Aspiciliopsis macrophthalma (Hook. f. & Taylor) B. de Lesd., Orceolina antarctica (Müll. Arg.) R. S. Poulsen & Søchting and O. kerguelensis (Tuck.) Hertel.
The New Taxa
Ducatina umbilicata Ertz & Søchting gen. et sp. nov.
MycoBank No.: MB 817845 (genus) and MB 817846 (species)
Taxon belonging to the Trapeliaceae and phylogenetically related to Aspiciliopsis macrophthalma, Orceolina antarctica and Orceolina kerguelensis but differing by the combination of the following characters: an umbilicate thallus, the presence of cephalodia and different unknown chemical secondary compounds.Type: Crozet Islands, Île de la Possession, partie amont de la rivière de la crique de la Chaloupe, lieu-dit ‘Le Donjon’, au nord-ouest de la base Alfred Faure, le long du transit vers la Baie Américaine, 46°25'01''S, 51°50'15''E, c. 200 m, grosse dalle rocheuse, 23 November 2015, D. Ertz 20595 (BR—holotype!; REN, PC—isotypes!).

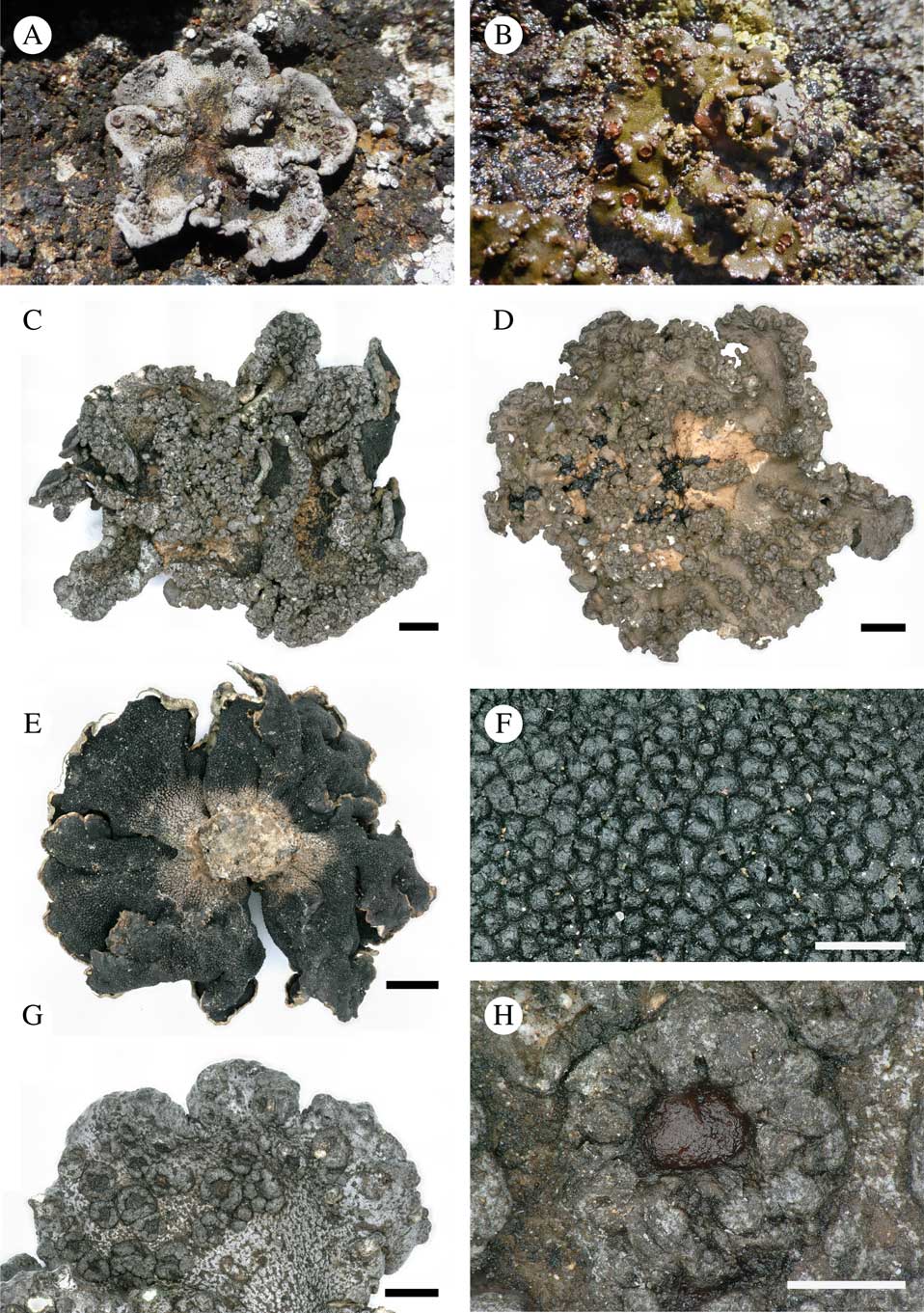
Fig. 2 Habitat of Ducatina umbilicata on Île de la Possession. In colour online.

