Introduction
Cetraria aculeata (Schreb.) Fr. s. l. (including C. steppae (Savicz) Kärnef.) (Parmeliaceae, Lecanoromycetes) is a widespread, terricolous, fruticose lichen adapted to arctic, temperate and semi-arid conditions (Printzen et al. Reference Printzen, Domaschke, Fernández-Mendoza and Pérez-Ortega2013). Its taxonomy, biogeography, phylogeny and symbiotic interactions were recently studied (e.g. Fernández-Mendoza et al. Reference Fernández-Mendoza, Domaschke, García, Jordan, Martin and Printzen2011; Printzen et al. Reference Printzen, Fernández-Mendoza, Muggia, Berg and Grube2012; Nadyeina et al. Reference Nadyeina, Lutsak, Blum, Grakhov and Scheidegger2013; Lutsak et al. Reference Lutsak, Fernández-Mendoza, Nadyeina, Şenkardeşler and Printzen2017). Despite the wide distribution of this bipolar lichen (Printzen et al. Reference Printzen, Domaschke, Fernández-Mendoza and Pérez-Ortega2013), data regarding its associated fungi are limited. So far six lichenicolous species have been reported: Clypeococcum cetrariae Hafellner (Khodosovtsev & Darmostuk Reference Khodosovtsev and Darmostuk2017), Endococcus parmeliarum Etayo (Brackel Reference Brackel2015), Katherinomyces cetrariae Khodos., Sphaerellothecium aculeatae Khodos. et al. (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016), Lichenopeltella cetrariicola (Nyl.) R. Sant. (Suija Reference Suija2005; Brackel Reference Brackel2011; Kukwa et al. Reference Kukwa, Kowalewskà, Śliwa, Czarnota, Czyżewska, Flakus, Kubiak, Wilk, Dimos-Zychm and Kolanko2012) and Taeniolella rolfii Diederich & Zhurb. (Zhurbenko Reference Zhurbenko2009).
In Southern Ukraine, Cetraria aculeata s. l. is one of the most frequent components of psammophytic lichen communities, which are indicators of deflation processes (Khodosovtsev et al. Reference Khodosovtsev, Boіko, Nadyeina and Khodosovtseva2011). During fieldwork in a sand dune region in the Ukraine, we noted several types of damage (bleaching, turning reddish, etc.) or even death of thalli of Cetraria aculeata caused by various lichenicolous fungi. The spread of fungal infections was especially evident in wet seasons, while in dry seasons we did not observe any symptoms. We previously reported three species from Ukrainian sand dunes, viz. Clypeococcum cetrariae, Katherinomyces cetrariae and Sphaerellothecium aculeatae (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016; Khodosovtsev & Darmostuk Reference Khodosovtsev and Darmostuk2017), two of which were described as new. Yet in different parts of the sand dunes of Lower Dnipro, an additional coelomycetous fungus with large hyaline aseptate conidia was collected that at first glance resembled Didymocyrtis foliaceiphila (Diederich et al.) Ertz & Diederich. Both the morphology and ITS sequences of this novel fungus were used to clarify its phylogeny and it is here described as a new species. We also describe as new the genus Katherinomyces, present additional records of lichenicolous species growing on C. aculeata found during the study and provide an identification key to 11 lichenicolous fungi known to grow on Cetraria aculeata.
Materials and Methods
Sampling and cultures
Ninety specimens of Cetraria aculeata were collected in different habitats in the Black Sea lowland (Ukraine). During collections taking place in the the Lower Dnepr sand dunes between 2015 and 2017, we found the largest concentration of lichenicolous fungi on Cetraria aculeata. 28 infected specimens were examined in detail using standard light microscopy techniques under LOMO microscopes MBS–1 and MICROMED–2. Microscopical examination was performed on material mounted in water; 10% KOH (K), Lugol’s iodine (I) either without or after a KOH pretreatment (K/I), or Brilliant Cresyl blue (BCr). Measurements were made in water with a precision of 0·1 μm for microscopical structures and 5 μm for ascomata, basidiomata and pycnidia. Measurements are given as
![]() $$\left( {{\rm min}-} \right)\bar{x}-{\rm SD}-\bar{x}{\plus}{\rm SD}\left( {-{\rm max}} \right)$$
, where
$$\left( {{\rm min}-} \right)\bar{x}-{\rm SD}-\bar{x}{\plus}{\rm SD}\left( {-{\rm max}} \right)$$
, where
![]() $$\bar{x}$$
is the mean and SD is the standard deviation. Photographs were taken with a Levenhuk C510 NG camera. All specimens examined are deposited in the herbaria of Kherson State University, Ukraine (KHER) and the Natural History Museum of the University of Tartu (TU).
$$\bar{x}$$
is the mean and SD is the standard deviation. Photographs were taken with a Levenhuk C510 NG camera. All specimens examined are deposited in the herbaria of Kherson State University, Ukraine (KHER) and the Natural History Museum of the University of Tartu (TU).
DNA extraction, amplification and sequencing
For the extraction of genomic DNA, three to five conidiomata per specimen of the unknown Didymocyrtis were removed from the host thallus and placed into a 1·5 ml test tube. Total DNA was extracted using the High Pure PCR Template Preparation Kit (Roche Applied Science®) following the protocol provided by the manufacturer. The internal transcribed spacer (ITS) region was amplified and sequenced using the primers ITS0F and LA-W (Tedersoo et al. Reference Tedersoo, Jairus, Horton, Abarenkov, Suvi, Saar and Kõljalg2008), and ITS4 and ITS5 (White et al. Reference White, Bruns, Lee and Taylor1990). The PCR reaction mix consisted of 5 μl 5× HOT FIREPol® Blend Master Mix (Solis BioDyne, Tartu, Estonia), 0·5 μl of both primers (both 20 μM), 3 μl of target DNA and distilled water added to a total volume of 25 μl. The PCR cycle included 36 cycles and the annealing temperature was set at 57 °C.
PCR products were visualized on a 1% agarose gel stained with ethidium bromide. For the purification of PCR products, 1 μl of FastAP and 0·5 μl of Exonuclease I (Thermo Scientific, Waltham, MA, USA) were added to each tube and the tubes were then incubated at 37 °C for 45 min; the enzymes were deactivated by heating at 85 °C for 15 min. Both complementary strands were sequenced by Macrogen Inc. (Amsterdam, the Netherlands).
We used Sequencher 4.10.1. (GeneCodes Corp.®, Ann Arbor, MI, USA) to check, assemble and manually adjust the resulting sequence fragments. The consensus sequences were compared with those publicly available in NCBI (https://www.ncbi.nlm.nih.gov/genbank) using a BLAST search to confirm their identity. The newly generated sequences are accessible in NCBI under Accession numbers MG519610–MG519614 (Table 1).
Table 1 GenBank Accession numbers, laboratory codes and voucher information (herbarium code, collectors and the collection date) for the newly generated sequences of Didymocyrtis trassii. The ITS barcoding sequence from the isotype is marked in bold

Sequence analysis, alignment and phylogenetic reconstructions
We performed a nucleotide-to-nucleotide comparison and sequence-based clustering including INSD (International Nucleotide Sequence Databases), UNITE and environmental sequences using UNITE Phylogenetic Module massBLASTer in PlutoF workbench (Abarenkov et al. Reference Abarenkov, Tedersoo, Nilsson, Vellak, Saar, Veldre, Parmasto, Prous, Aan and Ots2010) based on the species hypothesis (SH) concept (Kõljalg et al. Reference Kõljalg, Nilsson, Abarenkov, Tedersoo, Taylor, Bahram, Bates, Bruns, Bengtsson-Palme and O’Callaghan2013). To visualize the relationships, the five newly generated ITS sequences were aligned together with 62 Didymocyrtis sequences (Lawrey et al. Reference Lawrey, Diederich, Nelsen, Freebury, Van den Broeck, Sikaroodi and Ertz2012; Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015) derived from the National Center for Biotechnology Information (NCBI) using MUSCLE (Edgar Reference Edgar2004). The sequences were later manually trimmed with SeaView 4.6 (Gouy et al. Reference Gouy, Guindon and Gascuel2010) and thus the final alignment consisted of 574 sites, of which 70 were variable and 59 informative. The best-fit model of DNA evolution for the ITS data set, TrN+I+G, was chosen based on the Akaike Information Criterion (AIC; Akaike Reference Akaike1973) as implemented in jModelTest 2.0.2 (Posada Reference Posada2008). Phylogenetic reconstruction on the resulting alignment was carried out using the Metropolis-coupled Markov chain Monte Carlo (MCMC) approach in MrBayes v.3.2.6. (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) at the CIPRES Science Gateway (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Two parallel simultaneous runs, each using four independent chains and starting from a random tree, were performed over 10 000 000 generations; tree sampling was carried out every 1000th generation. The first 25% of saved data was discarded as burn-in and the 50% majority-rule consensus tree and posterior probabilities (PP) were calculated from the rest. We used Tracer v.1.6.0 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2014) in order to test if the stationarity of log-likelihood values was reached to stable equilibrium. As an alternative, a maximum likelihood (ML) approach was applied to the same data using PhyML (Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010) with the GTR evolutionary model chosen and bootstrap support (BS) calculated over 500 replicates. The phylogenetic tree was visualized and edited using FigTree 1.4.2 (Rambaut Reference Rambaut2014). We used ITSx (Bengtsson-Palme et al. Reference Bengtsson-Palme, Veldre, Ryberg, Hartmann, Branco, Wang, Godhe, Bertrand, De Wit and Sanchez2013) to extract variable ITS1, ITS2 and conserved 5.8S subregions from full rDNA ITS sequences.