Fig. 3 Ducatina umbilicata. A–D, upper surface of thallus showing the variability of the species, from rather smooth to strongly verrucose thalli; A, abundantly fertile dry thallus (field observation); B, damp fertile thallus (field observation); C, strongly verrucose thallus (holotype); D, verrucose thallus with clearly visible pale brown cephalodia in the centre of the thallus (Ertz 20572). E & F, lower surface of thallus; E, holotype; F, showing the black verrucae in detail (Ertz 20671); G, abundantly fertile thallus lobe (holotype); H, detail of one apothecium (holotype). Scales: C–E=2 mm; F & H=500 µm; G=1 mm. In colour online.
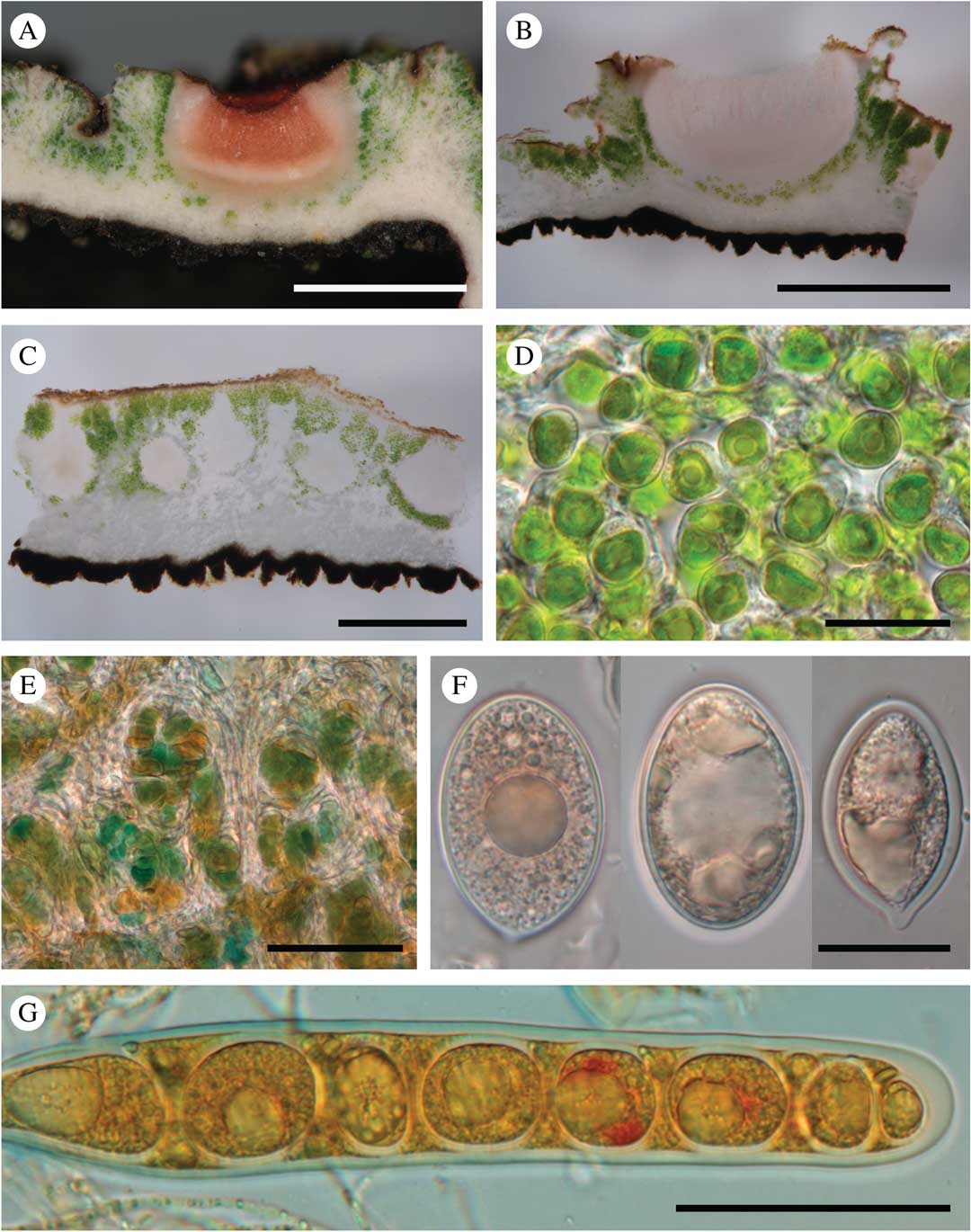
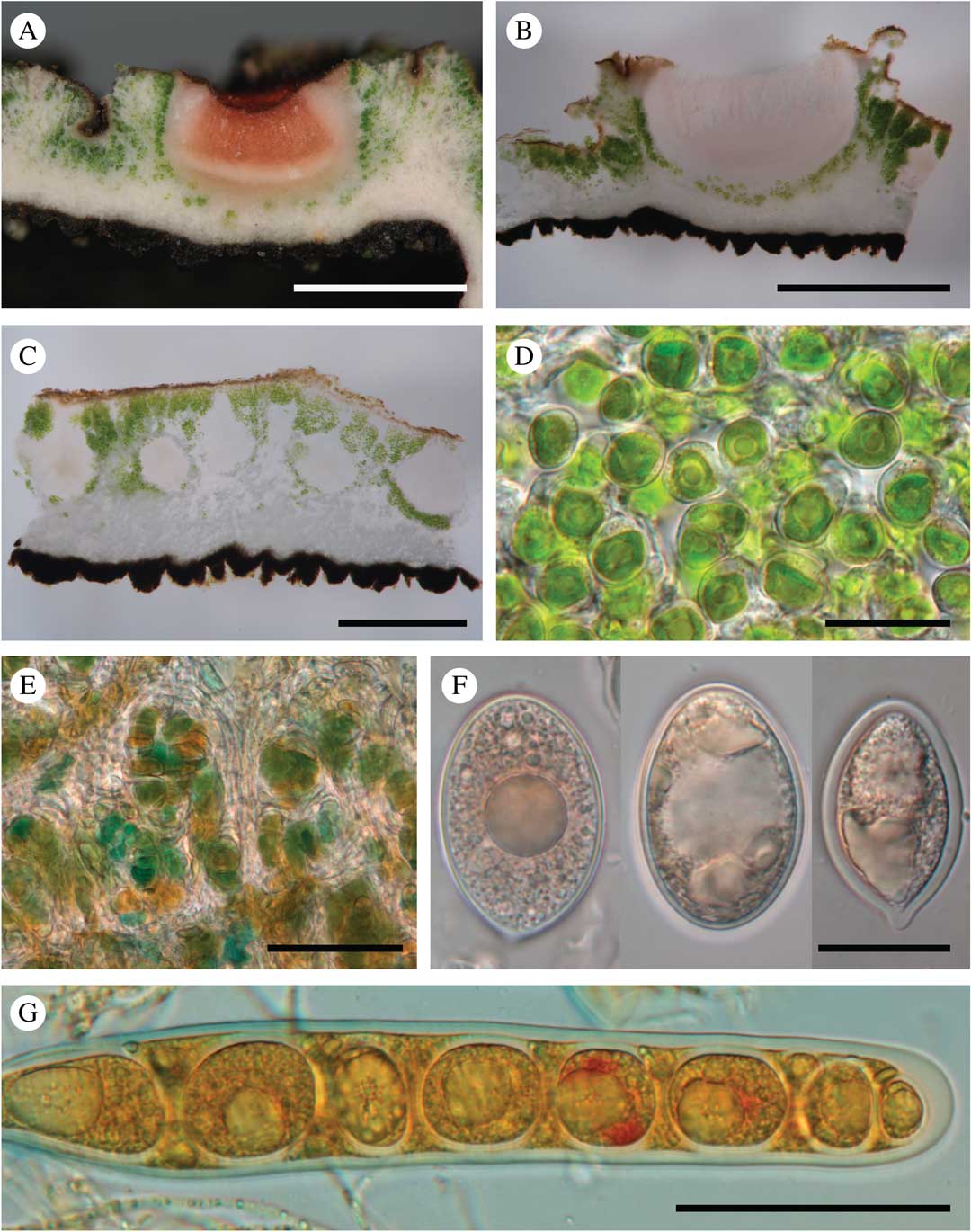
Fig. 4 Ducatina umbilicata. A & B, section through an ascoma (Ertz 20671); C, section through the thallus showing several pycnidia (Ertz 