Results
The initial SH-clustering settled the newlygenerated sequences into species hypothesis SH527899.07FU (with reference sequence KT383833 representing Didymocyrtis pseudeverniae (Etayo & Diederich) Ertz & Diederich) (https://unite.ut.ee/bl_forw_sh.php? sh_name=SH527899.07FU#fndtn-panel1). The nucleotide-to-nucleotide comparison showed sequence similarity of 98·78–98·95% with constant single nucleotide polymorphisms (SNP) in six positions (Table 2): position 180 (T–G) of ITS1, and positions 39 (T–C), 119 (G–T), 125 (T–C), 144 (indel: T) and 146 (C–T) of ITS2. The analysis of ITS sequences supports a close relationship with D. pseudeverniae (PP=0·99, BS=80; Fig. 2).
Table 2 The results of sequence-based clustering and nucleotide-to-nucleotide comparison. Query is equivalent to the Laboratory code (see Table 1 for voucher information). The assignment to species hypothesis is indicated by reference sequence (Ref. seq.; indicated by NCBI code) and UNITE SH-code (Kõljalg et al. Reference Kõljalg, Nilsson, Abarenkov, Tedersoo, Taylor, Bahram, Bates, Bruns, Bengtsson-Palme and O’Callaghan2013). The similarity percentage (Prcnt), number of mismatched nucleotide positions (MisM), and the lengths of the Query (Qend) and Reference sequences (Rend) are provided

We found that both taxa have relatively long and multiguttulate conidia in contrast to other Didymocyrtis species, which have smaller conidia typically with 1–2 apical guttules (Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015). For comparison we examined a single specimen of D. pseudeverniae (TU75667) and found that conidia, similar to those of specimens growing on Cetraria aculeata, are surrounded by a thin halo (c. 1 μm; observed in BCr). Besides the host choice (Pseudevernia vs. Cetraria), we found that pycnidia and conidia were smaller in specimens growing on C. aculeata compared to those of D. pseudeverniae: pycnidia are 60–110 μm vs. 130–170 μm (Etayo & Diederich Reference Etayo and Diederich1996), conidia 12·0–20·5×4·2–8·5 μm vs. 14–26×6–9 μm (Etayo & Diederich Reference Etayo and Diederich1996). Didymocyrtis foliaceiphila is sister to both (PP=0·95, BS=86; Fig. 2) but conidia of this species are biguttulate and much smaller, (5·0–)5·8–7·1(–7·5)×(2·0–)2·2–2·7(–3·0) μm (Diederich et al. Reference Diederich, Kocourková, Etayo and Zhurbenko2007).
Thus, taking into account the differences in morphology and ITS sequences, as well as the specialization of the host lichen with a different ecology, we describe a new lichen-inhabiting species growing on C. aculeata.
Taxonomy
Didymocyrtis trassii Suija, Darmostuk & Khodos. sp. nov.
MycoBank No.: MB 823931
Lichenicolous asexual fungus on Cetraria aculeata, similar to Didymocyrtis pseudeverniae in having large, obpyriform to clavate, multiguttulate and halonate conidia but differing by its smaller pycnidia, (60–)70–100(–110) μm diam., and smaller conidia (12·0–)14·2–18·2(–20·5)×(4·2–)5·0–7·2(–8·3) μm.
Type: Ukraine, Kherson Oblast, Goloprystansky District, way between villages Burkuty and Promin, 46°21'53·1"N, 32°46'22"E, alt. 42 m, on Cetraria aculeata, in sand dunes, 21 November 2015, A. Khodosovtsev (KHER 9327—holotype; KHER 9326, 9325, TU84808—isotypes; ITS barcoding sequence Accession MG519611).
(Fig. 1)
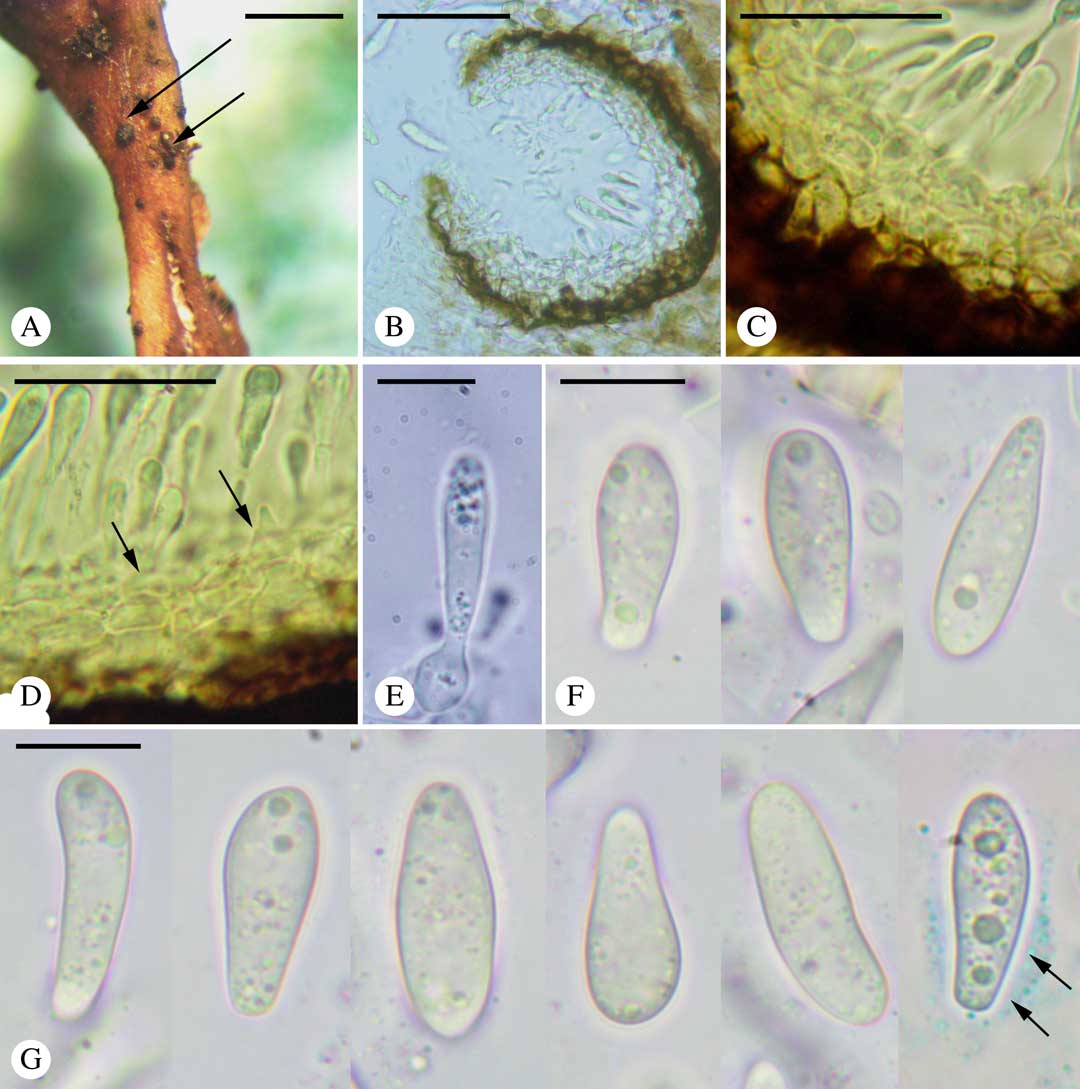
Fig. 1 Didymocyrtis trassii Suija, Darmostuk & Khodos. A, conidiomata (arrows) on the thallus of Cetraria aculeata; B, section through a conidioma; C, conidiomatal wall; D, conidiogenous cells (arrows); E, conidium with conidiogenous cell; F & G, conidia in water (last one in BCr with visible halo (arrows)). Scales: A=1 mm; B=50 μm; C & D=25 μm; E–G=10 μm. In colour online.
Ascomata unknown. Conidiomata pycnidial, Phoma-type. Vegetative hyphae light brown, immersed in the host thallus, 1–2 μm thick.