20671); D, chlorococcoid photobiont of the thallus (Ertz 20671); E, cyanobacteria of the cephalodia (holotype); F, ascospores (Ertz 20671, left; Ertz 20594, right two); G, ascus with ascospores (Ertz 20671). Scales: A & B=1 mm; C=0·5 mm; D & F=20 µm; E & G=50 μm. In colour online.

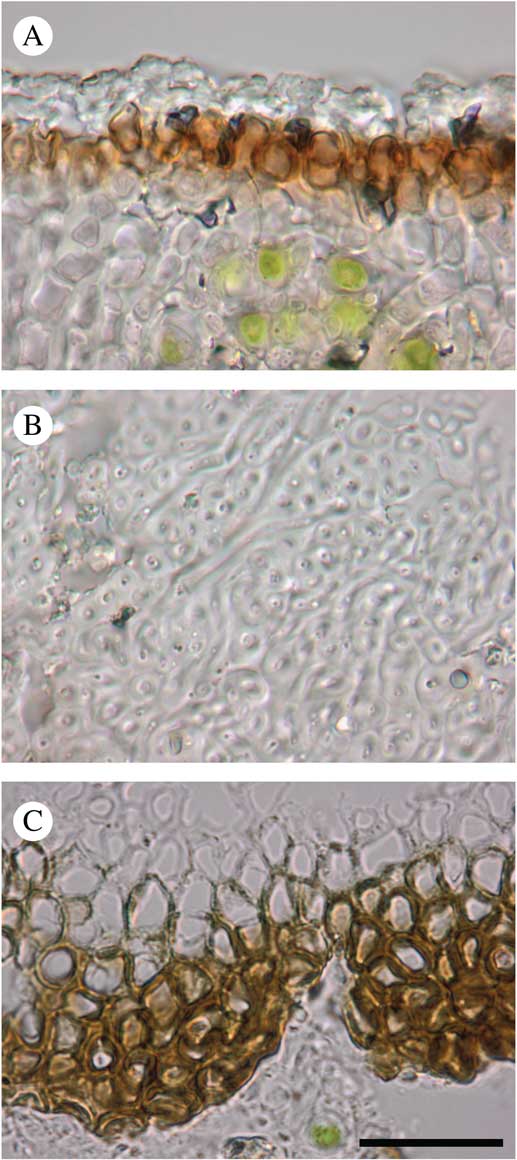
Fig. 5 Ducatina umbilicata sections (holotype). A, upper cortex; B, medulla; C, lower cortex. Scale=20 μm. In colour online.
Thallus up to 2 cm diam., 0·17–0·60(–1·00) mm thick, stout, umbilicate, irregularly incised up to c. 2/3 of the thallus length in lobes; lobes ascending, radiating in one layer from the centre to periphery, rarely with secondary smaller lobes, undulating, concave or flat, occasionally folded, c. 0·5–1·5 cm wide, usually contiguous to somewhat overlapping; margin entire, often with the outermost edge slightly recurved; upper surface greyish to dark olivaceous when dry, epruinose, green-olivaceous when wet, esorediate, smooth or frequently with subglobose to globose, smooth, solid, stout verrucae, 0·5–1·0 mm diam., solitary to densely clustered; lower surface uniformly black, except near the holdfast where brownish probably due to the dirty substratum, uniformly and densely rimose-warted, without rhizines. Medulla white, rather dense, 50–200 µm thick; upper cortex c. 10–25 µm, paraplectenchymatous, made of hyaline to dark brown, isodiametric to rectangular cells, c. 3–5 µm diam., covered by an epinecral hyaline layer of c. 3–10 µm; lower cortex formed by triangular warts in cross-section, dark brown to carbonaceous, 45–200 µm wide at base and 40–140 µm tall, paraplectenchymatous, made of isodiametric cells, 3–8 µm diam. or of slightly elongated cells, c. 6–10×4–6 µm diam., with brownish wall c. 1 µm thick. Photobiont chlorococcoid, forming a layer c. 75–375 µm thick, sometimes interrupted at irregular intervals by hyaline vertical hyphal strands c. 8–38 µm thick; individual algal cells globose, 7–10 µm diam. Cephalodia present at the base of the thallus lobes, only visible on the upper surface of the thallus, pale brown, cream or rusty, flat to slightly convex, smooth, immersed in thallus or semi-sessile, 2–3 mm diam.; photobiont filamentous cyanobacteria without a distinct gelatinous sheath, cells usually wider than long, olivaceous-green to orange, 2–9×6–15 µm. Hyphal cells of cephalodia hyaline becoming pale brown near the surface, in cross-section usually elongate, 10–18×3–5 µm, rarely isodiametric, 3–5 µm diam.
Apothecia scattered on the upper surface of the thallus lobes, solitary or groups of 2–5, immersed to half-sessile, rounded, 0·5–1·0 mm diam.; disc shallowly concave to plane, epruinose, pale to dark red-brown when dry. Thalline margin prominent, persistent, concolorous with thallus, smooth, often with 2–6 transversal cracks to crenate, 0·15–0·30 mm thick. Proper excipulum hyaline, laterally 50–75 µm thick, prosoplectenchymatous (±textura porrecta) with cells 5–15×2–4 µm; basally 45–100 µm thick and paraplectenchymatous with cells of 4–7 µm diam. Hymenium hyaline (thin sections) to pinkish (thick sections), 290–375 µm. Epihymenium poorly differentiated, yellowish to pale brown, c. 5–10 µm. Paraphyses abundant, sparsely branched with few anastomoses, except in upper part of the hymenium where paraphyses are more branched-anastomosing, 1·5–2·0 µm thick, not or slightly enlarged at the apex, 3 µm. Subhymenium hyaline, c. 100 µm thick. Asci narrowly cylindrical, 8-spored (uniseriate; sometimes 1–2 ascospores degenerated), 205–225×26–28 µm, wall not or slightly thickened at the apex. Ascospores ellipsoid to subglobose, sometimes with a more acute to mucronate end, hyaline, thick-walled, (23·0–)29·5–38·0(–42·0)×(12–)17–23(–25)µm (n=32), without gelatinous sheath.
Pycnidia immersed in the thallus, pyriform, with a hyaline wall of textura intricata, c. 325–375×200–275 µm. Conidiogenous cells ampulliform, hyaline, 16–20×1·5–2·0 µm. Conidia filiform, straight, simple, hyaline, 11–17×1 µm.