Fig. 2 Internal transcribed spacer (ITS)-based 50% majority-rule unrooted consensus tree based on a Bayesian approach and showing relationships between Didymocyrtis trassii and other Didymocyrtis species. The NCBI Accession numbers are given after each species, posterior probabilities (PP)≥0·95 (before the slash) and Bootstrap support (BS)≥75 (after the slash) are given on the branches.
Pycnidia scattered, semi-immersed, black, subglobose, ostiolate, (60–)70–100(–110) μm diam. (n=15); wall 25–35 μm thick, composed of 3–4 external brownish layers and 2–3 internal hyaline layers; cells pseudoparenchymatous, thick-walled, (3·5–)3·5–5·5(–7·5) μm (n=20); brown pigment turning olivaceous black in K. Conidiophores absent. Conidiogenous cells ampulliform, aseptate, hyaline, smooth-walled, (5·0–)6·4–9·2(–10·0)×(3·0–)3·5–5·5(–6·0) μm (n=20). Conidia holoblastic, obpyriform to clavate, straight or slightly curved, usually narrow in the lower part, hyaline, smooth-walled, halonate, apex rounded, base rounded to abruptly truncate, multiguttulate, (12·0–)14·2–18·2(–20·5)×(4·2–)5·0–7·2(–8·3) μm (n=100), halo up to 2 μm thick, length/width (l/w) ratio (1·8–)2·2–3·2(–3·9).
Etymology. The epithet honours the late Estonian lichenologist and botanist Hans-Voldemar Trass (2 May 1928–14 February 2017).
Host and pathogenicity. The fungus is known only on Cetraria aculeata. According to Ertz et al. (Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015), the lichenicolous species of Didymocyrtis may be host-specific (e.g. D. pseudeverniae, D. xanthomendozae) or not (e.g. D. consimilis, D. cladoniicola, D. foliaceiphila, D. melanelixiae). The new species seems to belong to the former group. Didymocyrtis trassii usually grows on the lower branches of thick cushions of C. aculeata on sand and does not cause any visible damage to the host thallus.
Distribution. Didymocyrtis trassii is known only from sand dunes in Southern Ukraine.
Notes. Some basally abruptly truncate conidia of D. trassii resemble conidia of Abrothallus, but the conidiogenous cells in this genus show one to three annellations and a refractive periclinal rim at the base of the conidia (Hawksworth Reference Hawksworth1981).
Recently, Ertz et al. (Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015) showed that some Polycoccum species represent the sexual stage of Didymocyrtis. There are two Polycoccum species described from Parmeliaceae, viz. Polycoccum montis-wilhelmii Diederich on Hypotrachyna (Aptroot et al. Reference Aptroot, Diederich, Sérusiaux and Sipman1997) and P. crespoae Váczi & D. Hawksw. on Chondropsis (Váczi & Hawksworth Reference Váczi and Hawksworth2001). Both species are known from the Southern Hemisphere and their asexual stages are not yet known.
Specimens examined (all on Cetraria aculeata on sand dunes). Ukraine: Kherson Oblast: Goloprystansky District, Chalbas’ka arena, village of Burkuty, 46°22'02·9"N, 32°46'29·7"E, alt. 26 m, 9 iv 2008, A. Khodosovtsev (KHER 8734); 46°23'38·6"N, 32°48'35·7"E, alt. 13 m, 18 xi 2016, A. Khodosovtsev, V. Darmostuk (KHER 10329, 10330, 10675; TU84809); ibid., 28 iv 2017, A. Khodosovtsev (KHER 10765; TU84813); village of Gladkovka, 46°25'14·3"N, 32°35'38·8"E, alt. 15 m, 15 vi 2017, A. Khodosovtsev (KHER 10672; TU84810); village of Ivanivka, Botanical Reserve “Khrestova Saga”, 46°24'37·2"N, 32°04'59·9"E, alt. 11 m, 25 vi 2017, V. Darmostuk (KHER 10673; TU84812); Black Sea Biosphere Reserve, Solonoozerny, 46°27'33"N, 31°57'38"E, 6 v 2017, A. Khodosovtsev, V. Darmostuk (KHER 10674; TU84811); Oleshkivsky District, village of Radensk, 46°20'56·2"N, 32°34'19·5"E, alt. 22 m, 1 xi 2015, A. Khodosovtsev (KHER 8734); Kozachelagerska arena, 46°37'04·7"N, 32°58'12·4"E, alt. 23 m, pine forest, 5 iv 2008, A. Khodosovtsev (KHER 3665).