Chemistry
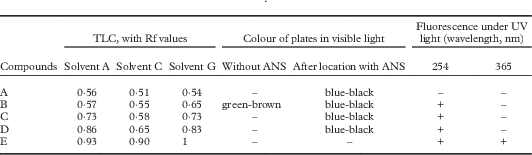
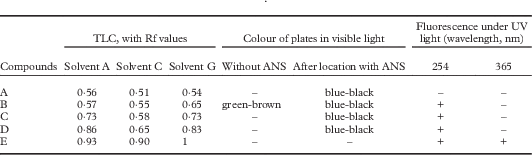
Thallus surface K−, C−, KC−, PD−, UV−; medulla K+ pale yellow, C−, KC−, PD−, UV+ pale yellow. Medulla, cortex, excipulum, ascospores I−, KI−. Hymenium and subhymenium I+ red or I+ pale blue turning quickly red, KI+ dark blue. Ascus wall KI−, except for a thin KI+ blue layer on its surface. TLC revealed several substances (Table 2). The molecular mass of the main substance (compound B in Table 2) was determined by DART-MS as being 441·3224, with C28H43NO3 as proposed formula. This substance might be attributed to steroids, possibly N-methylbuxene, a metabolite not yet recorded from lichens, but a confirmation by nuclear magnetic resonance (NMR) is needed.
Table 2 Characterization of the extrolites of Ducatina umbilicata
Etymology
The generic name is derived from ‘ducatus’ and refers to the thallus morphology being reminiscent of a weathered metal coin (with the idea that it was hidden on remote islands by some unknown pirates). The specific epithet refers to the umbilicate thallus.
Distribution and ecology
Known from Île de la Possession (Crozet) where it is widespread and abundant on exposed horizontal rock surfaces at low altitude (Fig. 2), and from Îles Kerguelen where it is known from only two localities on Peninsule Courbet and Île Foch. In both localities on Îles Kerguelen the habitat is boulder-dominated, ±vegetation-free polygonal soils on an exposed alpine plateau c. 600 m a.s.l. where it grows on basaltic rock in the ±protected crevices between boulders.
Additional specimens examined. Îles Crozet: Île de la Possession: Partie supérieure du versant gauche de la Rivière du Camp, au nord de la base Alfred Faure, le long du transit vers la Baie Américaine, 46°25'14''S, 51°50'59''E, c. 150 m, grosse dalle rocheuse près du niveau du sol, 2015, D. Ertz 20594 (BR); ibid., près de la Pointe du Bougainville, 46°26'22''S, 51°50'58''E, c. 150 m, rocher exposé dans un petit vallon, 2015, D. Ertz 20572 (BR); ibid., crête rocheuse située juste au nord de la Baie Américaine et à l’ouest du Rocher Pyramidal, lieu-dit ‘Crête Alouette’, dalle rocheuse, 2012, D. Ertz 17785 (BR); ibid., 46°22'56''S, 51°48'04''E, c. 120 m, rocher, 2015, D. Ertz 20671 (BR).—Îles Kerguelen: Peninsule Courbet: Mt. du Chateau, c. 500 m NE of Les Créneaux, 49°14'6''S, 70°08'0''E, c. 640 m, on basaltic rock on plateau, 1998, R. S. Poulsen 446a (C) and U. Søchting 9492 (C). Île Foch: c. 200 m NE of summit of Mt. R. Bureau, 48°58'5''S, 69°20'4''E, c. 610 m, on basaltic rock on plateau, 1999, R. S. Poulsen 916 (C).
Discussion
Ducatina umbilicata is characterized by its umbilicate thallus having a black verrucose lower surface and cannot be confused with any other species of Trapeliaceae. Other lichen genera with an umbilicate growth form and a chlorococcoid photobiont such as Dermatocarpon Eschw., Glypholecia Nyl., Lasallia Mérat, Omphalora T. H. Nash & Hafellner, Rhizoplaca Zopf and Umbilicaria Hoffm. are phylogenetically unrelated, and usually have different fruiting bodies and no cephalodia. Because the umbilicate growth form is dispersed over the taxonomic system, including different classes and orders, it has evolved independently multiple times, implying that this thallus shape has an intrinsic adaptive value. Shift in growth form has been shown to be involved in adaptive radiation in various groups, such as angiosperms (e.g. Agrawal et al. Reference Agrawal, Fishbein, Halitschke, Hastings, Rabosky and Rasmann2009), and has been suggested in lichen lineages too (e.g. Divakar et al. Reference Divakar, Kauff, Crespo, Leavitt and Lumbsch2013). Ecological drivers that might be responsible for this shift are difficult to assess. The umbilicate growth form is mainly present in various saxicolous habitats, in particular in cold to temperate climates (e.g. Umbilicaria), sometimes in humid-aquatic habitats (e.g. Dermatocarpon), and might have played a role in the colonization of these habitats by lichens. Lichens often grow in dense mixed populations covering most of the rock surface where the thalli compete for light and space (e.g. Pentecost Reference Pentecost1980; Harris Reference Harris1996; Hestmark Reference Hestmark1997). The umbilicate thallus is an intrinsic advantage in interactions with other foliose or crustose lichens. Indeed, such thalli might bend over and overlap the other lichens with their unattached margins, capturing most of the incoming light (Hestmark Reference Hestmark1997). In humid-aquatic habitats, the umbilicate forms might provide a higher competitive value against bryophytes, algal or microbial mats. Moreover, by reducing the contact with the rock surface to a single point corresponding to the central holdfast and elevating the margins above the substratum, the umbilicate forms might reduce the impact of large temperature variations that often occur on exposed rocky surfaces. However, an umbilicate thallus might sometimes have a negative impact on lichen survival. This could be the case on subantarctic islands, where the frequent strong winds might detach thalli not firmly attached to the substratum and increase exposure to the abrasive action of wind-blown mineral particles and ice crystals. This, in combination with other factors, might explain why most of the saxicolous species in this region have a crustose or placodioid thallus. While adaptive radiation has led to species-rich umbilicate genera (e.g. Dermatocarpon, Umbilicaria), Ducatina umbilicata is the only umbilicate species known so far from the family Trapeliaceae, and thus deserves further ecological study.
The generic placement of the new species is problematic because of its phylogenetic position between the genera Aspiciliopsis and Orceolina. Because of the strongly supported sister relationship with Orceolina, a description in that genus was first investigated. However, Orceolina strongly differs by its crustose-effigurate habit, distinct cortex anatomy with very thick cortical layers, orange thallus colour, urceolate apothecia and the lack of cephalodia and secondary chemical compounds (Lumbsch Reference Lumbsch1997; Poulsen et al. Reference Poulsen, Schmitt, Søchting and Lumbsch2001). An enlarged concept of Aspiciliopsis to encompass the new species and Orceolina was also considered. However, although that option would make sense from a biogeographical and phylogenetic point of view, Aspiciliopsis would then be a small, very heterogeneous genus and any chemical or morphological characters would hardly support it. In addition to the differences between Orceolina and the new species, the monotypic genus Aspiciliopsis mainly differs from the latter by a placodioid, pale pinkish grey to grey-white thallus, numerous cephalodia and the gyrophoric acid chemosyndrome. Another option would be to merge Aspiciliopsis macrophthalma, Orceolina and the new species in Placopsis. However, this would render Placopsis morphologically heterogeneous by the inclusion of more distantly related taxa. Due to the strongly deviating anatomical, morphological and chemical characters, Schmitt et al. (Reference Schmitt, Lumbsch and Søchting2003) decided to keep Orceolina separate, a position that is followed here. Since attempts at emending the genera Aspiciliopsis, Orceolina and Placopsis to include the new taxon are problematic, we prefer to describe a new genus. This solution will also let the very peculiar combination of morphological and chemical characters of this stunning member of Trapeliaceae be highlighted. It is remarkable that the clade consisting of Aspiciliopsis, Ducatina and Orceolina includes as much thallus variation as the rest of the family combined.