Specimen of Didymocyrtis pseudeverniae examined. Norway: Nord-Trøndelag County: Steinkjer municipality, Mokk, 64°02'27.6"N, 12°29'51.7"E, alt. 354 m., on Pseudevernia furfuracea, 4 viii 2015, A. Suija (TU75667).
Other lichenicolous species on Cetraria aculeata collected in sand dunes in Southern Ukraine
Acremonium lichenicola Gams s. lat.
Notes. Specimen KHER 10286 is characterized by conidiogenous cells 30–50 μm long, absent or very short unbranched conidiophores c. 2–5 μm long, and 0(–1) septate conidia (4·1–)5·1–7·3(–9·3)×(1·5–)1·8–2·8(–3·0) μm (n=40). The relative frequency of 1-septate conidia was 6%. As lichenicolous species of Acremonium are in need of revision involving molecular data, we use the name A. lichenicola s. l. here. Cetraria aculeata is a new host for A. lichenicola.
Specimens examined. Ukraine: Kherson Oblast: Oleschkivsky District, landscape reserve “Sagi”, 46°37'04·03"N, 32°50'03·13"E, alt. 15 m, 5 x 2016, A. Khodosovtsev, V. Darmostuk (KHER 10286, 10288); village of Radensk, 46°33'57·1"N, 32°52'40.2"E, alt. 32 m, 20 v 2016, A. Khodosovtsev (KHER 9763).
Clypeococcum cetrariae Hafellner
Note. This species was recently collected on Cetraria aculeata in Lower Dnipro dunes (Khodosovtsev & Darmostuk Reference Khodosovtsev and Darmostuk2017). The fungus is recognizable by its black necrotic spots covered by numerous pseudothecia.
Didymocyrtis cladoniicola (Diederich, Kocourk. & Etayo) Ertz & Diederich

Fig. 3 A–F, Eonema pyriforme (KHER 9763); A, basidiocarp; B, section of basidiocarp (in water); C, hymenium and subhymenium (in water); D, basidium (in water); E, basidium (in cotton blue); F, basidiospores (in water and cotton blue). G & H, Didymocyrtis cladoniicola (KHER 8735): G, conidiogenous cell (in water); H, conidia (in water). Scales: A=1 mm; B & C=50 μm; D–F & H=10 μm; G=5 μm. In colour online.
Notes. The conidial size of our specimen KHER 10328 (Fig. 3) fits D. cladoniicola: (4·5–)4·9–5·7(–6·3)×(2·4–)2·6–3·2(–3·8) μm (n=30), l/w ratio (1·4–)1·6–2·1(–2·3) on Cetraria aculeata vs. (3·8–)4·7–5·9(–7·3)×(2·0–)2·4–3·0(–3·5) μm, l/w ratio (1·4–)1·7–2·2(–2·8) in the original description on Cladonia species (Diederich et al. Reference Diederich, Kocourková, Etayo and Zhurbenko2007). The species has been reported on Flavoparmelia caperata, Parmelina tiliacea, Ramalina pollinaria, R. polymorpha and Squamarina cartilaginea (Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015). Cetraria aculeata is a new host for this species. The C. aculeata specimen (KHER 10328) infected by D. cladoniicola was found in a lichen community where the fungus was prevalent on Cladonia rangiformis and C. foliacea.
Specimens examined. Ukraine: Kherson Oblast: Goloprystansky District, Black Sea Reserve, Solonoozerny, 29 ii 2008, O. Umanets (KHER 8735); Oleshkivsky District, village of Radensk, 46°33'57·1"N, 32°52'40·2"E, alt. 32 m, 20 xi 2016, V. Darmostuk (KHER 10328).
Eonema pyriforme (M. P. Christ.) Redhead, Lücking & Lawrey
Notes. In addition to the basionym Xenasma pyriforme M. P. Christ., the species was known as Athelia pyriformis (M. P. Christ.) Jülich or Athelidium pyriforme (M. P. Christ.) Oberw. Larsson (Reference Larsson2007) explained the problems of using any of these three names and suggested that an independent generic status be implemented for this resupinate member of Agaricales. This idea was eventually implemented by Lawrey et al. (Reference Lawrey, Lücking, Sipman, Chaves, Redhead, Bungartz, Sikaroodi and Gillevet2009) who introduced the generic name Eonema Redhead et al. We follow the latter nomenclatural concept in the present study.
In our specimen, basidiospores are slightly smaller, (5·3–)5·5–6·5(–7·0)×(3·3–)3·5–4·0(–4·5) μm (n=20), than in the material studied by other authors, cf. 7·0–8·5×3·5–5·0 μm in the type collection (Christiansen Reference Christiansen1960), 7·0–9·5×3·6–5·5 μm (Jülich Reference Jülich1972) and 7–10×4–5 μm (Eriksson & Ryvarden Reference Eriksson and Ryvarden1973). However, all the measurements mentioned originate from Northern European collections (i.e. from a region much narrower than the distribution range of the species) and may not reflect the whole range of variability in the species. Athelia phycophila Jülich, a species believed to be lichenized or associated with mosses, has pyriform spores of a size that better matches our description (5·0–6·5×3·5–4·2 μm) but it has short clavate basidia (13–16 μm long) with short sterigmata (3·5–4·0 μm), typical in Athelia, and is known only from the type locality in Venezuela (Jülich Reference Jülich1972).
Eonema pyriforme has been reported from Belgium, the Czech Republic, Denmark, France, Germany, Italy, Portugal, the Netherlands, Norway, Russia (European part and Siberia), Spain, Sweden, Switzerland, the United Kingdom (Bernicchia & Gorjón Reference Bernicchia and Gorjón2010) and Canada (Lawrey et al. Reference Lawrey, Lücking, Sipman, Chaves, Redhead, Bungartz, Sikaroodi and Gillevet2009) and is here reported from the Ukraine for the first time. The current distribution data may be incomplete due to the rarity on wood, a substratum usually inspected by corticiologists. Instead, E. pyriforme prefers forming fruit bodies on ferns, grasses and herbs (e.g. Arenaria serpyllifolia, Poa annua, Pteridium aquilinum) (Jülich Reference Jülich1972; Lawrey et al. Reference Lawrey, Lücking, Sipman, Chaves, Redhead, Bungartz, Sikaroodi and Gillevet2009). We report E. pyriforme for the first time from lichen thalli. The exact nutrition mode of the species is still unclear but such a broad phylogenetic spectrum of substrata may suggest a saprotrophic lifestyle in forest debris and the use of various vertical supports for more efficient spore dispersal. Eonema pyriforme may therefore be considered a facultative lichenicolous fungus.
Specimen examined. Ukraine: Kherson Oblast: Oleshkivsky District, village of Burkuty, Lake Shelemetske, 46°33'57·1"N, 32°52'40·2"E, alt. 32 m, 20 v 2016, A. Khodosovtsev (KHER 9763).
Katherinomyces cetrariae Khodos. gen. et sp. nov.
MycoBank No.: MB 823932 (genus) and MB 823933 (species)
(= Katherinomyces cetrariae Khodos. nom. inval. (Art. 40.1) in Khodosovtsev et al., Nova Hedwigia 103: 48 (2016)).
Lichenicolous fungus on Cetraria aculeata. Vegetative hyphae light brownish, immersed. Conidiomata stromatic with brownish walls. Conidiophores short, poorly developed, brown, 4–7×3–4 μm. Conidiogenous cells broadly ellipsoid, bacilliform or polygonal, brown, (5·7–)6·3–7·8(–9·0)×(2·8–)3·3–5·1(–5·5) μm. Conidia irregular in shape, bacilliform, broadly ellipsoid, ovoid or rarely polygonal, holoblastic, aseptate, (4·3–)6·6–12·6(–16·3)×(2·8–)3·5–5·2(–6·0) μm.
Type: Ukraine, Kherson Oblast, Goloprystansky District, Chalbas’ka arena, village of Burkuty, alt. 26 m, 46°22′02·9″N, 32°46′29·7″E, 9 April 2008, A. Khodosovtsev (KHER 5461—holotype).
Notes. The names Katherinomyces and K. cetrariae were invalidly published (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016) because the cited MycoBank identifier was that for the genus, while no identifier had been issued for the species (Art. 40.1). Here we validate the names following the new version of the Melbourne Code (McNeill et al. Reference McNeill, Barrie, Buck, Demoulin, Greuter, Hawksworth, Herendeen, Knapp, Marhold and Prado2012). A detailed description and comparison of Katherinomyces with similar genera is provided in Khodosovtsev et al. (Reference Khodosovtsev, Gavrylenko and Klymenko2016). The species is noticeable in the field owing to the reddish coloured branches of the host thallus.
Lichenoconium erodens M. S. Christ. & D. Hawksw.
Note. Lichenoconium erodens is one of the few lichenicolous species having a broad host spectrum (Hawksworth Reference Hawksworth1977). Cetraria aculeata is a new host for this species.