Interestingly, the clade Aspiciliopsis-Orceolina includes only austral species (Fig. 1). Ducatina umbilicata and Orceolina antarctica are known only from Îles Crozet and Îles Kerguelen, O. kerguelensis has also been reported from Marion, Prince Edward and Heard Islands (Poulsen et al. Reference Poulsen, Schmitt, Søchting and Lumbsch2001) while Aspiciliopsis macrophthalma is widely distributed in the Southern Hemisphere (Galloway Reference Galloway2013). This contrasts with the genus Placopsis, sister to the clade Aspiciliopsis-Orceolina (Fig. 1), where an adaptive radiation has led to more than 60 recognized species (Schneider et al. Reference Schneider, Resl and Spribille2016), including some extending to the Northern Hemisphere. The subclade Ducatina-Orceolina even includes taxa known only from the subantarctic Indian Ocean, pointing to a possible origin of the lineage in this region. In relation to that, it must be noted that Îles Kerguelen is considered to be of very old volcanic origin compared to other oceanic islands, possibly with rocks up to 40 million years old (Giret et al. Reference Giret, Grégoire, Cottin and Michon1997; Wallace et al. Reference Wallace, Frey, Weis and Coffin2002).
Ducatina is one of the few genera of Trapeliaceae to have cephalodia. This symbiotic association with two different photobionts (green algae and cyanobacteria) is a characteristic of Aspiciliopsis, Ducatina and Placopsis within the Trapeliaceae, while cephalodia are lacking in the related genera Orceolina and Trapelia. Placopsis roseonigra Brodo was recently shown to be closely related to Trapelia by Schneider et al. (Reference Schneider, Resl and Spribille2016), but that species does not have real cephalodia but instead only a cyanobacterial bed associated with the vegetative areoles, named ‘paracephalodia’ (Poelt & Mayrhofer Reference Poelt and Mayrhofer1988; Brodo Reference Brodo1995). Because cyanobacteria possess the ability to fix nitrogen from the air, and the frequency of heterocysts is higher in tripartite lichens than in bipartite lichens (Rai et al. Reference Rai, Söderbäck and Bergman2000), presence of cephalodia is a great advantage for lichen growth, in particular for early colonizers of nitrogen-poor substrata such as bare rock or soil (e.g. Raggio et al. Reference Raggio, Green, Crittenden, Pintado, Vivas, Pérez-Ortega, De los Ríos and Sancho2012). For instance, Placopsis perrugosa has the highest annual growth rate for both crustose and placodial lichens (Sancho et al. Reference Sancho, Palacios, Green, Vivas and Pintado2011). It is therefore surprising that, in contrast to Aspiciliopsis, Ducatina and Placopsis, cephalodia are lacking in Orceolina, members of which are growing on nutrient-poor rocks of fell-fields. The discovery of Ducatina supports the idea that the absence of cephalodia in Orceolina is a secondary loss.
This research is supported by the French Polar Institute (Institut Polaire Français Paul-Émile Victor) within the context of the program 136 ‘Subanteco’. We are indebted to Toby Spribille and an anonymous referee for their constructive suggestions. We thank Aude Boutet, Julien Tomasino and Tiphaine Lefebvre, civil volunteers who helped with data collection during field trips. RSP and US thank Yves Frenot for arranging the field trip to Crozet and Kerguelen Islands in 1998. We wish to thank very warmly Cyrille Gerstmans, Myriam Dehaan and Wim Baert for their help with the molecular work and other technical parts of the manuscript. AG thanks Solenn Ferron, Anne-Cécile Le Lamer and Francoise Le Dévéhat who helped with the chemistry analysis. DART-MS analyses were performed by Tiffanie Ziajjko in the Centre Régional de Mesures Physique de l’Ouest (CRMPO). DE was financially supported by the Fonds National de la Recherche Scientifique, Belgium (FNRS) to visit the Crozet archipelago.