Specimen examined. Ukraine: Mykolayiv Oblast: Ochakovsky District, village of Pokrovka, Regional Landscape Park “Kinburns’ka Kosa”, 46°28'48·4"N, 31°39'55·9"E, alt. 2 m, 18 vii 2016, V. Darmostuk (KHER 10113).
Sphaerellothecium aculeatae Khodos., Klymenko & Gavrylenko
Note. This species was recently described on C. aculeata from Lower Dnipro dunes in the Ukraine (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016). It is more aggressive in wet seasons and causes bleaching and eventually death of the lichen thallus (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016).
Taeniolella rolfii Diederich & Zhurb.
Note. This species was first described on Cetraria nigricans from the Siberian Arctic (Diederich & Zhurbenko Reference Diederich and Zhurbenko1997) but is now known on different Cetraria species (including C. aculeata) from the British Isles, Canada, Finland, Greenland, Mongolia, Poland, Russia, Sweden and the USA (Diederich & Zhurbenko Reference Diederich and Zhurbenko2001; Hawksworth Reference Hawksworth2003; Zhurbenko Reference Zhurbenko2009; Kukwa et al. Reference Kukwa, Czarnota and Perz2010). The specimens studied form gall-like swellings on the tips of C. aculeata similar to lichen soralia. Taeniolella rolfii is reported here as new for the Ukraine.
Specimens examined. Ukraine: Kherson Oblast: Oleshkivsky District, near village of Luch, 46°22'41·7"N, 32°47'10·3"E, alt. 35 m, 16 iv 2017, A. Khodosovtsev (KHER 10911); near village of Burkuty, 46°22'34·1"N, 32°47'40·9"E, alt. 37 m, 25 iv 2017, A. Khodosovtsev (KHER 10912).
Discussion
The widely distributed Cetraria aculeata has been the subject of extensive studies ranging from ecophysiology to phylogeography (review by Printzen et al. Reference Printzen, Domaschke, Fernández-Mendoza and Pérez-Ortega2013). One further aspect for future studies is the severity and frequency of fungal infections of this lichen which can cover large areas of the landscape. We did not find any infected specimens of C. aculeata from carbonaceous substrata in the Black Sea region, while nine lichenicolous species were identified from Lower Dnipro sand dunes. It is possible that this observation reflects the small proportion of thalli in C. aculeata populations in petrophytic grasslands and its relatively large coverage (20–80%) of sand dunes. In total, eleven lichenicolous species are known to grow on C. aculeata (Suija Reference Suija2005; Brackel Reference Brackel2011, Reference Brackel2015; Kukwa et al. Reference Kukwa, Kowalewskà, Śliwa, Czarnota, Czyżewska, Flakus, Kubiak, Wilk, Dimos-Zychm and Kolanko2012) and only two of these, Endococcus parmeliarum and Lichenopeltella cetrariicola, were not found in this study.
The severity of fungal infections is dependent on the infecting species but also on environmental (seasonal) conditions (Gilbert Reference Gilbert1988; Beck et al. Reference Beck, Peršoh and Rambold2014). The most common species in the study area, Sphaerellothecium aculeatae, is also the most aggressive, especially in wet seasons, and causes thallus bleaching and eventually death (Khodosovtsev et al. Reference Khodosovtsev, Gavrylenko and Klymenko2016). Taeniolella rolfii, growing on different Cetraria species (Diederich & Zhurbenko Reference Diederich and Zhurbenko1997), induces sporodochia on the tips of C. aculeata giving a sorediate appearance to the host thallus. Clypeococcum cetrariae, a rare species in the study area, forms blackish, necrotic spots on thallus branches. Infections by Katherinomyces cetrariae are recognizable by their reddish thallus branches. Acremonium lichenicola, Didymocyrtis trassii and Eonema pyriforme, which usually grow on the lower branches of thick cushions of C. aculeata over sand, do not cause visible damage to the lichen thallus. The lack of visible symptoms may be the reason why the newly described species Didymocyrtis trassii has remained unnoticed until recently.

We express our gratitude to Paul Diederich for discussion on the manuscript, Vitaliy Klymenko for photographic assistance and Nataliya Maluga for managing herbarium collections. Rasmus Puusepp (Tartu, Estonia) is thanked for performing lab work. This study was financially supported by the project of Ministry of Science and Education of the Ukraine No. 0116U004735. The contribution by AS was financed by IUT 20-30 and by the European Regional Development Fund (Centre of Excellence EcolChange).







